Abstract
This study tested the hypothesis that bioactive glass used for implant coatings enhance the expression of key osteoblast-specific markers associated with osteoblast progenitor differentiation. The ions from experimental bioactive glass (6P53-b) and commercial Bioglass (45S5) were added to osteoblast progenitor (MC3T3-E1.4) cultures as a supplemented ion extract (glass conditioned medium (GCM)). Ion extracts (Si: 47.9 ± 10.4 ppm, Ca: 69.8 ± 14.0 for 45S5; Si: 33.4 ± 3.8 ppm, Ca: 57.1 ± 2.8 ppm for 6P53-b) and control extract (Si: < 0.1 ppm, Ca: 49.0 ppm in α-MEM) were supplemented (10%FBS, 1% pen-strep) and added to pre-osteoblast (MC3T3-E1.4) cultures. Cell proliferation rate was enhanced (150% of control) within the first three days after adding 45S5 and 6P53-b GCM. For differentiating cultures (GCM or control medium + ascorbic acid (50 mg L−1)), osteocalcin protein expression increased to 40×−70× control after 10 days exposure to GCM with corresponding expression level increases at the gene level (day 3, 45S5 GCM: 14× control; day 5, 6P53-b GCM: 19× control). Runx2 levels were approximately 2× control after 7 days exposure to GCM. Finally, collagen type 1 expression was enhanced after 1 day of exposure (Col1α1, 45S5 GCM: 3x of control; 6P53-b GCM: 4× control) and on through the course of differentiation (day 5, Col1α2, 45S5 GCM: 3.15× control; 6P53-b GCM: 2.35× control)
Keywords: bioactive glass ions, osteogenesis, osteoblasts, silicon, calcium
INTRODUCTION
Implants are used in dental applications to replace missing teeth or in orthopaedic and craniofacial applications to replace lost bone. These implants must restore the physiological structure and function while also facilitating complete bone apposition [1]. Currently, Ti implants are used successfully for tooth replacement in mandibular or maxillary bone (86% and 76%, respectively [2]) and nearly 93% for bone replacement in the cranium [3]. However, faster osteointegration and improvements in implant success rate and longevity are still desired.
Various coatings technologies to improve the implant-bone interface have been attempted. Hydroxyapatite (HA) was used for a number of years, however, loss of HA occurs from the implant-ceramic interface [4] or bone-ceramic interface [5]. Commerical Bioglass™ (Hench and colleagues [6–11]). is widely known for its benefits in various bone substitutes [12] and periodontal procedures [13], however, it is difficult to use as an implant coating because it cracks at the Ti-glass interface when cyclically loaded [14]. Both hydroxyapatite and Bioglass cracking is due primarily to a large thermal expansion mismatch with Ti [14]
Improvement in glass coating technology led to the development of a family of bioactive glasses (50–59 wt.% SiO2) to enhance the osteointegration potential of Ti (E. Saiz and A. P. Tomsia and colleagues [14–26]) or other applications involving polymer-bioactive glass composites to build scaffolds in hard and soft tissue engineering [27]. By doping the glass with additional constituents and partial substitution of CaO with MgO and Na2O with K2O (Table 1, 6P53-b vs. 45S5), improved adhesion to Ti alloy was achieved during the coating process. In previous in vitro studies, these glasses facilitated direct mineralized tissue attachment (as compared to mechanical attachment of mineralized tissue to Ti surfaces [25]). The bioactive glass coating forms a hydroxyapatite (HA) surface layer which facilitates the direct bond to bone [25, 28].
Table 1.
Composition (wt. %) of Bioglass (45S5) and experimental bioactive glass (6P53-b)
| SiO2 | Na2O | K2O | MgO | CaO | P2O5 | |
|---|---|---|---|---|---|---|
| 45S5 (Mo-Sci) | 45.0 | 24.5 | 24.5 | 6.0 | ||
| 6P53-b (LBL) | 52.7 | 10.3 | 2.8 | 10.2 | 18.0 | 6.0 |
In general, the surface of the glass material and its corrosion behavior in the physiological environment influence its apposition to bone. In this study, we focus on the corrosion of ionic products, isolated from the bioactive glass surface, as they influence osteoblast behavior. This procedure will be to separate the effect of ions released during in vitro dissolution from the bioactive glass surface texture changes that occur.
Interestingly, recent studies have shown that ions from bioactive glass dissolution influence osteoblast intracellular and extracellular marker expression. Hench and colleagues have suggested that these ions play an active role in osteoblast behavior in that they alter the expression of osteoblast differentiation markers associated with bone matrix formation [29–38], while other studies have focused on individual ion effects on osteoblast function [39–43]. For example, commercial Bioglass in several studies was shown to influence Runx2 and osteocalcin expression [44], which are expressed within the first 10 days of the differentiation compartment of the osteoblast progentior cell cycle. Their expression indicates the maturation of the osteoblast progenitor to the mineralizing phenotype. Furthermore, Foppiano et al. [45] found that these dissolution products promoted the up-regulation of Runx2. Yet, no significant effort has been attempted to connect the enhanced gene expression (within 7 days) as it impacts downstream matrix protein expression (within 10 days) by osteoblasts under the influence of these bioactive glass corrosion products. To determine the effect of these ions on osteoblasts, a materials extract was isolated as a dissolved product from the bioactive glass surface.
In another study [46], we attempted to separate the role of the surface in contrast to the dissolved ion products. We found the initial in vitro dissolution of the glass was rapid, which increased the pH of the in vitro environment to which osteolast progenitors were exposed. This increased pH appeared to decrease the amount of cell proliferation and alkaline phosphatase activity. For these reasons, we pre-soaked the glass materials in simulated body fluid for a period of 10 days. This initial soaking period was seen to stabilize the in vitro pH (7.0–7.4) to near physiological [46]. The enhanced marker expression observed by Foppiano et al. [45] in fact occurred when the bioactive glass was pre-soaked in vitro.
In this study, we test the hypothesis that bioactive glass ions enhance osteoblast differentiation. The goals of this study are to (1) demonstrate that specific osteoblast markers are enhanced in the presence of bioactive coating glass (6P53-nb) ions; (2) demonstrate that similar osteoblast behavior is observed for commercially available Bioglass™ (45S5) ions; and (3) determine a direct correlation between gene and matrix protein expression.
The key osteogenic markers to be investigated here are collagen type 1 alpha 1 (Col1α1, Col1α2), core binding factor a (Cbfa1/Runx2), alkaline phosphatase (ALP), and osteocalcin. Collagen type 1 forms the biological support to which mineralized tissue binds for bone formation [47–50]. Runx2 is a key transcription factor associated with early expression of the osteoblast phenotype [51]. Alkaline phosphatase is a key dephosphorylating enzyme expressed by osteoblasts to turnover expressed collagen into a form that is amenable for bone matrix formation [41, 47–50]. Osteocalcin is a key non-collageneous protein that binds extracellular calcium to bone matrix [52].
