Abstract
What is more interesting about brown adipose tissue (BAT) is its ability to provide thermogenesis, protection against obesity by clearing triglycerides, releasing batokines, and mitigating insulin resistance. White adipose tissue (WAT) on the other hand stores excess energy and secretes some endocrine factors like leptin for regulating satiety. For the last decade there has been an increasing interest in the browning of fat keeping in view its beneficial effects on metabolic disorders and protection in the form of perivascular fat. Obesity is one such metabolic disorder that leads to significant morbidity and mortality from obesity-related disorders such as type 2 diabetes mellitus (T2D) and cardiovascular disease risk. Browning of white fat paves the way to restrict obesity and obesity related disorders. Although exercise has been the most common factor for fat browning; however, there are other factors that involve: (I) beta aminoisobutyric acid (BAIBA); (2) gamma amino butyric acid (GABA); (3) PPARy agonists; (4) JAK inhibition; and (5) IRISIN. In this review, we propose two novel factors musclin and TFAM for fat browning. Musclin a myokine released from muscles during exercise activates PPARy which induces browning of WAT that has beneficial metabolic and cardiac effects. TFAM is a transcription factor that induces mitochondrial biogenesis. Since BAT is rich in mitochondria, higher expression of TFAM in WAT or TFAM treatment in WAT cells can induce browning of WAT. We propose that fat browning can be used as a therapeutic tool for metabolic disorders and cardiovascular diseases.
During the past decade scientists have become more and more interested in fat browning owing to its beneficial effects in metabolic disorders apart from its thermogenic property. It is well known today that mammals have two types of adipose tissue, white adipose tissue (WAT) and brown adipose tissue (BAT) (Gil et al., 2011). Whereas WAT is most common and found as subcutaneous tissue, around the abdomen, thighs, and waist, the BAT is found particularly as perivascular, epicardial, supra-adrenal, and supra-scapular tissue (Fig. 1). The major difference between WAT and BAT is that BAT has more number of mitochondria and small fat droplets whereas WAT has a big fat droplet and less mitochondria (Fig. 2). High accumulation of WAT leads to obesity. Obesity is a complex disorder involving an excessive amount of body fat and is the result of an energy imbalance between calories consumed and calories expended (Hill et al., 2012). It is very concerning that in modern world it is wide spread and even more concerning is that this condition leads to significant morbidity and mortality from obesity related diseases such as type 2 diabetes mellitus (T2D) and cardiovascular disease (Cypess and Kahn, 2010). Fatty liver and visceral adipose tissue (VAT) which are related to obesity are also associated with cardiovascular risk factors and cardiometabolic disorders (Lee et al., 2016). Metabolic impact and role of WAT as an endocrine organ became well known in mammalian physiology initially because of capability of adipocyte and adipose tissue (AT) to secrete hormones and cytokines which are involved in glucose metabolism, lipid metabolism, inflammation, and coagulation (Hajer et al., 2008; Vazquez-Vela et al., 2008). Thus, it will lead to regulation of metabolism of cells located in many organs and tissues including: muscle, liver, vasculature, and brain (Hajer et al., 2008). Adipocytes secrete adipokines, which include hormones, cytokines, and other proteins with specific biological functions and in that manner adipose tissue should maintain its functionality, which is highly affected by obesity (Jeanson et al., 2015). Therefore, for many years the role of some adipokines and their importance in the control of energy balance and in the development of obesity are being studied. Current literature suggests a clear connection between obesity and increased level of AT macrophages in mouse models as well as humans (Gutierrez et al., 2009). Extensive research is being performed to understand the factors that initiate macrophage infiltration into AT. Many possible AT-specific cells and molecules contribute to macrophage infiltration of AT, including apoptotic adipocytes, chemokines, other immune cells, leptin, and preadipocytes with special reference to adiponectin. Adiponectin is a hormone which is present greatly in adipose tissue (Greenberg and Obin, 2006; Hajer et al., 2008) and is downregulated in obesity (Ouchi and Walsh, 2007,2008; Fantuzzi, 2008) but on the other hand this hormone is increased in inflammatory process that are not in connection with increasing of adipose tissue mass (Fantuzzi, 2008; Robinson et al., 2011). Some clinical studies showed that levels of adiponectin are higher in patients with systemic lupus erythematosus, cystic fibrosis, inflammatory bowel disease, and rheumatoid arthritis; however, the mechanisms are still unknown (Robinson et al., 2011). Apart from obesity the levels of adiponectin are also reduced in insulin resistance, metabolic syndrome, and T2D (Greenberg and Obin, 2006; Yamamoto et al., 2014). Additionally, adiponectin shows anti-atherosclerotic effects and improves insulin sensitivity (Hajer et al., 2008; Yamamoto et al., 2014). Yamamoto et al. (2014) showed that adiponectin is also beneficial in Japanese population without insulin resistance. Though the mechanism is unclear, but it is speculated to be mediated by the inhibition of hepatic glucose output and amelioration of glucose uptake in the muscle which leads to increasing fatty acid oxidation and higher energy consumption in vitro, probably by increased uncoupling of adenosine triphosphate (ATP) generation in mitochondria (Berg et al., 2001; Fruebis et al., 2001; Hajer et al., 2008). Other endocrine factors that are secreted by adipose tissues include fibroblast growth factor 21 (Liu et al., 2013a), interleukin 6 (Fontes et al., 2015), free fatty acids (Yore et al.,2014), and nerve growth factors (Zheng et al., 2015) (Fig. 3) which are beneficial and cardioprotective. One of the important roles of the BAT is to provide non-shivering thermogenesis by uncoupling the electron transport chain from ATP synthesis using uncoupling protein I (UCPI) (Seale and Lazar, 2009). BAT is present as perivascular fat which secretes vasorelaxing/vasoconstriction factors and maintains vascular tone (Xu et al., 2010). Keeping in view the beneficial effects of BAT, the factors that mediate browning of fat are of immense importance. These factors include beta-amino isobutyric acid (BAIBA), gamma amino butyric acid (GABA), PPARY, exercise, IRISIN and micorRNAs (Fig. 2). This review summarizes the current knowledge about BAT, browning of WAT, and factors that underline the conversion of WAT to BAT, as well as their use as therapeutics for metabolic diseases such obesity and T2D.
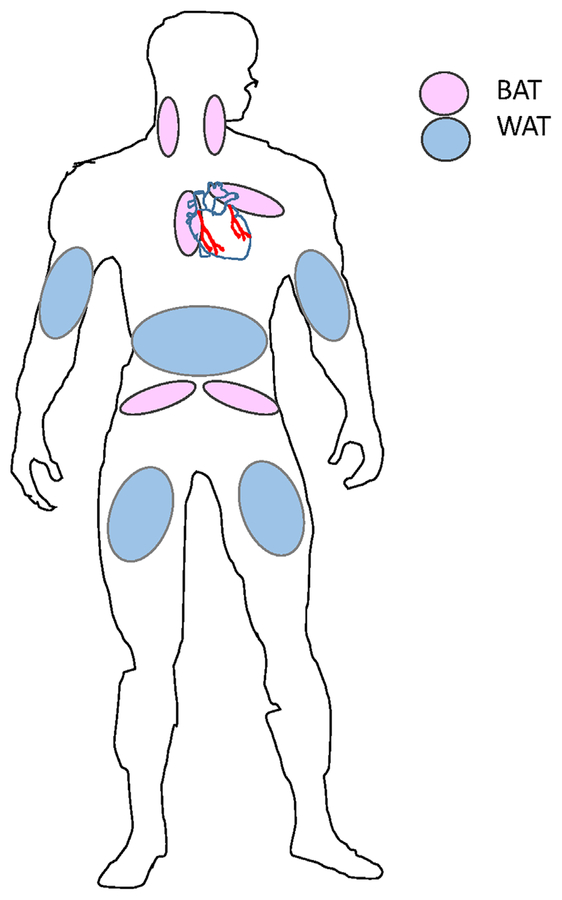
Fig. 1.
