This cohort study assesses and validates the Dutch Crosslinking for Keratoconus (DUCK) score, a novel clinical assessment tool for crosslinking treatment in patients with keratoconus.
Key Points
Question
Can adhering to the Dutch Crosslinking for Keratoconus (DUCK) score improve clinical decision making in patients with progressive keratoconus?
Findings
This cohort study of 504 eyes and 388 patients found that adhering to the DUCK score was associated with a reduction in the overall crosslinking treatment rate without increasing the risk of disease progression.
Meaning
This DUCK score may better identify eyes that were rightly withheld treatment and may prevent unnecessary exposure to treatment risks and improve overall effectiveness.
Abstract
Importance
Defining keratoconus progression is fundamental in clinical decision making because crosslinking treatments are indicated when the disease is considered progressive. Currently, there is no consensus which parameters should be used to define progression.
Objective
To assess and validate a novel clinical scoring system as an easy-to-use assessment tool for crosslinking treatment in patients with keratoconus.
Design, Setting, and Participants
Prospective cohort study at 2 academic treatment centers. Patients with keratoconus referred between January 1, 2012, and June 30, 2014, with 2-year follow-up were included. Analysis began March 2017.
Interventions
The Dutch Crosslinking for Keratoconus (DUCK) score is based on changes in 5 clinical parameters that are routinely assessed: age, visual acuity, refraction error, keratometry, and subjective patient experience. The DUCK score is derived by scoring 0 to 2 points per item, and cutoffs were determined by clinical experience. We compared the DUCK scores to the conventional 1.0-diopter increase in maximum keratometry criterion, within the last 12 months, in a longitudinal discovery and a validation cohort. Sensitivity analyses and intraitem correlations were performed.
Main Outcomes and Measures
Overall treatment rate reduction and the duly withheld treatment rate.
Results
A total of 504 eyes of 388 patients were available for analysis on disease progression in the course of 12 and 24 months. Baseline patient characteristics of the discovery cohort and the validation cohort were comparable in terms of age (mean [SD], 26.8 [8.3] years vs 26.3 [9.1]), sex (216 of 332 [65%] vs 123 of 172 [72%] men), and maximum keratometry (mean [SD], 53.5 [7.1] vs 52.7 [6.3]). Adhering to the DUCK score, rather than maximum keratometry, was associated with a reduction in overall treatment rate by 23% (95% CI, 18%-30%), without increasing the risk of disease progression (ie, the rate of progression for both groups was equal; ±0%). The DUCK score appears to better identify eyes that were duly withheld treatment by 35% (95% CI, 22%-49%).
Conclusions and Relevance
These results provide validation of the DUCK score as a tool to determine whether a crosslinking treatment might be warranted. Compared with the conventional maximum keratometry criterion of more than 1.0 diopter, the DUCK score may better select patients who might benefit from crosslinking treatment. Potentially, it may prevent unnecessary treatments, reduce exposure to treatment risks, and improve the cost effectiveness of crosslinking.
Introduction
Keratoconus is a corneal condition that can lead to refractive myopia, irregular astigmatism, corneal thinning, and poor visual acuity due to the hallmark conelike shape of the cornea and in advanced cases, corneal scarring.1 Keratoconus has an annual incidence of 1:7500, with an estimated prevalence of 1:375.2 Its multifactorial etiology is an enigma, although current insights pivot toward an inflammatory origin.3,4 Keratoconus typically develops in the second decade of life until progress gradually halts.5 However, progression is generally more severe in younger patients.6Corneal crosslinking (CXL), a combination of local UV radiation and riboflavin photosensitizing eyedrops, is regarded to be an established treatment to prevent disease progression.7 The clinical effects of CXL appear to last, although long-term follow-up is limited owing to the novelty of the treatment.8 Corneal crosslinking is a cost-effective treatment to prevent vision loss, and it reduces the need for corneal transplant surgery.9,10
Currently, global consensus dictates that the main indication for CXL treatment is progression of keratoconus, regardless of visual acuity or age.11 However, guidelines lack a clear definition of disease progression. Three seminal randomized clinical trials12,13,14,15 on CXL effectiveness–based keratoconus progression on changes in keratometry readings (>1.0-diopter [D] increase in steepest meridian), a change in visual acuity, and/or a change in refractive error (>1.0-D increase in manifest cylinder).
Keratometry is an important parameter with adequate repeatability.16,17 Keratoconus grading systems are available, although the Amsler-Krumeich classification is poorly suited for assessing disease progression,18 and the new ABCD grading is increasingly dependent on corneal tomography readings.19 Importantly, none of the above-mentioned trials, guidelines, or grading systems incorporates extraocular factors such as eye rubbing,20 age,21 hormonal changes during pregnancy,22 contact lens tolerability,23 ethnic origin,24 or pericorneal diseases such as atopia and allergic conjunctivitis.25 Furthermore, patient preferences are not explicitly considered.