MATERIALS AND METHODS
Study Design
Glass conditioned media of 6P53-b, 45S5 and control were prepared and measured for their ion concentrations by inductively coupled plasma mass spectrometry (ICP-MS). The glass conditioned media and control were then added to pre-osteoblast cells (MC3T3-E1.4) seeded in multi-well plates, and studied for their influence on osteoblast proliferation and differentiation. Differentiation studies were evaluated using protein assays and quantitative polymer chain reaction (qPCR) to quantify the level of expressed genes associated with osteogenesis.
Specimen and Media Preparation
Preparation of experimental bioactive coating glass (6P53-b, Table 1) was performed as described previously [25]. In brief, 6P53-b powders were commercially purchased (SEM-COM, Toledo, OH), placed into a Pt crucible, and melted in air in a Pt crucible for 5 h between 1400oC and 1500oC. The melt was cast in a pre-heated (200oC) graphite mold yielding glass bars and annealed (500oC, 6 h). The annealed bars were then cut into square samples (~ 1 × 1 × 0.2 cm) with a low speed diamond saw (Isomet, Buehler, Ltd., Lake Bluff, IL) and exposed to gamma radiation, to complete sterilization. All glass specimens (45S5 and 6P53-b) were then soaked for 10 d prior to study in vitro.
Bioactive glass conditioned media were prepared by soaking glass specimens in α-MEM for an additional 2 d (volume:surface area = 3 mL: per?? cm2), and was recovered as an ion extract. Samplings of this ion extract and control (α-MEM) were analyzed using ICP-MS. The ion extract (0.2 µm filter-sterilized) was supplemented (10% FBS, 1% pen-strep, final concentration) to make glass conditioned medium (GCM), with the reported concentrations of ions within the GCM at 89% of the concentrations determined from ICP-MS. Control medium was prepared by supplementing un-conditioned α-MEM, with 10% FBS and 1% pen-strep (final concentration). The above media preparation was used for proliferation studies while further supplementation of glass-conditioned and control medium with ascorbic acid (50 μg/mL, final concentration) was used for differentiation studies.
ICP-MS
Ion extracts and control were collected from as follows. Three each of 45S5 and 6P53-b glass specimens were used to make ion extracts for analysis. In addition, three separate control media containers were samples for analysis (error less than 1%). The collected ion extract and control samples were analyzed using ICP-MS (University of California at Davis Center for Plasma Mass Spectrometry). ICP-MS analysis was performed by introducing ion extract aliquots?? samples into a mass spectrometer (250 amu 7500a, Agilent Technologies, Palo Alto, CA). Solutions were sprayed through a high solid type nebulizer as a plasma into a thermoelectrically controlled spray chamber. This instrument has four mass-flow controllers (Plasma, Auxiliary, carrier gas lines) and also contains a 27.12 MHz solid state (1600 W) ICP source on a three stage vacuum system. An ion optic system (Omega II off-axis lens system) was used to direct the plasma through the quadrupole mass filter (hyperbolic cross-sectional rod system, 3 MHz) and onto the electron multiplier detector. Data acquisition was performed using a simultaneous dual-mode electron multiplier (nine order dynamic range, 100 µs dwell time). Results of analysis were then compared to a standard for identification and quantitation of ion concentration. The average and standard deviation of the ion concentrations per set of samples was reported.
Cell Culture
Glass conditioned media (2 mL) were used to treat mouse pre-osteoblast (MC3T3-E1.4) cultures. This cell line is derived from mouse calvaria and expresses the osteoblast phenotype when differentiated. The cells were cultured in 150 cm2 flasks (passage 25–30) prior to seeding(50 000 cells cm −2) in 6-well tissue culture plastic plates and incubated overnight. After the cell line doubling time (12–16 h), these cells were synchronized (α-MEM, 1% FBS, 1% pen-strep) for an additional 48 h. The media was then replaced with the glass conditioned media (6P53-b and 45S5) or control medium for proliferation studies. For differentiation studies, additional supplementation of ascorbic acid (50 μg mL −1) was used. For both studies, media changes were made every 48 h.
Cell Proliferation Assay
Cell proliferation was assayed using the MTS Assay (Promega, Madison, WI). This assay is derived from the MTT assay, which measures the mitochondrial activity of living cells by measuring the turnover of tetrazolium dye reagent by cells into a formazen product. The color of the formazen product was measured using a spectrophotometer (SpectraMax Plus, Molecular Devices, San Jose, CA) and quantified based on a calibration curve for formazan absorbtion. Cells were also assayed to determine if some proliferation may occur during differentiation. To perform this experiment, the cells were allowed to remain in their respective media (GCM and control medium) and ascorbic acid was added to the cultures. Cells were then assayed using the MTS protocol to determine cell number, as mentioned above. The cell population was then recorded as a cell density (cells cm−2). All experiments were conducted in triplicate.
Protein Expression Assays.
At the desired time point of assay, cell cultures were collected in lysis buffer (CelLytic-M, Sigma Co., St. Louis, MO) and lysed according to the manufacturer’s protocol. Total protein quantification (BCA protocol, Pierce Biotechnology, Rockford Illinois) was conducted for all samples and normalized to the same total protein concentration prior to assay.
The concentration of alkaline phosphatase (Procedure 104) and osteocalcin (Mouse Osteocalcin EIA Kit, Biomedical Technologies Inc., Stoughton, MA) were measured. Adjustment of Procedure 104 was performed by reaction of sample alkaline phosphatase with pre-incubated (37oC) nitrophenyl phosphate (0.4% w:v) and amino-2-methyl-1-propanol (1.5 M, pH = 10). The reaction was then stopped using 0.02 N NaOH. The reaction produces inorganic phosphate and nitrophenol [53], which produces a yellow color and its intensity is assayed using a spectraphotometer [53].
Expression of these proteins was assayed at intervals of 1,3, 5, 6, and 10 days. The first week of time points was compared with gene expression during this same time period. The last time point was chosen to examine a long-term effect of GCM on osteocalcin and alkaline phosphatase production. All experiments were conducted in triplicate and samples were assayed twice for confirmation. All results of analyzed proteins were normalized to total protein concentration and relative to control.
qRT-PCR
To quantify levels of gene expression, qRT-PCR was used. Cells were cultured as described above and pelleted at the selected time point in the experiment and mRNA was extracted (RNeasy, Quiagen, Valencia, CA). Extracts were converted to cDNA using reverse transcriptase (Reverse Transcription System, Promega, Madison, WI) according to the manufacturer’s protocol. Absorbance (A) measurements of mRNA and cDNA samples were performed using a full-spectrum UV/Vis nanodrop volume analyzer (ND-1000, Nanodrop Technologies, Wilmington, Delaware) to quantify total cDNA concentration (A260) and quality (A260/A280). The measurement of nucleic acid absorbance at A260/A280 is used to assess the purity of nucleic acids after retrieval from cell and tissue cultures. The purity range used for this work was between 1.8–2.0, which is recommended from the manufacturer’s protocol. All cDNA samples were diluted to the same concentration (100–1000 ng μl −1),
For quantitative PCR, samples were mixed with reagents for PCR as follows (final concentration): cDNA sample (10%), FastStart Taqman Master Mix (Rox, 2x, 50%) (Roche Applied Sciences, Mannheim, Germany), forward primer (900 nM), reverse primer (900 nM), and hydrolysis probe (250 nM) mixture (Table 2) (10%), and PCR grade water (company, city, state,?? 30%). Sample reaction was performed using a real time PCR machine (ABI7500, Applied Biosystems inc., Foster City, CA).
