This randomized clinical trial assesses the effect of afatinib vs placebo after definitive chemoradiotherapy on disease-free survival in patients with head and neck squamous cell carcinoma.
Key Points
Question
Does afatinib as adjuvant therapy after definitive chemoradiotherapy improve disease-free survival in head and neck cancer?
Findings
This randomized clinical trial of 617 patients found that afatinib therapy after definitive chemoradiotherapy in patients with intermediate- to high-risk unresected head and neck cancer did not improve disease-free survival vs placebo. In addition, afatinib therapy did not confer any health-related quality-of-life benefit, and changes over time in global health status and pain scores favored placebo.
Meaning
These study findings indicate that use of adjuvant afatinib therapy after concurrent chemoradiotherapy is not recommended in head and neck cancer.
Abstract
Importance
Locoregionally advanced head and neck squamous cell cancer (HNSCC) is treated curatively; however, risk of recurrence remains high among some patients. The ERBB family blocker afatinib has shown efficacy in recurrent or metastatic HNSCC.
Objective
To assess whether afatinib therapy after definitive chemoradiotherapy (CRT) improves disease-free survival (DFS) in patients with HNSCC.
Design, Setting, and Participants
This multicenter, phase 3, double-blind randomized clinical trial (LUX-Head & Neck 2) studied 617 patients from November 2, 2011, to July 4, 2016. Patients who had complete response after CRT, comprising radiotherapy with cisplatin or carboplatin, with or without resection of residual disease, for locoregionally advanced high- or intermediate-risk HNSCC of the oral cavity, hypopharynx, larynx, or oropharynx were included in the study. Data analysis was of the intention-to-treat population.
Interventions
Patients were randomized (2:1) to treatment with afatinib (40 mg/d) or placebo, stratified by nodal status (N0-2a or N2b-3) and Eastern Cooperative Oncology Group performance status (0 or 1). Treatment continued for 18 months or until disease recurrence, unacceptable adverse events, or patient withdrawal.
Main Outcomes and Measures
The primary end point was DFS, defined as time from the date of randomization to the date of tumor recurrence or secondary primary tumor or death from any cause. Secondary end points were DFS at 2 years, overall survival (defined as time from the date of randomization to death), and health-related quality of life.
Results
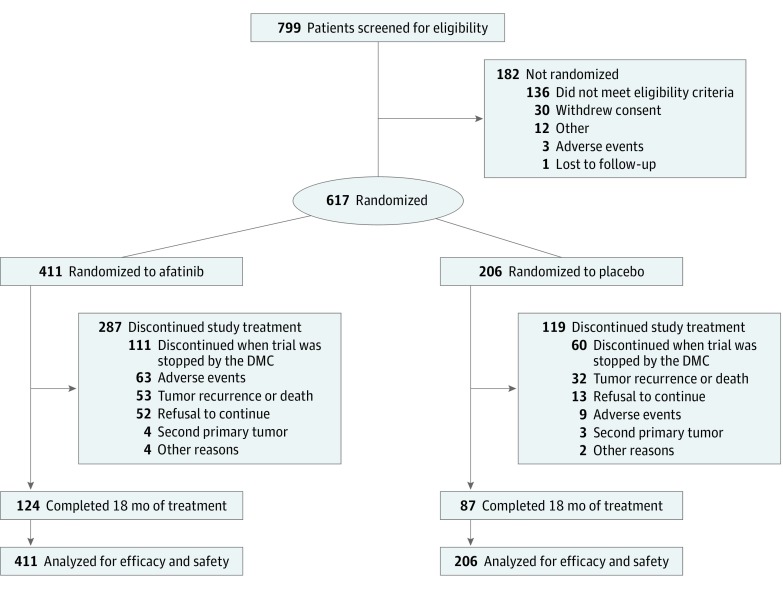
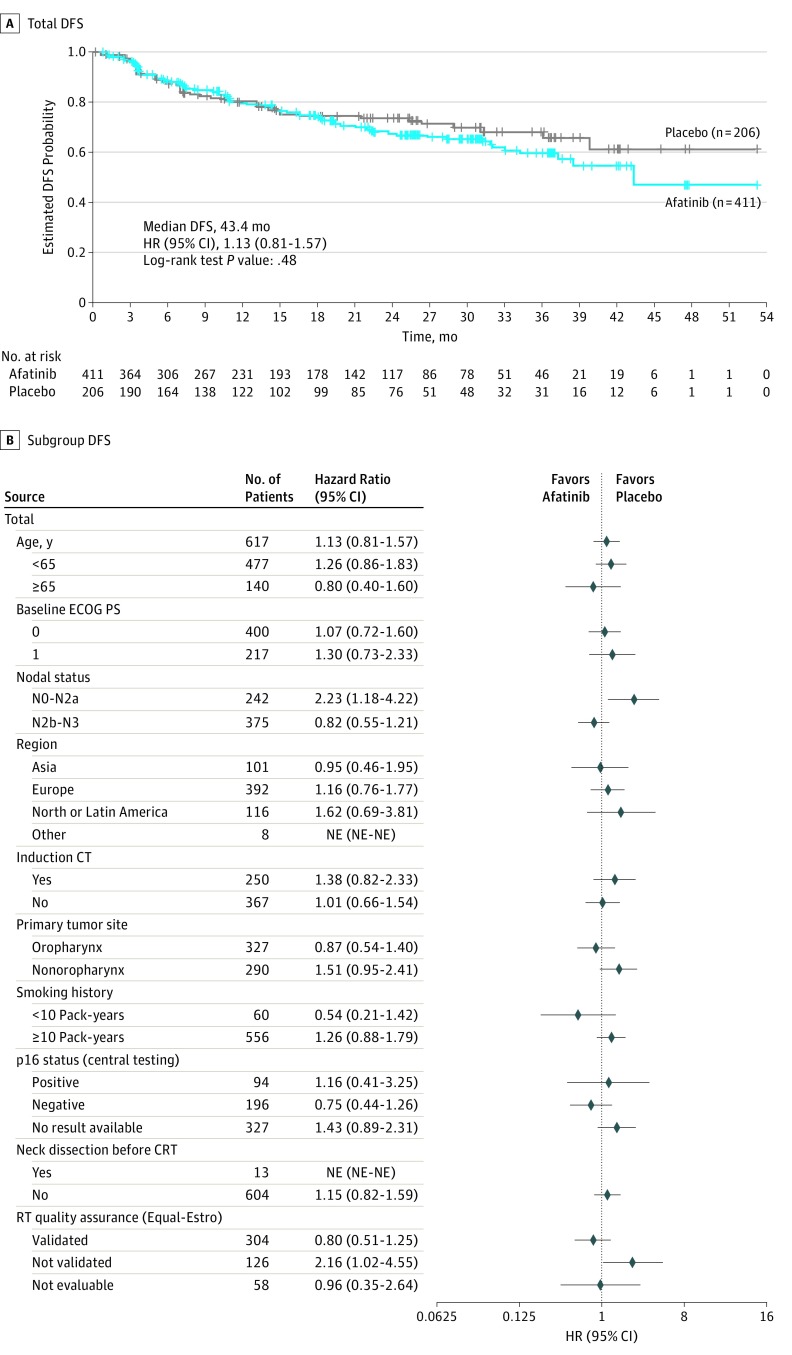
A total of 617 patients were studied (mean [SD] age, 58 [8.4] years; 528 male [85.6%]). Recruitment was stopped after a preplanned interim futility analysis on July 4, 2016, on recommendation from an independent data monitoring committee. Treatment was discontinued. Median DFS was 43.4 months (95% CI, 37.4 months to not estimable) in the afatinib group and not estimable (95% CI, 40.1 months to not estimable) in the placebo group (hazard ratio, 1.13; 95% CI, 0.81-1.57; stratified log-rank test P = .48). The most common grade 3 and 4 drug-related adverse effects were acneiform rash (61 [14.8%] of 411 patients in the afatinib group vs 1 [0.5%] of 206 patients in the placebo group), stomatitis (55 [13.4%] in the afatinib group vs 1 [0.5%] in the placebo group), and diarrhea (32 [7.8%] in the afatinib group vs 1 [0.5%] in the placebo group).
Conclusions and Relevance
This study’s findings indicate that treatment with afatinib after CRT did not improve DFS and was associated with more adverse events than placebo in patients with primary, unresected, clinically high- to intermediate-risk HNSCC. The use of adjuvant afatinib after CRT is not recommended.
Trial Registration
