Abstract
The critical role of the left atrium (LA) in cardiovascular homoeostasis is mediated by its reservoir, conduit, systolic, and neurohormonal functions. Atrial fibrillation is generally a reflection of underlying disease of the LA, especially in patients with heart failure. Disease-related LA remodelling leads to a decline in both atrial contractility and distensibility along with an impairment in the control of neurohormonal systems that regulate intravascular volume. Catheter ablation can lead to further injury to the atrial myocardium, as evidenced by post-procedural troponin release and tissue oedema. The cardiomyocyte loss leads to replacement fibrosis, which may affect up to 30–35% of the LA wall. These alterations further impair atrial force generation and neurohormonal functions; the additional loss of atrial distensibility can lead to a ‘stiff LA syndrome’, and the fibrotic response predisposes to recurrence of the atrial arrhythmia. Although it intends to restore LA systole, catheter ablation often decreases the chamber’s transport functions. This is particularly likely in patients with long-standing atrial fibrillation and pre-existing LA fibrosis, especially those with increased epicardial adipose tissue (e.g. patients with obesity, diabetes and/or heart failure with a preserved ejection fraction). Although the fibrotic LA in these individuals is an ideal substrate for the development of atrial fibrillation, it may be a suboptimal substrate for catheter ablation. Such patients are not likely to experience long-term restoration of sinus rhythm, and catheter ablation has the potential to worsen their haemodynamic and clinical status. Further studies in this vulnerable group of patients are needed.
Keywords: Left atrium, Catheter ablation, Atrial fibrillation, Heart failure
The left atrium (LA) plays a pivotal role in maintaining the physiological integrity of the cardiovascular system in three ways. First, the chamber acts as a reservoir for pulmonary venous return during left ventricular (LV) systole and as a conduit from the pulmonary veins to the LV during early diastole.1 Its ability to enlarge without increasing chamber pressures is critical to preventing deleterious increases in pulmonary venous and arterial pressures. Second, LA contraction boosts the filling of the LV at end-diastole. This action—through the Frank–Starling mechanism—enhances the strength of ventricular systole, without the need to maintain a continuously high LA pressure during diastole.2 Third, the LA is the nexus of the interplay of several neurohormonal systems that are activated by LA stretch. The chamber is richly supplied by adrenergic and cholinergic nerves; its distension stimulates mechanoreceptors, leading to inhibition of central sympathetic outflow to the kidneys, and to natriuresis.3 Atrial stretch also triggers the release of natriuretic peptides from the LA, which plays an additional role in volume homoeostasis.
Derangement of homoeostatic functions of the left atrium in cardiac disease
All three critical physiological actions are impaired in patients who have LA disease, which often becomes clinically manifest as atrial fibrillation (AF). Atrial fibrillation is a reflection of extensive abnormalities in the LA that precede the development of and progress with the duration of the arrhythmia. Atrial remodelling and fibrosis can be assessed by speckle tracking echocardiography and by magnetic resonance imaging, respectively. The haemodynamically stressed atrium enlarges and becomes more spherical; this deformational change predisposes to the development of AF and to recurrent AF following catheter ablation.4,5
As AF becomes persistent and long-standing, progressive LA fibrosis exerts deleterious effects on the chamber’s reservoir, conduit, and booster functions (Figure 1).6 With the loss of atrial capacitance, pulmonary venous return leads to disproportionate increases in LA pressure.7 These structural and pathophysiological derangements advance in parallel with the duration of AF; they are independent of LA chamber size, but are related to the quantity of fibrosis.6 The greater the severity of these abnormalities, the lower the likelihood that electrical, chemical, or ablative cardioversion can result in sustained restoration of sinus rhythm.8 Although it is commonly believed that AF itself accelerates the development of these derangements, it is difficult to separate the contribution of the arrhythmia from the role played by the underlying disease. The direct contribution of AF to the progression of LA disease remains uncertain.
Figure 1.
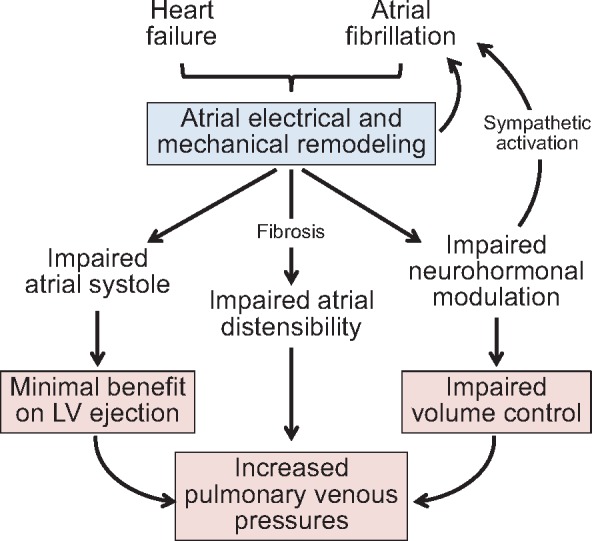
Impairment of left atrium function in atrial fibrillation and heart failure. Left atrium electrical and mechanical remodelling impairs the chamber’s contractility, distensibility and modulation of neurohormonal systems, leading to deranged volume control, sympathetic activation, and a limited ability of left atrium systole to enhance left ventricular ejection.