Distribution of WAT and BAT in human body. The accumulation of WAT is around the waist and hips, arms and legs, and it presents the largest percentage of adipose tissue in human body. Subcutaneous BAT includes depots lying between the anterior neck muscles; in the anterior abdominal wall, and in the inguinal area. Perivascular BAT can be found around the aorta, common carotid artery, and brachiocephalic artery; in anterior pericardial fat and around epicardial coronary artery. BAT can be found also around kidney, adrenal gland, pancreas, and liver.
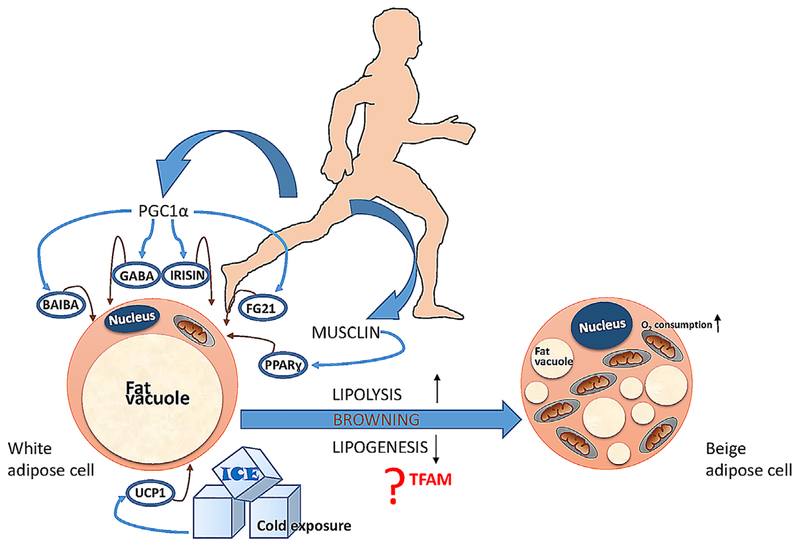
Fig. 2.
A comprehensive view of the factors involved in the process of browning. Exercise has beneficial effects on browning but many other factors are also promoted according to physical activity such as (I) beta aminoisobutyric acid (BAIBA); (2) gamma aminobutyric acid (GABA); (3) IRISIN; (4) fibroblast growth factor 21 (FGF2I); and (5) musclin (PPARγ agonist). Beside all these factors cold exposure can lead to browning through UCPI. The possible mechanisms involve lipogenic pathways.
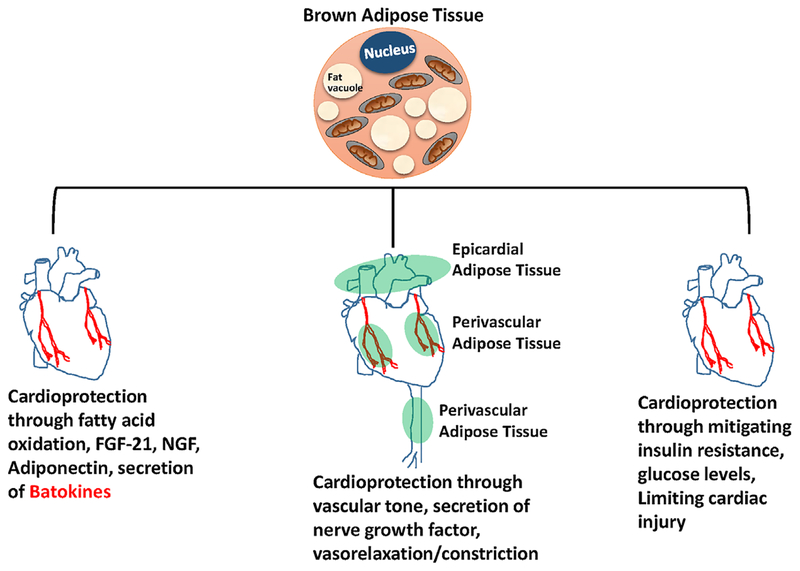
Fig. 3.
Cardioprotection through browning of fat. Browning of fat can lead to cardioprotection in three ways: (I) by secreting batokines like FGF2I, adiponectin, nerve growth factors, and free fatty acids; (2) by maintaining vascular tone through secretion of vasorelaxing/vasoconstricting factors from perivascular and epicardial tissues; and (3) by reducing the levels of triglycerides and mitigating insulin resistance and limiting cardiac injury.
White and Brown Adipose Tissue
Modern life style that includes most time during the day in seated position, with consumption of calorie-dense food and with little or totally without physical activity is a very big step closer to obesity. This condition poses a severe threat to worldwide public health. Also, it is particularly alarming because of the pathological accumulation of excess dysfunctional adipose tissue that characterizes obesity and it is a major risk factor for many other diseases, including T2D, heart disease, insulin resistance, hyperglycemia, dyslipidemia, hypertension, and some types of cancer (Seale and Lazar, 2009; Harms and Seale, 2013; Reddy et al., 2014). The major role of WAT according to classical view of its function is providing a long term metabolic fuel which is especially convenient when there are long periods of some physical efforts. In times of caloric need, during fasting or absence of energy intake, storage of triglycerides (TGs), and releasing of fatty acids (FAs) for oxidation will be a key in maintaining of energy balance in organism (Trayhurn and Beattie, 2001; Vazquez-Vela et al., 2008; Wang et al., 2008). WAT has two depots in body: visceral white adipose tissue (vWAT) and subcutaneous white adipose tissue (scWAT) (Stanford et al., 2015). Both type of WAT are very much involved in functions such as: lipid storage, hormone production, immune function, and local tissue architecture. However, development of type 2 diabetes mellitus (T2DM) and cardiovascular complications is more connected with increased amount of vWAT (Roman et al., 2015; Stanford et al., 2015) while accumulation of scWAT is associated with improved insulin sensitivity and a reduced risk for developing T2DM (Stanford et al., 2015).
Brown adipose tissue (BAT) is specialized in energy expenditure and it is present in nearly all mammalian species (Giralt and Villarroya, 2013; Nedergaard and Cannon, 2014). BAT has an important thermogenic function as a natural defense system against cold stress in newborn mammals and in adults. BAT has not only crucial role against hypothermia but also has a power to modulate the proton gradient by uncoupling cellular respiration from mitochondrial ATP synthesis. After many years of research, studies have shown that the expression of uncoupling protein I (UCPI) in response to cold stress, or food intake resulting in regulation of energy balance (increased glucose and free fatty acid oxidation) leads to the generation of heat and serves to protect an organism from hypothermia (Algire et al., 2013; Roman et al., 2015; Sidossis and Kajimura, 2015). BAT is characterized by small lipid droplets (LDs) and microstructurally high number of mitochondria (Kiefer et al., 2012; Rezaee, 2013). Storage of energy in the form of triglycerides (TGs) in LDs is accessible for rapid hydrolysis and oxidation of fatty acids (FAs) (Rezaee, 2013; Warner and Mittag, 2016). BAT is derived from precursor cells, which share the same lineage as skeletal muscle cells and are positive for Myf5 (Zafrir, 2013; Giralt and Villarroya, 2016). Contrary to the skeletal muscle cells, BAT progenitors initiate the expression of PRDM16 and BMP7 during development, which drives them to become mature brown adipocytes (Ohno et al., 2012; Zafrir, 2013). BAT is present in adult humans and its beneficial properties like inverse correlation with body mass index (BMI), high vascularization to enhance the transfer of the thermogenic effect throughout the body, results in metabolisation of enough TGs to have an impact on overall body weight, and metabolic health (Reddy et al., 2014; Roman et al., 2015; Tharp and Stahl, 2015).
