Abstract
Aims
This trial aimed to evaluate the safety and efficiency of a common and simplified protocol for the surveillance of cardiac implantable electronic devices based on remote monitoring (RM) in patients with pacemakers (PMs) and implantable cardiac defibrillators (ICDs) for at least 24 months.
Methods and results
The RM-ALONE is a multicentre prospective trial that randomly assigned 445 patients in two groups, both followed by RM: the home monitoring-only (HMo) based on RM + remote interrogations (RIs) every 6 months and the HM + IO that adds in-office evaluations every 6 months to RM. Four hundred and forty-five patients were enrolled in the study, 294 PMs and 151 ICDs recipients. In the HMo group, 20% of patients experienced ≥1 major adverse cardiac event (MACE) vs. 19.5% in HM + IO group (P = 0.006 for non-inferiority). The proportion of patients with a PM/ICD who experienced ≥1 MACE was 15.2/29.3% in HMo group and 16.1/26.3% in HM + IO group (hazard ratio 0.95/1.15, 95% confidence interval 0.53–1.70/0.62–2.10). There were 789 in-office evaluations (136 in the HMo and 653 in the HM + IO; P < 0.001). There was a 79.2% reduction of in-office evaluations with no significant differences in unscheduled visits between groups: 122 (54.5%) in HMo and 101 (45.3%) in HM + IO; P = 0.15. The time a physician/nurse spent per patient/follow-up was significantly reduced in the HMo group: 4/5 min (0–30)/(1–30) vs. 10/10 min (0–40)/(1–40) in HM + IO (P < 0.0001).
Conclusion
The RM-ALONE protocol common for ICD and PM surveillance, consisting of RM + RI every 6 months has proven safe and efficient in reducing hospital visits and staff workload.
Keywords: Remote monitoring, Remote interrogation, Pacemaker , Implantable cardiac defibrillator, Telemedicine
See page 1847 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz101)
Introduction
The surveillance of patients carrying cardiac implantable electronic devices (CIEDs) is crucial for the early detection of clinical and technical problems that may arise but places a significant workload on the staff of the institutions that manage them. A Heart Rhythm Society (HRS) survey on physician workforce trends showed that the follow-up of CIEDs was the most frequent activity reported by cardiac electrophysiologists.1
Different trials have shown, besides their reliability, the multiple advantages of remote monitoring (RM: Automated transmission of data based on prespecified alerts; provides rapid detection of arrhythmias or device malfunction) in the management of CIEDs by studying pacemakers (PMs) and implantable cardiac defibrillators (ICDs), mainly separately.2,3
In terms of safety, RM is non-inferior to conventional follow-up in ICDs4,5 and PMs,6 reduces the time to clinical decision, and can therefore prevent many worsening conditions in ICDs4,7,8 and even in PMs using only remote interrogation (RI: Routine, scheduled remote device interrogation structured to mirror in-office follow-ups).9 Moreover, RM reduces inappropriate shocks and spares ICD’s batteries5 and, unlike RI, has shown improvement in survival.10–13
Regarding efficiency, RM and RI reduce the face-to-face visits in PMs and ICDs,4,6,14 and RM has proven to be a cost-effective approach in ICDs,15,16 resulting in a high degree of patient satisfaction.7,11
Despite the wide range of data supporting this technology, the adoption of RM yet remains suboptimal.11 The current expert consensus17,18 recommends after the first visit 2–12 weeks post-implantation, an annual in-person evaluation in addition to continuous RM + RI every 3–12 months for PMs and every 3–6 months for ICDs.19
The main objective of this study is to demonstrate, assuming that RM is the standard follow-up method, that it is possible to completely and safely dispense with face-to-face visits by maintaining the same RI structure every 6 months for both PM- and ICD-bearing patients.
Methods
Study design
RM-ALONE was a prospective, randomized, multicentre clinical trial conducted at 16 Spanish institutions (Supplementary material online, Appendix SA) comparing the safety and efficiency of a RM-only approach (HMo group) vs. RM plus standard in-office follow-up (HM + IO group) in single- or dual-chamber PM and ICD recipients.
The study was designed by a steering committee, composed of physicians who also conducted the study, in collaboration with the sponsor, Biotronik that also participated to the study design and data monitoring (Supplementary material online, Appendixes SB and SC).
The trial was carried out in compliance with Good Clinical Practice and the Declaration of Helsinki and was approved by the Independent ethics committees of each centre. Patient enrolment started on May 2010 and finished on December 2013.
Patients were enrolled after 3–6 months of device implantation (single- or dual-chamber PM or ICD) (Supplementary material online, Appendix D) and setup of device parameters.
Patients were eligible for inclusion in the RM-ALONE trial if: they were ≥18 years, had an implanted CIED equipped with Home Monitoring®, had medical/psychic status controlled, provided written informed consent and had stable Global System for Mobile communications (GSM) network coverage. Patients were excluded if had a replaced implant or upgraded to cardiac resynchronization therapy (CRT). Cardiac resynchronization therapies were excluded in the absence of reliable tools to automatically optimize QRS narrowing at the time of study design requiring an electrocardiogram (ECG) in the follow-ups for this.
Unlike previous studies, no patient was excluded because of PM dependency or ICD indication for secondary prevention.4,6,8,14
Home monitoring20 is a RM system that transmits automatically and daily the data stored in the CIED to the Biotronik HM-Service centre. The staff responsible for the patient’s care can check this information on a secure website, where the patients are automatically classified and flagged for attention. Additionally, physicians are notified on prespecified alerts (Supplementary material online, Appendix online).