Table 2.
Gene Expression Assay Primers and Probes (from ABI)
| gene | Accession number | Gene Bank mRNAs | Amplicon length |
|---|---|---|---|
| GAPDH | NM_008084.2 | 31 | 107 |
| AKP2 | NM_007431.1 | 8 | 90 |
| OCN | NM_031368.3 | 6 | 89 |
| RUNX2 | NM_009820.2 | 2 | 115 |
| COL1α2 | NM_00773.2 | 18 | 140 |
All amplification was compared to a control gene (glyceraldehyde 3-phosphate dehydrogenase or GAPDH) for relative quantification. Data acquisition was performed using the Applied Biosystems 7500 thermal cycler software. Threshold cycle (CT) was determined using a four parameter sigmoidal fit of the amplification curve (Qiu et al. [54]) and relative expression was determined using the delta CT method. Genes to be studied are given below. Gene expression was studied within the first 7 days of differentiation because this period marks the early expression of the osteoblast phenotype. All experiments were conducted in triplicate and all assays were repeated twice for confirmation of results.
Statistics
Data analysis was conducted to determine statistical significance (p < 0.05). two-way ANOVA testing was used to compare the treatments (45S5 GCM, 6P53-b GCM, control) and temporal effects on MC3T3-E1.4 cells. For gene-related studies, genes expressed as a result of exposure of cells to different treatments were reported relative to the control treatment (100%).
RESULTS
Ion concentrations in the cell culture media changed as a result of bioactive glass dissolution in vitro (without addition of pen-strep and FBS). Results (Table 3) of ICP-MS analysis showed (1) increased concentrations of Si and Ca for both glasses and (2) Na, K, Mg and phosphate concentrations were relatively close or slightly decreased relative to α-MEM.
Table 3.
Ion Extract Concentration (mg L −1)
| Si4+ | Na+ | K+ | Ca2+ | Mg2+ | P043− | |
|---|---|---|---|---|---|---|
| α-MEM | - | 4300 | 325.0 | 49.0 | 37.6 | 28.8 |
| 45S5+ α-MEM | 47.9 ± 10.4 | 4168 ± 404 | 299.8 ± 26.6 | 69.8 ± 14.0 | 27.8 ± 2.5 | 23.6 ± 4.7 |
| 6P53b+ α-MEM | 33.4 ± 3.8 | 4458 ± 186 | 298.3 ± 34.9 | 57.1 ± 2.8 | 32.7 ± 5.4 | 32.6 ± 9.3 |
Direct exposure of glass conditioned media (GCM) enhanced the proliferation rate of pre-osteoblasts. Results (Figure 1) of proliferation experiments showed increased numbers of cells in both glass conditioned media within 24–72 h (after cell synchronization (t = 0 days), p < 0.05). For example, 1 day after treatment, cell numbers were significantly higher in 45S5 and 6P53-b GCM (120% and 130% of control, respectively) and a similar result was seen on day 3 (140% and 150% of control, respectively). Significant differences were not observed after 72, h with an observed plateau of the cell density after 7 days in culture for all treatments. (probably due to limited MTS reagent exposure to overcrowded cell layers within the well plate).
Figure 1.
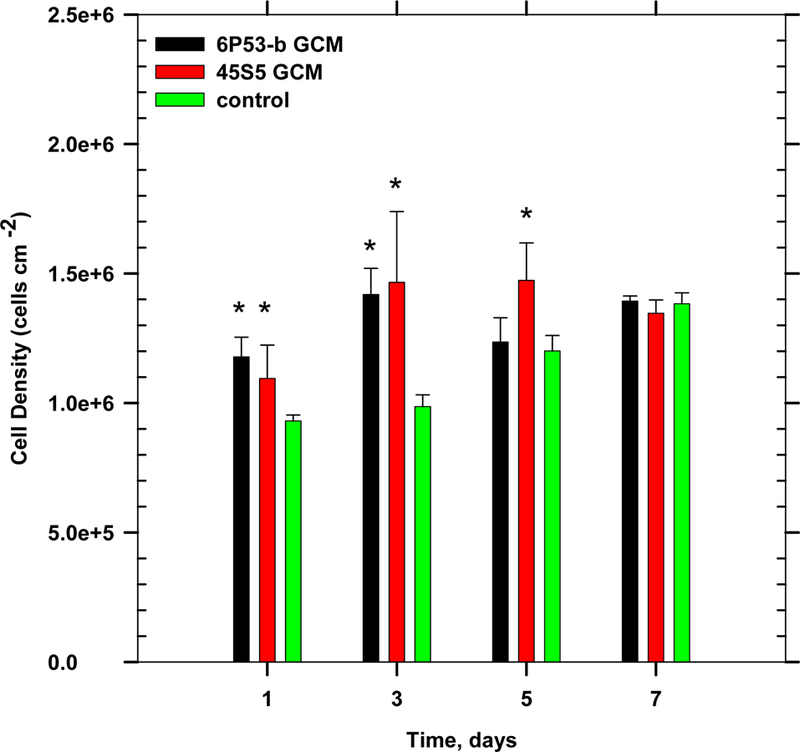
Effect of glass conditioned media on osteoblast progenitor proliferation (ANOVA, p < 0.05, * denotes statistical significance).
Bioactive glass corrosion products enhanced the expression of several key osteogenic markers during MC3T3-E1.4 differentiation. Alkaline phosphatase expression in GCM-treated cells was significantly increased. For example, after 10 days of exposure, a 2× increase (relative to control) in alkaline phosphatase expression was seen in GCM-treated cells as compared to control (Figure 2). However, on earlier days of exposure there was no significant enhanced expression for alkaline phosphatase.
Figure 2.
