Abstract
Background:
Pulmonary exacerbations (PEx) in cystic fibrosis (CF) patients decrease lung function, increase symptoms and reduce health-related quality of life (HRQoL). We evaluated associations between 8 symptom-based questions from the Cystic Fibrosis Respiratory Symptom Diary - Chronic Respiratory Infection Symptom Score (CFRSD-CRISS) and the 5-level EuroQOL-5 Dimensions (EQ-5D-5L) summary score and hypothesized the CFRSD-CRISS would be a meaningful way to asses quality-of-life in future cost effectiveness studies among CF patients with PEx.
Methods:
CF patients who had CFRSD-CRISS and EQ-5D-5L measurements on the day of the initial PEx, 7 days later, and at the end of intravenous antibiotic treatment were included. We examined age-stratified (<18 versus ≥18 years old) characteristics, including the percent predicted of forced expiratory volume in 1 second (ppFEV1), CFRSD-CRISS measurements, and domains of the EQ-5D. We also calculated age-stratified Pearson correlation coefficients between the EQ-5D-5L and CFRSD-CRISS items at each of the 3 time points.
Results:
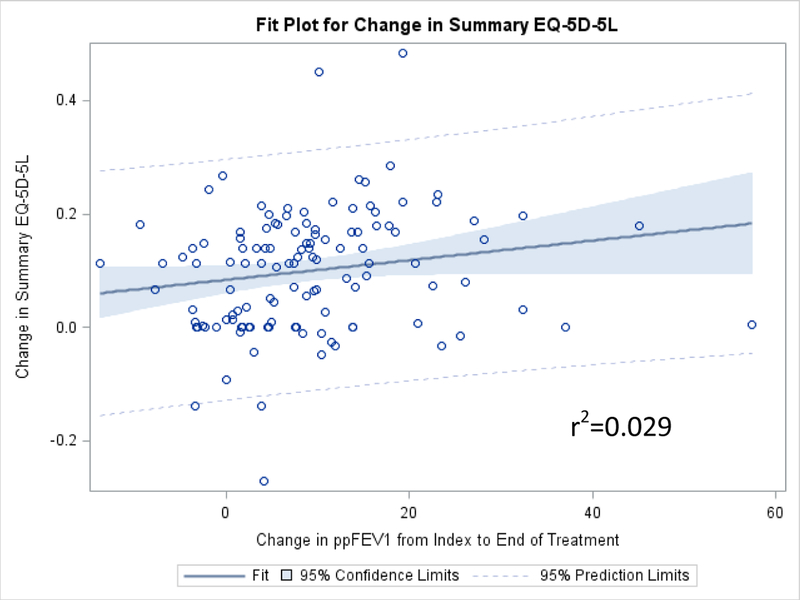
A total of 169 patients were analyzed. Patients reported having problems performing usual activities and with pain/discomfort on the first day of the PEx and these measures improved by the end of treatment. PpFEV1 improved in both age categories by the end of PEx treatment but was not associated with the change in summary EQ-5D-5L over the time of PEx treatment (r-squared=0.029). Correlations were weak (generally < .4) between the elements of the EQ-5D-5L versus the CFRSD-CRISS.
Conclusions:
Value assessment of treatments for CF PEx will require the collection of preference-weighted measures rather than only the symptom-based questions of the CFRSD-CRISS.
Keywords: cystic fibrosis, pulmonary exacerbations, lung function, Cystic Fibrosis Respiratory Symptom Diary - Chronic Respiratory Infection Symptom Score, 5-level EuroQOL-5 Dimensions, cost effectiveness analysis
Introduction
Cystic fibrosis (CF) affects an estimated 33,000 to 34,000 people in the United States, with 900–1000 new cases diagnosed each year.1 In recent years, improvements in nutrition, antibiotic therapies, transporter modifying drug therapies, and chest physiotherapies have extended the lifespan of CF patients to a median survival of about 47 years.2 However, this extended life expectancy leads to more opportunities for pulmonary exacerbations (PEx) of CF symptoms. Relative to the general population, CF patients have reduced health-related quality of life (HRQoL),3,4 possibly due to symptoms, especially during times of PEx, such as dyspnea, cough, reduced lung function, and repeated respiratory infections and hospitalizations.1
A preference-weighted measure of function and well-being that is sensitive to the HRQoL changes that occur during the course of PEx treatments and that is equally effective in children and adults would be ideal for estimating the value of novel PEx interventions. Preference-weighted measures are the standard for health economic analyses of various medical treatments. One instrument that has been developed, the 5-Level EuroQOL-5 Dimensions (EQ-5D-5L) score, is one of the most commonly used measures of preference-weighted HRQoL in cost-effectiveness studies for many disease states.5–7 However, CF-specific questionnaires have been more commonly used to assess symptoms and function in the CF population. One of these, the Cystic Fibrosis Respiratory Symptom Diary-Chronic Respiratory Infection Symptom Score (CFRSD-CRISS),8 designed for CF patients age 12 and over, contains 16 items on the timing and severity of CF symptoms and their impacts. A subset of these questions, the 8-question CFRSD-CRISS sub-score, assesses only the severity of eight CF-related symptoms. The Cystic Fibrosis Questionnaire-Revised (CFQ-R), also commonly used to assess HRQoL in CF patients,9–12 measures functioning in twelve domains, including vitality, health perceptions, respiratory symptoms, treatment burden, and physical, emotional, and social functioning. Finally, the percent predicted of forced expiratory volume in 1 second (ppFEV1), is commonly used as a measure of lung function of CF patients and has been used as a surrogate for HRQoL in some cost-effectiveness studies.13–15
The Standardized Treatment Of Pulmonary exacerbations (STOP) study was conducted as a pilot, observational trial to determine the feasibility of adopting a standardized protocol for treating CF PEx.16 The STOP study collected clinical and HRQoL measures, including the EQ-5D-5L and the 8 symptom-based questions from the CFRSD-CRISS, over the course of each patient’s PEx treatment. The purpose of this paper was to evaluate how well the EQ-5D-5L corresponded to symptoms of CF patients who were experiencing exacerbations. A previous study17 that used the 3-level version of the EQ-5D implied that elements of the EQ-5D, such as usual activities, pain/discomfort, and anxiety/depression, were reduced during pulmonary exacerbations. We theorized that experiencing these problems would be related to worsened CF symptoms. We hypothesized that our analyses would reveal that the EQ-5D-5L was highly correlated to the 8 symptom-based measures of the CFRSD-CRISS and thus the CFRSD-CRISS would be a meaningful way to asses quality-of-life in future cost effectiveness studies among CF patients with pulmonary exacerbations. Secondarily, we examined the association between the EQ-5D-5L and ppFEV1 over the course of treatment of the PEx events, which would allow us to determine the degree to which the EQ-5D-5L tracks with improvement in the most often used clinical measure related to CF PEx.
Methods
Study Population: STOP Study
The population of CF patients for these analyses was collected from the STOP study, which is described in detail elsewhere.16 Briefly, this multi-center, prospective observational cohort study enrolled CF patients who were hospitalized for treatment of PEx with the purpose of identifying clinical endpoints that would be useful for future studies that would determine the most efficacious treatments for PEx.18 The STOP study enrolled a total of 220 CF patients age 12 years and older from 11 US CF centers who were admitted to an inpatient service for PEx treatment with intravenous (IV) antibiotics from January 2014-January 2015.16 The duration and specific antibiotic treatment regimens were determined by the treating physicians and not influenced by the STOP study protocols. Patients were followed for 28 days following their index PEx. Institutional Review Board (IRB) approval was obtained from each participating CF center for the parent STOP study and all participants or their guardians provided written informed consent where required; this analysis was approved by the University of Washington IRB (approval number 00000484).