Efforts to improve patient selection for any health intervention should focus on reducing overtreatment while preventing undertreatment. The ultimate aim is to reduce exposure to potential health risks associated with unnecessary treatments and to improve overall cost effectiveness.26 Analogous to existing algorithms that aid in clinical decision making such as the Glasgow Coma Scale score27or Apgar score,28 we conceptualized an easy-to-use compound score to assess disease progression in keratoconus: the Dutch Crosslinking for Keratoconus (DUCK) score. The DUCK score is an assessment tool that provides quick insight in disease activity (Table 1). It incorporates age, patient-reported quality of vision, objective visual acuity, manifest refraction, and keratometry. The goal of this study is to compare the DUCK score with current practice, ie, assessing indication for CXL based on keratometry readings.12 We validated our findings in an independent cohort from another academic hospital.
Table 1. The DUCK Scorea.
| Variable | Score 0 | Score 1 | Score 2 |
|---|---|---|---|
| Age, y | >35 | 18-35 | <18 |
| Quality of vision | No impact on daily life | Mild impact on daily life | Severe impact on daily life |
| UDVA difference, Snellen line | <1 | 1-2 | >2 |
| Refraction difference (SE), D | <0.5 | 0.5-1.0 | >1.0 |
| Maximum keratometry difference, D | <1 | 1-2 | >2 |
Abbreviations: D, diopter; DUCK, Dutch Crosslinking for Keratoconus; SE, spherical equivalent; UDVA, uncorrected distance visual acuity.
To assess differences, parameters should be documented at 2 separate moments. Always control extraocular inflammation and advise not to rub the eyes prior to treatment. A DUCK score more than 5 indicates a crosslinking treatment for progressive keratoconus.
Methods
Study Design
The data were collected from an ongoing prospective cohort of patients with keratoconus referred to a tertiary academic hospital (University Medical Center Utrecht, Utrecht, the Netherlands). All patients referred between January 1, 2012, and June 30, 2014, were included in this study. Suitable patients were identified through International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10), registration codes (H18.6), and all records from the included patients were searched for relevant information. An independent validation cohort was collected from the Maastricht University Medical Center using the same methods. The data analyses were commenced in March 22, 2017, and finalized September 24, 2018.
All patients underwent an ophthalmic evaluation including corneal topography. Eyes were excluded when a diagnosis other than keratoconus was established (eg, pellucid marginal degeneration, post–laser-assisted in situ keratomileusis ectasia), when prior corneal treatments had been performed (eg, CXL, intracorneal ring segment implantation), when eyes were not eligible for CXL (eg, corneal scarring, pachymetry below 400 μm), or when a patient visited our clinic only once and thus evaluation over time was not possible. The DUCK study adhered to the Declaration of Helsinki29 and relevant local laws and was approved by the University Medical Center Utrecht Ethics Review Board (METC no° 15/157). Patients provided written and oral consent.
Determining Keratoconus Progression: Current Practice vs DUCK Score
Two methods to establish keratoconus progression were compared in this study: the DUCK score and the conventional change in maximum keratometry. The DUCK score quantifies disease progression and was based on 5 separate domains: age, decrease in visual acuity, increase in refractive error, increase in keratometry readings, and perceived quality of vision (Table 1). These parameters were specifically chosen for their considered association with disease progression, their ease of assessment, their generalizability, and independence of specific equipment or devices. Age is considered (and proven) as a surrogate of corneal biomechanics owing to lower rates of disease progression at increasing age. Inversely, younger cases are in general more severe, and higher rates of disease progression are reported. Cutoff points for age were therefore chosen at younger than 18 years, 18 to 35 years, and older than 35 years.6,30,31 Quality of vision is arguably the most relevant ophthalmic parameter from a patient perspective. However, its quantification is cumbersome, and the association between patient-reported outcomes and refractive state is subject of debate.32 High-contrast visual acuity testing has limitations, but it is embedded in clinical practice and therefore the most practical proxy of visual functioning.33 Uncorrected distance visual acuity (UDVA) was considered the most sensitive parameter for changes in visual functioning for several reasons. First and foremost, early keratoconus can be corrected with glasses. Cases of progressive keratoconus amenable to a phoropter/spectacle correction would lead to a stable corrected distance visual acuity assessment and thus yield no viable data on keratoconus disease progression. A confounding effect of developing axial myopia on UDVA was considered negligible, based on the relatively short interval in measurements (12 months) and the age of typical keratoconus patients (older than 13 years; the Sorsby criterion).34
Likewise, maximum keratometry was considered the most relevant reading for changes in keratometry because of its intrinsic volatility in progressive keratoconus with adequate repeatability16,17 and its widespread use in clinical trials and treatment guidelines.12,13,14 Alternative tomographic parameters either change less pronounced (corneal pachymetry, mean keratometry) or are less embedded in clinical practice (posterior curvature).35
Changes in manifest refractive error were converted to spherical equivalents because both spherical and cylindrical power can increase with disease progression.36 Patient-reported subjective quality of vision was scored as 0 (no complaints), 1 (complaints mildly affecting quality of life), or 2 (complaints severely affecting quality of life). All other domains were also scored 0, 1, or 2 points based on predefined cutoffs motivated by clinical experience and existing literature (Table 1). This leads to a DUCK score range of 0 to 10 points, with 10 indicating the highest rate of disease progression. According to current practice, differences in maximum keratometry were calculated by subtracting follow-up measurements from baseline measurements and an increase of more than 1.0 D over 12 months was used as a cutoff to qualify for CXL. In cases where follow-up fell short of 12 months and maximum keratometry progression was less than 1.0 D (eg, 0.8-D progression over 9 months), expert opinion was used to judge progression. The expert (R.P.L.W.) was aware of only the degree of progression and the time frame over which progression had occurred.
