Abstract
Protein delivery into cells is a potentially transformative tool for treating “undruggable” targets in diseases associated with protein deficiencies or mutations. The vast majority of these targets are accessed via the cytosol, a challenging prospect for proteins with therapeutic and diagnostic relevance. In this review we will present promising non-viral approaches for intracellular and ultimately cytosolic delivery of proteins using nanocarriers. We will also discuss the mechanistic properties that govern the efficacy of nanocarrier-mediated protein delivery, applications of nanomaterials, and key challenges and opportunities in the use of nanocarriers for intracellular protein delivery.
Keywords: intracellular protein delivery, supramolecular assemblies, protein therapeutics, endosomal escape, membrane fusion
1. Introduction
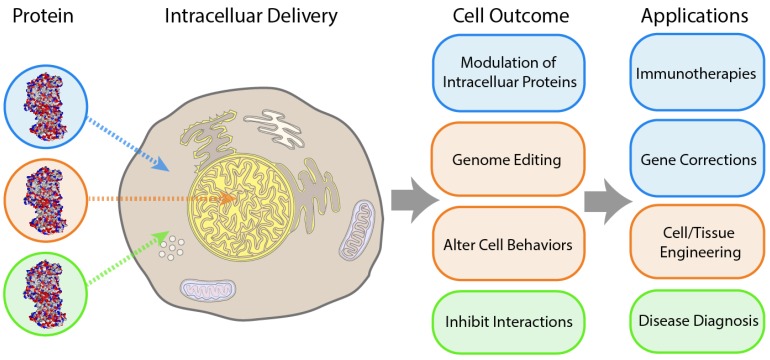
Controlled delivery of proteins into cells is a key tool for biological research and therapeutic applications 1. Intracellular delivery of recombinant proteins has fast-acting consequences 2, 3 and has been successfully demonstrated as a potential strategy for handling a wide range of diseases 4. However, trafficking protein species across the cellular membrane is a challenge, and as such nearly all commercially available protein therapeutics have been employed against extracellular targets. Intracellular delivery presents a major hurdle to the applicability of these therapeutic approaches, and advancements in this field would substantially broaden the utility of protein therapeutics. The challenges associated with intracellular delivery of recombinant proteins are in large part due to membrane impermeability. Endosomal uptake provides a means of overcoming this barrier, but presents an even greater obstacle for most applications, with the exception of lysosomal diseases, through entrapment followed by endo/lysosomal degradation pathways 5, 6. Cytosolic delivery is required for general intracellular activity, as well as for therapeutics targeted at specific organelles, such as the nucleus (for genome editing 7, 8 or antisense therapy 9), and mitochondria (for pro-apoptotic anticancer drugs 10). Platforms capable of cytosolic delivery of protein-based therapeutics would provide new strategies for addressing diseases associated with cancer 11, inflammatory diseases 12, diabetes 13, and neurodegenerative 14 and oxidative stress-related disorders (Figure 1) 15.
Figure 1.
Key outcomes and applications enabled by cytosolic delivery of proteins.
Given the potential impact of intracellular protein therapeutics, the development of effective delivery systems for proteins has been an intense focus for research. Either direct delivery into the cytosol or escape from endosomal pathways has, however, remained a challenge due to numerous factors, including the charge 16, molecular weight, and polarity of proteins 17, as well as biocompatibility of carriers 18 and the necessity to maintain structure and activity in many delivery cargos 19, 20.
Due to the challenges presented by protein delivery into cells, researchers have introduced proteins of interest indirectly by delivering their respective nucleic acids (DNA or mRNA) 21, 22, allowing the cell to produce the protein on its own. Viruses provide effective vectors for these nucleic acid-based deliveries, having evolved naturally to be capable of delivering their genetic cargo to infect the host cell and express new gene sequences 23. This route has been extensively used for the introduction and incorporation of genes of interest into a host cell 24. While largely outside the scope of this review, viral delivery methods pose significant issues for therapeutic applications, including inherently associated toxicity and immunogenicity, which challenges their feasibility as clinically safe and translatable systems 25. Alternative transient delivery methods include use of cationic transfection agents such as Lipofectamine 26, 27 or polyethyleneimine (PEI) 28, or through mechanical methods such as electroporation 29 or membrane deformation 30. However, in general approaches involving the delivery of DNA and mRNA do not offer control over the level of protein expressed by the cell or the timeframe over which protein expression occurs, typically resulting in a distribution of expression levels 25, 31. This heterogeneity presents challenges for both fundamental biological studies and therapeutic dosing.
Direct delivery of proteins to cells can avoid the temporal and expression level issues presented by nucleic acid delivery strategies 25. However, intracellular delivery has remained a challenge for researchers, and to date, this issue remains a generally unmet goal. Direct delivery to the cytosol is challenging 32, and endocytic uptake typically results in substantial sequestration and concomitant degradation by proteases, in particular the cathepsins 33. Substantial effort has focused on the release of protein payloads from the endosome, as will be discussed further in Section 2. Additionally, a number of approaches have focused on direct delivery to the cytosol 34. Physical methods such as electroporation have been used extensively and are well-documented approaches to achieving cytosolic protein introduction; however, there are critical limitations to their use for in vivo application 32.
Approaches utilizing supramolecular interactions have become prominent tools for achieving intracellular delivery of proteins of interest, with notable successes demonstrated in a variety of applications 25. Several platforms have been developed for protein delivery, the foremost being nanoparticles, lipids, and polymeric systems 32. In this review, we will highlight recent and landmark approaches to the intracellular delivery of proteins, focusing on supramolecular nanoassemblies utilized for intracellular protein delivery. We will discuss mechanistic aspects of delivery, along with applications, challenges, and outlooks for intracellular protein therapeutics.
2. Strategies for protein delivery
The cell membrane separates the interior of the cells from the extracellular environment and thus creates a discrete partition between internal and external environments 35 that limits direct intracellular delivery of most compounds to only small molecules and ions capable of membrane diffusion 36. Large molecules such as proteins, polypeptides, and monoclonal antibodies are generally impermeable to the cell due to their high molecular weight, limiting access to intracellular targets 37, 38.
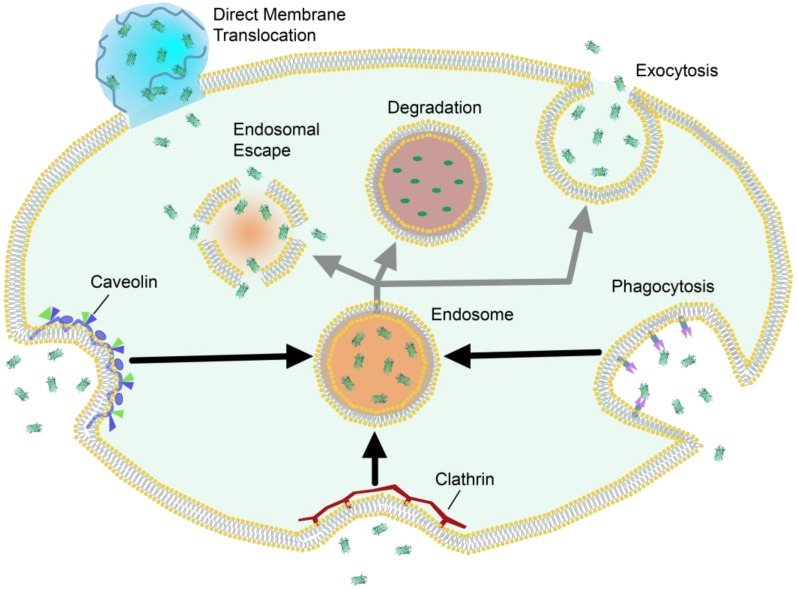
Cells access nutrients, signaling molecules and other exogenous species through endosomal uptake. This is the major cellular mechanism for trafficking across the plasma membrane, and often precedes either exocytosis or sequestration and degradation of the trafficked species (Figure 2). Cells can internalize foreign species through active transport “engulfing” mechanisms such as clathrin-mediated endocytosis 39, 40, phagocytosis 41, 42, micropinocytosis 43, and caveolae-mediated endocytosis 44, which result in varying levels of degradation, exocytosis, or endosomal release 45. Nanocarrier systems have been studied as an avenue by which to promote cellular uptake of exogenous, potentially therapeutic cargo. Due to the overall anionic nature of the phospholipid bilayer, nanomaterials are commonly functionalized to carry cationic charge, which promotes general electrostatic interaction and uptake through endosomal mechanisms 32. Certain nanomaterials are hypothesized to promote direct membrane translocation using cell-penetrating peptides or synthetic delivery vehicles. Unlike endosomal uptake, these approaches are considered passive mechanisms for trafficking across the lipid bilayer 46.