Timing of Assessments and Description of Measures
Patients may have had up to four visits for this study: 1) the day of the initial pulmonary exacerbation (hereafter termed “index day”), 2) 7 days later, 3) at the end of IV antibiotic treatment, and 4) 28 days after the index day. Because not all patients had completed IV treatment by day 28, we used data from 3 time points: 1) index day; 2) day 7; and 3) the end of antibiotic treatment (or day 28 if treatment continued past day 28). At each of these time points, patients answered the 5 questions from the EQ-5D-5L pertaining to mobility, self-care, usual activities, pain/discomfort, and anxiety/depression.5,19 We used US-based population weights to convert domain-specific EQ-5D-5L scores to summary EQ-5D-5L index scores ranging from −0.11 (health status worse than death) to 1 (perfect health).20 Additionally, patients completed the 8 items of the CFRSD-CRISS questionnaire, which assessed CF-specific symptoms such as difficulty breathing, feeling feverish, feeling tired, having chills/sweats, cough severity, sputum production, chest tightness, and wheezing.8,21 These scores were totaled to obtain Rasch-derived summary scores ranging from 0–100, with higher scores indicating greater symptom severity.18 Although these questions were assessed every day that patients were enrolled in the STOP study, we used only data that was assessed on the same days as the EQ-5D-5L to ensure correspondence between the two measurements and the subjects’ immediate health status.
Spirometry measures were also taken at each of the three time points, but because we were interested in the change in ppFEV1 over the course of treatment for the PEx, we only used spirometry measurements at the index day and at the end of antibiotic treatment. We calculated the ppFEV1 using the Global Lung Initiative equations.22
Statistical Analysis
We examined demographic (age, race, ethnicity, gender, insurance status, education) and clinical (duration of IV antibiotic treatment, IV therapy after completion of initial therapy, ppFEV1 at the index day and at the end of treatment, antibiotic classes used to treat PEx) variables of the cohort. We determined whether the patients’ summary EQ-5D-5L and CFRSD-CRISS scores improved by a minimally clinically important amount (determined to be ≥0.074 for the EQ-5D23 and ≥11 points for the CFRSD-CRISS24) between the index day and the end of PEx treatment. We reported means, medians, and standard deviations for continuous variables and counts and percentages for categorical variables. For normally distributed variables such as patient age and mean ppFEV1, we used t-tests and chi-square tests (or Fisher’s exact test if n<5) to examine the significance of differences between continuous and categorical variables, respectively.
In order to examine trends in measures over the time the PEx were being treated, we created histograms of the summary CFRSD-CRISS measurements, the 5 domains of the EQ-5D-5L, and the summary EQ-5D-5L index score on the index day, 7 days later, and the end of PEx antibiotic treatment. To test whether the CFRSD-CRISS and EQ-5D-5L scores were significantly different between the index day and the end of PEx antibiotic treatment, we used the Kruskal-Wallis test. We also created a histogram of the ppFEV1 on the index day and at the end of PEx treatment and a scatter plot of the change in ppFEV1 from the index day until the end of PEx treatment versus the change in summary EQ-5D-5L score over the same period of time. We also created scatter plots of the absolute (rather than changes over time) summary EQ-5D-5L versus the ppFEV1 at both index and at the end of PEx treatment. We calculated the Spearman correlation coefficients25 between each of the individual EQ-5D-5L and individual symptom-based CFRSD-CRISS items at each of the three time points.
Finally, we created 3 generalized linear mixed models with gamma distributions, all with the change in EQ-5D-5L summary scores between the index day and the end of PEx treatment as the outcome variable. We chose to evaluate the changes in these variables because we hypothesized that changes over time would be more likely to be correlated than the absolute values of the measures at each of the time points. The independent variables were: 1) the change in summary CFRSD-CRISS scores from the index day until the end of PEx treatment; 2) the change in ppFEV1 from the index day until the end of PEx treatment; and 3) both the change in summary CFRSD-CRISS scores and the change in ppFEV1 from the index day until the end of PEx treatment. All three models were adjusted for gender, ppFEV1 on the index day, CFRSD-CRISS on the index day, and whether the patient received gram positive, MRSA, anaerobic, and broad spectrum antibiotics for their PEx. We examined point estimates and 95% confidence intervals (95% CIs) of these three models to determine whether the combination of the changes in CFRSD-CRISS scores and ppFEV1 over time would improve prediction of the change in EQ-5D-5L compared to either measurement alone.
Because previous research has shown that HRQoL tends to vary by age in CF patients,12 we stratified all analyses into those <18 versus those who were ≥18 years of age. SAS version 9.4 (SAS Institute, Inc., Cary, North Carolina, 2017) was used for all analyses.
Results
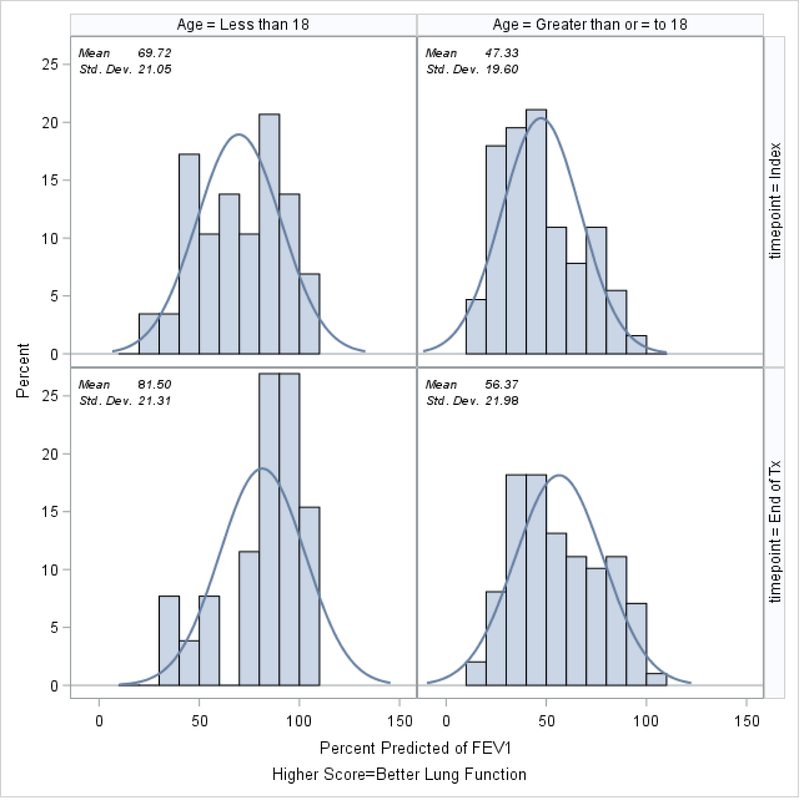
Demographic and clinical characteristics of the 169 patients who had EQ-5D-5L and CFRSD-CRISS measurements on the same day on at least 3 separate visits, stratified by age, are shown in Table 1. Because we were interested in assessing the relationship of EQ-5D-5L with the 8 symptom-based questions of the CFRSD-CRISS over time as PEx were being treated, we excluded patients (n=51; 23% of the patients in the STOP study) who did not have EQ-5D-5L and CFRSD-CRISS measurements on the same day at the 3 time points. Patients who were <18 at the time of their PEx were similar to those who were ≥18 in terms of duration of IV treatment, whether any IV therapy occurred after completion of initial treatment, gender, race, parent education status, and whether their EQ-5D-5L and CFRSD-CRISS scores improved by minimally clinically significant amounts by the end of antibiotic therapy. Relative to patients ≥18 years, patients who were <18 at the time of their exacerbation tended to have statistically significantly higher ppFEV1 both at the index day and at the end of treatment (70% ± 21% versus 47% ± 20% on the index day, p<0.0001 and 81% ± 21% versus 56% ± 22% at the end of PEx treatment, p<0.0001). Patients who were <18 were less likely to have taken pseudomonas antibiotics (94% vs 100%, p=0.04) and more likely to have taken a steroid while they were taking antibiotics (9% vs 1%, p=0.02).
Table 1.