Data Collection
Data were collected at multiple points to determine keratoconus progression and treatment effects. The first consultation and subsequent follow-up of 12 months were used to determine disease progression. If no CXL was performed after the first 12 months of follow-up, another 12 months of follow-up data were collected. If no CXL was performed, follow-up was continued up to 24 months. If, within 24 months after the first consultation CXL was performed, follow-up data were collected until 12 months after treatment (eFigure in the Supplement).
If progressive keratoconus was diagnosed during the first hospital visit in the participating centers, relevant prior measurements and data were collected from the referring center. Because both the discovery and validation cohort represented real-life clinical practice, the decision to perform CXL was not always based on objective, measurable criteria. Therefore, it was possible that eyes with a maximum keratometry increase of more than 1.0 D did not undergo CXL and vice versa.
The following data were collected: age, sex, left or right eye, date of first consultation, UDVA, corrected distance visual acuity, manifest refraction, corneal tomography (maximum, mean, steep, and flat keratometry, cone location [x/y]) and pachymetry (thinnest point, apex, center) (Pentacam HR type 70900; Oculus Optikgerate GmbH), endothelial cell count (SP-3000P; Topcon Corporation), and intraocular pressure (CT-80; Topcon Corporation). Furthermore we recorded any remarks on subjective quality of vision as described to the practitioner, contact lens use, CXL date, specifics of the treatment, and a complete ophthalmic evaluation with focus on periocular eczema, blepharitis, papillary conjunctivitis, and clinical signs of keratoconus. In case of ocular allergy or patients with periocular eczema were treated appropriately (mest-cell stabilizers, antihistamines, tacrolimus ointment) prior to a CXL treatment. Patients were instructed not to rub their eyes and to discontinue contact lens wear 2 weeks prior to the hospital visits. In case data were collected from referring centers, data on corneal tomography were only included if the measurements were performed using corneal tomography to avoid bias due to significant interdevice discrepancies.37,38
Surgical Procedure
Conventional nonaccelerated epithelial-off CXL procedures were performed as described previously by Wollensak et al.39 In summary, after the application of topical anesthetics, a corneal abrasion was made using a blunt knife, and 0.1% riboflavin (Peschke Meditrade GmbH) was applied every 3 minutes for 30 minutes. The cornea was subsequently exposed to a 3-mW 365-nm UV light source for 30 minutes (UV-X; Peschke Meditrade GmbH). Afterwards, a bandage contact lens (Pure Vision, Bausch & Lomb) was placed on the cornea.
Data Analysis
Snellen UDVA and corrected distance visual acuity measurements were converted to logMAR values for analysis. To ensure adequate calculation of DUCK scores at all points, missing values were imputed using multiple imputation.40 Multiple imputation was performed for 3 parameters included in the DUCK score (maximum keratometry, UDVA, and refractive error), and all other variables in the data set were used as possible predictors for those parameters. The 95% confidence intervals of overall treatment rate reduction and duly withheld treatment rates were calculated using a Wilson test for confidence limits for proportions.41 An independent-samples t test was used to analyze baseline differences between the discovery cohort and the validation cohort. A sensitivity analysis was performed to determine the cutoff point for the DUCK score at which the eye was eligible for CXL, and mutual interitem correlations between all 5 parameters of the DUCK score were calculated to assess validity of the chosen values. Interitem correlations were defined as low when Pearson ρ was smaller than 0.2. A 2-sided P value less than .05 was considered statistically significant. Data were analyzed using SPSS version 21.0 (IBM).
Results
In the discovery cohort, 388 eyes of 206 patients were identified between January 1, 2012, and June 30, 2014. After reviewing all individual records, 332 eyes (189 patients) were considered eligible for further analysis. The flowchart (eFigure in the Supplement) provides further details on these numbers and the times at which progression calculations were performed. In the validation cohort, 172 eyes (111 patients) were identified, resulting in a total of 504 eyes eligible for study participation. A total of 230 eyes (46%) underwent CXL during follow-up. Baseline data for both discovery and validation cohorts are shown in Table 2.