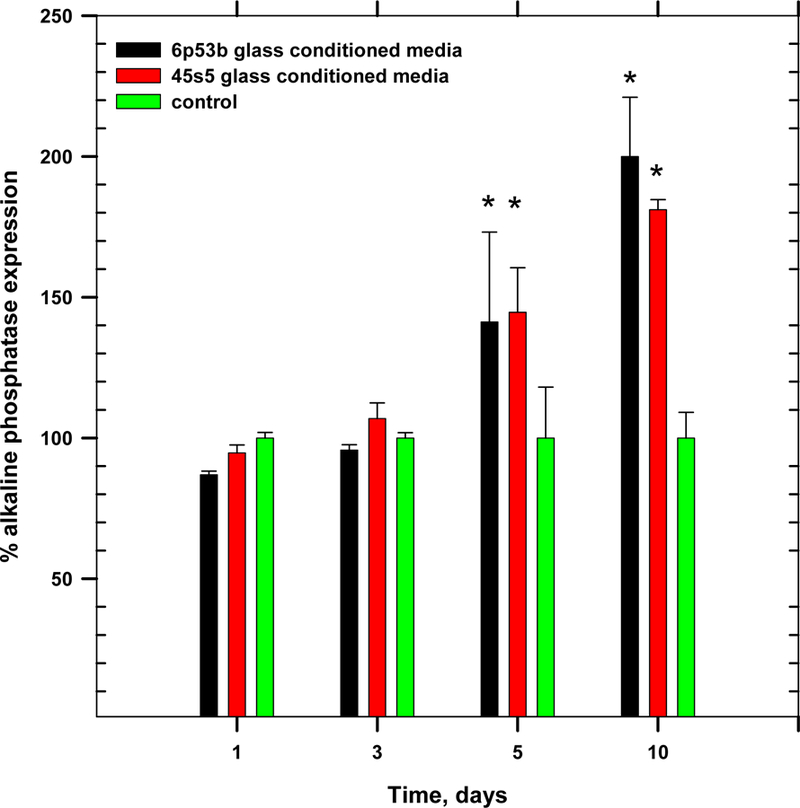
Effect of glass conditioned media on osteoblast progenitor alkaline phosphatase expression (ANOVA, p < 0.05, * denotes statistical significance)
GCM treatment also enhanced osteocalcin expression. For example, the expression of osteocalcin peaked (Figure 3) for cells treated with ascorbic acid and 6P53-b GCM (40× control after 10 days) and 45S5 GCM (70× control after 6 days). This result is markedly higher than the result obtained in a separate control study (results not shown). In that study, MC3T3-El.4 cells were cultured in control media and ascorbic acid treatment. After 21 days, osteocalcin expression was maximally expressed (30 ng μL−1), which was approximately 1.5× higher than the measured osteocalcin expression after 10 days of control media and ascorbic acid treatment in this work (Figure 3).
Figure 3.

Osteocalcin expression over 10 days of differentiation for cells cultured in the presence of GCM and control media (ANOVA, p < 0.05, * denotes statistical significance).
This significantly increased protein expression of osteocalcin correlated with the gene expression of osteocalcin within 7 days of treatment. While MC3T3-E1.4 cells differentiated, osteocalcin gene expression reached a maximum after 5 days in 6P53-b GCM (14× control) and after 3 days in 45S5 GCM (19× control) (Figure 4). These significant increases in osteocalcin gene and protein expression indicate the enhanced effect of GCM on differentiating osteoblasts.
Figure 4.

Osteocalcin gene expression in the presence of GCM and control treated cell cultures over 7 days during the course of differentiation (ANOVA, p < 0.05, * denotes statistical significance).
It is well-known that collagen expression and AA treatment are pre-requisites for osteocalcin and alkaline phosphatase expression [47, 48, 55]. Collagen type 1 expression (Col1α1 and Col1α2) was enhanced in GCM-treated cultures as compared to control cultures. For example, Col1α1 was maximally increased after 1 day of exposure to GCM (45S5: 4× control; 6P53-b: 3× control, Figure 5), while Col1α2 expression was maximally increased after 5 days of GCM exposure (45S5: 3.15× control; 6P53-b: 2.35× control, Figure 6). Besides the maximal expression just mentioned, overall collagen type 1 expression was observed throughout differentiation. The increased expression of collagen type 1 probably contributed to the enhanced osteocalcin expression.
Figure 5.

Collagen type 1 alpha 1 expression in the presence of GCm and control treated cells (ANOVA, p < 0.05, * denotes statistical significance).
Figure 6.
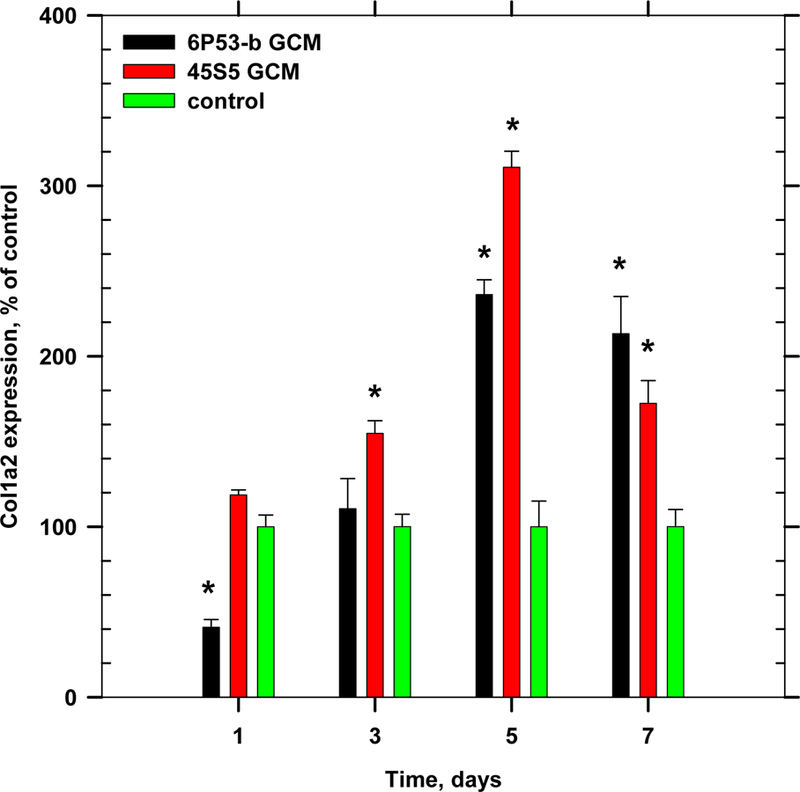
Gene expression of collagen type 1 (col1α2) during time course of differentiation (ANOVA, p < 0.05, * denotes statistical significance).
GCM exposure also enhanced Runx2 expression. Runx2, an early transcription factor expressed by osteoblasts during early differentiation [56], was observed to be 2× control treated cells (Figure 7).
Figure 7.
