Key Points
Question
Does combination therapy with niraparib plus pembrolizumab provide any clinical change or safety benefit in patients with advanced or metastatic triple-negative breast cancer?
Findings
Among 47 of 55 patients enrolled in this open-label, single-arm, phase 2 study who were eligible for efficacy evaluation, combination niraparib plus pembrolizumab achieved an objective response rate of 21% and a disease control rate of 49%, with a median duration of response not yet reached.
Meaning
Combination niraparib plus pembrolizumab offers promising antitumor activity in patients with advanced or metastatic triple-negative breast cancer, warranting further investigation.
This open-label phase 2 clinical trial evaluates the clinical activity and safety of combination niraparib and pembrolizumab treatment in women with advanced or metastatic triple-negative breast cancer.
Abstract
Importance
Poly(adenosine diphosphate–ribose) polymerase inhibitor and anti–programmed death receptor-1 inhibitor monotherapy have shown limited clinical activity in patients with advanced triple-negative breast cancer (TNBC).
Objective
To evaluate the clinical activity (primary) and safety (secondary) of combination treatment with niraparib and pembrolizumab in patients with advanced or metastatic TNBC.
Design, Setting, and Participants
This open-label, single-arm, phase 2 study enrolled 55 eligible patients with advanced or metastatic TNBC irrespective of BRCA mutation status or programmed death-ligand 1 (PD-L1) expression at 34 US sites. Data were collected from January 3, 2017, through October 29, 2018, and analyzed from October 29, 2018, through February 27, 2019.
Interventions
Patients were administered 200 mg of oral niraparib once daily in combination with 200 mg of intravenous pembrolizumab on day 1 of each 21-day cycle.
Main Outcomes and Measures
The primary end point was objective response rate (ORR) per the Response Evaluation Criteria in Solid Tumors, version 1.1. Secondary end points were safety, disease control rate (DCR; complete response plus partial response plus stable disease), duration of response (DOR), progression-free survival (PFS), and overall survival.
Results
Within the full study population of 55 women (median age, 54 years [range, 32-90 years]), 5 patients had confirmed complete responses, 5 had confirmed partial responses, 13 had stable disease, and 24 had progressive disease. In the efficacy-evaluable population (n = 47), ORR included 10 patients (21%; 90% CI, 12%-33%) and DCR included 23 (49%; 90% CI, 36%-62%). Median DOR was not reached at the time of the data cutoff, with 7 patients still receiving treatment at the time of analysis. In 15 evaluable patients with tumor BRCA mutations, ORR included 7 patients(47%; 90% CI, 24%-70%), DCR included 12 (80%; 90% CI, 56%-94%), and median PFS was 8.3 months (95% CI, 2.1 months to not estimable). In 27 evaluable patients with BRCA wild-type tumors, ORR included 3 patients (11%; 90% CI, 3%-26%), DCR included 9 (33%; 90% CI, 19%-51%), and median PFS was 2.1 months (95% CI, 1.4-2.5 months). The most common treatment-related adverse events of grade 3 or higher were anemia (10 [18%]), thrombocytopenia (8 [15%]), and fatigue (4 [7%]). Immune-related adverse events were reported in 8 patients (15%) and were grade 3 in 2 patients (4%); no new safety signals were detected.
Conclusions and Relevance
Combination niraparib plus pembrolizumab provides promising antitumor activity in patients with advanced or metastatic TNBC, with numerically higher response rates in those with tumor BRCA mutations. The combination therapy was safe with a tolerable safety profile, warranting further investigation.
Trial Registration
ClinicalTrials.gov identifier: NCT02657889
Introduction
Triple-negative breast cancer (TNBC) is an aggressive breast cancer subtype that lacks estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (ERBB2/HER2 [formerly HER2 or HER2/neu]; OMIM 164870) expression. Triple-negative breast cancer carries a poorer prognosis than other subtypes, with 10-year survival rates of less than 50%.1 Targeted therapies are not currently available for non–BRCA-mutated TNBC, and chemotherapy remains the standard of care despite its limited benefit.2 In clinical trials, patients with advanced TNBC treated with single-agent taxane- or platinum-based chemotherapy had a median progression-free survival (PFS) of 4 to 6 months3,4,5,6,7 and a median overall survival of 11 to 17 months.3,7 Addition of anti–programmed death-ligand 1 (PD-L1) antibody atezolizumab to chemotherapy with albumin-bound paclitaxel in patients with untreated metastatic TNBC improved PFS and numerically improved overall survival vs albumin-bound paclitaxel alone.8
Programmed death receptor-1 (PD-1) limits autoimmunity by inhibiting effector T lymphocytes and is activated by the immunosuppressive PD-L1.9 Tumor cell–expressed PD-L1/2 ligands can bind PD-1 receptors to inactivate T cells, thus evading immune system–mediated destruction.10,11,12 Expression of PD-L1 positively correlates with the presence of tumor-infiltrating lymphocytes, and expression of both is higher in TNBC tumors than in other breast cancer subtypes.13,14,15,16 Response rates to anti–PD-1 and anti–PD-L1 antibodies alone range from 5% to 23%, with higher rates observed when these are used as first-line therapy and among patients with PD-L1–positive tumors.17,18,19,20 Although these clinical activities are modest at best, the few patients who respond have shown long durations of response and survival.19,20
Poly(adenosine diphosphate–ribose) polymerase (PARP) enzymes act to detect and repair DNA damage, and blocking this process with PARP inhibitors leads to cell death through synthetic lethality, particularly in cells already deficient in homologous recombination repair (HRR).21 Tumor mutations in BRCA1 (OMIM 113705) and BRCA2 (OMIM 600185) (tBRCAmut) cause defects in HRR and are estimated to be present in 20% to 25% of patients with basal-like TNBC.21,22 In the registrational phase 3 trial of the PARP inhibitor olaparib,23,24 the subgroup of patients with germline BRCAmut TNBC had an objective response rate (ORR) of 55% and experienced a benefit in PFS compared with patients receiving the physician’s choice of treatment (5.6 vs 2.9 months). In the registrational phase 3 trial of talazoparib tosylate,25,26 patients with germline BRCA mutation TNBC had an ORR of 62% and a PFS of 5.8 months. Monotherapy with PARP inhibitors has not shown activity outside patients with BRCA mutations. In a phase 2 study of olaparib,27 no responses to olaparib occurred among 21 patients with TNBC irrespective of BRCA mutation status, and PFS was only 54 days. Monotherapy with PARP inhibitors has not been well studied in tumors with DNA repair defects other than BRCA.