Table 2. Baseline Characteristics of 504 Eyes .
| Variable | Cohort, Mean (SD) | P Value | Missing, % | |
|---|---|---|---|---|
| Discovery (n = 332) | Validation (n = 172) | |||
| Age, y | 26.8 (8.3) | 26.3 (9.1) | .54 | 0 |
| Male, No. (%) | 216 (65) | 123 (72) | .71 | 0 |
| Laterality, right eye, No. (%) | 163 (49) | 88 (51) | .16 | 0 |
| Crosslinking treatment, No. % | 159 (47.8) | 72 (41.8) | .20 | 0 |
| Maximum keratometry, D | 53.5 (7.1) | 52.7 (6.3) | .19 | 19.8 |
| Mean keratometry, D | 46.4 (3.7) | 45.8 (3.3) | .15 | 20.8 |
| Spherical equivalent, D | −2.5 (3.3) | −3.0 (3.0) | .12 | 23.8 |
| UDVA logMAR | .64 (.50) | .44 (.40) | <.05a | 35.3 |
| Subjective quality of visionb | ||||
| Mean | 0.7 | 0.3 | <.05a | 33.3 |
| Median | 1 | 0 | ||
Abbreviations: D, diopter; UDVA, uncorrected distance visual acuity.
Calculated using an independent-samples t test and considered statistically significant for P < .05.
Scored as 0 (no complaints), 1 (complaints mildly affecting quality of life), and 2 (complaints severely affecting quality of life).
Sensitivity Analysis
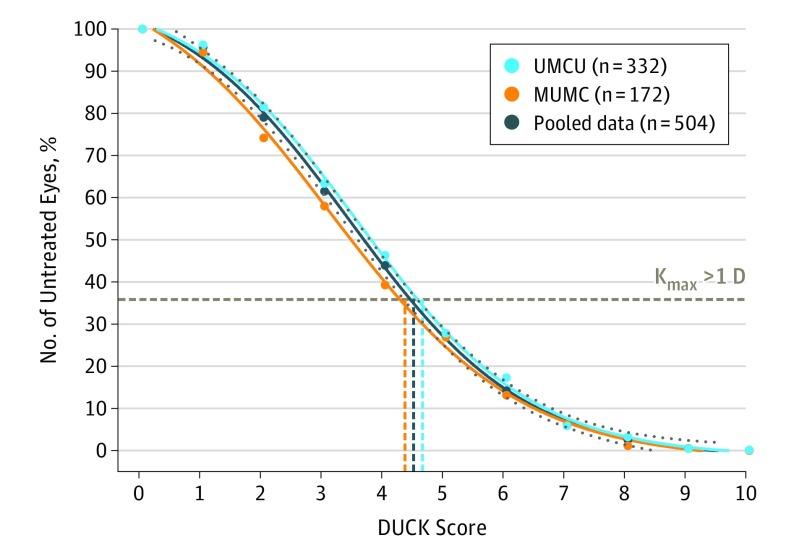
A cutoff value for the DUCK score was determined to assess its validity associated with conventional assessment of disease progression based on changes in maximum keratometry readings only. In the Figure, the DUCK score is plotted against the hypothetical number of CXL treatments performed, where a higher cutoff leads to fewer eyes indicated for CXL. The dotted line represents the percentage of eyes undergoing CXL if treatment decision were based on the conventional criterion of more than 1.0-D increase in maximum keratometry. For the discovery cohort, the steepest part of the curve corresponds to a DUCK score of 4.55, and therefore a cutoff of 5 of 10 or more was chosen as indicative of disease progression warranting CXL treatment. Incorporating the data from the validation cohort did not materially alter these results.
Figure. Sensitivity Analysis of the DUCK Score Compared With Actual Crosslinking Treatments.
A score of more than 5 of 10 was considered indicative of disease progression warranting crosslinking treatment. DUCK incidates Dutch Crosslinking for Keratoconus score; Kmax, maximum keratometry; MUMC, Maastricht University Medical Center; UMCU, University Medical Center Utrecht.
Interitem Correlation Analysis
When different items in a score such as the DUCK score are strongly correlated, the additional information per single item is limited. Therefore, relatively low correlations between items are desirable. All 5 parameters of the DUCK score (age, patient-reported outcomes, keratometry, refractive error, visual acuity) were analyzed for mutual correlations. Table 2 indicates that these mutual correlations in general are very low, albeit significant. Only the association between refraction and quality of vision has a 0.40 correlation (P = .045).
DUCK Score vs Maximum Keratometry Alone
To assess the added value of the DUCK score over the conventional maximum keratometry criterion, a tabulation was created and several subsequent calculations were performed. Table 3 is double layered and reports on (1) the consequences of basing the decision to treat on a DUCK cutoff score of 5, (2) the consequences of basing the decision to treat on maximum keratometry progression of more than 1.0 D, (3) the actual amount of CXL treatments performed, and (4) whether disease progression occurred at follow-up (based on the maximum keratometry progression). For instance, the top left field reports that 109 eyes had a DUCK score lower than 5, a change in maximum keratometry less than 1.0 D, did not undergo CXL, and were considered stable at follow-up. Results are reported for the pooled data only because per-cohort analyses did not reveal substantially different outcomes (eAppendix 1 and 2 in the Supplement). Table 3 (bottom line) shows that 230 (109 + 121) eyes (46%) underwent CXL.
