Abstract
Macrocyclic hosts, such as cyclodextrins, calixarenes, cucurbiturils, and pillararenes, exhibit unparalleled advantages in disease diagnosis and therapy over the past years by fully taking advantage of their host-guest molecular recognitions. The dynamic nature of the non-covalent interactions and selective host-guest complexation endow the resultant nanomaterials with intriguing properties, holding promising potentials in theranostic fields. Interestingly, the differences in microenvironment between the abnormal and normal cells/tissues can be employed as the stimuli to modulate the host-guest interactions, realizing the purpose of precise diagnosis and specific delivery of drugs to lesion sites. In this review, we summarize the progress of supramolecular theranostics on the basis of host-guest chemistry benefiting from their fantastic topological structures and outstanding supramolecular chemistry. These state-of-the-art examples provide new methodologies to overcome the obstacles faced by the traditional theranostic systems, promoting their clinical translations.
Keywords: Host-Guest Chemistry, Supramolecular Theranostics
1. Introduction
Different from traditional molecular chemistry on the basis of the covalent bonding of atoms, supramolecular chemistry, “chemistry beyond the molecule”, is based on the intermolecular interactions, in which the building blocks are linked together by non-covalent bonds 1-6. The origin of supramolecular chemistry is from the living biological systems, nucleic acid assembly, phospholipid membranes, protein folding, ribosomes, and microtubules are the representative examples of supramolecular self-assemblies, playing critical roles in a variety of biological processes 7-11. The most fantastic property of supramolecular chemistry is the dynamic nature, endowing the resultant architectures with interesting stimuli-responsiveness 12-17. A series of non-covalent interactions, such as π-π stacking interactions, hydrogen bonding, metal-ligand coordinations, electrostatic interactions, Van der Waals force, and charge-transfer interactions, have been extensively employed to construct sophisticated materials by hierarchically organizing the building blocks 17-24. Among them, the supramolecular systems fabricated from host-guest interactions exhibit some extraordinary properties arising from the introduction of macrocylic hosts, exhibiting promising potentials in various fields, especially in biomedical applications 25-29.
The binding affinities of the host-guest complexes can be adjusted by external stimuli, such as temperature, ion, pH, redox, enzyme, and light 30-34. Coincidentally, these stimuli are the differences in the microenvironment between the normal cells/tissues and the lesion ones. Therefore, smart supramolecular systems possessing theranostic functions can be developed that are sensitive to the specific stimulus, realizing the purpose of precise diagnosis and targeting delivery of cargoes to lesion sites 35-39. Indeed, some limitations faced by the traditional theranostic platforms are effectively overcome by taking full advantage of host-guest chemistry 40-43. For example, the formation of host-guest inclusion complexes greatly enhances the solubility/stability of anticancer drugs in physiological environment and maintains their activity, such as paclitaxel (PTX), camptothecin (CPT), and cisplatin 44-46. Additionally, host-guest complexations allow the incorporation of functional groups into one platform through “Lego-like” approaches. The imaging probes, targeting ligands, and therapeutic agents can be conjugated on the hosts or guests separately, avoiding tedious and time-consuming synthesis 47-51. The pharmacokinetic profiles and excretion pathway of the low molecular weight drugs can be optimized through supramolecular strategy, favorable to increasing their therapeutic outcome and diminish the side effect. More interestingly, the loaded cargoes in the supramolecular theranostic systems can be released in the sites of active due to the dissociation of the host-guest linkages triggered by the specific stimulus in abnormal lesions.
Benefiting from the distinct advantages, several breakthroughs have been achieved in theranostic fields using host-guest chemistry, and some of them are already in clinical trials or approved by the U.S. Food & Drug Administration (FDA). It should be pointed out that host-guest complexes are used industrially in pharmaceutical and allied applications decades ago mainly using cyclodextrins as macrocyclic hosts, while these hosts are typically “one trick ponies”, their sole role is to complex and deliver the drugs. Attributing to the vigorous development of nanotechnology and imaging modalities, smart theranostic systems are fabricated recently combining therapeutic and diagnostic functions, making the host-guest systems visible in vitro and in vivo 52. Their delivery, internalization, and excretion processes can be monitored, and the therapeutic results can be assessed using various imaging methods, including fluorescence imaging, positron emission tomography (PET)/single-photon emission computed tomography (SPECT), X-ray computed tomography (CT), ultrasound imaging (US), magnetic resonance imaging (MRI), and photoacoustic imaging (PA). Considering the promising potentials in clinical translations, supramolecular theranostics are attracting more and more attentions from chemists, materials scientists, biologists, and physicians. In this review, we summarise the progresses of supramolecular theranostics over the past years on the basis of host-guest chemistry, especially in cancer theranostics. Since various macrocycles are used in the construction of supramolecular theranostics including cyclodextrins, calixarenes, cucurbiturils, and pillararenes, the following discussions are classified according to the types of macrocyclic molecules involved.
2. Cyclodextrin-based supramolecular theranostics
Cyclodextrins (CDs) including α-, β-, and γ-cyclodextrins, are the macrocyclic oligosaccharides containing six, seven and eight D-glucoses, respectively, which are connected by α-1,4-glucosidic linkers to form a toroidal structure with a primary and a secondary rim 53-57. CDs are feasible hosts for hydrophobic guests in aqueous media driving by the hydrophobic and van der Waals interactions in the nonpolar cavities 58, 59. The solubility of most drugs is extremely poor in water, so their applications are greatly limited and several clinical trials are failed for this reason. Fortunately, this issue can be solved by the formation of CD-drug inclusion complex, bringing new hopes for the failed drugs. Compared with other macrocycles, CDs are the most popular additives in pharmaceutical products for the following reasons: (1) CDs are seminatural products that are produced in thousands of tons per year from starch with low cost. (2) CDs are highly biocompatible that can be directly used as ingredients of foods, drugs, or cosmetics. (3) The binding affinities between CDs and specific guests are strong enough to stabilize the complexes in physiological environment, while the loaded cargoes can be released from the cavity when they arrive to the destination. (4) The cavity provides a hydrophobic environment to protect the drugs from enzymatic hydrolysis during circulation and delivery processes, facilitating to maintain their bioactivity.
2.1. Supramolecular imaging
Molecular imaging is an indispensable tool in modern diagnostics, which provides important biological information in living systems at the molecular level 60-65. On the other hand, the different aspects of the drug delivery processes, such as pharmacokinetics, biodistribution, accumulation at the target sites, kinetics of drug release, and treatment efficacy can be quantitatively monitored using non-invasive imaging techniques. Over the past decades, a series of sophisticated probes have been exploited, which have profoundly improved the performance of imaging modalities. However, several limitations still exist for the commonly used imaging probes. For example, the quantum yields of the fluorophores are always unsatisfactory and the fluorescence can be quenched during the delivery and imaging processes. For gadolinium-based contrasts, their relatively weak contrast effect and nephrotoxicity greatly limit their clinical applications. These shortages can be possibly solved or overcome through supramolecular formulations using host-guest chemistry by changing the properties and excretion of the probes.
Due to the non-invasive nature, non-ionizing radiation, and excellent spatial resolution, magnetic resonance imaging (MRI) has extensive applications in the diagnosis of diseases and the understanding of biological processes over the past decades 66-69. Among various contrast agents, Gd3+ chelates are widely accepted by physicians, because they are easy to administer and provide positive longitudinal (T1) weighted. Unfortunately, the low longitudinal relaxivity (r1) and nephrotoxicity greatly limit the application of the commercial Gd3+-based agents 70-76. Thompson et al. grafted the contrast (Gd3+-DO3A) to 2-hydroxypropyl-β-cyclodextrin (HPCD) and further prepared a polyrotaxane (Gd3+-DO3A-HPCD/Pluronic PR) through host-guest chemistry, in which Pluronic F127 worked as the axle and Gd3+-DO3A-HPCD was the wheel locked by two cholesterol stoppers on both sides 77. Through chemical modification and supramolecular self-assembly, the enhancement ratio in the blood of Gd3+-DO3A-HPCD/Pluronic PR was approximately 2-fold of Gd3+-DO3A-HPCD, while the amount in the kidneys was effectively reduced. The alteration in pharmacokinetics and excretion pathway facilitated to avoid the toxicity of Gd3+-based agents. Relaxivity measurements indicated that the r1 values of Gd3+-DO3A-HPCD and Gd3+-DO3A-HPCD/Pluronic PR were 7.82 and 23.83 mM-1 s-1 (1.5 T, 37 °C) per Gd chelate respectively. Compared with the Gd3+-DO3A-HPCD wheel, the formation of polyrotaxane resulted in a 3-fold improvement in ionic relaxivity stemming from the reduced rotational motion of cyclodextrin units and increased polymer rigidity in this mechanical interlocked molecule.
Hasenknopf et al. utilized cyclodextrin polyrotaxanes as a platform to develop imaging agents 78. α-CDs on the axle could be modified by both fluorescence probe BODIPY and MRI contrast Gd-DOTA for bimodal imaging through a copper-catalyzed azide-alkyne cycloaddition (CuAAC). Compared with the commercial Gd-DOTA (3.83 mM-1 s-1 at 20 MHz), the r1 values were increased to 7.06 and 8.57 mM-1 s-1 by conjugating one and two Gd-DOTA to α-CD. Interestingly, the r1 values of the polyrotaxanes increased to 17.43 and 20.95 mM-1 s-1 using CD-Gd and CD-Gd2 as the wheel. Notably, the existence of BODIPY did not influence the molar relaxivity of the polyrotaxane, even a slight enhancement in r1 value was observed (18.60 mM-1 s-1). Due to the increase of the water exchange rate at relatively high temperature, the relaxivities of the polyrotaxanes were further increased at 37 oC than at 25 oC. Apart from the formation of polyrotaxane, relaxivity also remarkably enhanced upon the formation of the dendrimeric macromolecular host-guest adducts driving by the interactions between β-CD and adamantly (Ada) group 79, which resulted from the decrease of the molecular tumbling rate and fast water-exchange. These examples demonstrated the supramolecular approaches were effective for improving the relaxivity properties of the Gd3+ complexes as compared with the small molecules, which were not available in other architectures.
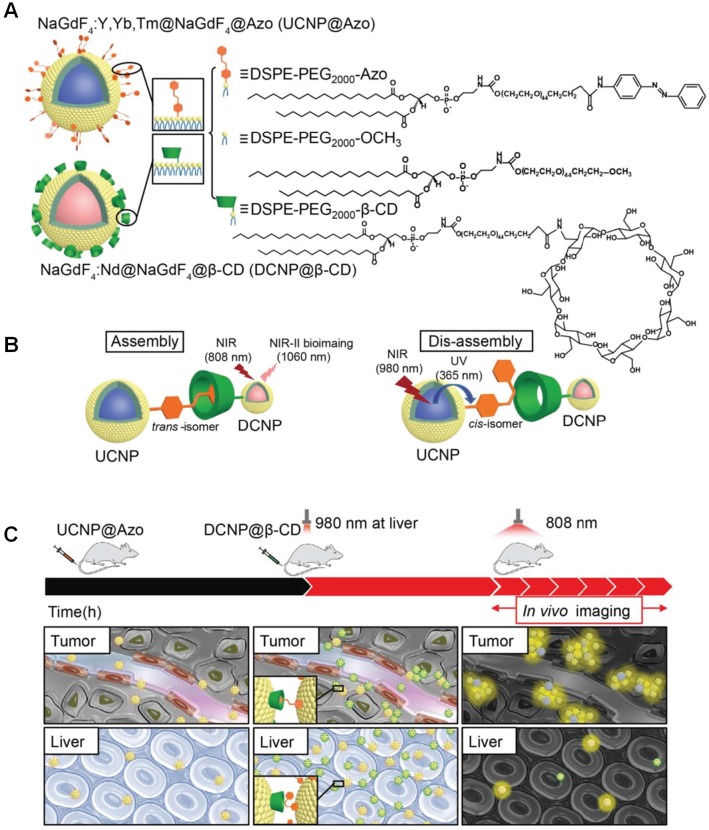
Compared with traditional NIR imaging (~700-900 nm), fluorescence imaging in the second biological transparency window (NIR-II, 1000-1700 nm) exhibits great advantages, including high signal-to-noise ratio, high resolution, and deep tissue penetration 80-84. Until now, a series of organic/inorganic nanomaterials and organic small molecules have been prepared in the last couple of years, in order to collect high-definition NIR-II images at wavelengths well in excess of 1000 nm. Zhang et al. utilized the recognition motif of azobenzne (Azo) and β-CD to realize the assembly and disassembly between Azo modified lanthanide upconversion nanoparticles (UCNP@Azo) and β-CD modified downconversion nanoprobes (DCNP@β-CD) 85, achieving precision bioimaging of tumors (Figure 1). Azo is a photo-responsive groups, the isomerization can be reversibly controlled by UV and visible light 86-89. However, the tissue penetration of the short wavelength light is poor and the UV irradiation may cause photodamage to tissues. These problems can be solved using UCNP to convert NIR light into the UV/vis light, which can induce the isomerization of Azo group. The interval between two injections of UCNP@Azo (first injection) and DCNP@β-CD (second injection) was optimized to 10 h to obtain stable superior signal-to-noise ratio (Figure 1c). The host-guest complexation in tumor site significantly prolonged the retention time of DCNP@β-CD to afford a stable bioimaging window from 18 to 24 h post injection of the second injection. Importantly, NIR irradiation at 980 nm induced the isomerization transformation from trans state to cis state, resulting in the in vivo disassembly between the UCNP@Azo and DCNP@β-CD. Through this supramolecular strategy, the accumulation of the NIR II probes in the reticuloendothelial system (RES) (liver and spleen) remarkably lowered. The bioimaging background was reduced through NIR-triggered disassembly, the liver retention decreased 2.3 times than that of the assembly strategy, which was also favourable to accelerate the RES clearance rate to avoid long-term systemic toxicity.
Figure 1.
(a) Chemical structure of the building blocks and cartoon representation of UCNP@Azo and DCNP@β-CD. (b) Assembly and disassembly between UCNP@Azo and DCNP@β-CD controlled by host-guest recognition. (c) In vivo assembly of UCNP@Azo and DCNP@β-CD with improved tumor targeting and NIR-triggered disassembly with rapid clearance in liver. Reproduced with permission from 85, copyright 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
2.2. Supramolecular chemotherapy
As a systemic anticancer therapy, chemotherapy is widely used in cancer treatment using toxic agents to destroy cancer cells by stopping or slowing their growth. More frequently, chemotherapy is used along with other treatments to improve antitumor outcomes, such as surgery, radiation therapy, phototherapy or immunotherapy. Unfortunately, conventional chemotherapy using the low molecular weight drugs is greatly limited by several issues including poor solubility/stability of the drugs, drug resistance, low antitumor efficacy, and severe side effects. The drawbacks faced by the traditional chemotherapy can be effectively overcome by taking advantages of nanotechnology. Nonspecific distributions of the nanomedicines can be avoided and high tumor accumulation can be realized by exploiting the enhanced permeability and retention (EPR) effect and active targeting, which are helpful to improve antitumor performance and reduce side effect. Recently, host-guest systems on the basis of cyclodextrin recognitions are extensively used as delivery vehicles or drug containers in the fabrications of nanomedicines 90-92. Considering the stimuli-responsiveness of the non-covalent interactions, these supramolecular nanomedicines exhibited several advantages in cancer therapy.
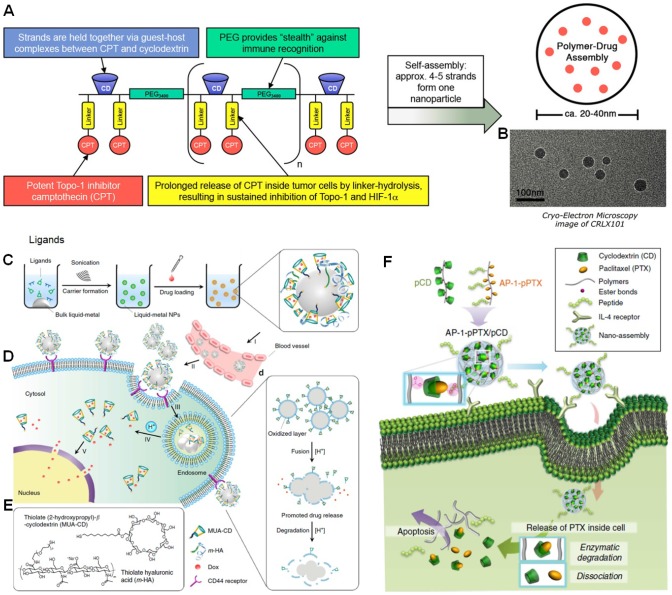
The size and structure of CDs is suitable for some anticancer drugs to form inclusion complex, such as CPT, PTX, doxorubicin (DOX) and so on. Indeed, a lot of work have been reported and several breakthroughs have been achieved using CDs as containers to complex and deliver the drugs. One successful example (CRLX-101) came from Davis and co-workers 93-96, who synthesized a PEG-containing polymer containing disubstituted β-CD and CPT grafted to the backbone through a glycine ester linkage with a molecular weight and drug loading capacity of 97 kDa and 6.8% (Figure 2a). The host-guest interactions between β-CD and CPT provided driving forces to form and stabilize the nanoparticles 20-40 nm in diameter (Figure 2b). The pharmacokinetics of CRLX-101 was comprehensively in different models including mice, rats, dogs, and humans, demonstrating that the area under the curves (AUC) was linearly with milligrams of CPT per square meter for all species. The loaded CPT was effectively cleaved from the polymer in tumor tissue led to a tumor to plasma ratio of 2.5 at 24 h further increasing to 21.2 at 48 h post injection. The drug amount in tumor was higher than that in any other organs. In vivo antitumor studies were carried out on different tumor models, such as HT29 colon cancer, LS174T colon cancer, MDA-MB-231 breast cancer, H69 small cell lung cancer, H1299 non-small cell lung cancer, or Panc-1 pancreatic cancer xenografts. All of investigations indicated superior efficacy of this supramolecular drug. More excitingly, this drug has been applied in human phase II clinical trial. The plasma concentration of released CPT in humans was consistent with the results in animals. The data from human patients indicated that CRLX-101 was highly accumulated in tumor site and the active drug released successfully over a period of several days to give inhibition of its target in the tumors of humans. Recent studies suggested that the antitumor performance was further improved by combining CRLX-101 with other drugs, such as bevacizumab, creating complete tumor regressions, reducing metastasis, and extending survival rate of the mice with metastatic disease 97-99.
Figure 2.
(a) Schematic of CRLX101 and study design. (b) Cryo-TEM image of CRLX101. Reproduced with permission from 94, copyright 2016 National Academy of Sciences. (c) Preparation route of LM-NP/Dox-L. (d) pH-Responsive delivery of Dox by LM-NP/Dox-L to the nuclei for the targeted cancer therapy. (e) Chemical structures of MUA-CD and m-HA. Reproduced with permission from 100, copyright 2015 Nature Publishing Group. (f) Schematic representation of nano-assembly-mediated PTX delivery. Reproduced with permission from 101, copyright 2014 Nature Publishing Group.
For inorganic nanocarriers, the biodegradability is an obstacle impeding their clinical translations. Gu et al. innovatively developed a transformable liquid-metal nanomedicine (LM-NP/Dox-L) through a simple sonication-mediated method (Figure 2c), in which the liquid-phase eutectic gallium-indium was the core 100. The shell was composed of thiolated (2-hydroxypropyl)-β-cyclodextrin (MUA-CD) and thiolated hyaluronic acid (m-HA), which served as drug container for DOX and active targeting moiety, respectively. Interestingly, the resultant nanoparticles fused and subsequently degraded in acidic endosomes after cellular internalization, facilitating the drug release and efficient elimination. Attributing to the EPR effect and active targeting mechanisms, LM-NP/Dox-L exhibited extended circulation time and high tumor accumulation, responsible for its supreme tumour inhibition activity. Toxicology evaluations confirmed that the biomarkers related to the liver function, renal function, and haematological assessment were in normal range, indicating the systemic toxicity of LM-NP/Dox-L was low. The strategy established in this work offers a novel method to fabricate theranostic agents with low toxicity.
The solubility of PTX can be significantly enhanced by the formation of inclusion host-guest complex with β-CD. In order to maximize the in vivo anticancer efficacy of PTX and inhibit burst release during circulation, Kim et al. designed a novel nano-assembly for effective anticancer therapy 101. A polymer-cyclodextrin conjugate (pCD) and a polymer-paclitaxel conjugate (pPTX) were synthesized as the building block (Figure 2f). Highly stable nanoparticles were prepared through multivalent host-guest interactions between β-CD and PTX. The binding affinity of pPTX/pCD was 104-fold higher than the monovalent host-guest complex, beneficial to preventing the premature drug release and increasing its circulation time in the blood. AP-1 peptide acting as a targeting ligand towards interleukin-4 (IL-4) receptor was further introduced, endowing the nanomedicine (AP-1-pPTX/pCD) with ability to specifically deliver PTX to MDA-MB-231 cells through receptor-mediated endocytosis. PTX was conjugated to the polymer backbone through ester linkages, which allowed efficient drug release by enzymatic degradation after cellular uptake. Administration of AP-1-pPTX/pCD significantly suppressed the tumor growth and extended the survival rate of the mice bearing MDA-MB-231 tumor. The therapeutic result of this supramolecular nanomedicine was much higher than that of the commercially used anticancer drug Taxol, exhibiting promising potential in cancer therapy.
For the nanocarriers no matter organic or inorganic ones, their degradability impeded the clinical translation of nanomedicines. The poor metabolism and inefficient clearance of the exogenous vehicles lead to the interactions with various components of the immune system, inevitably resulting in adverse reactions and long-term immunotoxicity. For example, Cremophor EL, a formulation vehicle in Taxol, is the main cause of severe anaphylactoid hypersensitivity reactions, hyperlipidaemia, aggregation of erythrocytes, abnormal lipoprotein patterns, and peripheral neuropathy. Although the widely used copolymers in nanomedicines are claimed to be biocompatible and biodegradable, such as polycaprolactone and poly(lactic acid), the degradation always takes several weeks or even several months, which is still too long to avoid their potential toxicity. To overcoming nanomaterials-induced toxicity, it is urgent to develop new nanoplatforms that can efficiently deliver drugs to tumors and completely degraded into small molecules for fast clearance from body after drug release.
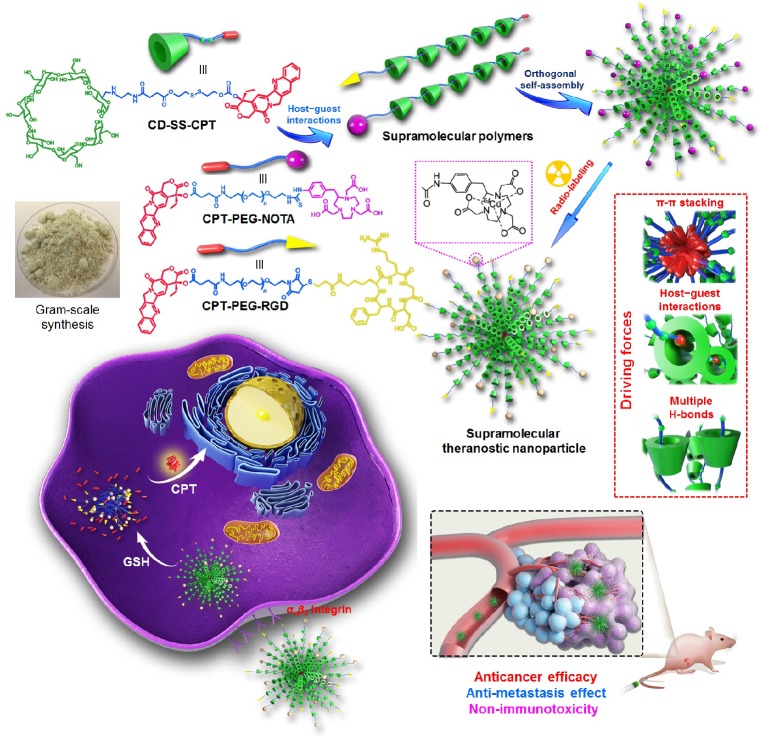
Chen et al. developed a supramolecular polymer-based nanotheranostics by using CD-SS-CPT as supramolecular monomer 102, in which the β-CD host and CPT guest was connected by a glutathione (GSH)-responsive disulfide (SS) group (Figure 3). Targeting ligand and imaging contrast were incorporated into the nanoparticles by orthogonal self-assembly in aqueous solution using CPT-PEG-RGD or CPT-PEG-NOTA as supramolecular polymerization initiators. By optimizing the ratio between CD-SS-CPT and CPT-PEG-RGD, supramolecular nanoparticles (SNPs) with relatively high stability and suitable size were prepared due to the dynamic nature of host-guest interactions. On account of the different GSH concentration in the bloodstream (1-10 μM) and inside cells (1-10 mM), SNPs were stable during circulation, while active CPT was release triggered by the intracellular GSH through a cascade reaction. Through this supramolecular strategy, the solubility of CPT was significantly improved by a factor of 232, and the lactone form of CPT was maintained by the formation of inclusion complex. Positron emission tomography (PET) imaging was utilized to monitor the delivery and distribution of SNPs, indicating high tumor accumulation was realized benefiting from nanotechnology and supramolecular chemistry. Superior antitumor performs were observed against HeLa subcutaneous xenograft and orthotopic breast tumor models. Additionally, this supramolecular nanomedicine exhibited excellent anti-metastasis effect. The byproduct produced after drug release could be effectively eliminated from body mainly through renal pathway, thus remarkably reducing the systemic toxicity and long-term immunotoxicity of SNPs.