Preclinical models have shown that PARP inhibitors and anti–PD-1 antibodies show synergistic antitumor activity irrespective of BRCA mutation status and PD-L1 expression.28,29,30 The TOPACIO/KEYNOTE-162 (Niraparib in Combination With Pembrolizumab in Patients With Triple-Negative Breast Cancer or Ovarian Cancer) trial evaluated the hypothesis that combination treatment of niraparib plus pembrolizumab would be a safe and effective therapy for patients with advanced or metastatic TNBC.
Methods
Study Design and Participants
TOPACIO is a multicenter, open-label, single-arm, phase 2 study with a phase 1 lead-in portion evaluating the safety and efficacy of combination treatment with niraparib and pembrolizumab in patients with metastatic TNBC. Patients were enrolled at 34 sites in the United States. Safety data for all patients participating in the phase 1 lead-in portion of the study have been previously reported.31 In the phase 2 TNBC portion of the study, patients received the recommended phase 2 dose of 200 mg of oral niraparib once daily and 200 mg of intravenous pembrolizumab on day 1 of each 21-day cycle.31 Data were collected from January 3, 2017, through October 29, 2018. Target enrollment of 48 patients was estimated to provide 82% power to rule out the null hypothesis (ORR ≤15%) when the true ORR was 30% at the 1-sided 5% type I error rate. Assuming that the true ORR was 35%, enrollment of 48 patients was estimated to provide 94% power.
The protocol (available in Supplement 1) was approved by the institutional review board or the independent ethics committee at each study site (listed in eMethods in Supplement 2). The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practices Guideline, the principles of the Declaration of Helsinki, and national and local regulatory requirements. Before enrollment in the study, written informed consent was obtained from each patient by the local investigator.
Eligible patients had advanced or metastatic breast cancer that was negative for estrogen receptor, progesterone receptor, and ERBB2/HER2.32,33 Full inclusion and exclusion criteria are provided in eMethods in Supplement 2.
Procedures
Patients began treatment with 200 mg of oral niraparib once daily and 200 mg of intravenous pembrolizumab on day 1 of every 21-day cycle based on findings from the phase 1 dose-finding portion of this study.31 Radiographic evaluations to assess the extent of disease were conducted during treatment every 9 weeks for the first year and every 12 weeks thereafter. Per Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1), patients who achieved a complete response or a partial response had the response confirmed. Details of biomarker testing are provided in eMethods in Supplement 2.
Outcomes
The primary objective of the phase 2 TNBC cohort study was to assess the clinical activity of combination treatment with niraparib and pembrolizumab using the primary end point of ORR, as assessed by the investigators per RECIST 1.1. Secondary end points included the duration of response (DOR) per RECIST 1.1; disease control rate (DCR), defined as the proportion of patients achieving a complete response, a partial response, or stable disease as per RECIST 1.1; PFS; and overall survival. Full definitions of outcome measures, including exploratory objectives and safety variables, are in eMethods in Supplement 2.
Statistical Analysis
Data were analyzed from October 29, 2018, to February 27, 2019. Demographics, baseline characteristics, and safety results were summarized descriptively. Efficacy was evaluated by determining confirmed ORR using RECIST 1.1. Response end points were evaluated using the full analysis set, defined as all patients who received any amount of the study treatment, as well as the efficacy-evaluable analysis set, which included all patients who received any amount of the study treatment and who had at least 1 evaluable postbaseline tumor assessment. Point estimates and 2-sided 90% CIs were provided for the analysis of ORR and DCR. For time-to-event end points, the median and corresponding 2-sided 95% CIs were obtained using Kaplan-Meier methods. Safety was evaluated in all patients who received any amount of the study treatment. Exploratory subgroup analyses were performed by biomarker status (BRCA, HRR, and PD-L1) using descriptive methods; no inferential analyses were performed on any subgroup. All statistics were performed using SAS software, version 9.4 (SAS Institute Inc).