ClinicalTrials.gov identifier: NCT01345669
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer worldwide.1 Approximately 50% of patients present with locoregionally advanced disease,2 and many patients receive definitive concurrent chemoradiotherapy (CRT) as primary therapy. Outcomes for patients treated with primary CRT are comparable to those for surgery, and many patients treated with surgery require combined-modality postoperative therapy.3 Risk of recurrence remains high among some subsets of patients, even among those who attain a complete response with CRT or who have no evidence of disease after surgery to resect residual disease.4 Strategies to reduce recurrence and death have largely focused on intensification of conventional treatment, with limited success for altered fractionation radiotherapy together with chemotherapy5 or induction chemotherapy.6,7
The epidermal growth factor receptor (EGFR) has an important role in progression and treatment resistance in HNSCC8; targeting of EGFR with the monoclonal antibody cetuximab improves chemotherapy and radiotherapy responsiveness and improves survival in the locoregionally advanced and metastatic settings.9,10,11 However, the small-molecule inhibitors of EGFR tyrosine kinase activity, gefitinib and erlotinib, have limited activity in HNSCC.12,13 Other members of the ERBB receptor family may also be aberrantly expressed in HNSCC, may contribute to resistance to EGFR targeting, and may be targets themselves.14 Afatinib, an irreversible ERBB family inhibitor, has demonstrated efficacy in recurrent or metastatic HNSCC after failure of platinum-based therapy.15 Targeting of EGFR and other ERBB family members has been explored as maintenance or adjuvant therapy after definitive treatment.16,17 Thus, this study examines whether the orally available, active, tolerable, irreversible ERBB family inhibitor afatinib could prevent or delay recurrence in patients with clinical features of intermediate- to high-risk disease.
Methods
Study Design and Participants
In this double-blind, placebo-controlled, phase 3 randomized clinical trial (LUX-Head & Neck 2), eligible patients had histologically or cytologically confirmed, locoregionally advanced HNSCC. Unfavorable risk was defined as a nonoropharyngeal primary site or oropharyngeal cancer in heavy smokers (>10 pack-years). Patients had unresected disease before CRT. Definitive CRT must have been completed no longer than 24 weeks before randomization. Previous treatment with EGFR-targeted agents was not permitted. Patients with primary tumor of the base of tongue and/or tonsil together with a smoking history of 10 pack-years or less were ineligible. Full eligibility criteria are listed in the eMethods in Supplement 1. The study protocol was designed in accordance with the Declaration of Helsinki,18 the International Conference on Harmonization Guideline for Good Clinical Practice, and applicable region-specific regulatory requirements and was approved by independent ethics committees at each center. All patients provided written informed consent. An independent data monitoring committee (DMC) monitored study conduct. The trial protocol can be found in Supplement 2.
Randomization and Masking
Between November 2, 2011, and July 4, 2016, a total of 617 patients were randomized 2:1 to receive afatinib or placebo and stratified based on nodal status (N0-N2a vs N2b-N3) and Eastern Cooperative Oncology Group (ECOG) performance status (0 vs 1). The randomization list was generated using a validated pseudorandom number generator (block size, 3). Patient assignment to a treatment group was by an interactive voice or web-based response system. Patients, investigators, and the sponsor trial team were blinded to the randomized treatment until database lock.
