Abstract
Purpose of review
Current clinical pathological classifications of glomerular diseases are inadequate at predicting patient disease progression or response to therapy. With the advent of precision medicine and its successes in oncology, it is important to understand if similar approaches in glomerular diseases can improve patient management. The purpose of this review is to summarize approaches to obtain comprehensive molecular profiles from human biopsies and utilize them to define the pathophysiology of glomerular failure.
Recent findings
Multicenter research networks have provided the framework to capture both prospective clinical disease course and patterns of end organ damage in biopsy cohorts. With these sample and data sets in hand, efforts are progressing towards molecular disease characterization, identification of novel prognostic marker, development of more precise clinical trials and discovery of predictive biomarkers to more effectively stratify patients to appropriate treatment regiments. Partnerships between academia, public funding agencies and private companies seek to improve timelines and maximize resources while also leveraging domain expertise in an integrated framework to holistically understand disease.
Summary
The application of system biology techniques within team science frameworks across disciplines and continents will seek to realize the impact of precision medicine to bring urgently needed novel therapeutic options to patients with glomerular disease.
Keywords: glomerular disease, kidney disease, precision medicine, system biology
INTRODUCTION
Patients with glomerular disease present with a disease driven by dysfunction of specific segments of the nephron, but with nonspecific, syndromic disease presentations. Access to kidney tissue via renal biopsy has been an essential tool to link nonspecific clinical presentations to distinct structural damage pattern in the glomerulus and along the nephron. These damage patterns are subsequently linked to systemic autoimmune (i.e. systemic lupus erythematosus, vasculitis) or metabolic (diabetic nephropathy) diseases or aggregated into groups of intrinsic renal diseases [i.e. focal segmental glomerulosclerosis (FSGS), membranous nephropathy, and minimal change disease (MCD)]. However, the current disease classification has limited ability to predict an individual patient’s response to therapy or disease progression, as it does not accurately explain the underlying molecular disease heterogeneity. A molecular-pathophysiological characterization will help to understand the underlying mechanistic causes of the disease and abnormal biology to inform novel intervention strategies with increased efficacy and reduced toxicity [1–3].
The development of molecular profiling techniques underlying precision medicine married with the fact that renal tissue is readily available presents a unique opportunity for precision medicine to advance our understanding of glomerular disease and help improve patient care.
Precision medicine is essentially the generation, analysis, and understanding of molecular characterization data to more accurately diagnose and treat a patient (i.e. the right drug at the right time for the right patient). Precision medicine is often driven by large-scale molecular profiling technologies (also referred to as omics technologies), which can define a prospective tissue at the different layers of molecular characterization, including the genome, transcriptome, proteome, metabolome, lipidome, and epigenome level. These types of data paint a multidimensional image of a patient’s disease and can help to define the specific mechanism and the molecular abnormalities underlying its progression (Fig. 1).
FIGURE 1.