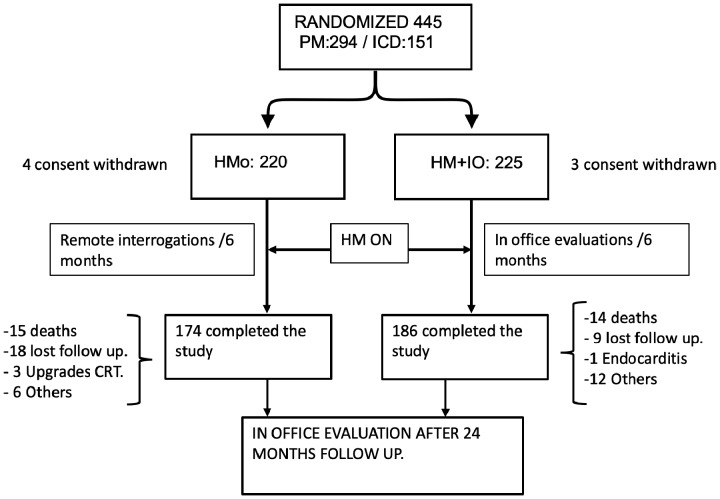
After a 12-week post-implant face-to-face visit to reprogramme the CIED and check the wound, patients were randomly assigned to the HMo group or to the HM + IO group in a 1:1 ratio (Figure 1). To prevent bias, the randomizations blocks and schedule were generated before the study implantation by an external consultant. Randomization was stratified by site and by device, so two randomization schemes (one for ICDs and one for PM) were produced for each site according to these specifications: two groups (HMo and HM + IO groups), allocation ratio (1:1), and block size of 4.
Figure 1.
A flowchart of patients between enrolment and end of follow-up. CRT, cardiac resynchronization therapy; HM + IO, home monitoring plus in-office evaluations; HMo, home monitoring only; ICD, implantable cardiac defibrillator; PM, pacemaker.
Both groups had HM programmed ON, and all the alert events generated were checked at the discretion of each centre according to their usual practice. Patients in the HMo group were scheduled for a RI every 6 months without any patient intervention. Patients in the HM + IO group were scheduled for in-office-visits for device check only every 6 months.19
Patients in both groups attended an in-office evaluation after at least 24 months (end of study visit). All programmed visits with other health providers were unmodified and patients received standard treatment for their underlying diseases. Additional visits could be programmed upon patients and/or physician request or by the occurrence of an alert notified by HM and were considered unscheduled visits.
After every follow-up, patients in both groups received a report summarizing his/her health and device status. While this is a common practice in face-to-face visits, in the remote group this report served to reassure patients.
Study objectives
Primary objective
The primary safety objective was to demonstrate non-inferiority of following up CIEDs with a single remote protocol for PMs and ICDs with RI every 6 months in addition to RM (HMo group) compared to the monitoring with face-to-face visits every 6 months plus RM follow-up (HM + IO group).
The primary study outcome was the proportion of patients with ≥1 major adverse cardiac event (MACE) over 24 months of follow-up.
The following events were considered MACEs: death of any cause, stroke, hospitalization related to device or cardiac cause, and device-related surgical intervention.
Adverse events were reviewed and classified by the Clinical Events Committee (Supplementary material online, Appendix D) composed of an expert cardiologist who was not involved in the trial and was not aware of the treatment allocation groups. The ultimate decision to qualify the events as MACE relied on this committee.
Secondary objectives
The secondary safety objective was to demonstrate that differences in terms of MACE occurrence were not statistically significant between the HMo and HM + IO groups in each type of device (PM and ICD).
The secondary efficiency objectives were to measure the decrease in the number of in-office follow-ups in the HMo group, and to compare the workload of healthcare professionals in following up patients of the HMo and HM + IO groups.
The efficiency was evaluated by means of three parameters: number of in-office evaluations, time per patient in each followup, and total workload.
The time dedicated by each healthcare professional per patient in each follow-up resulted from the total time dedicated by clinicians and nurses to the following activities: (a) follow-up of patients through HM, (b) follow-up of patients through in-office-visits, (c) review of HM alerts, (d) follow-up requested by the patient, and (e) follow-up triggered by HM alerts.
The total workload of each healthcare professional per patient and day was calculated as the total time invested by the staff (a + b + c + d + e) on the surveillance of the patients divided by the number of patients and total days they remained within the study. As the fraction obtained from each staff member per patient was multiplied by 100 patients and 22 working days, the workload of each staff is presented in minutes/100 patients/month.
Statistical analyses
The primary study hypothesis was that patient safety was not inferior in the HMo group compared to the HM + IO group. Non-inferiority was established using a 10% non-inferiority margin for the lower limit of the one-sided 97.5% confidence interval (CI) on the difference in the proportion of patients with ≥1 MACE between groups.
The non-inferiority margin was defined considering data from principal trials on RM. A non-inferiority margin of 5% was defined in TRUST (ICD population)4 and of 7% in COMPAS (PM population).6 In contrast, RM-ALONE trial combined both PMs and ICDs (ratio of 2:1), increasing the heterogeneity of the sample population. Consequently, and given the random nature of the onset of arrhythmic events, the non-inferiority margin was set at 10%, so this had an effect of reducing the total population sample size. Furthermore, the ICD population was further reduced as the event rate in this subgroup of patients was expected to be higher than in those having a PM. That was the reason we had a proportion of 2:1 of PMs vs. ICDs.
According to published studies, the frequency of MACE is 14.9% and, based on these data, 201 patients per arm were required to demonstrate non-inferiority between groups with 80% power. Assuming a drop-out rate of 5%, 424 patients were required (212 per arm). The study was powered only for the primary analysis; secondary endpoints were considered as supportive analysis with an exploratory purpose.
Statistical analyses were performed in the intention-to-treat population comprised by all randomized patients.
Continuous variables were described by the number of available and missing data, mean, 95% CI, median, standard deviation (SD), extremes (min–max); and categorical variables were described by n (%).
The distribution of variables was assessed using the Kolmogorov–Smirnov test, and equality of variances was verified by the Levene’s test. Comparisons between groups for the number of patients with ≥1 MACE were performed using the non-inferiority test and Cox-Regression (hazard ratio [HR] and 95% CI), the log-rank test to evaluate the differences in the time to first MACE, the χ2 test for the number of patients with MACE, and the Analysis of Variance (ANOVA) test for the mean number of MACEs per patient. Comparisons between groups for workload were performed using the ANOVA test or the Mann–Whitney test if abnormal distribution was met.
The level of statistical significance was set at P < 0.05. Statistical analyses were performed using SAS software (SAS Institute, Cary, SC, USA) for Windows, version 9.2. nQuery Advisor 6.2 software was used for sample size calculation.