Figure 3.
Supramolecular polymers constructed from the host-guest complexations between CPT and β-CD orthogonally self-assemble into SNPs for cancer theranostics. Reproduced with permission from 102, copyright 2018 American Chemical Society.
Nanomedicines can be high accumulated in the primary tumor through EPR effect after intravenous injection, however they hardly reach early-stage metastatic tumors that are always nonvascularized and incapable of conforming the EPR effect. Recently, Zhao et al. utilized a hollow polymer-silica nanohybrid to deliver DOX for the treatment of intra-abdominal metastasis 103. β-CD was introduced onto the surface as a gatekeeper through the host-guest interactions with Ada groups grafted on the hybrid through a disulfide bond to achieve GSH-responsive release of the loaded DOX. The nanomedicine could be enriched in metastatic tumor through intraperitoneal injection, which was more efficient than intravenous injection, thus resulting in superior therapeutic performance in vivo.
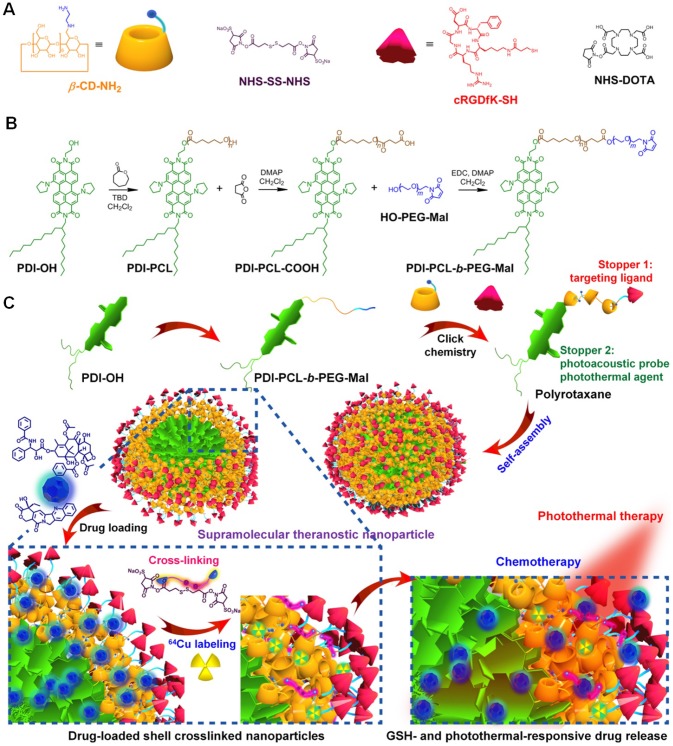
Several breakthroughs have achieved in cancer chemotherapy by increasing the therapeutic efficacy and inhibiting the side effects of the loaded anticancer drugs. DOXiL (DOX liposome), Onivyde (irinotecan liposome), and Abraxane (PTX protein-bound particles) have been approved by FDA to treat several types of cancers as first-line drugs. However, the dilution-induced premature drug release during systemic circulation greatly reduced drug concentration at the target site and increased toxicity towards normal tissues, remaining the major obstacle for their clinical translation. Development of a smart nanomedicine allowing for tailored release profiles with precise spatial and temporal dosage control is urgently desired. Chen et al. developed a novel biomaterial inspired from mechanically interlocked molecules and supramolecular chemistry (Figure 4) 104. A polyrotaxane was prepared and used as a theranostic platform, where the amphiphilic diblock copolymer acted as the axle and the primary-amino-containing β-CD acted as the wheels (Figure 4a and b). Different from traditional copolymers, nanoparticles formed from this polyrotaxane like a “nanosponge” to encapsulate hydrophobic anticancer drugs with high loading efficient and stability, such as paclitaxel and camptothecin. Interestingly, the “doors” of the nanomedicines were closed through a shell-crosslink strategy by connecting the wheels, thus sealing the anticancer drugs inside the nanoparticles to inhibit the premature drug leakage during circulation. GSH that highly expressed in cancerous cells is the “key” of these “doors”, which can specifically open the “doors” and release the drugs after the cancer cells internalized the nanomedicines (Figure 4c). Additionally, the release rate could be further accelerated using photothermal effect, thus decreasing the side effects. The other stopper of the polyrotaxane (cyclic peptide, cRGDfk) was further utilized to endow the nanomedicines with excellent targeting ability, selectively delivering drugs to cancer cells overexpressing αvβ3 integrin receptor. As a consequence, the maximum tolerated dose of the supramolecular nanomedicine is significantly increased by fully taking advantage of the smart topological structure of the polyrotaxane. PET imaging and PA imaging are allowed to trace the delivery, accumulation, bio-distribution, and excretion of the naomedicine. In vivo antitumor investigations confirmed that the combination of chemotherapy with laser-irradiation-active photothermal therapy (PTT) completely eliminated the tumors without any recurrence after a single-dose injection. Moreover, the nanomedicines exhibited excellent anti-metastasis by completely killing the cancer cells in the primary tissue using photochemotherapy. This supramolecular theranostic platform provides a blueprint to guide the design of the next generation of nanomedicines for safe and effective cancer treatment.
Figure 4.
Synthesis and fabrication schematics of SCNPs for supramolecular theranostics. (a) Chemical structures and cartoon representations of the building blocks. (b) Synthetic route to the polyrotaxane. (c) Schematic illustrations of the preparation of drug-loaded SCNPs and dual-responsive drug release. Reproduced with permission from 104, copyright 2018 Nature Publishing Group.
2.3. Supramolecular gene therapy
Gene therapy, a promising approach for the treatment of many inheritable or acquired diseases, can modify the expression of an individual's genes or that correct abnormal genes through the administration of a specific DNA (or RNA).105-111 The main challenge to realizing the application of gene therapy is the demand for effective and safe delivery methods to transport short cargoes to the site of action in the cells of target tissues. Naked DNA (or RNA) molecules are negative charged and high soluble, greatly restricting membrane permeation and access to the cytoplasm. On the other hand, the DNA (or RNA) can stimulate the innate immune system and are easily degraded by serum nucleases in the bloodstream. Therefore, new materials/methods are urgently required to assist the delivery of DNA (or RNA) with high efficiency and low side effects. Compared with viral vectors, non-viral materials have attracted tremendous interest, because they are simple to prepare, easy to modify, rather stable, and relatively safe. A broad diversity of non-viral vectors has been employed, such as peptides, polymers, aptamers, lipids, and antibodies.
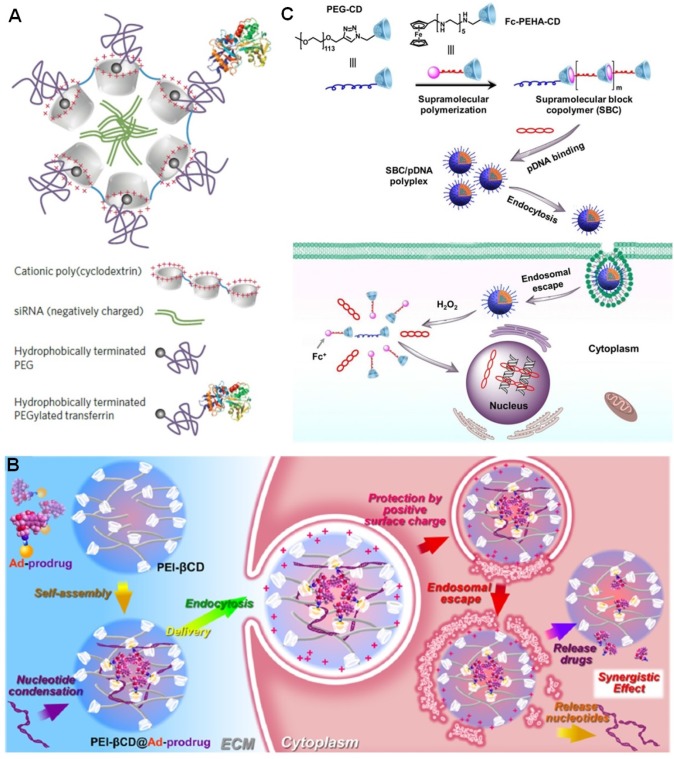
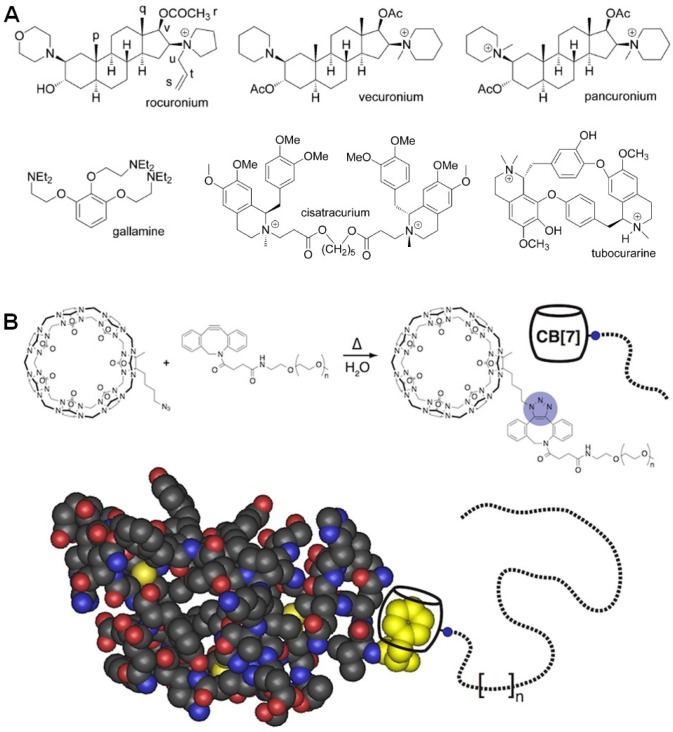
Davis et al. developed a cationic polycationic oligomers (n ≈ 5) containing β-CD in the backbone to deliver siRNA 112-120. In order to avoid nonspecific clearance of the resultant sub-100-nm nanoparticles, adamantine-PEG (AD-PEG) and adamantine-PEG-transferrin (AD-PEG-Tf) were incorporated by the host-guest interactions (Figure 5a). PEGylation was favorable to prevent protein absorption, but also reduced cellular uptake and silencing efficacy. Due to the overexpression of transferrin receptor in many tumors, this hurdle can be solved by the introduction of transferrin into this supramolecular system. Indeed, a doubling of tumor-specific knockdown of gene expression was realized through transferrin targeting in mouse model, in comparison with the EPR effect alone. The tolerated dosage of the nanoparticles can be reach 27 mg siRNA per kg of body weight in cynomolgus monkeys, and the translated efficacy located in the range of 0.6-1.2 mg siRNA per kg. More importantly, this product (CALAA-01) is in clinical trials at Arrowhead Research Corporation using a siRNA sequence to block expression of the M2 subunit of ribonucleotide reductase. In human clinical studies, CALAA-01 exhibited tumor specific knockdown in patients with metastatic melanoma. This was the first targeted nanoparticle siRNA delivery system to enter clinical trials for cancer, attributing to the low toxicity and excellent condensation ability of the cationic polymer, the steric stabilization by PEGylation and the introduction of targeting protein through host-guest chemistry.
Figure 5.
(a) Schematic illustrations of CALAA-01 for siRNA delivery. Reproduced with permission from 118, copyright 2013 Nature Publishing Group. (b) Schematic illustrations of self-assembled host-guest complexes PEI-β-CD@Ad-prodrug with nucleotide condensation ability for co-delivery of drugs and genes. Reproduced with permission from 123, copyright 2014 American Chemical Society. (c) Formation of a cationic supramolecular block copolymer and its pDNA binding and H2O2-triggered pDNA release in vitro. Reproduced with permission from 124, copyright 2017 American Chemical Society.
In gene therapy, the cationic polymer polyethylenimine (PEI) is a golden standard and has been widely utilized for in vitro and in vivo transfection, because of its stronger compaction capacity than other polycationic vectors. Additionally, the loaded DNA (or RNA) can escape from endosome through proton sponge effect to prevent themselves from being degraded, thus improve their transfection efficient. Tang et al. covalently modified β-CD by PEI to obtain an interesting gene delivery vector (PEI-β-CD) (Figure 5b) 121-123. Through host-guest chemistry, PEG segments, targeting ligands, and drugs were easily integrated without time-consuming synthesis. Codelivery of drug/prodrug and gene into the cytoplasm was realized by fully exploiting the cavity of β-CD, resulting in synergistic anticancer efficacy in vivo after release of drugs and nucleotides. For example, PTX was modified by Ada group to afford a prodrug that could be loaded into the nanoparticles self-assembled from PEI-β-CD and short hairpin RNAs (shRNA). The sophisticated delivery system was favorable to codeliver PTX for chemotherapy and shRNA for gene therapy by downregulating the expression of the survivin and Bcl-2. In vivo studies confirmed this supramolecular strategy suppresses cancer growth more effectively than delivery of either paclitaxel or shRNA in ovarian cancer therapy.
Although PEI was extensively used, its applications were greatly impeded by its toxicity. Typically, the toxicity of low molecular weight PEI is low, while the condensation capability also significantly decreases. Novel materials with high gene transfection efficient and low toxicity are required in clinical gene therapy. Based on the β-CD/ferrocene host-guest recognition, Zhu et al. fabricated a smart supramolecular gene vector combining the merits of conventional polymers and supramolecular polymers ranging from structures to functions (Figure 5c) 124. The supramolecular polymerization occurred in aqueous solution triggered by host-guest complexation to afford a cationic supramolecular copolymer in the presence of PEG-CD. The condensed pDNA could be fully released due to the dissociation of supramolecular copolymer arising from the oxidation of ferrocene by H2O2. Different from traditional PEI vectors, this supramolecular copolymer possessed high stability, excellent biodegradability, low cytotoxicity, and intelligent responsiveness, attributing to the dynamic nature of host-guest connections. The high gene transfection efficiency further suggested this supramolecular platform will be a promising nonviral carrier for gene therapy in the future.
The host-guest recognitions were also fully used to construct smart nanoplatforms to codelivery of anticancer drugs and genes. For example, Zhao et al. developed theranostic prodrug vesicles for imaging guided codelivery of CPT and siRNA for synergetic cancer therapy 125. β-CD was modified by an amino dendrimer through click reaction to give a hydrophilic host molecule. The prodrug guest was synthesized by the conjugation of adamantane (Ada)-modified naphthalimide with CPT via a GSH-responsive disulfide linkage. A supramolecular amphiphile was obtained via host-guest complexation between Ada and β-CD, which self-assembled into vesicles in aqueous solution. The fluorescence recovered accompanied by the release of CPT by cleaving the disulfide bond, allowing for intracellular imaging and simultaneous monitoring of drug release. siRNA (siPlK1) loaded on the vesicle was efficiently transported into cancer cells to improve cancer therapeutic efficacy. Xu et al. applied the host-guest chemistry in the fabrication of a hybrid nanomaterial to codeliver DOX and pDNA 126. The degradable silica nanoparticles embedding DOX were functionalized by Ada groups on the surface. Polycation comprising one β-CD core and two ethanolamine-functionalized poly(glycidyl methacrylate) arms worked as a pDNA delivery vector, which was further introduced using the β-CD/Ada host-guest recognition. The DOX encapsulated in the hybrid nanoparticles significantly facilitated the cellular uptake and the subsequent gene transfection.
2.4. Supramolecular immunotherapy
Cancer immunotherapy referring to the employment of the body's immune system to fight against cancer cells, opens a new field in cancer treatment and has gained prominence over the past few decades 127-129. The current clinical success of cancer immunotherapies, including immune checkpoint blockade, cytokines treatment, and chimeric antigen receptor T cell immunotherapy, expands the treatment modality of cancer, successfully conferring remission upon patients with previously bleak outcomes. Therefore, the development of an effective treatment with low side effects against tumors is highly demanded for cancer immunotherapy. Considering the distinct physiochemical properties and advantages in targeting delivery, enhanced cellular internalization, and stimuli-responsiveness, nanoscale delivery systems hold great promise in cancer immunotherapy, such as metallic, polymeric, and liposomal formulations.
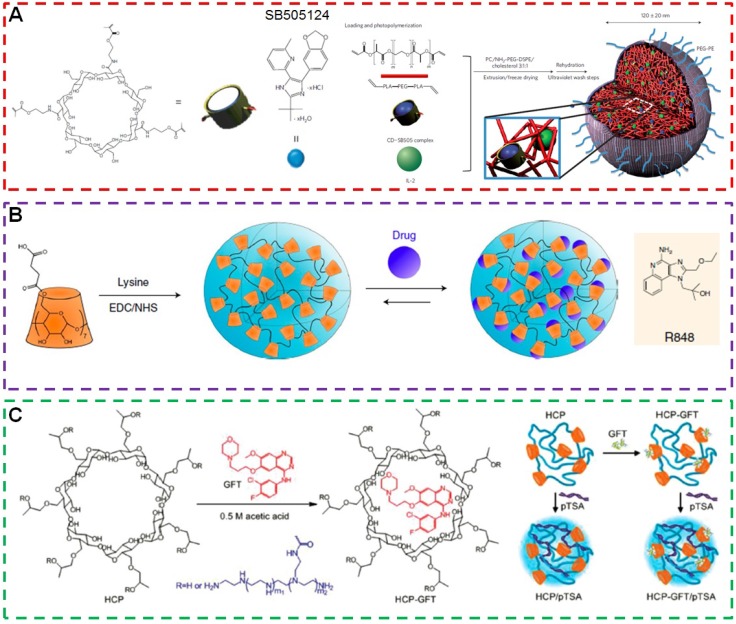
Immunotherapy has been approved as an effective therapeutic modality for patients with advanced melanoma. However, the secretion of the transforming growth factor-β (TGF-β) stunts local tumour immune responses, possibly by decreasing the number and activity of natural killer (NK) cells, reducing the activity of cytotoxic T lymphocytes while increasing the number of regulatory T lymphocytes (Tregs). Fahmy et al. developed nanoscale liposomal polymeric gels (nanolipogels; nLGs) with the ability to codeliver TGF-β receptor-I inhibitor (SB505124) and cytokine (IL-2) to overcome the immunoinhibitory nature of the tumour microenvironment (Figure 6a) 130. β-CD in the core of nLGs provided a container for the hydrophobic molecular inhibitor and allowed sustained and simultaneous release of both hydrophobic SB505124 and hydrophilic IL-2 to the tumor microenvironment, which was extremely difficult for conventional delivery systems. In vivo studies demonstrated codelivery of SB505124 and IL-2 by nLGs displayed potent immune responses in a B16/B6 mouse model of melanoma results from a crucial mechanism by activating both innate and adaptive immunity. The combination therapy increased the activity of NK cells and of intratumoral-activated CD8+ T-cell infiltration. As a result, tumor growth was significantly delayed and the survival of tumor-bearing mice was greatly prolonged.
Figure 6.
(a) Schematic of the fabrication of the nLG particle system. Reproduced with permission from 130, copyright 2012 Nature Publishing Group. (b) Schematic of CDNP preparation by lysine crosslinking of succinyl-β-cyclodextrin and subsequent drug loading by guest-host complexation of R848. Reproduced with permission from 131, copyright 2018 Nature Publishing Group. (c) Chemical structures of HCP and GFT and schematic illustration of the formation of HCP/pTSA complexes and HCP-GFT/pTSA complexes. Reproduced with permission from 132, copyright 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Tumor-associated macrophages (TAMs) are attracting more and more attentions from scientists because of their important roles in tumor metastasis and therapeutic resistance. The enhancement in TAMs counts always accelerates the progression of untreated tumors and lowers the anticancer efficacy of checkpoint blockade immunotherapy. Fortunately, the TAMs are highly plastic, which can be converted from tumor-supportive M2-like cells into tumoricidal M1-like cells using small molecules to inhibit receptors, tyrosine kinases or other transduction pathways in TAMs. However, it remains a challenging task to preferentially deliver low molecular weight inhibitors to TAMs in vivo. For many nanoformulations, like copolymers, liposomes and modified dextrans, the loading capacity of small molecule TAM modifying agents is unsatisfactory. In order to address these challenges, Weissleder et al. prepared β-cyclodextrin nanoparticles (CDNP) with a diameter of about 30 nm through the reaction between succinyl-β-cyclodextrin and L-lysine (Figure 6b) 131. β-CD in CDNP was used as a supramolecular drug reservoir to encapsulate R848 (CDNP-R848), an agonist of the toll-like receptors TLR7 and TLR8. This nanoformulation led to efficient delivery of CDNP-R848 to TAMs in vivo and facilitated the TAM uptake. The administration of CDNP-R848 altered the functional orientation of the tumor immune microenvironment towards an M1 phenotype in multiple tumor models. More impressively, improved immunotherapy response rates were monitored in an anti-PD-1 resistant tumor model when CDNP-R848 was administrated with immune checkpoint inhibitor anti-PD-1. This pioneering work provided a valuable methodology for the rational design of sophisticated delivery systems for cancer immunotherapy.
Gefitinib (GFT), is a tyrosine kinase inhibitor, is widely used to inhibit the proliferation of cancer cells by targeting epidermal growth factor receptors. Unfortunately, the anticancer efficacy of GFT diminished and tumor recurrence always appeared after certain periods due to mutations in the epidermal growth factor receptors or other mutations. Tang et al. combined immunotherapy and chemotherapy to overcome drug resistance and improve antitumor performance (Figure 6c) 132. A cationic polymer 2-hydroxypropyl-β-cyclodextrin- polyethylenimine (HCP) containing β-CD and PEI arms was used as the delivery vehicle, in which β-CD and PEI were used to load GFT and superantigen (plasmid DNA encoding transmembrane staphylococcal enterotoxin A protein, pTSA) through host-guest and electrostatic interactions. Interestingly, compared with monotherapy (GFT or pTSA) and simultaneous treatment (simultaneous codelivery of GFT and pTSA), the dual sequential treatment significantly enhanced T cell immunity, promoted cytokine production, inhibited tumor growth, and prolonged survival time in tumor models with lung carcinoma xenografts. This work confirmed the significant contribution of immunotherapy to chemotherapy and opens up new possibilities for treating a wide spectrum of cancers.
2.5. Supramolecular strategies for other diseases
Apart from cancer theranostics, cyclodextrin-based host-guest chemistry also finds extensive applications in other fields. Due to the difference in cavity size, CDs show different binding affinities and selectivities towards specific guests. For example, aliphatic chains and small molecules such as polyethylene glycol (PEG) are suitable for α-CD to form inclusion complexes. Alprostadil alphadex, a marketed product, is a sterile, pyrogen-free powder containing alprostadil in an alfadex (α-CD) inclusion complex, which is used to relax smooth muscle and increase blood flow, in order to treat peripheral circulatory disorders. For the free drug, its poor solubility requires intra-arterial administration to obtain useful clinical results. In addition to solubility, the host-guest complexation also improves its stability, making this supramolecular drug be available for parenteral use. For β-CD with larger cavity, it can form host-guest complex with prostaglandin E2, greatly increasing the stability of the loaded drug. The resultant product Prostarmon E is highly effective for the induction of labour in oxytocin-insensitive individuals and for avoidance bleeding after delivery.