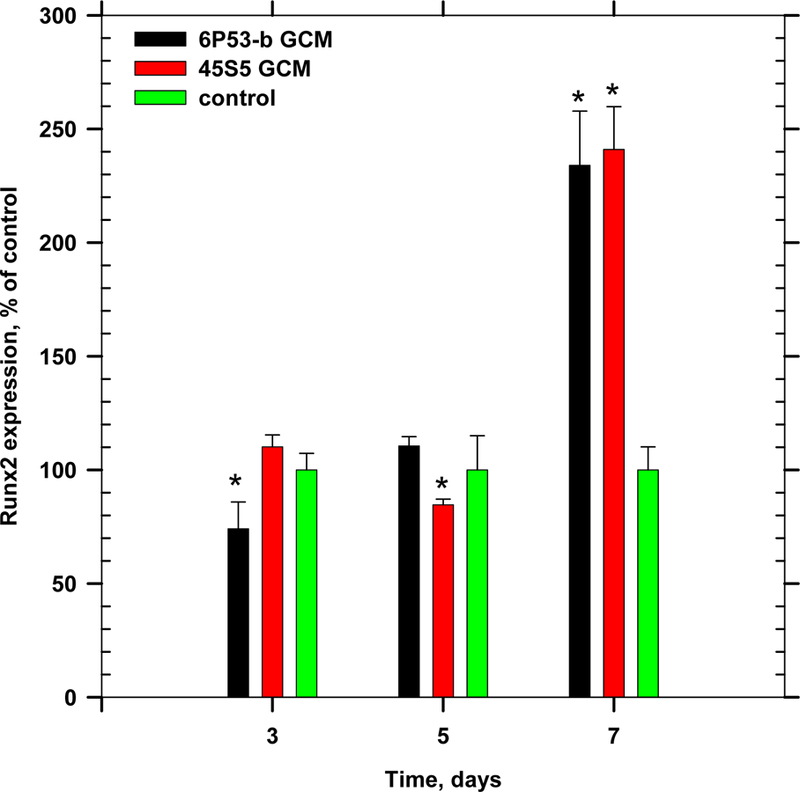
Gene expression of Runx2 during time course of differentiation (ANOVA, p < 0.05, * denotes statistical significance).
DISCUSSION
This study tested the hypothesis that bioactive coating glass ions enhanced the expression of genes and associated proteins that are related to osteoblast differentiation and production of bone extra cellular matrix (ECM). The results from this study indicate that osteoblast behavior was enhanced by these ions derived from both bioactive glasses.
The dissolution of bioactive glass 6P53-b in α-MEM was found to be similar to that of Bioglass [10]. The pre-soaking period of 10 d in vitro was intended to prevent MC3T3-E1.4 cultures from incidental exposure to higher than physiological in vitro pH [28, 46]. Without this pre-soaking period, the GCM takes on a basic pH, which we found decreased alkaline phosphatase expression [46]. Subsequent soaking of the glass in α-MEM yielded increased Si and Ca concentrations, indicating that the HA layer formed during initial soaking period does not totally block further ion release and is probably also porous. In a similar glass system (57 wt.% SiO2), Saiz et al. [14] reported continued silicon and calcium dissolution over a 30 day period in vitro. They found that after 10 days of pre-soaking in SBF, Ca2+ concentrations increased (15–20 ppm higher than SBF Ca concentration after 8 days of soaking) and Si4+ concentrations increased (40–50 ppm higher than SBF Si concentration after 8 days of soaking) [14]. Dissolution over an additional 2 day soak in a-MEM (to make GCM) showed continued dissolution of Si and Ca, consistent with the observed trend reported by Saiz et al. [14].
Previously, Foppiano et al. [45] found that collagen type 1 (Col1α1) was expressed at a lower level (80% of control) after 7 days of exposure of MC3T3-E1.4 cells to GCM and ascorbic acid. Under similar conditions in this work, Col1α1 was 1.4× control in 6P53-b GCM and 0.9× control in 45S5 GCM. Considering that our results show markedly higher expression level increases (> 2× control), the small changes in expression may not greatly influence osteoblast behavior. Thus, a 20% decrease or increase in relative expression may not appreciably alter downstream matrix production.
Furthermore, Foppiano et al. [45] found that increased expression of Runx2 (2× control) was observed for cells cultured in a similar glass system after 7 d exposure of MC3T3-E1.4 cells in the presence of bioactive glass corrosion products. This was in agreement with the present study that showed Runx2 expression was increased compared with controls for both bioactive glasses. The increased expression of Runx2 and collagen type 1 probably increased expression of osteocalcin and alkaline phosphatase beyond the initial 5 day treatment.
Increased levels of osteocalcin were observed as a result of osteoblast exposure to GCM. The expression of osteocalcin occurs as a direct result of the expression of collagen type 1 and Runx2 [52, 56], Osteocalcin is a critical Ca binding protein for bone ECM development and marks terminal differentiation, at which point osteoblasts begin to form calcified tissue. The increased level of osteocalcin was significant after 5–6 days of GCM exposure, which coincided with Runx2 and collagen type 1 expression in MC3T3-E1.4 cells. Expression of Runx2 mRNA was seen after 5–7 days in culture. Thus, the observed enhanced behavior of osteoblasts in the presence of GCM coincides with the well-known temporal mechanisms of expressed Runx2, collagen type 1, and osteocalcin in osteoblast progenitors.
The timeline of events that occurs with these osteoblasts was reviewed by Xiao et al. [57]. In their explanation, they note that osteoblasts must be in contact with a collagen-containing matrix. Our results show that collagen type 1 expression is maximally expressed after 1 day in culture, consistent with the work of Quarles et al. [50] and Franceschi et al. [47–49, 58, 59], and suggests that osteoblasts must express collagen type 1 in the absence of an existing collagen type 1 containing ECM. The binding of the osteoblast to ECM collagen type 1 is α2β1 integrin mediated. Integrin binding at the osteoblast membrane induces the expression of mitogen-activated protein kinases (MAPKs) that transduce signals to the osteoblast nucleus. These MAPKs phosphorylate and activate Runx2 and alkaline phosphatase, which then binds to the promoter region of downstream genes such as osteocalcin.
Considering the mechanism above and the results in this work showing an enhanced effect on osteoblast differentiation, two inferences can be made. First, the earlier collagen is expressed or the faster the attachment of the osteoblast to collagen type 1 in the ECM starts the temporal cascade of events that lead to other downstream markers. Second, these ions may catalyze the signal pathways in the osteoblast by simply activating cell receptors (such as the integrins mentioned above) that mediate the signal pathways therein to the nucleus. Finally, inhomogeneity in the data (e.g. osteocalcin protein (Figure 3) and gene expression (Figure 4)) could be due to the regulation of these ions across the plasma membrane or nuclear membrane that can then alter these signaling pathways.