Procedures
Patients received oral afatinib, 40 mg once daily; the dose was escalated to 50 mg after 4 weeks or more with no treatment-related adverse events (AEs) other than grade 1 rash. In the event of grade 3 or higher treatment-related AEs, grade 2 or higher diarrhea, nausea and/or vomiting, or grade 1 or higher reduced renal function, treatment was interrupted until severity reduced to grade 1 or lower. Tolerability-guided dose reduction was then permitted in 10-mg decrements to a minimum of 20 mg. Patients who required further reductions were removed from therapy. Treatment continued for 18 months or until disease recurrence or secondary primary tumor, unacceptable AEs, or patient withdrawal.
Images of the head, neck, and chest were assessed by the investigator and independent central review, a central team independent of the trial investigators. Disease status was assessed using computed tomography, magnetic resonance imaging, or positron emission tomography–computed tomography every 16 weeks for 2 years and every 24 weeks thereafter until disease recurrence, unavailability or loss to follow-up, or trial completion. Radiotherapy data were independently reviewed through a central quality assurance unit (EQUAL-ESTRO). Health-related quality of life (HRQoL) was assessed using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (QLQ-C30) and its associated head and neck cancer–specific module (QLQ-HN35).19 Incidence and severity of AEs were evaluated according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 3.0.20 Prespecified tumor biomarker assessment of p16 status, PTEN, and ERBB2 expression was conducted on archival tumor tissue samples from patients who provided separate consent (see eMaterial and eMethods in the Supplement).
Outcomes
The primary end point was investigator-assessed disease-free survival (DFS), defined as time from the date of randomization to the date of tumor recurrence or secondary primary tumor or death from any cause. Secondary end points were DFS at 2 years, overall survival (OS) (time from the date of randomization to death), and HRQoL.
Statistical Analysis
The trial was powered to detect a prolonged median DFS with afatinib of 48 months compared with the assumed DFS of 34 months with placebo. This assumption was based on data from a trial investigating lapatinib vs placebo during CRT and for up to 12 months as maintenance (MAINTYNANCE),21 which suggested median DFS with placebo was likely to be approximately 34 months. Assuming exponential distribution for the time to tumor recurrence or secondary primary tumor (or death), our trial was powered to detect a prolonged median DFS of 14 months with afatinib. Randomization of 669 patients was therefore required to detect a difference in DFS (with a hazard ratio [HR] of 0.71) at a power of 80% with a 1-sided type I error of α = .025. P < .05 was considered to be statistically significant.
Efficacy analyses included all randomized patients (intention-to-treat population). Safety analyses included all treated patients (received at least 1 dose of study drug). Disease-free survival was analyzed using a stratified log-rank test (2-sided, .05 significance level), with stratification factors of nodal status (N0-N2a vs N2b-N3) and ECOG performance status (0 vs 1). The Kaplan-Meier method was used to estimate DFS for each treatment group; HRs were derived using a stratified Cox proportional hazards regression model. The SAS statistical software, version 9.4 (SAS Institute Inc) was used for all statistical analyses.
Results
Patients and Treatment Exposure
A total of 617 patients were studied (mean [SD] age, 58 [8.4] years; 528 male [85.6%]) (Figure 1). A preplanned futility analysis, performed by the DMC at approximately 40% of DFS events, revealed that the study was unlikely to demonstrate a significant advantage with afatinib. There were no major safety concerns, but more treatment-related AEs were observed with afatinib therapy. Therefore, based on the independent DMC recommendation, the trial was halted on July 4, 2016. Patients were discontinued from treatment, and follow-up for disease recurrence and survival was stopped. At the time of trial cessation, 171 patients (27.7%) were receiving study treatment (111 [27.0%] in the afatinib group and 60 [29.1%] in the placebo group); 211 (34.2%) had completed 18 months of treatment (124 [30.2%] in the afatinib group and 87 [42.2%] in the placebo group). Overall, patient demographics and tumor characteristics at baseline were well balanced between groups (Table 1).
