Abstract
Hyperthyroidism, thyrotoxicosis and thyroid storm are a continuum of disease. A life-threatening and potentially fatal manifestation of thyrotoxicosis is thyroid storm. Thyroid storm is considered rare with an occurrence rate of 1–2% of all patients with hyperthyroidism, making a high index of suspicion important in the early recognition of this debilitating complication. We present the case of a 63-year-old female with a significant history of being non-compliant with her hyperthyroidism regimen and presented to the emergency department in severe respiratory distress. She was ultimately diagnosed with thyroid storm induced high-output congestive heart failure, intubated, had a cardiac arrest and was transferred to the intensive care unit in a guarded condition. Her hospital course was unremarkable and she was discharged on Day 12.
Introduction
The most common underlying etiology of a thyroid storm is Grave’s disease, an autoimmune disorder characterized by thyrotropin receptor antibodies. As an endocrine emergency and sepsis mimic, thyroid storm becomes a must recognize disease process, with an untreated mortality of 100% and 10–50% with treatment [1]. The clinical presentation of thyroid storm is diverse, presents within the spectrum of thyrotoxicosis and requires a multifactorial treatment regimen. As a severe hypermetabolic condition, thyroid storm can challenge even the most skilled physician.
Case Report
A 63-year-old female presented to the emergency department (ED) in severe respiratory distress. She stated, ‘I had asthma as a kid, this kind of feels like that.’ Symptoms started abruptly 2 days ago while she was leaving her house to go shopping and continued to progressively get worse. Associated symptoms included dyspnea on exertion, pleuritic chest pain, diaphoresis and lightheadedness. She denied any recent travel, hormone replacement, prior deep vein thrombosis or pulmonary embolism, calf pain/leg pain or any other symptoms. Vitals on arrival to the ED are as follows: temperature of 101.2°F, blood pressure 220/114 mmHg, respiratory rate up to 42 breaths/min, heart rate of 124 beats/min and a pulse ox of 88% on a non-rebreather. Physical exam was notable for severe respiratory distress and diaphoresis with conversational dyspnea, intercostal retractions and supraclavicular retractions. The patient was not wanting to talk or move secondary to the shortness of breath. Examination of the head and neck revealed no evidence for proptosis; however, she did exhibit fullness to her thyroid bilaterally. Her cardiopulmonary exam revealed diffuse rales in all lung fields and no obvious murmur. Her lower extremities were significant for 1+ pitting edema bilaterally. The patient was placed on bi-level positive airway pressure on arrival, a high dose nitroglycerine infusion was started and furosemide was given. Her electrocardiogram (EKG) revealed sinus tachycardia at 119 with biatrial enlargement. Chest radiography, Fig. 1, shows bilateral airspace opacities, right greater than left. Findings consistent with diffuse pulmonary edema.
Figure 1.
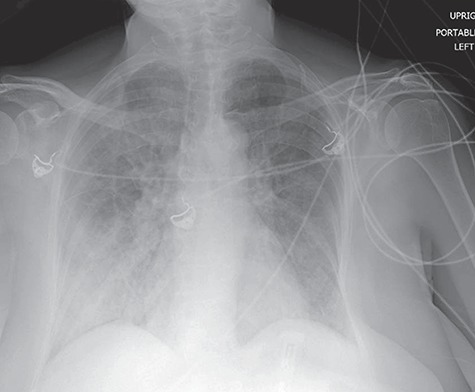
Chest radiography shows bilateral airspace opacities, right greater than left. Findings consistent with diffuse pulmonary edema.
Blood pressure improved to 170/70 mmHg, and the patient was able to communicate regarding her medical history. She noted intermittent palpitations for over 1 year, decreased appetite, an unintentional 10-lb weight loss and blurry vision. Chart review revealed that she had a thyroid stimulating hormone (TSH) level performed as an outpatient 2 months prior returning a value of <0.01. At that time, a thyroid ultrasound was performed and was concerning for a solid right thyroid nodule measuring up to 1.8 cm. A subsequent technetium thyroid study revealed a goiter with increased technetium trapping consistent with the clinical suspicion of Graves’ disease. The patient had refused radioiodine therapy. She was prescribed methimazole and propranolol as an outpatient, but missed multiple doses frequently.