Table 3. DUCK Score Compared With Actual CXL Treatments Done According to the Conventional Maximum Keratometry Criterion.
| DUCK Score | Maximum Keratometry Progression, No. (%) | Sum | |||
|---|---|---|---|---|---|
| Actual CXL Treatment <1.0 D | Actual CXL Treatment >1.0 D | ||||
| No | Yes | No | Yes | ||
| <5 | |||||
| Stablea | 109 (50.9) | 58 (53.2) | 26 (44.1)b | 37 (30.6) | 230 |
| Progressive | 86 (40.2)c | 31 (28.4) | 9 (15.2)c | 9 (7.4) | 135 |
| ≥5 | |||||
| Stablea | 10 (4.7)b | 10 (9.2) | 20 (33.9)b | 60 (49.6) | 100 |
| Progressive | 9 (4.2)c | 10 (9.2) | 4 (6.8) | 15 (12.4) | 38 |
| Sum | 214 | 109 | 59 | 121 | 504 |
Abbreviations: CXL, crosslinking; D, diopter; DUCK, Dutch Crosslinking for Keratoconus.
Stable disease: less than 1.0-D progression in maximum keratometry at 1-year follow-up. Progressive disease was defined as more than 1.0-D progression.
Eyes that had an indication for CXL using the maximum keratometry criterion or the DUCK score but were nonprogressive in the follow-up.
Eyes that received no CXL but follow-up showed progression.
Overtreatment and Undertreatment
If the decision to treat were based on progression in maximum keratometry only, 180 eyes (36%) would undergo CXL. This is shown in the right half of the Table 3 (59 + 121). When using a DUCK score of 5 or higher, 138 eyes (27%) would undergo CXL. This is shown in the bottom half of Table 3 (100 + 38). Basing the decision to treat on the DUCK score instead of progression in maximum keratometry would reduce the number of treatments with 23% (95% CI, 18%-30%) without inducing unwanted undertreatment. Both the maximum keratometrycriterion and the DUCK score did not treat 95 eyes (86 + 9, fields marked in Table 3 with a tablenote), which later appeared to be progressive.
For the maximum keratometrycriterion and the DUCK score, 46 (20 + 26) eyes (9.12%) and 30 (10 + 20) eyes (5.95%), respectively, had an indication for treatment but turned out to be nonprogressive during follow-up (these fields are marked with a tablenote in Table 3). Applying the DUCK score reduced the number of eyes that were duly not treated with 35% (95% CI, 22%-49%).
Overtreatment and undertreatment analyses were also performed using 1 eye per patient; the results using only right eyes or only left eyes in case of bilateral inclusion were virtually the same (eAppendix 3 in the Supplement).
Discussion
The DUCK score is a tool to determine whether a CXL treatment is warranted. The DUCK score was assessed in a discovery cohort of 332 eyes and validated in an independent cohort of 172 eyes. When compared with the conventional criterion of more than 1.0-D increase in keratometry, the DUCK score was associated with a lower overall treatment rate by 23%, without increasing the risk of disease progression (ie, the rate of progression for both groups was equal; ±0%). The DUCK score appeared to better identify eyes that should not be treated compared with a mere keratometry reading (+35%), and it takes the patient’s perspective into account.
The relatively high rate of disease progression after either criterion judged to refrain from treatment deserves attention. The DUCK score was associated with a reduction in the number of CXL treatments, but it appears that clinical criteria are not the sole determinants of disease progression. The emerging insights of an inflammatory activation that affects the ocular surface opens a promising diagnostic avenue.3,4 Future research might be aimed at developing biomarkers that assess keratoconus disease activity.
Strengths and Limitations
This study has several methodological strengths. First, these clinical results and their validation are based on 2 large cohorts, with practically all eyes of all patients included for analysis. The comparability of both cohorts was good, perhaps because of the similar settings: both academic clinics were in the same country and were subjected to the same local laws and health insurance regulations. The homogeneity of the data and a low amount of missing records (Table 2) justified for us to pool the data. The items included in the DUCK score, including the patient’s experience, were only modestly correlated, which means that individual items do add information, compared with maximum keratometry readings alone (Table 4).
Table 4. Interdependence of the 5 Dutch Crosslinking for Keratoconus Score Parameters Showing Significant but Modest Interitem Correlations (N = 504).