The enhanced behavior of pre-osteoblasts in the presence of GCM is probably due to the increased Ca and Si media concentrations. On one hand, the role of Ca is well-known on osteoblasts as evident by the vast array of commercially available dietary supplements and pharmaceuticals used to treat patients with low bone density or osteoporosis. Maeno et al. [39] found that increased Ca concentrations (8 mM higher than that of α-MEM Ca concentration) also enhanced the expression of osteocalcin (2× control) in primary mouse osteoblast monolayer cultures. In our work, the relative increased Ca concentrations for 6P53-b and 45S5 GCM were 8 and 20 ppm (0.2 and 0.5 mM, respectively) and may have led, in part, to increased levels of osteocalcin. The increased Ca concentration may trigger mechanisms in which MC3T3-E1 cells respond via a calcium receptor [60–63].
On the other hand, the role of Si on osteoblasts is still somewhat uncertain. In our work, increased Si concentrations for 6P53-b and 45S5 GCM (1.2 mM and 1.7 mM, respectively) may have had a greater impact on the observed enhanced osteocalcin expression, Runx2, and collagen expression. In MG-63 osteosarcoma cultures, Reffitt et al. [40] found that increased Si ion concentrations (10–50 μM, 3 d after cell confluence) increased the relative expression of collagen type 1 (180% of control), alkaline phosphatase (150% of control) and osteocalcin (150% of control). Further studies or evidence [64] is lacking in showing a direct effect of Si on osteoblasts and will be the focus of future work.
This work showed for the first time a direct link between GCM treatment and osteocalcin production. This means that there is a specificity to the osteoblast response to the presence of GCM. Hench and colleagues and Foppiano et al. found an effect of GCM treated cells on osteoblast gene expression, however, these researchers did not show a direct link between how this altered gene expression relates to matrix production. Without the downstream expression of matrix proteins related to expressed genes, the altered gene expression may not be meaningful. Such a specific link is critical as it shows how altered gene expression as a result of osteoblast exposure to GCM influences matrix production.
This study raises clinically relevant questions with regard to future implant preparation prior to implantation. In particular, is the pre-soaking period a necessary procedure to avoid potentially adverse localized conditions near the implant coating-bone interface. Although this could be potentially harmful, the initial rapid dissolution physiologically may not be deleterious since the physiological environment is highly buffered. However, such issues need to be addressed prior to clinical use.
CONCLUSIONS
Enhanced expression of four key osteogenic markers was found when culturing cells in the presence of glass conditioned media. (GCM). Markedly higher expression of osteocalcin was observed at the protein level with correlated increases at the gene level. The increased osteocalcin expression was related directly to the expression of three key genes (Col1α1, Col1α2, and Runx2). The enhanced expression most likely occurred due to the increased concentrations of both Ca and Si in the GCM from both bioactive glasses. Alkaline phosphatase expression remained comparable to controls and this observation was attributed to no significant increase or decrease in phosphate ion concentrations.
ACKNOWLEDGEMENTS
The authors would like to thank the following contributors to this paper: Tiffany Vallortigara, Janet Wong, Kelly Leong, Garrett Porteous. The authors would also like to thank the following for their kind advice related to the above work: Linda Prentice, Larry Watanabe, Grace Nonomura, Dr. Stuart Gansky, Professor Pamela DenBeston, Dr. Yuan Zhang, Dr. James Chen, Dr. Stefan Habelitz, Dr. Sunita P. Ho, Dr. Kuniko Saeki, and Dr. Huynh, Tri. Finally, the authors appreciate the financial support by the National Institutes of Health/National Institute of Dental and Craniofacial Research Grants K25 DE018230 Varanasi (PI) and R01 DE11289 Tomsia (PI)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sawyer JD, editor. The Quest for Nature’s Match. American Ceramic Society Bulletin; 2007.
- 2.Adell R, Lekholm U, Rockler B, Branemark PI. A 15-Year Study of Osseointegrated Implants in the Treatment of the Edentulous Jaw. Int J Oral Maxillof 1981; 10: 387–416. [DOI] [PubMed] [Google Scholar]
- 3.Miles BA, Sinn DP, Gion GG. Experience with cranial implant-based prosthetic reconstruction. J Craniofac Surg 2006; 17: 889–97. [DOI] [PubMed] [Google Scholar]
- 4.Baltag L, Watanabe K, Kusakari H, Taguchi N, Miyakawa O, Kobayashi M, et al. Long-term changes of hydroxyapatite-coated dental implants. J Biomed Mater Res 2000; 53: 76–85. [DOI] [PubMed] [Google Scholar]
- 5.Lee TM, Yang CY, Chang E, Tsai RS. Comparison of plasma-sprayed hydroxyapatite coatings and zirconia-reinforced hydroxyapatite composite coatings: in vivo study. J Biomed Mater Res A 2004; 71A: 652–60. [DOI] [PubMed] [Google Scholar]
- 6.Ogino M, Ohuchi F, Hench LL. Compositional Dependence of the Formation of Calcium-Phosphate Films on Bioglass. J Biomed Mater Res 1980; 14: 55–64. [DOI] [PubMed] [Google Scholar]
- 7.Ogino M, Hench LL. Formation of Calcium-Phosphate Films on Silicate-Glasses. Journal of Non-Crystalline Solids 1980; 38–9: 673–8. [Google Scholar]
- 8.Karlan MS, Hench LL, Madden M, Ogino M. Bone-Bonding Bioactive Material Implant in Head and Neck - Bioglass. Surg Forum 1978; 29: 575–7. [PubMed] [Google Scholar]
- 9.Hench LL, Wilson J. Surface-Active Biomaterials. Science 1984; 226: 630. [DOI] [PubMed] [Google Scholar]
- 10.Hench L. Bioceramics. Journal of the American Ceramic Society 1998; 81: 1705. [Google Scholar]