BAT cells possess large number of mitochondria that contain a unique protein called uncoupling protein I (UCPI) which is a very important regulatory factor in the thermogenesis (Seale and Lazar, 2009). Cold sensations signal neurons up to the preoptic area of the hypothalamus, further activating the sympathetic nervous system to release norepinephrine, which induces UCPI and BAT activity (Kiefer et al., 2012; Zafrir, 2013). The benefits of UCPI-expressing BAT may not be limited to their metabolic characteristics, but also their property as a potent secretome (Tharp and Stahl, 2015). It is well known that WAT and BAT do not have same precursor cells and that they share the same origin as skeletal muscle cells (Kiefer et al., 2012; Roman et al., 2015). In spite of the fact that classical BAT shares precursors with skeletal muscles and is present in interscapular areas, perirenal, and periaortic regions, we have beige (bright) tissue—BAT, that is, present mainly in WAT itself (Koppen and Kalkhoven, 2010; Kiefer et al., 2012; Roman et al., 2015). The evidence of existence of BAT in WAT reveals the real meaning of term “browning”. In addition, accumulation of both vWAT and scWAT lead to the development of T2D and obesity that subsequently lead to cardiovascular complications (Cypess et al., 2009; Cypess and Kahn, 2010). However, existence of BAT is beneficial and its role in animals as well as in humans much needs to be explored.
Role of WAT and BAT in Metabolic Disorders
The treatment of patients with metabolic disorders requires significant efforts on the first place in lifestyle modifications, which are often difficult to apply and they are only partially successful. Consequently, during the years a lot of time, money, and exertion were spent to find a potential therapeutic target to treat obesity and associated diseases (Zafrir, 2013; Warner and Mittag, 2016). However, today, some studies suggest that the solution might be adipose tissue (AT) itself. AT has been reported as the main energy reservoir organ in the body. Although insulin regulates metabolism in both brown and white adipocytes, the role of these tissues in energy storage and utilization is quite different. Moreover, today significant number of studies are showing how and in which manner especially BAT plays role in obesity and T2D. Despite a similar ability to undergo thermogenesis, brown and beige cells have many different characteristics and should be considered as distinct cell types for several reasons as described by Harms and Seale (2013). However, in both brown and beige adipocytes, very similar amounts of UCPI were found after activation which indicates same thermogenic capacity which shows that they have more than one similar function (Harms and Seale, 2013). UCPI, when activated, uncouples electron transport in the respiratory chain from generation of adenosine triphosphate (ATP) and thus releases the energy stored in the form of heat (Zafrir, 2013). Thus, it is now understandable that cold exposure of overweight patients can lead to better activation of BAT, but could also corroborate the inverse association with BMI. Finally, studies showed that BAT prevalence and activity were much lower in patients with severe obesity, and BAT activity was increased in this group after weight loss induced by bariatric surgery (Betz and Enerback, 2015). Although the idea of stimulating BAT activity in way of cold exposure to combat obesity is interesting approach, we should be realistic and see if it is also comfortable for patients. One study actually indicates that cold acclimation for 10 days (I4–I5°C) leads to increased peripheral insulin sensitivity by ~43% in eight subjects with T2D (Hanssen et al., 2015).
A recent study on mice shows that browning of WAT via cold exposure can greatly improve the anti-diabetic effect of T0901317 (potential agonist of liver × receptors) in diabetic mice. The results from this study suggested that browning of WAT via cold exposure or molecules such as fibroblast growth factor 21 (FGF2I), IRISIN, and Nrg4 would be an excellent strategy in managing insulin-dependent diabetes (Gao et al.,2015). Similarly, another group of investigators showed important role of FGF2I in the process of browning. They showed clear function for FGF21 in regulating chronic adaptive thermogenesis by increasing the transcription of thermogenic genes and enhancing the thermogenic capacity of the organism by “browning” white fat (Fisher et al., 2012). In last century, scientist tried to mimic the effect of activated UCPI using chemical uncoupler 2, 4-dinitrophenol (DNP) substance. However, when it is applied in high doses, it can cause irregularity of respiratory uncoupling in all cells causing dangerous side effects, including hyperthermia and death (Harms and Seale, 2013). Hence, there was an urge to investigate new factors that mediate browning of fat without having any side effects.
Factors Which Underline Browning
Since the end of the 20th century researchers are trying to define the process of browning. The important landmark in this field of research was studies on animals as well as on humans exposed to cold conditions. In these types of studies, it was obvious that BAT activity in man was acutely cold induced and was stimulated via the sympathetic nervous system (Huttunen et al., 1981; Nedergaard et al., 2007; Nedergaard and Cannon, 2014). Taking all that in consideration, recent studies on mice have reported that browning can be mediated by the emergence of UCPI -expressing cells in WAT not only by cold exposure or adrenergic stimulation but also by hormonal stimuli (Lo and Sun, 2013; Moisan et al., 2015). According to these mediators we now know few regulatory pathways of browning such as PGC-Iα, C/EBPα, PPARγ (peroxisome proliferator-activated receptor gamma), PRDMI6, SRC-1 (steroid receptor coactivator-1) and TIF2 (transcriptional intermediary factor-2), TBXI5 (T-box 15), and TFAM (mitochondrial transcription factor A). Other transcription factors and coregulators include FoxC2 (forkhead box protein C2), IRISIN (secreted protein), BMP7 (bone morphogenetic protein 7), FGF2I, 4E-BPI [elF4E (eukaryotic translation initiation factor 4E)-binding protein I], CNP (cardiac natriuretic peptide), TRPV4 (transient receptor potential vanilloid 4), COX (cyclo-oxygenase)-2, and miRNAs (microRNAs) (Fig. 2) (Lo and Sun, 2013).
PPARγ
PPARγ has a key and irreplaceable role in the regulation of differentiation of both WAT and BAT though they have similar transcription pathways (Koppen and Kalkhoven, 2010; Lo and Sun, 2013). The importance of PPARγ is evident from clinical implications of its agonists which lead to increased expression of adiponectins especially in therapy of insulin resistance and T2D (Koppen and Kalkhoven, 2010). Studies also show that patients with a dominant-negative mutation in the PPAR-γ gene have very serious diabetic condition which indicates a correlation between T2D and this signaling molecule (Barroso et al., 1999). According to these findings we can assume a very important impact of this factor on the process of browning in clinical cases such as T2D. Moreover, Ohno et al. (2012) in their study, showed that full PPARγ agonists, such as rosiglitazone, bind to and stabilize helix 12 of the ligand binding domain (LBD) of PPAR7 which leads to extended accumulation of PRDM16. PRDM16 has a pivotal role in the development of beige cells in subcutaneous WAT. Partial agonist of thiazolidinedione (TZD) such as non-TZD compounds (MRL24, nTZDpa) predominantly show effects on helix3 and the ß-sheet region, with minimum influence on helix 12 and based on this they have a very weak or no browning effect at all (Ohno et al., 2012). In present, browning and the search for beige progenitors in human WAT has become a burning issue for the scientific world.