The concurrent presence of heart failure heightens the severity of the structural and functional derangements of the LA seen in AF (Figure 1).9 In sinus rhythm, the force of atrial emptying increases as the LV undergoes hypertrophy,2 but the strength of atrial systole weakens in patients with heart failure with a reduced ejection fraction (HFrEF)10; the magnitude of this impairment has prognostic significance.11 In those with volume overload due to HFrEF, functional mitral regurgitation may lead to increased atrial distensibility.12 In contrast, atrial reservoir and conduit functions are typically diminished in patients with heart failure and a preserved ejection fraction (HFpEF).13 Accordingly, patients with HFrEF have larger LA volumes but lower peak LA pressures, whereas those with HFpEF have greater LA stiffness leading to smaller LA volumes despite higher peak pressures.14 Yet, despite their smaller LA volumes, patients with HFpEF are more likely to have AF, suggesting that atrial fibrosis (not dilatation) is the major determinant of AF during prolonged atrial stress. Fibrosis may result from the accumulation and inflammation of epicardial adipose tissue, which is a prominent feature of many patients with HFpEF and has been linked to foci of electrical derangements in the adjacent underlying atrial myocardium.15
Finally, the role of the LA in modulating the activity of neurohormonal systems is substantially impaired in patients with AF, especially with coexistent heart failure. LA remodelling leads to loss of normal sympathoinhibition; the resulting increase in autonomic activity to the LA can trigger AF, whereas restoration of central sympathetic inhibition ameliorates the risk of AF.16 Heart failure also disrupts the adaptive neurohormonal response to LA stretch, such that chamber distension no longer leads to reflex sympathoinhibition,16 but instead, causes paradoxical sympathetic activation, leading to AF.17 In addition, circulating natriuretic peptides are increased in heart failure, particularly in patients with AF, but heart failure impairs the cleavage of prohormones in the LA, thus limiting the release of biologically active peptides.18 Fibrosis also impairs atrial stretch, thereby, constraining the primary stimulus to natriuretic peptide synthesis even when LA pressures are increased.15 End-stage disease may lead to exhaustion of natriuretic peptide synthesis, particularly in patients with long-standing AF.19
Characterization of left atrial injury induced by catheter ablation
Catheter ablation disrupts with the structural and functional integrity of autonomic nerve circuits, particularly those surrounding the pulmonary veins. Using radiofrequency or temperature-mediated injury, the procedure seeks to focally abolish triggers that are responsible for AF. Early ablation procedures were limited to creating circumferential substrate-based lines around the pulmonary veins, but recently, the procedure has been expanded to include posterior or atrial roof ablation lines, ablation lines from the left inferior pulmonary vein to the mitral annulus, and additional empiric ablation lines in the LA wall.20
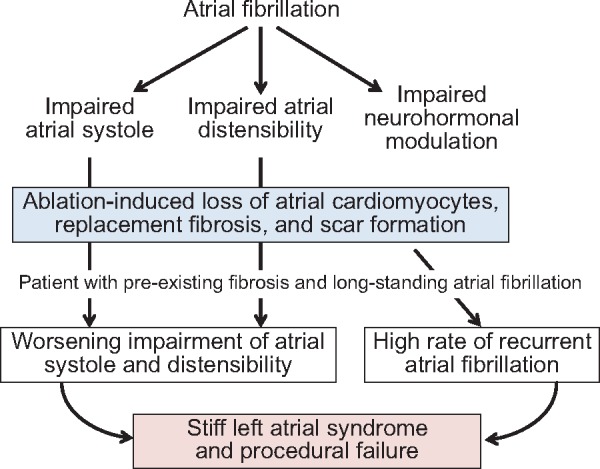
Although it intends to target neural circuits, ablation injures the atrial myocardium, as evidenced by post-procedural release of troponin21 and tissue oedema by imaging studies.22 In patients with healthy atria who typically have only paroxysmal AF, the consequences of the injury may be modest.23 However, the procedure-related cardiomyocyte loss eventually leads to replacement fibrosis, and in patients with long-standing AF and severely diseased atria, ablation-induced injury can have serious deleterious effects on LA structure and function (Take home figure).24 The quantity of loss of contractile tissue and replacement fibrosis depends on the extent or number of procedures, but 30–35% of the LA wall may be replaced by scar following ablation.25,26 These changes are not seen in the right atrium, which—although affected by AF—is typically not injured by the procedure.20
Take home figure.

Potential risks of catheter ablation in patients with long-standing atrial fibrillation and pre-existing left atrium fibrosis. With long-standing atrial fibrillation and meaningful left atrium fibrosis, the loss of cardiomyocytes and scar formation following ablation can exacerbate the pre-existing impairment of atrial contractility and distensibility. These sequelae are less likely in patients with relatively healthy atria and only paroxysmal atrial fibrillation.
Does the ablation-related injury affect the homoeostatic functions of the LA, which are already impaired in patients with AF, particularly those with heart failure? What are the clinical consequences of the loss of the chamber’s adaptive physiological actions?
Consequences of the effect of ablation on left atrial systolic function
Ablation is followed by impairment of LA systolic function, which is related to the magnitude of scar formation.20 During follow-up, 40–70% of patients who undergo ablation show a decrease in LA ejection fraction, particularly if they have demonstrated biochemical evidence of cardiomyocyte death (as reflected by CK-MB and troponin release); this is especially likely with extensive or multiple procedures.24 In one-third of patients, restoration of electrocardiographic P waves is not accompanied by haemodynamic evidence of meaningful atrial systole, i.e. ‘a waves’ on pressure tracings.25 Even in those who maintain an atrial systolic contribution to LV filling, the magnitude of the atrial-generated pressure wave is meaningfully diminished, which is disadvantageous if LV filling during diastole is already compromised.26 Conversely, attenuation of atrial contractile force could be adaptive, since the full restoration of atrial systole might promote retrograde flow into the pulmonary veins and exacerbate pulmonary hypertension.27 In any case, those who cannot generate atrial contractile force despite normal electrical activation have an increased risk of recurrent AF.25
Therefore, although ablation seeks to restore atrial contractile function, the ability of the LA to transport pulmonary venous blood (which includes the reservoir, conduit and systolic phases of atrial function) is decreased by the procedure,20 especially in patients with long-standing AF and pre-existing LA fibrosis.