Figure 2.
Schematic representation of selected intracellular trafficking mechanisms across the plasma membrane, and their generalized pathways.
2.1 Endosomal uptake/escape approaches
Engulfment and concomitant entrapment within the endosome is by far the most common uptake mechanism for intracellular delivery. As this mechanism often leads to degradation pathways, endosomal entrapment is a major barrier to intracellular delivery of biomacromolecules and therapeutics. Proteins and other biomacromolecules themselves have no inherent ability to escape the endosome, and thus researchers have taken a variety of approaches to trigger release of the entrapped cargo.
The proton sponge effect (pH-buffering) is a potential endosomal disruption approach mediated by synthetic vectors with a high buffering capacity. In this process, the endosomal membrane ruptures due to osmotic pressure promoted by influx of H+ followed by counterions such as Cl-, resulting in rupturing of the endosome and release of the contents to the cytosol. Vectors intending to employ the proton sponge effect typically feature secondary and tertiary amines, which buffer the acid influx and protect the therapeutic 47. It should be noted, however, that this mechanism has been questioned in recent reports 48.
Multiple additional strategies have been developed for triggering endosomal disruption 49, 50. Engineered peptide sequences, such as GALA have been used to self-assemble across the membrane to create defined nanoscale pores, followed by the release of small molecules or proteins from the endosome 51. The generally small size of these pores however limits diffusion to ions, small molecules, and proteins of up to ~5 kDa. Polymers can interact electrostatically with the lipid membrane and induce endosomal escape 52. PEI in particular has been shown by atomic absorption spectroscopy to be capable of thinning or disrupting membranes 53. This mechanism has been explored through molecular dynamics simulations with lipid membranes, showing a fundamental pathway for endosomal disruption by adsorption and insertion of the polymer into the membrane 54. Many of these approaches though, including those which incorporate PEI, have shown notable toxicity to the host. Approaches that involve the use of pH-responsive functionalities respond to the acidic environment of the endo/lysosome, and trigger interaction of disruptive groups like -COOH or anhydride with the endosomal membrane 55, 56, 57. However, the acidic environment required for these systems can also be harmful to the protein cargo, and furthermore the pH required for release may not be attainable until the cargo reaches the late endosome with its accompanying proteases. Regardless, endosomal escape approaches are typically hindered by low efficiency of release, which has to date limited their applicability.
2.2 Direct Membrane Translocation
Direct membrane translocation serves as an alternative strategy to endosomal uptake for intracellular delivery of proteins. By avoiding the endosomal pathway entirely, these approaches circumvent issues associated with degradation of cargo and limited cytosolic release, generally enhancing delivery efficiency. 25 Typically, translocation occurs through multiple interactions, including lipophilic/hydrophobic switches and electrostatic interactions between components from the protein or nanocarrier, and the membrane constituents, which can lead to temporary membrane disruption 58.
Arginine residues, and more specifically their guanidinium functional moieties, are often critical for efficient membrane penetration in CPPs 59, 60. As a result, guanidinium-functionalized delivery agents have been utilized to promote intracellular delivery. Based on the design of the materials, nano-scale vehicles with guanidinium-functionalities can create regions in the membrane that are locally repartitioned 61. This change in the plasma membrane composition directs the formation of micellar pores in the membrane, which promote the direct trafficking of delivery cargo. While extensively postulated, to date this mechanism is still not fully understood, and remains largely speculative.
3. Protein Delivery Strategies
Numerous approaches have been taken in recent years to achieve the intracellular and ultimately cytosolic delivery of proteins. Among these carrier approaches, supramolecular methods have achieved notable success, largely through the use of nanomaterials including nanoparticles, polymers, and lipids. In the following sections we will discuss advances that have been made in recent years for intracellular delivery of therapeutic proteins using supramolecular assemblies. The introduction of an antigen-specific immune response by polymeric carrier systems 62, 63, 64 is a key challenge in biomedicine, but is largely outside the scope of this review due to the numerous different pathways and immunological processes involved.
3.1 Nanoparticles
3.1.1 Silica
Nanostructures comprised of silica are among the most commonly-used nanomaterials for delivery, largely due to the versatility, flexibility, and relatively acute toxicity of silica itself 65, 66. Kane et al. reported the use of hydrophobic smooth silica nanoparticles to immobilize functional proteins on the nanoparticle surface and deliver them into the cell 67. In this work, 15 nm silica nanoparticles were functionalized with a hydrophobic moiety (n-octadecyltrimethoxysilane, n-ODMS) to encapsulate proteins through hydrophobic interaction. The authors delivered functional ribonuclease A (RNase A) and the antibody against phosphor-Akt (pAkt) to MCF-7 breast cancer cells as well as rat neural stem cells and demonstrated the initiation of cell death in both cases.
Mesoporous silica nanoparticles (MSNs) are nanoscale silica particles featuring networks of mesopores that are capable of encapsulating proteins and/or small molecules 68, 69. Their versatility, low inherent toxicity, and high loading capacity has made them attractive carrier vehicles for protein delivery. Mou et al. utilized modified MSNs for delivering two antioxidant enzymes, superoxide dismutase (SOD) and glutathione peroxidase (GPx), as a synergistic co-delivery approach to enhance scavenging of reactive oxygen species (ROS) 70.
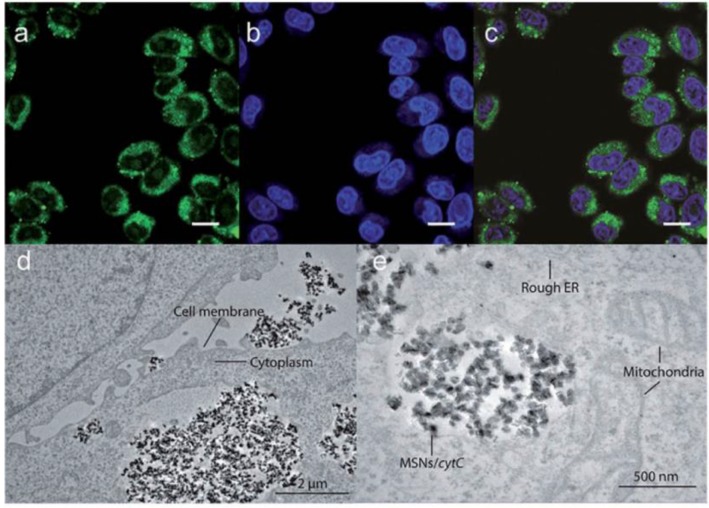
In a recent study by Kros et al., MSNs were developed that effectively delivered proteins 71. In this work, 230 nm MSN-based nanovehicles were developed using 10 nm MSNs coated with a lipid bilayer which contained lipopeptides to facilitate fusion with the cell membrane. The core MSNs were loaded with cytochrome C (CytC) as a delivery cargo and were then encapsulated within the bilayer. The lipid bilayers as well as the cell membrane were pre-treated with complementary coiled-coil lipopeptides, which led to efficient delivery of CytC and subsequent apoptosis of cells. (Figure 3) While the apoptosis indicated that some CytC was available in the cytosol, the punctate fluorescence and lack of protein in the nucleus indicated the majority of CytC remained sequestered in endosomes.
Figure 3.
Confocal images (a-c) and transmission electron microscopy images (d-e) showing endosomal delivery of Atto488-labeled CytC into HeLa cells by MSNs, scale bar = 25 μm. (Adapted with permission from Ref. 71 Copyright (2017) John Wiley and Sons).
Hollow MSNs (H-MSNs) are hollow core-mesoporous shell structures that offer increased loading capacity, low density, and large pore volume 72. In a study by Kim et al. H-MSNs were developed that were capable of endosomal delivery of FITC-labeled proteins of different size and charge to HeLa cells 73. Delivery efficiency in this study was shown to be over 20-fold higher than that of traditional MSNs, as evaluated through fluorescence, though this delivery was also primarily endosomal in nature.