Results
Patients
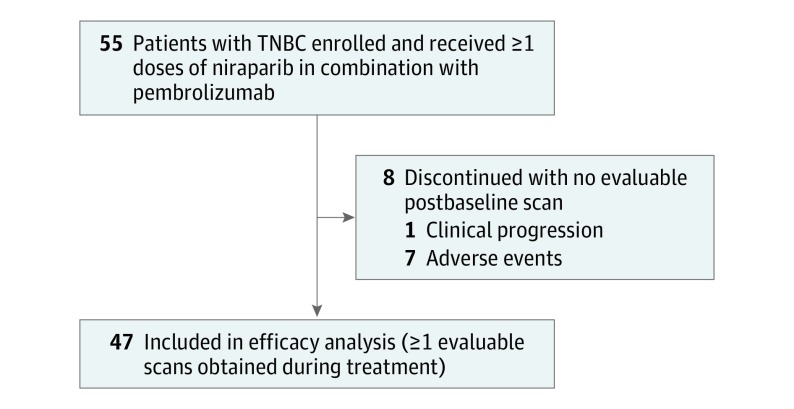
From January 3 through October 4, 2017, 55 women with TNBC were enrolled in phase 2 and received the initial dose of the study drugs (Figure 1). At the time of the October 29, 2018, data cutoff, 7 patients were receiving treatment. Overall, 48 patients had discontinued treatment, 37 because of radiologic disease progression, 2 because of clinical disease progression, and 9 because of adverse events. The median age in the TNBC cohort was 54 years (range, 32-90 years). Patients had received a median of 1 prior line of therapy (range, 0-3) in the metastatic setting, with 14 of 55 (25%) receiving 2 prior lines. Forty-three patients (78%) received previous adjuvant or neoadjuvant therapy that was not counted as a prior line of therapy (eTable 1 in Supplement 2). Among the 31 patients who received platinum-based chemotherapy at any time, 16 patients had a platinum chemotherapy–free interval (time from last platinum-based chemotherapy dose to progression) of no more than 8 weeks, and 15 patients had a platinum-free interval longer than 8 weeks. The biomarker status of enrolled patients is listed in eTable 2 in Supplement 2. The median duration of follow-up at the time of data cutoff was 14.8 months (range, 0.7-25.0 months).
Figure 1. Flow Diagram of Study Enrollment, Treatment, and Outcomes.
TNBC indicates triple-negative breast cancer.
Efficacy
In the full analysis population (n = 55), 5 patients had confirmed complete responses, 5 had confirmed partial responses, 13 had stable disease, and 24 had disease progression. Of the 8 patients who did not have an evaluable postbaseline scan, 1 discontinued owing to clinical progression that was not confirmed by scan, and the remaining 7 discontinued study treatment early owing to an adverse event regardless of causality. Three of the patients with stable disease had a partial response that was not confirmed by a subsequent scan. In the efficacy-evaluable population (n = 47), the confirmed ORR included 10 patients (21%; 90% CI, 12%-33%) with a complete response in 5 patients (11%), and the DCR included 23 (49%; 90% CI, 36%-62%) (Table 1).
Table 1. Best Overall Tumor Responses in the Full-Analysis and Efficacy-Evaluable Populations.
| Best Overall Response | Study Population | |
|---|---|---|
| Full Analysis (N = 55) | Efficacy Evaluable (n = 47) | |
| Complete response, No. (%) | 5 (9) | 5 (11) |
| Partial response, No. (%) | 5 (9) | 5 (11) |
| Stable disease, No. (%) | 13 (24) | 13 (28) |
| Progressive disease, No. (%) | 24 (44) | 24 (51) |
| Not performed or not evaluable, No. (%) | 8 (15) | NA |
| ORR, No. (%) [90% CI]a | 10 (18) [10-29] | 10 (21) [12-33] |
| DCR, No. (%) [90% CI]b | 23 (42) [31-54] | 23 (49) [36-62] |
Abbreviations: DCR, disease control rate; NA, not applicable; ORR, objective response rate.
Includes complete and partial responses.
Includes complete and partial responses and stable disease.
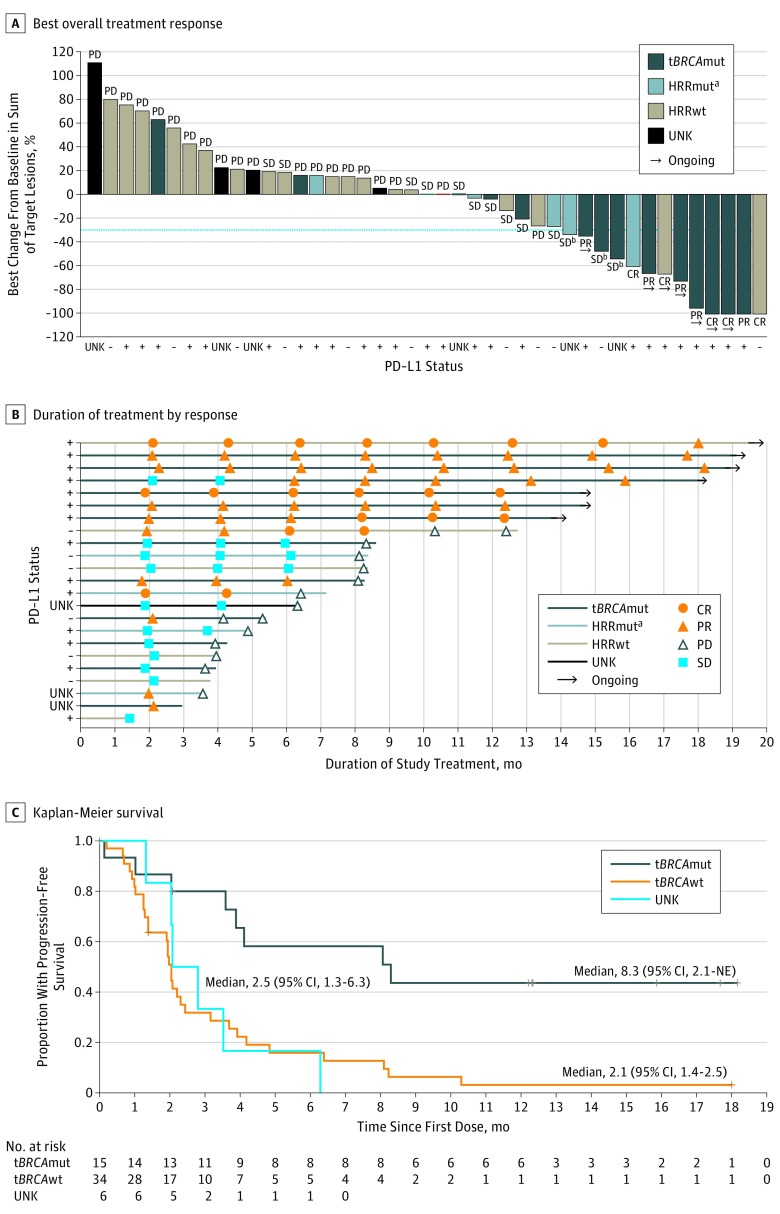
In patients with a confirmed complete or partial response, the median DOR had not been reached at the time of data cutoff (eFigure in Supplement 2). Duration of response ranged from 4.6 to 15.9 months, with 7 responders still receiving treatment at the time of the data cutoff (Figure 2A-B). Of the 10 responders, 3 patients (all with ongoing treatment) had a response duration longer than 1 year; 4 patients (all with ongoing treatment) had a response duration of 9 to 12 months; and 2 additional patients (none with ongoing treatment) had a response duration of 6 to 9 months (Figure 2B). Four of 13 patients with stable disease continued without progression for more than 6 months. In all treated patients, the median PFS was 2.3 months (95% CI, 2.1-3.9 months), with 6- and 12-month PFS estimated to be 28% and 14%, respectively. The overall survival data were not mature at the time of this analysis.