| Variable | Quality of Vision | P Value | Age | P Value | Keratometry | P Value | Refraction | P Value |
|---|---|---|---|---|---|---|---|---|
| Age | 0.155 | <.001 | ||||||
| Keratometry | 0.199 | <.001 | 0.067 | .001 | ||||
| Refraction | −0.40 | .045 | −0.003 | .87 | 0.002 | .91 | ||
| Visual acuity | 0.154 | <.001 | 0.002 | .93 | 0.157 | <.001 | 0.078 | <.001 |
A number of limitations deserve attention. First and foremost, this study was not designed as a randomized clinical trial. In eyes that underwent CXL, it is not known if progression had occurred if the treatment had not taken place. For this reason we cannot report reliably on overtreatment. Another factor is that state-of-the-art tomographical indices were deliberately excluded from the DUCK score. A myriad of indices are available in clinical practice, although many are either device specific or aimed toward early diagnosis of keratoconus rather than disease progression.20,38,39,40 Our consideration was that a corneal tomogram is an intermediate outcome parameter from a patient perspective; visual acuity, subjective complaints, and contact lens tolerance affect a patient’s quality of vision and quality of life more directly. Only corneal tomography (maximum keratometry) was chosen to reduce the clinical assessment being too dependent on anatomical (rather than functional) parameters. It was considered to incorporate other moderators of disease progression, such as uncontrolled ocular allergy, atopic constitution, eye rubbing, or contact lens wear. These data are difficult to quantify and Shetty et al42 have showed that proper control of extraocular inflammation prevents disease progression.18,19,43,44,45
Therefore, we recommend that the DUCK score be applied after extraocular morbidities and eye rubbing are addressed. Also, the effect of contact lens wear on disease progression is controversial, and the use of lenses was no determinant for CXL treatment.46,47 For these reasons, the factors mentioned above were not included in the DUCK score.
Conclusions
Defining keratoconus progression is fundamental for clinical decision making in keratoconus care. Optimizing patient selection for CXL treatment prevents unnecessary exposure to treatment risks and improves overall effectiveness. The DUCK score is a clinical tool that may aid in selection of patients that are most likely to benefit from CXL. Adhering to the DUCK score, rather than maximum keratometry, may reduce overall treatment rate between 18 and 30%, without increasing the risk of disease progression (±0%), based on 2 years of follow-up. These findings also suggest the DUCK score may be better able to identify eyes in which treatment was duly withheld.
eAppendix 1. Initial cohort data
eAppendix 2. Validation cohort data
eAppendix 3. Per-eye analyses
eFigure. Flowchart on longitudinal data acquisition of the DUCK study
References
- 1.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42(4):297-319. doi: 10.1016/S0039-6257(97)00119-7 [DOI] [PubMed] [Google Scholar]
- 2.Godefrooij DA, de Wit GA, Uiterwaal CS, Imhof SM, Wisse RPL. Age-specific incidence and prevalence of keratoconus: a nationwide registration study. Am J Ophthalmol. 2017;175:169-172. doi: 10.1016/j.ajo.2016.12.015 [DOI] [PubMed] [Google Scholar]
- 3.Wisse RPL, Kuiper JJW, Gans R, Imhof S, Radstake TRDJ, Van der Lelij A. Cytokine expression in keratoconus and its corneal microenvironment: a systematic review. Ocul Surf. 2015;13(4):272-283. doi: 10.1016/j.jtos.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 4.Ionescu C, Corbu CG, Tanase C, et al. Inflammatory biomarkers profile as microenvironmental expression in keratoconus. Dis Markers. 2016;2016:1243819. doi: 10.1155/2016/1243819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner H, Barr JT, Zadnik K. Collaborative Longitudinal Evaluation of Keratoconus (CLEK) study: methods and findings to date. Cont Lens Anterior Eye. 2007;30(4):223-232. doi: 10.1016/j.clae.2007.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soeters N, van der Valk R, Tahzib NG. Corneal cross-linking for treatment of progressive keratoconus in various age groups. J Refract Surg. 2014;30(7):454-460. doi: 10.3928/1081597X-20140527-03 [DOI] [PubMed] [Google Scholar]
- 7.Parker JS, van Dijk K, Melles GRJ. Treatment options for advanced keratoconus: a review. Surv Ophthalmol. 2015;60(5):459-480. doi: 10.1016/j.survophthal.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 8.Raiskup F, Theuring A, Pillunat LE, Spoerl E. Corneal collagen crosslinking with riboflavin and ultraviolet-A light in progressive keratoconus: ten-year results. J Cataract Refract Surg. 2015;41(1):41-46. doi: 10.1016/j.jcrs.2014.09.033 [DOI] [PubMed] [Google Scholar]