Roughening of the silica nanoparticle surface provides a strategy for increased protein cargo loading. Yang et al. used roughened particles with octadecyl-decorated hydrophobic surface modifications to achieve intracellular delivery 74. These rough silica nanoparticles (RSNs) showed an increased surface area of >200% as compared to smooth silica nanoparticles (SSNs) of similar overall size. In a comparative cell uptake study using MCF-7 and SCC-25 cells, RSNs loaded with RNase A triggered significantly decreased cell viability as compared to cells treated with RNase A-loaded SSNs, demonstrating the effect of increased delivery of active protein as an effect of enhanced loading capacity. In this study the authors also examined endosomal release within the cell and demonstrated that while surface functionalization and roughness both contributed to enhanced uptake, only surface functionalization could trigger enhanced endosomal release of protein into the cell.
3.1.2 Gold Nanoparticles
Gold nanoparticles (AuNPs) provide promising delivery platforms for proteins and small molecules due to the inherent non-toxicity of gold, and their ease of functionalization with a wide variety of ligands. 75, 76. One study by Rotello et al. utilized functionalized AuNPs for the intracellular delivery of a large membrane-impermeable enzyme, β-Galactosidase (β-Gal) 77. This enzyme has a high molecular weight (473 kDa), and is negatively charged, making its delivery into cells particularly challenging. The authors utilized gold nanoparticles with a 2.5 nm core, functionalized with ligands featuring an alkylic segment for core stability, a tetraethylene glycol (TEG) moiety to prevent non-specific interactions, and a cationic His-Lys-Arg-Lys (HKRK) peptide tag to promote protein binding. AuNP-β-Gal interaction was monitored through fluorescence quenching of fluorophore-labeled protein. These complexes delivered their protein cargo into HeLa cells and displayed enzymatic activity as monitored by X-Gal assay. Punctate fluorescence was observed with this approach however, indicating entrapment in either endosomes or other vesicular compartments.
New approaches that integrate the functionality of nanoparticles with the membrane-compatibility of lipids have shown success in a number of protein delivery applications. In further work by Rotello et al., nanoparticle-stabilized capsules (NPSCs) were developed using gold nanoparticles functionalized with the HKRK ligands. HKRK-AuNPs were used as interfacial stabilizers in a colloidal solution of linoleic acid-based micelles, to form Pickering-type emulsions, names nanoparticle stabilized nanocapsules (NPSCs) 78. NPSCs were loaded with protein cargo and demonstrated as effective vehicles for the delivery of model protein (green fluorescent protein, or GFP), and active therapeutic protein (caspase 3) as evidenced by confocal microscopy, and a significantly enhanced cellular apoptotic ratio, respectively. Notably, live cell video imaging was used to demonstrate that these NPSC systems deliver their protein cargo through a direct 'membrane-fusion'-type mechanism, avoiding endosomal entrapment without causing toxicity to the cell. Follow-up work demonstrated a modified version of this system to be capable of delivering the β -Gal enzyme as well 79.
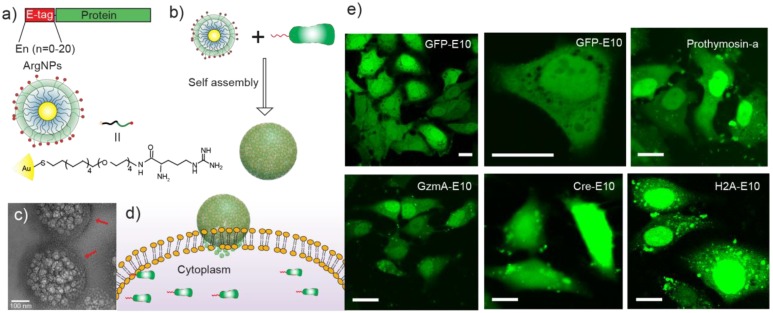
Rotello et al. more recently developed a two-component protein-nanoparticle system, achieving direct cytosolic protein delivery 80. In this report, 2 nm gold nanoparticles were functionalized with arginine-presenting ligands (ArgNPs) and electrostatically interact with co-engineered proteins modified to present an oligo-glutamic acid tag (E-tag) at the C-terminus. The localized anionic charge on these proteins was utilized as a complement to the cationic charge on the surface of the gold nanoparticles in a carboxylate-guanidinium interaction, to form self-assembled supramolecular 'nanoassemblies' which featured multiple layers of structural hierarchy. Notably, multiple proteins were evaluated for delivery, all with varying size and pI values, and five different fluorescently-labeled, E-tagged proteins were delivered into the cell (GFP, Histone 2A, Granzyme A, Cre recombinase, and Prothymosin-α), demonstrating this as a general system for direct cytosolic protein delivery. The authors observed fluorescent spread of these proteins throughout the cytosol within 30 s and into the nucleus within 90 s, confirming delivery through a transient, 'membrane-fusion'-type mechanism (Figure 4).
Figure 4.
(a) Engineered proteins carrying an E-tag on either N- or C-terminus, and the design of the arginine functionalized gold nanoparticles. (b) E-tag proteins and ArgNPs interact electrostatically and form hierarchical nanoassemblies. (c) Representative transmission electron micrograph (TEM) of GFP-E10:ArgNPs assemblies. Red arrows show the nanoparticle coating on the nanoassembly surface. (d) Delivery of Cas9-RNP via a membrane fusion mechanism. (e) Confocal microscopy images showing nanoassembly-mediated cytosolic delivery of E-tagged proteins in vitro. Scale bar = 20 μm. (Adapted with permission from Ref. 80 Copyright (2017) American Chemical Society).
Genome editing is an area of research in which gold nanoparticles have been extensively explored for delivery of active proteins. Clustered, regularly-interspaced short palindromic repeats (CRISPR) is a revolutionary technology that has been pioneered in recent years for its ability to selectively interrogate and edit the mammalian genome using a bacterially-derived nuclease protein. Cas9 protein needs to access the nucleus of the host cell to function as a nuclease, and endosomal entrapment is therefore a major barrier to CRISPR/Cas9 delivery. This challenge is exacerbated by the need for guide RNA and frequently a donor strand of DNA as well, each of which must be either co-delivered as a separate vector or delivered precomplexed with Cas9 32. In a follow-up to their previous study 80, Rotello et al. applied their ArgNPs-based protein delivery system for the cytosolic introduction of the CRISPR/Cas9 machinery into mammalian cells 81. In this approach, the authors modified the Cas9 protein with a poly-E-tag, as in their previous study, and pre-complexed this with the single guide RNA (sgRNA), forming the ribonucleoprotein (RNP). E-tagged Cas9 was complexed with ArgNPs utilizing the carboxylate-guanidinium interaction, to form self-assembled complexes of ~300 nm. Protein delivery was optimized through fluorescent conjugation and confocal microscopy analysis, achieving up to ~90% delivery in the cytosol and nucleus of HeLa cells. Delivery of the active protein achieved up to ~30% gene editing efficiency of the PTEN and AAVS1 genes.
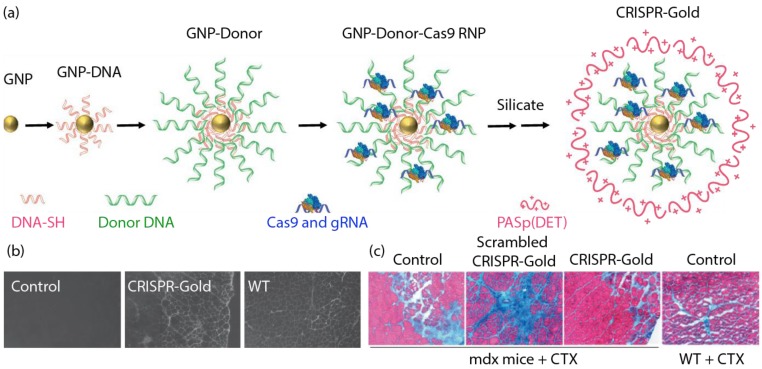
In a recent study, Murthy et al. developed a nanocarrier known as 'CRISPR-Gold' to introduce the CRISPR/Cas9 editing machinery into the cell through endosomal uptake and polymer-mediated release 82. CRISPR-Gold was constructed around a 15 nm AuNP core, functionalized with 5' thiol modified single-stranded DNA. These customized DNA strands held the donor DNA template through complementary base-pairing. Cas9 protein, pre-complexed with its sgRNA molecules were then associated to the complex through electrostatic interactions. Finally, the CRISPR-Gold complex was encapsulated within a silicate/polymer coating to provide stability in a biological environment, and endosomal disruptive ability (Figure 5a). CRISPR-Gold demonstrated >10% repair efficiency in a fluorescent reporter cell line, and 5.4% efficiency following direct injection in a murine model of Duchenne muscular dystrophy. The animals also showed a concurrent physiological response to the purported repair of the dystrophin mutation, with enhanced muscular capability (Figure 5b and 5c).