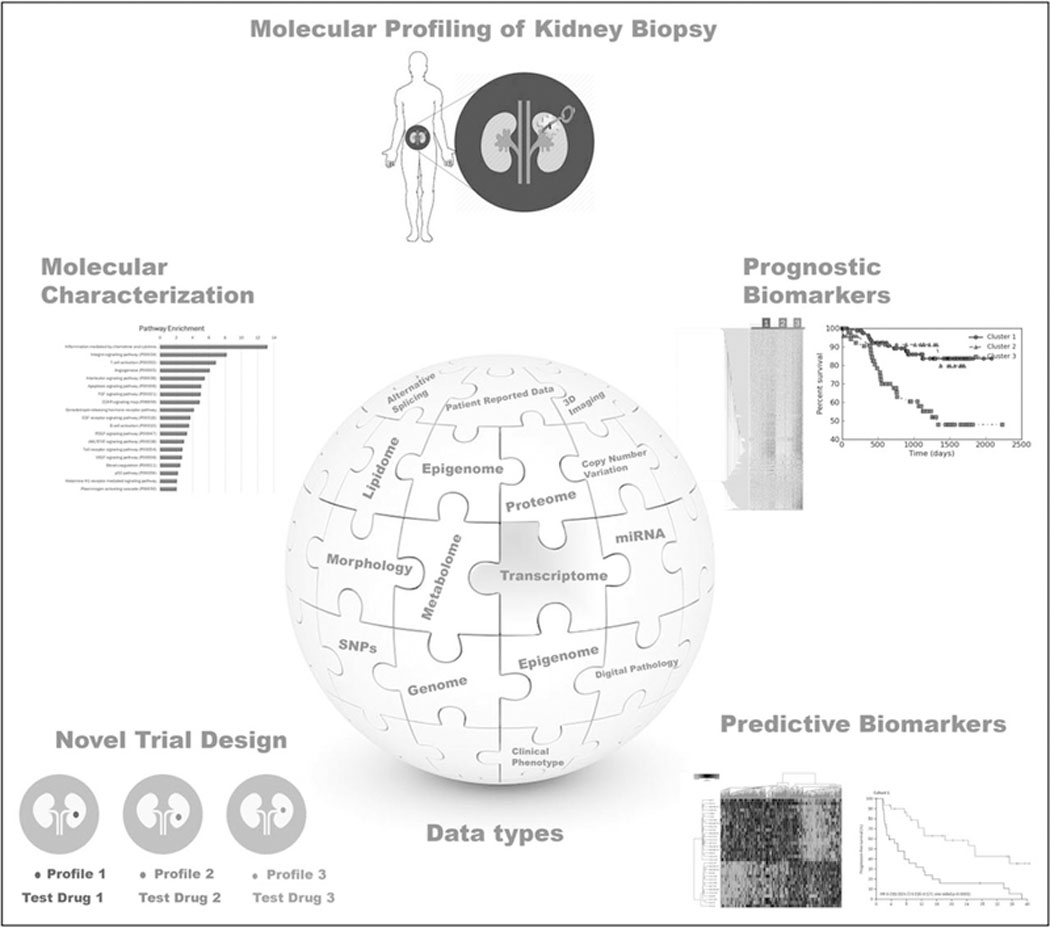
Precision medicine in glomerular diseases. In glomerular disease, molecular profiling technologies can be key to defining prospective tissue at the different layers of molecular characterization. This approach can paint a more global multidimensional image of a patient’s disease, which can lead to improved molecular disease characterization and trial design as well as leading to novel prognostic and predictive biomarkers.
One of the biggest challenges in precision medicine, however, is to define opportunities and challenges and manage expectations for this approach. The oncology field, which is generally regarded as the most advanced in the application of precision medicine may be used as an example to help contextualize the expectations of precision medicine by the kidney disease community. Substantial efforts and resources have been dedicated to precision medicine approaches in oncology, leading to initial successes, which can generally be summarized into four categories: molecular characterization of disease subtypes via improved understanding of the underlying molecular pathophysiology, improved clinical management through the use of prognostic biomarkers to define patient populations most likely to benefit from intervention, design of targeted clinical trials via molecularly informed patient stratification, and finally identification of predictive biomarkers to monitor individual patient’s responses to targeted therapies based on the patient’s disease biology.
MOLECULAR DISEASE CHARACTERIZATION
Large-scale consortia like The Cancer Genome Atlas (TCGA) and International Cancer Genome Consortium (ICGC) molecularly characterize cancer disease subtypes. The networks use multiple omics approaches including transcriptomic, proteomic, genetic, metabolomics, and epigenetic analysis for a deeper understanding of the disease’s molecular pathophysiology.