Figure 2. Antitumor Activity of Niraparib in Combination With Pembrolizumab by Biomarker Status.
A, Change is stratified by biomarker status as tumor BRCA mutation (tBRCAmut), homologous recombination repair mutation (HRRmut, including tBRCA wild type [tBRCAwt]), HRR wild type (HRRwt), or unknown (UNK). The broken line indicates a 30% decrease. Four patients with progressive disease due to a new lesion did not have target lesion measurements available and are not included in the plot. B, Overall response was determined using Response Evaluation Criteria in Solid Tumors, version 1.1, as confirmed complete response (CR), partial response (PR), progressive disease (PD), or stable disease (SD) and stratified by biomarker status. C, Progression-free survival was stratified by tBRCAmut or tBRCAwt biomarker status or UNK. NE indicates not evaluable; PD-L1, programmed death-ligand 1; minus sign, negative; and plus sign, positive.
aIncludes 1 patient with germline BRCAmut and unknown tBRCA status.
bPatient had a PR that was not confirmed by a subsequent scan and was therefore classified as SD.
Exploratory univariate analyses were conducted in biomarker-defined evaluable populations according to BRCA or HRR mutation status or PD-L1 status (eTable 2 in Supplement 2). Fifteen of the 47 patients (32%) in the evaluable population had tBRCAmut, 27 (57%) had tBRCA wild type (tBRCAwt), and the remaining 5 had unknown tBRCA status. Of the 15 patients with tBRCAmut, 8 mutations were germline, 2 were somatic, and 5 had unknown germline/somatic status. Of the 5 patients with unknown tBRCAmut status, 2 had germline BRCAwt mutations and 3 had unknown germline BRCA status. Overall, 28 patients (60%) had PD-L1–positive disease (combined proportion score, ≥1); 13 (28%), PD-L1–negative disease; and 6 (13%), unknown. Positivity for PD-L1 was higher in the tBRCAmut (12 of 15 [80%]) population compared with the tBRCAwt population (15 of 27 [56%]).
The response rate was numerically higher in patients with tBRCAmut than in those without confirmed tBRCAmut. The ORR included 7 of 15 patients with tBRCAmut (47%; 90% CI, 24%-70%), and the DCR, 12 of 15 (80%; 90% CI, 56%-94%), with 2 confirmed complete responses, 5 confirmed partial responses, and 5 with stable disease (Figure 2A-B and Table 2). Of the 2 patients with somatic tBRCAmut, 1 had a complete response and 1 had a partial response. One patient with tBRCAmut and stable disease that continued without progression for longer than 6 months and 6 patients with a complete or a partial response continued to receive treatment at the data cutoff date. The median PFS in patients with tBRCAmut was 8.3 months (95% CI, 2.1 months to not estimable) (Figure 2C).
Table 2. Response Rates in Biomarker-Defined, Efficacy-Evaluable Population.
| Biomarker Status | No. | ORR, No. (%) [90% CI] | DCR, No. (%) [90% CI] |
|---|---|---|---|
| BRCA | |||
| tBRCAmut | 15 | 7 (47) [24-70] | 12 (80) [56-94] |
| tBRCAwt | 27 | 3 (11) [3-26] | 9 (33) [19-51] |
| tBRCA unknown | 5 | 0 (0) [0-45] | 2 (40) [8-81] |
| HRRa | |||
| HRRmut | 20 | 8 (40) [22-61] | 16 (80) [60-93] |
| HRRwt | 22 | 2 (9) [2-26] | 6 (27) [13-47] |
| HRR unknown | 5 | 0 (0) [0-45] | 1 (20) [1-66] |
| PD-L1 | |||
| Positive | 28 | 9 (32) [18-49] | 14 (50) [33-67] |
| Negative | 13 | 1 (8) [0.4-32] | 6 (46) [22-71] |
| Unknown | 6 | 0 (0) [0-39] | 3 (50) [15-85] |
Abbreviations: DCR, disease control rate; HRR, homologous recombination repair; mut, mutation; ORR, objective response rate; PD-L1, programmed death-ligand 1; tBRCA, tumor BRCA; wt, wild type.