Clinical and demographic characteristics stratified by age.
| Variable (n (%) unless stated otherwise) | Age <18 (n=33) | Age ≥18 (n=136) | p-value |
|---|---|---|---|
| Age (mean (median) ± std dev) | 15.7 (16.0) ± 1.6 | 29.1 (27.2) ± 8.9 | <0.0001 |
| Height (cm, mean (median) ± std dev) | 161 (161.8) ± 10 | 166.8 (165.1) ± 9.5 | 0.002 |
| Duration of IV treatment (mean (median) ± std dev) | 14 (15.0) ± 4.2 | 15.7 (15.0) ± 5.4 | 0.20 |
| Missing | 3 | 8 | |
| Any IV therapy after completion of initial therapy | 18 (60%) | 80 (63%) | 0.80 |
| Missing | 3 | 8 | |
| ppFEV1 (mean (median) ± std dev); (n): | |||
| ±3 Days from Index Date | 69.7% (70.5%) ± 21.0% (29) | 47.3% (44.7%) ± 19.6% (136) | <0.0001 |
| ±3 Days from End of Tx day | 81.4% (85.7%) ± 21% (26) | 56.4% (54.1%) ± 22.0% (99) | <0.0001 |
| Change in FEV1 % Predicted from Index Date to End of Tx (± 3 days) | 14.5% (11.3%) ± 17.5% (24) | 7.6% (7.4%) ± 7.8% (96) | 0.07 |
| Female | 19 (58%) | 81 (60%) | 0.84 |
| Race | |||
| White | 31 (94%) | 131 (96%) | |
| Black | 2 (6%) | 3 (2%) | |
| Asian | 0 | 0 | 0.40 |
| Two or more races | 0 | 2 (1%) | |
| Hispanic | 3 (10%) | 5 (4%) | 0.18 |
| Unknown | 2 | 6 | |
| What type of insurance did the patient have?1 | |||
| Private | 20 (61%) | 76 (56%) | 0.65 |
| Medicare | 1 (3%) | 35 (26%) | 0.004 |
| Medicaid | 18 (55%) | 47 (35%) | 0.04 |
| State special needs | 4 (12%) | 11 (8%) | 0.47 |
| Military | 1 (3%) | 7 (5%) | 0.60 |
| Other | 1 (3%) | 7 (5%) | 0.60 |
| Missing | 0 | 1 | |
| Patient covered under his/her parent’s insurance | 21 (66%) | 33 (28%) | <0.0001 |
| Missing | 1 | 19 | |
| Patient covered under a patient assistance program | 6 (24%) | 23 (25%) | 0.93 |
| Missing | 8 | 43 | |
| Patient education | |||
| <High school | 32 (97%) | 9 (7%) | |
| High school diploma | 1 (3%) | 36 (28%) | |
| Some college | 0 | 44 (34%) | <0.0001 |
| College grad. | 0 | 38 (29%) | |
| Masters/doctoral | 0 | 2 (2%) | |
| Missing/Unknown | 0 | 7 | |
| Father’s education | |||
| <High school | 1 (8%) | 4 (6%) | |
| High school diploma | 5 (38%) | 21 (34%) | |
| Some college | 1 (8%) | 11 (18%) | 0.75 |
| College grad. | 6 (46%) | 22 (35%) | |
| Masters/doctoral | 0 | 4 (6%) | |
| Missing/Unknown | 20 | 74 | |
| Mother’s education | |||
| <High school | 1 (7%) | 5 (8%) | |
| High school diploma | 2 (14%) | 20 (32%) | |
| Some college | 6 (43%) | 12 (19%) | 0.11 |
| College grad. | 3 (21%) | 24 (38%) | |
| Masters/doctoral | 2 (14%) | 2 (3%) | |
| Missing/Unknown | 19 | 73 | |
| Antibiotic Classes received1 | |||
| Gram Positive Cocci | 31 (94%) | 119 (88%) | 0.29 |
| MRSA | 16 (48%) | 72 (53%) | 0.65 |
| Gram Negative | 33 (100%) | 136 (100%) | -- |
| Pseudomonas | 31 (94%) | 136 (100%) | 0.04 |
| Anaerobes | 96 (58%) | 77 (57%) | 0.67 |
| Patient took ≥1 Broad Spectrum Antibiotic | 30 (91%) | 111 (82%) | 0.20 |
| Patient did not take ≥1 Broad Spectrum Antibiotic | 3 (9%) | 25 (18%) | |