Parallel efforts are also underway in glomerular disease (NEPTUNE, KPMP, AMP and CureGN). A concern raised towards implementing a molecular disease characterization approach in kidney disease is that it is much more difficult to procure the same sample sizes as those obtained in the large cancer consortiums like TCGA (n = 11000) and ICGC (N = 17 000). Upon further analysis, the disease-specific subcohorts used for molecular analysis that resulted in the majority of the clinically relevant findings were significantly smaller. There were over 11 000 patient samples collected in the overall TCGA cohort but the average number of samples per disease cohort was 300. In fact, in a survey of the 212 study cohorts curated in the cancer cBioportal, 22% have less than 50 samples and the majority had less than 200 samples (65%), indicating that meaningful information can be derived from omics studies with less than 200 patients. Specifically, 99 out of the 137 studies (73%) in cBioportal with less than 200 patient led to meaningful novel discoveries that were published in high impact peer-reviewed journals.
It is, therefore, reasonable to expect meaningful results from studies with sample curation of 50 samples, which is very well within the sample resource constraints within the ongoing kidney disease cohorts. As an example, the Nephrotic Syndrome Study Network (NEPTUNE) consortium, which enrolls adults and pediatric patients presenting with a clinical diagnosis for FSGS, MCD, or membranous nephropathy, has already collected detailed prospective clinical data and biological material including biopsies for molecular profiling from more than 450 Nephrotic Syndrome research participants. This type of sample size is well above the average enrollment for any of the major cancer studies mentioned above. The long-term observational data gathered include tissue level molecular profile such as transcriptome analysis. These data sets can be used not only to help further molecular characterization of Nephrotic Syndrome but also identify molecular subtypes useful for novel therapeutic strategies. To date this study has led to 35 publications including clinical, molecular, and genetic findings regarding
Nephrotic Syndrome, comparable in size to the efforts in oncology and can advance our understanding of the molecular pathophysiology. For example, the molecular mechanism mediating the excessive risk linked to variance in apolipoprotein L1 (APOL1) for glomerular diseases [like HIV-associated nephropathy (HIVAN), FSGS and lupus nephritis) and end-stage renal disease (ESRD) in black patients [4–6] are being analyzed via integrative genomics strategies in human biopsy tissues [7].
The NIDDK’s Kidney Precision Medicine Project’s (KPMP, see www.kpmp.org) overarching goal is to display the molecular phenotypes of acute kidney injury (AKI) and chronic kidney disease (CKD) in their structural context and develop a readily accessible kidney tissue atlas for the renal research community and people suffering from kidney disease. The consortium will utilize next generation technologies for assignments of molecular states to renal tissue with high spatial resolution to aid in the development and implementation of targeted therapies.
The specific goal of these analyses is to address the tissue heterogeneity common to complex organ systems. The kidney shows high complexity in this context as each of the one million nephrons [8■] is a highly specialized unit requiring spatially tightly defined cellular functions to generate and process the glomerular ultrafiltrate [9]. The renal cells are distinguishably by gene expression patterns and vary in origin and exist in various states of activity and metabolism at one time. In-silico nano-dissection methods exist to deconvolute the bulk transcriptome to identify unique renal cell type signatures [10] but these approaches are most effective when paired with data derived from single-cell analyses to discern the cell-type-specific molecular markers associated with the unique cells. Both approaches can be useful for determining cell-type specific roles in disease causality and molecular subtypes.
Robust information can also be derived from meta analyses of heterogeneous studies. For example, in the TCGA ovarian cohort, when the researchers integrated data from three different gene expression platforms, only 11864 out of the more than 20 000 genes were used for transcriptional analysis and those were enough to molecularly characterize four novel subtypes of ovarian cancer that associated with clinical outcome [11]. The authors also needed to address batch effects between the studies and after combining all data identified ~1500 genes to perform their clustering analysis. Despite these challenges, four molecular subtypes of ovarian cancer were identified, which were subsequently validated independently using a well annotated cohort profiled on a single array platform with longer follow-up [12].
