Abstract
Approximately 70% of sudden sensorineural hearing loss (SSNHL) cases are idiopathic, but SSNHL may occasionally be related to fatality. Here, we report the case of a 54-year-old Asian female who complained of hearing loss as a primary symptom and was diagnosed with pyogenic ventriculitis (PV) derived from Streptococcus agalactiae endocarditis. Both PV and S. agalactiae endocarditis are rare and very severe infections with a high mortality rate. Rapid intensive treatment and surgery were required for successful clinical outcomes.
INTRODUCTION
A complaint of hearing loss can reflect a wide variety of abnormalities. Sudden or acute hearing loss in adults may result from a range of disease states: idiopathic (sudden idiopathic sensorineural hearing loss), iatrogenic, a side effect of medication, a complication of a viral or bacterial infection, a labyrinthine fluid abnormality, a vascular event, trauma, neoplasm, an autoimmune abnormality or a neurological disease [1].
Sudden sensorineural hearing loss (SSNHL), defined as the idiopathic loss of hearing of 30 dB over at least 3 test frequencies occurring over a period of less than 3 days, is considered an otolaryngologic emergency. Any age group can be affected, but the peak incidence occurs in the fifth or sixth decade of life, with an equal gender distribution. The overall incidence ranges from 5 to 20/100 000 people/year. Its severity ranges from difficulty with conversation to complete hearing loss [2, 3].
SSNHL is idiopathic in 70% of cases, infectious in 13% and is related to otologic disease, trauma, vascular disease, haematologic disorders or neoplasm in the vast majority of remaining cases [2, 3]. A delay in diagnosis is common because the patient may report ear fullness that is often attributed to cerumen impaction or congestion from upper respiratory infections. The likelihood of recovery is related to the severity of hearing loss, age of the patient and associated vestibular symptoms [2, 3].
Ventriculitis, a complication of meningitis, is a rare cerebral infection that has been variably referred to as ependymitis, intraventricular abscess, ventricular emphysema and pyocephalus [4, 5]. Pyogenic ventriculitis (PV) is an uncommon but very severe intracranial infection related to ruptured brain abscesses, ventricular catheter, trauma or meningitis. PV requires rapid diagnosis and therapy because of its high mortality [4, 5].
Here, we report the case of a patient with SSNHL as a major primary symptom, which turned out to be life-threatening, Streptococcus agalactiae (S. agalactiae) endocarditis that affected the central nervous system and destroyed mitral chordae tendineae, resulting in severe mitral regurgitation.
CASE REPORT
A 54-year-old Asian female was referred to the division of otolaryngology with suspected SSNHL. Hearing loss suddenly appeared 1 day before the first visit to our hospital. Her audiogram revealed profound and severe sensorineural hearing loss of the right and left ears, respectively (Fig. 1). Since she had been febrile, malaise and anorexic for approximately a week, she was referred to the internal medicine division. Her past medical history was significant for an approximate 5-year post-operative history of breast cancer. She had been taking oral tamoxifen citrate (20 mg/day) after the surgery. Her Glasgow Coma Scale rating was E2V5M6. No abnormalities were found in her physical and neurological findings including the following: height, 159.0 cm; body weight, 43.0 kg; body mass index, 17.0 kg/m2; heart rate, 90 beats/min with regularity; body temperature, 36.4°C; except for blood pressure, 77/42 mmHg (↓); oxygen saturation in room air, 90% (↓); and respiratory rate, 24 breaths/min (↑).
Figure 1.
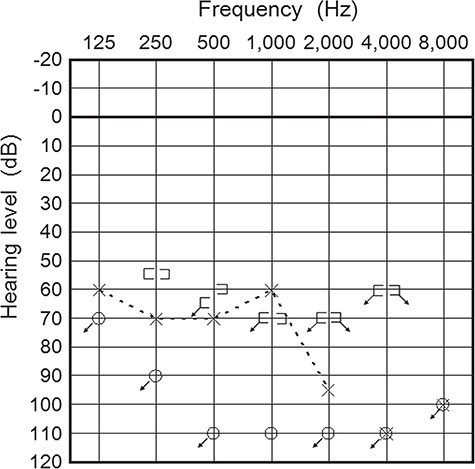
Pure tone audiogram shows profound and severe sensorineural hearing loss of the right (○) and left (×) ears. Three-frequency average hearing loss (the average thresholds at 500, 1000 and 2000 Hz) of the right and left ears was more than 115 and 75 dB, respectively.