Figure 5.
(a) Generation of CRISPR-Gold. (b) CRISPR-Gold-injected muscle of mdx mice showed dystrophin expression (immunofluorescence), whereas control mdx mice did not express dystrophin protein. (c) CRISPR-Gold reduces muscle fibrosis in mdx mice. Trichrome staining was performed on the tibialis anterior muscle cryo-sectioned to 10 μm two weeks after an injection of CRISPR-Gold. CTX was co-injected in all three groups of mdx mice. Images were acquired at the areas of muscle injury and regeneration. Fibrotic tissue appears blue, while muscle fibers appear red. Wild-type mice treated with CTX were analyzed five days after injection. (Adapted with permission from Ref. 82 Copyright (2017) Macmillan Publishers).
In a follow up study performed by Lee et al., CRISPR-gold was intracranially injected to a murine model of fragile X syndrome demonstrated ~15% knockout efficiency of the MGLUR5 gene, and significantly enhanced behavioral response compared to diseased mice 83. These studies collectively represent a significant advancement in the delivery of an active therapeutic protein, and the co-delivery of nucleic acids, which is often a challenge itself.
3.2 Polymers
Polymeric carrier systems can be modified in a highly reproducible and scalable manner to include moieties that promote specific functionality, including cellular uptake, biological stability, and substrate-specific affinity. Polymeric carriers can be designed to directly interact with the protein therapeutic, or to encapsulate the cargo within polymeric micelles, colloids, rods or gels. The following will discuss some noteworthy examples of polymeric nanocarrier systems for intracellular delivery.
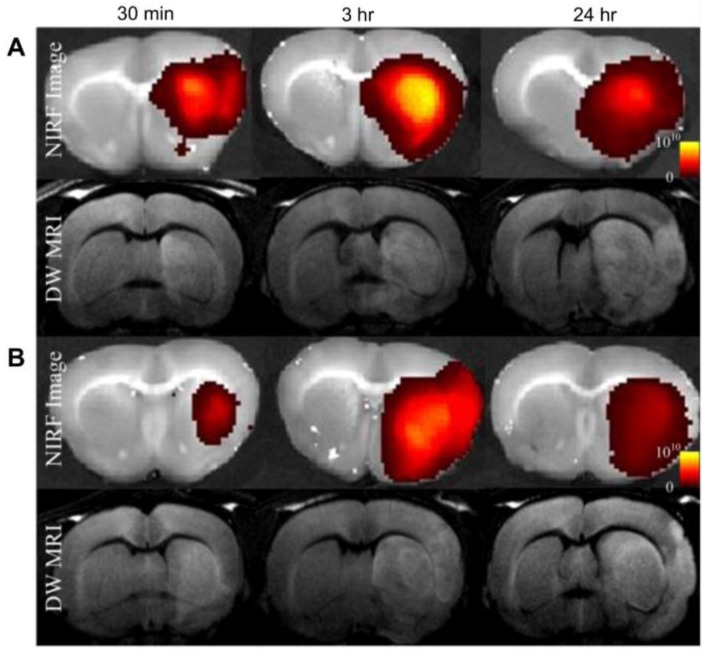
Self-assembly of amphiphilic polymers into micelles is one of the most commonly used supramolecular architectures for delivery systems. For protein delivery, the polymeric micelles are often designed to be responsive to environmental triggers such as pH, temperature, or chemical triggers such as glucose concentration 84, whereby the polymeric carrier disassembles in response, releasing the cargo. Liu et al. designed a pH-sensitive micelle using a degradable block copolymer 85. The authors designed the micelles to have a negative zeta potential in the physiological pH range, which could potentially enable increased circulation time and decreased non-specific uptake, but to switch to a positive charge under acidic conditions (such as are often associated with diseases like cancer and ischemia), allowing rapid cellular uptake in disease-relevant tissue. The authors labeled the polymer with Cy5.5, a far-red dye, and delivered albumin as a model protein to rats which exhibited focal cerebral ischemia, through intravenous injection. Near Infrared Fluorescence (NIRF) imaging of the brains demonstrated fluorescence intensity only in the ischemic area of the brain (Figure 6, right hemisphere).
Figure 6.
In vivo diffusion-weighted MRI (DW-MRI), and near-infrared fluorescence (NIRF) images show albumin-Cy5.5 accumulated at a significantly higher amount in group A (PEG-PAE-API-albumin-Cy5.5) as compared to group B (albumin-Cy5.5 only) at 30 min and 3 h after intravenous injection. (Adapted with permission from Ref. 85 Copyright (2012) Elsevier Science).
In an interesting study, Thayumanavan et al. took a different approach and used polymeric nanogels to bind and deliver proteins intracellularly 86. They used their nanogel to concurrently deliver protein and a small lipophilic molecule, the simultaneous delivery of which is typically challenging due to the two having opposing supramolecular host requirements. The authors achieved this codelivery by creating and utilizing the hydrophobic interior of the gel to host lipophilic guest molecules, with the proteins bound to the surface of the gel through complementary electrostatic interactions. Using this method, the authors were able to efficiently deliver functional β-gal to HeLa cells.
Tew et al. used a polyoxanorbornene-based system as a synthetic mimic of traditional CPPs to deliver therapeutic protein 87. In this study, the authors created a small library of these mimics with identical chemical composition but varied the hydrophobic/hydrophilic monomer segregation by creating a block copolymer, a gradient copolymer and a non-segregated homopolymers via ring opening metathesis polymerization (ROMP). This polymer contained both hydrophobic and cationic domains, with the hydrophobic region containing phenyl-functionalized repeat units, and the cationic segment containing guanidinium-rich regions to mimic the properties of a CPP. They established that the block copolymer was able to achieve better efficiencies in both protein binding and delivery. They then demonstrated this polymer's ability to efficiently deliver active Cre recombinase, which achieved gene recombination in a Jurkat T cell reporter line. Cheng et al. have functionalized phenyl and biguanide moieties onto branched polyethylenimine (bPEI) and successfully delivered proteins with reasonable efficiency to the cytosol 88. Taken together, guanidium functionalities provide the capability of translocating proteins with different molecular weights and pI into HeLa cells without degradation.
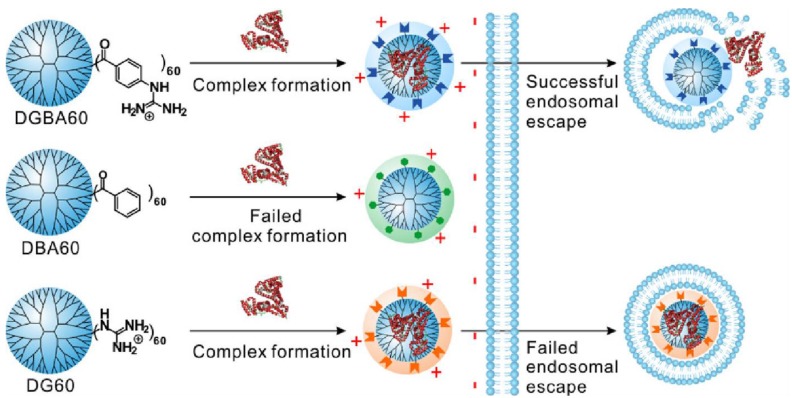
Dendrimers are repetitively branched spherical polymeric molecules that have been explored as delivery agents for drugs, nucleic acids and proteins. Cheng et al. have screened highly efficient fluoroalkyl or fluroaromatic functionalized amine-terminated poly(amidoamine) (PAMAM) dendrimers for in vitro protein delivery 89, 90. Fluoroalkyls or fluoroaromatic functional groups are functionalized onto these vehicles to take advantage of the fluorophilic effect, whereby they are able to self-assemble into nanomicelles or nanoaggregates in aqueous solutions 91. After screening, the best fluoroalkyl PAMAM was then coated with an anionic hyaluronic acid shell and demonstrated high efficacy for cancer therapy in a murine breast cancer model. These systems were used for delivery of proteins of varying size and pI, including BSA, β-Gal, saporin, and a cyclic hendecapepide. In another study, Cheng et al. demonstrated the delivery of R-phycoerythrin (R-PE, 240 kDa) and β-Gal by using guanidinobenzoic acid functionalized dendrimers 92. Benzoic acid and guanidyl functionalized dendrimers were also utilized as controls, demonstrating that both the guanidyl and phenyl groups were essential for intracellular protein delivery as mediated by the dendrimer (Figure 7).