Consequences of the ablation-related impairment of left atrial distensibility
By adding to the fibrotic burden of the diseased LA, catheter ablation impairs the capacitance of the chamber, often dramatically. The procedure characteristically leads to a decrease in LA volume and LA expansion index, which is related to the number of ablation lines or procedures, and thus, scar formation.20,28 The reduction in chamber distensibility following catheter ablation causes LA pressures to increase disproportionately as the chamber fills with pulmonary venous blood, even though sinus rhythm is restored. Post-ablation increases in LA pressures are seen in up to 40% of unselected patients29; such increases presage a high rate of recurrence of AF during follow-up.30,31
In some patients, the distensibility of the LA is so severely compromised that the chamber becomes patently stiff and has exceptional difficulty accommodating even modest levels of pulmonary venous return. Pulmonary venous and arterial pressures increase markedly, leading to worsening dyspnoea and exercise intolerance, despite sinus rhythm.28,31,32 Patients with pre-existing impairment of LA distensibility are at particular risk. The true incidence of this ‘stiff LA syndrome’ is not known. Although current estimates suggest that its occurrence in ≈10% of unselected patients who undergo ablation,30 there are few prospective studies of this complication, and the risk is likely to be meaningfully higher in those with heart failure and pre-existing LA fibrosis. Of note, these adverse effects on the mechanical functions of the LA are not seen following pharmacological cardioversion.33
The stiff LA syndrome should be distinguished from the development of heart failure immediately following an ablation procedure. New-onset or worsening heart failure is seen in 15–30% patients of patients shortly following ablation, especially if it involves numerous ablation lines.32 The volume load administered as a result of open irrigation can contribute to the occurrence of these events,34 as can the early necrosis and tissue oedema seen in many individuals.21,22
Electrophysiological and neurohormonal consequences of left atrial ablation
Left atrial fibrosis can destabilize the chamber’s electrophysiological properties. Ablation of septal lines and extensive septal complex fractionated electrograms can delay LA activation or even cause dissociation of biatrial activation.35 More commonly, ablation-mediated injury can result in areas with discordant voltage that predispose to recurrent AF.36 Although electrophysiologists often recommend a repeat ablation for recurrent AF, the incremental burden of additional post-procedural fibrosis may further destabilize the substrate and predispose to AF recurrence, leading to a diminishing chance of success with each procedure. Patients with substantial LA fibrosis by magnetic resonance imaging have evidence of increased re-entrant activity by high-resolution electrocardiographic mapping21 and a high rate of procedural failure.36,37
Ablation-mediated restoration of sinus rhythm leads to a sustained decrease in circulating natriuretic peptides, and re-elevation of peptide levels presages the recurrence of AF.37 These observations are primarily relevant to patients with relatively healthy LA and only paroxysmal AF. Long-term lowering of circulating natriuretic peptides probably reflects the restoration of sinus rhythm, since it is also observed following electrical cardioversion.38 However, in contrast to electrical cardioversion, ablation can lead to an immediate increase in natriuretic peptides due to acute cytolysis of LA tissue.39 Furthermore, in patients with a severely diseased LA and long-standing AF, the sustained decreases in natriuretic peptides levels following ablation may be related to a loss of LA cardiomyocytes,39 which are the primary source of peptide synthesis. This diminution of circulating natriuretic peptides might have adverse physiological consequences in chronic heart failure. The adaptive actions of these peptides on the heart and kidneys are already attenuated in these patients, as evidence by the benefits that accrue following long-term neprilysin inhibition. A disproportionate fall in natriuretic peptides following ablation-induced LA injury might contribute to post-procedural increases in cardiac filling pressures.30
Identification of high-risk phenotypes for catheter ablation
The adverse effects of catheter ablation on LA function may be particularly important in certain high-risk phenotypes. Patients who are obese or have Type 2 diabetes, or both, typically exhibit accumulation and inflammation of epicardial adipose tissue, which is often accompanied by injury and fibrosis of the underlying atrial myocardium, and thereby, to abnormalities of electrogram fractionation and AF.15 These regional interactions likely explain (in part) why patients with obesity or type 2 diabetes are at increased risk of AF.40,41 Poor glycaemic control increases the risk of AF41; conversely, dramatic degrees of weight loss and certain antihyperglycaemic drugs can ameliorate AF.42,43 Not surprisingly, patients with diabetes, obesity or increased epicardial fat have a high rate of procedural failure with catheter ablation44,45 and are particularly likely to develop stiff LA syndrome, especially following repeated procedures.46
Ablation-induced LA dysfunction may be particularly important in HFpEF. The syndrome is commonly accompanied by obesity and diabetes, and the prevalence of AF is extremely high (≈60%), most likely related to extensive atrial inflammation and fibrosis. Atrial fibrosis explains why LA systolic performance and reservoir functions are impaired in these individuals47; yet, because of impaired LA distensibility, LA volumes, and natriuretic peptides are not markedly increased, despite a meaningful elevation of LA pressures.15,47 Ablation-induced scar can add to the high pre-existing LA fibrotic burden. This fibrosis would be expected to presage a low rate of procedural success in HFpEF, and the post-ablation loss of LA capacitance can increase pulmonary venous pressures and worsen the clinical status of these patients.30
Evidence for left atrial dysfunction in trials of catheter ablation in heart failure
There have been six randomized controlled trials of catheter ablation for AF in HFrEF (Table 1)48–53, and the CABANA study54 also enrolled a small group of these patients. Although several trials reported favourable effects of ablation on functional capacity and exercise tolerance, such benefits have been difficult to interpret in these unblinded studies, since symptomatic assessments are influenced by knowledge of the treatment assignment. The effects of ablation on mortality have been reported in two trials (CASTLE-AF and AATAC)50,51, but the data have been sparse (<100 deaths combined). The former suffered from important methodological limitations,55 and in both trials, the comparator groups received membrane-active antiarrhythmic drugs that can increase the risk of death and heart failure.