Baseline laboratory results were as follows: white blood cell count, 21 700/μl (↑); haemoglobin, 12.5 g/dl; platelet count, 3.0 × 104/μl (↓); blood urea nitrogen, 38 mg/dl (↑); creatinine, 1.34 mg/dl (↑); sodium, 143 mEq/l; potassium, 3.8 mEq/l; chlorides, 105 mEq/l; total protein, 5.9 g/dl (↓); albumin, 2.9 g/dl (↓); C-reactive protein, 19.06 mg/dl (↑); procalcitonin, 7.47 ng/ml (↑); total bilirubin, 0.95 mg/dl; direct bilirubin, 0.57 mg/dl (↑); aspartate aminotransferase, 52 IU/l (↑); alanine aminotransferase, 50 IU/l (↑); lactate dehydrogenase, 396 IU/l (↑); alkaline phosphatase, 488 IU/l (↑); cholinesterase, 135 IU/l (↓); γ-glutamyl transpeptidase, 142 IU/l (↑); and glucose, 142 mg/dl (↑). Urinalysis revealed protein (1+), sugar (−) and ketones (±) with no blood cells or casts. Electrocardiogram revealed sinus tachycardia (101 beats/min) and an incomplete right bundle branch block. Chest X-ray revealed consolidation of both lower lung fields with air bronchogram and a reticular pattern (Fig. 2a). These findings suggested that the patient suffered from sepsis, specifically septic shock (quick sequential organ failure assessment [SOFA] score, 3 points).
Figure 2.
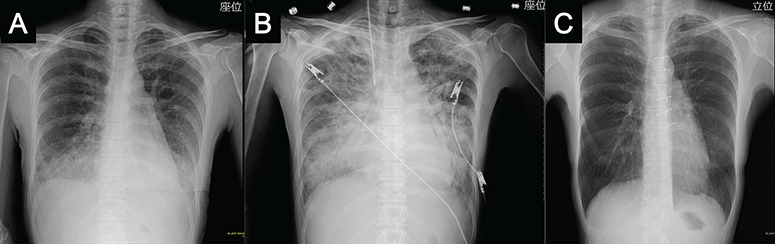
Chest X-ray shows consolidation of both lower lung fields on air bronchogram and a reticular pattern on admission (a); `butterfly shadow’ on the same day after admission (Day 1) (b); normal findings at ~6 months after mitral valve reconstruction surgery (c).
On the day of admission (Day 1), her respiratory failure was rapidly exaggerated following administration of ceftriaxone sodium hydrate (2 g q24hr), which was initiated immediately after taking 2 sets of blood cultures (Fig. 2b). On Day 2, platelet transfusion was performed because the platelet count decreased to 1.4 × 104/μl, and subcutaneous and oral bleeding as well as blood-stained sputum were present due to disseminated intravascular coagulation. Non-invasive positive pressure ventilation and intravenous noradrenaline were further administered for congestive heart failure and low blood pressure, respectively. Finally, mechanical ventilation was performed after intubation on Day 3, but her condition turned out to be acute respiratory distress syndrome (PaO2/FIO2 ratio was 184.8) caused by septic shock (SOFA score, 11 points) and congestive heart failure. Transesophageal echocardiography following transthoracic echocardiography revealed vegetation of the mitral valve and ruptured mitral chordae tendineae, resulting in severe mitral regurgitation (Video 1). The patient was immediately transferred to a highly specialized hospital, and mitral reconstructive surgery including removal of bacterial vegetation was performed on Day 3.
Magnetic resonance imaging (MRI) after surgery revealed hyper-intense lesions with a fluid–fluid level in the bilateral lateral ventricles (Fig. 3a), and the left side of the inner ear lesions was enhanced by gadolinium (Fig. 3b). These findings suggested that the patient suffered from PV and suppurative labyrinthitis, respectively [4, 5].
Figure 3.
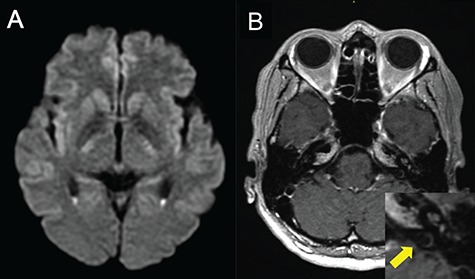
MRI shows PV (a) and suppurative labyrinthitis (b). (a) Diffusion-weighted image reveals hyper-intense lesions with a fluid–fluid level in the trigones of the bilateral lateral ventricles; (b) gadolinium-enhanced T1-weighted image reveals increased signals in the cochlea of the left inner ear lesions (yellow arrow of a zoomed-in image).
Two sets of blood cultures as well as bacterial vegetation yielded S. agalactiae. In summary, SSNHL was caused by PV, which originated from S. agalactiae endocarditis. Infectious endocarditis also destroyed the patient’s mitral valve, causing severe congestive heart failure. The patient fully recovered, except for hearing loss, ~6 months after surgery (Fig. 2c).
DISCUSSION
Bacterial and fungal meningitis may extend to the labyrinth and cause SSNHL [1–3]. One of the complications of meningitis is PV, characterized by the presence of suppurative fluid in the ventricles, caused suppurative labyrinthitis resulting in SSNHL of the present case. PV predominantly occurs in patients with previous head trauma or those undergoing prolonged intraventricular surgery or placement of drains and shunts into the ventricular space. Less frequent causes include ruptured brain abscesses, extension of dental abscesses and progression of meningitis into the ventricles [4, 5]. Furthermore, PV has rarely been reported as a secondary complication of infective endocarditis. Only two cases with PV complicating from infective endocarditis have been reported and were fatal [6, 7].