PROGNOSTIC BIOMARKERS
Prognostic biomarkers based on genomic tests derived from transcriptional signatures are now used to clinically manage patients and predict outcomes in breast and ovarian cancer. The landmark breast cancer article in which microarray gene expression was utilized to describe molecular subtypes in breast cancer included only 42 patient samples [13]. Perou’s seminal publication describing molecular subtypes in breast cancer has accumulated over 12000 citations. Although larger studies since then have further refined the subtypes, the original subtypes they described were meaningful to clinical decision-making in breast cancer and provided the basis for Food and Drug (FDA)-approved gene expression signatures as prognostic biomarkers [14]. These tools are now utilized to determine patients with good prognosis who are needed to be monitored rather than subjected to aggressive forms of therapies. Similar type of strategies could be envisioned in glomerular disease to identify molecular signatures to improve the clinical management of patients [15]. Those patients with a good prognosis can be managed with current clinical protocols and monitored regularly rather than being subjected to aggressive therapies like immunologics with known toxicities [16–18].
The hope is that the multiscalar data integration approaches in the glomerular disease consortia will lead to the development of molecular signatures useful for clinical decision-making [19]. Similarly, the Biomarker Enterprise to Attack diabetic kidney disease (BEAt-DKD) is a large international consortium with the objective of utilizing a system biology approach to identifying and validating novel predictive and prognostic biomarkers in DKD (for more on BEAt-DKD see the public and private partnership section below).
So far, the prognostic markers in the kidney disease are single markers. The hallmark finding that illustrates how a single study can change an entire disease area is the identification of podocyte membrane glycoprotein, N-type phospholipase A2 receptor (PLA2R), as the first and dominant autoantigen discovered in primary membranous nephropathy [20]. PLA2R-positive membranous nephropathy is now a molecular defined disease entity and PLA2R antibody levels are starting to be utilized as diagnostic biomarker predictive of lower risk of spontaneous or immunosuppressant-induced remission, higher risk of Nephrotic Syndrome and ESRD [21–23]. Kidney injury molecule 1 (KIM-1) is an epithelial transmembrane glycoprotein, which has emerged as a marker of tubular injury [24–26]. Neutrophil gelatinase-associated lipocalin (NGAL) levels can distinguish acute kidney injury (AKI) and prerenal AKI and associated with unfavorable outcome [27]. Epidermal growth factor was identified in a comprehensive transcriptomic-based CKD biopsy study from a list of candidate biomarker associated with CKD progression, kidney tissue specificity, and correlation between its mRNA levels in kidney biopsies and urinary protein level [28,29■].
NOVEL TRIAL DESIGN
Novel trial designs based on molecular profiling data are being implemented to test precision medicine-based therapies [LUNG-MAP (NCT02154490), NCI-MATCH (NCT02465060), TAPUR(NCT02693535), and ENTRECTINIB (NCT02568267)]. For example, the landmark TCGA study in squamous cell lung cancers that led to the Lung MAP clinical trial included 178 patient samples [30]. This ‘first-of-its-kind clinical trial model uses a multidrug, targeted screen approach to match investigational new treatments based on the unique tumor profiles of a patient’ (https://www.lung-map.org/about-lung-map) [31]. Molecular profile-informed clinical trial designs are coming within reach based on data emerging from the ongoing glomerular disease cohort studies like NEPTUNE. One challenge for patient stratification in clinical trials will be to balance recruitment of a preselected patient population based on molecular signature and eliminating likely nonresponders with the need to effectively accrue patients. Defining novel clinical trial end points like proteinuria remission or 40% reduction in eGFR is further improving feasibilty of clinical trials in glomerular diseases. The emergence of consortiums in renal disease, which mirror the large cancer consortium will help in patient accrual and implementing precision medicine trials in glomerular disease. Furthermore, a shift in paradigm towards public-private partnerships (see below) for efficient clinical development and implementation can enhance clinical trial capacity further.
PREDICTIVE BIOMARKERS
FDA-approved predictive biomarkers based on genomic tests utilizing mutational analysis are part of targeted therapies in lung (EGFR) [32], ovarian (BRCA) [33], and skin cancers (BRAF) [34] and help stratify patients to the appropriate therapy regimen. Although, it is well established that cancer is a disease of the genome, the discoveries in oncology are not necessarily related to mutational changes only. A landmark breast cancer study used transcriptional profile as a predictive biomarker. The cyclin dependent kinase (CDK) inhibitor palbociclib, which had failed to reach market, obtained FDA approval to treat hormone receptor positive HER2-advanced or metastatic breast cancer after clinical trials were designed based on a preclinical molecular profiling study that used gene expression data from 47 human breast cancer cell lines to help identify a responsive molecular subtype [35,36]. Iorio et al. [37■] demonstrated in a recent Cell article that gene expression data in contrast to mutation, copy number variation, or DNA methylation data were most predictive of response to drugs.