Table 1.
Randomized clinical trials of catheter ablation for atrial fibrillation in patients with chronic heart failure (ranked by severity of heart failure, least to most severe)
| Patients studied | Effect on LA and LV function | Effect on exercise tolerance and quality-of-life | Effect on morbidity and mortality | Limitations of evidence | |
|---|---|---|---|---|---|
| CAMERA-MRI48 | n = 66, EF ≈33%, BNP ≈260 pg/mL, most with long-standing AF | Increase in EF by MRI if minimal pre-existing fibrosis; decrease in LA volume after 6 months | No benefit on exercise tolerance or quality-of-life, despite lack of blinding | No meaningful data on clinical events | Levels of exercise tolerance and natriuretic peptides inconsistent with meaningful heart failure |
| ARC-HF49 | n = 52, EF ≈24%, BNP ≈350 pg/mL, most with long-standing AF | No significant increase in radionuclide EF; decrease in LA (but not right atrial) area after 1 year | Increase in exercise tolerance and quality-of-life, but lack of blinding | No meaningful data on clinical events | — |
| CASTLE-AF50 | n = 397, EF ≈30%, no baseline data on BNP; long-standing AF in only 30% | Reported increase in EF assessed by echocardiography; no data on LA function | Reported increase in exercise tolerance, but lack of blinding; no measures of quality-of-life | Reduced risk of death and of hospitalization for heart failure, but comparator group treated with membrane-active drugs | 20% of randomized patients not in primary analysis (more in ablation group); baseline imbalances at randomization (medical group had more severe disease) |
| AATAC51 | n = 203, EF ≈30%, BNP not reported, mean AF duration <1 year | Reported increase in EF assessed by echocardiography; no data on LA function | Increase in exercise tolerance and quality-of-life, but lack of blinding | Numerically fewer deaths in ablation group; but comparator group treated with amiodarone | No data on heart failure hospitalizations; sparse data on mortality |
| CAMTAF52 | n = 55, EF ≈32%, BNP ≈500 pg/mL, most with long-standing AF | Reported increase in EF assessed by echocardiography; no data on LA function | Increase in exercise tolerance and quality-of-life, but lack of blinding | No meaningful data on clinical events | — |
| MacDonald et al.53 | n = 41, EF ≈18%, N-terminal proBNP ≈2200 pg/mL, typically long-standing AF | No increase in EF by MRI; no data on LA function | No benefit on exercise tolerance or quality-of-life, despite lack of blinding | No meaningful data on clinical events | Baseline imbalances (medical group had less severe disease) |
AF, atrial fibrillation; BNP, brain natriuretic peptide; EF, ejection fraction; LA, left atrial; MRI, magnetic resonance imaging.
Only two of the six trials (CAMERA-MRI and ARC-HF)48,49 evaluated the potential for catheter ablation to exert adverse effects on LA structure and function, and in both studies, the patients had minimal evidence for or only mild degrees of heart failure prior to the procedure (Table 1). In both trials, AF ablation led to a decrease in LA (but not right atrial) volumes at 6–12 months. However, neither trial evaluated LA filling dynamics, pressure-volume relationships, or capacitance. Interestingly, the ARC-HF trial reported structural improvement in LV geometry following ablation, but only in patients without pre-procedural cardiac fibrosis. In contrast, MacDonald et al.53 evaluated patients who likely had extensive fibrosis, since they had long-standing AF, an EF of 18%, and a N-terminal proBNP >2000 pg/mL. Despite the open-label design of this trial, favourable effects on exercise tolerance and quality-of-life were not observed, and the rate of AF recurrence was high (Table 1).
The incidence of stiff LA syndrome in patients with meaningful pre-procedural LA fibrosis is unknown. Worsening dyspnoea in these patients is often (and perhaps mistakingly) attributed to worsening of LV function, since LA pressure–volume relationships and filling dynamics are not routinely evaluated in clinical practice. In particular, the potential adverse effects of ablation on LA function in patients with marked pre-existing cardiac fibrosis (i.e. those with HFpEF) are unknown, since such individuals have not been systematically evaluated for meaningful periods of time following one or more ablation procedures.
Only 15% of the patients enrolled in the CABANA trial54 had heart failure at the time of randomization, and primary endpoint events were observed in only 49 patients. The study included patients with HFrEF and HFpEF, but the effect of ablation on the progression of heart failure in these subgroups is not known. However, given the overall small number of participants and events, the trial is not likely to add meaningfully to the existing evidence base.