The statistics work was carried out by an external consultant and a biostatistician contracted by the sponsor.
Results
Study population
Between May 2010 and December 2013, 445 participants were randomized to the HMo group (n = 220) or to the HM + IO group (n = 225). Of them, 294 had an implanted PM and 151 had an ICD. Eighty-five patients (19.1%) finished the study prematurely (before Month 24): 46 (20.0%) in the HMo group and 39 (17.3%) in the HM + IO group (P = 0.337). Most common reasons for early termination were patient’s death (32.6% in the HMo vs. 38.5% in the HM +IO groups), lost to follow-up (26.1% vs. 23.1%), and other reasons (19.6% vs. 30.8%) (Figure 1).
The overall attrition rate (consent withdrawal, moving, and lost to follow-up) was 11.5% and for each group individually 12.7% (HMo) and 10.2% (HM + IO) (P = 0.461).
Baseline characteristics were balanced between both arms. Demographic and clinical characteristics are shown in Supplementary material online, Table S1. The mean duration of follow-up was 20.7 ± 7.1 months.
Mean age in the overall population was 68.9 years and 71% were men, with no marked differences between groups. Among all the participants included, 33.9% had an implanted ICD and 66.1% a PM, both showing a similar distribution in clinical and demographic baseline characteristics (Table 1).
Table 1.
Baseline characteristics in pacemaker and implantable cardiac defibrillator patients
| PMs | HMo (n: 145) | HM + IO (n: 149) | Total (n: 294) |
| Sex male/female (male%) | 89/56 (61.4) | 100/49 (67.1) | 189/105 (64.3) |
| Age (years), mean ± SD (min–max) | 72.2 ± 11 (25–89) | 73.1 ± 9.9 (19–87) | 72.7 ± 10.6 (19–89) |
| NYHA ≥III | 8 (5.5) | 4 (2.7) | 12 (4) |
| LVEF (%), mean ± SD | 62.2 ± 8 | 62 ± 9.7 | 62.2 ± 8.8 |
| Persistent/permanent AF | 23 (15.9) | 20 (13.4) | 43 (14.6) |
| Paroxysmal AF | 15 (10.3) | 27 (18.1) | 42 (14.28) |
| Pacing indication | |||
| Sick sinus syndrome | 43 (29.7) | 58 (39) | 101 (34.4) |
| Slow AF | 27 (18.6) | 27 (18.1) | 54 (18.4) |
| AV block | 94 (64.8) | 88 (59.1) | 182 (61.9) |
| Neuromediated syncope | 5 (3.4) | 6 (4) | 11 (3.7) |
| Others | 11 (7.6) | 10 (6.7) | 21 (7.1) |
| Underlying heart disease | |||
| Primary conduction system disease | 104 (71.7) | 104 (69.8) | 208 (70.7) |
| Ischaemic heart disease | 17 (11.7) | 27 (18.1) | 44 (15) |
| Valvular heart disease | 8 (5.51) | 12 (8) | 20 (6.8) |
| Single chamber | 30 (20.7) | 30 (20.13) | 60 (20.4) |
| Dual chamber | 115 (79.3) | 119 (79.9) | 234 (79.6) |
| ICDs | HMo (n: 75) | HM + IO (n: 76) | Total (n: 151) |
| Sex male/female (male%) | 63/12 (84) | 64/12 (84.2) | 127/24 (84) |
| Age (years), mean ± SD (min–max) | 62.5 ± 14.8 (18–89) | 60.4 ± 13.8 (28–85) | 61.5 ± 14.3 (18–89) |
| NYHA ≥III | 2 (2.7) | 4 (5.3) | 6 (4) |
| LVEF (%), mean ± SD | 38.3 ± 14 | 36.9 ± 15 | 37.6 ± 14.9 |
| Persistent/permanent AF | 17 (22.6) | 13 (17.1) | 30 (19.9) |
| Paroxysmal AF | 2 (2.66) | 4 (5.26) | 6 (3.4) |
| ICD indication | |||
| Primary prevention | 40 (53.3) | 44 (58) | 84 (55.6) |
| Secondary prevention | 35 (46.6) | 32 (42) | 67 (44.4) |
| Underlying heart disease | |||
| Ischaemic cardiomyopathy | 40 (56.3) | 43 (60.6) | 72 (58.5) |
| Dilated cardiomyopathy | 13 (17.3) | 16 (21) | 29 (19.2) |
| Hypertrophic cardiomyopathy | 13 (17.3) | 9 (11.8) | 22 (14.6) |
| Channelopathies | 2 (2.7) | 1 (1.3) | 3 (2) |
| Single chamber | 54 (72) | 55 (72.3) | 109 (72.2) |
| Dual chamber | 21 (28) | 21 (27.6) | 42 (27.8) |
Results are expressed as n (%).
AF, atrial fibrillation; HM + IO, HM + in-office; HMo, HM-only; ICD, implantable cardiac defibrillator; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PM, pacemaker.
No statistically significant differences were found for any variable (P < 0.05).
In PM patients, 79.6% were double chambered, 61.9% indicated by atrioventricular (AV) block, 70.7% diagnosed of primary disease of the conduction system, and 54% were PM dependent (>75% or time paced).
In ICD patients, 72.2% were single-chamber devices, 55.6% indicated in primary prevention, and 58.5% due to ischaemic cardiomyopathy (Table 1).
Safety assessments
Overall population
No significant differences in safety were observed between the HMo and the HM + IO groups either in the total population or in the PM and ICD subgroups.
By the end of the trial, 88 patients (19.8%) showed ≥1 MACE; 44 (20%) in the HMo group and 44 (19.5%) in the HM + IO group, confirming the non-inferiority for the primary endpoint (P = 0.006; HR 1.04, 95% CI 0.68–1.58, P = 0.838 χ2 test) (Table 2). The mean ± SD number of MACEs experienced per patient was statistically comparable between groups: 0.34 ± 0.79 and 0.36 ± 0.87 for patients followed by HMo and HM + IO, respectively (P = 0.894).
Table 2.