Within 5 minutes of arrival, she began to decompensate, with increasing diaphoresis, tachypnea, hypoxia, bradycardia and lethargy. Decision was thus made to intubate utilizing etomidate and rocuronium. Subsequent chest radiography post intubation, Fig. 2, shows a re-demonstration of diffuse pulmonary edema, in addition to small bilateral pleural effusions with associated atelectasis. Approximately 4 minutes later, her rhythm went from bradycardic into asystole. Advanced cardiac life support protocol was followed, with a resulting return of spontaneous circulation achieved after 5 minutes. Bedside cardiac ultrasound showed no evidence for a pericardial effusion or right heart strain. CT angiography of the chest, Figs 3 and 4, shows no filling defects within the pulmonary vasculature and re-demonstration of multifocal ill-defined patchy/fluffy airspace consolidative and ground glass opacities within the bilateral lungs, favored to represent pulmonary edema.
Figure 2.
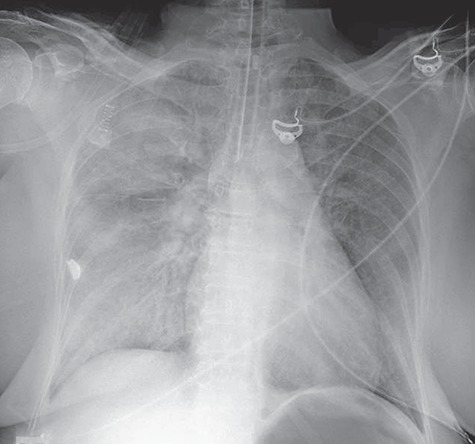
Chest radiography post intubation showing a re-demonstration of diffuse pulmonary edema, in addition to small bilateral pleural effusions with associated atelectasis.
Figure 3.
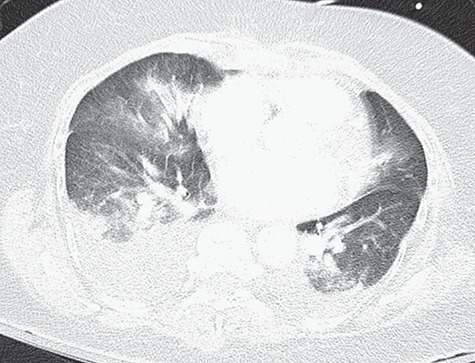
CT angiography of the chest with intravenous contrast showing no filling defects within the pulmonary vasculature, and re-demonstration of multifocal ill-defined patchy/fluffy airspace consolidative and ground glass opacities within the bilateral lungs, favored to represent pulmonary edema.
Figure 4.
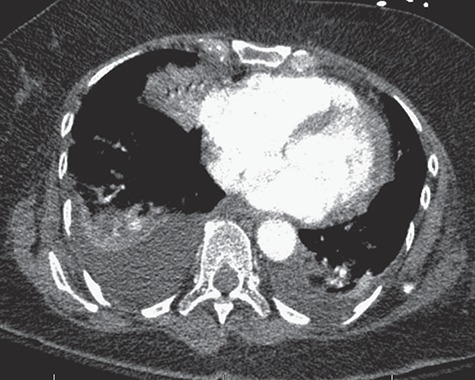
CT angiography of the chest with intravenous contrast showing no filling defects within the pulmonary vasculature, and re-demonstration of multifocal ill-defined patchy/fluffy airspace consolidative and ground glass opacities within the bilateral lungs, favored to represent pulmonary edema.
Laboratory evaluation was notable for a TSH <0.01 (0.40–4.50 mcIU/ml), free T3 of 10.2 (2.3–4.2 pg/ml), free T4 of 2.7 (0.8–1.8 ng/dL), brain natriuretic peptide of 566 (0–100 pg/ml), troponin of 0.01/0.12/0.24 (0.00–0.04 ng/ml) and blood glucose of 210. Arterial blood gas revealed a pH of 7.03, pCO2 89, p02 345, HCO3 23. The complete blood count and complete metabolic panel were unremarkable. No other acute lab abnormalities were found.