Summary and conclusions
AF reflects significant underlying long-standing structural and functional abnormalities of the LA, which are not resolved by the restoration of sinus rhythm. Efforts to interrupt neural circuits with catheter ablation can cause further injury to the LA, as evidenced acutely, by cardiomyocyte necrosis and tissue oedema, and chronically, by the loss of contractile and reservoir function as a result of replacement fibrosis. The extent of these changes depends on the number of ablation lines and ablation procedures. Up to 30–35% of the LA mass may be replaced by scar following catheter ablation.
In patients with a relatively healthy LA who typically have only paroxysmal AF, these deleterious changes in the LA may be well-tolerated, and restoration of sinus rhythm may have favourable effects on LV structure and function if there was minimal evidence for heart failure and the ventricular response had been rapid despite pharmacological treatments.56 In contrast, in patients with severe underlying LA disease (generally those with long-standing AF and particularly when associated with obesity, diabetes, and HFpEF), the injurious effects of catheter ablation can impair the reservoir, conduit, and transport functions of the LA, which can have important haemodynamic and clinical consequences. Although ablation is intended to restore atrial systole, the inflammatory and fibrotic response to the procedure has deleterious effects on total LA emptying. The most important consequence of ablation-induced fibrosis is to impair LA distensibility and cause a stiff LA syndrome, leading to worsening dyspnoea and heart failure. The risk of stiff LA syndrome is likely to be high when ablation is performed in patients who already have a fibrotic noncompliant LA chamber, particularly those who have HFpEF. Since the prevalence of AF in these patients is ≈60%, interest in using catheter ablation in HFpEF is growing, despite the need for repetitive procedures and the absence of demonstrable benefit on symptoms or outcomes in controlled clinical trials.
Although the fibrotic LA in chronic heart failure is an ideal substrate for the development of AF, it may be a suboptimal substrate for catheter ablation. Such patients are not likely to experience long-term restoration of sinus rhythm, and catheter ablation can worsen their haemodynamic and clinical status. Given the known consequences of catheter ablation, the benefit-to-risk in most patients with long-standing AF, advanced atrial remodelling, and underlying heart failure (both HFrEF and HFpEF) remains to be established. The cautious approach recommended in current ESC guidelines is warranted.57
Conflict of interest: M.P. has recently consulted for Abbott, Actavis, Akcea, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cardiorentis, Daiichi Sankyo, Gilead, Johnson & Johnson, NovoNordisk, Pfizer, Relypsa, Sanofi, Synthetic Biologics, and Theravance.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
References
- 1. Mehrzad R, Rajab M, Spodick DH.. The three integrated phases of left atrial macrophysiology and their interactions. Int J Mol Sci 2014;15:15146–15160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Imanishi J, Tanaka H, Sawa T, Motoji Y, Miyoshi T, Mochizuki Y, Fukuda Y, Tatsumi K, Matsumoto K, Okita Y, Hirata K.. Association of left atrial booster-pump function with heart failure symptoms in patients with severe aortic stenosis and preserved left ventricular ejection fraction. Echocardiography 2015;32:758–767. [DOI] [PubMed] [Google Scholar]
- 3. Myers BD, Peterson C, Molina C, Tomlanovich SJ, Newton LD, Nitkin R, Sandler H, Murad F.. Role of cardiac atria in the human renal response to changing plasma volume. Am J Physiol 1988;254:F562–F573. [DOI] [PubMed] [Google Scholar]
- 4. Ambale-Venkatesh B, Yoneyama K, Sharma RK, Ohyama Y, Wu CO, Burke GL, Shea S, Gomes AS, Young AA, Bluemke DA, Lima JA.. Left ventricular shape predicts different types of cardiovascular events in the general population. Heart 2017;103:499–507. [DOI] [PubMed] [Google Scholar]
- 5. den Uijl DW, Cabanelas N, Benito EM, Figueras R, Alarcón F, Borràs R, Prat S, Guasch E, Perea R, Sitges M, Brugada J, Berruezo A, Mont L.. Impact of left atrial volume, sphericity, and fibrosis on the outcome of catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2018;29:740–746. [DOI] [PubMed] [Google Scholar]
- 6. Habibi M, Lima JA, Khurram IM, Zimmerman SL, Zipunnikov V, Fukumoto K, Spragg D, Ashikaga H, Rickard J, Marine JE, Calkins H, Nazarian S.. Association of left atrial function and left atrial enhancement in patients with atrial fibrillation: cardiac magnetic resonance study. Circ Cardiovasc Imaging 2015;8:e002769.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ágoston G, Szilágyi J, Bencsik G, Tutuianu C, Klausz G, Sághy L, Varga A, Forster T, Pap R.. Impaired adaptation to left atrial pressure increase in patients with atrial fibrillation. J Interv Card Electrophysiol 2015;44:113–118. [DOI] [PubMed] [Google Scholar]