Rate of major adverse cardiac events in the population
| Overall population (n: 445) | HMo (n: 220) | HM + IO (n: 225) | P-value; HR (95% CI) |
| Number of patients ≥1 MACE (n: 88) | 44 (20) | 44 (19.5) | 0.838; 1.04 (0.68–1.58)a |
| Deaths: CV/non-CV/unknown | 15 (6.8): 7/8 | 15 (6.7): 8/6/1 | 0.942; 0.97 (0.47–1.99) |
| Stroke | 5 (2.2) | 4 (1.8) | 0.644 |
| Device-related surgery | 2 (0.9) | 7 (3.1) | 0.110 |
| Hospitalizations due to CIED or CV reasons | 53 | 55 | 0.708 |
| Total number of MACEs (mean ± SD) | 75 (0.34 ± 0.79) | 81 (0.36 ± 0.87) | 0.894 |
| Number of patients AF detected | 44 (20) | 47 (20.8) | 0.816 |
| PM-bearing patients (n: 294) | HMo (n: 145) | HM + IO (n: 149) | |
| Number of patients ≥1 MACE (n: 46) | 22 (15.2) | 24 (16.1) | 0.876; 0.95 (0.53–1.70) |
| Deaths CV/non-CV/unknown | 8 (5.5): 4/4 | 12 (8): 6/5/1 | 0.402; 1.46 (0.59–3.57) |
| Stroke | 5 (3.4) | 1 (0.7) | 0.055 |
| Device-related surgery | 0 (0) | 2 (1.3) | 0.188 |
| Hospitalizations due to CIED or CV reasons | 25 | 30 | 0.933 |
| Total number of MACEs (mean ± SD) | 38 (0.26 ± 0.70) | 45 (0.3 ± 0.86) | 0.925 |
| Number of patients AF detected | 37 (25.5) | 40 (26.8) | 0.795 |
| ICD-bearing patients (n: 151) | HMo (n: 75) | HM + IO (n: 76) | |
| Number of patients ≥1 MACE | 22 (29.3) | 20 (26.3) | 0.649; 1.15 (0.62–2.10) |
| Deaths CV/non-CV/unknown | 7 (9.3): 3/4 | 3 (3.9): 2/1 | 0.173; 0.40 (0.1–1.56) |
| Stroke | 0 (0) | 3 (3.9) | 0.073 |
| Device-related surgery | 2 (2.6) | 5 (6.6) | 0.218 |
| Hospitalizations due to CIED or CV reasons | 28 | 25 | 0.551 |
| Total number of MACEs (mean ± SD) | 37 (0.49 ± 0.93) | 36 (0.47 ± 0.90) | 0.793 |
| Number of patients AF detected | 7 (9.3) | 7 (9.2) | 0.979 |
| ICD therapies delivered | |||
| Patients receiving ≥1 appropriate therapy delivery | 26 (34.6) | 21 (27.6) | 0.350 |
| Patients receiving ≥1 inappropriate therapy delivery | 7 (9.3) | 7 (9.2) | 0.979 |
| Number of appropriate shocks delivered (mean ± SD) | 32 (0.43 ± 1.45)b | 11 (0.14 ± 0.53) | 0.268 |
| Number of patients ≥1 appropriate shock delivered | 11 (14.6) | 7 (9.2) | 0.300 |
| Number of inappropriate shocks delivered (mean ± SD) | 9 (0.12 ± 0.63) | 3 (0.04 ± 0.25) | 0.394 |
| Number of patients ≥1 inappropriate shock delivered | 4 (5.3) | 2 (2.6) | 0.395 |
Results are expressed as n (%).
AF, atrial fibrillation; CIED, cardiac implantable electronic device; CV, cardiovascular; HM, home monitoring.
Non-inferiority P = 0.006.
In this group, three patients suffered an electrical storm (≥3 shocks within 24 h) and were delivered 10, 5, and 4 appropriate shocks, and other patient was delivered four shocks within the 24 months, so four patients received 72% of the total shocks in the HMo group.
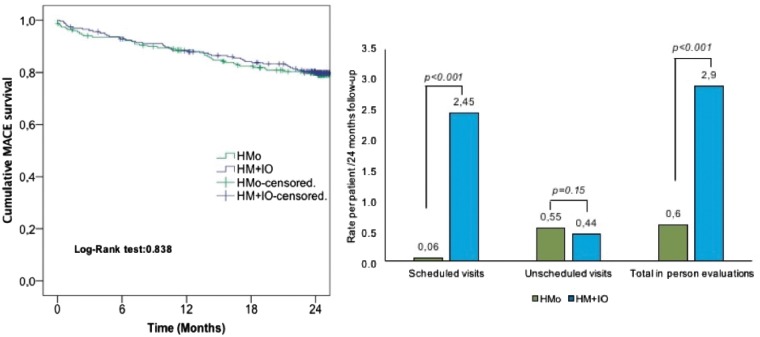
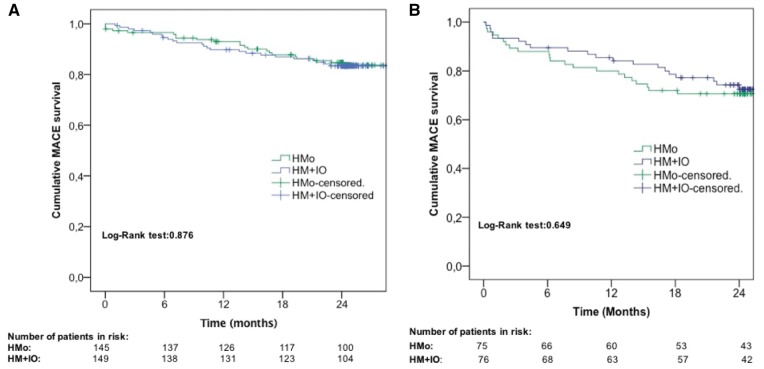
Time to first MACE did not significantly differ between both groups in the overall population and neither in PM- nor in ICD-implanted patients (Figures 2and3, Take home figure).
Figure 2.