| Other meds taken while on antibiotics | |||
| Mucolytic | 0 | 3 (2%) | 0.52 |
| Steroid | 3 (9%) | 1 (1%) | 0.02 |
| Days from exacerbation (mean (median) ± std dev) to: | |||
| 1st PRO measurement (Index) | 1.0 (1.0) ± 0 | 1.1 (1.0) ± 0.9 | 0.09 |
| 2nd PRO measurement (~Day 7) | 7.2 (7.0) ± 1.4 | 7.4 (7.0) ± 2.4 | 0.37 |
| 3rd PRO measurement (End of PEx treatment) | 15.8 (14.0) ± 6.4 | 17.6 (15.0) ± 6.8 | 0.19 |
| EQ-5D-5L improved2 by the end of antibiotic therapy | 20 (61%) | 85 (63%) | 0.84 |
| CFRSD-CRISS improved3 by the end of antibiotic therapy | 28 (85%) | 108 (79%) | 0.48 |
We also compared patients who had EQ-5D-5L and CFRSD-CRISS measurements on the same day on at least 3 separate visits with those who did not and found that the two groups were generally similar. However, patients who did not have at least 3 measures on the same day were less likely to have achieved minimally clinically important improvements in their EQ-5D-5L and CFRSD-CRISS scores by the end of treatment (Supplement).
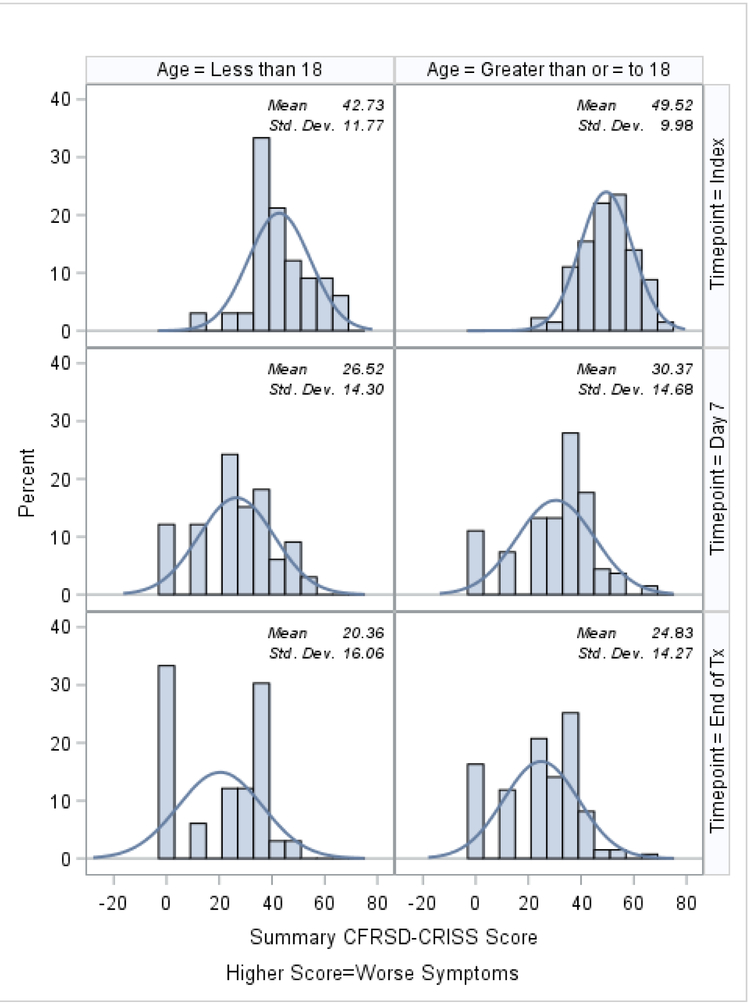
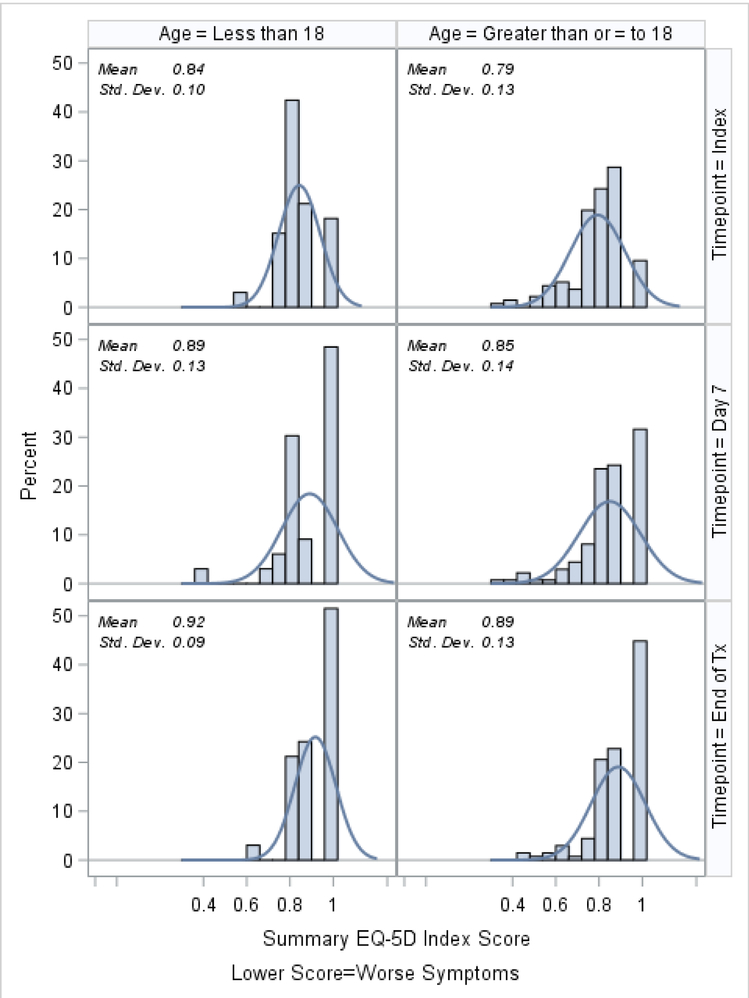
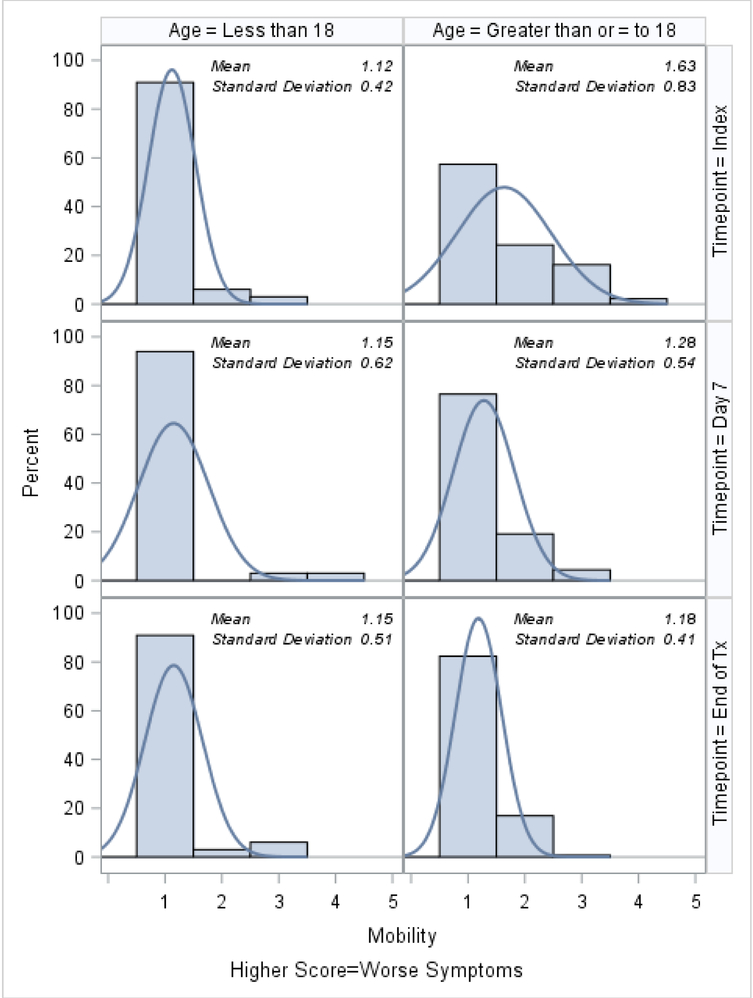
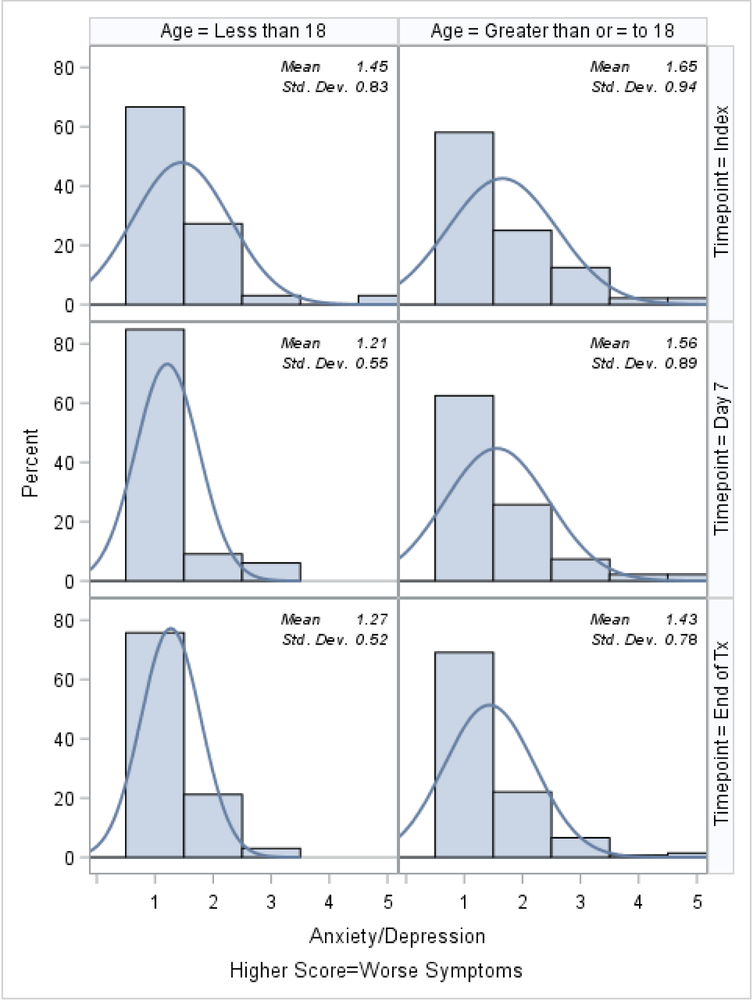
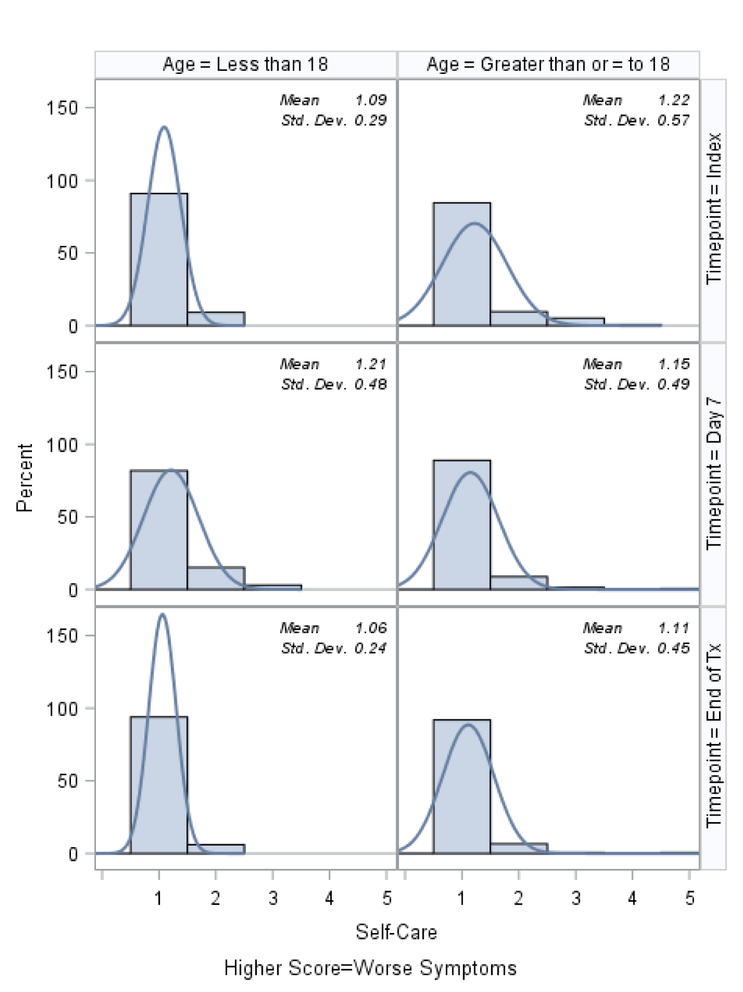
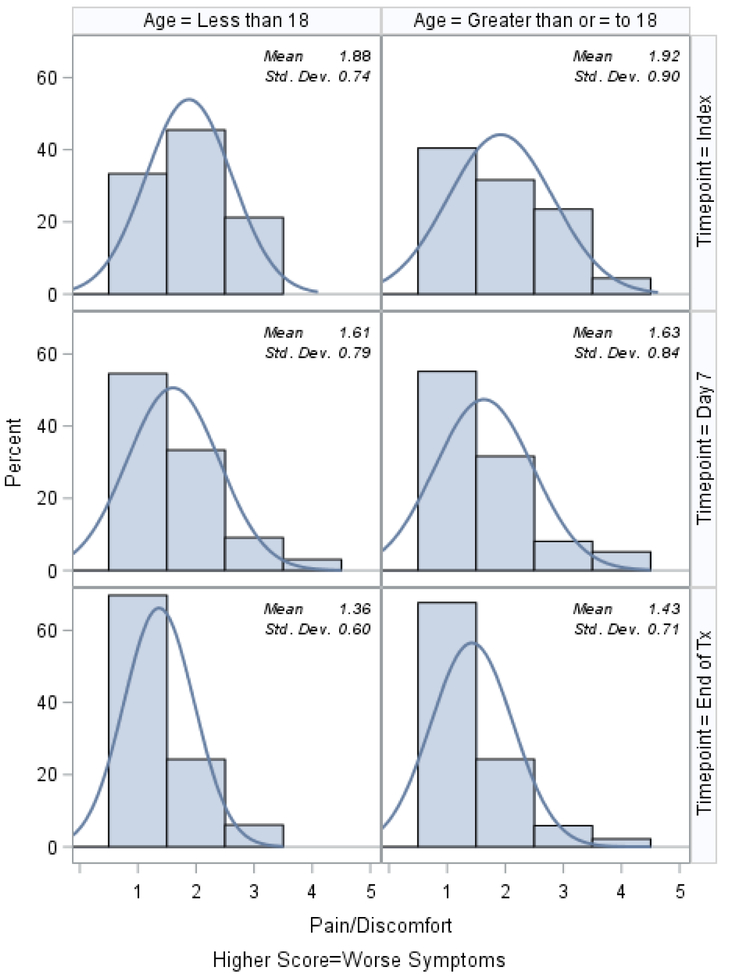
Histograms of the patient-reported outcomes on the index day, at day 7, and at the end of treatment, stratified by age, are shown in Figures 1–3. In general, patients who were ≥18 tended to report worse symptoms and quality-of-life indicators. For example, patients ≥18 tended to have higher (worse) CFRSD-CRISS summary scores compared to patients <18 (Figure 1). In both age groups, however, the mean CFRSD-CRISS scores had improved statistically significantly by then end of PEx treatment (Kruskal-Wallis p-value for patients <18 and patients ≥18 <0.0001). Patients <18 tended not to have had problems with mobility (Figure 2a) at any time during their PEx, but patients ≥18 reported having some problems with these on the index day, though they had improved by the end of treatment (Kruskal-Wallis test for difference in mean between index day and end of PEx treatment for patients <18=0.90; for patients ≥18 p<0.0001). Similarly, patients <18 had few problems with anxiety/depression at any time (p-value for Kruskal-Wallis test for difference in mean between index day and end of PEx treatment for patients <18=0.26) but patients ≥18 reported having some problems with anxiety/depression on the index day that improved by the end of treatment, but not statistically significantly (p-value for Kruskal-Wallis test for difference in mean between index day and end of PEx treatment for patients ≥18 =0.11; Figure 2e). Neither age category reported having problems with self-care at any point during their PEx (Figure 2b) and the EQ-5D-5L scores for self-care did not improve by statistically significant amounts in either age group over time (Kruskal-Wallis