MicroRNAs
MicroRNAs which are small 18–25 nucleotide entities, are interesting candidates affecting browning of fat. Zheng et al., in their investigations showed important role of microRNAs (miRNAs) in the process of WAT browning especially epididymal adipose tissue. Their findings suggest a potential UCPI-targeting miR-9 and miR-338–3p to be involved in the process of browning of epididymal adipose tissue through posttranscriptional suppression of UCP I gene expression (Zheng et al., 2014). Liu et al. showed that miR-l33a inhibits white adipocyte browning and negatively regulates PRDMI6. According to this, they found that miR-133a KO mice have dramatic phenotype including adipocyte browning, improved glucose metabolism, and insulin sensitivity (Liu et al., 2013b). Also, another group of authors suggested that miR-34a may provide an effective therapeutic option for combating obesity-related metabolic disorders, especially because of the effects of anti-miR-34a on increased expression of UCP I in all types of adipose tissues. This is due to the fact that downregulation of miR-34a also improves hepatic FGF2I signaling and lipid oxidation (Fu et al., 2014). One of the possible targets in treatment of obesity and T2D could be miR-155 though it is in the initial stage of research. Studies conducted on mice suggest that reduction of miR-155 for 50% already enhances recruitment of beige fat cells and differentiation of BAT. According to this, therapeutic strategies to reduce miR-155 might be a promising strategy in treatment of metabolic disorders (Chen et al., 2013). Finally, as miRNA is a huge family of molecules, only some of them are described in literature affecting metabolic disorders. For example, miR-196a, miR-26, and miR-30 act as positive regulators and miR-155, miR-133, miR-27b, and miR-34 as negative regulators in complex diseases like obesity and diabetes (Karbiener and Scheideler, 2014; Arias et al., 2015).
Transplantation of BAT
Newest way to improve quantity of BAT is transplantation. Some studies show very impressive results of this technique resulting in improved glucose tolerance and reduced body weight while some others show that transplanted BAT activates only BAT and muscle (Villarroya and Giralt, 2015). However, one question still remains, could this method be applied on humans too? Stanford et al. showed that transplantation of scWAT from trained mice can improve metabolic homeostasis. Their conclusion is that adipose tissue from exercise-trained mice is also a source of circulating factors (adipokines), and these exercise-induced adipokines may have beneficial effects on systemic metabolism. As an underlined mechanism they mentioned that a single bout of exercise can increase the expression of PGCI α mRNA in both scWAT and vWAT. When compared to vWAT, scWAT has higher expression of many genes involved in glucose homeostasis and insulin action such as Glut I, lgf-1, Igfbp3, PPARγ, as well as genes involved in lipid metabolism such as Hsl, ß-adrenergic receptors, hydroxymethylglutaryl CoA synthase (Stanford et al., 2015). Although scWAT has increased expression of PRDM16 (a transcriptional coregulatory protein responsible for the development of brown adipocytes in both BAT and scWAT), it should be mentioned that it is possible to develop PPAR7 ligands that affect PRDM 16 protein accumulation without full agonist activity and such compounds can have plausible therapeutic activity toward obesity and diabetes (Bostrom et al., 2012; Ohno et al., 2012; Stanford et al., 2015). Five proteins were identified as PGCI α target genes in muscle and likely to be secreted: IL-15, Fndc5, VEGFß, Lrgl, and TIMP4 (Bostrom et al., 2012; Stanford et al., 2015).
Exercise
As we can see from literature, one of the most beneficial things of exercise except browning is to improve the state of the whole body itself. Studies have shown that exercise training can increase the expression of most important metabolic proteins such as GLUT4 and PGC-I α (Fig. 2). Many of these metabolic adaptations to adipose tissue can occur independently of significant changes in weight loss. It is also showed that during exercise, SNS can be activated which promotes response from catecholamine. Therefore, adrenergic-receptor stimulation (B-AR stimulation) has chronic (mitochondrial biogenesis, recruitment of brown adipocytes in WAT) and acute (lipolysis) promotion of UCP I (Sanchez-Delgado et al., 2015). Stanford et al. (2015) showed that exercise training results in profound changes to WAT, including increased expression of genes involved in mitochondrial biogenesis, increased mitochondrial activity, increased browning of scWAT, and an altered adipokine profile of WAT. Some studies showed that various myokines released from skeletal muscle during exercise can be responsible for browning such as meteorin-like I, myostatin, and ß-aminoisobutyric acid (BAIBA) in rodents as well as in humans (Zafrir, 2013; Stanford et al., 2015). On the other hand, first study on humans showed that both PGCI α and Fndc5 mRNA expression was increased in skeletal muscle in response to 12 weeks of training and the plasma concentration of IRISIN was reduced. There were little or no effects of long-term training on selected browning genes and also this group of scientist didn’t notice correlation between circulating IRISIN with UCP I mRNA in subcutaneous adipose tissue. The role of IRISIN in the process of browning especially in humans is for serious debate and has been reviewed by Elsen et al. (Elsen et al., 2014; Norheim et al., 2014).
Musclin
Musclin—“exercise factor” or “exercise hormone” is a peptide produced by skeletal muscle as a response to exercise and it can be found in bloodstream. In a study done by Subbotina et al. (2015), it was shown that musclin as an exercise-responsive factor promotes skeletal muscle mitochondrial biogenesis and exercise endurance. Although the studies about musclin are scarce, the role of musclin seems to be very promising. Liu et al. had some interesting findings, where they reported that musclin reduced glucose uptake in rat skeletal muscle through mechanisms involving the inhibition of Akt/PKB. Moreover, they are proposing that PPARγ and liver × receptor a (LXRα) have a role in the reduction of glucose uptake in skeletal muscle exposed to musclin (Liu et al., 2008). Very similar findings were reported by Nishizawa et al. (2004). As we can conclude, possible role of mitochondria biogenesis and PPARγ in mechanism of musclin indicate that this peptide most probably has effects on the process of browning. In future, more studies should be done, especially with T2D patients in focus.
IRISIN
In last couple of years, special attention was given to IRISIN (a cleavage product of Fndc5 gene) as one of the factors which can help browning of fat. IRISIN is released into the bloodstream during exercise and promotes fat browning stimulated by UCP I expression. High UCP I expression caused by IRISIN is seen when it is injected in adenoviral particles to mice and finally this process leads to increase in energy expenditure and improvement in obesity and glucose homeostasis (Bostrom et al., 2012; Zafrir, 2013; Kurdiova et al., 2014; Rodriguez et al.,2015). However, a controversy exists concerning IRISIN origin, regulation and function in humans. Kurdiova et al. explored Fndc5 gene and IRISIN protein in two clinical studies: a cross-sectional study (effects of T2D in drug-naive men) and an intervention study (exercise effects in sedentary, overweight/obese individuals). They concluded the following: first, complex clinical studies combined with cell culture work revealed that Fndc5/IRISIN was decreased in T2D in vivo, but not in muscle cells in vitro, indicating that diabetes-related factor(s) regulate Fndc5/IRISIISI in vivo, second, several attributes of T2D, such as hyperglycaemia, triglyceridaemia, visceral adiposity, and extramyocellular lipid deposition were negatively associated with adipose tissue Fndc5 mRNA and circulating IRISIN. Moreover, mimicking diabetic status in vitro by treating muscle cells with palmitate and glucose, lowered Fndc5 mRNA. However, IRISIN was positively linked to muscle mass, strength, and metabolism, pointing to common regulatory factors and/or the potential for IRISIN to modify muscle phenotype (Kurdiova et al., 2014). Myostatin and leptin have also been shown to negatively regulate IRISIN-induced fat browning (Rodriguez et al., 2015).