- 8. Schneider C, Malisius R, Krause K, Lampe F, Bahlmann E, Boczor S, Antz M, Ernst S, Kuck KH.. Strain rate imaging for functional quantification of the left atrium: atrial deformation predicts the maintenance of sinus rhythm after catheter ablation of atrial fibrillation. Eur Heart J 2008;29:1397–1409. [DOI] [PubMed] [Google Scholar]
- 9. Triposkiadis F, Pieske B, Butler J, Parissis J, Giamouzis G, Skoularigis J, Brutsaert D, Boudoulas H.. Global left atrial failure in heart failure. Eur J Heart Fail 2016;18:1307–1320. [DOI] [PubMed] [Google Scholar]
- 10. Dernellis JM, Stefanadis CI, Zacharoulis AA, Toutouzas PK.. Left atrial mechanical adaptation to long-standing hemodynamic loads based on pressure-volume relations. Am J Cardiol 1998;81:1138–1143. [DOI] [PubMed] [Google Scholar]
- 11. Pellicori P, Zhang J, Lukaschuk E, Joseph AC, Bourantas CV, Loh H, Bragadeesh T, Clark AL, Cleland JG.. Left atrial function measured by cardiac magnetic resonance imaging in patients with heart failure: clinical associations and prognostic value. Eur Heart J 2015;36:733–742. [DOI] [PubMed] [Google Scholar]
- 12. Maiello M, Sharma RK, Matteo CM, Reddy HK, Palmiero P.. Differential left atrial remodeling in LV diastolic dysfunction and mitral regurgitation. Echocardiography 2009;26:772–778. [DOI] [PubMed] [Google Scholar]
- 13. von Roeder M, Rommel KP, Kowallick JT, Blazek S, Besler C, Fengler K, Lotz J, Hasenfuß G, Lücke C, Gutberlet M, Schuler G, Schuster A, Lurz P.. Influence of left atrial function on exercise capacity and left ventricular function in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging 2017;10:pii:e005467. doi:10.1161/CIRCIMAGING.116.005467. [DOI] [PubMed] [Google Scholar]
- 14. Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA.. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail 2015;8:295–303. [DOI] [PubMed] [Google Scholar]
- 15. Packer M. The epicardial adipose inflammatory triad: coronary athero-sclerosis, atrial fibrillation, and heart failure with a preserved ejection fraction. Eur J Heart Fail 2018;20:1567–1569. [DOI] [PubMed] [Google Scholar]
- 16. Dibner-Dunlap ME, Thames MD.. Control of sympathetic nerve activity by vagal mechanoreflexes is blunted in heart failure. Circulation 1992;86:1929–1934. [DOI] [PubMed] [Google Scholar]
- 17. Giannopoulos G, Kossyvakis C, Efremidis M, Katsivas A, Panagopoulou V, Doudoumis K, Raisakis K, Letsas K, Rentoukas I, Pyrgakis V, Manolis AS, Tousoulis D, Stefanadis C, Deftereos S.. Central sympathetic inhibition to reduce postablation atrial fibrillation recurrences in hypertensive patients: a randomized, controlled study. Circulation 2014;130:1346–1352. [DOI] [PubMed] [Google Scholar]
- 18. Ibebuogu UN, Gladysheva IP, Houng AK, Reed GL.. Decompensated heart failure is associated with reduced corin levels and decreased cleavage of pro-atrial natriuretic peptide. Circ Heart Fail 2011;4:114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van den Berg MP, Tjeerdsma G, Jan de Kam P, Boomsma F, Crijns HJ, van Veldhuisen DJ.. Longstanding atrial fibrillation causes depletion of atrial natriuretic peptide in patients with advanced congestive heart failure. Eur J Heart Fail 2002;4:255–262. [DOI] [PubMed] [Google Scholar]
- 20. Wylie JV Jr, Peters DC, Essebag V, Manning WJ, Josephson ME, Hauser TH.. Left atrial function and scar after catheter ablation of atrial fibrillation. Heart Rhythm 2008;5:656–662. [DOI] [PubMed] [Google Scholar]
- 21. Yoshida K, Yui Y, Kimata A, Koda N, Kato J, Baba M, Misaki M, Abe D, Tokunaga C, Akishima S, Sekiguchi Y, Tada H, Aonuma K, Takeyasu N.. Troponin elevation after radiofrequency catheter ablation of atrial fibrillation: relevance to AF substrate, procedural outcomes, and reverse structural remodeling. Heart Rhythm 2014;11:1336–1342. [DOI] [PubMed] [Google Scholar]
- 22. Okada T, Yamada T, Murakami Y, Yoshida N, Ninomiya Y, Shimizu T, Toyama J, Yoshida Y, Ito T, Tsuboi N, Kondo T, Inden Y, Hirai M, Murohara T.. Prevalence and severity of left atrial edema detected by electron beam tomography early after pulmonary vein ablation. J Am Coll Cardiol 2007;49:1436–1442. [DOI] [PubMed] [Google Scholar]
- 23. Dagres N, Hindricks G, Kottkamp H, Varounis C, Bode K, Arya A, Sommer P, Kremastinos DT, Piorkowski C.. Effect of atrial fibrillation ablation on left atrial contractile function in patients with paroxysmal atrial fibrillation and a relatively well preserved atrial function. Acta Cardiol 2009;64:167–169. [DOI] [PubMed] [Google Scholar]
- 24. Kim JS, Im SI, Shin SY, Kang JH, Na JO, Choi CU, Kim SH, Kim EJ, Rha SW, Park CG, Seo HS, Oh DJ, Hwang C, Kim YH, Yong HS, Lim HE.. Changes in left atrial transport function in patients who maintained sinus rhythm after successful radiofrequency catheter ablation for atrial fibrillation: a 1-year follow-up multislice computed tomography study. J Cardiovasc Electrophysiol 2017;28:167–176. [DOI] [PubMed] [Google Scholar]