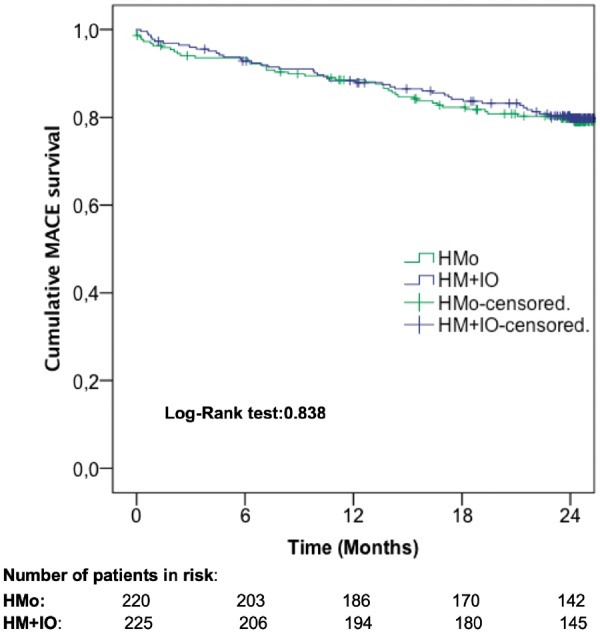
Cumulative major adverse cardiac event survival in the overall population. HM + IO, home monitoring plus in-office evaluations; HMo, home monitoring only.
Figure 3.
Cumulative major adverse cardiac event survival in the pacemaker (A) and implantable cardiac defibrillator (B) population. HM + IO, home monitoring plus in-office evaluations; HMo, home monitoring only.
Take home figure.
In an additional analysis, we found no significant differences between the two groups for each independent component of the composite MACE.
Fifteen patients (6.8%) died in the HMo group and 15 (6.6%) in the HM + IO group (P = 0.942; HR 0.97, 95% CI 0.47–1.99). Among them, three were due to heart failure (HF), three to strokes, one to sudden unexplained death (SCD) (one PM-bearing patient), and eight to non-cardiac causes in the HMo group, whereas in the HM + IO four patients died from HF, one from an acute myocardial infarction, two from a SCD (one PM-and one ICD-bearing patients), one from stroke, six from non-cardiac causes, and one from unknown cause. The number of MACEs is listed in Table 2.
Pacemaker-implanted patients
By the end of the trial, 46 patients (15.6%) showed ≥1 MACE; 22 (15.2%) in the HMo group and 24 (16.1%) in the HM + IO group, confirming no statistically significant differences between groups (P = 0.876; HR 0.95, 95% CI 0.53–1.70).
The mean ± SD number of MACEs experienced per patient was statistically comparable between groups: 0.26 ± 0.70 and 0.3 ± 0.86 for patients followed by HMo and HM + IO, respectively; P = 0.925.
Eight (5.4%) deaths were registered in the HMo group and 12 (8%) in the HM + IO group (Table 2).
Implantable cardiac defibrillator-implanted patients
In patients with an implanted ICD, ≥1 MACE occurred in 22 patients (29.3%) in the HMo group and in 20 patients (26.3%) in the HM + IO group (P = 0.649; HR 1.15, 95% CI 0.62–2.10).
On average 0.49 ± 0.93 and 0.47 ± 0.90 MACEs were observed per patient in the HMo and HM + IO groups, respectively (P = 0.793).
The number of deaths were 7 (9.3%) in the HMo group and 3 (3.9%) in the HM + IO group (Table 2 andSupplementary material online, Table S2).
Efficiency assessment
Overall population
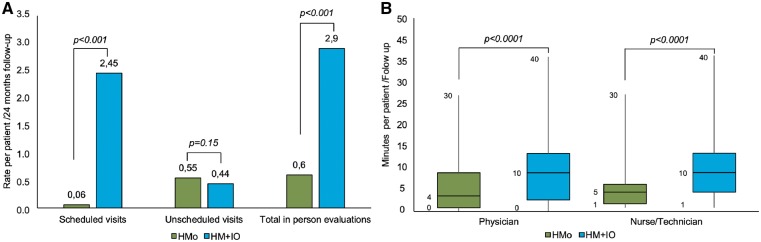
Excluding the start and closing visits, the overall population attended 136 face-to-face visits in the HMo group and 653 in the HM + IO group, representing a 79.2% reduction in in-office visits. If start and closing visits were not excluded the reduction would be 47.8%.
The proportion of unscheduled visits was statistically comparable between groups, with 122 visits in the HMo group and 101 visits in the HM + IO group (P = 0.160). Unscheduled visits were triggered by device alert (28.7% in the HMo group and 23.8% in the HM + IO group), patient request (15.6% in the HMo group and 8.9% in the HM + IO group) and other reasons, mainly by scheduling mistakes (55.7% in the HMo group and 67.3% in the HM + IO group). Differences in the proportions of reasons for unscheduled visits did not reach statistical significance (P = 0.159).
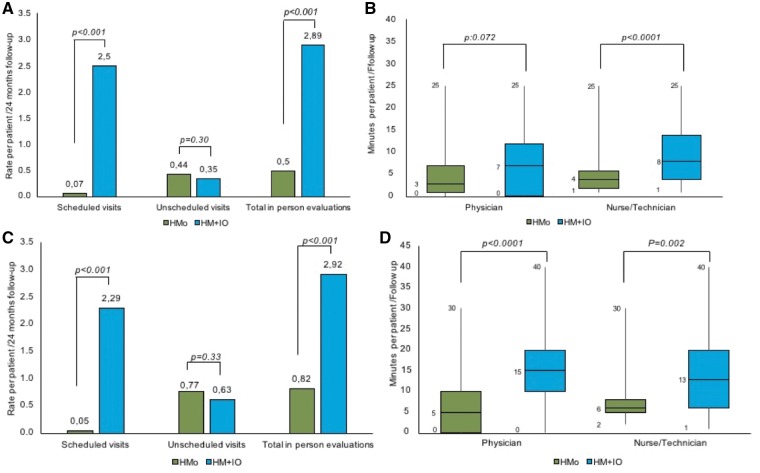
We observed a significant reduction in the rate of scheduled and total in-office visits per patient in the HMo group at 24-month follow-up, without this leading to a significant increase in unscheduled visits (P = 0.15) (Figure 4A, Take home figure).
Figure 4.