Figure 7.
Schematic illustration showing the structure of the guanidinobenzoic acid functionalized dendrimers and their features in intracellular protein delivery. (Adapted with permission from Ref. 92 Copyright (2017) American Chemical Society).
3.3 Lipid-Based Delivery
The mammalian cell membrane is an inherently lipid-based barrier. Lipid-based strategies for intracellular protein delivery are thus advantageous not only for their composition, but further for their ability to encapsulate delivery cargo, which often circumvents the need for cargo modification. There are however limitations to lipid-based systems in a therapeutic context, including low stability in physiological conditions and potential toxicity 93.
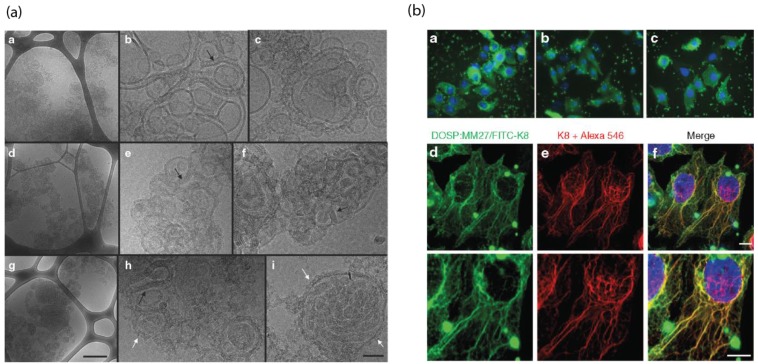
Pitard et al. reported a guanidinium-cholesterol cationic lipid within a polymer shell to deliver active β-gal and the anti-cytokeratin8 (K8) antibody intracellularly 94. Notably, different lipid/protein ratios showed different formations of supramolecular assemblies, as observed by cryo-transmission electron microscopy, with the optimized liposomal construct demonstrating intracellular delivery of K8 antibody to 67% of HeLa cells. This system was evaluated for its therapeutic capability in a cystic fibrosis model HeLa cell line carrying the characteristic ΔF508 mutation in the cystic fibrosis transmembrane regulator gene (CFTR) and its associated protein. Delivery of the active K8 antibody to these cells has demonstrated to prevent disease progression through inhibition of the misfolded CFTR protein. Using this delivery system, the authors demonstrated regulated chloride efflux, consistent with regulation of the cystic fibrosis disease state, providing a tool for the study of CFTR protein channel functionality. In this study, a lipid endosomal disruption agent was used, but it was still postulated that many of these protein lipoplexes were trapped within endosomes, as indicated by the highly punctate fluorescence. (Figure 8).
Figure 8.
(a) Cryo TEM images demonstrate the structure of cationic lipid/K8 antibody complexes, scale bar = 0.5 μm. (b) Endosomal cellular delivery of the FITC-K8 antibody by liposome, scale bar = 10 μm. (Adapted with permission from Ref. 94 Copyright (2015) Elsevier Science).
A recent report by Xu et al. utilized lipid-like nanoparticles to facilitate CRISPR/Cas9 delivery to the brain for therapeutic gene editing 95. In this work, lipid-like nanoparticles were developed which were self-assembled between lipid and protein through electrostatic interaction. Twelve bioreducible lipids molecules were synthesized via Michael addition of various amine and acrylate moieties that featured disulfide functional groups and long hydrophobic alkyl carbon chains. These bioreducible lipids interact electrostatically with recombinant proteins, which were fused with superpositively-charged GFP, to form lipid/protein nanocomplexes. These lipid-like NPs were purported to deliver into the cell through clathrin-mediated endocytosis, based on the results of endocytic inhibitor assays. Following endosomal escape, the cargo was released from the carrier following cytosolic reduction of the disulfide moiety and was able to efficiently traffic into the nucleus. This strategy was utilized for the intracellular delivery of the CRISPR/Cas9 RNP in GFP-expressing human embryonic kidney (HEK) cells, with a knockout efficiency of over 50% in vitro. This strategy was particularly notable because the inherent anionic charge of the Cas9 RNP eliminated the need for the supernegative GFP in the assembly. The authors further expanded this study to perform Cre recombinase-mediated gene editing of brain cells in a Rosa26tdTomato mouse model. The assemblies were formed and directly injected to various regions of the brain, 6 days after which cells were extracted, and it was found that ~350 cells in a 0.5-mm2 brain area were tdTomato+, confirming effective intracellular delivery of active Cre recombinase to brain cells in a murine model. Localized injection, as mentioned, provides an effective method for in vivo introduction of therapeutics and is especially useful for labile lipid-based approaches. However, these methods are also limited in translatability and practicality as compared to systemic administration. To date however, a limited number of approaches have been developed which can achieve specificity for a specific locale or cell type in vivo.
Jiang et al. approached CRISPR/Cas9 delivery by adding a functionalized HIV-1-transactivator of transcription peptide (TAT peptide) onto the core of gold nanoclusters (TAT-GNs) 96. The cationic TAT-GNs were mixed with Cas9 proteins and plasmids containing sgRNA to form a ternary complex (GCP) through electrostatic interactions. The GCP were further encapsulated in an anionic lipid shell for endosomal escape and followed by post-functionalization with polyethylene glycol‐phospholipids (DSPE-PEG) to form LGCP (Polyethylene glycol-lipid/GNs/Cas9 protein/sgRNA plasmid). The LGCP nanoparticles achieved more than 70% down-regulation of the Plk1 protein, which resulted in 19.4% apoptosis in an A375 cell line. In addition, the progress of tumor growth was reduced by 75% as compared to a control group after intratumoral injections of LGCP nanoparticle on melanoma model BALB/c mice.
Cell specificity for non-local administration of protein therapeutics in vivo is a significant challenge to intracellular delivery approaches, and it is notable that several systems have recently been reported to have achieved success in this field. Anderson et al. employed the use of cationic lipidoids to deliver the anionic oligonucleotide-attached horseradish peroxidase and NeutrAvidin (a deglycosylated version of the avidin protein) in vitro 97. Furthermore, this LNPs-based treatment showed specific uptake in dendritic cells, macrophages, and monocytes within the spleen of C57BL/6 mice after repeated intravenous injection. In vivo targeting to disease-relevant tissue has been explored extensively in recent years, and remains a significant issue in therapeutic protein delivery, however is largely outside the scope of this review.
4. Summary, Challenges and Outlook
The surge of new technologies in the field of protein delivery has opened the potential of protein therapeutics to treat various diseases including diabetes, cancer, and inflammatory diseases. As compared with traditional small-molecule drugs, protein therapeutics offer high specificity, and the ability to treat “undruggable” targets, in a wide range of diseases. Currently, nearly all existing commercially available protein therapeutics are developed for extracellular targets, and while intracellular delivery of proteins would significantly broaden their utility, protein internalization poses a significant limitation to the field due to inefficient membrane permeability and/or endosomal escape. This hurdle has inspired a number of intracellular protein delivery strategies.
Proteins are labile, and susceptible to denaturation, degradation and aggregation - making it challenging to design effective protein delivery methods. Additionally, proteins have limited ability to cross cell membranes due to their large size and surface change distribution, and so require incorporation into a delivery vehicle to aid intracellular delivery. As protein structure is: a) highly specific, b) required for pharmacological activity, and c) variable between proteins, designing generalized delivery methods becomes complicated. Even for many of the most effective intracellular delivery platforms, endosomal entrapment remains a major hurdle, limiting access to the cytosol to a fraction of the total cargo.
Methods to control and design specific supramolecular interactions via protein engineering, such as the introduction of E-tags, have proven a simple method to allow for the delivery of various proteins using a single carrier platform. This is an exciting step forward for the field as it inherently simplifies the design of the delivery vehicle and makes it widely applicable. While challenges remain to the field of intracellular protein delivery, great advances such as these have been made in recent years in the development of new carrier systems capable of unprecedented efficacy in biologically-relevant situations and should prove revolutionary in scope to therapeutic and diagnostic purposes in the years to come.
Acknowledgments
This work was supported by the NIH (GM008515 (DCL); GM077173 and EB022641, (VMR), Research Corporation for Science Advancement (TREE award to VMR) and the Australian Cancer Council (JAK). JAK would like to acknowledge the support of the Australian-American Fulbright Association and the Cancer Council WA Top-Up Scholarship.