In renal disease, the Janus kinase signal transducer and activator of transcription (JAK-STAT) pathway was identified as biological target for pharmacological intervention from a transcriptomic study in diabetic kidney disease (DKD) biopsies [38]. JAK and STAT are important regulators of the inflammatory signaling molecule such as IL-16, IL-12, IL-23, and interferon-alpha. The binding of such ligands to their receptors leads to an activation of the signaling pathway that results in the transcription of pro-inflammatory target genes like CCL2, IL-24, and SOC1, which are a major genetic signature in DKD and lupus nephritis [38–40]. Differential gene expression analysis comparing transcriptomes collected from the tubulointerstitial and glomerular compartment from early and late stage DKD with controls identified the activation of the pathway, specifically differential regulation of the downstream transcriptional targets [38]. This discovery led to a phase II clinical trial [41■], which demonstrated that a JAK1/2 inhibitor can significantly reduce albuminuria in patients with DKD.
Subsequently, expression and urinary excretion of CCL2 [42–44], a target of the JAK STAT pathway, is increased in patients with diabetic nephropathy and blockage of its interaction with CC-chemokine receptor 2 (CCR2) in animal models reduces progression of the disease [45,46]. High urinary CCL2 levels are also associated with worse outcomes [47]. Thus, several studies have explored CCR2 inhibitors in human DKD, testing their ability to reduce proteinuria in patients (NCT017120161, NCT01447147).
PUBLIC-PRIVATE PARTNERSHIP AS THE NEXT LEVEL OF SYSTEM BIOLOGY
Just as there is a continuum of data types from the clinical data to the phenotype and genotype, a similar continuum exists for the drug development processes that stretches from public academic entities and funding institutions, nonprofit disease advocacy groups, and private entities like molecular diagnostic companies and biopharmaceutical companies. Each data type provides a unique insight into the molecular pathophysiology of the disease. Linking data with each other in an integrated analytic approach can lead to enhanced molecular characterization of the disease for therapeutic target identification. Similarly, each entity along the drug development process has established domain expertise. Public–private partnership in precision medicine aims to integrate the diverse expertise with the goal to enhance the utility of the systems biology approach and further accelerate the development of novel therapies.
The Accelerating Medicine Partnership (AMP) Rheumatoid Arthritis (RA)/Lupus Network (LN) is a prime example of this type of initiative and is a collaborative group effort involving the National Institute of Health (NIH), the US FDA, biopharmaceutical companies, and an investigative community from many nonprofit academic organizations. AMP RA/LN aims to leverage systems biology combined with single cell-profiling approaches to accelerate the discovery of novel biomarkers and relevant targets for the treatment of RA, lupus and related autoimmune disease.
BEAt-DKD is an international public–private partnership specifically developed for diabetic kidney disease with the aim of utilizing systems biology approaches to develop prognostic and predictive biomarkers to improve the clinical outcome for patients. BEAt-DKD is leveraging insight gained by preceding European Union-funded activities in systems biology of renal disease (SysKid and Summit) and is now integrating these large cohort data sets including clinical, pathological, and biochemical parameters. The group has already identified five novel subtypes of diabetes [48■], which robustly predict of risks for diabetic complications like DKD with the potential to identify early treatment options for patients at high risk of complications.
The Renal Pre-Competitive Consortium (RPC2) is a public–private partnership with the specific aim of accelerating drug development in chronic kidney disease (CKD) through the use of a systems biology approach to identify both prognostic and predictive biomarkers as well identify targets for novel drug discovery. The data types include clinical data from routine diagnostics, morphometry digital disease, gene expression, genome-wide association studies (GWAS) from kidney biopsies, and proteomic and metabolomics data from blood and urine specimen [49■].