GABA and BAIBA
Very novel thing in literature is the effect of gamma amino butyric acid (GABA) on process of browning. GABA has a long history in commercial use as a food supplement according to its health functions. Recent studies show that GABA is a potential antioxidant and can regulate hyperglycemia but unfortunately accurate mechanism is still unclear. Tian et al. (2011) have shown that it can be by regulating the redox status of muscle and plasma free amino acids (pFAAs). Moreover, according to these findings, Xie et al. did some experiments with different GABA supplements in three different doses on mice model on high fat diet. Their findings showed that treatment with GABA restored the redox status in muscle and HFD-induced pFAAs disorders to different extents, which may be responsible for the decrease of fasting blood glucose levels. However, the authors suggestto be careful with the dose of GABA, especially in preventing obesity (Xie et al., 2015). Long before that, Braun et al. (2004) showed that GABA by activation of GABAB receptors inhibits insulin secretion by suppression of exocytosis. GABABRI subunit can be novel target for treatment of many different modern life style diseases, which has been shown by a study from Nakamura et al. In their experiments, they noticed that GABABRI subunit is constitutively expressed from adipocytes to primarily regulate leptin expression at the transcriptional level. This explains the possible functional role of GABA in adipocytes (Nakamura et al., 2011).
One of the newest things that are investigated in recent time are the effects of ß-aminoisobutyric acid (BAIBA) on the process of browning and therefore its role in treatment of obesity and associated diseases. Roberts et al. (2014) first showed that BAIBA could be a new small molecular myokine. This group of scientists proved that BAIBA is regulated by PGC-I α and increases the expression of brown adipocyte-specific genes. According to these findings they tested new hypothesis whether BAIBA would induce a browning response in human pluripotent stem cells (PSCs) during their differentiation to mature white adipocytes. Their data indicated that BAIBA activates a browning gene program and increases the mitochondrial activity of human I PSCs differentiated into white adipocytes. The mechanism was unraveled by higher degree of UCP-I staining in the PPARγ2 programmed white adipocytes treated with BAIBA when compared to untreated cells. Expression analysis of inguinal WAT using qPCR revealed significant increase in brown adipocyte-specific genes UCP-I and CIDEA. Expression of PGC-I α and Cytochrome C were also increased following BAIBA treatment and demonstrated that BAIBA induces increased expression of brown/beige adipocyte-specific genes in vivo. Finally, we can say that BAIBA has been identified as a novel small molecular myokine representing the first in its class of non-adrenergic activators of the thermogenic program in WAT. The identification of BAIBA as a PGC-I a mediated and exercise triggered signal has significant implications not only for understanding of exercise and its protective role against the development of metabolic diseases, but also as potential therapeutics for T2D and the metabolic syndrome (Roberts et al., 2014).
One step closer to discovery of new facts about BAIBA and its role in T2D comes from Xiang Shi et al. in their experimental study. They showed that, oral administration of BAIBA attenuates hepatic endoplasmic reticulum (ER) stress, apoptosis, and glucose/lipid metabolic disturbance in mice with STZ/HFD-induced T2D. AMP-activated protein kinase (AMPK) signaling contributes to the role of BAIBA in attenuating ER stress. These findings regarding BAIBA provided new insights toward the development of therapeutic agents aimed at effectively reducing hepatic ER stress and glucose/lipid metabolic disturbance in T2D or other metabolic disorders (Shi et al., 2016). Previously, Jung et al. (2015) reported that BAIBA attenuates insulin resistance, suppresses inflammation, and induces fatty acid oxidation via the AMPK-PPAR6 pathway in skeletal muscle. Although we have reviewed some of the most important factors for process of browning and show possible targets in developing a treatment for metabolic disorders with special reference to obesity and T2D, we should also highlight importance of browning and BAT for cardiovascular system especially because most common outcome of obesity and T2D are cardiovascular diseases.
Is Browning of Fat Cardioprotective?
There has been an increasing scientific interest in the BAT during the last few years since the discovery of BAT in adult humans. Although the thermogenic properties of BAT have been well established but their role in cardioprotection is unknown. The thermogenic property of BAT is attributed to high number of mitochondria and uncoupling of the electron transport chain due to high expression of uncoupling protein UCPI (Cannon and Nedergaard, 2004). The mice which are deficient in UCPI are obese, cold sensitive, and have increased oxidative stress which suggests that UCP-I has a systemic effect on the body (Feldmann et al., 2009). However, the link between brown fat, UCP-I, and cardiovascular function is unclear. In an attempt to find a link between brown fat and cardiac injury, Thoonen et al. (2015) used UCPI knock out mice and induced cardiomyopathy using isoproterenol. Both WT and UCPI knock out mice exhibited similar cardiac parameters like echocardiography, cardiac fibrosis, cardiac troponin, without isoproterenol treatment. However, when isoproterenol was administered, UCPI knock out mice showed augmented myocardial injury, fibrosis, and decreased survival rates. Interestingly, when BAT from WT was transplanted to UCPI knock out mice with isoproterenol treatment; it improved cardiac parameters and survival. This study suggested that BAT is cardioprotective and can minimize cardiac injury in ischemic condition. Another interesting study by Subhadra et al. demonstrated that surgical transplantation of BAT reversed type I diabetes in streptozotocin treated mice (Gunawardana and Piston, 2012). Transplantation of BAT resulted in normalized glucose tolerance and reduced tissue inflammation showing BAT to be beneficial for glucose metabolism.
Interestingly, the authors showed that BAT transplant increased levels of leptin and adiponectin. Secretion of these two endocrine factors suggested that BAT can behave as endocrine system and the endocrine factors secreted (‘batokines’) can be favorable against cardiovascular risk. Some of the ‘batokines’ to be listed which have a cardioprotective role are: (I) fibroblast growth factor 21 (FGF2I) (Liu et al., 2013a; Planavila et al., 2013; Gimeno and Moller, 2014); (2) Interleukin 6 (IL-6) (Stanford et al., 2013; Fontes et al., 2015); (3) free fatty acids (Yore et al., 2014); and (4) nerve growth factor (NGF) (Wei et al., 2015; Zheng et al., 2015). In view of the fact that BAT activation and batokines can contribute to cardioprotection (Fig. 3) and improve metabolic disorders, it can be proposed as a promising tool in clinical translation. Since BAT is present as a perivascular tissue, it has a profound effect on vascular tone and heart. Most vessels in the body are surrounded by perivascular adipose tissue (PVAT) except the cerebral vessels (Gao, 2007). The PVAT mostly share brown adipose like properties like high mitochondrial density and high expression of UCPI (Chang et al., 2013). The epicardial adipose tissue (EAT) exhibits high UCPI expression but its function still needs to be clear. The PVAT around the aorta is of significant interest, as it has been shown to promote vasorelaxation in aortic rings (Chang et al., 2013). The PVAT secretes a number of vasorelaxing factors such as adiponectin (Xu et al., 2010); gasotransmitters hydrogen sulfide (Wojcicka et al., 201 I); nitric oxide (Gao et al., 2007), palmitic methyl ester (Lee et al., 201 I). These vasorelaxing factors can modulate local blood pressure in the microvasculature (Houben et al., 2012). The UCPI expression in the PVAT also contributes to blood pressure in humans (Li et al., 2015). PVAT is modestly anti-inflammatory and enhances endothelial— dependent and—independent vasorelaxation (Houben et al., 2012; Fitzgibbons and Czech, 2014). However, in metabolic diseases like obesity and diabetes, the role of PVAT may be altered from beneficial in lean and healthy to harmful in obese and diabetic (Lastra and Manrique, 2015). For example, in obesity PVAT secretes less adiponectin and more pro-inflammatory adipokines such as leptin, angiotensinogen, resistin, IL-6, TNF-a, and MCP-I (Fitzgibbons and Czech, 2014). Overall, the BAT like property of PVAT and its ability to contribute to vasorelaxation suggests that PVAT is an important factor in regulating vascular tone. Keeping in view the properties of BAT to protect from cardiac injury and vascular tone in the form of perivascular fat, it can be speculated that browning of white fat can be cardioprotective (Fig. 3). Hence, the factors that govern browning of fat have immense importance and can be targeted for future therapies.