Measured in BRCA1/2 and 16 other DNA repair genes.
Among the 27 patients with tBRCAwt status, the ORR included 3 (11%; 90% CI, 3%-26%) and the DCR included 9 (33%; 90% CI, 19%-51%), with 3 complete responses and 6 with stable disease. Two patients with tBRCAwt status and stable disease continued without progression for longer than 6 months; 1 patient continued to receive treatment at the time of the data cutoff (Figure 2B). Median PFS in patients with tBRCAwt was 2.1 months (95% CI, 1.4-2.5 months). Mutations in the HRR pathway genes other than BRCA were observed in 5 patients, for whom the ORR included 1 (20%; 95% CI, 1%-66%) and DCR included 4 (80%; 95% CI, 34%-99%). For the 20 patients with BRCA1/2 or other HRR mutations (15 tBRCAmut and 5 other HRR mutations), the ORR included 8 patients (40%; 90% CI, 22%-61%), and the DCR included 16 (80%; 90% CI, 60%-93%) (Table 2). eTable 3 in Supplement 2 has additional information about specific HRR gene mutations and responses.
The response rate was also numerically higher in patients with PD-L1–positive disease than in those with PD-L1–negative disease. Among 28 patients with PD-L1–positive tumors, 9 were included in the ORR of 32% (90% CI, 18%-49%) compared with 1 of 13 patients in the ORR of 8% (90% CI, 0.4%-32%) with PD-L1–negative tumors. Best treatment responses for individual evaluable patients are shown in Figure 2A. The ORR was numerically greater in patients receiving no more than 1 line of previous treatment vs 2 or more lines and in patients without prior platinum-based chemotherapy use; in patients with prior platinum-based chemotherapy, the ORR was numerically greater in those patients with a platinum chemotherapy–free interval (days from the last platinum-based dose to disease progression) greater than 56 days. However, the number of patients was small and the CIs overlapped for these subgroup analyses (eTable 4 in Supplement 2). Response rates by prior platinum-based chemotherapy use and biomarker status (tBRCA and HRR mutation and PD-L1 expression) are shown in eTable 5 in Supplement 2.
Safety
All patients with TNBC who received the study treatment (N = 55) were evaluable for safety outcomes. Treatment-related adverse events of any grade were reported in 51 patients (93%), the most common of which were nausea (30 [55%]), fatigue (24 [44%]), anemia (19 [35%]), thrombocytopenia (14 [25%]), and constipation (13 [24%]) (Table 3). In general, nausea was controlled using standard antiemetics. The most common treatment-related adverse events of grade 3 or greater were anemia (10 [18%]), thrombocytopenia (8 [15%]), and fatigue (4 [7%]). Seven patients received platelet transfusion(s) for thrombocytopenia, and 15 received red blood cell transfusion(s) for anemia. In addition, adverse events of grade 3 or greater that were most common in laboratory findings included decreased platelet count (6 [11%]), decreased neutrophil count (4 [7%]), and decreased lymphocyte count (4 [7%]). Treatment-related serious adverse events were reported in 11 patients (20%); only thrombocytopenia (3 patients) and pyrexia (2 patients) were reported in more than 1 patient. The most common adverse events leading to treatment discontinuation were increased levels of alkaline phosphatase, bilirubin, alanine aminotransferase, and aspartate aminotransferase and fatigue. One death resulted from acute respiratory distress syndrome, deemed by the investigator to be possibly related to treatment.
Table 3. Treatment-Related Adverse Events.
| Adverse Event | No. (%) of Patients by Adverse Event | |
|---|---|---|
| Any Grade (N = 55) | Grade ≥3 (N = 55) | |
| Any treatment-related | 51 (93) | 32 (58) |
| Treatment-related occurring in >10% of patients | ||
| Nausea | 30 (55) | 0 |
| Fatigue | 24 (44) | 4 (7) |
| Anemia | 19 (35) | 10 (18) |
| Thrombocytopenia | 14 (25) | 8 (15) |
| Constipation | 13 (24) | 0 |
| Diarrhea | 10 (18) | 0 |
| Decreased appetite | 9 (16) | 0 |
| Vomiting | 8 (15) | 0 |
| Prespecified treatment-related and immune-related | ||
| Any | 8 (15) | 2 (4) |
| Adrenal insufficiency | 1 (2) | 1 (2) |
| Hyperglycemia | 1 (2) | 0 |
| Hyperthyroidism | 1 (2) | 0 |
| Hypothyroidism | 4 (7) | 0 |
| Pneumonitis | 1 (2) | 0 |
| Polymyalgia rheumatica | 1 (2) | 1 (2) |
Immune-related adverse events were those known to be associated with anti–PD-1 inhibitors.34 The immune-related adverse events deemed to be associated with treatment by the investigators occurred in 8 patients (15%); the only such event reported in more than 1 patient was hypothyroidism (4 [7%]) (Table 3). Two patients (4%) had grade 3 immune-related adverse events associated with the study treatment. One patient had grade 3 adrenal insufficiency, which resolved after treatment with corticosteroids and interruption of pembrolizumab therapy, and 1 patient had polymyalgia rheumatica, which resolved after treatment with corticosteroids, interruption of niraparib therapy, and discontinuation of pembrolizumab therapy. No treatment-associated grade 4 or 5 immune-related adverse events occurred, and no niraparib treatment discontinuations occurred because of immune-related adverse events.