- 9.Godefrooij DA, Mangen MJJ, Chan E, et al. Cost-effectiveness analysis of corneal collagen crosslinking for progressive keratoconus. Ophthalmology. 2017;124(10):1485-1495. doi: 10.1016/j.ophtha.2017.04.011 [DOI] [PubMed] [Google Scholar]
- 10.Godefrooij DA, Gans R, Imhof SM, Wisse RPL. Nationwide reduction in the number of corneal transplantations for keratoconus following the implementation of cross-linking. Acta Ophthalmol. 2016;94(7):675-678. doi: 10.1111/aos.13095 [DOI] [PubMed] [Google Scholar]
- 11.Gomes JAP, Tan D, Rapuano CJ, et al. ; Group of Panelists for the Global Delphi Panel of Keratoconus and Ectatic Diseases . Global consensus on keratoconus and ectatic diseases. Cornea. 2015;34(4):359-369. doi: 10.1097/ICO.0000000000000408 [DOI] [PubMed] [Google Scholar]
- 12.Wittig-Silva C, Chan E, Islam FMA, Wu T, Whiting M, Snibson GR. A randomized, controlled trial of corneal collagen cross-linking in progressive keratoconus: three-year results. Ophthalmology. 2014;121(4):812-821. doi: 10.1016/j.ophtha.2013.10.028 [DOI] [PubMed] [Google Scholar]
- 13.O’Brart DPS, Chan E, Samaras K, Patel P, Shah SP. A randomised, prospective study to investigate the efficacy of riboflavin/ultraviolet A (370 nm) corneal collagen cross-linkage to halt the progression of keratoconus. Br J Ophthalmol. 2011;95(11):1519-1524. doi: 10.1136/bjo.2010.196493 [DOI] [PubMed] [Google Scholar]
- 14.Hersh PS, Stulting RD, Muller D, Durrie DS, Rajpal RK; United States Crosslinking Study Group . United States multicenter clinical trial of corneal collagen crosslinking for keratoconus treatment. Ophthalmology. 2017;124(9):1259-1270. doi: 10.1016/j.ophtha.2017.03.052 [DOI] [PubMed] [Google Scholar]
- 15.Hersh PS, Greenstein SA, Fry KL. Corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. J Cataract Refract Surg. 2011;37(1):149-160. doi: 10.1016/j.jcrs.2010.07.030 [DOI] [PubMed] [Google Scholar]
- 16.McAlinden C, Khadka J, Pesudovs K. A comprehensive evaluation of the precision (repeatability and reproducibility) of the oculus pentacam HR. Invest Ophthalmol Vis Sci. 2011;52(10):7731-7737. doi: 10.1167/iovs.10-7093 [DOI] [PubMed] [Google Scholar]
- 17.Shetty R, Arora V, Jayadev C, et al. Repeatability and agreement of three Scheimpflug-based imaging systems for measuring anterior segment parameters in keratoconus. Invest Ophthalmol Vis Sci. 2014;55(8):5263-5268. doi: 10.1167/iovs.14-15055 [DOI] [PubMed] [Google Scholar]
- 18.Krumeich JH, Daniel J, Knülle A. Live-epikeratophakia for keratoconus. J Cataract Refract Surg. 1998;24(4):456-463. doi: 10.1016/S0886-3350(98)80284-8 [DOI] [PubMed] [Google Scholar]
- 19.Belin MW, Duncan JK. Keratoconus: the ABCD grading system. Klin Monbl Augenheilkd. 2016;233(6):701-707. doi: 10.1055/s-0042-100626 [DOI] [PubMed] [Google Scholar]
- 20.Naderan M, Shoar S, Rezagholizadeh F, Zolfaghari M, Naderan M. Characteristics and associations of keratoconus patients. Cont Lens Anterior Eye. 2015;38(3):199-205. doi: 10.1016/j.clae.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 21.Léoni-Mesplié S, Mortemousque B, Touboul D, et al. Scalability and severity of keratoconus in children. Am J Ophthalmol. 2012;154(1):56-62.e1. doi: 10.1016/j.ajo.2012.01.025 [DOI] [PubMed] [Google Scholar]
- 22.Naderan M, Jahanrad A. Topographic, tomographic and biomechanical corneal changes during pregnancy in patients with keratoconus: a cohort study. Acta Ophthalmol. 2017;95(4):e291-e296. doi: 10.1111/aos.13296 [DOI] [PubMed] [Google Scholar]
- 23.Ünlü M, Yüksel E, Bilgihan K. Effect of corneal cross-linking on contact lens tolerance in keratoconus. Clin Exp Optom. 2017;100(4):369-374. doi: 10.1111/cxo.12470 [DOI] [PubMed] [Google Scholar]
- 24.Cozma I, Atherley C, James NJ. Influence of ethnic origin on the incidence of keratoconus and associated atopic disease in Asian and white patients. Eye (Lond). 2005;19(8):924-925. doi: 10.1038/sj.eye.6701677. [DOI] [PubMed] [Google Scholar]
- 25.Georgiou T, Funnell CL, Cassels-Brown A, O’Conor R. Influence of ethnic origin on the incidence of keratoconus and associated atopic disease in Asians and white patients. Eye (Lond). 2004;18(4):379-383. doi: 10.1038/sj.eye.6700652 [DOI] [PubMed] [Google Scholar]
- 26.Hsu M. From laboratory to clinic and back: connecting neuroeconomic and clinical measures of decision-making dysfunctions In: Diefenbach MA, Miller-Halegoua S, Bowen DJ, eds. Handbook of Health Decision Science. Berlin, Germany: Springer;2016. [Google Scholar]
- 27.Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;2(7872):81-84. doi: 10.1016/S0140-6736(74)91639-0 [DOI] [PubMed] [Google Scholar]