test for difference in mean between index day and end of PEx treatment for patients <18=0.25; for patients ≥18 p=0.14). Both age categories reported having problems performing usual activities and problems with pain and discomfort at the index day and these measures improved in both age categories by the end of treatment (Figures 2c–2d; Kruskal-Wallis test for difference in mean usual activity scores between index day and end of PEx treatment for patients <18 p=0.004; for patients ≥18 p<0.0001; for the difference in mean pain/discomfort scores between index day and end of PEx treatment for patients <18 p=0.01; for patients ≥18 p<0.0001). Finally, histograms of the summary EQ-5D-5L index scores over time are shown in Figure 3. Relative to patients ≥18, these tended to be higher (indicating better quality-of-life) at the index day in patients <18, and scores in both groups had improved by statistically significant amounts by the end of treatment (Kruskal-Wallis test for difference in mean between index day and end of PEx treatment for patients <18=0.006; for patients ≥18 p<0.0001).
Figure 1.
8-Question CFRSD-CRISS summary score histograms at index, day 7 and the end of PEx treatment, stratified by age.
Figure 3.
EQ-5D-5L summary score histograms at index, day 7 and the end of IV antibiotic treatment, stratified by age.
Figure 2a.
EQ-5D-5L: Problems walking (Mobility) histograms at index, day 7 and the end of IV antibiotic treatment, stratified by age.
Figure 2e.
EQ-5D-5L: Problems with anxiety or depression histograms at index, day 7 and the end of IV antibiotic treatment, stratified by age.
Figure 2b.
EQ-5D-5L: Problems washing or dressing yourself (Self-care) histograms at index, day 7 and the end of IV antibiotic treatment, stratified by age.
Figure 2c.
EQ-5D-5L: Problems with usual activities histograms at index, day 7 and the end of IV antibiotic treatment, stratified by age.
Figure 2d.
EQ-5D-5L: Problems with pain or discomfort histograms at index, day 7 and the end of IV antibiotic treatment, stratified by age.
In Figure 4, we show the ppFEV1 at the index day compared to the end of PEx treatment, stratified by age. This measure improved by statistically significant amounts in both age categories by the end of PEx treatment (Kruskal-Wallis test for difference in mean ppFEV1 between index day and end of PEx treatment for patients <18=0.03; for patients ≥18 p=0.003).
Figure 4.
ppFEV1 at the time of the index exacerbation versus at the end of treatment.
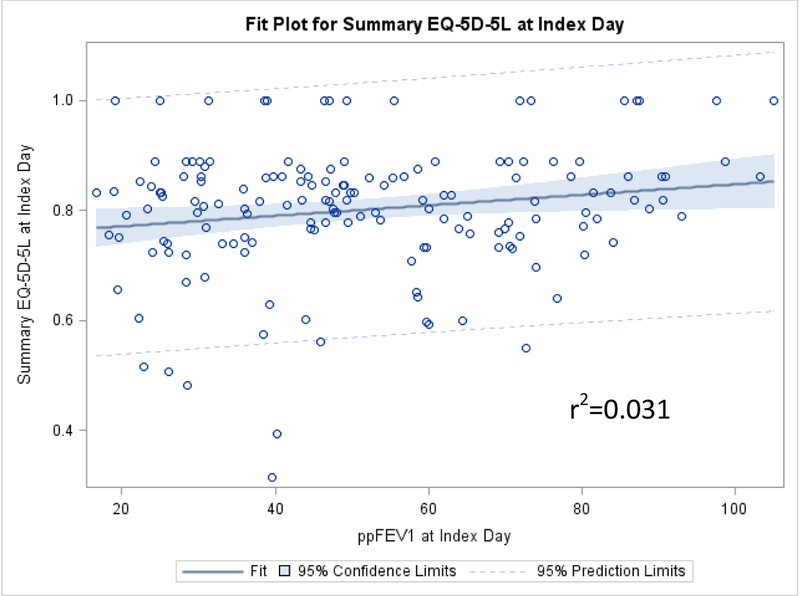
The scatter plots comparing the change in the summary EQ-5D-5L index score versus the change in ppFEV1 from the index day to the end of PEx treatment, as well as plots showing the comparisons of these measures on the index day and at the end of PEx treatment, are shown in Figures 5a–5c. The two variables were very poorly associated, with an r-squared values ranging from 0.010 to 0.031.