β-CD with a max internal diameter of 7.8 Å possesses high affinity to the guests with larger size, such as cholesterol, which is mainly responsible for atherosclerosis, an inflammatory disease linked to elevated blood cholesterol concentrations. Huge attentions have been paid in the prevention and treatment of atherosclerosis, but cardiovascular disease still remains the leading cause of death worldwide. The overabundance of cholesterol in the subendothelial space easily forms cholesterol crystals, thus leading to a complex inflammatory response. Latz et al. attempted to use HPCD to complex and eliminate the deposited cholesterol, thus preventing and reversing atherosclerosis 133. Indeed, the atherosclerotic plaque size and cholesterol crystals load were reduced by the administration of HPCD. The reason was that the administration of HPCD increased the production of oxysterol in both macrophages and human atherosclerotic plaques. On the other hand, this supramolecular treatment promoted liver X receptor-mediated transcriptional reprogramming, significantly improving cholesterol efflux and exerting anti-inflammatory effects. Considering the biocompatibility of cyclodextrins, this supramolecular therapeutic method shows bright future in the prevention and treatment of human atherosclerosis.
Onychomycosis, a clinically stubborn disease with high prevalence and low cure rates, significantly reduces quality of life. However, the existing approaches to onychomycosis are unsatisfactory. Ketoconazole (KTZ) is a commonly used antifungal drug, but its efficacy is poor through oral administration, because the drug concentration at the sites of fungal infection is hardly maintained for a long time. Other therapeutic modalities, such as topical cream and laser-based treatments, are also ineffective to cure the disease. Tseng et al. fabricated KTZ-encapsulated cross-linked fluorescent supramolecular nanoparticles (KTZ/c-FSMNPs) to increase therapeutic results by controlling the in vivo release behavior of KTZ 134. Ad-PEG, Ad-PAMAM, and CD-PEI were used as the molecular building blocks to construct supramolecular nanoparticles through a multivalent molecular recognition between Ada and β-CD. Benefiting from the high KTZ loading efficiency/capacity, optimal fluorescent property, and sustained KTZ release profile, KTZ/c-FSMNPs 4800 nm in diameter exhibited satisfactory results in the treatment of onychomycosis. The existence of a fluorescent conjugated polymer (MPS-PPV) in KTZ/c-FSMNPs allowed to monitor their intradermal retention properties by in vivo fluorescent imaging. The biocompatibility and finite intradermal retention of this supramolecular therapeutic approach make it a promising candidate for intradermal controlled release of antifungal drug to treat onychomycosis.
3. Calixarene-based supramolecular theranostics
Calix[n]arenes (C[n]As) are versatile macrocyclic hosts prepared from the reaction between phenols and formaldehyde, in which the phenolic units are connected by methylene bridges at the meta-positions (generally composed of 4, 5, 6 or 8 phenolic units), endowing the C[n]As with basket-like structures and well-defined cavities 135-139. The upper and lower rims of C[n]As can be modified by functional groups to prepare water soluble C[n]As for biomedical applications. Driven by the ion-dipole interaction, electrostatic interaction, hydrophobic effect, and hydrogen-bonding, C[n]As are useful hosts to complex ions, sugars, amino acids, proteins, peptides, drugs, hormones, and nucleic acids. Recently, several theranostic systems have been developed by using C[n]As as the feasible platforms, showing promising applications in early-stage diagnosis and precise therapy.
3.1. Supramolecular cancer theranostics
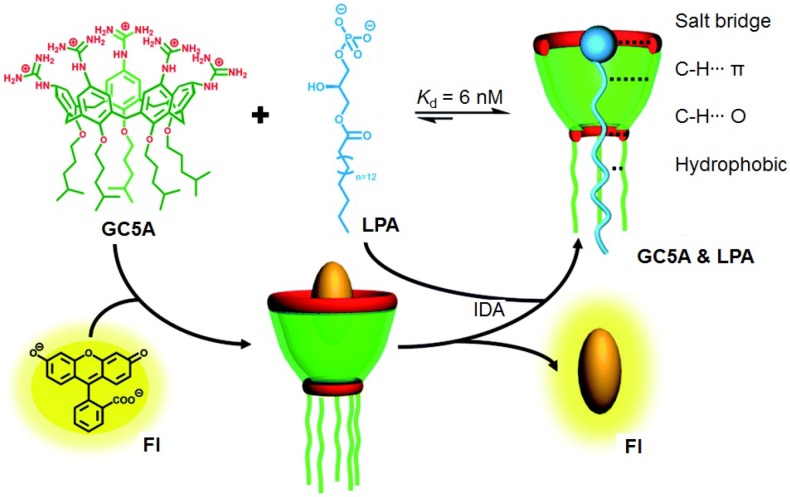
Lysophosphatidic acid (LPA) is a biomarker that is widely used to detect ovarian and other gynecologic cancers in their early stage. The concentration of LPA in healthy human plasma locates in the range between 0.1 to 6.3 mM, while the concentration rapidly increases to >63.2 mM for the patients have gynecologic cancers. For the existed detection methods, such as capillary electrophoresis, tandem mass spectroscopy, and radioenzymatic assays, the sensitivity and selectivity are unsatisfactory. It is urgently desired to find new receptors with high affinity and selectivity towards LPA to overcome the bottleneck in LPA detection. Through an indicator displacement assay, Guo et al. realized ultrasensitive fluorescence detection of LPA using a water-soluble guanidinium-modified calix[5]arene (GC5A) as the macrocyclic scaffold (Figure 7) 140. The cavity size of GC5A fits well with LPA, which effectively threads GC5A. The alkyl chains on the lower rim of GC5A provided additional hydrophobic interaction with the tail of LPA. Furthermore, the guanidinium groups on upper rime donated charge-assisted hydrogen bonds with the phosphate head of LPA. The binding affinity was determined to be (1.6 ± 0.1) × 108 M-1, attributing to the effective host-guest complexation. The detection limit of LPA was as low as 1.7 μM in the untreated serum. Notably, the other biologically important species in plasma did not affect the specific detection of LPA, confirm its feasibility in the complicated physiological milieu. This work provides an encouraging candidate to diagnose ovarian cancer and other gynecologic cancers at early stages.
Figure 7.
Schematic illustration of the binding between LPA and GC5A and the detection of LPA using IDA principle. Reproduced with permission from 140, copyright 2018 Royal Society of Chemistry.
By full taking advantages of the C[n]As-based host-guest recognitions, macrocyclic amphiphiles and supramolecular amphiphiles were build up, which were further utilized to deliver anticancer drugs and photosensitizers. Aldrich-Wright et al. modified the C[4]As scaffold to afford a water soluble p-sulfonatocalix[4]arenes (C4AS) 141, showing the ability to complex platinum(II)-based anticancer complexes, such as [(5,6-dimethyl-1,10-phenanthroline)(1R,2R-diaminocyclohexane) platinum(II)]2+ and ([(5,6-dimethyl-1,10-phenanthroline) (1S,2S-diaminocyclohexane) platinum(II)]2+. The host‒guest complexation effectively protected drugs from being degraded by GSH and remarkably decreased the diffusion rate of metal complexes, thus maintaining their anticancer activity.
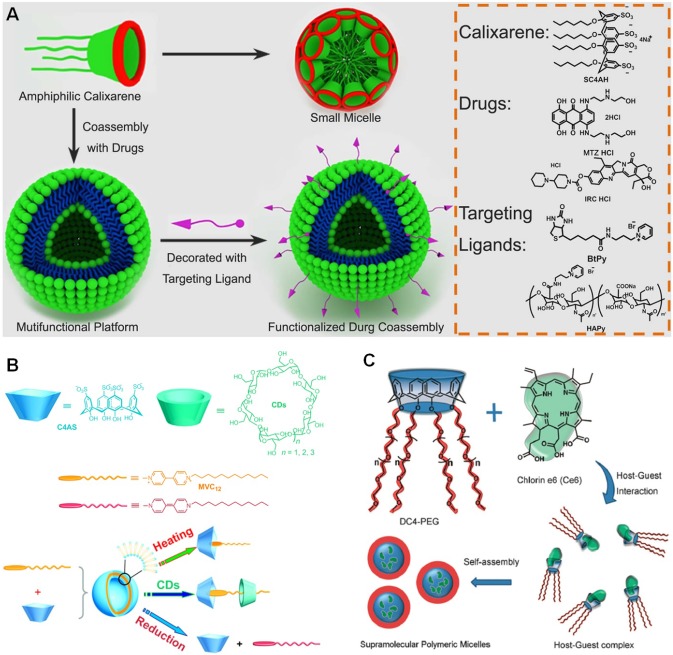
Amphiphilic calixarenes were prepared by introducing hydrophobic and hydrophilic groups on different rim. The resultant macrocyclic amphiphiles could self-assemble into well-defined nanostructures, such as vesicles, micelles, and nanoparticles, suitable carriers for drugs. A novel amphiphilic tetrahexyloxy-tetra-p-aminocalix[4]arene was synthesized by Lim and coworkers, in which the lower rim was modified by alkyl chains and the upper rim was functionalized by amine groups 142. The prepared nanoparticles from an emulsion evaporation technique were used to load PTX with a drug loading efficiency of 69.1 ± 5.3 μg drug/mg carrier, comparable with that of the commercially available Abraxane. Liu et al. used an amphiphilic p-sulfonatocalixarene as a “drug chaperone” to coassemble with cationic drugs (mitoxantrone∙HCl and irinotecan∙HCl) into a multifunctional platform driven by multivalent charge interactions (Figure 8a) 143. The loaded drugs were protected by the amphiphilic drug chaperone premature degradation upon formation of nanostructures. Interestingly, the surface of the coassemblies was further decorated by targeting ligands (biotin or hyaluronic acid) through host-guest chemistry due to the existence of calixarene cavities. In vitro studies validated that the anticancer activities of the drugs were significantly improved by this supramolecular strategy.
Figure 8.
(a) Functionalization protocol of the “drug chaperone” strategy and chemical structures of anticancer drugs, SC4AH and targeting ligands. Reproduced with permission from 143, copyright 2015 Nature Publishing Group. (b) Molecular structure of the building blocks and schematic illustration of the construction of a supramolecular binary vesicle via complexation of calix[4]arene (C4AS) with MVC12. Reproduced with permission from 144, copyright 2011 American Chemical Society. (c) Representation of the structure of DC4-PEG, Ce6, and formation of the supramolecular polymeric micelles based on host-guest interaction. Reproduced with permission from 145, copyright 2018 Royal Society of Chemistry.
The hydrophobic and hydrophilic parts can also be linked by the host-guest complex, resulting in the formation of supramolecular amphiphiles. Based on the recognition between paraquat and p-sulfonatocalix[4]arene, Liu et al. reported a novel supramolecular binary nanovesicle 144, and used it as delivery vehicle to load DOX∙HCl in the interior of the vesicles (Figure 8b). On account of the stimuli-responsive host-guest complexation, this nanomedicine was sensitive to temperature, cyclodextrin hosts, and redox potential. The anticancer activity of the DOX encapsulated nanovesicles was greatly maintained, while the cytotoxicity towards normal cells was pronouncedly reduced.
Besides chemodrugs, the calixarene-based supramolecular systems also enable to encapsulate photosensitizers for photodynamic therapy (PDT). Zhu et al. employed hydrophobic chlorin e6 (Ce6) as the guest and hydrophilic pegylated calix[4]arene (DC4-PEG) as the host to construct a supramolecular amphiphile (Figure 8c) 145. Through orthogonal self-assembly, the amphiphile formed nanoparticles in aqueous solution with a diameter less than 200 nm. Compared with free Ce6, the PDT efficacy was improved through this supramolecular formulation, because the π-π stacking of the photosensitizer was dramatically inhibited by the host-guest complexation, conducive to enhance the singlet oxygen generation quantum yield.
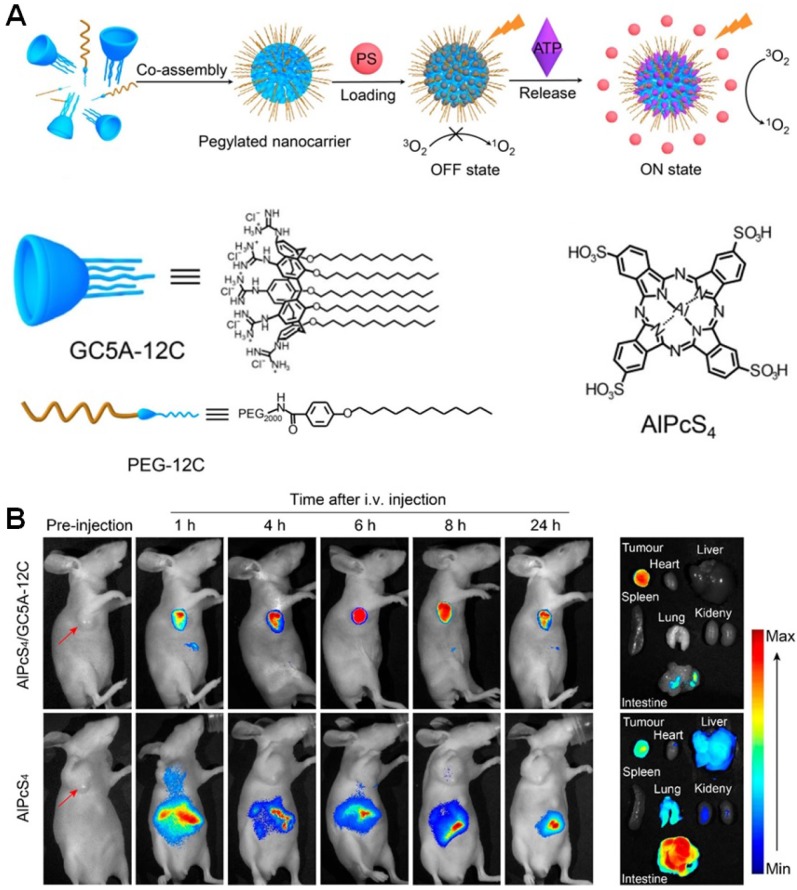
For in vivo applications, tumor selectivity and dark toxicity hinder become the main obstacles for PDT in clinical translation. Therefore, it is desirable to develop smart phototheranostics that are inert during circulation but can be activated in tumor microenvironment or in cancer cells induced by specific stimulus. Recently, Guo et al. designed an activatable phototheranostic platform through a methodology termed as biomarker displacement activation (BDA) (Figure 9) 146. An amphiphilic guanidinium-modified calix[5]arene pentadodecyl ether (GC5A-12C) was firstly used to pre-load the anionic photosensitizer (sulfonated aluminum phthalocyanine, AlPcS4) through host-guest interactions. As a consequence, the fluorescence and photoactivity of AlPcS4 were completely annihilated (OFF state) by the formation of nanoparticles with the assistance of 4-(dodecyloxy)benzamido-terminated methoxy poly(ethyleneglycol) (PEG-12C). The imaging and therapeutic properties of AlPcS4 were completely recovered (ON state) after being displaced by adenosine triphosphates (ATP) when the nanocarriers accumulated into tumor tissues via EPR effect. Due to the huge difference in ATP concentration between tumor tissues (> 100 μM) and normal tissues (1-10 nM), this sophisticated phototheranostics exhibited superior tumor selectivity as confirmed by in vivo fluorescence imaging. Compared with free AlPcS4, the tumor growth was dramatically inhibited after the treatment of nanoparticles followed by laser irradiation, suggesting the macrocyclic amphiphilic was indispensable in this targeted phototheranostics.
Figure 9.
(a) The cartoon representation of the BDA strategy. (b) In vivo fluorescence imaging of the 4T1 tumor-bearing nude mice. Reproduced with permission from 146, copyright 2018 American Chemical Society.
3.2. Host-guest chemistry for detoxification
Viologens including paraquat (PQ) and diquat (DQ), are showing an increasing number of scientific and technical applications as herbicides, DNA probes, prooxidants, and so on, which have been widely used by millions of growers in over 120 countries all over the world. However, their high toxicity is the potential risks to animals, the environment, and human, especially for PQ that can easily lead to various diseases or even death after absorption through digestive tract, respiratory tract and skin. Accidental or suicidal ingestion of PQ resulted in rapid multi-organ failure with a mortality rate exceeding 60%, while no effective antidotes are available. Liu et al. proposed a brilliant protocol to inhibit the toxicity of viologens by the host-guest complexation 147. Both guests formed stable inclusion complexes with the anionic hosts, including p-sulfonatocalix[4]arene (C4AS), p-sulfonatocalix[5]arene (C5AS), and p-sulfonatothiacalix[4]arene. Among these host-guest systems, the binding affinity in PBS at pH 7.2 between C5AS and PQ was the highest. The formation of inclusion complex induced the reduction potentials of PQ to shift to more negative values, highly inhibiting the production of reactive oxygen species in vivo. Further investigations demonstrated that the ingestion of C4AS and C5AS significantly prolonged the survival rate of viologen poisoned mice with lung and liver protection. Qi et al. studied the antidotic mechanism of C4AS for PQ by pharmacokinetics in vivo 148. The host-guest complexation effectively reduced the area under the concentration-time curve and maximum concentration of PQ. The other detoxication mechanism was that the inclusion complex was more difficult to pass through the biomembrane (such as the mucous membrane of the small intestine) than free PQ originating from its strong hydrophilicity, large molecular volume, and three-dimensional molecular structure.
The organophosphorus (OP) compounds comprising a labile P-X group (X = F, CN, SR, etc.) are highly toxic substances. For example, O-ethyl S-[2-(diisopropylamino) ethyl] methylphosphonothioate (VX) is able to react with the nucleophilic serine residue of the catalytic triad, inhibiting the action of acetylcholinesterase in neuromuscular junctions. As a result, the accumulation of the acetylcholine neurotransmitter leads to severe toxic effects on the central and peripheral nervous system, thereby causing respiratory malfunction and death. Proteins are used as bioscavengers to detoxify OPs before clinical signs occur, but limited by their low in vivo stability and immunogenicity. Therefore, it remains urgent demand to search artificial scavengers capable of trapping OPs in addition to encapsulation catalysts with a mode of action akin to enzymes. Kubik et al. synthesized sulfonatocalix[4]arenes with an appended hydroxamic acid residue and used these macrocyclic hosts as artificial scavengers to detoxify VX and related V-type neurotoxic Ops 149. The strong binding affinity to cationic nerve agents and the complex-induced cleavage of the P-S bond of the nerve agent were responsible for the detoxification. Compared with the free VX, the hydrolysis speed was rapidly accelerated by a factor of approximately 3500 in aqueous buffer at 37 oC and pH 7.4, involving phosphonylation of the hydroxamic acid on the calixarene hosts followed by a Lossen rearrangement. Although, the action mode of this supramolecular method is stoichiometric rather than catalytic, these hosts are the most efficient artificial scavengers for detoxifying persistent V-type nerve agents under mild conditions.
3.3. Host-guest chemistry for the treatment of Alzheimer's disease
Enzymes are responsible for a series of biochemical processes, and the dysfunction in the enzyme expression level always results in many diseases. Compared with other responsive systems sensitive to ion, temperature, light, or pH, the enzyme-based strategies exhibit elegant advantages in the fields of biotechnology, diagnostics, and targeted drug delivery. The entrapped cargoes can be delivered to the site of action and then released from the carriers induced by the specific enzymatic reactions. Cholinesterase, a key protein overexpressed in Alzheimer's disease (AD), is an ideal stimulus for the development of responsive delivery systems.
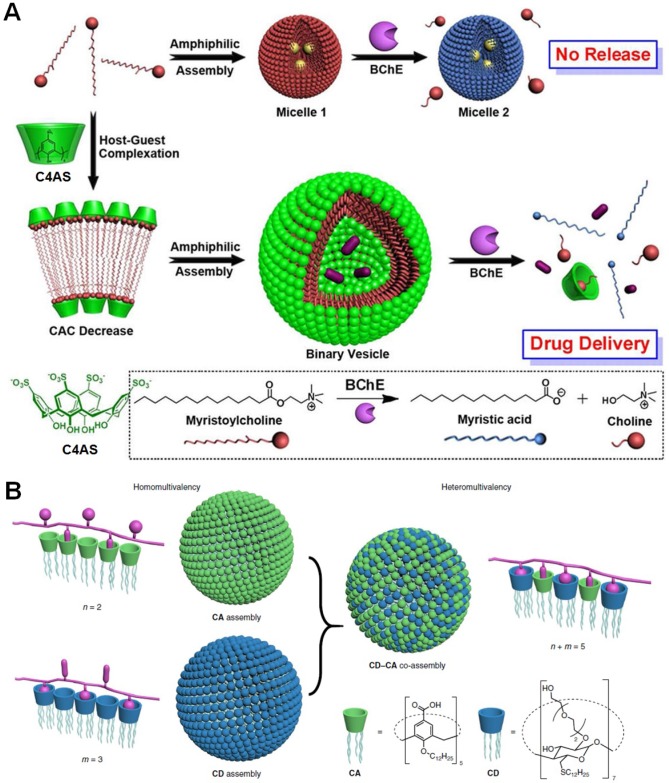
Liu et al. constructed a supramolecular amphiphile using p-sulfonatocalix[4]arene (C4AS) as the host and a natural myristoylcholine as the enzyme-cleavable guest (Figure 10a) 150. The host-guest complexation between the anionic host and cationic guest significantly decreased the critical aggregation concentration by a factor of ~100, reinforcing the complex stability in aqueous solution. The complexed head worked the hydrophilic part and the alkyl chain acted as the hydrophobic part. The formed amphiphile self-assembled into vesicles with a diameter ranging from 90 to 200 nm when the ratio of C4AS/myristoylcholine was optimized to 1:10. The vesicle completely disassembled in the presence of butyrylcholinesterase (BChE) due to the dissociation of the host-guest interactions by the hydrolysis of myristoylcholine into choline and myristic acid. Because of the high selectivity, this system kept stable in solution containing other enzymes, such as exonuclease I (Exo I), alkaline phosphatase (CIAP), and glucose oxidase (GOx). The vesicles were further employed to deliver tacrine, a water-soluble drug for the treatment of AD. Different from the total disassembly of the free vesicles, the existence of tacrine led to partial disassembly of the nanomedicine, eliminating the potential side effects caused by excessive drug release. Cytotoxicity evaluation confirmed the biocompatibility of the vesicles, paving the way for their future applications in the treatment of AD.
Figure 10.
(a) Schematic illustration of amphiphilic assemblies of myristoylcholine in the absence and presence of C4AS. Reproduced with permission from 150, copyright 2012 American Chemical Society. (b) Illustration of the heteromultivalent peptide recognition by co-assembly of CD and CA amphiphiles. Reproduced with permission from 151, copyright 2018 Nature Publishing Group.
Amyloid-β peptides (Aβ), comprised mainly of 40- and 42-residue peptides, plays a causative role in the memory loss, neurodegeneration, and dementia associated with AD. Considerable biochemical and genetic investigations demonstrate that the formation of senile plaques in the brain composed of the aggregated Aβ is the main pathological hallmarks of AD. Guo et al. designed a heteromultivalent platform (CD-CA) by co-assembling two different amphiphilic macrocycles 151, cyclodextrin (CD) and calixarene (CA), into one ensemble in view of their complementary binding behaviors in aqueous solution (Figure 10b). Benefiting from heteromultivalency and self-adaptability, the binding efficiency and selectivity of CD-CA were drastically improved due to the existence of two orthogonal, and non-covalent binding sites on the surface of the co-assembly. Compared with the homomeric CA assembly and CD assembly, CD-CA displayed extremely high avidity toward YKYKYK peptide (1010 times for CD, 100 times for CA). Excitingly, CD-CA exhibited specific recognition towards Aβ42 containing two K and one Y residues, the association constant was determined to be as high as (7.9 ± 0.4) × 107 M-1. The incubation of Aβ42 with an equivalent amount of CD-CA co-assembly significantly inhibited the β-sheet aggregation. Moreover, most of the mature fibrils (75%) can be dissociated by treating with CD-CA co-assembly, which was much effective than those of CD assembly (4%), the CA assembly (15%), and their simple mixture (22%). Different from the inhibition of fibrils formation, it's really challenging to break down the formed amyloid fibrils, emphasizing the importance of host-guest chemistry in AD treatments.