Summary
Through this review it is clear that adrenergic system is not the only factor for the regulation of BAT activation but there are so many new therapeutic possibilities. Unfortunately, for many of these agents the underlying mechanisms are unclear and might turn out to be unsuitable for humans as much as it is for rodents. Although, we need to recognize that in this field 21 st century leads in direction to define the organs with possible new endocrine role, but of course in the light of BAT. However, we say critically that many of those factors involved in the process of browning are discovered recently and their therapeutic activity is still a huge question mark. More studies should be conducted in future on humans with special emphasis on agents which can be used in therapeutic treatment for obesity and associated diseases especially because human body is still a mystery in so many ways.
Future Directions
Browning of fat can be a useful tool for treating metabolic disorders such as obesity and type 2 diabetes. With more and more research on factors that cause fat browning, it can lead to novel molecules with fewer side effects. High BAT prevalence indicates lean body mass, protection from obesity, healthy vascular tone, and less cardiovascular risk. Browning of epicardial fat can be a new target for cardioprotection which can provide energy in hypoxia. Since exercise leads to fat browning, stimulating GPCRs like GABA, PPARγ, BAIBA, and ß2AR can be novel therapeutic strategies for fat browning. Use of adipokines like FGF2I, NGF, and interleukins is another aspect for fat browning strategies. Since BAT contains more number of mitochondria, we propose that factors which promote mitochondrial biogenesis can lead to fat browning as well as enhanced energy production. TFAM is a transcription factor that promotes mitochondrial biogenesis by enhancing mtDNA copy number and mitigates ROS production in mitochondria. We speculate that treatment of TFAM can promote fat browning as well as mitochondrial efficiency in WAT and lead to cardioprotection. Not to overstate, in future, fat browning can be seen as a treatment for metabolic disorders as well as cardioprotection.
Acknowledgments
Contract grant sponsor: NIH;
Contract grant numbers: HL-74185, HL-108621.
Footnotes
Conflicts of interest: None.
Literature Cited
- Algire C, Medrikova D, Herzig S. 2013. White and brown adipose stem cells: From signaling to clinical implications. Biochim Biophys Acta 1831:896–904. [DOI] [PubMed] [Google Scholar]
- Arias N, Aguirre L, Fernandez-Quintela A, Gonzalez M, Lasa A, Miranda J, Macarulla MT, Portillo MP. 2015. MicroRNAs involved in the browning process of adipocytes. J Physiol Biochem [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK, O’Rahilly S. 1999. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature 402:880–883. [DOI] [PubMed] [Google Scholar]
- Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. 2001. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7:947–953. [DOI] [PubMed] [Google Scholar]
- Betz MJ, Enerback S. 2015. Human brown adipose tissue: What we have learned so far. Diabetes 64:2352–2360. [DOI] [PubMed] [Google Scholar]
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM. 2012. A PGCI-alpha-dependent myokine that drives brown-fat-1 ike development of white fat and thermogenesis. Nature 481:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M, Wendt A, Buschard K, Salehi A, Sewing S, Gromada J, Rorsman P. 2004. GABAB receptor activation inhibits exocytosis in rat pancreatic beta-cells by G-protein-dependent activation of calcineurin. J Physiol 559:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. 2004. Brown adipose tissue: Function and physiological significance. Physiol Rev 84:277–359. [DOI] [PubMed] [Google Scholar]
- Chang L, Milton H, Eitzman DT, Chen YE. 2013. Paradoxical roles of perivascular adipose tissue in atherosclerosis and hypertension. Circ J 77:1 1–18. [DOI] [PubMed] [Google Scholar]
- Chen Y, Siegel F, Kipschull S, Haas B, Fröhlich H, Meister G, Pfeifer A. 2013. MiR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nat Commun 4:1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Kahn CR. 2010. Brown fat as a therapy for obesity and diabetes. Curr Opin Endocrinol Diabetes Obes 17:143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfìne AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. 2009. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsen M, Raschke S, Eckel J. 2014. Browning of white fat: Does irisin play a role in humans? J Endocrinol 222: R25–R38. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G 2008. Adiponectin and inflammation: Consensus and controversy. j Allergy Clin Immunol 121:326–330. [DOI] [PubMed] [Google Scholar]
- Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. 2009. UCPI ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 9:203–209. [DOI] [PubMed] [Google Scholar]
- Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, Spiegelman BM. 2012. FGF21 regulates PGC-1 alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 26:271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgibbons TP, Czech MP. 2014. Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: Basic mechanisms and clinical associations. J Am Heart Assoc 3: e000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes JA, Rose NR, Cihakova D. 2015. The varying faces of IL-6: From cardiac protection to cardiac failure. Cytokine 74:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruebis J, Tsao TS, Ebbets-Reed D, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. 2001. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA 98:2005–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T, Seok S, Choi S, Huang Z, Suino-Powell K, Xu HE, Kemper B, Kemper JK. 2014. MicroRNA 34a inhibits beige and brown fat formation in obesity in part by suppressing adipocyte fibroblast growth factor 21 signaling and SIRTI function. Mol Cell Biol 34:4130–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Zhang C, Ma Y, Liu D. 2015. Cold exposure improves the anti-diabetic effect of T090I3I7 in streptozotocin-induced diabetic mice. AAPSJ 17:700–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ. 2007. Dual modulation of vascular function by perivascular adipose tissue and its potential correlation with adiposity/lipoatrophy-related vascular dysfunction. Curr Pharm Des 13:2185–2192. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Lu C, Su LY, Sharma AM, Lee RM. 2007. Modulation of vascular function by perivascular adipose tissue: The role of endothelium and hydrogen peroxide. Brj Pharmacol 151:323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil A, Olza J, Gil-Campos M, Gomez-Llorente C, Aguilera CM. 2011. Is adipose tissue metabolically different at different sites? Int J Pediatr Obes 6:13–20. [DOI] [PubMed] [Google Scholar]
- Gimeno RE, Moller DE. 2014. FGF2l-based pharmacotherapy—Potential utility for metabolic disorders. Trends Endocrinol Metab 25:303–311. [DOI] [PubMed] [Google Scholar]
- Giralt M, Villarroya F. 2013. White, brown, beige/brite: Different adipose cells for different functions? Endocrinology 154:2992–3000. [DOI] [PubMed] [Google Scholar]