Figure 5a.
Scatter plot of the change in EQ-5D-5L summary scores versus the change in ppFEV1 between index and the end of PEx treatment
Figure 5c.
Scatter plot of the EQ-5D-5L and ppFEV1 at the end of PEx treatment.
We examined Spearman correlation coefficients between each of the elements of the EQ-5D-5L versus the elements of the CFRSD-CRISS at the three time points of interest in Tables 2a–2c. In general, correlations between the two measures were weak. At the index day, the EQ-5D-5L question about pain/discomfort was moderately correlated with the CFRSD-CRISS question about feeling tired among the cohort who were <18 (r=0.62). At the day 7 measurement, the EQ-5D-5L questions about usual activities and pain/discomfort were moderately correlated with the CFRSD-CRISS question about chest tightness (r=0.52 and r=0.51, respectively) and the EQ-5D-5L question about usual activities was also moderately correlated with the CFRSD-CRISS question about breathing (r=0.51) and wheezing (r=0.44) among the cohort who were <18. The CFRSD-CRISS question about breathing as also moderately correlated with the EQ-5D-5L questions about mobility (r=0.41) and self-care (r=0.45) among those <18. Also, at the day 7 measurement, the CFRSD-CRISS question about feeling tired was modestly correlated with the EQ-5D-5L questions about usual activities (r=0.47), pain/discomfort (r=0.43), and anxiety/depression (r=0.40) among the ≥18 cohort. We also observed a moderate correlation among those <18 at the third measurement at the end of PEx treatment between the EQ-5D-5L measurement of anxiety/depression and the CFRSD-CRISS assessment of feeling tired (r=0.52) and coughing (r=0.52). In cohort members >18 at the end of PEx treatment, we observed moderate correlation between the EQ-5D-5L measurement of mobility and the CFRSD-CRISS assessment of breathing (r=0.43). Those members also had moderate correlation between the EQ-5D-5L measurements of usual activities and pain/discomfort with the CFRSD-CRISS assessment of breathing (r=0.44 for both EQ-5D-5L measures) and feeling tired (r=0.40 and r=0.44, respectively).
Table 2a.
Spearman correlation coefficients for EQ-5D-5L domains versus CFRSD-CRISS questions on the patients’ 1st measurement on the index day.
| AGE <18 | ||||||||
|---|---|---|---|---|---|---|---|---|
| CFRSD-CRISS Breathing |
CFRSD-CRISS Feverish |
CFRSD-CRISS Tired |
CFRSD-CRISS Chills |
CFRSD-CRISS Cough |
CFRSD-CRISS Mucus Quantity |
CFRSD-CRISS Chest Tightness |
CFRSD-CRISS Wheezing |
|
| EQ-5D-5L Mobility |
0.18 | −0.10 | 0.33 | −0.02 | 0.11 | 0.04 | 0.28 | 0.03 |
| EQ-5D-5L Self-Care |
−0.05 | −0.10 | −0.05 | 0.19 | −0.07 | −0.11 | −0.02 | 0.01 |
| EQ-5D-5L Usual Activities |
0.49 | −0.17 | 0.33 | −0.10 | 0.22 | 0.23 | 0.42 | 0.36 |
| EQ-5D-5L Pain/ Discomfort |
0.34 | 0.20 | 0.62 | 0.20 | 0.32 | 0.02 | 0.42 | 0.41 |
| EQ-5D-5L Anxiety/ Depression | 0.02 | −0.22 | −0.06 | −0.09 | 0.34 | 0.37 | −0.03 | 0.12 |
| AGE ≥18 | ||||||||
| CFRSD-CRISS Breathing |
CFRSD-CRISS Feverish |
CFRSD-CRISS Tired |
CFRSD-CRISS Chills |
CFRSD-CRISS Cough |
CFRSD-CRISS Mucus Quantity |
CFRSD-CRISS Chest Tightness |
CFRSD-CRISS Wheezing |
|
| EQ-5D-5L Mobility |
0.44 | 0.20 | 0.18 | 0.21 | 0.16 | 0.01 | 0.31 | 0.12 |
| EQ-5D-5L Self-Care |
0.22 | 0.19 | 0.10 | 0.12 | 0.05 | 0.004 | 0.07 | 0.08 |
| EQ-5D-5L Usual Activities |
0.48 | 0.19 | 0.40 | 0.08 | 0.34 | 0.24 | 0.39 | 0.22 |
| EQ-5D-5L Pain/ Discomfort |
0.39 | 0.36 | 0.35 | 0.40 | 0.21 | 0.21 | 0.42 | 0.30 |
| EQ-5D-5L Anxiety/ Depression | 0.31 | 0.11 | 0.28 | 0.17 | 0.11 | 0.17 | 0.26 | 0.06 |
Table 2c.
Spearman correlation coefficients for EQ-5D-5L domains versus CFRSD-CRISS questions on the patients’ 3rd measurement at the end of PEx treatment.
| AGE <18 | ||||||||
|---|---|---|---|---|---|---|---|---|
| CFRSD-CRISS Breathing |
CFRSD-CRISS Feverish |
CFRSD-CRISS Tired |
CFRSD-CRISS Chills |
CFRSD-CRISS Cough |
CFRSD-CRISS Mucus Quantity |
CFRSD-CRISS Chest Tightness |
CFRSD-CRISS Wheezing |
|
| EQ-5D-5L Mobility |
0.16 | 0.33 | 0.06 | 0.24 | 0.08 | 0.01 | 0.12 | −0.10 |
| EQ-5D-5L Self-Care |
0.19 | −0.06 | −0.07 | −0.08 | −0.01 | −0.21 | −0.12 | −0.08 |
| EQ-5D-5L Usual Activities |
0.19 | 0.20 | 0.32 | 0.38 | 0.18 | −0.14 | 0.20 | 0.13 |
| EQ-5D-5L Pain/ Discomfort |
0.02 | 0.09 | 0.13 | 0.01 | 0.09 | 0.10 | 0.36 | 0 |
| EQ-5D-5L Anxiety/ Depression | 0.32 | −0.14 | 0.52 | 0.06 | 0.52 | 0.18 | 0.33 | 0.14 |
| AGE ≥18 | ||||||||
| CFRSD-CRISS Breathing |
CFRSD-CRISS Feverish |
CFRSD-CRISS Tired |
CFRSD-CRISS Chills |
CFRSD-CRISS Cough |
CFRSD-CRISS Mucus Quantity |
CFRSD-CRISS Chest Tightness |
CFRSD-CRISS Wheezing |
|
| EQ-5D-5L Mobility |
0.43 | 0.15 | 0.21 | 0.24 | 0.19 | 0.19 | 0.32 | 0.03 |
| EQ-5D-5L Self-Care |
0.23 | −0.05 | 0.23 | −0.10 | 0.11 | 0.12 | 0.08 | −0.01 |
| EQ-5D-5L Usual Activities |
0.44 | 0.11 | 0.40 | 0.17 | 0.13 | 0.26 | 0.23 | 0.15 |
| EQ-5D-5L Pain/ Discomfort |
0.44 | 0.23 | 0.44 | 0.27 | 0.34 | 0.27 | 0.31 | 0.32 |
| EQ-5D-5L Anxiety/ Depression | 0.32 | 0.23 | 0.33 | 0.22 | 0.16 | 0.21 | 0.21 | 0.16 |
Finally, the results of the 3 adjusted generalized linear mixed models examining the relationships between the change in EQ-5D-5L summary scores from the index day until the end of PEx treatment versus the changes in CFRSD-CRISS and ppFEV1 are shown in Table 3 We found that the change in CFRSD-CRISS and ppFEV1 were not strongly affected by the change in EQ-5D-5L summary scores, with parameter estimates ranging from −0.02 to 0.03.
Table 3.