CONCLUSION
Precision medicine has helped to improve treatment and increase survival for cancer patients. In kidney disease, first successes of this approach are emerging defining molecular subtypes like PLA2R-positive membranous nephropathy or inflammatory subtypes in DKD. Looking at the landscape of precision medicine in kidney disease, a critical element of success will be to further develop collaborative approaches across disciplines and continents, which evidently have been so successful in cancer research. This strategy will not only benefit from pooling resources but also most importantly can accelerate the time window to bring urgently needed novel therapeutic options to patients with CKD.
KEY POINTS.
Multidisciplinary precision medicine consortiums in glomerular disease will help advance our understanding of the molecular pathophysiology in patients with glomerular disease.
The precision medicine efforts in glomerular disease are using next generation application of traditional omics technologies to untangle the molecular and cellular heterogeneity in kidney tissue.
Multiscalar data integration approaches are aimed to develop molecular signatures useful for therapeutic target identification, patient stratification and ultimately clinical decision-making.
Public-private partnership represents the next level of system biology application to improve the timeline of bench to bedside discoveries.
Acknowledgements
We would like to thank Dr Mark Tomilo for critical review of the manuscript.
Financial support and sponsorship
This work was supported by NEPTUNE study grants from the NIH Office of Rare Diseases, U54 DK083912; O’Brien Kidney Center Grant, P30 DK081943; Neph- Cure; and by the Intramural Research programs of the NCI and NIDDK and The University of Michigan.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Hingorani SR, Weiss NS, Watkins SL. Predictors of peritonitis in children with nephrotic syndrome. Pediatr Nephrol 2002; 17:678–682. [DOI] [PubMed] [Google Scholar]
- 2.Kerlin BA, Ayoob R, Smoyer WE. Epidemiology and pathophysiology of nephrotic syndrome-associated thromboembolic disease. Clin J Am Soc Nephrol 2012; 7:513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monach PA, Arnold LM, Merkel PA. Incidence and prevention of bladder toxicity from cyclophosphamide in the treatment of rheumatic diseases: a data-driven review. Arthritis Rheum 2010; 62:9–21. [DOI] [PubMed] [Google Scholar]
- 4.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 2010; 329: 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tzur S, Rosset S, Shemer R, et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet 2010; 128:345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams WW, Pollak MR. Health disparities in kidney disease–emerging data from the human genome. N Engl J Med 2013; 369:2260–2261. [DOI] [PubMed] [Google Scholar]
- 7.Sampson MG, Robertson CC, Martini S, et al. , Nephrotic Syndrome Study Network. Integrative genomics identifies novel associations with APOL1 risk genotypes in black NEPTUNE subjects. J Am Soc Nephrol 2016; 27: 814–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.■.Denic A, Mathew J, Lerman LO, et al. Single-nephron glomerular filtration rate in healthy adults. N Engl J Med 2017; 376:2349–2357. A discussion of the next generation of GFR analysis. Calculating the single-nephron GFR was more informative and associated with certain risk factors for chronic kidney disease and maybe more useful in the system biology analytical pipeline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JW, Chou CL, Knepper MA. Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol 2015; 26:2669–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ju W, Greene CS, Eichinger F, et al. Defining cell-type specificity at the transcriptional level in human disease. Genome Res 2013; 23:1862–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011; 474:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konecny GE, Wang C, Hamidi H, et al. Prognostic and therapeutic relevance of molecular subtypes in high-grade serous ovarian cancer. J Natl Cancer Inst 2014; 106:; pii: dju249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000; 406:747–752. [DOI] [PubMed] [Google Scholar]