4. Cucurbituril-based supramolecular theranostics
Cucurbit[n]urils (CB[n]s), mainly including CB5, CB6, CB7, CB8 and CB10, are prepared from the acid-catalyzed condensation reaction of glycoluril and formaldehyde, in which the glycoluril units are connected by methylene bridges 151-156. For CB[n]s family, their heights are some (9.1 Å), while the annular widths, equatorial widths, and cavity volumes gradually increase associated with the improvement of repeating units. Compared with the size of CB[n]s and CDs, it can be found that the cavity sizes of CB6, CB7, and CB8 are similar to α-, β-, and γ-CD, respectively. However, the binding behaviors of CB[n]s are quite different from those of CDs. Hydrophobic interaction is the main driving force to form inclusion complex between CDs and guest. Hydrogen bonding, ion-dipole interactions, and/or van der Waals force are responsible for CB[n]s-based host-guest chemistry, because CB[n]s possess highly symmetric pumpkin-like structure with a hydrophobic cavity and two negatively charged carbonyl lined portals. Due to the discrimination in portal and cavity sizes, the specific host-guest recognition motifs are constructed between CB[n]s and guests with different sizes.
4.1. Supramolecular theranostics using cucurbiturils as containers
The portal size of CB6 is 3.9 Å, suitable for the protonated diaminoalkanes to form stable inclusion complexes. For spermidine and spermine, the binding affinities to CB6 are higher than 1011 and 1012 M-1, respectively 157, stabilizing the formed complexes in a dynamic fluidic environment such as the blood stream in live animals. Based on the CB6/spermidine recognition, Kimoon et al. utilized CB6-based polymer nanocapsules (CB[6]PNs) as a non-covalent platform for multimodal imaging 158. The surface of CB[6]PNs was modified by different spermidine-conjugated tags through host-guest chemistry. Cyclic RGDyK, 64Cu-NOTA complexes, and cyanine 7 were introduced into CB[6]PNs for cancer targeting, PET imaging, and NIR imaging, respectively. In vivo studies confirmed the successful cancer-targeted multimodal imaging of tumors, suggesting potential applications of CB[6]PNs in cancer theranostics.
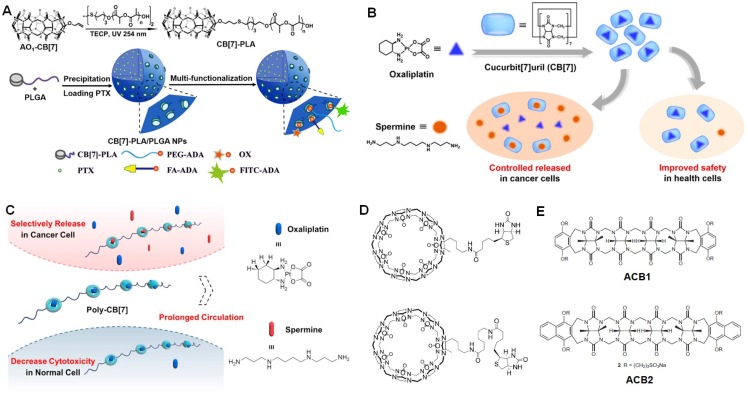
For CB7, methyl viologen dication, 2,6-bis(4,5-dihydro-1H-imidazol-2-yl) naphthalene, and protonated adamantanamine are ideal guests to form 1:1 inclusion complexes. Wang et al. conjugated CB7 to poly(lactic acid) (PLA)/poly(lactic-co-glycolic acid) (PLGA) to afford an amphiphilic copolymer 159, which self-assembled into nanoparticles (Figure 11a). Through a “Lego-like” approach, anticancer drug (oxaliplatin), targeting ligand (folic acid), fluorescence dye (FITC) and PEG linked with amantadine could decorate the nanoparticles surface on the basis of the host-guest interaction between amantadine and CB7 with an association constant of ~108 M-1. The hydrophobic core of the multifunctional nanoparticles was further used to load PTX as the secondary drug for synergistic therapy against cancer cells.
Figure 11.
(a) Synthesis of CB7-PLA and schematic illustration of CB7-PLA/PLGA NPs with non-covalently tailorable surface. Reproduced with permission from 159, copyright 2018 American Chemical Society. (b) Supramolecular chemotherapeutic drug constructed from the host-guest complexation between CB7 and oxaliplatin. Reproduced with permission from 160, copyright 2017 American Chemical Society. (c) Schematic illustration of supramolecular polymeric chemotherapy based on poly-CB7. Reproduced with permission from 161, copyright 2018 Elsevier. (d) Chemical structures of biotin modified CB7. Reproduced with permission from 162, copyright 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (e) Chemical structures of ACB1 and ACB2. Reproduced with permission from 163, copyright 2013 Nature Publishing Group.
Rather than traditional guests, CB7 also holds the ability to complex oxaliplatin. The binding affinity was determined to be 2.89 × 106 M-1 by Zhang et al. using isothermal titration calorimetry (Figure 11b) 160. The cytotoxicity of oxaliplatin-CB7 was significantly decreased upon formation of host-guest complex. Interestingly, the anticancer activity of oxaliplatin was waken up by intracellular spermine overexpressing in tumor due to its competitive complex with CB7. Additionally, the consumption of spermine further enhanced the anticancer activity than oxaliplatin itself, realizing a synergistic anticancer efficacy. For oxaliplatin-CB7, the circulation time will be an obstacle for its in vivo applications, because the complex is easily eliminated from body, thus reducing the antitumor outcome. In order to solve this issue, Zhang et al. synthesized a water-soluble polymer bearing CB7 (poly-CB7) in the backbone through click reactions and used poly-CB7 to deliver oxaliplatin (Figure 11c) 161. The high association constant guaranteed the efficient complexation under the administration condition and high drug loading content (12.1 %). It should emphasized that over 90% oxaliplatin was wrapped by CB7 in blood within the injection dose of 5 mg oxaliplatin per kg, preventing premature drug release during circulation. Using poly-CB7 as the carrier, the area under the curve of oxaliplatin/poly-CB7 group was 21.6-fold higher than oxaliplatin group. The efficient release of oxaliplatin and depletion of spermine in cancer cells cooperatively inhibit the growth of tumor with higher bioactivity than oxaliplatin. Another advantage of this supramolecular system was that the systemic toxicity was reduced.
The platinum-based anticancer drugs are widely used as first line drugs for the treatment of various cancers. However, the severe side effects limit their clinical usage. It is necessary to develop an improved version of oxaliplatin with enhanced anticancer efficacy and reduced toxicity against normal tissues. Hence, Isaacs et al. conjugated a biotin ligand to a monofunctionalized CB7 derivatives by amide bond formation (Figure 11d) 162, aiming to endow the supramolecular containers with targeting ability. The modification of CB7 did not affect the host-guest properties, the synthesized hosts were able to complex a series of anticancer agents. In vitro anticancer evaluation verified that the targeted container·oxaliplatin exhibited approximately an order of magnitude higher activity against L1210FR cells. Besides the symmetrical CB[n]s, Isaacs et al. also developed acyclic cucurbit[n]urils (ACBs) 163, a novel class of solubilizing agents with extremely low toxicity (Figure 11e). The ACBs significantly enhanced the solubility of ten insoluble drugs by a factor of between 23 and 2,750 upon formation of container-drug complexes. Very excitingly, the neutral PTX gave the largest solubility enhancement (2,750-fold) ascribing to the flexibility of the acyclic structure and the efficient host-guest interactions, including π-π stacking, the hydrophobic interaction, and hydrogen bonds. Compared with the free drug, the complexation did not interfere the anticancer activity of PTX, but increase the cancer killing ability. In vivo studies indicated that the administration of ACB-albendazole complex effectively inhibit the tumor growth and extended the survival of the mice bearing SKOV-3 xenograft tumors 164.
4.2. Supramolecular gate-keepers using cucurbiturils-based recognitions
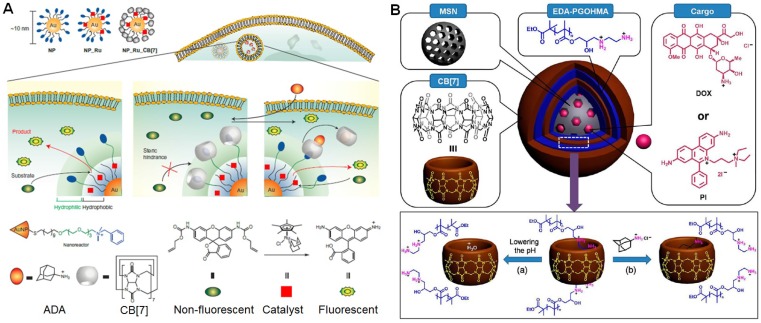
Cucurbiturils-based recognitions were fully used as the “switches” to control the drug release by adjusting the association and dissociation of the host-guest complexation. Recently, Rotello et al. used CB7-based recognition to regulate the bioorthogonal catalysis in cells (Figure 12a) 165. The catalytic activity was inhibited by embedding the hydrophobic transition metal catalysts in the monolayer of water-soluble gold nanoparticles followed by the blockage of the “gate-keeper” using host-guest chemistry, because the substrates unable to touch the catalysts. The activity of these catalysts completely restored by removing CB7 on the surface upon addition of a competing guest (1-adamantylamine). Using this concept, the anticancer efficacy of propargyl-modified 5-fluorouracil (pro-5FU) was activated in cells through the palladium-mediated chemocatalysis, thus releasing the active 5FU to inhibit the nucleotide synthetic enzyme thymidylate synthase to induce cytotoxicity. This work provided a biomimic strategy to mimic the allosteric effect of natural enzymes.
Figure 12.
(a) Schematic illustration of bioorthogonal nanozyme design and supramolecular regulation of intracellular catalysis. Reproduced with permission from 165, copyright 2015 Nature Publishing Group. (b) Schematic diagram of the construction of LbL-MSNs using CB7-based recognition. Reproduced with permission from 167, copyright 2014 American Chemical Society.
Issacs et al. employed a metal organic polyhedron (MOP) as a delivery platform to loaded DOX 166. The MOP contains 18 covalently attached CB7 groups by controlling the ratio of the building blocks during the preparation process. The amphiphilic guest with an 1,6-hexanediamine (HDA) hydrophilic head and a hydrophobic C18H37 tail was using to modify MOP through the host-guest complexation between CB7 and HDA. The solubility of the resultant MOP was good because there were 12 Pd2+ ions and 18 dicationic HDA guests bound to CB7. The layer containing alkyl chains was a hydrophobic phase, suitable for the encapsulation of DOX. The host-guest complexes on the surface acted as “gate-keepers” to prevent the loaded drug from being release prematurely. Due to the difference in binding affinities between CB7/HAD (8.97 × 107 M-1) and CB7/1-adamantylamine (4.23 × 1012 M-1) recognition motifs, the “gate-keepers” were opened by the addition of 1-adamantylamine, and the drug escape from the platform. By changing the “locks” using different guests, smart delivery systems were obtained responsive to dual pH-chemical and photochemical-chemical stimuli. The presence of CB7 hosts on the surface enabled a wide range of plug-and-play non-covalent functionalization for diagnostic and therapeutic applications.
Mesoporous silica nanoparticles (MSNs) are promising drug carriers, however the release behaviors are hardly controlled without post-modifications. Yang et al. reported a supramolecular layer-by-layer method to coat the surface of MSNs (LBL-MSNs) by using CB7 and bis-aminated poly(glycerol methacrylate)s (BA-PGOHMAs) 167, in which CB7 worked as a molecular bridge to connect two bis-aminated polymers through host-guest interactions (Figure 12b). The premature release was inhibited at physiological pH (pH = 7.4) due to the blockage of the pores, attributing to the electrostatic interactions between negatively charged MSN surfaces and positively charged EDA-PGOHMA. In addition, the formation of host-guest complexes driven by cation-dipole interactions in the multiple layers stabilized the hybrid nanomedicine. The “gate-keepers” were opened to yield ways for the loaded drug upon acidifying the solution, because the CB7 in the supramolecular polymer coatings was occupied by the plentiful hydronium ions. Meanwhile, the addition of adamantaneamine hydrochloride can also accelerate the drug release resulted from the competitive host-guest complexation. In vivo studies showed the formulation of this supramolecular nanomedicine resulted in a highly efficient tumor-growth inhibition rate.
4.3. Supramolecular delivery systems using cucurbiturils-based recognitions
Different from other CB[n]s, CB8 can act as a “molecular handcuff” to complex two guest molecule inside its cavity because of its large portal size (6.9 Å) and cavity volume (479 Å3) 168-176. For example, electron-deficient methylviologen (MV) derivatives and electron-rich 2,6-dihydroxynaphthalene derivatives can be simultaneously encapsulated by CB8 in aqueous solution driven by multiple noncovalent interactions, forming an 1:1:1 ternary complex with an association constant up to 1015 M-2. Based on the ternary recognitions, dynamic diblock copolymers and brush copolymers have been prepared that are suitable for the construction of nanocarriers for drugs. The stimuli-responsiveness of the host-guest recognitions makes the resultant nanomedicines sensitive to the specific tumor-related triggers, selectively releasing the loaded drugs in cancer cells after accumulations and internalizations.
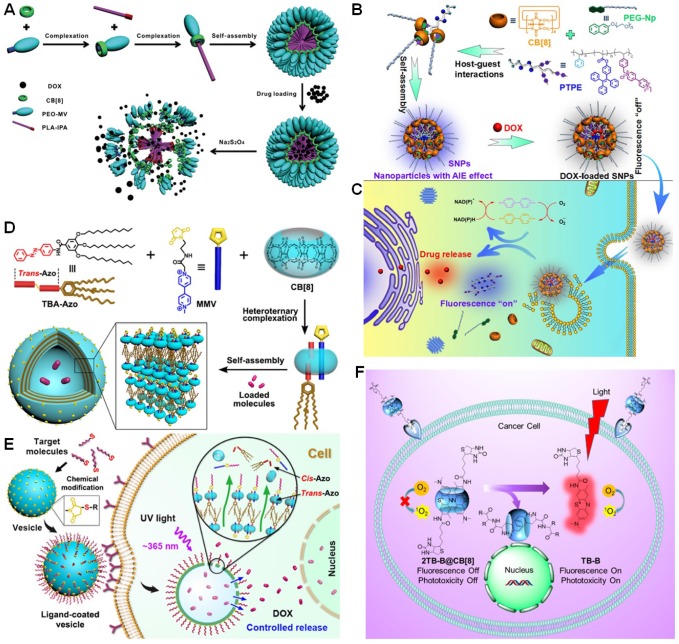
Using the ternary recognition between CB8, MV, and indole, Ji et al. established an amphiphilic diblock copolymer (PEO-MV/CB8/PLA-IPA) using indole-terminated PLA (PLA-IPA) and MV-terminated PEO (PEO-MV) as the building blocks (Figure 13a) 177. PEO-MV/CB8/PLA-IPA self-assembled into nanoparticles that were utilized to carry DOX in the hydrophobic core. The host-guest complexation could be effectively controlled by the redox chemistry of MV, allowing reduction-triggered release of anticancer drugs. Another redox-responsive ternary recognition between CB8, 4,4'-bipyridinium derivative, and PEGylated naphthol (PEG-Np) was used by Chen et al. to construct a supramolecular brush copolymer. Different from other polymeric vehicles 178, aggregation-induced emissive fluorophore (tetraphenylethene, TPE) was grafted on the polymer, making the delivery system visible (Figure 13b and c). Interestingly, the fluorescence of TPE and DOX was both quenched after loading the drug due to energy transfer relay, in which the emission transferred from TPE to DOX and then self-quenched by the aggregation-caused quenching (ACQ) effect of DOX. Triggered by reducing agents and low pH, the “silenced” fluorescence related to TPE and DOX was recovered due to the disassembly of the supramolecular nanoparticles and release of DOX, realizing in situ visualization of the drug release.
Figure 13.
(a) Schematic illustration of the formation, drug loading and reduction-triggered drug release of CB8-based supramolecular assemblies. Reproduced with permission from 177, copyright 2014 Royal Society of Chemistry. (b) Chemical structures of the building blocks and the preparation of nanoparticles. (c) Schematic illustration of the imaging-guided drug delivery. Reproduced with permission from 178, copyright 2017 American Chemical Society. (d) Schematic representation of the assembly strategy to construct supramolecular vesicles. (e) Schematic representation of targeted and photo-controlled drug delivery. Reproduced with permission from 179, copyright 2018 American Chemical Society. (f) CB8-regulated supramolecular photosensitizer and its mechanism for targeted cancer imaging and PDT. Reproduced with permission from 181, copyright 2016 American Chemical Society.
Azo can also work as the secondary guest for CB8 to form 1:1:1 ternary complex with MV in the cavity of CB8. Luo et al. constructed highly stable giant supramolecular vesicles through hierarchical self-assembly of CB8-based supramolecular amphiphiles (Figure 13d and e) 179. The hollow cavity of the vesicles enabled to capture DOX∙HCl with loading efficiency of 62.1%. The maleimide groups on the external surface of the supramolecular vesicles could be modified by iRGD peptide and bovine serum albumin through click reactions, promoting the resultant multifunctional vesicles targeting specificity and cell internalization. Photo-responsive drug release was observed by manipulating the heteroternary complexation resulting from the morphological changes of the assemblies caused by the trans to cis isomerization of Azo group.
By rationally choosing the guests, CB8 can complex two same molecules to form 1:2 host-guest complexes. For example, a series of tricyclic basic dyes can complex with CB7 and CB8, such as methylene blue, toluidine blue, and acridine orange 180. Due to the different complexation modes, the changes in physical or chemical property of some dyes are totally distinct. The formation of 1:1 inclusion complex with CB7 always enhances the fluorescence, while the fluorescence is quenched after forming an 1:2 complex with CB8 possibly for the π-π stacking interactions of the dyes in the cavity. Inspired by this phenomenon, Zhang et al. used a supramolecular approach to fabricate an activatable photosensitizer for simultaneous cancer imaging and selective ablation of cancer by PDT (Figure 13f) 181. Toluidine blue functionalized by biotin (TB-B) was chosen as the guest, which formed a stable [3]pseudorotaxane-type host-guest complex with CB8 (2TB-B@CB8). The N-terminated aromatic peptides exhibited extremely high binding constant with CB8 (109-1011 M-2), which was strong enough able to trigger the release of TB-B from the cavity of CB8. This established system incorporating a variety of smart functions such as targeting, protection, transport, delivery, imaging, and therapy selectively accumulated in tumor, lit-up the tumor site, and enhanced cancer ablation ability.
4.4. Supramolecular systems for the treatment of other diseases
Neuromuscular blocking agents (NMBAs) are extensively utilized during anesthesia in operating rooms. It is reported that more than 400 million patients receive curare-type NMBAs every year, including atracurium, pancuronium, rocuronium, cisatracurium, and vecuroniumnd. At the end of the surgery, it is necessary to use reversal agents (neostigmine and edrophonium) to reverse the biological effect of NMBAs to prevent residual neuromuscular block and recover muscle function. However, the utilization of commercially used reversal agents always causes cardiovascular side effects or even result in a (depolarizing) neuromuscular block in clinical practice. Sugammadex, the first selective relaxant binding agent, is a modified γ-cyclodextrin that has been widely used for the reversal of neuromuscular blockade after administration of the aminosterioid non-depolarizing neuromuscular-blocking agents such as rocuronium or vecuronium.
Inspired by this, Isaacs et al. employed water soluble ACBs to reverses neuromuscular block using host-guest chemistry (Figure 14a) 182. ACBs formed 1:1 host-guest complexes with steroidal and benzyl isoquinoline type NMBAs, and the binding constants located within the range of 2.2 × 105-3.4 × 109 M-1. In vivo studies showed that ACB2 was able to tightly complex with rocuronium in bloodstream (association constant = 3.4 × 109 M-1) and the formed complex excreted from body quickly, which decreased the concentration of rocuronium at the neuromuscular junction, thus reversing deep neuromuscular block. The administration of ACB2 accelerated recovery of both train-of four (TOF) ratio to 0.9 (26 s) and spontaneous breathing (32 s), which were much faster than the mice treated with placebo (21 min and 12.5 min for TOF and spontaneous breathing, respectively), suggesting that ACBs were the promising candidates as reversal agents for neuromuscular block.
Figure 14.
(a) Chemical structures of NMBAs. Reproduced with permission from 182, copyright 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (b) Strategy for supramolecular PEGylation. Reproduced with permission from 183, copyright 2016 National Academy of Sciences.
Over the past decades, biomolecules especially for proteins have attracted more and more attentions. However, these biopharmaceuticals continuously cause a series of attendant complications due to the poor chemical and/or structural stability in physiological environment, where the active drugs are converted into inactive and/or potentially immunogenic forms. Typically, PEGylation of protein drugs through covalent modification is chosen to increase the solubility, promote the stability, inhibit aggregation, reduce glomerular filtration, and improve pharmacological activity. Although the direct covalent modification is feasible, several issues still exist. The modified proteins need purification to eliminate exogenous compounds that are possibly cause immunogenicity. Over-modification may also result in a deleterious effect on proteins activity and function. Anderson et al. proposed a supramolecular route to modify the properties of therapeutic proteins using CB7-based host-guest chemistry (Figure 14b) 183, endowing the supramolecular proteins with prosthetic functionality. Non-covalent modification substantially increased the stability of three distinct protein drugs (insulin, glucagon, and an antibody) and prolonged their pharmacological activity. CB7 exhibited high affinity to an N-terminal aromatic residue on insulin, thus allowing supramolecular modification of insulin by CB7-PEG. In vivo evaluations demonstrated that the duration of insulin activity could be controlled by change the molecular weight of CB7-PEG without a need for direct modification to the therapeutic protein. Compared with conventional PEGylation, supramolecular PEGylation of therapeutic proteins possesses evolutionary advantages. Firstly, the non-covalent modifications are clear, isolation and purification are unnecessary. Secondly, the therapeutic entities remain unmodified, facilitating to maintain their activities and functions. This can simplify regulatory approval of a previously approved biopharmaceuticals. Thirdly, the supramolecular building blocks can be easily excreted from body, greatly reducing the risk of immunogenicity.
5. Pillararene-based supramolecular theranostics
Pillar[n]arenes (PA[n]s), a new type of macrocycles reported in 2008, is a rising star in supramolecular chemistry. Different from C[n]As with a basket-shaped structure, PA[n]s are linked by methylene bridges at para-positions of 2,5-dialkoxybenzene rings, forming a symmetric and rigid pillar-shaped structure 184-193. The electron-rich and hydrophobic cavity make PA[n]s prefer complexing neutral or electron-withdrawing guests, such as alkyl chains, pyridinium salts, imidazoliums, diamines, and viologen moieties 194-203. The unique structures and easy functionalization of PA[n]s afford them excellent properties in host-guest chemistry 204-214. Water-soluble P[n]As bearing trimethylammonium, carboxylate, or imidazolium groups have been fabricated that are able to wrap diverse drug molecules to enhance their solubility and stability in aqueous media. Wheate et al. studied the host-guest complexation between carboxylated pillar[6,7]arene (CP6A and CP7A) with different drugs including chlorhexidine hydrochloride, memantine, and proflavine 215. Hydrophobic interactions, hydrogen bonding, and electrostatic interactions are cooperatively responsible for the formation of inclusion complexes. Oxaliplatin strongly bond to CP6A in PBS at pH 7.4 with a binding constant of (1.02 ± 0.05) × 104 M-1 216. Interestingly, this host-guest complexation was pH-responsive, the binding constant decreased significantly associated with the reduction of solution pH, which was helpful to release the loaded drug in tumor tissue. The improvement in stability and circulation time of oxaliplatin by the formation of supramolecular complex greatly enhanced its anti-tumor performance in vivo.