Figure 1. CONSORT Study Design.
DMC indicates data monitoring committee.
Table 1. Patient Baseline Demographics and Tumor Characteristicsa.
| Characteristic | Afatinib (n = 411) | Placebo (n = 206) |
|---|---|---|
| Sex | ||
| Male | 350 (85.2) | 178 (86.4) |
| Female | 61 (14.8) | 28 (13.6) |
| Age, median (range), y | 58.0 (25.0-83.0) | 57.0 (25.0-79.0) |
| ECOG performance status | ||
| 0 | 267 (65.0) | 133 (64.6) |
| 1 | 144 (35.0) | 73 (35.4) |
| Region | ||
| Asia | 71 (17.3) | 30 (14.6) |
| Europe | 260 (63.2) | 132 (64.1) |
| North or Latin America | 75 (18.2) | 41 (19.9) |
| Other | 5 (1.2) | 3 (1.5) |
| Smoking status | ||
| Current smoker | 114 (27.7) | 45 (21.8) |
| Current nonsmoker | 297 (72.3) | 161 (78.2) |
| Smoking pack-yearsb | ||
| <10 | 42 (10.2) | 18 (8.7) |
| ≥10 | 368 (89.5) | 188 (91.3) |
| Alcohol consumption | ||
| Nondrinker | 256 (62.3) | 129 (62.6) |
| ≤7 Units per week | 75 (18.2) | 37 (18.0) |
| >7 Units per week | 74 (18.0) | 39 (18.9) |
| Primary tumor site | ||
| Oral cavity | 35 (8.5) | 21 (10.2) |
| Oropharynx | 216 (52.6) | 111 (53.9) |
| Hypopharynx | 85 (20.7) | 48 (23.3) |
| Larynx | 73 (17.8) | 25 (12.1) |
| >1 Site | 2 (0.5) | 1 (0.5) |
| T stage for primary tumor | ||
| T0 | 0 | 0 |
| T1 | 26 (6.3) | 11 (5.3) |
| T2 | 99 (24.1) | 55 (26.7) |
| T3 | 159 (38.7) | 67 (32.5) |
| T4 | 127 (30.9) | 73 (35.4) |
| N stage for primary tumor | ||
| N0 to N2a | 159 (38.7) | 83 (40.3) |
| N2b to N3 | 252 (61.3) | 123 (59.7) |
| Time since first diagnosis, median (range), moc | 7.8 (3.4-16.1) | 7.8 (4.3-80.9) |
| Clinical stage at diagnosis | ||
| III | 72 (17.5) | 40 (19.4) |
| IVa | 309 (75.2) | 141 (68.4) |
| IVb | 30 (7.3) | 25 (12.1) |
| Differentiation grade | ||
| Well differentiated | 50 (12.2) | 29 (14.1) |
| Moderately differentiated | 153 (37.2) | 74 (35.9) |
| Poorly differentiated | 90 (21.9) | 45 (21.8) |
| Undifferentiated | 7 (1.7) | 0 |
| Not specified or not assessable | 111 (27.0) | 58 (28.2) |
| p16 Status (central testing) | ||
| Positive | 53 (12.9) | 41 (19.9) |
| Negative | 135 (32.8) | 61 (29.6) |
| No result available | 223 (54.3) | 104 (50.5) |
| Induction chemotherapy | ||
| Yes | 166 (40.4) | 84 (40.8) |
| No | 245 (59.6) | 122 (59.2) |
| Chemotherapy type | ||
| Cisplatin based | 311 (75.7) | 157 (76.2) |
| Carboplatin based | 32 (7.8) | 19 (9.2) |
| Both | 68 (16.5) | 29 (14.1) |
| Radiotherapy dose, median (range), Gy, | 70.0 (39.6-74.2) | 70.0 (45.0-76.0) |
| Neck dissection before CRT | ||
| Yes | 10 (2.4) | 3 (1.5) |
| No | 401 (97.6) | 203 (98.5) |
| R0 resection and/or neck dissection after CRT | ||
| Yes | 32 (7.8) | 9 (4.4) |
| No | 379 (92.2) | 197 (95.6) |
| Time from CRT end to randomization, median (range), wk | 16.9 (3.9-27.3) | 16.9 (4.8-26.0) |
Abbreviations: CRT, concurrent chemoradiation; ECOG, Eastern Cooperative Oncology Group;
Data are presented as number (percentage) of patients unless otherwise indicated.
Smoking pack-years were summarized for ex- and current smokers who reported pack-years at the screening visit. The less than 10 pack-years group includes nonsmokers.
Sample sizes are 409 for the afatinib group and 205 for the placebo group.
Median treatment duration was 300.0 days (interquartile range [IQR], 92.0-559.0 days) with afatinib and 455.5 days (IQR, 228.0-560.0 days) with placebo; 85.3% of patients in the afatinib group and 98.5% of patients in the placebo group had taken at least 80% of the planned doses of study medication.
Efficacy
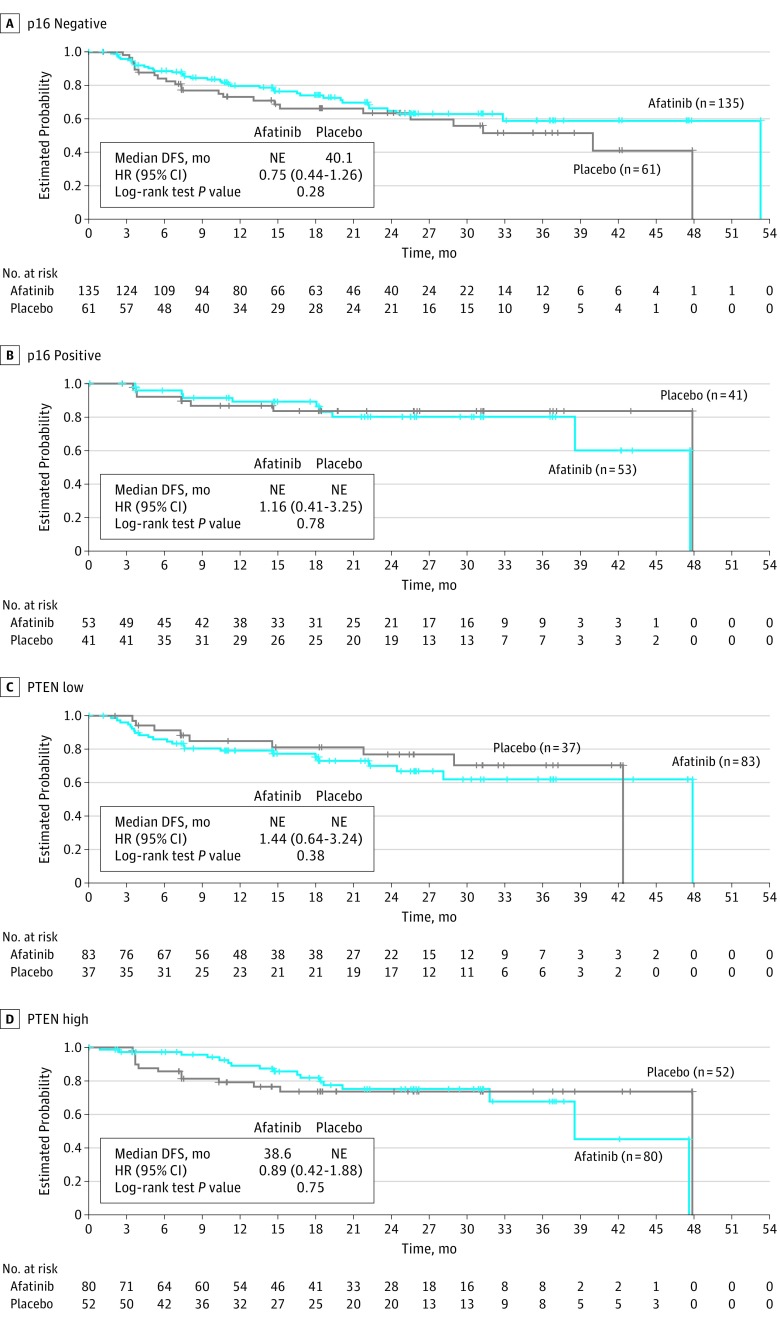