Results of generalized linear mixed models of change in EQ-5D-5L summary scores versus change in summary Cystic Fibrosis Respiratory Symptom Diary-Chronic Respiratory Infection Symptom Score (CFRSD-CRISS), the change in percent predicted of forced expiratory volume in 1 second (ppFEV1), and the change in CFRSD-CRISS plus the change in ppFEV1, stratified by age.
| Age <18 (n=33) | Age ≥18 (n=136) | |
|---|---|---|
| Adjusted1 Estimate (95% CI) | Adjusted Estimate (95% CI) | |
| Model 1 | ||
| Change in CFRSD-CRISS | −0.002 (−0.006, 0.001) | −0.01 (−0.02, −0.0002) |
| Model 2 | ||
| Change in ppFEV1 | −0.02 (−0.05, −0.002) | 0.03 (0.008, 0.04) |
| Model 3 | ||
| Change in CFRSD-CRISS | −0.0003 (−0.03, 0.03) | −0.0004 (−0.02, 0.01) |
| Change in ppFEV1 | 0 (−0.05, 0.001) | 0.03 (0.0006, 0.04) |
All models adjusted for gender, ppFEV1 and CFRSD-CRISS on the index day, and whether the patient received gram positive, MRSA, anaerobic, or broad spectrum antibiotics for their PEx.
Discussion
We observed large improvements in the symptom-based questions of the CFRSD-CRISS and the EQ-5D-5L index over the course of PEx treatment, in addition to a clinically relevant and appropriate change in the ppFEV1. However, contrary to our hypothesis, we did not find significant correlations between the CFRSD-CRISS and EQ-5D-5L summary scores. We did find weak correlations between several of the individual items of the CFRSD- CRISS and EQ-5D, particularly among the cohort that was <18, but the correlations we observed were not strong enough to justify use of the 8 symptom-based CFRSD-CRISS questions as a preference-weighted measure of HRQoL for cost-effectiveness analysis.
Other studies have examined the EQ-5D as a measure of HRQoL in CF patients and have attempted to link this profile measure with disease-specific instruments. Solem and colleagues analyzed data from a multi-center randomized controlled trial of ivacaftor and assessed EQ-5D (the 3-level version of the questionnaire) every 8 weeks in CF patients for a year.17 They found that the EQ-5D-3L decreased proximal to a PEx, with decreased scores in usual activities, pain/discomfort, and anxiety/depression most frequently reported. We similarly found that patients in our study, especially those ≥18, reported difficulty with usual activities and pain/discomfort during their index PEx; however we observed fewer reports of problems with anxiety/depression. Others have evaluated the relationship between the EQ-5D-5L and the CFQ-R, the twelve-domain disease-specific instrument that was described above. Bradley and colleagues evaluated an observational cohort similar to ours in the United Kingdom and found a correlation coefficient of 0.72 between the CFQ-R and EQ-5D.10 These authors also reported that patients experiencing severe PEx were likely to report problems with usual activities and pain/discomfort, as we did, and their population also reported some problems with mobility, which we did not observe. A subsequent cross-sectional survey by Acaster et al. used regression mapping techniques with a sample of 401 patients in order to predict EQ-5D-3L scores adequately from the CFQ-R’s domains in a two-part model.9 These authors did not report results on the specific items of the EQ-5D-3L.
Prior research on the relationship between ppFEV1 and EQ-5D scores has been mixed, with some studies finding moderate correlations between the two17,26 and others finding minimal associations.27,28 We found that the ppFEV1 improvement was not well-correlated with the change in EQ-5D-5L over the course of treatment. A hypothesis for the weak link between ppFEV1 and HRQoL (as measured by the EQ-5D-5L, which is not specific to any particular disease) is that the severity of CF, as measured by ppFEV1, is less relevant to HRQoL than the symptoms and disruptions that accompany PEx.17,29 That is, CF patients have adapted to poor lung function because they have had it their entire lives and are accustomed to it. This lack of association also emphasizes the fact that CF symptoms alone are not necessarily correlated with function.
Limitations
Certain limitations exist that should be considered when interpreting our findings. First, the sample size available from the STOP study was relatively small considering the different instruments and changes over time, particularly since our results were stratified by age. It is possible, though unlikely, that a larger sample of CF patients may yield differences in the correlation between the outcome measures. Second, our analyses used data from an observational study that allowed PEx treatment to be carried out at the discretion of the treating clinicians. To the extent that treatment-specific differences in outcomes may exist, heterogeneity in patient outcomes may have attenuated our ability to detect associations. Also, the fact that we excluded patients who were missing assessments at certain time points may have affected our results. While we found few differences between those who were and were not missing data at all time points (Supplement), we cannot be certain that our findings would have been the same if we had had data on the full cohort. Finally, to minimize the burden on the CF patients undergoing PEx, we collected only 8 symptom-based items of the CFRSD-CRISS, rather than the full 16-item questionnaire which included much more detailed information on the timing and severity of CF symptoms. It is possible that a more detailed assessment of CF symptoms might have had stronger correlations with the EQ-5D-5L in our population.
Conclusion
We demonstrated mostly weak correlations, especially among patients who were ≥18 years, between the 8 symptom-based questions of the CFRSD-CRISS and the EQ-5D-5L in a population of CF patients who experienced PEx. We therefore conclude that the CFRSD-CRISS alone is not a meaningful way to assess quality-of-life in future cost-effectiveness studies among CF patients with pulmonary exacerbations. Future research should examine how well the EQ-5D-5L correlates to the full CFRSD-CRISS, which contains 16-items on CF symptoms, their timing, and their impact. In the meantime, other preference-weighted measures besides the EQ-5D-5L could be explored, such as the Short Form-6 Dimension (SF-6D),30 the Health Utilities Index Mark 3,31 or perhaps a CF-specific instrument that is preference-weighted.
Supplementary Material
Figure 5b.
Scatter plot of the EQ-5D-5L and ppFEV1 on the index day.
Table 2b.
Spearman correlation coefficients for EQ-5D-5L domains versus CFRSD-CRISS questions on the patients’ 2nd measurement 7 days after the index day.
| AGE <18 | ||||||||
|---|---|---|---|---|---|---|---|---|
| CFRSD-CRISS Breathing |
CFRSD-CRISS Feverish |
CFRSD-CRISS Tired |
CFRSD-CRISS Chills |
CFRSD-CRISS Cough |
CFRSD-CRISS Mucus Quantity |
CFRSD-CRISS Chest Tightness |
CFRSD-CRISS Wheezing |
|
| EQ-5D-5L Mobility |
0.41 | --* | 0.14 | −0.14 | 0.24 | 0.26 | 0.40 | 0.47 |
| EQ-5D-5L Self-Care |
0.45 | --* | 0.21 | 0.29 | 0.28 | 0 | 0.40 | 0.25 |
| EQ-5D-5L Usual Activities |
0.51 | --* | 0.11 | 0.12 | 0.22 | −0.02 | 0.52 | 0.44 |
| EQ-5D-5L Pain/ Discomfort |
0.38 | --* | 0.24 | 0.23 | 0.23 | 0.24 | 0.51 | 0.34 |
| EQ-5D-5L Anxiety/ Depression | 0.29 | --* | 0 | 0.02 | 0.28 | 0.18 | 0.37 | 0.29 |
| AGE ≥18 | ||||||||
| CFRSD-CRISS Breathing |
CFRSD-CRISS Feverish |
CFRSD-CRISS Tired |
CFRSD-CRISS Chills |
CFRSD-CRISS Cough |
CFRSD-CRISS Mucus Quantity |
CFRSD-CRISS Chest Tightness |
CFRSD-CRISS Wheezing |
|
| EQ-5D-5L Mobility |
0.37 | 0.07 | 0.31 | 0.08 | 0.22 | 0.18 | 0.31 | 0.02 |
| EQ-5D-5L Self-Care |
0.21 | 0.25 | 0.37 | 0.32 | 0.18 | 0.07 | 0.30 | 0.18 |
| EQ-5D-5L Usual Activities |
0.41 | 0.15 | 0.47 | 0.21 | 0.24 | 0.35 | 0.34 | 0.18 |
| EQ-5D-5L Pain/ Discomfort |
0.37 | 0.20 | 0.43 | 0.38 | 0.17 | 0.09 | 0.31 | 0.12 |
| EQ-5D-5L Anxiety/ Depression | 0.27 | 0.23 | 0.40 | 0.24 | 0.24 | 0.24 | 0.28 | 0.18 |
All patients <18 scored a 1, indicating that they did not feel feverish, so correlation coefficients could not be calculated.