- Giralt M, Villarroya F. 2016. Mitochondrial uncoupling and the regulation of glucose homeostasis. Curr Diabetes Rev [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Obin MS. 2006. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr 83:461S–465S. [DOI] [PubMed] [Google Scholar]
- Gunawardana SC, Piston DW. 2012. Reversal of type I diabetes in mice by brown adipose tissue transplant. Diabetes 61:674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez DA, Puglisi MJ, Hasty AH. 2009. Impact of increased adipose tissue mass on inflammation, insulin resistance, and dyslipidemia. Curr Diab Rep 9:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajer GR, van Haeften TW, Visseren FL. 2008. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 29:2959–2971. [DOI] [PubMed] [Google Scholar]
- Hanssen MJ, Hoeks J, Brans B, van der Lans AA, Schaart G, van den Driessche JJ, Jorgensen JA, Boekschoten MV, Hesselink MK, Havekes B, Kersten S, Mottaghy FM, van Marken Lichtenbeit WD, Schrauwen P. 2015. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med 21:863–865. [DOI] [PubMed] [Google Scholar]
- Harms M, Seale P. 2013. Brown and beige fat: Development, function and therapeutic potential. Nat Med 19:1252–1263. [DOI] [PubMed] [Google Scholar]
- Hill JO, Wyatt HR, Peters JC. 2012. Energy balance and obesity. Circulation 126:126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben AJ, Eringa EC, Jonk AM, Serne EH, Smulders YM, Stehouwer CD. 2012. Perivascular fat and the microcirculation: Relevance to insulin resistance, diabetes, and cardiovascular disease. Curr Cardiovasc Risk Rep 6:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen P, Hirvonen J, Kinnula V. 1981. The occurrence of brown adipose tissue in outdoor workers. EurJ Appi Physiol Occup Physiol 46:339–345. [DOI] [PubMed] [Google Scholar]
- Jeanson Y, Carriere A, Casteilla L. 2015. A new role for browning as a redox and stress adaptive mechanism? Front Endocrinol (Lausanne) 6:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung TW, Hwang HJ, Hong HC, Yoo HJ, Baik SH, Choi KM. 2015. BAIBA attenuates insulin resistance and inflammation induced by palmitate or a high fat diet via an AMPK-PPARdelta-dependent pathway in mice. Diabetologia 58:2096–2105. [DOI] [PubMed] [Google Scholar]
- Karbiener M, Scheideier M. 2014. MicroRNA functions in brite/brown fat—Novel perspectives towards anti-obesity strategies. Comput Struct Biotechnol J 11:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer FW, Cohen P, Plutzky J. 2012. Fifty shades of brown: Perivascular fat, thermogenesis, and atherosclerosis. Circulation 126:1012–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppen A, Kalkhoven E. 2010. Brown vs white adipocytes: The PPARgamma coregulator story. FEBS Lett 584:3250–3259. [DOI] [PubMed] [Google Scholar]
- Kurdiova T, Balaz M, Vician M, Maderova D, Vlcek M, Valkovic L, Srbecky M, Imrich R, Kyselovicova O, Belan V, Jelok I, Wolfrum C, Klimes I, Krssak M, Zemkova E, Gasperikova D, Ukropec J, Ukropcova B. 2014. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: In vivo and in vitro studies. J Physiol 592:1091–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastra G, Manrique C. 2015. Perivascular adipose tissue, inflammation and insulin resistance: Linkto vascular dysfunction and cardiovascular disease. Horm Mol Biol Clin Invest 22:19–26. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Britton KA, Pedley A, Massaro JM, Speliotes EK, Murabito JM, Hoffmann U, Ingram C, Keaney JF Jr, Vasan RS, Fox CS. 2016. Adipose tissue depots and their cross-sectional associations with circulating biomarkers of metabolic regulation. J Am Heart Assoc 5: pii: e002936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YC, Chang HH, Chiang CL, Liu CH, Yeh Jl, Chen MF, Chen PY, Kuo JS, Lee TJ. 2011. Role of perivascular adipose tissue-derived methyl palmitate in vascular tone regulation and pathogenesis of hypertension. Circulation 124:1160–1171. [DOI] [PubMed] [Google Scholar]
- Li X, Liu J, Wang G, Yu J, Sheng Y, Wang C, Lv Y, Lv S, Qi H, Di W, Yin C, Ding G. 2015. Determination of UCP I expression in subcutaneous and perirenal adipose tissues of patients with hypertension. Endocrine 50:413–423. [DOI] [PubMed] [Google Scholar]
- Liu SQ, Roberts D, Kharitonenkov A, Zhang B, Hanson SM, Li YC, Zhang LQ, Wu YH. 2013a. Endocrine protection of ischemic myocardium by FGF21 from the liver and adipose tissue. Sci Rep 3:2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Bi P, Shan T, Yang X, Yin H, Wang YX, Liu N, Rudnicki MA, Kuang S. 2013b. MiR-133a regulates adipocyte browning in vivo. PLoS Genet 9: e 1003626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Huo X, Pang XF, Zong ZH, Meng X, Liu GL. 2008. Musclin inhibits insulin activation of Akt/protein kinase B in rat skeletal muscle. J Int Med Res 36:496–504. [DOI] [PubMed] [Google Scholar]
- Lo KA, Sun L. 2013. Turning WAT into BAT: A review on regulators controlling the browning of white adipocytes. Biosci Rep 33: pii: e00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisan A, Lee YK, Zhang JD, Hudak CS, Meyer CA, Prummer M, Zoffmann S, Truong HH, Ebeling M, Kiialainen A, Gerard R, Xia F, Schinzel RT, Amrein KE, Cowan CA. 2015. White-to-brown metabolic conversion of human adipocytes byJAK inhibition. Nat Cell Biol 17:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Hinoi E, Takarada T, Takahata Y, Yamamoto T, Fujita H, Takada S, Hashizume S, Yoneda Y. 2011. Positive regulation by GABA(B)RI subunit of leptin expression through gene transactivation in adipocytes. PLoS ONE 6: e20l67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J, Bengtsson T, Cannon B. 2007. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 293: E444–E452. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Cannon B. 2014. The browning of white adipose tissue: Some burning issues. Cell Metab 20:396–407. [DOI] [PubMed] [Google Scholar]
- Nishizawa H, Matsuda M, Yamada Y, Kawai K, Suzuki E, Makishima M, Kitamura T, Shimomura I.2004. Musclin, a novel skeletal muscle-derived secretory factor. J Biol Chem 279:19391–19395. [DOI] [PubMed] [Google Scholar]
- Norheim F, Langleite TM, Hjorth M, Holen T, Kielland A, Stadheim HK, Gulseth HL, Birkeland Kl, Jensen J, Drevon CA. 2014. The effects of acute and chronic exercise on PGC-1 alpha, irisin and browning of subcutaneous adipose tissue in humans. FEBSJ 281:739–749. [DOI] [PubMed] [Google Scholar]
- Ohno H, Shinoda K, Spiegelman BM, Kajimura S. 2012. PPARgamma agonists induce a white-to-brown fat conversion through stabilization of PRDM 16 protein. Cell Metab 15:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi N, Walsh K. 2007. Adiponectin as an anti-inflammatory factor. Clin Chim Acta 380:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi N, Walsh K. 2008. A novel role for adiponectin in the regulation of inflammation. Arterioscler Thromb Vase Biol 28:1219–1221. [DOI] [PubMed] [Google Scholar]