5.1. Supramolecular amphiphiles for cancer treatment
A variety of supramolecular amphiphiles were constructed by taking full advantages of PA[n]s-based host-guest chemistry 216-221. Functional groups can be integrated into the supramolecular systems, endowing the delivery platforms with stimuli-responsive properties. Wang et al. constructed a dual-responsive supramolecular amphiphile on the basis of the recognition between lysine and a water-soluble pillar[5]arene (WP5) (Figure 15a) 222. An amphiphilic guest was utilized, in which the hydrophilic head and hydrophobic tail were linked by a disulfide group. The amphiphile self-assembled into vesicles in aqueous solution about 112 nm in diameter. A hydrophilic anticancer drug, mitoxantrone (MTZ), was loaded in the cavity of the supramolecular vesicles. Burst drug release could be achieved in the acidic tumor microenvironment containing high concentration of GSH, attributing to the GSH-responsiveness of the guest and the pH-responsiveness of the host. In order to enhance the biocompatibility of supramolecular carriers, Wang et al. used phosphate-based pillar[5]arene (WP5P) and pillar[6]arene (WP6P) to fabricate supramolecular amphiphiles 223. Due to the different binding modes, WP5P-based supramolecular amphiphile self-assembled into micelles while WP6P-based supramolecular amphiphile formed vesicles, which were suitable to load hydrophobic DOX and hydrophilic MTZ, respectively. The loaded drugs could be efficiently released by lowering the solution pH and adding Zn2+. Unlike conventional delivery systems, the properties of the supramolecular amphiphiles could be easily regulated by changing the functional groups. For example, a photo-responsive drug delivery system was established by introducing Azo group into the amphiphilic guest 224. Moreover, PDT and chemotherapy were combined using a boron-dipyrromethene (BODIPY) photosensitizer as the hydrophobic part of the guest, greatly increasing the anticancer results in a synergistic approach 225.
Figure 15.
(a) Schematic illustration of the formation of supramolecular vesicles and their stimuli-responsive drug release. Reproduced with permission from 222, copyright 2015 Royal Society of Chemistry. (b) Cartoon representation of the multifunctional supramolecular vesicles for chemophotothermal synergistic therapy. Reproduced with permission from 226, copyright 2018 American Chemical Society. (c) Illustration of the formation of vesicles and their redox/pH dual-responsive drug release. Reproduced with permission from 228, copyright 2016 Royal Society of Chemistry.
Based on the recognition between WP5 and trimethylammonium, Fan et al. constructed bola-type multifunctional supramolecular amphiphiles for multimodal therapy (Figure 15b) 226. Unlike liposomes, the membrane of the vesicles self-assembled from the supramolecular amphiphile was composed of conjugated NIR-absorbing dyes (perylene diimide, PDI) and alkyl chains, which provided an additional driving force (π-π stacking) for the stabilization of the vesicles. On the other hand, the existence of PDI was helpful to encapsulate DOX in the hydrophobic membrane with high-loading efficiency. PDI also worked as a photothermal agent to enrich the functions of the supramolecular nanomedicine. The combination of PTT and chemotherapy exhibited more remarkable antitumor efficacy than single treatment. Additionally, PTT and PDT were simultaneously incorporated using an NIR-absorbing diketopyrrolopyrrole (DPP) as the bridge of the supramolecular amphiphile 227. The cargo loaded in the vesicles was special, tirapazamine (TPZ) was a hypoxia-activated prodrugs that converted into cytotoxic oxidizing radicals in hypoxic environment, which could be produced by PDT process due to the continuous consumption of O2. The cascade activation of chemotherapy cooperated with PDT and PTT to synergistically combat against cancer.
A ferrocenecarboxylic acid capped pillar[5]arene (FACP5) was developed that self-assembled into single-layered vesicles (Figure 15c) 228. Supramolecular guest with a galactose residue was plugged in the cavity of FACP5 in the membrane was through the host-guest complexation between pyridinium and FACP5. Galactose on the surface worked as targeting ligand to specifically deliver DOX∙HCl to MCF-7 cells overexpressing galectin via the carbohydrate-protein interactions. The supramolecular vesicles exhibited an AND logic gate function to fine-tune drug release. The vesicles disassembled at low solution pH in the presence of GSH, while the structure was maintained either by adding GSH or by regulating the solution pH to 4.0 alone. The host-guest interaction was vanished after the COO- was protonated into COOH and the cationic ferrocenium was reduced into neutral ferrocenyl group. Additionally, Pei et al. intelligently prepared another type of supramolecular vesicles based on the complexation between a tryptophan-modified pillar[5]arene (TP5) and the same guest for synergistic and targeted drug delivery 229. It should be pointed out that the multivalent interactions between the ten indole rings of tryptophan groups on the macrocyclic platform and the intracellular DNA created synergistic effect with the drug loaded in the cavity of supramolecular vesicles, showing potential in overcoming drug resistance.
Another part of supramolecular amphiphiles were also prepared by the scientists by using anticancer drugs as the hydrophobic parts, such as DOX and CPT 230-234. The solubility of the drugs was effectively enhanced and the cellular internalization was adjusted. For example, large aggregated were formed by CPT along several micrometers in size, which was unfavorable for cellular uptake 235. While the size of the self-assemblies decrease to ~100 nm by the formation of therapeutic supramolecular amphiphile, significantly increasing the uptake by the cells. More importantly, the anticancer activity of the drugs was maintained by rationally using pH-, and GSH-responsive linkages. In vitro studies confirmed that the anticancer efficacy of these supramolecular drugs was satisfactory.
5.2. Supramolecular polymeric drug delivery systems
For the water soluble P[n]As modified by anionic carboxylate or phosphate groups, the negatively charged property is unfavorable for cellular internalization. In order to solve this problem, Huang et al. exploited a ternary supramolecular system using a hydrophilic diblock copolymer methoxy-poly(ethylene glycol)114-block-poly(L-lysine hydrochloride)200 to balance the charges (Figure 16a and b) 236. The solubility of a photo-cleavable prodrug (Py-Cbl) was firstly improved by loading it in the hydrophobic cavity of WP6. The host-guest complex was further utilized as supramolecular crosslink to prepare ternary polyion complex (PIC) micelles through multiple electrostatic interactions. The neutral PEG shell not only stabilized the PIC micelles, but also facilitated cellular endocytosis of the ternary micelles. As anticipated, the core-shell structured PIC micelles were uptaken by cancer cells, and the active anticancer drug chlorambucil was successfully release upon irradiation.
Figure 16.
(a) Chemical structures of the building blocks. (b) Cartoon representation of the self-assembly process of a ternary complex micelle. Reproduced with permission from 236, copyright 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (c) Illustration of the assembly and disassembly of thermo- and oxidation-responsive supramolecular polymeric vesicles. Reproduced with permission from 237, copyright 2016 Royal Society of Chemistry. (d) Chemical structures and cartoon representations of the building blocks. (e) Schematic illustration of the formation of polymersomes self-assembled from the amphiphilic supramolecular dicopolymer and their use as reduction-responsive drug delivery vehicles. Reproduced with permission from 238, copyright 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (f) Chemical structures and cartoon representations of the building blocks. (g) Schematic illustration of the formation of supramolecular nanoparticles self-assembled from the amphiphilic supramolecular brush copolymer and their use as drug delivery vehicles. Reproduced with permission from 239, copyright 2016 Royal Society of Chemistry.
Considering the stimuli-responsive property of the host-guest chemistry, sophisticated supramolecular drug delivery systems have been fabricated using the non-covalent bonds as the linkers. On the basis of pillar[6]arene-ferrocene recognition, Wang et al. prepared an amphiphilic supramolecular diblock copolymer using pillar[6]arene-terminal-modified poly(N-isopropylacrylamide) (PNIPAM-P6) and ferrocene-terminal-modified methoxy-poly(ethylene glycol) (mPEG-Fc) and the building blocks (Figure 16c) 237. Supramolecular vesicles were obtained through hierarchical self-assembly, which exhibited thermo and oxidation dual-responsiveness. Accelerated drug release was monitored by lowering the temperature or adding oxidizing agent caused by the solubility changes of the host polymer and the oxidation of the ferrocene moiety of the guest polymer, respectively.
The recognition between pillar[5]arene and MV is frequently used in the construction of functional supramolecular architectures. A supramolecular diblock copolymer and a supramolecular brush copolymer were prepared by Huang et al. using the concept of ''block-copolymer-free'' strategy with pillar[5]arene-based host-guest chemistry (Figure 16d-g) 238, 239. For example, P5-PEG-Biotin referred to poly(ethylene glycol) (PEG) containing a triglycol monomethyl ether-modified pillar[5]arene host and a biotin targeting group on each side and PCL-C2V referred to poly(caprolactone) with a viologen terminator were connected by the host-guest complex with a binding constant of (1.14 ± 0.11) × 104 M-1. The obtained supramolecular amphiphile P5-PEG-Biotin⊃PCL-C2V self-assembled into polymersomes in water, which were further utilized to encapsulate DOX. The biotin decorating the surface endowed supramolecular polymersomes with excellent targeting ability, preferentially delivering DOX to biotin receptor-positive HeLa cancer cells. The reduction of the viologen group by the intracellular reductase NAD(P)H resulted in the release of DOX arising from the disassembly of the polymersomes. Using the aggregation-induced emissive polymer as a scaffold, a supramolecular brush copolymer was fabricated utilizing pillar[5]arene-based host-guest chemistry, which was further used as a self-imaging delivery vehicle. In situ drug release was monitored by rationally exploiting the Förster resonance energy transfer and aggregation-caused quenching phenomenon. Compared with free DOX, the circulation time and anti-tumor efficacy of these two sophisticated supramolecular nanomedicines were profoundly promoted, while the systematic toxicity was decreased through flexible and modular supramolecular strategy.
5.3. Supramolecular hybrid nanomaterials for drug delivery
Over the past decades, organic-inorganic hybrid materials have attracted increasing attention because of their excellent properties and processability that can enormously differ from those of the separate ones 240-247. These hybrids widely applied in bio-relevant applications based on controlled delivery of therapeutic, diagnostic, and pharmaceutical agents. Combining the merits of the fine-tuning properties of hybrid nanomaterials and the dynamic nature of supramolecular chemistry, supramolecular hybrids provide unique ways to bridge the gap between materials sciences, supramolecular chemistry, and nanotechnology, offering novel perspectives for the application of supramolecular hybrids in theranostics.
On account of their rigid structure, desirable surface area, high porosity, excellent biocompatibility, and exceptional stability, metal-organic frameworks (MOFs) are promising candidates for drug delivery. Unfortunately, the poor water dispersibility and difficult post-modification of MOFs greatly limit their biomedical applications. Pei et al. designed a supramolecular hybrid delivery system using WP6 to assemble with DOX-loaded zeolitic imidazolate frameworks (ZIF-8@DOX) to enhance its water dispersibility through the coordination between the metal nodes and the carboxyl group of WP6 248. Host-guest chemistry was employed to introduce targeting ligand on the hybrid surface, thus improving the selectivity in killing hepatoma (HepG2) cancer cells. Inspired by designable layer-by-layer assembly concept and facile surface engineering, Yang et al. reported a proof-of-concept multifunctional nanoplatform (PUWPFa) for synergistic cancer chemophotothermal therapy (Figure 17a and b) 249. In this supramolecular hybrid, the polypyrrole nanoparticles (PPy NPs) acting as the core were excellent photothermal agent. The MOF-PPy NPs scaffold allowed the hybrid to load 5FU with a high loading efficiency. Interestingly, pillar[6]arene-based [5]pseudorotaxanes were pH/temperature dual-responsive gatekeepers, allowing targeted drug delivery and precise drug release, minimizing collateral damage to normal tissues. Folic acid grafted polyethyleneimine (PEI-Fa) at the outmost layer was able to enhance tumor accumulation and therapeutic performance. In vivo anti-tumor investigations demonstrated the satisfactory therapeutic outcomes of this newly developed multifunctional nanoplatform.
Figure 17.
(a) Schematic illustration of the preparation of PUWPFa nanoparticles and (b) their application for dual targeted chemophotothermal therapy. Reproduced with permission from 249, copyright 2018 American Chemical Society. (c) Schematic illustration of the fabrication process and operation of Fe3O4@UiO-66@WP6 theranostic nanoplatform and the structures of the representative building blocks. Reproduced with permission from 251, copyright 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Cerium oxide nanomaterials have been approved to possess anticancer effect mainly by inducing oxidative stress and causing lipid peroxidation and cell membrane leakage. Pei et al. non-covalently modified cerium oxide nanorods (CeONRs) by a galactose functionalized pillar[5]arene (GP5) for target drug delivery 250. Anticancer drug was trapped in the porous pores of the inorganic support, and 2-((3-(trimethoxysilyl)propyl)disulfanyl) pyridine (TPDP) endowed the multi-component delivery system with GSH responsiveness. As expected, this supramolecular system multiple functions enhanced the anticancer efficacy in a synergistic manner.
Other inorganic nanomaterials can also be employed to develop multifunctional supramolecular hybrids, such as Fe3O4, CuS, and so on, significantly enriching the properties for cancer theranostics. For example, Yang et al. constructed a pillar[6]arene nanovalve operated supramolecular hybrid (Fe3O4@UiO-66@WP6) combining the merits of multi-stimuli responsive drug release (Figure 17c) 251, MRI guidance, and effective chemotherapy. Fe3O4 particles acted as the inner core with superior abilities of MRI and the UiO-66 MOF shell was designed to load anticancer drug 5FU with a high loading capacity. The surface of the inorganic core-shell nanocomposite was further installed by pillararene-based pseudorotaxanes as tightness-adjustable nanovalves through host-guest complexation with the aim to adjust drug release in response to the external-stimuli, such as pH, temperature, and ions. In vitro studies indicated that the drug-loaded Fe3O4@UiO-66@WP6 with high anticancer efficacy and low toxicity against normal cell showed potential in cancer theranostics. Yu et al, functionalized CuS nanoparticles by WP5 through a facile one-pot supramolecular capping method 252. Through host-guest interactions, the surface was further modified by a pyridinium-based guest containing a galactose as a targeting ligand. Driven by electrostatic interactions between negatively charged WP5 and positively charged DOX∙HCl, the anticancer drug could be attached on the smart supramolecular hydrid (CuS@CPG-DOX) with a high drug loading capacity (48.4%). The combination of PTT and chemotherapy exhibited a remarkably enhanced therapeutic effect, as well as excellent biocompatibility.
Recently, Chen et al. developed a supramolecular hybrid material (GO@CP6⊃PyN) using a pillar[6]arene-based host-guest complex to decorate the surface of graphene oxide (GO) through non-covalent interaction (Figure 18a and b) 253. Compared with free GO, the absorption of the hybrid was greatly improved by supramolecular formulation, which was favorable to enhancing its photothermal effect and PA signal by improving the light absorption efficiency. More excitingly, the bicarbonate counterions on the surface of GO@CP6⊃PyN were decomposed into CO2 nanobubbles upon laser irradiation, which acted as supramolecular chaperone to effectively amplify the US and PA signals of the supramolecular hybrid material. This work suggested that the introduction of supramolecular chemistry not only remedied the disadvantages of pristine GO, but also introduced novel functions into the hybrid, confirming supramolecular method was an exceedingly exquisite strategy to fabricate multifunctional hybrid materials for precise diagnosis/imaging and highly effective therapy.
Figure 18.
(a) Chemical structures of the building blocks (b) Schematic representation of the preparation of supramolecular hybrid material exhibiting NIR light-triggered PA and US imaging enhancement. Reproduced with permission from 253, copyright 2018 Royal Society of Chemistry.
6. Conclusions and perspectives
As discussed above, a variety of sophisticated supramolecular theranostics have been developed and applied in theranostic fields. Some of them have already been approved by FDA for clinical use, and some of them are in clinical trial. Benefiting from the host-guest chemistry, the stability/solubility of the drugs are effectively enhanced, the pharmacokinetics behaviors and duration of activity are significantly improved, thus fulfilling the requirements from patients and doctors. Owing to the dynamic nature, the preparations of the functional supramolecular architectures are easy and feasible through “Lego-like” self-assembly, avoiding time-consuming synthesis and purification. By rationally exploiting the stimuli-responsive properties of host-guest chemistry, spatial and temporal release of the loaded cargoes can be released, greatly enhancing the therapeutic results and reducing the side effects. Although supramolecular theranostics on the basis of host-guest chemistry have been extensively developed over the past years and achieved a series of charming progresses, there still remain several issues that researchers are facing. For example, although various supramolecular systems have been developed for the treatment of Alzheimer's disease, these investigations are stuck in vitro. It is extremely hard for the nanosystems to efficiently deliver drugs to brain by crossing blood brain barrier. This challenge has impeded the scientist for decades and will continue to be an obstacle. How to rationally employ supramolecular strategy on the basis of their dynamic properties to remove this block on the way will be an interesting topic. Additionally, the syntheses of some macrocycles are difficult to scale up, which may also be a problem for clinical use.
New macrocyclic hosts are urgently demanded for the fabrication of smart supramolecular theranostics. The reported hosts mainly include crown ethers, cyclodextrins, cyclodextrins, calixarenes, cucurbiturils, and pillararenes. Recently, supramolecular metal-based systems including MOF and supramolecular coordination complexes are utilized as hosts to loaded cargoes, and the theranostic results are quite exciting. 254-267 Apart from cyclodextrins, almost no products have been approved or even in clinical trials using the other macrocycles due to their potential toxicity and immunogenicity. If these artificial compounds cannot be cleared from body quickly and effectively, immunotoxicities are hardly avoided. More attentions need to pay during the development of novel theranostic host-guest systems. Biocompatibility and degradability are required for the next-generation of hosts, while their host-guest complexation capability and stimuli-responsiveness should be maintained.
For the mostly reported photo-responsive groups, they are sensitive to UV and visible light. Unfortunately, the tissue penetration of the light with short wavelengths is extremely poor, closing the door to in vivo applications. Although upconversion nanomaterials and two photon responsive groups have been utilized, the results are unsatisfactory due to the low conversion efficiency. Considering the non-limited penetration depth highly localized control of ultrasound and X-ray, novel responsive groups sensitive to these stimuli will be promising candidates in the future applications. Simultaneously, ultrasound therapy and radiotherapy can be integrated into the supramolecular systems, achieving synergistic efficacy.
The complexation and decomplexation of the host-guest systems need to be reasonably balanced during the delivery process and after cellular internalization, which is favorable to enhance anticancer efficacy while reduce undesirable side effects. In order to inhibit the premature release, the host-guest interaction should tightly connect the building blocks to stabilize the supramolecular delivery systems. Triggered by the tumor-specific biomarkers, burst release is required upon endocytosis by the cancer cells arising from the dissociation of the host-guest complexation. For most of the host-guest recognitions, the association constants are lower than 105 M-1, which indicated that a large portion of the complex will disassemble after being injected into the bloodstream caused by the dilution-induced dissociation. Therefore, it is essential to introduce other driving forces to maintain the complexation during circulation. For example, the host-guest complexes can be embedded into polymeric scaffolds to fabricate supramolecular polymeric nanoformulations, thus significantly improving their stability.
The understanding of intractable disease especially for cancers is rapidly growing, which requires more collaboration with the multidisciplinary researchers from chemistry, materials engineering, cancer biology, pharmacology, and oncology. New biomaterials are urgently desired to develop personalized theranostic platforms for precise diagnosis and intelligent therapy. Supramolecular chemistry provides a new choice for the scientist to construct smart and effective nanotheranostics. The inherent shortages of some drugs that lead to their failure in clinical trials can be possibly solved by taking full advantages of supramolecular chemistry and nanotechnology, possibly changing the trash (failed drugs) into treasures. In view of the significant research efforts being dedicated, we believe that humanity will greatly benefit from supramolecular theranostics in the near future.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Biomedical Imaging and Bioengineering, and National Institutes of Health.