- 14.Ross JS, Hatzis C, Symmans WF, et al. Commercialized multigene predictors of clinical outcome for breast cancer. Oncologist 2008; 13:477–493. [DOI] [PubMed] [Google Scholar]
- 15.Simon R Roadmap for developing and validating therapeutically relevant genomic classifiers. J Clin Oncol 2005; 23:7332–7341. [DOI] [PubMed] [Google Scholar]
- 16.Vanderlaan BF, Broder MS, Chang EY, et al. Cost-effectiveness of 21-gene assay in node-positive, early-stage breast cancer. Am J Manag Care 2011; 17:455–464. [PubMed] [Google Scholar]
- 17.Albanell J, Gonzalez A, Ruiz-Borrego M, et al. Prospective transGEICAM study of the impact of the 21-gene Recurrence Score assay and traditional clinicopathological factors on adjuvant clinical decision making in women with estrogen receptor-positive (ER+) node-negative breast cancer. Ann Oncol 2012; 23:625–631. [DOI] [PubMed] [Google Scholar]
- 18.Cusumano PG, Generali D, Ciruelos E, et al. European inter-institutional impact study of MammaPrint. Breast 2014; 23:423–428. [DOI] [PubMed] [Google Scholar]
- 19.Gadegbeku CA, Gipson DS, Holzman LB, et al. Design of the Nephrotic Syndrome Study Network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int 2013; 83:749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beck LH Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 2009; 361:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofstra JM, Beck LH Jr, Beck DM, et al. Antiphospholipase A(2) receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 2011; 6:1286–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoxha E, Thiele I, Zahner G, et al. Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J Am Soc Nephrol 2014; 25:1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim YG, Choi YW, Kim SY, et al. Anti-phospholipase A2 receptor antibody as prognostic indicator in idiopathic membranous nephropathy. Am J Nephrol 2015; 42:250–257. [DOI] [PubMed] [Google Scholar]
- 24.Vaidya VS, Niewczas MA, Ficociello LH, et al. Regression of microalbuminuria in type 1 diabetes is associated with lower levels of urinary tubular injury biomarkers, kidney injury molecule-1, and N-acetyl-beta-D-glucosaminidase. Kidney Int 2011; 79:464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tramonti G, Kanwar YS. Review and discussion of tubular biomarkers in the diagnosis and management of diabetic nephropathy. Endocrine 2013; 43:494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nielsen SE, Andersen S, Zdunek D, et al. Tubular markers do not predict the decline in glomerular filtration rate in type 1 diabetic patients with overt nephropathy. Kidney Int 2011; 79:1113–1118. [DOI] [PubMed] [Google Scholar]
- 27.Singer E, Elger A, Elitok S, et al. Urinary neutrophil gelatinase-associated lipocalin distinguishes prerenal from intrinsic renal failure and predicts outcomes. Kidney Int 2011; 80:405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ju W, Nair V, Smith S, et al. , ERCB, C-PROBE, NEPTUNE, and PKU-IgAN Consortium. Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med 2015; 7:; 316ra193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.■.Betz BB, Jenks SJ, Cronshaw AD, et al. Urinary peptidomics in a rodent model of diabetic nephropathy highlights epidermal growth factor as a biomarker for renal deterioration in patients with type 2 diabetes. Kidney Int 2016; 89:1125–1135. The study discusses the utility of peptidomics analysis in animal models to identify novel biomarkers of diabetic nephropathy and the results confirmed an earlier study which identified the same biomarker using transcriptomic analysis. This is an example of two independent studies reaching the same conclusion. [DOI] [PubMed] [Google Scholar]
- 30.Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012; 489:519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herbst RS, Gandara DR, Hirsch FR, et al. Lung Master Protocol (Lung-MAP)- a biomarker-driven protocol for accelerating development of therapies for squamous cell lung cancer: SWOG S1400. Clin Cancer Res 2015; 21:1514–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khozin S, Blumenthal GM, Jiang X, et al. U. S. Food and Drug Administration approval summary: erlotinib for the first-line treatment of metastatic nonsmall cell lung cancer with epidermal growth factor receptor exon 19 deletions or exon 21 (L858R) substitution mutations. Oncologist 2014; 19:774–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balasubramaniam S, Beaver JA, Horton S, et al. FDA approval summary: rucaparib for the treatment of patients with deleterious BRCA mutation-associated advanced ovarian cancer. Clin Cancer Res 2017; 23: 7165–7170. [DOI] [PubMed] [Google Scholar]