Acknowledgements
Study concept and design: Drs. Patrick, Hansen, Goss, and Kessler
Acquisition, analysis, and interpretation of data: All authors
Drafting of the manuscript: Dr. Gold
Critical revision of the manuscript for important intellectual content: All authors
Statistical analysis: Drs. Gold and Hansen
Obtained funding: Dr. Kessler
Guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article: Dr. Gold
This work was supported by a Cystic Fibrosis Foundation Therapeutic (CFFT) award: “Cost Effectiveness Analysis and Comparative Effectiveness Research Component of STOP 2.” Award Number: KESSLE17AB0.
Abbreviation List
- CF
Cystic fibrosis
- US
United States
- PEx
Pulmonary exacerbations
- HRQoL
Health-related quality of life
- EQ-5D-5L
5-Level EuroQOL-5 Dimensions
- CFRSD-CRISS
Cystic Fibrosis Respiratory Symptom Diary- Chronic Respiratory Infection Symptom Score
- CFQ-R
Cystic Fibrosis Questionnaire-Revised
- ppFEV1
Percent predicted of forced expiratory volume in 1 second
- STOP
Standardized treatment of pulmonary exacerbations
- IV
Intravenous
Footnotes
Conflict of interest Statement: none
References
- 1.O’Sullivan BP, Freedman SD. Cystic fibrosis. Lancet. 2009;373(9678):1891–1904. [DOI] [PubMed] [Google Scholar]
- 2.Keogh RH, Stanojevic S. A guide to interpreting estimated median age of survival in cystic fibrosis patient registry reports. J Cyst Fibros. 2018;17(2):213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angelis A, Kanavos P, Lopez-Bastida J, et al. Social and economic costs and health-related quality of life in non-institutionalised patients with cystic fibrosis in the United Kingdom. BMC Health Serv Res. 2015;15:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson JA, Connolly M, Zuberbuhler P, Brown NE. Health-related quality of life for adults with cystic fibrosis: a regression approach to assessing the impact of recombinant human DNase. Pharmacotherapy. 2000;20(10):1167–1174. [DOI] [PubMed] [Google Scholar]
- 5.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–343. [DOI] [PubMed] [Google Scholar]
- 6.Norman R, Cronin P, Viney R, King M, Street D, Ratcliffe J. International comparisons in valuing EQ-5D health states: a review and analysis. Value Health. 2009;12(8):1194–1200. [DOI] [PubMed] [Google Scholar]
- 7.Payakachat N, Ali MM, Tilford JM. Can The EQ-5D Detect Meaningful Change? A Systematic Review. Pharmacoeconomics. 2015;33(11):1137–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goss CH, Edwards TC, Ramsey BW, Aitken ML, Patrick DL. Patient-reported respiratory symptoms in cystic fibrosis. J Cyst Fibros. 2009;8(4):245–252. [DOI] [PubMed] [Google Scholar]
- 9.Acaster S, Pinder B, Mukuria C, Copans A. Mapping the EQ-5D index from the cystic fibrosis questionnaire-revised using multiple modelling approaches. Health Qual Life Outcomes. 2015;13:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradley JM, Blume SW, Balp MM, Honeybourne D, Elborn JS. Quality of life and healthcare utilisation in cystic fibrosis: a multicentre study. Eur Respir J. 2013;41(3):571–577. [DOI] [PubMed] [Google Scholar]
- 11.Habib AR, Manji J, Wilcox PG, Javer AR, Buxton JA, Quon BS. A systematic review of factors associated with health-related quality of life in adolescents and adults with cystic fibrosis. Ann Am Thorac Soc. 2015;12(3):420–428. [DOI] [PubMed] [Google Scholar]
- 12.van Horck M, Winkens B, Wesseling G, et al. Factors associated with changes in health-related quality of life in children with cystic fibrosis during 1-year follow-up. Eur J Pediatr. 2017;176(8):1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tappenden P, Sadler S, Wildman M. An Early Health Economic Analysis of the Potential Cost Effectiveness of an Adherence Intervention to Improve Outcomes for Patients with Cystic Fibrosis. Pharmacoeconomics. 2017;35(6):647–659. [DOI] [PubMed] [Google Scholar]
- 14.Tappenden P, Harnan S, Uttley L, et al. The cost effectiveness of dry powder antibiotics for the treatment of Pseudomonas aeruginosa in patients with cystic fibrosis. Pharmacoeconomics. 2014;32(2):159–172. [DOI] [PubMed] [Google Scholar]
- 15.Schechter MS, Trueman D, Farquharson R, Higuchi K, Daines CL. Inhaled aztreonam lysine versus inhaled tobramycin in cystic fibrosis. An economic evaluation. Ann Am Thorac Soc. 2015;12(7):1030–1038. [DOI] [PubMed] [Google Scholar]
- 16.Sanders DB, Solomon GM, Beckett VV, et al. Standardized Treatment of Pulmonary Exacerbations (STOP) study: Observations at the initiation of intravenous antibiotics for cystic fibrosis pulmonary exacerbations. J Cyst Fibros. 2017;16(5):592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solem CT, Vera-Llonch M, Liu S, Botteman M, Lin FJ, Castiglione B. Impact of Pulmonary Exacerbations On Eq-5d Measures In Patients With Cystic Fibrosis. Value Health. 2014;17(7):A535. [DOI] [PubMed] [Google Scholar]
- 18.West NE, Beckett VV, Jain R, et al. Standardized Treatment of Pulmonary Exacerbations (STOP) study: Physician treatment practices and outcomes for individuals with cystic fibrosis with pulmonary Exacerbations. J Cyst Fibros. 2017;16(5):600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.EuroQol Research Foundation. EQ-5D. 2017; 2017. Available at: https://euroqol.org/. Accessed December 15
- 20.Agency for Healthcare Research and Quality. Calculating the U.S. Population-based EQ-5D™ Index Score. 2005; https://archive.ahrq.gov/professionals/clinicians-providers/resources/rice/EQ5Dscore.html. Accessed Oct 1, 2017.
- 21.University of Washington. SEAQOL Instruments. 2017; http://depts.washington.edu/seaqol/instruments. Accessed January 11, 2018.
- 22.Stanojevic S GLI-2012 SAS Macro. 2013; http://www.ers-education.org/guidelines/global-lung-function-initiative/spirometry-tools/sas-macro.aspx. Accessed Oct 1, 2017. [Google Scholar]
- 23.Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res. 2005;14(6):1523–1532. [DOI] [PubMed] [Google Scholar]
- 24.VanDevanter DR, Heltshe SL, Spahr J, et al. Rationalizing endpoints for prospective studies of pulmonary exacerbation treatment response in cystic fibrosis. J Cyst Fibros. 2017;16(5):607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearson K Determination of the Coefficient of Correlation. Science. 1909;30(757):23–25. [DOI] [PubMed] [Google Scholar]
- 26.Hong JY, Lee SA, Kim SY, et al. Factors associated with quality of life measured by EQ-5D in patients with nontuberculous mycobacterial pulmonary disease. Qual Life Res. 2014;23(10):2735–2741. [DOI] [PubMed] [Google Scholar]
- 27.Aburuz S, Gamble J, Heaney LG. Assessment of impairment in health-related quality of life in patients with difficult asthma: psychometric performance of the Asthma Quality of Life Questionnaire. Respirology. 2007;12(2):227–233. [DOI] [PubMed] [Google Scholar]
- 28.Gee L, Abbott J, Hart A, Conway SP, Etherington C, Webb AK. Associations between clinical variables and quality of life in adults with cystic fibrosis. J Cyst Fibros. 2005;4(1):59–66. [DOI] [PubMed] [Google Scholar]
- 29.Britto MT, Kotagal UR, Hornung RW, Atherton HD, Tsevat J, Wilmott RW. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest. 2002;121(1):64–72. [DOI] [PubMed] [Google Scholar]
- 30.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21(2):271–292. [DOI] [PubMed] [Google Scholar]
- 31.Feeny D, Furlong W, Torrance GW, et al. Multiattribute and single-attribute utility functions for the health utilities index mark 3 system. Med Care. 2002;40(2):113–128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.