References
- 1.Aida T, Meijer EW, Stupp SI. Functional supramolecular polymers. Science. 2012;335:813–7. doi: 10.1126/science.1205962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehn JM. Perspectives in Supramolecular Chemistry- from Molecular Recognition Towards Molecular Information Processing and Self-Organization. Angew Chem Int Ed Engl. 1990;29:1304–19. [Google Scholar]
- 3.Lehn JM. Supramolecular chemistry: Where from? Where to? Chem Soc Rev. 2017;46:2378–9. doi: 10.1039/c7cs00115k. [DOI] [PubMed] [Google Scholar]
- 4.Yang L, Tan X, Wang Z, Zhang X. Supramolecular Polymers: Historical Development, Preparation, Characterization, and Functions. Chem Rev. 2015;115:7196–239. doi: 10.1021/cr500633b. [DOI] [PubMed] [Google Scholar]
- 5.Yu G, Jie K, Huang F. Supramolecular Amphiphiles Based on Host-Guest Molecular Recognition Motifs. Chem Rev. 2015;115:7240–303. doi: 10.1021/cr5005315. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Wang C. Supramolecular amphiphiles. Chem Soc Rev. 2011;40:94–101. doi: 10.1039/b919678c. [DOI] [PubMed] [Google Scholar]
- 7.Horwich AL, Farr GW, Fenton WA. GroEL-GroES-mediated protein folding. Chem Rev. 2006;106:1917–30. doi: 10.1021/cr040435v. [DOI] [PubMed] [Google Scholar]
- 8.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294:1684–8. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 9.Grabow WW, Jaeger L. RNA Self-Assembly and RNA Nanotechnology. Acc Chem Res. 2014;47:1871–80. doi: 10.1021/ar500076k. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Kladwang W, Lee M, Cantu D, Azizyan M, Kim H. et al. RNA design rules from a massive open laboratory. Proc Nat Acad Sci USA. 2014;111:2122–7. doi: 10.1073/pnas.1313039111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chworos A, Severcan I, Koyfman AY, Weinkam P, Oroudjev E, Hansma HG. et al. Building programmable jigsaw puzzles with RNA. Science. 2004;306:2068–72. doi: 10.1126/science.1104686. [DOI] [PubMed] [Google Scholar]
- 12.Appel EA, del Barrio J, Loh XJ, Scherman OA. Supramolecular polymeric hydrogels. Chem Soc Rev. 2012;41:6195–214. doi: 10.1039/c2cs35264h. [DOI] [PubMed] [Google Scholar]
- 13.Su H, Zhang PC, Cheetham AG, Koo JM, Lin R, Masood A. et al. Supramolecular Crafting of Self-Assembling Camptothecin Prodrugs with Enhanced Efficacy against Primary Cancer Cells. Theranostics. 2016;6:1065–74. doi: 10.7150/thno.15420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian R, Jacobson O, Niu G, Kiesewetter DO, Wang ZT, Zhu GZ. et al. Evans Blue Attachment Enhances Somatostatin Receptor Subtype-2 Imaging and Radiotherapy. Theranostics. 2018;8:735–45. doi: 10.7150/thno.23491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H, Wang ST, Su H, Chen KJ, Armijo AL, Lin WY. et al. A Supramolecular Approach for Preparation of Size-Controlled Nanoparticles. Angew Chem Int Ed Engl. 2009;48:4344–8. doi: 10.1002/anie.200900063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang PF, Chen YX, Zeng Y, Shen CG, Li R, Guo ZD. et al. Virus-mimetic nanovesicles as a versatile antigen-delivery system. Proc Nat Acad Sci USA. 2015;112:E6129–E38. doi: 10.1073/pnas.1505799112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu C, Ren E, Zhang Y, Yu J, Lin H, Pang X. et al. Zinc(II)-Dipicolylamine Coordination Nanotheranostics: Toward Synergistic Nanomedicine by Combined Photo/Gene Therapy. Angew Chem Int Ed Engl. 2018 doi: 10.1002/anie.201812482. DOI: 10.1002/anie.201812482. [DOI] [PubMed] [Google Scholar]
- 18.Brunsveld L, Folmer BJ, Meijer EW, Sijbesma RP. Supramolecular polymers. Chem Rev. 2001;101:4071–98. doi: 10.1021/cr990125q. [DOI] [PubMed] [Google Scholar]
- 19.Fang L, Olson MA, Benitez D, Tkatchouk E, Goddard WA 3rd, Stoddart JF. Mechanically bonded macromolecules. Chem Soc Rev. 2010;39:17–29. doi: 10.1039/b917901a. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Li Y, Zhang X, Xu X, Zhang Z, Hu C. et al. Supramolecular PEGylated Dendritic Systems as pH/Redox Dual-Responsive Theranostic Nanoplatforms for Platinum Drug Delivery and NIR Imaging. Theranostics. 2016;6:1293–305. doi: 10.7150/thno.15081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terao J, Tang A, Michels JJ, Krivokapic A, Anderson HL. Synthesis of poly(para-phenylenevinylene) rotaxanes by aqueous Suzuki coupling. Chem Commun; 2004. pp. 56–7. [DOI] [PubMed] [Google Scholar]
- 22.Wang S, Yu G, Wang Z, Jacobson O, Tian R, Lin LS, Hierarchical Tumor Microenvironment-Responsive Nanomedicine for Programmed Delivery of Chemotherapeutics. Adv Mater; 2018. e1803926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu G, Ma Y, Han C, Yao Y, Tang G, Mao Z. et al. A Sugar-Functionalized Amphiphilic Pillar[5]arene: Synthesis, Self-Assembly in Water, and Application in Bacterial Cell Agglutination. J Am Cheml Soc. 2013;135:10310–3. doi: 10.1021/ja405237q. [DOI] [PubMed] [Google Scholar]
- 24.Zhang DW, Zhao X, Li ZT. Aromatic Amide and Hydrazide Foldamer-Based Responsive Host-Guest Systems. Acc Chem Res. 2014;47:1961–70. doi: 10.1021/ar5000242. [DOI] [PubMed] [Google Scholar]
- 25.Hu J, Liu S. Engineering responsive polymer building blocks with host-guest molecular recognition for functional applications. Acc Chem Res. 2014;47:2084–95. doi: 10.1021/ar5001007. [DOI] [PubMed] [Google Scholar]
- 26.Karim AA, Dou Q, Li Z, Loh XJ. Emerging Supramolecular Therapeutic Carriers Based on Host-Guest Interactions. Chem Asian J. 2016;11:1300–21. doi: 10.1002/asia.201501434. [DOI] [PubMed] [Google Scholar]
- 27.Yang H, Yuan B, Zhang X, Scherman OA. Supramolecular chemistry at interfaces: host-guest interactions for fabricating multifunctional biointerfaces. Acc Chem Res. 2014;47:2106–15. doi: 10.1021/ar500105t. [DOI] [PubMed] [Google Scholar]
- 28.Yang YW, Sun YL, Song N. Switchable host-guest systems on surfaces. Acc Chem Res. 2014;47:1950–60. doi: 10.1021/ar500022f. [DOI] [PubMed] [Google Scholar]
- 29.Zhang M, Yan X, Huang F, Niu Z, Gibson HW. Stimuli-responsive host-guest systems based on the recognition of cryptands by organic guests. Acc Chem Res. 2014;47:1995–2005. doi: 10.1021/ar500046r. [DOI] [PubMed] [Google Scholar]
- 30.Cook TR, Zheng YR, Stang PJ. Metal-organic frameworks and self-assembled supramolecular coordination complexes: comparing and contrasting the design, synthesis, and functionality of metal-organic materials. Chem Rev. 2013;113:734–77. doi: 10.1021/cr3002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harada A. Cyclodextrin-based molecular machines. Acc Chem Res. 2001;34:456–64. doi: 10.1021/ar000174l. [DOI] [PubMed] [Google Scholar]
- 32.Spa SJ, Welling MM, van Oosterom MN, Rietbergen DDD, Burgmans MC, Verboom W. et al. A Supramolecular Approach for Liver Radioembolization. Theranostics. 2018;8:2377–86. doi: 10.7150/thno.23567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan X, Wang F, Zheng B, Huang F. Stimuli-responsive supramolecular polymeric materials. Chem Soc Rev. 2012;41:6042–65. doi: 10.1039/c2cs35091b. [DOI] [PubMed] [Google Scholar]
- 34.Yu G, Yan X, Han C, Huang F. Characterization of supramolecular gels. Chem Soc Rev. 2013;42:6697–722. doi: 10.1039/c3cs60080g. [DOI] [PubMed] [Google Scholar]
- 35.Cafeo G, Carbotti G, Cuzzola A, Fabbi M, Ferrini S, Kohnke FH. et al. Drug delivery with a calixpyrrole-trans-Pt(II) complex. J Am Chem Soc. 2013;135:2544–51. doi: 10.1021/ja307791j. [DOI] [PubMed] [Google Scholar]
- 36.Challa R, Ahuja A, Ali J, Khar RK. Cyclodextrins in drug delivery: an updated review. AAPS Pharm Sci Tech. 2005;6:E329–57. doi: 10.1208/pt060243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laza-Knoerr AL, Gref R, Couvreur P. Cyclodextrins for drug delivery. J Drug Target. 2010;18:645–56. doi: 10.3109/10611861003622552. [DOI] [PubMed] [Google Scholar]
- 38.Tibbitt MW, Dahlman JE, Langer R. Emerging Frontiers in Drug Delivery. J Am Chem Soc. 2016;138:704–17. doi: 10.1021/jacs.5b09974. [DOI] [PubMed] [Google Scholar]
- 39.Yu G, Yung BC, Zhou Z, Mao Z, Chen X. Artificial Molecular Machines in Nanotheranostics. ACS Nano. 2018;12:7–12. doi: 10.1021/acsnano.7b07851. [DOI] [PubMed] [Google Scholar]
- 40.Bae Y, Fukushima S, Harada A, Kataoka K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: polymeric micelles that are responsive to intracellular pH change. Angew Chem Int Ed Engl. 2003;42:4640–3. doi: 10.1002/anie.200250653. [DOI] [PubMed] [Google Scholar]
- 41.Shen J, Kim HC, Su H, Wang F, Wolfram J, Kirui D. et al. Cyclodextrin and polyethylenimine functionalized mesoporous silica nanoparticles for delivery of siRNA cancer therapeutics. Theranostics. 2014;4:487–97. doi: 10.7150/thno.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Webber MJ, Langer R. Drug delivery by supramolecular design. Chem Soc Rev. 2017;46:6600–20. doi: 10.1039/c7cs00391a. [DOI] [PubMed] [Google Scholar]
- 43.Zhou J, Yu G, Huang F. Supramolecular chemotherapy based on host-guest molecular recognition: a novel strategy in the battle against cancer with a bright future. Chem Soc Rev. 2017;46:7021–53. doi: 10.1039/c6cs00898d. [DOI] [PubMed] [Google Scholar]
- 44.Wang L, Li LL, Fan YS, Wang H. Host-guest supramolecular nanosystems for cancer diagnostics and therapeutics. Adv Mater. 2013;25:3888–98. doi: 10.1002/adma.201301202. [DOI] [PubMed] [Google Scholar]
- 45.Webber MJ, Appel EA, Meijer EW, Langer R. Supramolecular biomaterials. Nat Mater. 2016;15:13–26. doi: 10.1038/nmat4474. [DOI] [PubMed] [Google Scholar]
- 46.Zhang J, Ma PX. Cyclodextrin-based supramolecular systems for drug delivery: recent progress and future perspective. Adv Drug Deliv Rev. 2013;65:1215–33. doi: 10.1016/j.addr.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cabral H, Nishiyama N, Kataoka K. Supramolecular nanodevices: from design validation to theranostic nanomedicine. Acc Chem Res. 2011;44:999–1008. doi: 10.1021/ar200094a. [DOI] [PubMed] [Google Scholar]
- 48.Ma X, Zhao Y. Biomedical Applications of Supramolecular Systems Based on Host-Guest Interactions. Chem Rev. 2015;115:7794–839. doi: 10.1021/cr500392w. [DOI] [PubMed] [Google Scholar]
- 49.Peng L, Liu S, Feng A, Yuan J. Polymeric Nanocarriers Based on Cyclodextrins for Drug Delivery: Host-Guest Interaction as Stimuli Responsive Linker. Mol Pharm. 2017;14:2475–86. doi: 10.1021/acs.molpharmaceut.7b00160. [DOI] [PubMed] [Google Scholar]
- 50.Tu Z, Guday G, Adeli M, Haag R. Multivalent Interactions between 2D Nanomaterials and Biointerfaces. Adv Mater. 2018;30:e1706709. doi: 10.1002/adma.201706709. [DOI] [PubMed] [Google Scholar]
- 51.Yu G, Zhang M, Saha ML, Mao Z, Chen J, Yao Y. et al. Antitumor Activity of a Unique Polymer That Incorporates a Fluorescent Self-Assembled Metallacycle. J Am Chem Soc. 2017;139:15940–9. doi: 10.1021/jacs.7b09224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov. 2004;3:1023–35. doi: 10.1038/nrd1576. [DOI] [PubMed] [Google Scholar]
- 53.Chen Y, Liu Y. Cyclodextrin-based bioactive supramolecular assemblies. Chem Soc Rev. 2010;39:495–505. doi: 10.1039/b816354p. [DOI] [PubMed] [Google Scholar]
- 54.Gonzalez-Gaitano G, Isasi JR, Velaz I, Zornoza A. Drug Carrier Systems Based on Cyclodextrin Supramolecular Assemblies and Polymers: Present and Perspectives. Curr Pharm Des. 2017;23:411–32. doi: 10.2174/1381612823666161118145309. [DOI] [PubMed] [Google Scholar]
- 55.Harada A, Hashidzume A, Yamaguchi H, Takashima Y. Polymeric rotaxanes. Chem Rev. 2009;109:5974–6023. doi: 10.1021/cr9000622. [DOI] [PubMed] [Google Scholar]
- 56.Nepogodiev SA, Stoddart JF. Cyclodextrin-Based Catenanes and Rotaxanes. Chem Rev. 1998;98:1959–76. doi: 10.1021/cr970049w. [DOI] [PubMed] [Google Scholar]
- 57.Uekama K, Hirayama F, Irie T. Cyclodextrin Drug Carrier Systems. Chem Rev. 1998;98:2045–76. doi: 10.1021/cr970025p. [DOI] [PubMed] [Google Scholar]
- 58.Cheng N, Chen Y, Yu J, Li JJ, Liu Y. Enhanced DNA Binding and Photocleavage Abilities of β-Cyclodextrin Appended Ru(II) Complex through Supramolecular Strategy. Bioconjug Chem. 2018;29:1829–33. doi: 10.1021/acs.bioconjchem.8b00191. [DOI] [PubMed] [Google Scholar]
- 59.Yang Y, Zhang YM, Chen Y, Chen JT, Liu Y. Targeted polysaccharide nanoparticle for adamplatin prodrug delivery. J Med Chem. 2013;56:9725–36. doi: 10.1021/jm4014168. [DOI] [PubMed] [Google Scholar]
- 60.Boros E, Packard AB. Radioactive Transition Metals for Imaging and Therapy. Chem Rev. 2019;119:870–901. doi: 10.1021/acs.chemrev.8b00281. [DOI] [PubMed] [Google Scholar]
- 61.Jokerst JV, Gambhir SS. Molecular imaging with theranostic nanoparticles. Acc Chem Res. 2011;44:1050–60. doi: 10.1021/ar200106e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kunjachan S, Ehling J, Storm G, Kiessling F, Lammers T. Noninvasive Imaging of Nanomedicines and Nanotheranostics: Principles, Progress, and Prospects. Chem Rev. 2015;115:10907–37. doi: 10.1021/cr500314d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee S, Xie J, Chen X. Peptides and peptide hormones for molecular imaging and disease diagnosis. Chem Rev. 2010;110:3087–111. doi: 10.1021/cr900361p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith BR, Gambhir SS. Nanomaterials for In Vivo Imaging. Chem Rev. 2017;117:901–86. doi: 10.1021/acs.chemrev.6b00073. [DOI] [PubMed] [Google Scholar]
- 65.Guo D-S, Geng W-C, Jia S, Zheng Z, Li Z, Ding D. A Noncovalent Fluorescence Turn-on Strategy for Hypoxia Imaging. Angew Chem Int Ed Engl. 2019;58:2377–81. doi: 10.1002/anie.201813397. [DOI] [PubMed] [Google Scholar]
- 66.Gao X, Yue Q, Liu Y, Fan D, Fan K, Li S. et al. Image-guided chemotherapy with specifically tuned blood brain barrier permeability in glioma margins. Theranostics. 2018;8:3126–37. doi: 10.7150/thno.24784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Konopka CJ, Wozniak M, Hedhli J, Ploska A, Schwartz-Duval A, Siekierzycka A. et al. Multimodal imaging of the receptor for advanced glycation end-products with molecularly targeted nanoparticles. Theranostics. 2018;8:5012–24. doi: 10.7150/thno.24791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou Z, Tian R, Wang Z, Yang Z, Liu Y, Liu G. et al. Artificial local magnetic field inhomogeneity enhances T2 relaxivity. Nat Commun. 2017;8:15468. doi: 10.1038/ncomms15468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou Z, Wang L, Chi X, Bao J, Yang L, Zhao W. et al. Engineered iron-oxide-based nanoparticles as enhanced T1 contrast agents for efficient tumor imaging. ACS Nano. 2013;7:3287–96. doi: 10.1021/nn305991e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gale EM, Atanasova IP, Blasi F, Ay I, Caravan P. A Manganese Alternative to Gadolinium for MRI Contrast. J Am Chem Soc. 2015;137:15548–57. doi: 10.1021/jacs.5b10748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Louie A. Multimodality imaging probes: design and challenges. Chem Rev. 2010;110:3146–95. doi: 10.1021/cr9003538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.MacDonald TD, Liu TW, Zheng G. An MRI-sensitive, non-photobleachable porphysome photothermal agent. Angew Chem Int Ed Engl. 2014;53:6956–9. doi: 10.1002/anie.201400133. [DOI] [PubMed] [Google Scholar]
- 73.Mi P, Kokuryo D, Cabral H, Wu H, Terada Y, Saga T. et al. A pH-activatable nanoparticle with signal-amplification capabilities for non-invasive imaging of tumour malignancy. Nat Nanotechnol. 2016;11:724–30. doi: 10.1038/nnano.2016.72. [DOI] [PubMed] [Google Scholar]
- 74.Yu G, Yu S, Saha ML, Zhou J, Cook TR, Yung BC. et al. A discrete organoplatinum(II) metallacage as a multimodality theranostic platform for cancer photochemotherapy. Nat Commun. 2018;9:4335. doi: 10.1038/s41467-018-06574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun M, Zhang H-Y, Liu B-W, Liu Y. Construction of a supramolecular polymer by bridged bis (permethyl-β-cyclodextrin) s with porphyrins and its highly efficient magnetic resonance imaging. Macromolecules. 2013;46:4268–75. [Google Scholar]
- 76.Sun M, Zhang H-Y, Zhao Q, Hu X-Y, Wang L-H, Liu B-W. et al. A supramolecular brush polymer via the self-assembly of bridged tris (β-cyclodextrin) with a porphyrin derivative and its magnetic resonance imaging. J Mater Chem B. 2015;3:8170–9. doi: 10.1039/c5tb01537e. [DOI] [PubMed] [Google Scholar]
- 77.Zhou Z, Mondjinou Y, Hyun SH, Kulkarni A, Lu ZR, Thompson DH. Gd3+-1,4,7,10-Tetraazacyclododecane-1,4,7-triacetic-2-hydroxypropyl-βcyclodex trin/Pluronic Polyrotaxane as a Long Circulating High Relaxivity MRI Contrast Agent. ACS Appl Mater Interfaces. 2015;7:22272–6. doi: 10.1021/acsami.5b05393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fredy JW, Scelle J, Guenet A, Morel E, de Beaumais SA, Menand M. et al. Cyclodextrin polyrotaxanes as a highly modular platform for the development of imaging agents. Chemistry. 2014;20:10915–20. doi: 10.1002/chem.201403635. [DOI] [PubMed] [Google Scholar]
- 79.Martinelli J, Thangavel K, Tei L, Botta M. Dendrimeric β-cyclodextrin/Gd(III) chelate supramolecular host-guest adducts as high-relaxivity MRI probes. Chemistry. 2014;20:10944–52. doi: 10.1002/chem.201402418. [DOI] [PubMed] [Google Scholar]
- 80.Tian R, Ma H, Yang Q, Wan H, Zhu S, Chandra S. et al. Rational design of a super-contrast NIR-II fluorophore affords high-performance NIR-II molecular imaging guided microsurgery. Chem Sci. 2018;10:326–32. doi: 10.1039/c8sc03751e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ding F, Zhan Y, Lu X, Sun Y. Recent advances in near-infrared II fluorophores for multifunctional biomedical imaging. Chem Sci. 2018;9:4370–80. doi: 10.1039/c8sc01153b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hong G, Antaris AL, Dai H. Near-infrared fluorophores for biomedical imaging. Nat Biomed Eng. 2017;1:0010. [Google Scholar]
- 83.Smith AM, Mancini MC, Nie S. Bioimaging: second window for in vivo imaging. Nat Nanotechnol. 2009;4:710–1. doi: 10.1038/nnano.2009.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhu S, Yung BC, Chandra S, Niu G, Antaris AL, Chen X. Near-Infrared-II (NIR-II) Bioimaging via Off-Peak NIR-I Fluorescence Emission. Theranostics. 2018;8:4141–51. doi: 10.7150/thno.27995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao M, Li B, Wang P, Lu L, Zhang Z, Liu L, Supramolecularly Engineered NIR-II and Upconversion Nanoparticles In Vivo Assembly and Disassembly to Improve Bioimaging. Adv Mater. 2018; 1804. 982. [DOI] [PubMed] [Google Scholar]
- 86.Jin H, Zheng Y, Liu Y, Cheng H, Zhou Y, Yan D. Reversible and large-scale cytomimetic vesicle aggregation: light-responsive host-guest interactions. Angew Chem Int Ed Engl. 2011;50:10352–6. doi: 10.1002/anie.201103164. [DOI] [PubMed] [Google Scholar]
- 87.Tomatsu I, Hashidzume A, Harada A. Contrast viscosity changes upon photoirradiation for mixtures of poly(acrylic acid)-based alpha-cyclodextrin and azobenzene polymers. J Am Chem Soc. 2006;128:2226–7. doi: 10.1021/ja058345a. [DOI] [PubMed] [Google Scholar]
- 88.Xia D, Yu G, Li J, Huang F. Photo-responsive self-assembly based on a water-soluble pillar[6]arene and an azobenzene-containing amphiphile in water. Chem Commun. 2014;50:3606–8. doi: 10.1039/c3cc49686d. [DOI] [PubMed] [Google Scholar]
- 89.Yu G, Han C, Zhang Z, Chen J, Yan X, Zheng B. et al. Pillar[6]arene-based photoresponsive host-guest complexation. J Am Chem Soc. 2012;134:8711–7. doi: 10.1021/ja302998q. [DOI] [PubMed] [Google Scholar]
- 90.Chen XM, Chen Y, Hou XF, Wu X, Gu BH, Liu Y. Sulfonato-β-Cyclodextrin Mediated Supramolecular Nanoparticle for Controlled Release of Berberine. ACS Appl Mater Interfaces. 2018;10:24987–92. doi: 10.1021/acsami.8b08651. [DOI] [PubMed] [Google Scholar]
- 91.Li J, Loh XJ. Cyclodextrin-based supramolecular architectures: syntheses, structures, and applications for drug and gene delivery. Adv Drug Deliv Rev. 2008;60:1000–17. doi: 10.1016/j.addr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 92.Yang Y, Jia X, Zhang YM, Li N, Liu Y. Supramolecular nanoparticles based on β-CD modified hyaluronic acid for DNA encapsulation and controlled release. Chem Commun. 2018;54:8713–6. doi: 10.1039/c8cc04783a. [DOI] [PubMed] [Google Scholar]
- 93.Cheng J, Khin KT, Jensen GS, Liu A, Davis ME. Synthesis of linear, beta-cyclodextrin-based polymers and their camptothecin conjugates. Bioconjug Chem. 2003;14:1007–17. doi: 10.1021/bc0340924. [DOI] [PubMed] [Google Scholar]
- 94.Clark AJ, Wiley DT, Zuckerman JE, Webster P, Chao J, Lin J. et al. CRLX101 nanoparticles localize in human tumors and not in adjacent, nonneoplastic tissue after intravenous dosing. Proc Natl Acad Sci USA. 2016;113:3850–4. doi: 10.1073/pnas.1603018113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eliasof S, Lazarus D, Peters CG, Case RI, Cole RO, Hwang J. et al. Correlating preclinical animal studies and human clinical trials of a multifunctional, polymeric nanoparticle. Proc Natl Acad Sci USA. 2013;110:15127–32. doi: 10.1073/pnas.1309566110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schluep T, Hwang J, Hildebrandt IJ, Czernin J, Choi CH, Alabi CA. et al. Pharmacokinetics and tumor dynamics of the nanoparticle IT-101 from PET imaging and tumor histological measurements. Proc Natl Acad Sci USA. 2009;106:11394–9. doi: 10.1073/pnas.0905487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pham E, Birrer MJ, Eliasof S, Garmey EG, Lazarus D, Lee CR. et al. Translational impact of nanoparticle-drug conjugate CRLX101 with or without bevacizumab in advanced ovarian cancer. Clin Cancer Res. 2015;21:808–18. doi: 10.1158/1078-0432.CCR-14-2810. [DOI] [PubMed] [Google Scholar]