Data cutoff for analysis of DFS was October 25, 2016, after a median follow-up of 21.9 months (IQR, 11.0-31.3 months); 109 (26.5%) of 411 patients in the afatinib group and 52 (25.2%) of 206 patients in the placebo group had experienced a DFS event. Median DFS was 43.4 months (95% CI, 37.4 months to not estimable) with afatinib therapy and could not be estimated (95% CI, 40.1 months to not estimable) with placebo (HR, 1.13; 95% CI, 0.81-1.57; stratified log-rank test P = .48) (Figure 2A). Preplanned subgroup analyses of median DFS (Figure 2B) suggested that afatinib resulted in a worse DFS in patients with nodal status N0 to N2a (HR, 2.23; 95% CI, 1.18-4.22) and no benefit in patients with nodal status N2b to N3 (HR, 0.82; 95% CI, 0.55-1.21). In the biomarker-based analyses, the DFS HR for afatinib vs placebo was 0.75 (95% CI, 0.44-1.26) in patients with centrally confirmed p16-negative status and 0.89 (95% CI, 0.42-1.88) among those with tumors expressing high levels of PTEN (Figure 3). There was no difference between afatinib therapy and placebo based on ERBB3 expression levels (HR, 0.94; 95% CI, 0.32-2.80) (eFigure 1 in Supplement 1). Time from CRT to randomization was balanced between arms (Table 1) and did not affect DFS (HR, 0.94 [95% CI, 0.51-1.72] for patients with time from CRT to randomization ≤3 months and 1.24 [95% CI, 0.84-1.85] for those with time from CRT to randomization of >3 months; Cox proportional hazards regression model P = .43).
Figure 2. Analysis of Disease-Free Survival (DFS).
A, Kaplan-Meier estimates of DFS for all randomized patients. B, Forest plot of DFS according to predefined subgroups. CRT indicates chemoradiotherapy; CT, chemotherapy; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; NE, not estimable; and RT, radiotherapy.
Figure 3. Disease-Free Survival (DFS) According to p16 Status and PTEN Status by Central Testing.
Kaplan-Meier estimates of DFS in patients with p16-negative tumors (A), patients with p16-positive tumors (B), patients with tumors expressing low levels of PTEN (immunohistochemistry [IHC] H score ≤150), and patients with tumors expressing high levels of PTEN (IHC H score >150). HR indicates hazard ratio; NE, not estimable.
The probability of being disease free at 2 years was 67.2% in the afatinib group and 73.5% in the placebo group (estimated difference, −6.3%; 95% CI, −15.0 to 2.5; P = .16). At data cutoff, OS data were immature (only approximately 15% of the expected OS events had occurred); 62 patients (15.1%) had died in the afatinib group and 23 (11.2%) in the placebo group. Median OS could not be estimated for either group.
Health-Related Quality of Life
Among patients in the randomized population, QLQ-C30 and QLQ-HN35 questionnaire completion rates were high during the treatment visits (approximately 90%), decreasing from 50% to 60% for the end of treatment visit (eTable 1 in Supplement 1).
No significant difference was found in the proportions of patients with improving or worsening global health status or QoL between the 2 groups (odds ratio [OR] for improved vs not improved, 0.8; 95% CI, 0.58-1.16; P = .26) or for subscales of overall health or QoL. Similarly, no significant differences were found in the proportions of patients with an improving or worsening overall pain score (OR for improved vs not improved, 1.4; 95% CI, 1.0-2.10; P = .052) or swallowing score (OR, 1.4; 95% CI, 0.99-2.07; P = .06).
Time to deterioration (time to a ≥10-point worsening in score from baseline22) was significantly shorter in the afatinib group than in the placebo group for global health status and QoL as well as pain (eFigure 2 in Supplement 1). No significant difference was found in time to deterioration in swallowing scores for afatinib vs placebo. Changes in global health status (mean [SE] difference = −3.4 [0.98]; P < .001) and pain scores (mean [SE] difference = 3.2 [1.08], P = .003) over time significantly favored placebo, whereas no significant difference was found in swallowing scores (mean [SE] difference = 1.3 [1.08]; P = .22) (eTable 2 in Supplement 1).
Safety
Treatment-related AEs were reported in 396 patients (96.4%) in the afatinib group and 114 patients (55.3%) in the placebo group. The most common grade 3 to 4 treatment-related AEs with afatinib were rash or acne (61 [14.8%]), diarrhea (32 [7.8%]), and stomatitis (55 [13.4%]) (Table 2 and eTable 3 in Supplement 1).
Table 2. All-Grade Treatment-Related AEs (≥5% Incidence in Either Treatment Group).