Efficiency in the overall population. (A) Difference between groups in the rate of in-person evaluations per patient for the whole follow-up. (B) Difference between groups in the mean time spent by physicians and nurses per patient and follow-up.
Pacemaker-bearing patients
The PM-recipient population underwent 74 in-office evaluations in the HMo group and 431 in the HM + IO group, representing an 82.8% reduction in face-to-face visits. Of the total number of visits, the proportion of unscheduled visits was 86.5% in the HMo group and 12.3% in the HM + IO group.
We observed a significant reduction in the number of scheduled and total in-office visits that did not result in a significant increase in unscheduled visits (P = 0.309) (Figure 5A).
Figure 5.
Efficiency in the pacemaker (A, B) and implantable cardiac defibrillator (C, D) population. (A and C) Difference between groups in the rate of in-person evaluations per patient for the whole follow-up. (B and D) Difference between groups per patient and follow-up measuring the mean time (min) spent by staff members on each patient in any of the follow-up activities.
Implantable cardiac defibrillator-bearing patients
The ICD population underwent 62 in-office evaluations in the HMo group and 222 in the HM + IO group, with a 72% reduction in face-to-face visits in the HMo group. Unscheduled visits represented a 93.5% of the total number of visits in the HMo group and a 21.6% in the HM + IO group.
We observed a significant reduction in the number of scheduled and total in-office visits and no significant increase in unscheduled visits in the HMo group (P = 0.33) (Figure 5C).
Staff workload
The mean time required by the clinician per patient on the total amount of follow-ups (remote + in-office) was significantly shorter in the HMo group than in the HM + IO group; 5.9 ± 6.7 min/follow-up vs. 10.2 ± 8.1 min/follow-up, respectively (P < 0.0001). Similarly, the mean time spent by nurses for the monitoring of patients with HMo was significantly lower (6.3 ± 5.7 min/follow-up) than for HM + IO (11.1 ± 7.2 min/follow-up) (P < 0.0001) (Figure 4B). These differences are maintained in both PM or ICD devices (Figure 5B and D, respectively).
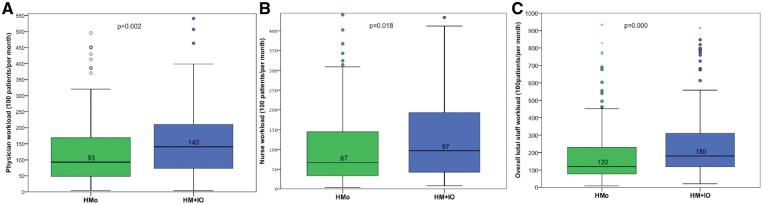
The median time (min) spent by nurses to review 100 patients/month was 92.86 (3.99–1172) in the HMo group and 140.3 (3.65–1764.7) in the HM + IO group (P = 0.002), and by the physician was 67.10 (3.9–610.4) in the HMo group and 96.9 (8.05–1666.6) in the HM + IO group (P = 0.018). The median workload of the staff was statistically lower in the HMo group than in the HM + IO group [92.86 (3.99–1172) vs. 140.36 (3.65–1764.70); P = 0.002] (Figure 6).
Figure 6.
Staff workload. Minutes per 100 patients and month spent by the whole staff (C) and separately by each member: nurses (A) and physicians (B).
Discussion
Main findings
The large number and complexity of CIEDs makes the work overload unmanageable in many cases. Here, we provide a simplified, safe, and efficient approach for the management of PM- and ICD-implanted patients.
The RM-ALONE protocol consisting of continuous RM and RI every 6 months, demonstrated non-inferiority in terms of safety with respect to continuous RM associated with on-site visits every 6 months for the overall population of PM and ICD.
In addition, RM reduced 79.2% the number of face-to-face visits, without a significant increase in unscheduled follow-ups, and significantly decreased the workload of the personnel involved.
Attrition rate
In a previous trial,4 many scheduled visits were missed in the conventional group, and some data suggested that RM increased patient engagement during follow-up.21 Despite these results, follow-up lengthening was associated with an increase in attrition rate and was one of the main concerns in REFORM reaching 20.7%.14 In our trial, the low attrition rate observed in the HMo group (12.7%) can be plausibly explained by the frequent contact with the patient, even if remotely, which helps them comply with therapy and not leave the follow-ups.
Safety aspects
The main concern about extending the time between in-office visits is that safety may be compromised. In this regard, we have proven non-inferiority in terms of safety in the overall population. Previous randomized trials have shown that RM is non-inferior to conventional follow-ups in terms of safety, but studying independently patients with ICDs and PMs with different follow-up schemes.4–7 To the best of our knowledge, RM-ALONE is the first randomized trial surveilling PMs and ICDs with the same follow-up pattern and using RM as a gold standard in both groups.
RM-ALONE is distinct from prior studies in that patients that were dependent on pacing4,6 or had an ICD for secondary prevention14 were not excluded; therefore, these study attributes are more reflective of a ‘real world’ population.
In RM-ALONE, the number of cardiovascular deaths in ICD patients predominantly followed by RM are slightly higher than those in TRUST4 (2% vs. 1%), even considering that in TRUST only 25% were secondary prevention indications while in RM-ALONE this reached a 46%. However, in the ECOST trial, the percentage of secondary prevention was similar to that in RM-ALONE, so was the total number of cardiovascular deaths in the RM group.5
The proportion of patients with ≥1 appropriate therapy was 34.6% in the HMo group and 27.6% in the HM + IO group (P = 0.350); 58% of the total amount were in secondary prevention ICD-recipients.
The proportion of patients suffering ≥1 appropriate shock in the HMo group (14.6%) was lower than that in the RM group of the ECOST5 (16.3%), whereas the proportion receiving ≥1 inappropriate shock in the HMo group (5.3%) is nearly identical to that in ECOST (5%), maintaining a substantial reduction with respect to the in-person group of the latter (10%). Therefore, compared to conventional in-office follow-up, RM protects from inappropriate therapies.
However, and though there were very few shocks to come to any conclusion, the proportion of patients with ≥1 inappropriate shock in the HMo group is higher than in the HM + IO group (5.3 vs. 2.7%; P = 0.395), and all of them occurred in patients with secondary prevention.