Discussion
TOPACIO is the first study, to our knowledge, to report the safety and efficacy of combining PARP inhibitors and immuno-oncology checkpoint therapy in patients with metastatic or advanced TNBC with or without BRCA mutation. Among enrolled patients, 78% had received prior adjuvant or neoadjuvant chemotherapy; two-thirds of the patients had received chemotherapy in the metastatic setting, of whom half had received platinum-based chemotherapy. Although the prespecified statistical criterion for the primary objective was not met (null ≤15%), combination treatment with niraparib and an anti–PD-1 antibody provided promising, durable clinical benefit. Disease control was achieved in half of the evaluable patients, and nearly one-quarter of evaluable patients experienced an objective response, with the median DOR not yet reached. Niraparib plus pembrolizumab provided responses of meaningful durability; of the 10 patients with treatment responses, 7 were still receiving treatment at the time of the data cutoff, and remarkably, 8 patients continued to receive treatment for 1 year or longer. These findings suggest that PARP inhibitors plus PD-1 blockade may provide clinically relevant improvements in DOR.
Of particular importance is that the combination treatment demonstrated clinical activity in patients irrespective of BRCA mutation or PD-L1 status, although the clinical activity is more pronounced in patients with tBRCAmut or those with PD-L1–positive tumors. The 21% ORR in all evaluable patients is numerically higher than the single-digit ORRs reported for anti–PD-1 and anti–PD-L1 agents in similar patient populations.17,18,19 This increase in response rate does not appear to be completely driven by stronger activity in the population with tBRCAmut because we observed 3 complete responses in patients with tBRCAwt status, and 2 of the 3 had no mutation in other HRR pathway genes.
The 47% ORR observed in patients with tBRCAmut treated with the niraparib plus pembrolizumab combination is similar to the ORR reported for olaparib monotherapy in patients with germline BRCAmut TNBC. However, the median PFS of 8.3 months in these patients in the present study was nearly 3 months longer than that observed for olaparib (5.6 months)23 or talazoparib (5.8 months)25 in patients with germline BRCAmut TNBC. The observation that PD-L1 was more frequently expressed in patients with tBRCAmut compared with tBRCAwt is consistent with previous publications in other cancer types.35,36 Breast cancers in patients with BRCA mutations lack effective DNA repair and are genomically unstable with a high mutational load, and treatment may rely on immune checkpoint inhibition via the PD-1/PD-L1 pathway to avoid immune destruction.37 The benefit from immunotherapy, given as monotherapy or combination therapy, can also manifest itself via long response durations or prolonged periods of stable disease. Median DOR was not reached at the time of data cutoff (range, 4.6-15.9 months), whereas for talazoparib, the median DOR was 4.3 months in patients with TNBC.25 Of the 10 responders, 2 patients (both with ongoing treatment) had a response duration longer than 1 year; 4 patients (all with ongoing treatment) had a response duration of 9 to 12 months; and 3 additional patients (1 with ongoing treatment) had a response duration of 6 to 9 months. Furthermore, 8 patients continued to receive treatment for longer than 1 year. Two patients with tBRCAwt disease, 1 with tBRCAmut disease, and 1 with tBRCA status unknown and stable disease continued to receive treatment without progression for longer than 6 months.
Although patients without prior platinum-based chemotherapy had numerically higher response rates than those with prior platinum-based chemotherapy, the CIs overlap. This finding is consistent with a previous trial of talazoparib in patients with breast cancer,38 which suggested higher response rates in patients without prior platinum-based chemotherapy.
No new safety signals were identified with the combination of niraparib plus pembrolizumab compared with monotherapy. The frequency of nausea is consistent with previous studies of niraparib.39,40 Indeed, nausea is one of the most frequently reported adverse events associated with PARP inhibitors in patients with breast cancers.41 Most events of grade 3 or greater were hematologic and consistent with the class effects of PARP inhibitors41,42; these were treated with transfusion as clinically indicated. We found no increase in the incidence of immune-related adverse events compared with that observed with niraparib treatment in the registrational trial,39 indicating that the addition of the checkpoint inhibitor pembrolizumab was not associated with immune-related tolerability of niraparib.
Limitations
This phase 2 study had a single-arm, open-label design and as such lacked a comparator arm. Therefore, the findings presented herein will need to be validated in a larger clinical trial. In addition, although the findings regarding patients with tBRCAwt disease and those with HRR mutations are noteworthy, owing to lack of randomization and small patient numbers, we cannot draw strong conclusions on the role of synergy between niraparib and pembrolizumab vs either agent as monotherapy.
Conclusions
These data suggest that the combination of a PARP inhibitor and an anti–PD-1 antibody has a tolerable safety profile in patients with advanced or metastatic TNBC and promising antitumor activity, irrespective of BRCA mutation status. To confirm the findings of this trial, further clinical development of niraparib in combination with PD-1 inhibition in larger-scale studies is under consideration.