- 25. Kishima H, Mine T, Takahashi S, Ashida K, Ishihara M, Masuyama T.. Left atrial pressure pattern without a-wave in sinus rhythm after cardioversion affects the outcomes after catheter ablation for atrial fibrillation. Heart Vessels 2018;33:1365–1372. [DOI] [PubMed] [Google Scholar]
- 26. Krezowski JT, Wilson BD, McGann CJ, Marrouche NF, Akoum N.. Changes in left ventricular filling parameters following catheter ablation of atrial fibrillation. J Interv Card Electrophysiol 2016;47:83–89. [DOI] [PubMed] [Google Scholar]
- 27. Schwartzman D, Kanzaki H, Bazaz R, Gorcsan J 3rd.. Impact of catheter ablation on pulmonary vein morphology and mechanical function. J Cardiovasc Electrophysiol 2004;15:161–167. [DOI] [PubMed] [Google Scholar]
- 28. Cochet H, Scherr D, Zellerhoff S, Sacher F, Derval N, Denis A, Knecht S, Komatsu Y, Montaudon M, Laurent F, Pieske BM, Hocini M, Haïssaguerre M, Jaïs P.. Atrial structure and function 5 years after successful ablation for persistent atrial fibrillation: an MRI study. J Cardiovasc Electrophysiol 2014;25:671–679. [DOI] [PubMed] [Google Scholar]
- 29. Kishima H, Mine T, Takahashi S, Ashida K, Ishihara M, Masuyama T.. The impact of elevated left atrial pressure in sinus rhythm after cardioversion on outcomes after catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2016;27:813–819. [DOI] [PubMed] [Google Scholar]
- 30. Witt CM, Fenstad ER, Cha YM, Kane GC, Kushwaha SS, Hodge DO, Asirvatham SJ, Oh JK, Packer DL, Powell BD.. Increase in pulmonary arterial pressure after atrial fibrillation ablation: incidence and associated findings. J Interv Card Electrophysiol 2014;40:47–52. [DOI] [PubMed] [Google Scholar]
- 31. Kumar S, Michaud GF.. The inconvenient truth of elevated left atrial pressure and AF recurrence despite catheter ablation. Heart Rhythm 2014;11:961–962. [DOI] [PubMed] [Google Scholar]
- 32. Huang HD, Waks JW, Contreras-Valdes FM, Haffajee C, Buxton AE, Josephson ME.. Incidence and risk factors for symptomatic heart failure after catheter ablation of atrial fibrillation and atrial flutter. Europace 2016;18:521–530. [DOI] [PubMed] [Google Scholar]
- 33. Escudero EM, San Mauro M, Lauglé C.. Bilateral atrial function after chemical cardioversion of atrial fibrillation with amiodarone: an echo-Doppler study. J Am Soc Echocardiogr 1998;11:365–371. [DOI] [PubMed] [Google Scholar]
- 34. Seiler J, Steven D, Roberts-Thomson KC, Inada K, Tedrow UB, Michaud GF, Stevenson WG.. The effect of open-irrigated radiofrequency catheter ablation of atrial fibrillation on left atrial pressure and B-type natriuretic peptide. Pacing Clin Electrophysiol 2014;37:616–623. [DOI] [PubMed] [Google Scholar]
- 35. Jiang CX, Sang CH, Dong JZ, Liu XP, Long DY, Yu RH, Tang RB, Wu JH, Ning M, Liu C, Ma CS.. Significant left atrial appendage activation delay complicating aggressive septal ablation during catheter ablation of persistent atrial fibrillation. Pacing Clin Electrophysiol 2010;33:652–660. [DOI] [PubMed] [Google Scholar]
- 36. Chelu MG, King JB, Kholmovski EG, Ma J, Gal P, Marashly Q, AlJuaid MA, Kaur G, Silver MA, Johnson KA, Suksaranjit P, Wilson BD, Han FT, Elvan A, Marrouche NF.. Atrial fibrosis by late gadolinium enhancement magnetic resonance imaging and catheter ablation of atrial fibrillation: 5-year follow-up data. J Am Heart Assoc 2018;7:e006313.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zografos TA, Katritsis DG.. Natriuretic peptides as predictors of atrial fibrillation recurrences following electrical cardioversion. Arrhythm Electrophysiol Rev 2013;2:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wozakowska-Kaplon B. Changes in plasma natriuretic peptide levels in patients with atrial fibrillation after cardioversion. Int J Cardiol 2010;144:436–437. [DOI] [PubMed] [Google Scholar]
- 39. Gould PA, Gula LJ, Bhayana V, Subbiah RN, Bentley C, Yee R, Klein GJ, Krahn AD, Skanes AC.. Characterization of cardiac brain natriuretic peptide release in patients with paroxysmal atrial fibrillation undergoing left atrial ablation. Circ Arrhythm Electrophysiol 2010;3:18–23. [DOI] [PubMed] [Google Scholar]
- 40. Grundvold I, Bodegard J, Nilsson PM, Svennblad B, Johansson G, Östgren CJ, Sundström J.. Body weight and risk of atrial fibrillation in 7,169 patients with newly diagnosed type 2 diabetes; an observational study. Cardiovasc Diabetol 2015;14:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dublin S, Glazer NL, Smith NL, Psaty BM, Lumley T, Wiggins KL, Page RL, Heckbert SR.. Diabetes mellitus, glycemic control, and risk of atrial fibrillation. J Gen Intern Med 2010;25:853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, Twomey D, Elliott AD, Kalman JM, Abhayaratna WP, Lau DH, Sanders P.. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol 2015;65:2159–2169. [DOI] [PubMed] [Google Scholar]