- 98.Pham E, Yin M, Peters CG, Lee CR, Brown D, Xu P. et al. Preclinical Efficacy of Bevacizumab with CRLX101, an Investigational Nanoparticle-Drug Conjugate, in Treatment of Metastatic Triple-Negative Breast Cancer. Cancer Res. 2016;76:4493–503. doi: 10.1158/0008-5472.CAN-15-3435. [DOI] [PubMed] [Google Scholar]
- 99.Tian X, Nguyen M, Foote HP, Caster JM, Roche KC, Peters CG. et al. CRLX101, a Nanoparticle-Drug Conjugate Containing Camptothecin, Improves Rectal Cancer Chemoradiotherapy by Inhibiting DNA Repair and HIF1α. Cancer Res. 2017;77:112–22. doi: 10.1158/0008-5472.CAN-15-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lu Y, Hu Q, Lin Y, Pacardo DB, Wang C, Sun W. et al. Transformable liquid-metal nanomedicine. Nat Commun. 2015;6:10066. doi: 10.1038/ncomms10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Namgung R, Mi Lee Y, Kim J, Jang Y, Lee BH, Kim IS. et al. Poly-cyclodextrin and poly-paclitaxel nano-assembly for anticancer therapy. Nat Commun. 2014;5:3702. doi: 10.1038/ncomms4702. [DOI] [PubMed] [Google Scholar]
- 102.Yu G, Zhao X, Zhou J, Mao Z, Huang X, Wang Z. et al. Supramolecular Polymer-Based Nanomedicine: High Therapeutic Performance and Negligible Long-Term Immunotoxicity. J Am Chem Soc. 2018;140:8005–19. doi: 10.1021/jacs.8b04400. [DOI] [PubMed] [Google Scholar]
- 103.Zhao L, Yuan W, Li J, Yang L, Su Y, Peng J, Independent of EPR Effect: A Smart Delivery Nanosystem for Tracking and Treatment of Nonvascularized Intra-Abdominal Metastases. Adv Funct Mater. 2018: 28; 1806. 162. [Google Scholar]
- 104.Yu G, Yang Z, Fu X, Yung BC, Yang J, Mao Z. et al. Polyrotaxane-based supramolecular theranostics. Nat Commun. 2018;9:766. doi: 10.1038/s41467-018-03119-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guo X, Huang L. Recent advances in nonviral vectors for gene delivery. Acc Chem Res. 2012;45:971–9. doi: 10.1021/ar200151m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jin L, Zeng X, Liu M, Deng Y, He N. Current progress in gene delivery technology based on chemical methods and nano-carriers. Theranostics. 2014;4:240–55. doi: 10.7150/thno.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kumar V, Palazzolo S, Bayda S, Corona G, Toffoli G, Rizzolio F. DNA Nanotechnology for Cancer Therapy. Theranostics. 2016;6:710–25. doi: 10.7150/thno.14203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li L, Li X, Wu Y, Song L, Yang X, He T. et al. Multifunctional Nucleus-targeting Nanoparticles with Ultra-high Gene Transfection Efficiency for In Vivo Gene Therapy. Theranostics. 2017;7:1633–49. doi: 10.7150/thno.17588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Son S, Namgung R, Kim J, Singha K, Kim WJ. Bioreducible polymers for gene silencing and delivery. Acc Chem Res. 2012;45:1100–12. doi: 10.1021/ar200248u. [DOI] [PubMed] [Google Scholar]
- 110.Zhou Z, Liu X, Zhu D, Wang Y, Zhang Z, Zhou X. et al. Nonviral cancer gene therapy: Delivery cascade and vector nanoproperty integration. Adv Drug Deliv Rev. 2017;115:115–54. doi: 10.1016/j.addr.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 111.Li Y, Wang H, Wang K, Hu Q, Yao Q, Shen Y. et al. Targeted Co-delivery of PTX and TR3 siRNA by PTP Peptide Modified Dendrimer for the Treatment of Pancreatic Cancer. Small. 2017;13:1602697. doi: 10.1002/smll.201602697. [DOI] [PubMed] [Google Scholar]
- 112.Bartlett DW, Davis ME. Physicochemical and biological characterization of targeted, nucleic acid-containing nanoparticles. Bioconjug Chem. 2007;18:456–68. doi: 10.1021/bc0603539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc Natl Acad Sci USA. 2007;104:15549–54. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Davis ME. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol Pharm. 2009;6:659–68. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 115.Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA. et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–70. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Heidel JD, Yu Z, Liu JY, Rele SM, Liang Y, Zeidan RK. et al. Administration in non-human primates of escalating intravenous doses of targeted nanoparticles containing ribonucleotide reductase subunit M2 siRNA. Proc Natl Acad Sci USA. 2007;104:5715–21. doi: 10.1073/pnas.0701458104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hubbell JA, Langer R. Translating materials design to the clinic. Nat Mater. 2013;12:963–6. doi: 10.1038/nmat3788. [DOI] [PubMed] [Google Scholar]
- 118.Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12:967–77. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- 119.Zuckerman JE, Davis ME. Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat Rev Drug Discovery. 2015;14:843–56. doi: 10.1038/nrd4685. [DOI] [PubMed] [Google Scholar]
- 120.Zuckerman JE, Gritli I, Tolcher A, Heidel JD, Lim D, Morgan R. et al. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proc Natl Acad Sci USA. 2014;111:11449–54. doi: 10.1073/pnas.1411393111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fan H, Hu QD, Xu FJ, Liang WQ, Tang GP, Yang WT. In vivo treatment of tumors using host-guest conjugated nanoparticles functionalized with doxorubicin and therapeutic gene pTRAIL. Biomaterials. 2012;33:1428–36. doi: 10.1016/j.biomaterials.2011.10.043. [DOI] [PubMed] [Google Scholar]
- 122.Hu Q, Li W, Hu X, Hu Q, Shen J, Jin X. et al. Synergistic treatment of ovarian cancer by co-delivery of survivin shRNA and paclitaxel via supramolecular micellar assembly. Biomaterials. 2012;33:6580–91. doi: 10.1016/j.biomaterials.2012.05.060. [DOI] [PubMed] [Google Scholar]
- 123.Hu QD, Tang GP, Chu PK. Cyclodextrin-based host-guest supramolecular nanoparticles for delivery: from design to applications. Acc Chem Res. 2014;47:2017–25. doi: 10.1021/ar500055s. [DOI] [PubMed] [Google Scholar]
- 124.Yasen W, Dong R, Zhou L, Wu J, Cao C, Aini A. et al. Synthesis of a Cationic Supramolecular Block Copolymer with Covalent and Noncovalent Polymer Blocks for Gene Delivery. ACS Appl Mater Interfaces. 2017;9:9006–14. doi: 10.1021/acsami.6b15919. [DOI] [PubMed] [Google Scholar]
- 125.Chen H, Jia H, Tham HP, Qu Q, Xing P, Zhao J. et al. Theranostic Prodrug Vesicles for Imaging Guided Codelivery of Camptothecin and siRNA in Synergetic Cancer Therapy. ACS Appl Mater Interfaces. 2017;9:23536–43. doi: 10.1021/acsami.7b06936. [DOI] [PubMed] [Google Scholar]
- 126.Zhang Q, Shen C, Zhao N, Xu FJ. Redox-Responsive and Drug-Embedded Silica Nanoparticles with Unique Self-Destruction Features for Efficient Gene/Drug Codelivery. Adv Funct Mater. 2017;27:1606229. [Google Scholar]
- 127.Adams JL, Smothers J, Srinivasan R, Hoos A. Big opportunities for small molecules in immuno-oncology. Nat Rev Drug Discovery. 2015;14:603–22. doi: 10.1038/nrd4596. [DOI] [PubMed] [Google Scholar]
- 128.Musetti S, Huang L. Nanoparticle-Mediated Remodeling of the Tumor Microenvironment to Enhance Immunotherapy. ACS Nano. 2018;12:11740–55. doi: 10.1021/acsnano.8b05893. [DOI] [PubMed] [Google Scholar]
- 129.Wang Z, Liu W, Shi J, Chen N, Fan C. Nanoscale delivery systems for cancer immunotherapy. Mater Horiz. 2018;5:344–62. [Google Scholar]
- 130.Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R. et al. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat Mater. 2012;11:895–905. doi: 10.1038/nmat3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rodell CB, Arlauckas SP, Cuccarese MF, Garris CS, Li R, Ahmed MS. et al. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat Biomed Eng. 2018;2:578–88. doi: 10.1038/s41551-018-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li D, Li Y, Xing H, Guo J, Ping Y, Tang G. Synergistic Enhancement of Lung Cancer Therapy Through Nanocarrier-Mediated Sequential Delivery of Superantigen and Tyrosin Kinase Inhibitor. Adv Funct Mater. 2014;24:5482–92. [Google Scholar]
- 133.Zimmer S, Grebe A, Bakke SS, Bode N, Halvorsen B, Ulas T. et al. Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci Transl Med. 2016;8:333ra50. doi: 10.1126/scitranslmed.aad6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang F, Yang P, Choi JS, Antovski P, Zhu Y, Xu X. et al. Cross-Linked Fluorescent Supramolecular Nanoparticles for Intradermal Controlled Release of Antifungal Drug-A Therapeutic Approach for Onychomycosis. ACS Nano. 2018;12:6851–9. doi: 10.1021/acsnano.8b02099. [DOI] [PubMed] [Google Scholar]
- 135.Casnati A, Sansone F, Ungaro R. Peptido- and glycocalixarenes: playing with hydrogen bonds around hydrophobic cavities. Acc Chem Res. 2003;36:246–54. doi: 10.1021/ar0200798. [DOI] [PubMed] [Google Scholar]
- 136.Danil De Namor AF, Cleverley RM, Zapata-Ormachea ML. Thermodynamics of Calixarene Chemistry. Chem Rev. 1998;98:2495–526. doi: 10.1021/cr970095w. [DOI] [PubMed] [Google Scholar]
- 137.Dondoni A, Marra A. Calixarene and calixresorcarene glycosides: their synthesis and biological applications. Chem Rev. 2010;110:4949–77. doi: 10.1021/cr100027b. [DOI] [PubMed] [Google Scholar]
- 138.Gutsche CD, Dhawan B, No KH, Muthukrishnan R. Calixarenes. 4. The synthesis, characterization, and properties of the calixarenes from p-tert-butylphenol. J Am Chem Soc. 1981;103:3782–92. [Google Scholar]
- 139.Kim JS, Quang DT. Calixarene-derived fluorescent probes. Chem Rev. 2007;107:3780–99. doi: 10.1021/cr068046j. [DOI] [PubMed] [Google Scholar]
- 140.Zheng Z, Geng W-C, Gao J, Wang Y-Y, Sun H, Guo D-S. Ultrasensitive and specific fluorescence detection of a cancer biomarker via nanomolar binding to a guanidinium-modified calixarene. Chem Sci. 2018;9:2087–91. doi: 10.1039/c7sc04989g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Krause-Heuer AM, Wheate NJ, Tilby MJ, Pearson DG, Ottley CJ, Aldrich-Wright JR. Substituted beta-cyclodextrin and calix[4]arene as encapsulatory vehicles for platinum(II)-based DNA intercalators. Inorg Chem. 2008;47:6880–8. doi: 10.1021/ic800467c. [DOI] [PubMed] [Google Scholar]
- 142.Weeden C, Hartlieb KJ, Lim LY. Preparation and physicochemical characterization of a novel paclitaxel-loaded amphiphilic aminocalixarene nanoparticle platform for anticancer chemotherapy. J Pharm Pharmacol. 2012;64:1403–11. doi: 10.1111/j.2042-7158.2012.01518.x. [DOI] [PubMed] [Google Scholar]
- 143.Wang YX, Guo DS, Duan YC, Wang YJ, Liu Y. Amphiphilic p-sulfonatocalix[4]arene as "drug chaperone" for escorting anticancer drugs. Sci Rep. 2015;5:9019. doi: 10.1038/srep09019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang K, Guo DS, Wang X, Liu Y. Multistimuli responsive supramolecular vesicles based on the recognition of p-Sulfonatocalixarene and its controllable release of doxorubicin. ACS Nano. 2011;5:2880–94. doi: 10.1021/nn1034873. [DOI] [PubMed] [Google Scholar]
- 145.Tu C, Zhu L, Li P, Chen Y, Su Y, Yan D. et al. Supramolecular polymeric micelles by the host-guest interaction of star-like calix[4]arene and chlorin e6 for photodynamic therapy. Chem Commun. 2011;47:6063–5. doi: 10.1039/c0cc05662f. [DOI] [PubMed] [Google Scholar]
- 146.Gao J, Li J, Geng WC, Chen FY, Duan X, Zheng Z. et al. Biomarker Displacement Activation: A General Host-Guest Strategy for Targeted Phototheranostics in Vivo. J Am Chem Soc. 2018;140:4945–53. doi: 10.1021/jacs.8b02331. [DOI] [PubMed] [Google Scholar]
- 147.Wang K, Guo DS, Zhang HQ, Li D, Zheng XL, Liu Y. Highly effective binding of viologens by p-sulfonatocalixarenes for the treatment of viologen poisoning. J Med Chem. 2009;52:6402–12. doi: 10.1021/jm900811z. [DOI] [PubMed] [Google Scholar]
- 148.Wang GF, Ren XL, Zhao M, Qiu XL, Qi AD. Paraquat detoxification with p-sulfonatocalix-[4]arene by a pharmacokinetic study. J Agric Food Chem. 2011;59:4294–9. doi: 10.1021/jf104571q. [DOI] [PubMed] [Google Scholar]
- 149.Schneider C, Bierwisch A, Koller M, Worek F, Kubik S. Detoxification of VX and Other V-Type Nerve Agents in Water at 37 oC and pH 7.4 by Substituted Sulfonatocalix[4]arenes. Angew Chem Int Ed Engl. 2016;55:12668–72. doi: 10.1002/anie.201606881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Guo DS, Wang K, Wang YX, Liu Y. Cholinesterase-responsive supramolecular vesicle. J Am Chem Soc. 2012;134:10244–50. doi: 10.1021/ja303280r. [DOI] [PubMed] [Google Scholar]
- 151.Xu Z, Jia S, Wang W, Yuan Z, Jan Ravoo B, Guo DS. Heteromultivalent peptide recognition by co-assembly of cyclodextrin and calixarene amphiphiles enables inhibition of amyloid fibrillation. Nat Chem. 2018;11:86–93. doi: 10.1038/s41557-018-0164-y. [DOI] [PubMed] [Google Scholar]
- 152.Kim K, Selvapalam N, Ko YH, Park KM, Kim D, Kim J. Functionalized cucurbiturils and their applications. Chem Soc Rev. 2007;36:267–79. doi: 10.1039/b603088m. [DOI] [PubMed] [Google Scholar]
- 153.Lagona J, Mukhopadhyay P, Chakrabarti S, Isaacs L. The cucurbit[n]uril family. Angew Chem Int Ed Engl. 2005;44:4844–70. doi: 10.1002/anie.200460675. [DOI] [PubMed] [Google Scholar]
- 154.Lee JW, Samal S, Selvapalam N, Kim HJ, Kim K. Cucurbituril homologues and derivatives: new opportunities in supramolecular chemistry. Acc Chem Res. 2003;36:621–30. doi: 10.1021/ar020254k. [DOI] [PubMed] [Google Scholar]
- 155.Li X, Bai H, Yang Y, Yoon J, Wang S, Zhang X. Supramolecular Antibacterial Materials for Combatting Antibiotic Resistance. Adv Mater. 2018;31:e1805092. doi: 10.1002/adma.201805092. [DOI] [PubMed] [Google Scholar]
- 156.Ni XL, Xiao X, Cong H, Zhu QJ, Xue SF, Tao Z. Self-assemblies based on the "outer-surface interactions" of cucurbit[n]urils: new opportunities for supramolecular architectures and materials. Acc Chem Res. 2014;47:1386–95. doi: 10.1021/ar5000133. [DOI] [PubMed] [Google Scholar]
- 157.Kim Y, Kim H, Ko YH, Selvapalam N, Rekharsky MV, Inoue Y. et al. Complexation of aliphatic ammonium ions with a water-soluble cucurbit[6]uril derivative in pure water: isothermal calorimetric, NMR, and X-ray crystallographic study. Chemistry. 2009;15:6143–51. doi: 10.1002/chem.200900305. [DOI] [PubMed] [Google Scholar]
- 158.MiáLee Y, JongáKim W, HyunáKang J, YoungáKim J, MináPark K. Cucurbit[6]uril-based polymer nanocapsules as a non-covalent and modular bioimaging platform for multimodal in vivo imaging. Mater Horiz. 2017;4:450–5. [Google Scholar]
- 159.Sun C, Zhang H, Li S, Zhang X, Cheng Q, Ding Y. et al. Polymeric Nanomedicine with "Lego" Surface Allowing Modular Functionalization and Drug Encapsulation. ACS Appl Mater Interfaces. 2018;10:25090–8. doi: 10.1021/acsami.8b06598. [DOI] [PubMed] [Google Scholar]
- 160.Chen Y, Huang Z, Zhao H, Xu JF, Sun Z, Zhang X. Supramolecular Chemotherapy: Cooperative Enhancement of Antitumor Activity by Combining Controlled Release of Oxaliplatin and Consuming of Spermine by Cucurbit[7]uril. ACS Appl Mater Interfaces. 2017;9:8602–8. doi: 10.1021/acsami.7b01157. [DOI] [PubMed] [Google Scholar]
- 161.Chen H, Chen Y, Wu H, Xu JF, Sun Z, Zhang X. Supramolecular polymeric chemotherapy based on cucurbit[7]uril-PEG copolymer. Biomaterials. 2018;178:697–705. doi: 10.1016/j.biomaterials.2018.02.051. [DOI] [PubMed] [Google Scholar]
- 162.Cao L, Hettiarachchi G, Briken V, Isaacs L. Cucurbit[7]uril containers for targeted delivery of oxaliplatin to cancer cells. Angew Chem Int Ed Engl. 2013;52:12033–7. doi: 10.1002/anie.201305061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ma D, Hettiarachchi G, Nguyen D, Zhang B, Wittenberg JB, Zavalij PY. et al. Acyclic cucurbit[n]uril molecular containers enhance the solubility and bioactivity of poorly soluble pharmaceuticals. Nat Chem. 2012;4:503–10. doi: 10.1038/nchem.1326. [DOI] [PubMed] [Google Scholar]
- 164.Hettiarachchi G, Samanta SK, Falcinelli S, Zhang B, Moncelet D, Isaacs L. et al. Acyclic Cucurbit[n]uril-Type Molecular Container Enables Systemic Delivery of Effective Doses of Albendazole for Treatment of SK-OV-3 Xenograft Tumors. Mol Pharm. 2016;13:809–18. doi: 10.1021/acs.molpharmaceut.5b00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tonga GY, Jeong Y, Duncan B, Mizuhara T, Mout R, Das R. et al. Supramolecular regulation of bioorthogonal catalysis in cells using nanoparticle-embedded transition metal catalysts. Nat Chem. 2015;7:597–603. doi: 10.1038/nchem.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Samanta SK, Quigley J, Vinciguerra B, Briken V, Isaacs L. Cucurbit[7]uril Enables Multi-Stimuli-Responsive Release from the Self-Assembled Hydrophobic Phase of a Metal Organic Polyhedron. J Am Chem Soc. 2017;139:9066–74. doi: 10.1021/jacs.7b05154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li QL, Sun Y, Sun YL, Wen J, Zhou Y, Bing QM. et al. Mesoporous Silica Nanoparticles Coated by Layer-by-Layer Self-assembly Using Cucurbit[7]uril for in Vitro and in Vivo Anticancer Drug Release. Chem Mater. 2014;26:6418–31. doi: 10.1021/cm503304p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Barrow SJ, Kasera S, Rowland MJ, del Barrio J, Scherman OA. Cucurbituril-Based Molecular Recognition. Chem Rev. 2015;115:12320–406. doi: 10.1021/acs.chemrev.5b00341. [DOI] [PubMed] [Google Scholar]
- 169.Chen C-J, Li D-D, Wang H-B, Zhao J, Ji J. Fabrication of dual-responsive micelles based on the supramolecular interaction of cucurbit[8]uril. Poly Chem. 2012;4:242–5. [Google Scholar]
- 170.Datta S, Misra SK, Saha ML, Lahiri N, Louie J, Pan D. et al. Orthogonal self-assembly of an organoplatinum(II) metallacycle and cucurbit[8]uril that delivers curcumin to cancer cells. Proc Natl Acad Sci USA. 2018;115:8087–92. doi: 10.1073/pnas.1803800115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fang R, Zhang H, Yang L, Wang H, Tian Y, Zhang X. et al. Supramolecular Self-Assembly Induced Adjustable Multiple Gating States of Nanofluidic Diodes. J Am Chem Soc. 2016;138:16372–9. doi: 10.1021/jacs.6b09601. [DOI] [PubMed] [Google Scholar]
- 172.Huang Z, Qin K, Deng G, Wu G, Bai Y, Xu JF. et al. Supramolecular Chemistry of Cucurbiturils: Tuning Cooperativity with Multiple Noncovalent Interactions from Positive to Negative. Langmuir. 2016;32:12352–60. doi: 10.1021/acs.langmuir.6b01709. [DOI] [PubMed] [Google Scholar]
- 173.Kang Y, Tang X, Yu H, Cai Z, Huang Z, Wang D. et al. Supramolecular catalyst functions in catalytic amount: cucurbit[8]uril accelerates the photodimerization of Brooker's merocyanine. Chem Sci. 2017;8:8357–61. doi: 10.1039/c7sc04125j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Samanta SK, Moncelet D, Briken V, Isaacs L. Metal-Organic Polyhedron Capped with Cucurbit[8]uril Delivers Doxorubicin to Cancer Cells. J Am Chem Soc. 2016;138:14488–96. doi: 10.1021/jacs.6b09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tang X, Huang Z, Chen H, Kang Y, Xu JF, Zhang X. Supramolecularly Catalyzed Polymerization: From Consecutive Dimerization to Polymerization. Angew Chem Int Ed Engl. 2018;57:8545–9. doi: 10.1002/anie.201803749. [DOI] [PubMed] [Google Scholar]
- 176.Xu W, Song Q, Xu JF, Serpe MJ, Zhang X. Supramolecular Hydrogels Fabricated from Supramonomers: A Novel Wound Dressing Material. A ACS Appl Mater Interfaces. 2017;9:11368–72. doi: 10.1021/acsami.7b02850. [DOI] [PubMed] [Google Scholar]
- 177.Zhao J, Chen C, Li D, Liu X, Wang H, Jin Q. et al. Biocompatible and biodegradable supramolecular assemblies formed with cucurbit[8]uril as a smart platform for reduction-triggered release of doxorubicin. Poly Chem. 2014;5:1843–7. [Google Scholar]
- 178.Wu D, Li Y, Yang J, Shen J, Zhou J, Hu Q. et al. Supramolecular Nanomedicine Constructed from Cucurbit[8]uril-Based Amphiphilic Brush Copolymer for Cancer Therapy. ACS Appl Mater Interfaces. 2017;9:44392–401. doi: 10.1021/acsami.7b16734. [DOI] [PubMed] [Google Scholar]
- 179.Hu C, Ma N, Li F, Fang Y, Liu Y, Zhao L. et al. Cucurbit[8]uril-Based Giant Supramolecular Vesicles: Highly Stable, Versatile Carriers for Photoresponsive and Targeted Drug Delivery. ACS Appl Mater Interfaces. 2018;10:4603–13. doi: 10.1021/acsami.8b00297. [DOI] [PubMed] [Google Scholar]
- 180.Montes-Navajas P, Corma A, Garcia H. Complexation and fluorescence of tricyclic basic dyes encapsulated in cucurbiturils. ChemPhysChem. 2008;9:713–20. doi: 10.1002/cphc.200700735. [DOI] [PubMed] [Google Scholar]
- 181.Wang XQ, Lei Q, Zhu JY, Wang WJ, Cheng Q, Gao F. et al. Cucurbit[8]uril Regulated Activatable Supramolecular Photosensitizer for Targeted Cancer Imaging and Photodynamic Therapy. ACS Appl Mater Interfaces. 2016;8:22892–9. doi: 10.1021/acsami.6b07507. [DOI] [PubMed] [Google Scholar]
- 182.Ma D, Zhang B, Hoffmann U, Sundrup MG, Eikermann M, Isaacs L. Acyclic cucurbit[n]uril-type molecular containers bind neuromuscular blocking agents in vitro and reverse neuromuscular block in vivo. Angew Chem Int Ed Engl. 2012;51:11358–62. doi: 10.1002/anie.201206031. [DOI] [PubMed] [Google Scholar]