Abbreviations
- mRNA)
Messenger RNA
- CPPs)
cell penetrating peptides
- SSNs)
smooth silica nanoparticles
- n-ODMS)
n-octadecyltrimethoxysilane
- RNAse A)
ribonuclease A
- pAkt)
phosphor-Akt
- MSNs)
mesoporous silica nanoparticles
- SOD)
superoxide dismutase
- GPx)
glutathione peroxidase
- ROS)
reactive oxygen species
- H-MSNs)
hollow MSNs
- FITC)
fluorescein isothiocyanate
- BSA)
bovine serum albumin
- RSNs)
rough silica nanoparticles
- AuNPs)
gold nanoparticles
- TEM)
transmission electron microscopy
- β-Gal)
β-Galactosidase
- TEG)
tetraethylene glycol
- HKRK)
His-Lys-Arg-Lys peptide
- NPSCs)
nanoparticle-stabilized capsules
- GFP)
green fluorescent protein
- eGFP)
enhanced GFP
- CRISPR)
clustered, regularly-interspaced short palindromic repeats
- Cas9)
CRISPR-associated protein 9
- PEG)
polyethylene glycol
- NIRF)
Near Infrared Fluorescence
- ROMP)
ring opening polymerization
- GSH)
glutathione
- PAMAM)
amine-terminated poly(amidoamine
- R-PE)
R-phycoerythrin
- CTX)
cardiotoxin
References
- 1.Akhtar MJ, Ahamed M, Kumar S, Siddiqui H, Patil G, Ashquin M. et al. Nanotoxicity of pure silica mediated through oxidant generation rather than glutathione depletion in human lung epithelial cells. Toxicology. 2010;276:95–102. doi: 10.1016/j.tox.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt S, Gonzalez D, Derendorf H. Significance of protein binding in pharmacokinetics and pharmacodynamics. J Pharm Sci. 2010;99:1107–1122. doi: 10.1002/jps.21916. [DOI] [PubMed] [Google Scholar]
- 3.Stewart MP, Sharei A, Ding X, Sahay G, Langer R, Jensen KF. In vitro and ex vivo strategies for intracellular delivery. Nature. 2016;538(7624):183–192. doi: 10.1038/nature19764. [DOI] [PubMed] [Google Scholar]
- 4.Dobson CM. Protein misfolding, evolution and disease. Trends Biochem Sci. 1999;24:329–332. doi: 10.1016/s0968-0004(99)01445-0. [DOI] [PubMed] [Google Scholar]
- 5.Stewart MP, Langer R, Jensen KF. Intracellular Delivery by Membrane Disruption: Mechanisms, Strategies, and Concepts. Chem Rev. 2018;118(16):7409–7531. doi: 10.1021/acs.chemrev.7b00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bareford LM, Swaan PW. Endocytic mechanisms for targeted drug delivery. Adv Drug Deliv Rev. 2007;59:748–758. doi: 10.1016/j.addr.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swiech L, Heidenreich M, Banerjee A, Naomi H, Li Y, Trombetta J. et al. In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9. Nat Biotechnol. 2015;33:102–106. doi: 10.1038/nbt.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lostalé-Seijo I, Louzao I, Juanes M, Montenegro J. Peptide/Cas9 nanostructures for ribonucleoprotein cell membrane transport and gene edition. Chem Sci. 2017;8:7923–7931. doi: 10.1039/c7sc03918b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scholz C, Wagner E. Therapeutic plasmid DNA versus siRNA delivery: common and different tasks for synthetic carriers. J Control Release. 2012;161:554–565. doi: 10.1016/j.jconrel.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Wadia JS, Dowdy SF. Transmembrane delivery of protein and peptide drugs by TAT-mediated transduction in the treatment of cancer. Adv Drug Deliv Rev. 2005;57:579–596. doi: 10.1016/j.addr.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Peer D, Karp JM, Hong S, Farkhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 12.Patterson H, Nibbs R, McInnes I, Siebert S. Protein kinase inhibitors in the treatment of inflammatory and autoimmune diseases. Clin Exp Immunol. 2014;176:1–10. doi: 10.1111/cei.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steele JA, Hallé JP, Poncelet D, Neufeld RJ. Therapeutic cell encapsulation techniques and applications in diabetes. Adv Drug Deliv Rev. 2014;67-68:74–83. doi: 10.1016/j.addr.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Li C, Gӧtz J. Tau-based therapies in neurodegeneration: opportunities and challenge. Nat Rev Drug Discov. 2017;16:863–883. doi: 10.1038/nrd.2017.155. [DOI] [PubMed] [Google Scholar]
- 15.Srivastava A, Yadav T, Sharma S, Nayak A, Kumari AA, Mishra N. Polymers in Drug Delivery. J Biosci Med. 2016;4:69–84. [Google Scholar]
- 16.Frokjaer S, Otzen DE. Protein drug stability: a formulation challenge. Nat Rev Drug Discov. 2005;4:298–306. doi: 10.1038/nrd1695. [DOI] [PubMed] [Google Scholar]
- 17.Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov. 2014;13:655–672. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naahidi S, Jafari M, Edalat F, Raymond K, Khademhosseini, Chen P. Biocompatibility of engineered nanoparticles for drug delivery. J Control Release. 2013;166:182–194. doi: 10.1016/j.jconrel.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Jawahar N, Meyyanathan SN. Polymeric nanoparticles for drug delivery and targeting: A comprehensive review. Int J Health Allied Sci. 2012;1:217–223. [Google Scholar]
- 20.Puri A, Loomis K, Smith B, Lee JH, Yavlovich A, Heldman E. et al. Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit Rev Ther Drug Carrier Syst. 2009;26:523–580. doi: 10.1615/critrevtherdrugcarriersyst.v26.i6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo D, Saltzman WM. Synthetic DNA delivery systems. Nat Biotechnol. 2000;18:33–37. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- 22.Kormann MS, Hasenpusch G, Aneja MK, Nica G, Flemmer AW, Herber-Jonat S. et al. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat Biotechnol. 2011;29:154–157. doi: 10.1038/nbt.1733. [DOI] [PubMed] [Google Scholar]
- 23.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 24.Hawley RG, Lieu FH, Fong AZ, Hawley TS. Versatile retroviral vectors for potential use in gene therapy. Gene Ther. 1994;1:136–138. [PubMed] [Google Scholar]
- 25.Mout R, Ray M, Lee YW, Scaletti F, Rotello VM. In vivo delivery of CRISPR/Cas9 for therapeutic gene editing: progress and challenges. Bioconjug Chem. 2017;28(4):880–884. doi: 10.1021/acs.bioconjchem.7b00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Felgner PL, Tsai YJ, Sukhu L, Wheeler CJ, Manthorpe M, Marshall J. et al. Improved cationic lipid formulations for in vivo gene therapy. Ann NY Acad Sci. 1995;772:126–139. doi: 10.1111/j.1749-6632.1995.tb44738.x. [DOI] [PubMed] [Google Scholar]
- 27.Lonez C, Vandenbranden M, Ruysschaert JM. Cationic liposomal lipids: from gene carriers to cell signaling. Prog Lipid Res. 2008;47:340–347. doi: 10.1016/j.plipres.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Lungwitz U, Breunig M, Blunk T, Göpferich A. Polyethylenimine-based non-viral gene delivery systems. Eur J Pharm Biopharm. 2005;60:247–266. doi: 10.1016/j.ejpb.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Heller LC, Ugen K, Heller R. Electroporation for targeted gene transfer. Expert Opin Drug Deliv. 2005;2:255–268. doi: 10.1517/17425247.2.2.255. [DOI] [PubMed] [Google Scholar]
- 30.Farsad K, De Camilli P. Mechanisms of membrane deformation. Curr Opin Cell Boil. 2003;15:372–381. doi: 10.1016/s0955-0674(03)00073-5. [DOI] [PubMed] [Google Scholar]
- 31.Chiper M, Niederreither K, Zuber G. Transduction Methods for Cytosolic Delivery of Proteins and Bioconjugates into Living Cells. Adv Healthc Mater. 2018;7(6):e1701040. doi: 10.1002/adhm.201701040. [DOI] [PubMed] [Google Scholar]
- 32.Scaletti F, Hardie J, Lee YW, Luther D, Ray M, Rotello VM. Protein delivery into cells using inorganic nanoparticle-protein supramolecular assemblies. Chem Soc Rev. 2018;47(10):3421–3432. doi: 10.1039/c8cs00008e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helenius A, Mellman I, Wall D, Hubbard A. Endosomes. Trends Bioichem Sci. 1983;8(7):245–250. [Google Scholar]