Therefore, we need larger trials to address if dismissing in-office evaluations increases therapies in secondary prevention ICD patients.
Comparing the PM subgroup, we evidenced that RM alone is as safe as adding face-to-face visits. In terms of survival, we found very similar results to COMPASS, and even considering that 54.3% of our patients were PM-dependents, which was a reason for exclusion in the former trial. There were fewer device-related complications in RM-ALONE, which may be due to a later randomization in our trial (3 months vs. 1 month after device implantation).6 It is noteworthy that we can only compare our results with previous trials that use RM (automatic daily monitoring),6 the only system with proven clinical improvement, even in the survival of patients,11 unlike RI that has not shown such improvement in other trial.9
RM-ALONE has been performed using a single proprietary system, as most of the RM trials,4,7,14 but a non-randomized multiproprietary prospective trial has shown that the use of RM is safe in ICD recipients and improves survival in cardiac resynchronization therapy defibrillator (CRTDs).22
Reduction of follow-ups and workload of the staff
Scheduled visits, regardless they are face-to-face or remotely driven, are very often futile and frequently result in no action.6,23 In fact, in RM-ALONE there were 102 significant reprogramming changes, 80% of them due to HM alerts.
We observed a reduction of 79.2% in total interim follow-ups, further more pronounced than in most of the previous trials (ranging from 45% to 56%).4,6 The single randomized prospective trial using only RM alerts as follow-up of PM lasted 18 months6 and the remaining studies either had a duration of <1 year or had some face-to-face visits at 12–15 months.4,8 Therefore, RM-ALONE is, to the best of our knowledge, the trial with the longest surveillance period of CIEDs supported exclusively by RM.
Unlike other trials4,14 showing an increase in unscheduled visits in the group with fewer face-to-face visits, in RM-ALONE we have not observed this trend. We believe that continuous ‘remote’ contact with patients, reassuring them by sending clinical reports avoided many unnecessary visits often caused by patient anxiety.
Limitations
RM-ALONE did not include CRT recipients, so the results might be not transferrable to this group. Though we believe that RM is quite useful from the implant, RM-ALONE protocol is only recommended for ‘stable’ (at least 3 months post-implantation) devices, and lasted 24 months so it could not capture late complications after this period.
RM-ALONE results can be only transferred to platforms capable of continuous RM, and since each proprietary system has its own peculiarities,24 we advise RM + RI every 6 months or occasionally every 3 months depending on the manufacturer characteristics.
Conclusions
The surveillance protocol common for single- and dual-chamber PMs and ICDs described in ‘RM-ALONE’, consisting of continuous RM and RI every 6 months, has proven to be safe for at least 2 years of follow-up and very efficient in terms of reducing hospital visits and staff workload (Take home figure).
Supplementary Material
Acknowledgements
The authors thank Alicia Moreno for her outstanding support. Carla Granados (Trialance SCCL) provided Medical Writing assistance.
Funding
This trial was fully supported by an unrestricted grant Biotronik SE & Co. KG, Berlin, Germany.
Conflict of interest: F.J.G-F. received a research grant from Biotronik and advisor/consulting fees from Medtronic and Boston Scientific. I.F.L. is the recipient of institutional grants from the Spanish Society of Cardiology and the Fundación para Investigación Cardiovascular and advisor/consulting and speaker personal fees from Microport, Biotronik, Medtronic, and Abbott. R.O.L. received consulting fees from Biotronik. F.J.T. received a grant from the Spanish Society of Cardiology. M.M.K. received research grant from Biotronik. All the other authors have no conflicts of interest to disclose.
References
- 1. Deering TF, Clair WK, Delaughter MC, Fisher WG, Garlitski AC, Wilkoff BL, Gillis AM.. A Heart Rhythm Society Electrophysiology Workforce study: current survey analysis of physician workforce trends. Heart Rhythm 2010;7:1346–1355. [DOI] [PubMed] [Google Scholar]
- 2. Ricci RP, Morichelli L, Santini M.. Home monitoring remote control of pacemaker and implantable cardioverter defibrillator patients in clinical practice: impact on medical management and health-care resource utilization. Europace 2008;10:164–170. [DOI] [PubMed] [Google Scholar]
- 3. Vogtmann T, Stiller S, Marek A, Kespohl S, Gomer M, Kuhlkamp V, Zach G, Loscher S, Baumann G.. Workload and usefulness of daily, centralized home monitoring for patients treated with CIEDs: results of the MoniC (Model Project Monitor Centre) prospective multicentre study. Europace 2013;15:219–226. [DOI] [PubMed] [Google Scholar]
- 4. Varma N, Epstein AE, Irimpen A, Schweikert R, Love C; TRUST Investigators. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation 2010;122:325–332. [DOI] [PubMed] [Google Scholar]
- 5. Guedon-Moreau L, Lacroix D, Sadoul N, Clementy J, Kouakam C, Hermida JS, Aliot E, Boursier M, Bizeau O, Kacet S; ECOST trial Investigators. A randomized study of remote follow-up of implantable cardioverter defibrillators: safety and efficacy report of the ECOST trial. Eur Heart J 2013;34:605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mabo P, Victor F, Bazin P, Ahres S, Babuty D, Da Costa A, Binet D, Daubert JC; CONNECT Investigators. A randomized trial of long-term remote monitoring of pacemaker recipients (the COMPAS trial). Eur Heart J 2012;33:1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crossley GH, Boyle A, Vitense H, Chang Y, Mead RH; COMPAS Trial Investigators. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) trial: the value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol 2011;57:1181–1189. [DOI] [PubMed] [Google Scholar]