- 11.Fujishiro Y, Hench LL, Oonish H. Quantitative rates of in vivo bone generation for Bioglassâ and hydroxyapatite particles as bone graft substitute. Journal of Materials Science: Materials in Medicine 1997; 8: 649–52. [DOI] [PubMed] [Google Scholar]
- 12.Moreira-Gonzalez A, Lobocki C, Barakat K, Andrus L, Bradford M, Gilsdorf M, et al. Evaluation of 45S5 bioactive glass combined as a bone substitute in the reconstruction of critical size calvarial defects in rabbits. J Craniofac Surg 2005; 16: 63–70. [DOI] [PubMed] [Google Scholar]
- 13.Wilson J, Low SB. Bioactive Ceramics for Periodontal Treatment - Comparative-Studies in the Patus Monkey. J Appl Biomater 1992; 3: 123–9. [DOI] [PubMed] [Google Scholar]
- 14.Saiz E, Goldman M, Gomez-Vega JM, Tomsia AP, Marshall GW, Marshall SJ. In vitro behavior of silicate glass coatings on Ti6Al4V. Biomaterials 2002; 23) 3749–56. [DOI] [PubMed] [Google Scholar]
- 15.Pazo A, Saiz E, Tomsia AP. Silicate glass coatings on Ti-based implants. Acta Mater 1998; 46: 2551–8. [Google Scholar]
- 16.Oku T, Suganuma K, Wallenberg LR, Tomsia AP, Gomez-Vega JM, Saiz E. Structural characterization of the metal/glass interface in bioactive glass coatings on Ti-6Al-4V. J Mater Sci-Mater M 2001; 12: 413–7. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Esteban S, Saiz E, Fujino S, Oku T, Suganuma K, Tomsia AP. Bioactive glass coatings for orthopedic metallic implants. J Eur Ceram Soc 2003; 23: 2921–30. [Google Scholar]
- 18.Gomez-Vega JM, Saiz E, Tomsia AP, Oku T, Suganuma K, Marshall GW, et al. Novel bioactive functionally graded coatings on Ti6Al4V. Adv Mater 2000; 12: 894–8. [Google Scholar]
- 19.Gomez-Vega JM, Saiz E, Tomsia AP, Marshall GW, Marshall SJ. Bioactive glass coatings with hydroxyapatite and Bioglass (R) particles on Ti-based implants. 1. Processing. Biomaterials 2000; 21: 105–11. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Vega JM, Saiz E, Tomsia AP. Glass-based coatings for titanium implant alloys. J Biomed Mater Res 1999; 46: 549–59. [DOI] [PubMed] [Google Scholar]
- 21.Gomez-Vega JM, Hozumi A, Saiz E, Tomsia AP, Sugimura H, Takai O. Bioactive glass-mesoporous silica coatings on Ti6Al4V through enameling and triblock-copolymer-templated sol-gel processing. J Biomed Mater Res 2001; 56: 382–9. [DOI] [PubMed] [Google Scholar]
- 22.Fujino S, Tokunaga H, Saiz E, Tomsia AP. Fabrication and characterization of bioactive glass coatings on Co-Cr implant alloys. Mater Trans 2004; 45: 1147–51. [Google Scholar]
- 23.Fujino S, Morinaga K, Saiz E, Tomsia AP. Bioactive glass coatings on Co-Cr implant alloys. Glass Sci Technol 2002; 75: 221–6. [Google Scholar]
- 24.Foppiano S, Marshall SJ, Saiz E, Tomsia AP, Marshall GW. Functionally graded bioactive coatings: Reproducibility and stability of the coating under cell culture conditions. Acta Biomaterialia 2006; xxx: xxx–xxx. [DOI] [PubMed] [Google Scholar]
- 25.Foppiano S, Marshall SJ, Marshall GW, Saiz E, Tomsia AP. The influence of novel bioactive glasses on in vitro osteoblast behavior. J Biomed Mater Res A 2004; 71A: 242–9. [DOI] [PubMed] [Google Scholar]
- 26.Bloyer DR, Gomez-Vega JM, Saiz E, McNaney JM, Cannon RM, Tomsia AP. Fabrication and characterization of a bioactive glass coating on titanium implant alloys. Acta Mater 1999; 47: 4221–4. [Google Scholar]
- 27.Russias J, Saiz E, Deville S, Gryn K, Liu G, Nalla RK, et al. Fabrication and in vitro characterization of three-dimensional organic/inorganic scaffolds by robocasting. J Biomed Mater Res A 2007; 83A: 434–45. [DOI] [PubMed] [Google Scholar]
- 28.Varanasi VG, Vallortigara T, Loomer PM, Saiz E, Tomsia AP, Marshall SJ, et al. , editors. Improving Biomaterials from a Cellular Point of View. Materials Research Society Symposium Proceedings; 2006; San Francisco, CA: Materials Research Society. [Google Scholar]
- 29.Xynos ID, Hukkanen MVJ, Hench LL, Polak JM. Bioglass 45S5 induces mineralisation in osteoblast cultures in vitro. J Pathol 1999; 187: 38a-a. [Google Scholar]
- 30.Xynos ID, Hukkanen MVJ, Batten JJ, Buttery LD, Hench LL, Polak JM. Bioglass (R) 45S5 stimulates osteoblast turnover and enhances bone formation in vitro: Implications and applications for bone tissue engineering. Calcified Tissue Int 2000; 67: 321–9. [DOI] [PubMed] [Google Scholar]
- 31.Xynos ID, Edgar AJ, Ramachandran M, Buttery LDK, Hench LL, Polak JM. Biochemical characterisation and gene expression profiling of human trabecular bone derived osteoblasts. J Pathol 2001; 193: 31a-a. [Google Scholar]
- 32.Xynos ID, Edgar AJ, Buttery LDK, Hench LL, Polak JM. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass (R) 45S5 dissolution. J Biomed Mater Res 2001; 55: 151–7. [DOI] [PubMed] [Google Scholar]
- 33.Xynos ID, Edgar AJ, Buttery LDK, Hench LL, Polak JM. Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochem Bioph Res Co 2000; 276: 461–5. [DOI] [PubMed] [Google Scholar]
- 34.Jones JR, Sepulveda P, Hench LL. Dose-Dependent Behavior of Bioactive Glass Dissolution. J Biomed Mater Res 2001; 58: 720–6. [DOI] [PubMed] [Google Scholar]
- 35.Jones JR, Hench LL. Regeneration of trabecular bone using porous ceramics. Curr Opin Solid St M 2003; 7: 301–7. [Google Scholar]
- 36.Hench LL, Polak JM. Third-generation biomedical materials. Science 2002; 295: 1014. [DOI] [PubMed] [Google Scholar]
- 37.Bielby RC, Pryce RS, Hench LL, Polak JM. Enhanced derivation of osteogenic cells from murine embryonic stem cells after treatment with ionic dissolution products of 58S bioactive sol-gel glass. Tissue Eng 2005; 11: 479–88. [DOI] [PubMed] [Google Scholar]
- 38.Bielby RC, Christodoulou IS, Pryce RS, Radford WJP, Hench LL, Polak JM. Time- and concentration-dependent effects of dissolution products of 58S sol-gel bioactive glass on proliferation and differentiation of murine and human osteoblasts. Tissue Eng 2004; 10: 1018–26. [DOI] [PubMed] [Google Scholar]
- 39.Maeno S, Niki Y, Matsumoto H, Morioka H, Yatabe T, Funayama A, et al. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials 2005; 26: 4847–55. [DOI] [PubMed] [Google Scholar]