- 34.Hubbell JA, Chilkoti A. Nanomaterials for drug delivery. Science. 2012;337(6092):303–305. doi: 10.1126/science.1219657. [DOI] [PubMed] [Google Scholar]
- 35.Wickner W, Schekman R. Protein translocation across biological membrane. Science. 2005;310(5753):1452–1456. doi: 10.1126/science.1113752. [DOI] [PubMed] [Google Scholar]
- 36.Langer R. Drug delivery and targeting. Nature. 1998;392(6679):5–10. [PubMed] [Google Scholar]
- 37.Dolezal P, Likic V, Tachezy J, Lithgow T. Evolution of the molecular machines for protein import into mitochondria. Science. 2006;313(5758):314–318. doi: 10.1126/science.1127895. [DOI] [PubMed] [Google Scholar]
- 38.Torchilin VP. Recent approaches to intracellular delivery of drugs and DNA and organelle targeting. Annu Rev Biomed Eng. 2006;8:343–375. doi: 10.1146/annurev.bioeng.8.061505.095735. [DOI] [PubMed] [Google Scholar]
- 39.Kaksonen M, Toret CP, Drubin DG. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Boil. 2006;7:404–414. doi: 10.1038/nrm1940. [DOI] [PubMed] [Google Scholar]
- 40.Chen H, Fre S, Slepnev VI, Capua MR, Takei K, Butler MH. et al. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature. 1998;394(6695):793–797. doi: 10.1038/29555. [DOI] [PubMed] [Google Scholar]
- 41.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 42.Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF. et al. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010;11(5):675–687. doi: 10.1111/j.1600-0854.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- 43.Kerr MC, Teasdale RD. Defining macropinocytosis. Traffic. 2009;10(4):364–371. doi: 10.1111/j.1600-0854.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- 44.Nabi IR, Le PU. Caveolae/raft-dependent endocytosis. J Cell Biol. 2003;161(4):673–677. doi: 10.1083/jcb.200302028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cuervo AM, Dice JF. A receptor for the selective uptake and degradation of proteins by lysosomes. Science. 1996;273:501–503. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]
- 46.Kauffman WB, Fuselier T, He J, Wimley WC. Mechanism matters: a taxonomy of cell penetrating peptides. Trends Biochem Sci. 2015;40(12):749–764. doi: 10.1016/j.tibs.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Varkouhi AK, Scholte M, Storm G, Haisma HJ. Endosomal escape pathways for delivery of biologicals. J Control Release. 2011;151(3):220–228. doi: 10.1016/j.jconrel.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Benjaminsen RV, Mattebjerg MA, Henriksen JR, Moghimi SM, Andresen TL. The possible "proton sponge " effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol Ther. 2012;21(1):149–57. doi: 10.1038/mt.2012.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Danial M, Perrier S, Jolliffe KA. Effect of the amino acid composition of cyclic peptides on their self-assembly in lipid bilayers. Org Biomol Chem. 2015;13(8):2464–2473. doi: 10.1039/c4ob02041c. [DOI] [PubMed] [Google Scholar]
- 50.Selby LI, Cortez-Jugo CM, Such GK, Johnston APR. Nanoescapology: progress toward understanding the endosomal escape of polymeric nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(5):Epub. doi: 10.1002/wnan.1452. 2017 Feb 3. [DOI] [PubMed] [Google Scholar]
- 51.Li W, Nicol F, Szoka FC Jr. GALA: a designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery. Adv Drug Deliv Rev. 2004;56(7):967–985. doi: 10.1016/j.addr.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 52.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4(7):581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 53.Leroueil PR, Berry SA, Duthie K, Han G, Rotello VM, McNerny DQ. et al. Wide varieties of cationic nanoparticles induce defects in supported lipid bilayers. Nano Lett. 2008;8(2):420–424. doi: 10.1021/nl0722929. [DOI] [PubMed] [Google Scholar]
- 54.Hong S, Leroueil PR, Janus EK, Peters JL, Kober MM, Islam MT. et al. Interaction of polycationic polymers with supported lipid bilayers and cells: nanoscale hole formation and enhanced membrane permeability. Bioconjug Chem. 2006;17(3):728–734. doi: 10.1021/bc060077y. [DOI] [PubMed] [Google Scholar]
- 55.Murthy N, Robichaud JR, Tirrell DA, Stayton PS, Hoffman AS. The design and synthesis of polymers for eukaryotic membrane disruption. J Control Release. 1999;61(1-2):137–143. doi: 10.1016/s0168-3659(99)00114-5. [DOI] [PubMed] [Google Scholar]
- 56.Cheung CY, Murthy N, Stayton PS, Hoffman AS. A pH-sensitive polymer that enhances cationic lipid-mediated gene transfer. Bioconjug Chem. 2001;12(6):906–910. doi: 10.1021/bc0100408. [DOI] [PubMed] [Google Scholar]
- 57.Cheung CY, Stayton PS, Hoffman AS. Poly(propylacrylic acid)-mediated serum stabilization of cationic lipoplexes. J Biomater Sci Polym Ed. 2005;16(2):163–179. doi: 10.1163/1568562053115390. [DOI] [PubMed] [Google Scholar]
- 58.Kelley SO, Stewart KM, Mourtada R. Development of novel peptides for mitochondrial drug delivery: amino acids featuring delocalized lipophilic cations. Pharm Res. 2011;28(11):2808–2819. doi: 10.1007/s11095-011-0530-6. [DOI] [PubMed] [Google Scholar]
- 59.Rothbard JB, Jessop TC, Lewis RS, Murray BA, Wender PA. Role of membrane potential and hydrogen bonding in the mechanism of translocation of guanidinium-rich peptides into cells. J Am Chem Soc. 2004;126(31):9506–9507. doi: 10.1021/ja0482536. [DOI] [PubMed] [Google Scholar]
- 60.Schröder T, Niemeier N, Afonin S, Ulrich AS, Krug HF, Bräse S. Peptoidic amino- and guanidinium-carrier systems: targeted drug delivery into the cell cytosol or the nucleus. J Med Chem. 2008;51(3):376–379. doi: 10.1021/jm070603m. [DOI] [PubMed] [Google Scholar]
- 61.Sakai N, Matile S. Anion-mediated transfer of polyarginine across liquid and bilayer membranes. J Am Chem Soc. 2003;125(47):14348–14356. doi: 10.1021/ja037601l. [DOI] [PubMed] [Google Scholar]
- 62.Rosalia RA, Cruz LJ, van Duikeren S, Tromp AT, Silva AL, Jiskoot W. et al. CD40-targeted dendritic cell delivery of PLGA-nanoparticle vaccines induce potent anti-tumor responses. Biomaterials. 2015;40:88–97. doi: 10.1016/j.biomaterials.2014.10.053. [DOI] [PubMed] [Google Scholar]
- 63.Silva AL, Rosalia RA, Varypataki E, Sibuea S, Ossendorp F, Jiskoot W. Poly-(lactic-co-glycolic-acid)-based particulate vaccines: particle uptake by dendritic cells is a key parameter for immune activation. Vaccine. 2015;33(7):847–854. doi: 10.1016/j.vaccine.2014.12.059. [DOI] [PubMed] [Google Scholar]
- 64.Ferreira SA, Gama FM, Vilanova M. Polymeric nanogels as vaccine delivery systems. Nanomedicine. 2013;9(2):159–173. doi: 10.1016/j.nano.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 65.Lim EK, Kim T, Paik S, Haam S, Huh YM, Lee K. Nanomaterials for theranostics: recent advances and future challenges. Chem Rev. 2015;115(1):327–394. doi: 10.1021/cr300213b. [DOI] [PubMed] [Google Scholar]
- 66.Wang Y, Zhao Q, Han N, Bai L, Li J, Liu J. et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine. 2015;11(20):313–327. doi: 10.1016/j.nano.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 67.Bale SS, Kwon SJ, Shah DA, Banerjee A, Dordick JS, Kane RS. Nanoparticle-mediated cytoplasmic delivery of proteins to target cellular machinery. ACS Nano. 2010;4(3):1493–1500. doi: 10.1021/nn901586e. [DOI] [PubMed] [Google Scholar]