The case was discussed with endocrinology and she was started on the following regimen: propranolol 1 mg IV followed by 60 mg PO every 6 hours via her nasogastric tube, methimazole 20 mg loading dose followed by 20 mg every 6 hours, SSKI Potassium Iodine Oral Solution 5 gtts q6–8 hours, hydrocortisone 100 mg IV q8 hours and cholestyramine powder 4 gm via NG tube q6 hours. The patient was admitted to the ICU in guarded condition for suspected high output congestive heart failure secondary to thyroid storm from a non-compliance with her hyperthyroidism treatment. The elevated troponin was deemed likely a type II mechanism related to hypoxia, bradycardia and cardiopulmonary arrest.
By Day 2, the patient was alert on the ventilator, answering yes or no questions and doing well on spontaneous breathing trials. A formal 2D echocardiography showed mild eccentric mitral regurgitation, trace pulmonic insufficiency, with a left ventricular ejection fraction of 60%. By Day 5, she was transferred out of the ICU. By Day 7, her free T3 was approaching normal at 2.1 (2.3–4.2 pg/ml) and her free T4 normalized at 2.4 (0.8–1.8 ng/dL). It will take several weeks for the TSH to return to normal. Her remaining hospital course was unremarkable and she was discharged home on Day 12. She later underwent radioactive iodine ablation as an outpatient. There will likely be a need for lifelong thyroid hormone replacement therapy once the patient develops an underactive thyroid. At her most recent endocrinology follow-up appointment, she remains compliant, has had a full return to baseline activities and enjoys spending time with her family.
Discussion
Thyroid storm is a severe hypermetabolic condition resulting in increased sensitivity and expression of beta-adrenergic receptors, leading to a higher response to endogenous catecholamines [2]. There are number of causes, including, but not limited to the following: medical non-compliance, untreated thyroid disease, trauma, myocardial ischemia, infection, overdose of thyroid hormone and diabetic ketoacidosis [2]. In our patient, her thyroid storm was secondary to medical non-compliance with her hyperthyroid medication and non-compliance with the recommended radioactive iodine treatment.
For the emergency physician, laboratory evaluation will reveal a TSH that is usually low or undetectable with an associated elevated T3 and T4. Other laboratory findings can include anemia, lower serum creatinine, hypercalcemia or thrombocytopenia. If adrenal insufficiency is also present, the patient may have hyperkalemia and hyponatremia [2]. The clinical presentation is extremely diverse, with similarities to a hyperadrenergic state, with multiple systems being affected.
Central nervous system manifestations (altered mental status, seizures, agitation, lethargy, psychosis and coma)
Cardiovascular manifestations (sinus tachycardia, palpitations, atrial fibrillation, congestive heart failure, chest pain, wide pulse-pressure and dyspnea on exertion)
Gastrointestinal manifestations (nausea, vomiting, abdominal pain and diarrhea)
Skin manifestations (flushed, warm, moist and possible vitiligo and hyperpigmentation)
Thyroid gland manifestations (diffuse enlargement, neck fullness and thyroid tenderness)
As the disease process progresses, excess amounts of thyroid hormone begin to have a significant impact on cardiovascular hemodynamics. The result is high-output congestive heart failure (an uncommon type of heart failure) as the cardiac tissue has an increased sensitivity to catecholamines. Characteristic features of a high-output congestive heart failure include an increased cardiac output and decreased systemic vascular resistance. In this particular form of heart failure, the increased cardiac output is greater than the metabolic demand. Patients will present with an increased stroke volume, increased myocardial contractility, increased ejection fraction, atrial arrhythmia and systolic hypertension with an associated wide pulse pressure [5]. If left untreated, the result is eventual dilated cardiomyopathy, lethal dysrhythmias and death [5]. This concept of high-output congestive heart failure is counterintuitive to the typical congestive heart failure secondary to other causes, such as a myocardial infarction, that results in decreased myocardial contractility, low cardiac output and ventricular arrhythmia [5].