- 8. Varma N, Michalski J, Stambler B, Pavri BB; TRUST Investigators. Superiority of automatic remote monitoring compared with in-person evaluation for scheduled ICD follow-up in the TRUST trial—testing execution of the recommendations. Eur Heart J 2014;35:1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crossley GH, Chen J, Choucair W, Cohen TJ, Gohn DC, Johnson WB, Kennedy EE, Mongeon LR, Serwer GA, Qiao H, Wilkoff BL; PREFER Study Investigators. Clinical benefits of remote versus transtelephonic monitoring of implanted pacemakers. J Am Coll Cardiol 2009;54:2012–2019. [DOI] [PubMed] [Google Scholar]
- 10. Hindricks G, Varma N, Kacet S, Lewalter T, Sogaard P, Guedon-Moreau L, Proff J, Gerds TA, Anker SD, Torp-Pedersen C.. Daily remote monitoring of implantable cardioverter-defibrillators: insights from the pooled patient-level data from three randomized controlled trials (IN-TIME, ECOST, TRUST). Eur Heart J 2017;38:1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Varma N, Piccini JP, Snell J, Fischer A, Dalal N, Mittal S.. The relationship between level of adherence to automatic wireless remote monitoring and survival in pacemaker and defibrillator patients. J Am Coll Cardiol 2015;65:2601–2610. [DOI] [PubMed] [Google Scholar]
- 12. Hindricks G, Taborsky M, Glikson M, Heinrich U, Schumacher B, Katz A, Brachmann J, Lewalter T, Goette A, Block M, Kautzner J, Sack S, Husser D, Piorkowski C, Sogaard P; IN-TIME study group. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet 2014;384:583–590. [DOI] [PubMed] [Google Scholar]
- 13. Saxon LA, Hayes DL, Gilliam FR, Heidenreich PA, Day J, Seth M, Meyer TE, Jones PW, Boehmer JP.. Long-term outcome after ICD and CRT implantation and influence of remote device follow-up: the ALTITUDE survival study. Circulation 2010;122:2359–2367. [DOI] [PubMed] [Google Scholar]
- 14. Hindricks G, Elsner C, Piorkowski C, Taborsky M, Geller JC, Schumacher B, Bytesnik J, Kottkamp H.. Quarterly vs. yearly clinical follow-up of remotely monitored recipients of prophylactic implantable cardioverter-defibrillators: results of the REFORM trial. Eur Heart J 2014;35:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ricci RP, Vicentini A, D’Onofrio A, Sagone A, Rovaris G, Padeletti L, Morichelli L, Fusco A, De Vivo S, Lombardi L, Denaro A, Pollastrelli A, Colangelo I, Santini M.. Economic analysis of remote monitoring of cardiac implantable electronic devices: results of the Health Economics Evaluation Registry for Remote Follow-up (TARIFF) study. Heart Rhythm 2017;14:50–57. [DOI] [PubMed] [Google Scholar]
- 16. Guedon-Moreau L, Lacroix D, Sadoul N, Clementy J, Kouakam C, Hermida JS, Aliot E, Kacet S; ECOST trial Investigators. Costs of remote monitoring vs. ambulatory follow-ups of implanted cardioverter defibrillators in the randomized ECOST study. Europace 2014;16:1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Slotwiner D, Varma N, Akar JG, Annas G, Beardsall M, Fogel RI, Galizio NO, Glotzer TV, Leahy RA, Love CJ, McLean RC, Mittal S, Morichelli L, Patton KK, Raitt MH, Ricci RP, Rickard J, Schoenfeld MH, Serwer GA, Shea J, Varosy P, Verma A, Yu CM.. HRS Expert Consensus Statement on remote interrogation and monitoring for cardiovascular implantable electronic devices. Heart Rhythm 2015;12:e69–e100. [DOI] [PubMed] [Google Scholar]
- 18. Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas PEESC Committee for Practice Guidelines (CPG)Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Document R, Kirchhof P, Blomstrom-Lundqvist C, Badano LP, Aliyev F, Bansch D, Baumgartner H, Bsata W, Buser P, Charron P, Daubert JC, Dobreanu D, Faerestrand S, Hasdai D, Hoes AW, Le Heuzey JY, Mavrakis H, McDonagh T, Merino JL, Nawar MM, Nielsen JC, Pieske B, Poposka L, Ruschitzka F, Tendera M, Van Gelder IC, Wilson CM.. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 2013;34:2281–2329. [DOI] [PubMed] [Google Scholar]
- 19. Wilkoff BL, Auricchio A, Brugada J, Cowie M, Ellenbogen KA, Gillis AM, Hayes DL, Howlett JG, Kautzner J, Love CJ, Morgan JM, Priori SG, Reynolds DW, Schoenfeld MH, Vardas PE, Heart Rhythm Society; European Heart Rhythm Association; American College of Cardiology; American Heart Association; European Society of Cardiology; Heart Failure Association of ESC; Heart Failure Society of America. HRS/EHRA expert consensus on the monitoring of cardiovascular implantable electronic devices (CIEDs): description of techniques, indications, personnel, frequency and ethical considerations. Heart Rhythm 2008;5:907–925. [DOI] [PubMed] [Google Scholar]
- 20. Lazarus A. Remote, wireless, ambulatory monitoring of implantable pacemakers, cardioverter defibrillators, and cardiac resynchronization therapy systems: analysis of a worldwide database. Pacing Clin Electrophysiol 2007;30:S2–S12. [DOI] [PubMed] [Google Scholar]
- 21. Varma N, Ricci RP.. Telemedicine and cardiac implants: what is the benefit? Eur Heart J 2013;34:1885–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. De Simone A, Leoni L, Luzi M, Amellone C, Stabile G, La Rocca V, Capucci A, D'Onofrio A, Ammendola E, Accardi F, Valsecchi S, Buja G.. Remote monitoring improves outcome after ICD implantation: the clinical efficacy in the management of heart failure (EFFECT) study. Europace 2015;17:1267–1275. [DOI] [PubMed] [Google Scholar]
- 23. Facchin D, Baccillieri MS, Gasparini G, Zoppo F, Allocca G, Brieda M, Verlato R, Proclemer A.. Findings of an observational investigation of pure remote follow-up of pacemaker patients: is the in-clinic device check still needed? Int J Cardiol 2016;220:781–786. [DOI] [PubMed] [Google Scholar]
- 24. Soth-Hansen M, Witt CT, Rasmussen M, Kristensen J, Gerdes C, Nielsen JC.. Time until diagnosis of clinical events with different remote monitoring systems in implantable cardioverter-defibrillator patients. Heart Rhythm 2018;15:1648–1654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.