Trial Protocol
eMethods. Inclusion/Exclusion Criteria and Further Information
eTable 1. Patient Demographics and Baseline Characteristics
eTable 2. Biomarker Status
eTable 3. Disease Control in tBRCAwt/Unknown Patients
eTable 4. ORR Subgroup Analysis in Efficacy Evaluable Population
eTable 5. ORR Analysis by Biomarker Status and Prior Platinum Use
eFigure. Duration of Response for All Patients Who Achieved Confirmed CR or PR
eReferences.
References
- 1.Malorni L, Shetty PB, De Angelis C, et al. . Clinical and biologic features of triple-negative breast cancers in a large cohort of patients with long-term follow-up. Breast Cancer Res Treat. 2012;136(3):795-804. doi: 10.1007/s10549-012-2315-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network Clinical practice guidelines in oncology: breast cancer version 1.2018. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed July 2, 2018.
- 3.Gradishar WJ, Kaklamani V, Sahoo TP, et al. . A double-blind, randomised, placebo-controlled, phase 2b study evaluating sorafenib in combination with paclitaxel as a first-line therapy in patients with HER2-negative advanced breast cancer. Eur J Cancer. 2013;49(2):312-322. doi: 10.1016/j.ejca.2012.08.005 [DOI] [PubMed] [Google Scholar]
- 4.Kim SB, Dent R, Im SA, et al. ; LOTUS Investigators . Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017;18(10):1360-1372. doi: 10.1016/S1470-2045(17)30450-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miles D, Cameron D, Bondarenko I, et al. . Bevacizumab plus paclitaxel versus placebo plus paclitaxel as first-line therapy for HER2-negative metastatic breast cancer (MERIDIAN): a double-blind placebo-controlled randomised phase III trial with prospective biomarker evaluation. Eur J Cancer. 2017;70:146-155. doi: 10.1016/j.ejca.2016.09.024 [DOI] [PubMed] [Google Scholar]
- 6.Miller K, Wang M, Gralow J, et al. . Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666-2676. doi: 10.1056/NEJMoa072113 [DOI] [PubMed] [Google Scholar]
- 7.O’Shaughnessy J, Schwartzberg L, Danso MA, et al. . Phase III study of iniparib plus gemcitabine and carboplatin versus gemcitabine and carboplatin in patients with metastatic triple-negative breast cancer. J Clin Oncol. 2014;32(34):3840-3847. doi: 10.1200/JCO.2014.55.2984 [DOI] [PubMed] [Google Scholar]
- 8.Schmid P, Adams S, Rugo HS, et al. ; IMpassion130 Trial Investigators . Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108-2121. doi: 10.1056/NEJMoa1809615 [DOI] [PubMed] [Google Scholar]
- 9.Gibbons Johnson RM, Dong H. Functional expression of programmed death-ligand 1 (B7-H1) by immune cells and tumor cells. Front Immunol. 2017;8(961):961. doi: 10.3389/fimmu.2017.00961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264. doi: 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Telli ML, Stover DG, Loi S, et al. . Homologous recombination deficiency and host anti-tumor immunity in triple-negative breast cancer. Breast Cancer Res Treat. 2018;171(1):21-31. doi: 10.1007/s10549-018-4807-x [DOI] [PubMed] [Google Scholar]
- 12.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24(2):207-212. doi: 10.1016/j.coi.2011.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghebeh H, Mohammed S, Al-Omair A, et al. . The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8(3):190-198. doi: 10.1593/neo.05733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mittendorf EA, Philips AV, Meric-Bernstam F, et al. . PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2(4):361-370. doi: 10.1158/2326-6066.CIR-13-0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ali HR, Glont SE, Blows FM, et al. . PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann Oncol. 2015;26(7):1488-1493. doi: 10.1093/annonc/mdv192 [DOI] [PubMed] [Google Scholar]
- 16.Loi S, Sirtaine N, Piette F, et al. . Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31(7):860-867. doi: 10.1200/JCO.2011.41.0902 [DOI] [PubMed] [Google Scholar]
- 17.Adams S, Schmid P, Rugo HS, et al. . Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30(3):397-404. doi: 10.1093/annonc/mdy517 [DOI] [PubMed] [Google Scholar]
- 18.Dirix LY, Takacs I, Jerusalem G, et al. . Avelumab, an anti–PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN solid tumor study. Breast Cancer Res Treat. 2018;167(3):671-686. doi: 10.1007/s10549-017-4537-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emens LA, Cruz C, Eder JP, et al. . Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol. 2019;5(1):74-82. doi: 10.1001/jamaoncol.2018.4224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nanda R, Chow LQ, Dees EC, et al. . Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34(21):2460-2467. doi: 10.1200/JCO.2015.64.8931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papadimitriou M, Mountzios G, Papadimitriou CA. The role of PARP inhibition in triple-negative breast cancer: unraveling the wide spectrum of synthetic lethality. Cancer Treat Rev. 2018;67:34-44. doi: 10.1016/j.ctrv.2018.04.010 [DOI] [PubMed] [Google Scholar]