Being within the spectrum of thyrotoxicosis, distinguishing severe thyrotoxicosis from thyroid storm can challenge even the most skilled physician. One set of diagnostic criteria that can be used to aid in diagnosing thyroid storm is the Burch-Wartofsky Scale [2]. Components include the degree of fever, tachycardia, presence of atrial fibrillation, presence of congestive heart failure, any gastrointestinal dysfunction and any central nervous system dysfunction [2]. A score greater than 45 points is diagnostic of thyroid storm, 25–44 points is associated with an impending thyroid storm and <25 points is unlikely. Our patient received 10 points for a fever of 101.2°F, 15 points for a heart rate of 124 beats/min, 20 points for congestive heart failure and 10 points for agitation, for a total score of 55 points. Another diagnostic aid that can be utilized in diagnosing thyroid storm are the criteria of the Japan Thyroid Association (presence of thyrotoxicosis in addition to at least one central nervous system dysfunction in combination with fever, CHF, tachycardia or gastrointestinal abnormality) [3].
Treatment is multifactorial and involves a specific order to the treatment regimen as outlined below.
Beta blocker is used to block the peripheral effects of the thyroid hormones and prevent the peripheral conversion of T4 to T3. Propranolol, a non-selective beta blocker, is used for this reason to treat the systemic effects (starting dose is often 1 mg IV push, titrating 1–3 mg q10 minutes and PO formulations dosed at 60–80 mg q6 hours) [2,3]. In our patient, she received 1 mg IV of propranolol and started on a PO formulation.
Administration of propylthiouracil (PTU) or methimazole is utilized to inhibit the de novo synthesis of T3 and T4. PTU is typically given as a 600–1000 mg loading dose followed by a maintenance dose of 300 mg q6. Methimazole is typically given as a 20–30 mg loading dose followed by a maintenance dose of 20–30 mg q6 [2].
Administration of SSKI Potassium Iodine Oral solution 5 gtts q6–8 hours or Lugol’s solution 4–8 gtts q6 hours [2]. These will block the release of pre-formed thyroid hormones.
Administration of corticosteroids (hydrocortisone 100 mg IV q8 hours or Dexamethasone 2–4 mg q6 hours) as thyroid storm causes depression of the hypothalamic pituitary axis. In addition, corticosteroids have the ability inhibit the peripheral conversion of T4 to T3 [2, 3].
Administration of cholestyramine powder (4 g PO q6 hours) aids in reducing thyroid hormone levels by interfering with anterior hepatic circulation and recycling of thyroid hormones [2, 3].
Definitive treatment often post-hospitalization involves surgery or radioactive iodine, as our patient had received. The Journal of Clinical Apheresis published an article in 2018 regarding patients that cannot undergo thyroidectomy or fail pharmacotherapy. In these patients, plasma exchange has been suggested as another treatment option; however, the optimal role is not established and there are only a limited number of cases within the medical literature [6].
To decrease the morbidity and mortality associated with thyroid storm, an emergency medicine physician must consider this diagnosis in any patient presenting to the ED in a hypermetabolic state [5]. The most common cause of death in thyroid storm is cardiopulmonary failure [4]. As a result, quick recognition and treatment is critical for patient survival.
Conflict of interest
None declared.
Funding
No financial support was received for this study.
Ethical Approval
No approval is required.
Consent
Informed patient consent was obtained.
Guarantor
G.T. is the guarantor of this study.
References
- 1. Idrose A. Acute and emergency care for thyrotoxicosis and thyroid storm. Acute Med Surg 2015;2:147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burch HB, Wartofsky L. Life-threatening thyrotoxicosis. Thyroid storm. Endocrinol Metab Clin North Am 1993;22:263–77. [PubMed] [Google Scholar]
- 3. Satoh T, Isozaki O, Suzuki A,et al. 2016 Guidelines for the management of thyroid storm from The Japan Thyroid Association and Japan Endocrine Society (First edition). Endocr J 2016;63:1025–64. [DOI] [PubMed] [Google Scholar]
- 4. Nai Q, Ansari M, Pak S, Tian Y, Amzad-Hossain M, Zhang Y, et al. Cardiorespiratory failure in thyroid storm: a case report and literature review. J Clin Med Res 2018 April;10:351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Osuna P, Udovcic M, Sharma M. Hyperthyroidism and the heart. Methodist Debakey Cardiovasc J 2017 April–June;13:60–3http://europepmc.org/articles/PMC5512680/#i1947-6094-13-2-60-b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McGonigle A, Tobian A, Zink J, King K. Perfect storm: therapeutic plasma exchange for a patient with thyroid storm. J Clin Apher 2018 February;33:113–6. [DOI] [PubMed] [Google Scholar]