- 34.Odogwu L, Mathieu L, Blumenthal G, et al. FDA approval summary: dabrafenib and trametinib for the treatment of metastatic non-small cell lung cancers harboring BRAF V600E mutations. Oncologist 2018; 23: 740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res 2009; 11:R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015; 16:25–35. [DOI] [PubMed] [Google Scholar]
- 37.■■.Iorio F, Knijnenburg TA, Vis DJ, et al. A landscape of pharmacogenomic interactions in cancer. Cell 2016; 166:740–754. A compendia of valuable omics tools for analyzing and leveraging multiscaler data. The study also compares the utility of different omics data in predicting response to drugs as well as assessing the value gain of integrating multidata types. The supplementary information contains valuable tools. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berthier CC, Zhang H, Schin M, et al. Enhanced expression of Janus kinasesignal transducer and activator of transcription pathway members in human diabetic nephropathy. Diabetes 2009; 58:469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hodgin JB, Nair V, Zhang H, et al. Identification of cross-species shared transcriptional networks of diabetic nephropathy in human and mouse glomeruli. Diabetes 2013; 62:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neusser MA, Lindenmeyer MT, Moll AG, et al. Human nephrosclerosis triggers a hypoxia-related glomerulopathy. Am J Pathol 2010; 176:594–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.■■.Brosius FC, Tuttle KR, Kretzler M. JAK inhibition in the treatment of diabetic kidney disease. Diabetologia 2016; 59:1624–1627. A good example of the use of molecular targeted therapies in glomerular diseases. The study vetted the JAK-STAT findings from transcriptomic analysis and animal studies to generate a hypothesis and design a clinical trial involving patients with DKD. They tested the hypothesis successfully in a phase II clinical trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marisa C, Lucci I, Di Giulio C, et al. MCP-1 and MIP-2 expression and production in BB diabetic rat: effect of chronic hypoxia. Mol Cell Biochem 2005; 276(1–2):105–111. [DOI] [PubMed] [Google Scholar]
- 43.Wada T, Furuichi K, Sakai N, et al. Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int 2000; 58:1492–1499. [DOI] [PubMed] [Google Scholar]
- 44.Mine S, Okada Y, Tanikawa T, et al. Increased expression levels of monocyte CCR2 and monocyte chemoattractant protein-1 in patients with diabetes mellitus. Biochem Biophys Res Commun 2006; 344:780–785. [DOI] [PubMed] [Google Scholar]
- 45.Seok SJ, Lee ES, Kim GT, et al. Blockade of CCL2/CCR2 signalling ameliorates diabetic nephropathy in db/db mice. Nephrol Dial Transplant 2013; 28:1700–1710. [DOI] [PubMed] [Google Scholar]
- 46.Awad AS, Kinsey GR, Khutsishvili K, et al. Monocyte/macrophage chemokine receptor CCR2 mediates diabetic renal injury. Am J Physiol Renal Physiol 2011; 301:F1358–F1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Titan SM, Vieira JM Jr, Dominguez WV, et al. Urinary MCP-1 and RBP: independent predictors of renal outcome in macroalbuminuric diabetic nephropathy. J Diabetes Complications 2012; 26:546–553. [DOI] [PubMed] [Google Scholar]
- 48.■.Ahlqvist E, Storm P, Karajamaki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol 2018; 6:361–369. A great example of how the integration of multiscalar data can impact patient management in a complex disease like diabetes. [DOI] [PubMed] [Google Scholar]
- 49.■■.Tomilo M, Ascani H, Mirel B, et al. Renal Pre-Competitive Consortium (RPC(2)): discovering therapeutic targets together. Drug Discov Today 2018. [Epub ahead of print] An informative discussion about the establishment of a specific public private partnerships (RPC2) and how the partnership can strengthen the utility of multiscalar data. [DOI] [PubMed] [Google Scholar]