- 28.Apgar V. A proposal for a new method of evaluation of the newborn infant. Curr Res Anesth Analg. 1953;32(4):260-267. [PubMed] [Google Scholar]
- 29.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 30.Koller T, Mrochen M, Seiler T. Complication and failure rates after corneal crosslinking. J Cataract Refract Surg. 2009;35(8):1358-1362. doi: 10.1016/j.jcrs.2009.03.035 [DOI] [PubMed] [Google Scholar]
- 31.Miki A, Maeda N, Ikuno Y, Asai T, Hara C, Nishida K. Factors associated with corneal deformation responses measured with a dynamic scheimpflug analyzer. Invest Ophthalmol Vis Sci. 2017;58(1):538-544. doi: 10.1167/iovs.16-21045 [DOI] [PubMed] [Google Scholar]
- 32.Kandel H, Khadka J, Goggin M, Pesudovs K. Patient-reported outcomes for assessment of quality of life in refractive error: a systematic review. Optom Vis Sci. 2017;94(12):1102-1119. doi: 10.1097/OPX.0000000000001143 [DOI] [PubMed] [Google Scholar]
- 33.Kniestedt C, Stamper RL. Visual acuity and its measurement. Ophthalmol Clin North Am. 2003;16(2):155-170. [DOI] [PubMed] [Google Scholar]
- 34.Fledelius HC, Christensen AS, Fledelius C. Juvenile eye growth, when completed? an evaluation based on IOL-Master axial length data, cross-sectional and longitudinal. Acta Ophthalmol. 2014;92(3):259-264. doi: 10.1111/aos.12107 [DOI] [PubMed] [Google Scholar]
- 35.Fujimoto H, Maeda N, Shintani A, et al. Quantitative evaluation of the natural progression of keratoconus using three-dimensional optical coherence tomography. Invest Ophthalmol Vis Sci. 2016;57(9):oct169-oct175. doi: 10.1167/iovs.15-18650 [DOI] [PubMed] [Google Scholar]
- 36.Wisse RPL, Godefrooij DA, Soeters N, Imhof SM, Van der Lelij A. A multivariate analysis and statistical model for predicting visual acuity and keratometry one year after cross-linking for keratoconus. Am J Ophthalmol. 2014;157(3):519-525. doi: 10.1016/j.ajo.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 37.Hashemi H, Mehravaran S. Corneal changes after laser refractive surgery for myopia: comparison of Orbscan II and Pentacam findings. J Cataract Refract Surg. 2007;33(5):841-847. doi: 10.1016/j.jcrs.2007.01.019 [DOI] [PubMed] [Google Scholar]
- 38.Crawford AZ, Patel DV, McGhee CNJ. Comparison and repeatability of keratometric and corneal power measurements obtained by Orbscan II, Pentacam, and Galilei corneal tomography systems. Am J Ophthalmol. 2013;156(1):53-60. doi: 10.1016/j.ajo.2013.01.029 [DOI] [PubMed] [Google Scholar]
- 39.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620-627. doi: 10.1016/S0002-9394(02)02220-1 [DOI] [PubMed] [Google Scholar]
- 40.Donders ART, van der Heijden GJMG, Stijnen T, Moons KGM. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59(10):1087-1091. doi: 10.1016/j.jclinepi.2006.01.014 [DOI] [PubMed] [Google Scholar]
- 41.Brown LD, Cai TT, DasGupta A. Interval estimation for binomial proportion. Stat Sci. 2001;16(2):101-117. doi: 10.1214/ss/1009213286 [DOI] [Google Scholar]
- 42.Shetty R, Kaweri L, Pahuja N, et al. Current review and a simplified “five-point management algorithm” for keratoconus. Indian J Ophthalmol. 2015;63(1):46-53. doi: 10.4103/0301-4738.151468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ambrósio R Jr, Caiado ALC, Guerra FP, et al. Novel pachymetric parameters based on corneal tomography for diagnosing keratoconus. J Refract Surg. 2011;27(10):753-758. doi: 10.3928/1081597X-20110721-01 [DOI] [PubMed] [Google Scholar]
- 44.Hashemi H, Beiranvand A, Yekta A, Maleki A, Yazdani N, Khabazkhoob M. Pentacam top indices for diagnosing subclinical and definite keratoconus. J Curr Ophthalmol. 2016;28(1):21-26. doi: 10.1016/j.joco.2016.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goebels S, Eppig T, Wagenpfeil S, Cayless A, Seitz B, Langenbucher A. Complementary keratoconus indices based on topographical interpretation of biomechanical waveform parameters: a supplement to established keratoconus indices. Comput Math Methods Med. 2017;2017:5293573. doi: 10.1155/2017/5293573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gasset AR, Houde WL, Garcia-Bengochea M. Hard contact lens wear as an environmental risk in keratoconus. Am J Ophthalmol. 1978;85(3):339-341. doi: 10.1016/S0002-9394(14)77725-6 [DOI] [PubMed] [Google Scholar]
- 47.McMahon TT, Edrington TB, Szczotka-Flynn L, Olafsson HE, Davis LJ, Schechtman KB; CLEK Study Group . Longitudinal changes in corneal curvature in keratoconus. Cornea. 2006;25(3):296-305. doi: 10.1097/01.ico.0000178728.57435.df [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Initial cohort data
eAppendix 2. Validation cohort data
eAppendix 3. Per-eye analyses
eFigure. Flowchart on longitudinal data acquisition of the DUCK study