- 40.Reffitt DM, Ogston N, Jugdaohsingh R, Cheung HFJ, Evans BAJ, Thompson RPH, et al. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast- ike cells in vitro. Bone 2003; 32: 127–35. [DOI] [PubMed] [Google Scholar]
- 41.Beck GR, Zerler B, Moran E. Phosphate is a specific signal for induction of osteopontin gene expression. P Natl Acad Sci USA 2000; 97: 8352–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zreiqat H, Shakibaei ME, Evans P, Knabe C, Howlett CR. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J Bone Miner Res 2001; 16: S328-S. [DOI] [PubMed] [Google Scholar]
- 43.Zreiqat H, Howlett CR, Zannettino A, Evans P, Schulze-Tanzil G, Knabe C, et al. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J Biomed Mater Res 2002; 62: 175–84. [DOI] [PubMed] [Google Scholar]
- 44.Hattar S, Asselin A, Greenspan D, Oboeuf M, Berdal A, Sautier JM. Potential of biomimetic surfaces to promote in vitro osteoblast-like cell differentiation. Biomaterials 2005; 26: 839–48. [DOI] [PubMed] [Google Scholar]
- 45.Foppiano S, Marshall SJ, Marshall GW, Saiz E, Tomsia AP. Bioactive glass coatings affect the behavior of osteoblast-like cells. Acta Biomaterialia 2007; 3: 765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varanasi V, Uritani N, Ancheta B, Saiz E, Loomer P, Tomsia A, et al. Osteomimetic coatings for Titanium Implants. Journal of Materials Science 2008; submitted. [Google Scholar]
- 47.Franceschi RT, Iyer BS. Relationship between Collagen-Synthesis and Expression of the Osteoblast Phenotype in Mc3t3-E1 Cells. J Bone Miner Res 1992; 7: 235–46. [DOI] [PubMed] [Google Scholar]
- 48.Franceschi RT, Iyer BS, Cui YQ. Effects of Ascorbic-Acid on Collagen Matrix Formation and Osteoblast Differentiation in Murine Mc3t3-E1 Cells. J Bone Miner Res 1994; 9: 843–54. [DOI] [PubMed] [Google Scholar]
- 49.Franceschi RT, Wilson JX, Dixon SJ. Requirement for Na+-Dependent Ascorbic-Acid Transport in Osteoblast Function. Am J Physiol-Cell Ph 1995; 37: C1430–C9. [DOI] [PubMed] [Google Scholar]
- 50.Quarles LD, Yohay DA, Lever LW, Caton R, Wenstrup RJ. Distinct Proliferative and Differentiated Stages of Murine Mc3t3-E1 Cells in Culture - an Invitro Model of Osteoblast Development. J Bone Miner Res 1992; 7: 683–92. [DOI] [PubMed] [Google Scholar]
- 51.Byers BA, Pavlath GK, Murphy TJ, Karsenty G, Garcia AJ. Cell-type-dependent up-regulation of in vitro mineralization after overexpression of the osteoblast-specific transcription factor Runx2/Cbfa1. J Bone Miner Res 2002; 17: 1931–44. [DOI] [PubMed] [Google Scholar]
- 52.Xiao GZ, Cui YQ, Ducy P, Karsenty G, Franceschi RT. Ascorbic acid-dependent activation of the osteocalcin promoter in MC3T3-E1 preosteoblasts: Requirement for collagen matrix synthesis and the presence of an intact OSE2 sequence. Mol Endocrinol 1997; 11: 1103–13. [DOI] [PubMed] [Google Scholar]
- 53.Sestini S, Notarantonio L, Cerboni B, Alessandrini C, Fimiani M, Nannelli P, et al. In vitro toxicity evaluation of silver soldering, electrical resistance, and laser welding of orthodontic wires. European Journal of Orthodontics 2006; 28: 567–72. [DOI] [PubMed] [Google Scholar]
- 54.Qiu H, Durand K, Rabinovitch-Chable H, Rigaud M, Gazaille V, Clavere P, et al. Gene expression of HIF-1 alpha and XRCC4 measured in human samples by real-time RT-PCR using the sigmoidal curve-fitting method. Biotechniques 2007; 42: 355–62. [DOI] [PubMed] [Google Scholar]
- 55.D’Alonzo RC, Kowalski AJ, Denhardt DT, Nickols GA, Partridge NC. Regulation of collagenase-3 and osteocalcin gene expression by collagen and osteopontin in differentiating MC3T3-E1 cells. J Biol Chem 2002; 277: 24788–98. [DOI] [PubMed] [Google Scholar]
- 56.Xiao GZ, Jiang D, Ge CX, Zhao ZR, Lai YM, Boules H, et al. Cooperative interactions between activating transcription factor 4 and Runx2/Cbfa1 stimulate osteoblast-specific osteocalcin gene expression. J Biol Chem 2005; 280: 30689–96. [DOI] [PubMed] [Google Scholar]
- 57.Xiao GZ, Gopalakrishnan R, Jiang D, Reith E, Benson MD, Franceschi RT. Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3-E1 cells. J Bone Miner Res 2002; 17: 101–10. [DOI] [PubMed] [Google Scholar]
- 58.Franceschi RT, Ahmed AJ, Iyer BS. Regulation of Osteoblast Gene-Expression by Ascorbic-Acid. Faseb J 1992; 6: A353-A. [Google Scholar]
- 59.Franceschi RT, Iyer BS, Parikh UK, Pinero GJ. Extracellular-Matrix Synthesis and Osteoblast-Specific Gene-Expression. J Bone Miner Res 1992; 7: S218-S. [Google Scholar]
- 60.Yamaguchi T, Chattopadhyay N, Kifor O, Butters RR, Sugimoto T, Brown EM. Mouse osteoblastic cell line (MC3T3-E1) expresses extracellular calcium (Ca-0(2+))-sensing receptor and its agonists stimulate chemotaxis and proliferation of MC3T3-E1 cells. J Bone Miner Res 1998; 13: 1530–8. [DOI] [PubMed] [Google Scholar]
- 61.Yamaguchi T, Chattopadhyay N, Kifor O, Sanders JL, Brown EM. Activation of p42/44 and p38 mitogen-activated protein kinases by extracellular calcium-sensing receptor agonists induces mitogenic responses in the mouse osteoblastic MC3T3-E1 cell line. Biochem Bioph Res Co 2000; 279: 363–8. [DOI] [PubMed] [Google Scholar]
- 62.Yamauchi M, Yamaguchi T, Kaji H, Sugimoto T, Chihara K. Involvement of calcium-sensing receptor in osteoblastic differentiation of mouse MC3T3-E1 cells. Am J Physiol-Endoc M 2005; 288: E608–E16. [DOI] [PubMed] [Google Scholar]
- 63.Yamauchi M, Yamaguchi T, Sowa H, Yano S, Kaji H, Sugimoto T, et al. The extracellular calcium-sensing receptor (CaR) is indispensable for expression of alkaline phosphatase (ALP) and osteocalcin (OC) as well as mineralization in mouse osteoblastic MC3T3-E1 cells. J Bone Miner Res 2003; 18: S289-S. [Google Scholar]
- 64.Lyu K, Nathanson D, Chou L. Effects of silicon, calcium, and phosphorus on human osteoblast culture. J Dent Res 2000; 79: 220-. [Google Scholar]