- 22.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61-70. doi: 10.1038/nature11412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senkus-Konefka E, Domchek SM, Im SA, et al. . 101 (PB-002): subgroup analysis of olaparib monotherapy versus chemotherapy by hormone receptor and BRCA mutation status in patients with HER2-negative metastatic breast cancer and a germline BRCA mutation: OlympiAD. Eur J Cancer. 2018;92:S19-S20. doi: 10.1016/S0959-8049(18)30285-5 [DOI] [Google Scholar]
- 24.Robson M, Im SA, Senkus E, et al. . Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523-533. doi: 10.1056/NEJMoa1706450 [DOI] [PubMed] [Google Scholar]
- 25.Eiermann W, Rugo HS, Diab S, et al. . Analysis of germline BRCA1/2 mutated (gBRCAmut) hormone receptor-positive (HR+) and triple negative breast cancer (TNBC) treated with talazoparib (TALA). J Clin Oncol. 2018;36(15 suppl):1070. doi: 10.1200/JCO.2018.36.15_suppl.1070 [DOI] [Google Scholar]
- 26.Litton JK, Rugo HS, Ettl J, et al. . Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379(8):753-763. doi: 10.1056/NEJMoa1802905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gelmon KA, Tischkowitz M, Mackay H, et al. . Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12(9):852-861. doi: 10.1016/S1470-2045(11)70214-5 [DOI] [PubMed] [Google Scholar]
- 28.Huang J, Wang L, Cong Z, et al. . The PARP1 inhibitor BMN 673 exhibits immunoregulatory effects in a Brca1(−/−) murine model of ovarian cancer. Biochem Biophys Res Commun. 2015;463(4):551-556. doi: 10.1016/j.bbrc.2015.05.083 [DOI] [PubMed] [Google Scholar]
- 29.Jiao S, Xia W, Yamaguchi H, et al. . PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res. 2017;23(14):3711-3720. doi: 10.1158/1078-0432.CCR-16-3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang S, Sun K, Xiao Y, et al. . Evaluation of niraparib in combination with anti-PD1/anti–PD-L1 in preclinical models. Cancer Res. 2018;78(13)(suppl):abstract 1724. doi: 10.1158/1538-7445.AM2018-1724 [DOI] [Google Scholar]
- 31.Konstantinopoulos PA, Sachdev JC, Schwartzberg L, et al. . Dose-finding combination study of niraparib and pembrolizumab in patients (pts) with metastatic triple-negative breast cancer (TNBC) or recurrent platinum-resistant epithelial ovarian cancer (OC) (TOPACIO/Keynote-162). Ann Oncol. 2017;28(suppl 5):406. doi: 10.1093/annonc/mdx376.009 [DOI] [Google Scholar]
- 32.Wolff AC, Hammond ME, Hicks DG, et al. ; American Society of Clinical Oncology; College of American Pathologists . Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997-4013. doi: 10.1200/JCO.2013.50.9984 [DOI] [PubMed] [Google Scholar]
- 33.Hammond ME, Hayes DF, Dowsett M, et al. ; American Society of Clinical Oncology; College of American Pathologists . American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med. 2010;134(7):e48-e72. doi: 10.1043/1543-2165-134.7.e48 [DOI] [PubMed] [Google Scholar]
- 34.Wang PF, Chen Y, Song SY, et al. . Immune-related adverse events associated with anti–PD-1/PD-L1 treatment for malignancies: a meta-analysis. Front Pharmacol. 2017;8:730. doi: 10.3389/fphar.2017.00730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strickland KC, Howitt BE, Shukla SA, et al. . Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016;7(12):13587-13598. doi: 10.18632/oncotarget.7277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wieser V, Gaugg I, Fleischer M, et al. . BRCA1/2 and TP53 mutation status associates with PD-1 and PD-L1 expression in ovarian cancer. Oncotarget. 2018;9(25):17501-17511. doi: 10.18632/oncotarget.24770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Audeh MW, Dadmanesh F, Yearley J. PDL-1 expression in primary breast cancers with germline mutations in BRCA 1 and 2. Cancer Res. 2016;76(suppl 4):abstract P4-04-01. doi: 10.1158/1538-7445.SABCS15-P4-04-01 [DOI] [Google Scholar]
- 38.Turner NC, Telli ML, Rugo HS, et al. ; ABRAZO Study Group . A phase II study of talazoparib after platinum or cytotoxic nonplatinum regimens in patients with advanced breast cancer and germline BRCA1/2 mutations (ABRAZO). Clin Cancer Res. 2019;25(9):2717-2724. doi: 10.1158/1078-0432.CCR-18-1891 [DOI] [PubMed] [Google Scholar]
- 39.Mirza MR, Monk BJ, Herrstedt J, et al. ; ENGOT-OV16/NOVA Investigators . Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154-2164. doi: 10.1056/NEJMoa1611310 [DOI] [PubMed] [Google Scholar]
- 40.Sandhu SK, Schelman WR, Wilding G, et al. . The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2013;14(9):882-892. doi: 10.1016/S1470-2045(13)70240-7 [DOI] [PubMed] [Google Scholar]
- 41.Livraghi L, Garber JE. PARP inhibitors in the management of breast cancer: current data and future prospects. BMC Med. 2015;13:188. doi: 10.1186/s12916-015-0425-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou JX, Feng LJ, Zhang X. Risk of severe hematologic toxicities in cancer patients treated with PARP inhibitors: a meta-analysis of randomized controlled trials. Drug Des Dev Ther. 2017;11:3009-3017. doi: 10.2147/DDDT.S147726 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods. Inclusion/Exclusion Criteria and Further Information
eTable 1. Patient Demographics and Baseline Characteristics
eTable 2. Biomarker Status
eTable 3. Disease Control in tBRCAwt/Unknown Patients
eTable 4. ORR Subgroup Analysis in Efficacy Evaluable Population
eTable 5. ORR Analysis by Biomarker Status and Prior Platinum Use
eFigure. Duration of Response for All Patients Who Achieved Confirmed CR or PR
eReferences.