| Event | No. (%) of AEs | |||||||
|---|---|---|---|---|---|---|---|---|
| Afatinib Group (n = 411) | Placebo Group (n = 206) | |||||||
| Grade 1-2 | Grade 3 | Grade 4 | Grade 5 | Grade 1-2 | Grade 3 | Grade 4 | Grade 5 | |
| Total with related AEs | 234 (56.9) | 154 (37.5) | 7 (1.7) | 1 (0.2) | 105 (51.0) | 9 (4.4) | 0 | 0 |
| Rash or acnea | 267 (65.0) | 60 (14.6) | 1 (0.2) | 0 | 43 (20.9) | 1 (0.5) | 0 | 0 |
| Diarrhea | 291 (70.8) | 32 (7.8) | 0 | 0 | 26 (12.6) | 1 (0.5) | 0 | 0 |
| Stomatitisa | 150 (36.5) | 55 (13.4) | 0 | 0 | 22 (10.7) | 1 (0.5) | 0 | 0 |
| Paronychiaa | 73 (17.8) | 11 (2.7) | 0 | 0 | 4 (1.9) | 0 | 0 | 0 |
| Fatiguea | 75 (18.2) | 2 (0.5) | 0 | 0 | 16 (7.8) | 1 (0.5) | 0 | 0 |
| Dry skin | 65 (15.8) | 1 (0.2) | 0 | 0 | 10 (4.9) | 0 | 0 | 0 |
| Decreased appetite | 48 (11.7) | 5 (1.2) | 1 (0.2) | 0 | 8 (3.9) | 0 | 0 | 0 |
| Pruritus | 47 (11.4) | 4 (1.0) | 0 | 0 | 9 (4.4) | 0 | 0 | 0 |
| Nausea | 36 (8.8) | 0 | 0 | 0 | 11 (5.3) | 1 (0.5) | 0 | 0 |
| Epistaxis | 34 (8.3) | 0 | 0 | 0 | 1 (0.5) | 0 | 0 | 0 |
| Weight decreased | 31 (7.5) | 0 | 0 | 0 | 3 (1.5) | 0 | 0 | 0 |
| Palmar-plantar erythrodysesthesia syndrome | 28 (6.8) | 2 (0.5) | 0 | 0 | 0 | 0 | 0 | 0 |
| Dry mouth | 25 (6.1) | 1 (0.2) | 0 | 0 | 2 (1.0) | 0 | 0 | 0 |
| Vomiting | 24 (5.8) | 0 | 0 | 0 | 8 (3.9) | 2 (1.0) | 0 | 0 |
| Dysgeusia | 20 (4.9) | 1 (0.2) | 0 | 0 | 5 (2.4) | 0 | 0 | 0 |
| Dyspepsia | 20 (4.9) | 1 (0.2) | 0 | 0 | 4 (1.9) | 0 | 0 | 0 |
Abbreviation: AE, adverse event.
Grouped term.
Adverse events leading to dose reduction occurred in 217 patients (52.8%) receiving afatinib therapy and 10 (4.9%) receiving placebo; the most common AEs were diarrhea (83 [20.2%] in the afatinib group vs 1 [0.5%] in the placebo), rash or acne (72 [17.5%] in the afatinib group vs 1 [0.5%] in the placebo group), and stomatitis (53 [12.9%] in the afatinib group vs 2 [1.0%] in the placebo group). Sixty-nine afatinib-treated patients (16.8%) had an AE leading to permanent treatment discontinuation; the most common were diarrhea (14 [3.4%]), stomatitis (14 [3.4%]), and rash or acne (9 [2.2%]). Fourteen patients (6.8%) in the placebo group had an AE that led to treatment discontinuation (neoplasm recurrence in 2 patients [not considered treatment related]; other AEs occurred in 1 patient each).
Serious AEs occurred in 80 patients (19.5%) in the afatinib group and 51 patients (24.8%) in the placebo group; treatment-related serious AEs occurred in 22 patients (5.4%) in the afatinib group and 3 patients (1.5%) in the placebo group. The most common treatment-related serious AEs were anemia, decreased appetite, and interstitial lung disease (each affecting 3 patients [0.7%]) receiving afatinib therapy and ischemic stroke, pulmonary alveolar hemorrhage, and respiratory tract infections (each affecting 1 patient [0.5%]) receiving placebo. Nine patients (2.2%) in the afatinib group and 6 (2.9%) in the placebo group had a fatal AE. One in the afatinib group was considered treatment related: the patient had cachexia at baseline, and weight loss was reported as an AE.
Discussion
To our knowledge, LUX-Head & Neck 2 is the first trial to assess broad ERBB family blockade vs placebo as adjuvant therapy after definitive CRT in patients with primary unresected, locoregionally advanced high- to intermediate-risk HNSCC. The trial failed to demonstrate superiority in terms of DFS at a preplanned futility analysis and was closed prematurely. At trial cessation, a lower percentage of patients in the afatinib group (approximately 30%) had completed the planned treatment period than in the placebo group (approximately 42%); early termination will have likely limited the number of patients who completed the planned 18-month treatment. Median exposure to study treatment was markedly shorter in the afatinib group than in the placebo group.
Overall, the study found that afatinib after definitive CRT in patients with intermediate- to high-risk unresected HNSCC did not improve DFS vs placebo. Subgroup analyses of DFS found no significant benefits with afatinib, although the premature trial closure limits any interpretation of these results because of the high level of censoring. Afatinib did not confer any HRQoL benefit, and changes over time in global health status and pain scores favored placebo. Given that patients had undergone definitive CRT, that patients were disease free at the start of the study, and that afatinib therapy did not affect recurrence, a negative effect on HRQoL with afatinib treatment is not unexpected.
In oropharyngeal squamous cell carcinoma, evidence of human papillomavirus (HPV) association correlates with improved prognosis in the curative and recurrent or metastatic settings.23,24 p16 Protein is a surrogate marker for HPV infection in oropharyngeal squamous cell carcinoma.25 As such, DFS events would be expected to occur less frequently in p16/HPV-positive patients. At the time of study design, no validated p16 assay was available; hence, the study was enriched for high- and intermediate-risk patients (ie, p16/HPV-negative patients) by excluding patients with a smoking history of 10 pack-years or less with an oropharyngeal primary tumor site. However, p16 status was unknown for approximately half of the patients because biomarker testing was not mandatory. Nevertheless, for patients with known p16-negative status, the DFS HR was 0.75 (95% CI, 0.44-1.26; P = .28). This finding is consistent with data from the phase 3 LUX-Head & Neck 1 trial, which compared treatment with afatinib vs methotrexate in patients with recurrent or metastatic HNSCC.15 Analysis of tumor biomarkers from LUX-Head & Neck 1 found that patients with p16-negative disease derived increased benefit from afatinib.26 Patients with tumors that were EGFR amplified, ERBB3 low, or PTEN high also had increased benefit from afatinib in LUX-Head & Neck 1. In the present study (LUX-Head & Neck 2), although the early trial termination limited interpretation of subgroup analyses, we also found a suggestion (albeit a relatively weak signal) that preserved PTEN expression may be associated with a benefit of afatinib over placebo; however, there was no apparent difference between treatments based on ERBB3 expression.