- 43. Zhang Z, Zhang X, Korantzopoulos P, Letsas KP, Tse G, Gong M, Meng L, Li G, Liu T.. Thiazolidinedione use and atrial fibrillation in diabetic patients: a meta-analysis. BMC Cardiovasc Disord 2017;17:96.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Glover BM, Hong KL, Dagres N, Arbelo E, Laroche C, Riahi S, Bertini M, Mikhaylov EN, Galvin J, Kiliszek M, Pokushalov E, Kautzner J, Calvo N, Blomström-Lundqvist C, Brugada J; ESC-EHRA Atrial Fibrillation Ablation Long-Term Registry investigators. Impact of body mass index on the outcome of catheter ablation of atrial fibrillation. Heart 2019;105:244–250.30279268 [Google Scholar]
- 45. Lu ZH, Liu N, Bai R, Yao Y, Li SN, Yu RH, Sang CH, Tang RB, Long DY, Du X, Dong JZ, Ma CS.. HbA1c levels as predictors of ablation outcome in type 2 diabetes mellitus and paroxysmal atrial fibrillation. Herz 2015;40: 130–136. [DOI] [PubMed] [Google Scholar]
- 46. Gibson DN, Di Biase L, Mohanty P, Patel JD, Bai R, Sanchez J, Burkhardt JD, Heywood JT, Johnson AD, Rubenson DS, Horton R, Gallinghouse GJ, Beheiry S, Curtis GP, Cohen DN, Lee MY, Smith MR, Gopinath D, Lewis WR, Natale A.. Stiff left atrial syndrome after catheter ablation for atrial fibrillation: clinical characterization, prevalence, and predictors. Heart Rhythm 2011;8:1364–1371. [DOI] [PubMed] [Google Scholar]
- 47. Santos AB, Kraigher-Krainer E, Gupta DK, Claggett B, Zile MR, Pieske B, Voors AA, Lefkowitz M, Bransford T, Shi V, Packer M, McMurray JJ, Shah AM, Solomon SD; PARAMOUNT Investigators. Impaired left atrial function in heart failure with preserved ejection fraction. Eur J Heart Fail 2014;16:1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Prabhu S, Taylor AJ, Costello BT, Kaye DM, McLellan AJA, Voskoboinik A, Sugumar H, Lockwood SM, Stokes MB, Pathik B, Nalliah CJ, Wong GR, Azzopardi SM, Gutman SJ, Lee G, Layland J, Mariani JA, Ling LH, Kalman JM, Kistler PM. Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction: the CAMERA-MRI study. J Am Coll Cardiol 2017;70:1949–1961. [DOI] [PubMed] [Google Scholar]
- 49. Jones DG, Haldar SK, Hussain W, Sharma R, Francis DP, Rahman-Haley SL, McDonagh TA, Underwood SR, Markides V, Wong T.. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure.J Am Coll Cardiol 2013;61:1894–1903. [DOI] [PubMed] [Google Scholar]
- 50. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, Schunkert H, Christ H, Vogt J, Bänsch D; CASTLE-AF Investigators. Catheter ablation for atrial fibrillation with heart failure.N Engl J Med 2018;378:417–427. [DOI] [PubMed] [Google Scholar]
- 51. Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D, Reddy M, Jais P, Themistoclakis S, Dello Russo A, Casella M, Pelargonio G, Narducci ML, Schweikert R, Neuzil P, Sanchez J, Horton R, Beheiry S, Hongo R, Hao S, Rossillo A, Forleo G, Tondo C, Burkhardt JD, Haissaguerre M, Natale A. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial.Circulation 2016;133:1637–1644. [DOI] [PubMed] [Google Scholar]
- 52. Hunter RJ, Berriman TJ, Diab I, Kamdar R, Richmond L, Baker V, Goromonzi F, Sawhney V, Duncan E, Page SP, Ullah W, Unsworth B, Mayet J, Dhinoja M, Earley MJ, Sporton S, Schilling RJ.. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial).Circ Arrhythm Electrophysiol 2014;7:31–38. [DOI] [PubMed] [Google Scholar]
- 53. MacDonald MR, Connelly DT, Hawkins NM, Steedman T, Payne J, Shaw M, Denvir M, Bhagra S, Small S, Martin W, McMurray JJ, Petrie MC. Radiofrequency ablation for persistent atrial fibrillation in patients with advanced heart failure and severe left ventricular systolic dysfunction: a randomised controlled trial.Heart 2011;97:740–747. [DOI] [PubMed] [Google Scholar]
- 54. Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, Noseworthy PA, Rosenberg YD, Jeffries N, Mitchell LB, Flaker GC, Pokushalov E, Romanov A, Bunch TJ, Noelker G, Ardashev A, Revishvili A, Wilber DJ, Cappato R, Kuck KH, Hindricks G, Davies DW, Kowey PR, Naccarelli GV, Reiffel JA, Piccini JP, Silverstein AP, Al-Khalidi HR, Lee KL; CABANA Investigators. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019; doi:10.1001/jama.2019.0693. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Packer M, Kowey PR.. Building castles in the sky. Catheter ablation in patients with atrial fibrillation and chronic heart failure. Circulation 2018;138:751–753. [DOI] [PubMed] [Google Scholar]
- 56. Prabhu S, Costello BT, Taylor AJ, Gutman SJ, Voskoboinik A, McLellan AJA, Peck KY, Sugumar H, Iles L, Pathik B, Nalliah CJ, Wong GR, Azzopardi SM, Lee G, Mariani J, Kaye DM, Ling LH, Kalman JM, Kistler PM.. Regression of diffuse ventricular fibrosis following restoration of sinus rhythm with catheter ablation in patients with atrial fibrillation and systolic dysfunction: a substudy of the CAMERA MRI trial. JACC Clin Electrophysiol 2018;4:999–1007. [DOI] [PubMed] [Google Scholar]
- 57. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H-C, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GYH, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K; ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]