- 183.Webber MJ, Appel EA, Vinciguerra B, Cortinas AB, Thapa LS, Jhunjhunwala S. et al. Supramolecular PEGylation of biopharmaceuticals. Proc Natl Acad Sci USA. 2016;113:14189–94. doi: 10.1073/pnas.1616639113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cragg PJ, Sharma K. Pillar[5]arenes: fascinating cyclophanes with a bright future. Chem Soc Rev. 2012;41:597–607. doi: 10.1039/c1cs15164a. [DOI] [PubMed] [Google Scholar]
- 185.Jie K, Zhou Y, Li E, Huang F. Nonporous Adaptive Crystals of Pillararenes. Acc Chem Res. 2018;51:2064–72. doi: 10.1021/acs.accounts.8b00255. [DOI] [PubMed] [Google Scholar]
- 186.Kakuta T, Yamagishi TA, Ogoshi T. Stimuli-Responsive Supramolecular Assemblies Constructed from Pillar[n]arenes. Acc Chem Res. 2018;51:1656–66. doi: 10.1021/acs.accounts.8b00157. [DOI] [PubMed] [Google Scholar]
- 187.Li C. Pillararene-based supramolecular polymers: from molecular recognition to polymeric aggregates. Chem Commun. 2014;50:12420–33. doi: 10.1039/c4cc03170a. [DOI] [PubMed] [Google Scholar]
- 188.Ogoshi T, Kanai S, Fujinami S, Yamagishi T-a, Nakamoto Y. para-Bridged symmetrical pillar[5]arenes: their Lewis acid catalyzed synthesis and host-guest property. J Am Chem Soc. 2008;130:5022–3. doi: 10.1021/ja711260m. [DOI] [PubMed] [Google Scholar]
- 189.Ogoshi T, Yamagishi TA, Nakamoto Y. Pillar-Shaped Macrocyclic Hosts Pillar[n]arenes: New Key Players for Supramolecular Chemistry. Chem Rev. 2016;116:7937–8002. doi: 10.1021/acs.chemrev.5b00765. [DOI] [PubMed] [Google Scholar]
- 190.Strutt NL, Zhang H, Schneebeli ST, Stoddart JF. Functionalizing pillar[n]arenes. Acc Chem Res. 2014;47:2631–42. doi: 10.1021/ar500177d. [DOI] [PubMed] [Google Scholar]
- 191.Xue M, Yang Y, Chi X, Zhang Z, Huang F. Pillararenes, a new class of macrocycles for supramolecular chemistry. Acc Chem Res. 2012;45:1294–308. doi: 10.1021/ar2003418. [DOI] [PubMed] [Google Scholar]
- 192.Zhang H, Zhao Y. Pillararene-based assemblies: design principle, preparation and applications. Chemistry. 2013;19:16862–79. doi: 10.1002/chem.201301635. [DOI] [PubMed] [Google Scholar]
- 193.Zhu H, Shangguan L, Shi B, Yu G, Huang F. Recent progress in macrocyclic amphiphiles and macrocyclic host-based supra-amphiphiles. Mater Chem Front. 2018;2:2152–74. [Google Scholar]
- 194.Chen W, Zhang Y, Li J, Lou X, Yu Y, Jia X. et al. Synthesis of a cationic water-soluble pillar[6]arene and its effective complexation towards naphthalenesulfonate guests. Chem Commun. 2013;49:7956–8. doi: 10.1039/c3cc44328k. [DOI] [PubMed] [Google Scholar]
- 195.Chi X, Yu G, Ji X, Li Y, Tang G, Huang F. Redox-responsive amphiphilic macromolecular [2]pseudorotaxane constructed from a Water-Soluble Pillar[5]arene and a Paraquat-Containing Homopolymer. ACS Macro Lett. 2015;4:996–9. doi: 10.1021/acsmacrolett.5b00525. [DOI] [PubMed] [Google Scholar]
- 196.Guan Y, Ni M, Hu X, Xiao T, Xiong S, Lin C. et al. Pillar[5]arene-based polymeric architectures constructed by orthogonal supramolecular interactions. Chem Commun. 2012;48:8529–31. doi: 10.1039/c2cc33943a. [DOI] [PubMed] [Google Scholar]
- 197.Ke C, Strutt NL, Li H, Hou X, Hartlieb KJ, McGonigal PR. et al. Pillar[5]arene as a co-factor in templating rotaxane formation. J Am Chem Soc. 2013;135:17019–30. doi: 10.1021/ja407229h. [DOI] [PubMed] [Google Scholar]
- 198.Liu L, Wang L, Liu C, Fu Z, Meier H, Cao D. Dimerization control in the self-assembly behavior of copillar[5]arenes bearing omega-hydroxyalkoxy groups. J Org Chem. 2012;77:9413–7. doi: 10.1021/jo301779y. [DOI] [PubMed] [Google Scholar]
- 199.Ogoshi T, Shiga R, Yamagishi TA. Reversibly tunable lower critical solution temperature utilizing host-guest complexation of pillar[5]arene with triethylene oxide substituents. J Am Chem Soc. 2012;134:4577–80. doi: 10.1021/ja300989n. [DOI] [PubMed] [Google Scholar]
- 200.Strutt NL, Forgan RS, Spruell JM, Botros YY, Stoddart JF. Monofunctionalized pillar[5]arene as a host for alkanediamines. J Am Chem Soc. 2011;133:5668–71. doi: 10.1021/ja111418j. [DOI] [PubMed] [Google Scholar]
- 201.Wang Y, Xu JF, Chen YZ, Niu LY, Wu LZ, Tung CH. et al. Photoresponsive supramolecular self-assembly of monofunctionalized pillar[5]arene based on stiff stilbene. Chem Commun. 2014;50:7001–3. doi: 10.1039/c4cc02760d. [DOI] [PubMed] [Google Scholar]
- 202.Yu G, Hua B, Han C. Proton transfer in host-guest complexation between a difunctional pillar[5]arene and alkyldiamines. Org Lett. 2014;16:2486–9. doi: 10.1021/ol500857w. [DOI] [PubMed] [Google Scholar]
- 203.Yu G, Zhou J, Chi X. Pillar[10]arene-based size-selective host-guest complexation and its application in tuning the LCST behavior of a thermoresponsive polymer. Macromol Rapid Commun. 2015;36:23–30. doi: 10.1002/marc.201400570. [DOI] [PubMed] [Google Scholar]
- 204.Chi X, Yu G, Shao L, Chen J, Huang F. A Dual-Thermoresponsive Gemini-Type Supra-amphiphilic Macromolecular [3]Pseudorotaxane Based on Pillar[10]arene/Paraquat Cooperative Complexation. J Am Chem Soc. 2016;138:3168–74. doi: 10.1021/jacs.5b13173. [DOI] [PubMed] [Google Scholar]
- 205.Hou X, Ke C, Cheng C, Song N, Blackburn AK, Sarjeant AA. et al. Efficient syntheses of pillar[6]arene-based hetero[4]rotaxanes using a cooperative capture strategy. Chem Commun. 2014;50:6196–9. doi: 10.1039/c4cc00733f. [DOI] [PubMed] [Google Scholar]
- 206.Tan LL, Li H, Tao Y, Zhang SX, Wang B, Yang YW. Pillar[5]arene-based supramolecular organic frameworks for highly selective CO2-capture at ambient conditions. Adv Mater. 2014;26:7027–31. doi: 10.1002/adma.201401672. [DOI] [PubMed] [Google Scholar]
- 207.Wang K, Wang CY, Zhang Y, Zhang SX, Yang B, Yang YW. Ditopic pillar[5]arene-based fluorescence enhancement material mediated by [c2]daisy chain formation. Chem Commun. 2014;50:9458–61. doi: 10.1039/c4cc03992k. [DOI] [PubMed] [Google Scholar]
- 208.Wang X, Deng H, Li J, Zheng K, Jia X, Li C. A neutral supramolecular hyperbranched polymer fabricated from an AB2-type copillar[5]arene. Macromol Rapid Commun. 2013;34:1856–62. doi: 10.1002/marc.201300731. [DOI] [PubMed] [Google Scholar]
- 209.Xu JF, Chen YZ, Wu LZ, Tung CH, Yang QZ. Dynamic covalent bond based on reversible photo [4 + 4] cycloaddition of anthracene for construction of double-dynamic polymers. Org Lett. 2013;15:6148–51. doi: 10.1021/ol403015s. [DOI] [PubMed] [Google Scholar]
- 210.Yu G, Wu D, Li Y, Zhang Z, Shao L, Zhou J. et al. A pillar[5]arene-based [2]rotaxane lights up mitochondria. Chem Sci. 2016;7:3017–24. doi: 10.1039/c6sc00036c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yu G, Xue M, Zhang Z, Li J, Han C, Huang F. A water-soluble pillar[6]arene: synthesis, host-guest chemistry, and its application in dispersion of multiwalled carbon nanotubes in water. J Am Chem Soc. 2012;134:13248–51. doi: 10.1021/ja306399f. [DOI] [PubMed] [Google Scholar]
- 212.Yu G, Zhou J, Shen J, Tang G, Huang F. Cationic pillar[6]arene/ATP host-guest recognition: selectivity, inhibition of ATP hydrolysis, and application in multidrug resistance treatment. Chem Sci. 2016;7:4073–8. doi: 10.1039/c6sc00531d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yu G, Tang G, Huang F. Supramolecular enhancement of aggregation-induced emission and its application in cancer cell imaging. J Mater Chem C. 2014;2:6609–17. [Google Scholar]
- 214.Ogoshi T, Kayama H, Yamafuji D, Aoki T, Yamagishi T-a. Supramolecular polymers with alternating pillar[5]arene and pillar [6] arene units from a highly selective multiple host-guest complexation system and monofunctionalized pillar [6] arene. Chem Sci. 2012;3:3221–6. [Google Scholar]
- 215.Wheate NJ, Dickson KA, Kim RR, Nematollahi A, Macquart RB, Kayser V. et al. Host-Guest Complexes of Carboxylated Pillar[n]arenes With Drugs. J Pharm Sci. 2016;105:3615–25. doi: 10.1016/j.xphs.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 216.Li B, Meng Z, Li Q, Huang X, Kang Z, Dong H. et al. A pH responsive complexation-based drug delivery system for oxaliplatin. Chem Sci. 2017;8:4458–64. doi: 10.1039/c7sc01438d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Guo S, Song Y, He Y, Hu XY, Wang L. Highly Efficient Artificial Light-Harvesting Systems Constructed in Aqueous Solution Based on Supramolecular Self-Assembly. Angew Chem Int Ed Engl. 2018;57:3163–7. doi: 10.1002/anie.201800175. [DOI] [PubMed] [Google Scholar]
- 218.Shao L, Zhou J, Hua B, Yu G. A dual-responsive supra-amphiphile based on a water-soluble pillar[7]arene and a naphthalene diimide-containing guest. Chem Commun. 2015;51:7215–8. doi: 10.1039/c5cc00937e. [DOI] [PubMed] [Google Scholar]
- 219.Zuo M, Qian W, Li T, Hu XY, Jiang J, Wang L. Full-Color Tunable Fluorescent and Chemiluminescent Supramolecular Nanoparticles for Anti-counterfeiting Inks. ACS Appl Mater Interfaces. 2018;10:39214–21. doi: 10.1021/acsami.8b14110. [DOI] [PubMed] [Google Scholar]
- 220.Zuo M, Qian W, Xu Z, Shao W, Hu XY, Zhang D. et al. Multiresponsive Supramolecular Theranostic Nanoplatform Based on Pillar[5]arene and Diphenylboronic Acid Derivatives for Integrated Glucose Sensing and Insulin Delivery. Small. 2018;14:1801942. doi: 10.1002/smll.201801942. [DOI] [PubMed] [Google Scholar]
- 221.Hua B, Shao L, Zhou J, Yu G. A diols-responsive triple-component supra-amphiphile constructed from pillar[5]arene-based recognition. RSC Adv. 2016;6:47281–4. [Google Scholar]
- 222.Wu X, Li Y, Lin C, Hu X-Y, Wang L. GSH-and pH-responsive drug delivery system constructed by water-soluble pillar[5]arene and lysine derivative for controllable drug release. Chem Commun. 2015;51:6832–5. doi: 10.1039/c5cc01393c. [DOI] [PubMed] [Google Scholar]
- 223.Hu X-Y, Liu X, Zhang W, Qin S, Yao C, Li Y. et al. Controllable construction of biocompatible supramolecular micelles and vesicles by water-soluble phosphate pillar [5,6] arenes for selective anti-cancer drug delivery. Chem Mater. 2016;28:3778–88. [Google Scholar]
- 224.Hu XY, Jia K, Cao Y, Li Y, Qin S, Zhou F. et al. Dual Photo- and pH-Responsive Supramolecular Nanocarriers Based on Water-Soluble Pillar[6]arene and Different Azobenzene Derivatives for Intracellular Anticancer Drug Delivery. Chemistry. 2015;21:1208–20. doi: 10.1002/chem.201405095. [DOI] [PubMed] [Google Scholar]
- 225.Meng L-B, Zhang W, Li D, Li Y, Hu X-Y, Wang L. et al. pH-Responsive supramolecular vesicles assembled by water-soluble pillar[5]arene and a BODIPY photosensitizer for chemo-photodynamic dual therapy. Chem Commun. 2015;51:14381–4. doi: 10.1039/c5cc05785j. [DOI] [PubMed] [Google Scholar]
- 226.Wang Q, Zhang P, Xu J, Xia B, Tian L, Chen J. et al. NIR-Absorbing Dye Functionalized Supramolecular Vesicles for Chemo-Photothermal Synergistic Therapy. ACS Appl Bio Mater. 2018;1:70–8. [Google Scholar]
- 227.Wang Q, Tian L, Xu J, Xia B, Li J, Lu F. et al. Multifunctional supramolecular vesicles for combined photothermal/photodynamic/hypoxia-activated chemotherapy. Chem Commun. 2018;54:10328–31. doi: 10.1039/c8cc05560b. [DOI] [PubMed] [Google Scholar]
- 228.Chang Y, Hou C, Ren J, Xin X, Pei Y, Lu Y. et al. Multifunctional supramolecular vesicles based on the complex of ferrocenecarboxylic acid capped pillar[5]arene and a galactose derivative for targeted drug delivery. Chem Commun. 2016;52:9578–81. doi: 10.1039/c6cc03637f. [DOI] [PubMed] [Google Scholar]
- 229.Yang K, Chang Y, Wen J, Lu Y, Pei Y, Cao S. et al. Supramolecular vesicles based on complex of Trp-modified pillar[5]arene and galactose derivative for synergistic and targeted drug delivery. Chem Mater. 2016;28:1990–3. [Google Scholar]
- 230.Hu X, Gao L, Mosel S, Ehlers M, Zellermann E, Jiang H, From Supramolecular Vesicles to Micelles: Controllable Construction of Tumor-Targeting Nanocarriers Based on Host-Guest Interaction between a Pillar[5]arene-Based Prodrug and a RGD-Sulfonate Guest. Small. 2018: 14; 1803. 952. [DOI] [PubMed] [Google Scholar]
- 231.Wang Y, Du J, Wang Y, Jin Q, Ji J. Pillar[5]arene based supramolecular prodrug micelles with pH induced aggregate behavior for intracellular drug delivery. Chem Commun. 2015;51:2999–3002. doi: 10.1039/c4cc09274k. [DOI] [PubMed] [Google Scholar]
- 232.Liu X, Shao W, Zheng Y, Yao C, Peng L, Zhang D. et al. GSH-Responsive supramolecular nanoparticles constructed by β-d-galactose-modified pillar[5]arene and camptothecin prodrug for targeted anticancer drug delivery. Chem Commun. 2017;53:8596–9. doi: 10.1039/c7cc04932c. [DOI] [PubMed] [Google Scholar]
- 233.Cao Y, Zou X, Xiong S, Li Y, Shen Y, Hu X. et al. Supramolecular Prodrug Micelles Constructed by Drug-Drug Conjugate with Water Soluble Pillar[6]arene for Controllable and Rapid Drug Release. Chin J Chem. 2015;33:329–34. [Google Scholar]
- 234.Cao Y, Li Y, Hu X-Y, Zou X, Xiong S, Lin C. et al. Supramolecular nanoparticles constructed by DOX-based prodrug with water-soluble Pillar[6]arene for self-catalyzed rapid drug release. Chem Mater. 2015;27:1110–9. [Google Scholar]
- 235.Wu D, Li Y, Shen J, Tong Z, Hu Q, Li L. et al. Supramolecular chemotherapeutic drug constructed from pillararene-based supramolecular amphiphile. Chem Commun. 2018;54:8198–201. doi: 10.1039/c8cc04334e. [DOI] [PubMed] [Google Scholar]
- 236.Yu G, Yu W, Mao Z, Gao C, Huang F. A Pillararene-Based Ternary Drug-Delivery System with Photocontrolled Anticancer Drug Release. Small. 2015;11:919–25. doi: 10.1002/smll.201402236. [DOI] [PubMed] [Google Scholar]
- 237.Wang S, Yao C, Ni M, Xu Z, Cheng M, Hu X-Y. et al. Thermo-and oxidation-responsive supramolecular vesicles constructed from self-assembled pillar[6]arene-ferrocene based amphiphilic supramolecular diblock copolymers. Poly Chem. 2017;8:682–8. [Google Scholar]
- 238.Yu G, Yu W, Shao L, Zhang Z, Chi X, Mao Z. et al. Fabrication of a Targeted Drug Delivery System from a Pillar[5]arene-Based Supramolecular Diblock Copolymeric Amphiphile for Effective Cancer Therapy. Adv Funct Mater. 2016;26:8999–9008. [Google Scholar]
- 239.Yu G, Zhao R, Wu D, Zhang F, Shao L, Zhou J. et al. Pillar[5]arene-based amphiphilic supramolecular brush copolymers: fabrication, controllable self-assembly and application in self-imaging targeted drug delivery. Poly Chem. 2016;7:6178–88. doi: 10.1039/C6PY01402J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Nguyen KT, Zhao Y. Engineered Hybrid Nanoparticles for On-Demand Diagnostics and Therapeutics. Acc Chem Res. 2015;48:3016–25. doi: 10.1021/acs.accounts.5b00316. [DOI] [PubMed] [Google Scholar]
- 241.Xing P, Zhao Y. Multifunctional Nanoparticles Self-Assembled from Small Organic Building Blocks for Biomedicine. Adv Mater. 2016;28:7304–39. doi: 10.1002/adma.201600906. [DOI] [PubMed] [Google Scholar]
- 242.Zhang P, Wang J, Chen H, Zhao L, Chen B, Chu C. et al. Tumor Microenvironment-Responsive Ultrasmall Nanodrug Generators with Enhanced Tumor Delivery and Penetration. J Am Chem Soc. 2018;140:14980–9. doi: 10.1021/jacs.8b09396. [DOI] [PubMed] [Google Scholar]
- 243.Zhang YM, Cao Y, Yang Y, Chen JT, Liu Y. A small-sized graphene oxide supramolecular assembly for targeted delivery of camptothecin. Chem Commun. 2014;50:13066–9. doi: 10.1039/c4cc04533e. [DOI] [PubMed] [Google Scholar]
- 244.Yu G, Li J, Yu W, Han C, Mao Z, Gao C. et al. Carbon Nanotube/Biocompatible Bola-Amphiphile Supramolecular Biohybrid Materials: Preparation and Their Application in Bacterial Cell Agglutination. Adv Mater. 2013;25:6373–9. doi: 10.1002/adma.201302942. [DOI] [PubMed] [Google Scholar]
- 245.Lu F, Wang J, TAO C, Zhu J-J. Highly Monodisperse Beta-Cyclodextrin-Covellite Nanoparticles for Efficient Photothermal and Chemotherapy. Nanoscale Horiz. 2018;3:538–44. doi: 10.1039/c8nh00026c. [DOI] [PubMed] [Google Scholar]
- 246.Liu Y, Bhattarai P, Dai Z, Chen X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem Soc Rev. 2019 doi: 10.1039/c8cs00618k. doi: 10.1039/C8CS00618K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ke K, Yang W, Xie X, Liu R, Wang L-L, Lin W-W. et al. Copper Manganese Sulfide Nanoplates: A New Two-Dimensional Theranostic Nanoplatform for MRI/MSOT Dual-Modal Imaging-Guided Photothermal Therapy in the Second Near-Infrared Window. Theranostics. 2017;7:4763–76. doi: 10.7150/thno.21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Yang K, Yang K, Chao S, Wen J, Pei Y, Pei Z. A supramolecular hybrid material constructed from pillar [6] arene-based host-guest complexation and ZIF-8 for targeted drug delivery. Chem Commun. 2018;54:9817–20. doi: 10.1039/c8cc05665j. [DOI] [PubMed] [Google Scholar]
- 249.Wu M-X, Yan H-J, Gao J, Cheng Y, Yang J, Wu J-R. et al. Multifunctional Supramolecular Materials Constructed from Polypyrrole@UiO-66 Nanohybrids and Pillararene Nanovalves for Targeted Chemophotothermal Therapy. ACS Appl Mater Interfaces. 2018;10:34655–63. doi: 10.1021/acsami.8b13758. [DOI] [PubMed] [Google Scholar]
- 250.Wu X, Zhang Y, Lu Y, Pang S, Yang K, Tian Z. et al. Synergistic and targeted drug delivery based on nano-CeO2 capped with galactose functionalized pillar[5]arenevia host-guest interactions. J Mater Chem B. 2017;5:3483–7. doi: 10.1039/c7tb00752c. [DOI] [PubMed] [Google Scholar]
- 251.Wu M, Gao J, Wang F, Yang J, Song N, Jin X. et al. Multistimuli Responsive Core-Shell Nanoplatform Constructed from Fe3O4@MOF Equipped with Pillar[6]arene Nanovalves. Small. 2018;14:1704440. doi: 10.1002/smll.201704440. [DOI] [PubMed] [Google Scholar]
- 252.Li Q, Sun Y, Ren L, Wang X, Wang C, Li L. et al. Supramolecular Nanosystem Based on Pillararene-Capped CuS Nanoparticles for Targeted Chemo-Photothermal Therapy. ACS Appl Mater Interfaces. 2018;10:29314–24. doi: 10.1021/acsami.8b09330. [DOI] [PubMed] [Google Scholar]
- 253.Yu G, Yang J, Fu X, Wang Z, Shao L, Mao Z. et al. Supramolecular Hybrid Material Constructed from Graphene Oxide and Pillar[6]arene-Based Host-Guest Complex as a Ultrasound and Photoacoustic Signals Nanoamplifier. Mater Horiz. 2018;5:429–35. doi: 10.1039/C8MH00128F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Barry NP, Zava O, Dyson PJ, Therrien B. Excellent Correlation between Drug Release and Portal Size in Metalla-Cage Drug-Delivery Systems. Chem Eur J. 2011;17:9669–77. doi: 10.1002/chem.201003530. [DOI] [PubMed] [Google Scholar]
- 255.Barry NP, Zava O, Furrer J, Dyson PJ, Therrien B. Anticancer activity of opened arene ruthenium metalla-assemblies. Dalton Trans. 2010;39:5272–7. doi: 10.1039/c001521k. [DOI] [PubMed] [Google Scholar]
- 256.Cai W, Chu CC, Liu G, Wáng YXJ. Metal-organic framework-based nanomedicine platforms for drug delivery and molecular imaging. Small. 2015;11:4806–22. doi: 10.1002/smll.201500802. [DOI] [PubMed] [Google Scholar]
- 257.Cai W, Wang J, Chu C, Chen W, Wu C, Liu G. Metal-Organic Framework-Based Stimuli-Responsive Systems for Drug Delivery. Adv Sci. 2018;6:1801526. doi: 10.1002/advs.201801526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Della Rocca J, Liu D, Lin W. Nanoscale metal-organic frameworks for biomedical imaging and drug delivery. Acc Chem Res. 2011;44:957–68. doi: 10.1021/ar200028a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.He Z, Dai Y, Li X, Guo D, Liu Y, Huang X. et al. Hybrid Nanomedicine Fabricated from Photosensitizer-Terminated Metal-Organic Framework Nanoparticles for Photodynamic Therapy and Hypoxia-Activated Cascade Chemotherapy. Small. 2019;15:1804131. doi: 10.1002/smll.201804131. [DOI] [PubMed] [Google Scholar]
- 260.Horcajada P, Chalati T, Serre C, Gillet B, Sebrie C, Baati T. et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat Mater. 2010;9:172–8. doi: 10.1038/nmat2608. [DOI] [PubMed] [Google Scholar]
- 261.Lewis JE, Gavey EL, Cameron SA, Crowley JD. Stimuli-responsive Pd2L4 metallosupramolecular cages: towards targeted cisplatin drug delivery. Chem Sci. 2012;3:778–84. [Google Scholar]
- 262.McKinlay AC, Morris RE, Horcajada P, Férey G, Gref R, Couvreur P. et al. BioMOFs: metal-organic frameworks for biological and medical applications. Angew Chem Int Ed Engl. 2010;49:6260–6. doi: 10.1002/anie.201000048. [DOI] [PubMed] [Google Scholar]
- 263.Taylor-Pashow KM, Della Rocca J, Xie Z, Tran S, Lin W. Postsynthetic modifications of iron-carboxylate nanoscale metal-organic frameworks for imaging and drug delivery. J Am Chem Soc. 2009;131:14261–3. doi: 10.1021/ja906198y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Therrien B, Süss-Fink G, Govindaswamy P, Renfrew AK, Dyson PJ. The “Complex-in-a-Complex” Cations [(acac)2M⊂ Ru6(p-iPrC6H4Me)6(tpt)2(dhbq)3]6+: A Trojan Horse for Cancer Cells. Angew Chem Int Ed Engl. 2008;120:3833–6. doi: 10.1002/anie.200800186. [DOI] [PubMed] [Google Scholar]
- 265.Wu MX, Yang YW. Metal-organic framework (MOF)-based drug/cargo delivery and cancer therapy. Adv Mater. 2017;29:1606134. doi: 10.1002/adma.201606134. [DOI] [PubMed] [Google Scholar]
- 266.Yu G, Yu S, Saha ML, Zhou J, Cook TR, Yung BC. et al. A discrete organoplatinum (II) metallacage as a multimodality theranostic platform for cancer photochemotherapy. Nat Commun. 2018;9:4335. doi: 10.1038/s41467-018-06574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Zhou J, Zhang Y, Yu G, Crawley MR, Fulong CRP, Friedman AE. et al. Highly emissive self-assembled BODIPY-platinum supramolecular triangles. J Am Chem Soc. 2018;140:7730–6. doi: 10.1021/jacs.8b04929. [DOI] [PubMed] [Google Scholar]