- 68.Rahikkala A, Pereira SAP, Figueiredo P, Passos MLC, Araújo ARTS, Saraiva MLMFS. et al. Mesoporous Silica Nanoparticles for Targeted and Stimuli-Responsive Delivery of Chemotherapeutics: A Review. Adv Biosyst. 2018;2:1800020. [Google Scholar]
- 69.Alvarez-Berríos MP, Sosa-Cintron N, Rodriguez-Lugo M, Juneja R, Vivero-Escoto JL. Hybrid Nanomaterials Based on Iron Oxide Nanoparticles and Mesoporous Silica Nanoparticles: Overcoming Challenges in Current Cancer Treatments. J Chem; 2016. Article ID 2672740. [Google Scholar]
- 70.Lin YH, Chen YP, Liu TP, Chien FC, Chou CM, Chen CT, Approach to Deliver Two Antioxidant Enzymes with Mesoporous Silica Nanoparticles into Cells. ACS Appl Mater Interfaces. 2016, 8; 1794. 4-17954. [DOI] [PubMed] [Google Scholar]
- 71.Yang J, Tu J, Lamers GEM, Olsthoorn RCL, Kros A. Membrane Fusion Mediated Intracellular Delivery of Lipid Bilayer Coated Mesoporous Silica Nanoparticles. Adv Healthc Mater. 2017;6:Epub. doi: 10.1002/adhm.201700759. 2017 Sep 25. [DOI] [PubMed] [Google Scholar]
- 72.Han P, Ma N, Ren H, Xu H, Li Z, Wang Z. et al. Oxidation-Responsive Micelles Based on a Selenium-Containing Polymeric Superamphiphile. Langmuir. 2010;26(18):14414–14418. doi: 10.1021/la102837a. [DOI] [PubMed] [Google Scholar]
- 73.Lim JS, Lee K, Choi JN, Hwang YK, Yun MY, Kim HJ. et al. Intracellular protein delivery by hollow mesoporous silica capsules with a large surface hole. Nanotechnology. 2012;23:Epub. doi: 10.1088/0957-4484/23/8/085101. 2012 Feb 1. [DOI] [PubMed] [Google Scholar]
- 74.Niu Y, Yu M, Meka A, Liu Y, Zhang J, Yang Y. et al. Understanding the contribution of surface roughness and hydrophobic modification of silica nanoparticles to enhanced therapeutic protein delivery. J Mat Chem B. 2016;2:212–219. doi: 10.1039/c5tb01911g. [DOI] [PubMed] [Google Scholar]
- 75.Boisselier E, Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev. 38(6); 1759. -1782. [DOI] [PubMed] [Google Scholar]
- 76.Ghosh P, Han G, De M, Kim CK, Rotello VM. Gold nanoparticles in delivery applications. Adv Drug Deliv Rev. 2008;60(11):1307–1315. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 77.Ghosh P, Yang X, Arvizo R, Zhu ZJ, Agasti SS, Mo Z. et al. Intracellular Delivery of a Membrane-Impermeable Enzyme in Active Form Using Functionalized Gold Nanoparticles. J Am Chem Soc. 2010;132:2642–2645. doi: 10.1021/ja907887z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang R, Kim CS, Solfiell DJ, Rana S, Mout R, Velázquez-Delgado EM. et al. Direct delivery of functional proteins and enzymes to the cytosol using nanoparticle-stabilized nanocapsules. ACS Nano. 2013;7:6667–6673. doi: 10.1021/nn402753y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tang R, Jiang Z, Ray M, Hou S, Rotello VM. Cytosolic delivery of large proteins using nanoparticle-stabilized nanocapsules. Nanoscale. 2016;8:18038–18041. doi: 10.1039/c6nr07162g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mout R, Yesilbag Tonga G, Wang LS, Ray M, Roy T, Rotello VM. Programmed Self-Assembly of Hierarchical Nanostructures through Protein-Nanoparticle Coengineering. ACS Nano. 2017;11:3456–3462. doi: 10.1021/acsnano.6b07258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mout R, Ray M, Yesilbag Tonga G, Lee YW, Tay T, Sasaki K. et al. Direct Cytosolic Delivery of CRISPR/Cas9-Ribonucleoprotein for Efficient Gene Editing. ACS Nano. 2017;11(3):2452–2458. doi: 10.1021/acsnano.6b07600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee K, Conboy M, Park HM, Jiang F, Kim HJ, Dewitt MA. et al. Nanoparticle delivery of Cas9 ribonucleoprotein and donor DNA in vivo induces homology-directed DNA repair. Nat Biomed Eng. 2017;1:889–901. doi: 10.1038/s41551-017-0137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee B, Lee K, Panda S, Gonzales-Rojas R, Chong A, Bugay V. et al. Nanoparticle delivery of CRISPR into the brain rescues a mouse model of fragile X syndrome from exaggerated repetitive behaviours. Nat Biomed Eng. 2018;2:497–507. doi: 10.1038/s41551-018-0252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wen N, Lü S, Gao C, Xu X, Bai X, Wu C. et al. Glucose-responsive zwitterionic dialdehyde starch-based micelles with potential anti-phagocytic behavior for insulin delivery. Chem Eng J. 2018;335:52–62. [Google Scholar]
- 85.Gao GH, Park MJ, Li Y, Im GH, Kim JH, Kim HN. et al. The use of pH-sensitive positively charged polymeric micelles for protein delivery. Biomaterials. 2012;33(35):9157–9164. doi: 10.1016/j.biomaterials.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 86.González-Toro DC, Ryu JH, Chacko RT, Zhuang J, Thayumanavan S. Concurrent binding and delivery of proteins and lipophilic small molecules using polymeric nanogels. J Am Chem Soc. 2012;134(16):6964–6967. doi: 10.1021/ja3019143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sgolastra F, Backlund CM, Ozay EI, deRonde BM, Minter LM, Tew GN. Sequence segregation improves non-covalent protein delivery. J Control Release. 2017;254:131–136. doi: 10.1016/j.jconrel.2017.03.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu C, Tan E, Xu Y, Lv J, Cheng Y. A Guanidinium-Rich Polymer for Efficient Cytosolic Delivery of Native Proteins. Bioconjug Chem. 2019;30(2):413–417. doi: 10.1021/acs.bioconjchem.8b00753. [DOI] [PubMed] [Google Scholar]
- 89.Wang M, Liu H, Li L, Cheng Y. A fluorinated dendrimer achieves excellent gene transfection efficacy at extremely low nitrogen to phosphorus ratios. Nat Commun. 2014;5:3053. doi: 10.1038/ncomms4053. [DOI] [PubMed] [Google Scholar]
- 90.Lv J, He B, Yu J, Wang Y, Wang C, Zhang S. et al. Fluoropolymers for intracellular and in vivo protein delivery. Biomaterials. 2018;182:167–175. doi: 10.1016/j.biomaterials.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Z, Shen W, Ling J, Yan Y, Hu J, Cheng Y. The fluorination effect of fluoroamphiphiles in cytosolic protein delivery. Nat Commun. 2018;9(1):1377. doi: 10.1038/s41467-018-03779-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chang H, Lv J, Gao X, Wang X, Wang H, Chen H. et al. Rational Design of a Polymer with Robust Efficacy for Intracellular Protein and Peptide Delivery. Nano Lett. 2017;17(3):1678–1684. doi: 10.1021/acs.nanolett.6b04955. [DOI] [PubMed] [Google Scholar]
- 93.Whitehead KA, Langer R, Anderson, DG. Knocking down Barriers: Advances in SiRNA Delivery. Nat Rev Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chatin B, Mével M, Devallière J, Dallet L, Haudebourg T, Peuziat P. et al. Liposome-based formulation for intracellular delivery of functional proteins. Mol Ther Nucleic Acids. 2015;4:e244. doi: 10.1038/mtna.2015.17. [DOI] [PubMed] [Google Scholar]
- 95.Wang M, Zuris JA, Meng F, Rees H, Sun S, Deng P. et al. Efficient delivery of genome-editing proteins using bioreducible lipid nanoparticles. Proc Natl Acad Sci U S A. 2016;113:2868–2873. doi: 10.1073/pnas.1520244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang P, Zhang L, Xie Y, Wang N, Tang R, Zheng W. et al. Genome Editing for Cancer Therapy: Delivery of Cas9 Protein/sgRNA Plasmid via a Gold Nanocluster/Lipid Core-Shell Nanocarrier. Adv Sci. 2017;4:1700175. doi: 10.1002/advs.201700175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eltoukhy AA, Chen D, Veiseh O, Pelet JM, Yin H, Dong Y. et al. Nucleic acid-mediated intracellular protein delivery by lipid-like nanoparticles. Biomaterials. 2014;35:6454–6461. doi: 10.1016/j.biomaterials.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]