The DFS observed in the control group was prolonged relative to our estimates, which may have limited the ability of our study to show a benefit for the afatinib group. It is possible that HPV status could have influenced the median DFS if our study included a higher proportion of HPV-positive patients, for whom prognosis is usually more favorable. However, in the MAINTYNANCE study, 82% of patients in the placebo group were HPV positive (unknown status in only 5%),21 whereas in our study, only 19.9% of patients were known to be HPV positive, with 50.5% of patients having unknown status. This finding suggests that the differences in expected vs observed DFS were unlikely to be a result of HPV status.
Treatment of high- and intermediate-risk, locoregionally advanced HNSCC remains challenging; however, to date, adjuvant and maintenance therapies have not demonstrated improvements in DFS or OS when used in unselected or clinically selected patients. Although blockade of ERBB family members in HNSCC has strong scientific rationale and demonstrated efficacy in platinum-refractory, recurrent or metastatic HNSCC, these results have not translated into the adjuvant setting. The addition of lapatinib therapy, an EGFR/ERBB2 inhibitor, to postoperative CRT and as long-term maintenance did not improve outcomes when compared with placebo in patients with surgically treated high-risk HNSCC.21 Similarly, the addition of panitumumab therapy, an EGFR antibody, to CRT in patients with unresected, locoregionally advanced HNSCC did not confer any benefit vs CRT alone.27 Although there are differences in study designs, the consistency of results suggests the role of ERBB inhibition in the adjuvant setting may need to be reassessed. Differences between antibody- and tyrosine kinase inhibition–sensitive cancers may emerge from the biomarker characterization of these cancers, and future studies in molecularly enriched populations may be warranted. For example, for afatinib, the p16-negative, PTEN-expressing patients with high nodal stage may be most appropriate for future trials of adjuvant afatinib. Preclinical studies have recently identified potential approaches to enhance ERBB3 blockade in HNSCC, for example, via agents that lock ERBB3 in the inactive conformation,28 via dual targeting of ERBB3 and Trop2,29 or via targeting of cetuximab and bromodomain-containing protein 4.30 However, more work is required to assess these approaches in the clinic.
In the present study, the afatinib safety profile was in line with previous reports.15 No unexpected safety findings were observed during the median treatment period. As might be expected in a placebo-controlled trial, the frequency of AEs was higher in patients receiving active treatment; however, in general, afatinib could be tolerated with appropriate dose adjustment and AE management.
Limitations
This study has a number of limitations, not least the premature termination, which limit the conclusions that can be drawn. In addition, at the time of study design, HPV biomarkers were still being debated; hence, HPV status was not included as a stratification factor in randomization. Patients may also have harbored additional phosphoinositide 3-kinases pathway mutations that affected EGFR inhibition and therefore outcome. Furthermore, patients were eligible for enrollment up to 24 weeks after completion of CRT, during which time many high-risk patients may have relapsed, possibly leading to a selection bias toward favorable-risk patients.
Conclusions
In this study, treatment with afatinib did not improve DFS compared with placebo in patients with primary unresected, clinically high- to intermediate-risk HNSCC and was associated with more treatment-related AEs and reduced QoL. Afatinib maintenance therapy in this setting is not recommended based on these results.
eMaterials and eMethods. Supplementary Methods and Materials
eTable 1. Questionnaire Completion Rates for QLQ-C30 and QLQ-HN3
eTable 2. Change in Global Health Status/QoL, Pain and Swallowing Scores Over Time
eTable 3. All-Grade Treatment-Related AEs
eFigure 1. Kaplan-Meier Estimates of Disease-Free Survival According to HER3 Status by Central Testing. (A) HER3 IHC ≤50 and (B) HER3 IHC >50
eFigure 2. Kaplan-Meier Estimates of Time to Deterioration in (A) EORTC Global Health Status, (B) EORTC Pain and (C) EORTC Swallowing
Trial Protocol
Data Sharing Statement
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-E386. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Seiwert TY, Salama JK, Vokes EE. The chemoradiation paradigm in head and neck cancer. Nat Clin Pract Oncol. 2007;4(3):156-171. doi: 10.1038/ncponc0750 [DOI] [PubMed] [Google Scholar]
- 3.Kelly JR, Park HS, An Y, et al. Comparison of survival outcomes among human papillomavirus-negative cT1-2 N1-2b patients with oropharyngeal squamous cell cancer treated with upfront surgery vs definitive chemoradiation therapy: an observational study. JAMA Oncol. 2017;3(8):1107-1111. doi: 10.1001/jamaoncol.2016.5769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lango MN, Andrews GA, Ahmad S, et al. Postradiotherapy neck dissection for head and neck squamous cell carcinoma: pattern of pathologic residual carcinoma and prognosis. Head Neck. 2009;31(3):328-337. doi: 10.1002/hed.20976 [DOI] [PubMed] [Google Scholar]
- 5.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35. doi: 10.1056/NEJMoa0912217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen EE, Karrison TG, Kocherginsky M, et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. 2014;32(25):2735-2743. doi: 10.1200/JCO.2013.54.6309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haddad R, O’Neill A, Rabinowits G, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol. 2013;14(3):257-264. doi: 10.1016/S1470-2045(13)70011-1 [DOI] [PubMed] [Google Scholar]
- 8.Ang KK, Berkey BA, Tu X, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62(24):7350-7356. [PubMed] [Google Scholar]