Note that central nervous system complications of infective endocarditis occur frequently. They can arise through several mechanisms and are a leading cause of death due to infective endocarditis. The major mechanism of neurologic complications is cerebral embolization. Cerebral emboli, resulting from dislodgement or fragmentation of cardiac vegetations, followed by vessel occlusion, cause various degrees of ischemia and infarction [8].
The microorganisms that are most commonly associated with PV are gramme-negative bacteria, including Bacteroides, Enterobacter, Escherichia coli and Klebsiella, followed by gramme-positive microorganisms, commonly Staphylococcus species [4, 5]. Two fatal cases of PV complicating from infective endocarditis occurred in patients who suffered from infection of coagulase-negative Staphylococci or Aggregatibacter aphrophilus, a gramme-negative organism [6, 7]. Streptococci infection associated with PV is rare, but Streptococcus acidominimus or Streptococcus suis infections have been recently reported [9, 10].
S. agalactiae, a group B β-haemolytic streptococcus, is known to cause severe infective endocarditis. The occurrence of endocarditis is not common (~6% of S. agalactiae infections), but it has a high mortality rate (nearly 100%), especially if a valve prosthesis is present. Early diagnosis, rapid administration of specific antibiotic therapy and surgery in the first week of treatment for S. agalactiae endocarditis contribute to better clinical outcomes [11].
In summary, a patient complaining of hearing loss suffered from two rare but fatal illnesses of PV and S. agalactiae endocarditis. Rapid intensive treatment and successful surgery saved the patient’s life.
ACKNOWLEDGEMENTS
The authors thank Dr Takashi Ueda, Department of Cardiovascular Surgery, Sapporo Higashi Tokushukai Hospital, for performing the heart surgery.
Conflicts of interest statement
None declared.
FUNDING
There is no source of funding to report for this case report.
ETHICAL APPROVAL
No ethical approval was required, as this was a clinical case.
CONSENT
Patient permission was obtained prior to writing this report.
GUARANTOR
Dr Toshiki Ito.
Supplementary Material
References
- 1. Bauer CA, Jenkins HA. Otologic symptoms and syndromes In: Cummings Otolaryngology. Philadelphia: Saunders, 2015, 2401–10. [Google Scholar]
- 2. Pfaff JA, Moore GP. Otolaryngology In: Rosen’s Emergency Medicine: Concepts and Clinical Practice. Philadelphia: Elsevier, 2018, 820–31. [Google Scholar]
- 3. Chau JK, Lin JR, Atashband S, Irvine RA, Weterberg ED. Systematic review of the evidence for the etiology of adult sudden sensorineural hearing loss. Laryngosope 2016;120;1011–1021. doi:http://doi.org/10.1002/lary.20873. [DOI] [PubMed] [Google Scholar]
- 4. Fukui MB, Williams RL, Mudigonda S. CT and MR imaging features of pyogenic ventriculitis. AJNR Am J Neuroradiol 2001;22:1510–6. [PMC free article] [PubMed] [Google Scholar]
- 5. Mohan S, Jain KK, Arabi M, Shah GV. Imaging of meningitis and ventriculitis. Neuroimaging Clin N Am 2012;22:557–583. doi:http://doi.org/10.1016/j.nic.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 6. Kiyan S, Aksay E, Ersel M, Yanturali S. A rare diagnosis in ED: cerebral pyogenic ventriculitis due to infective endocarditis. Am J Emerg Med 2007;25:120–122. doi:http://doi.org/10.1016/j.ajem.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 7. Jung GW, Parkins MD, Church D. Pyogenic ventriculitis complicating Aggregatibacter aphrophilus infective endocarditis: a case report and literature review. Can J Infect Dis Med Microbiol 2009;20:e107–9. 10.1155/2009/971735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wolff M, Timsit JF, Mourvillier B. Infectious endocarditis In: Textbook of Critical Care. Philadelphia: Elsevier, 2017, 602–8. [Google Scholar]
- 9. Shah GS. Pyogenic ventriculitis and meningitis caused by Streptococcus acidominimus in humans: a case report. Am J Case Rep 2018;19:329–334. doi:http://doi.org/10.12659/AJCR.908000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yanase T, Morii D, Kamio S, Nishimura A, Fukao E, Inose Y, et al . The first report of human meningitis and pyogenic ventriculitis caused by Streptococcus suis: a case report. J Infect Chemother 2018;24:669–673. doi:http://doi.org/10.1016/j/jiac.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 11. Siciliano RF, Cais DP, Navarro RC, Strabelli TMV. Acute Streptococcus agalactiae endocarditis: outcomes of early surgical treatment. Heart Lung 2010;39:331–334. doi:http://doi.org/10.1016/j/hrtlng.29.06.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