- Planavila A, Redondo I, Hondares E, Vinciguerra M, Munts C, Iglesias R, Gabrielli LA, Sitges M, Giralt M, van Bilsen M, Villarroya F. 2013. Fibroblast growth factor 21 protects against cardiac hypertrophy in mice. Nat Commun 4:2019. [DOI] [PubMed] [Google Scholar]
- Reddy NL, Tan BK, Barber TM, Randeva HS. 2014. Brown adipose tissue: Endocrine determinants of function and therapeutic manipulation as a novel treatment strategy for obesity. BMC Obes 1:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaee F, Dashty M. 2013. Role of adipose tissue in metabolic system disorders: Adipose tissue is the initiator of metabolic diseases. J Diabetes Metab S13:008. doi: 10.4172/2155-6156.SI-008 [DOI] [Google Scholar]
- Roberts LD, Bostrom P, O’Sullivan JF, Schinzel RT, Lewis GD, Dejam A, Lee YK, Palma MJ, Calhoun S, Georgiadi A, Chen MH, Ramachandran VS, Larson MG, Bouchard C, Rankinen T, Souza AL, Clish CB, Wang TJ, Estall JL, Soukas AA, Cowan CA, Spiegelman BM, Gerszten RE. 2014. Beta-Aminoisobutyric acid induces browning of white fat and hepatic beta-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab 19:96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson K, Prins J, Venkatesh B. 2011. Clinical review: Adiponectin biology and its role in inflammation and critical illness. Crit Care 15:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Ezquerro S, Mendez-Gimenez L, Becerril S, Fruhbeck G. 2015. Revisiting the adipocyte: A model for integration of cytokine signaling in the regulation of energy metabolism. AmJ Physiol Endocrinol Metab 309: E691–E7I4. [DOI] [PubMed] [Google Scholar]
- Roman S, Agii A, Peran M, Alvaro-Galue E, Ruiz-Ojeda FJ, Fernandez-Vazquez G, Marchai JA. 2015. Brown adipose tissue and novel therapeutic approaches to treat metabolic disorders. Transi Res 165:464–479. [DOI] [PubMed] [Google Scholar]
- Sanchez-Delgado G, Martinez-Tellez B, Olza J, Aguilera CM, Gil A, Ruiz JR. 2015. Role of exercise in the activation of brown adipose tissue. Ann Nutr Metab 67:21–32. [DOI] [PubMed] [Google Scholar]
- Seale P, Lazar MA. 2009. Brown fat in humans: Turning up the heat on obesity. Diabetes 58:1482–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi CX, Zhao MX, Shu XD, Xiong XQ, Wang JJ, Gao XY, Chen Q, Li YH, Kang YM, Zhu GQ. 2016. Beta-aminoisobutyric acid attenuates hepatic endoplasmic reticulum stress and glucose/lipid metabolic disturbance in mice with type 2 diabetes. Sci Rep 6:21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidossis L, Kajimura S. 2015. Brown and beige fat in humans: Thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest 125:478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford Kl, Middelbeek RJ, Goodyear LJ. 2015. Exercise effects on white adipose tissue: Beiging and metabolic adaptations. Diabetes 64:2361–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford Kl, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K Hirshman MF, Tseng YH, Goodyear LJ. 2013. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 123:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbotina E, Sierra A, Zhu Z, Gao Z, Koganti SR, Reyes S, Stepniak E, Walsh SA, Acevedo MR, Perez-Terzic CM, Hodgson-Zingman DM, Zingman LV. 2015. Musclin is an activity-stimulated myokine that enhances physical endurance. Proc Natl Acad Sci USA 112:16042–16047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharp KM, Stahl A. 2015. Bioengineering beige adipose tissue therapeutics. Front Endocrinol (Lausanne) 6:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoonen R, Ernande L, Cheng J, Nagasaka Y, Yao V, Miranda-Bezerra A, Chen C, Chao W, Panagia M, Sosnovik DE, Puppala D, Armoundas AA, Hindle A, Bloch KD, Buys ES, Scherrer-Crosbie M. 2015. Functional brown adipose tissue limits cardiomyocyte injury and adverse remodeling in catecholamine-induced cardiomyopathy. J Mol Cell Cardiol 84:202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Dang HN, Yong J, Chui WS, Dizon MP, Yaw CK, Kaufman DL. 2011. Oral treatment with gamma-aminobutyric acid improves glucose tolerance and insulin sensitivity by inhibiting inflammation in high fat diet-fed mice. PLoS ONE 6: e25338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayhurn P, Beattie JH. 2001. Physiological role of adipose tissue: White adipose tissue as an endocrine and secretory organ. Proc Nutr Soc 60:329–339. [DOI] [PubMed] [Google Scholar]
- Vazquez-Vela ME, Torres N, Tovar AR. 2008. White adipose tissue as endocrine organ and its role in obesity. Arch Med Res 39:715–728. [DOI] [PubMed] [Google Scholar]
- Villarroya F, Giralt M. 2015. The beneficial effects of brown fat transplantation: Further evidence of an endocrine role of brown adipose tissue. Endocrinology 156:2368–2370. [DOI] [PubMed] [Google Scholar]
- Wang P, Mariman E, Renes J, Keijer J. 2008. The secretory function of adipocytes in the physiology of white adipose tissue. J Cell Physiol 216:3–13. [DOI] [PubMed] [Google Scholar]
- Warner A, Mittag J. 2016. Breaking BAT: Can browning create a better white? J Endocrinol 228: RI9–R29. [DOI] [PubMed] [Google Scholar]
- Wei K, Liu L, Xie F, Hao X, Luo J, Min S. 2015. Nerve growth factor protects the ischemic heart via attenuation of the endoplasmic reticulum stress induced apoptosis by activation of phosphatidylinositol 3-kinase. Int J Med Sci 12:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcicka G, Jamroz-Wisniewska A, Atanasova P, Chaldakov GN, Chylinska-Kula B, Beltowski J. 2011. Differential effects of statins on endogenous H2S formation in perivascular adipose tissue. Pharmacol Res 63:68–76. [DOI] [PubMed] [Google Scholar]
- Xie ZX, Xia SF, Qiao Y, Shi YH, Le GW. 2015. Effect of GABA on oxidative stress in the skeletal muscles and plasma free amino acids in mice fed high-fat diet. J Anim Physiol Anim Nutr (Beri) 99:492–500. [DOI] [PubMed] [Google Scholar]
- Xu A, Wang Y, Lam KS, Vanhoutte PM. 2010. Vascular actions of adipokines molecular mechanisms and therapeutic implications. Adv Pharmacol 60:229–255. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Matsushita Y, Nakagawa T, Hayashi T, Noda M, Mizoue T. 2014. Circulating adiponectin levels and risk of type 2 diabetes in the Japanese. Nutr Diab 4: el 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yore MM, Syed I, Moraes-Vieira PM, Zhang T, Herman MA, Homan EA, Patel RT, Lee J, Chen S, Peroni OD, Dhaneshwar AS, Hammarstedt A, Smith U, McGraw TE, Saghatelian A, Kahn BB. 2014. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 159:318–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafrir B 2013. Brown adipose tissue: Research milestones of a potential player in human energy balance and obesity. Horm Metab Res 45:774–785. [DOI] [PubMed] [Google Scholar]
- Zheng LR, Zhang YY, Han J, Sun ZW, Zhou SX, Zhao WT, Wang LH. 2015. Nerve growth factor rescues diabetic mice heart after ischemia/reperfusion injury via up-regulation of the TRPVI receptor. J Diab Complications 29:323–328. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Liu X, Zhao Q, Zhang L, Li C, Xue Y. 2014. Regulation of UCP I in the browning of epididymal adipose tissue by beta3-adrenergic agonist: A role for MicroRNAs. Int J Endocrinol 2014:530636. [DOI] [PMC free article] [PubMed] [Google Scholar]