- 9.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567-578. doi: 10.1056/NEJMoa053422 [DOI] [PubMed] [Google Scholar]
- 10.Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA; Eastern Cooperative Oncology Group . Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol. 2005;23(34):8646-8654. doi: 10.1200/JCO.2005.02.4646 [DOI] [PubMed] [Google Scholar]
- 11.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116-1127. doi: 10.1056/NEJMoa0802656 [DOI] [PubMed] [Google Scholar]
- 12.Stewart JS, Cohen EE, Licitra L, et al. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck [published correct appears in J Clin Oncol. 2009;27(20):3410]. J Clin Oncol. 2009;27(11):1864-1871. doi: 10.1200/JCO.2008.17.0530 [DOI] [PubMed] [Google Scholar]
- 13.Bauman JE, Austin MC, Schmidt R, et al. ERCC1 is a prognostic biomarker in locally advanced head and neck cancer: results from a randomised, phase II trial. Br J Cancer. 2013;109(8):2096-2105. doi: 10.1038/bjc.2013.576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D, Qian G, Zhang H, et al. HER3 targeting sensitizes HNSCC to cetuximab by reducing HER3 activity and HER2/HER3 dimerization: evidence from cell line and patient-derived xenograft models. Clin Cancer Res. 2017;23(3):677-686. doi: 10.1158/1078-0432.CCR-16-0558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machiels JP, Haddad RI, Fayette J, et al. ; LUX-H&N 1 investigators . Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): an open-label, randomised phase 3 trial. Lancet Oncol. 2015;16(5):583-594. doi: 10.1016/S1470-2045(15)70124-5 [DOI] [PubMed] [Google Scholar]
- 16.Argiris A, Heron DE, Smith RP, et al. Induction docetaxel, cisplatin, and cetuximab followed by concurrent radiotherapy, cisplatin, and cetuximab and maintenance cetuximab in patients with locally advanced head and neck cancer. J Clin Oncol. 2010;28(36):5294-5300. doi: 10.1200/JCO.2010.30.6423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C, Kane M, Song J, et al. Phase I trial of gefitinib in combination with radiation or chemoradiation for patients with locally advanced squamous cell head and neck cancer. J Clin Oncol. 2007;25(31):4880-4886. doi: 10.1200/JCO.2007.12.9650 [DOI] [PubMed] [Google Scholar]
- 18.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 19.Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A. The EORTC QLQ-C30 scoring manual. 3rd ed Brussels, Belgium: European Organisation for Research and Treatment of Cancer; 2001. [Google Scholar]
- 20.Cancer Therapy Evaluation Program Common Terminology Criteria for Adverse Events, version 3.0 (CTCAE). National Cancer Institute. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Published August 9, 2006. Accessed May 15, 2019.
- 21.Harrington K, Temam S, Mehanna H, et al. Postoperative adjuvant lapatinib and concurrent chemoradiotherapy followed by maintenance lapatinib monotherapy in high-risk patients with resected squamous cell carcinoma of the head and neck: a phase III, randomized, double-blind, placebo-controlled study. J Clin Oncol. 2015;33(35):4202-4209. doi: 10.1200/JCO.2015.61.4370 [DOI] [PubMed] [Google Scholar]
- 22.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139-144. doi: 10.1200/JCO.1998.16.1.139 [DOI] [PubMed] [Google Scholar]
- 23.Deng Z, Hasegawa M, Aoki K, et al. A comprehensive evaluation of human papillomavirus positive status and p16INK4a overexpression as a prognostic biomarker in head and neck squamous cell carcinoma. Int J Oncol. 2014;45(1):67-76. doi: 10.3892/ijo.2014.2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vermorken JB, Psyrri A, Mesía R, et al. Impact of tumor HPV status on outcome in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck receiving chemotherapy with or without cetuximab: retrospective analysis of the phase III EXTREME trial. Ann Oncol. 2014;25(4):801-807. doi: 10.1093/annonc/mdt574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus–associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24(5):736-747. doi: 10.1200/JCO.2004.00.3335 [DOI] [PubMed] [Google Scholar]
- 26.Cohen EEW, Licitra LF, Burtness B, et al. Biomarkers predict enhanced clinical outcomes with afatinib versus methotrexate in patients with second-line recurrent and/or metastatic head and neck cancer. Ann Oncol. 2017;28(10):2526-2532. doi: 10.1093/annonc/mdx344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mesía R, Henke M, Fortin A, et al. Chemoradiotherapy with or without panitumumab in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-1): a randomised, controlled, open-label phase 2 trial. Lancet Oncol. 2015;16(2):208-220. doi: 10.1016/S1470-2045(14)71198-2 [DOI] [PubMed] [Google Scholar]
- 28.Lee S, Greenlee EB, Amick JR, et al. Inhibition of ErbB3 by a monoclonal antibody that locks the extracellular domain in an inactive configuration. Proc Natl Acad Sci U S A. 2015;112(43):13225-13230. doi: 10.1073/pnas.1518361112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Redlich N, Robinson AM, Nickel KP, et al. Anti-Trop2 blockade enhances the therapeutic efficacy of ErbB3 inhibition in head and neck squamous cell carcinoma. Cell Death Dis. 2018;9(1):5. doi: 10.1038/s41419-017-0029-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leonard B, Brand TM, O’Keefe RA, et al. BET inhibition overcomes receptor tyrosine kinase-mediated cetuximab resistance in HNSCC. Cancer Res. 2018;78(15):4331-4343. doi: 10.1158/0008-5472.CAN-18-0459 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMaterials and eMethods. Supplementary Methods and Materials
eTable 1. Questionnaire Completion Rates for QLQ-C30 and QLQ-HN3
eTable 2. Change in Global Health Status/QoL, Pain and Swallowing Scores Over Time
eTable 3. All-Grade Treatment-Related AEs
eFigure 1. Kaplan-Meier Estimates of Disease-Free Survival According to HER3 Status by Central Testing. (A) HER3 IHC ≤50 and (B) HER3 IHC >50
eFigure 2. Kaplan-Meier Estimates of Time to Deterioration in (A) EORTC Global Health Status, (B) EORTC Pain and (C) EORTC Swallowing
Trial Protocol
Data Sharing Statement