Abstract
Aims
Device-related infection (DRI) is a severe complication to cardiac implantable electronic devices (CIED) therapy. Device-related infection incidence and its risk factors differ between previous studies. We aimed to define the long-term incidence and incidence rates of DRI for different types of CIEDs in the complete Danish device-cohort and identify patient-, operation- and device-related risk factors for DRI.
Methods and results
From the Danish Pacemaker (PM) and implantable cardioverter-defibrillator (ICD) Register, we included consecutive Danish patients undergoing CIED implantation or reoperation from January 1982 to April 2018, resulting in 97 750 patients, 128 045 operations and follow-up of in total 566 275 device years (DY). We identified 1827 DRI causing device removals. Device-related infection incidence during device lifetime was 1.19% (1.12–1.26) for PM, 1.91% (1.71–2.13) for ICD, 2.18% (1.78–2.64) for cardiac resynchronization therapy (CRT)-pacemakers (CRT-P), and 3.35% (2.92–3.83) for CRT-defibrillators (CRT-D). Incidence rates in de novo implantations were 2.04/1000 DY for PM, 3.84 for ICD, 4.38 for CRT-P, and 6.76 for CRT-D. Using multiple-record and multiple-event per subject proportional hazard analysis, we identified implantation of complex devices (ICD and CRT), reoperations, prior DRI, male sex, and younger age as significantly associated with higher DRI risk.
Conclusion
Overall risk of infection was low in PM implantations but considerably higher in CRT systems and after reinterventions. These data support the importance of evaluating all patients considered for CIED therapy thoroughly, in order to identify potential modifiable risk factors and reduce the risk of early reoperations.
Keywords: CIED, Device, Infection, Incidence, Pacemaker, ICD, CRT
See page 1870 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz407)
Introduction
Cardiac implantable electronic devices (CIED) are safe and effective for treating cardiac arrhythmias with increase in survival and quality of life.1–3 Implant rate is increasing due to widening of indications and increasing number of generator replacements.4 Device-related infections (DRI) are amongst the most dreaded complications causing significant morbidity and mortality and generating substantial healthcare costs.5–7 It has been claimed that DRI rate has risen disproportionally to the rate of implantations.8,9 Increasing proportions of implantable cardioverter-defibrillator (ICD) and cardiac resynchronization therapy (CRT) systems as well as implantations in patients at higher risk of infection may explain these findings.
Reported DRI rates vary considerably depending on definition of device infection, type of operation, device type, and the duration of follow-up. The risk of infection is highest early after the operation, estimated to 0.5–1% for PM patients within the first 12 months,10–12 0.7–1.7% for ICD patients within 6 months,13,14 and up to 4.3% within the first 2.5 years of CRT implantation.15,16 Long-time risk of DRI has been reported sparsely in the literature and often with mixed types of operations, making it difficult to interpret the results. We therefore examined the incidence and incidence rates of DRI in different types of CIED with lifelong follow-up. We also investigated patient-, operation-, and device-related risk factors to identify patients with the highest risk of DRI.
Methods
Study population and event definition
All Danish patients who received a CIED from January 1982 to April 2018 were identified in the Danish Pacemaker and ICD Register (DPIR) and included. Type of operation (first implant, replacement, or up-/downgrade) and all reoperations with changes in implanted hardware were recorded. In cases with CIED system removal, the reason was specified. We recorded DRI as CIED removals due to either device pocket infection or systemic infection. The patients were followed until DRI or right censored at death, emigration or lost to follow-up. Patients with more than one operation were censored at time of reoperation and had a new entry for time of risk with the new device. The study was approved by the Danish Data Protection Agency [Jrn. 18/20048(16/39520)] and performed according to declaration of Helsinki. According to Danish law, Ethics Committee approval is not required for registry-based studies.
Data sources
In Denmark, CIED operations are centralized to 14 centres. Pacemaker implantations are performed in all centres while ICD and CRT-operations are undertaken only in six, respectively five high-volume centres. The DPIR is a national clinical quality database founded in 1982 by physicians from all implanting centres. Clinical and technical information of all CIED operations in Denmark have been recorded prospectively by the treating physicians. The registry is co-financed by the device industry, but it is controlled by a steering committee of physicians appointed by the Working Group of Arrhythmia under the Danish Society of Cardiology. Data are audited regularly and data quality is considered of high standard.17 The entire Danish population is registered in the Danish Civil Registrations System and is continuously updated on vital status.18
Statistical analysis
All statistical analyses were performed using Stata software package (Stata/MP version 14.2. for Windows). Categorical variables are presented with number and/or frequencies while age is presented as median age with interquartile range (IQR). Absolute incidence and incidence rates were calculated for each device and operation type as number of DRI per device operation and over time as DRI per 1000 years of follow-up and presented with 95% confidence interval (CI). Median time to infection was calculated and presented with IQR.
Multiple-record and multiple-event per subject proportional hazards analyses were used to identify independent patient-, surgery- and device-related risk factors of DRI and were presented as hazard ratios (HR) with 95% CI. A surrogate variable (complexity) for the duration and complexity of the operation was constructed by counting the number of pieces of hardware implanted and removed during the same operation. Exact duration of surgery and body mass index (BMI) was available for a subset of patients. We stratified continuous numeric variables in groups and divided implantation centres into PM-centres and ICD-centres. [The three major university centres (1982–2018) and centres implanting ICDs from the year starting ICD implantations] Total number of operations and any previous infection were counted for each patient and included in the analysis. The multivariable regression analysis included sex, device type, operation type, age, implant year, prior infection, centre volume, and complexity. P-values of <0.05 were considered statistically significant.
Results
Study population
During the study period, 99 105 patients underwent a de novo CIED implantation and n = 23 092 received one or more replacement and/or an up-/downgrade operation adding up to a total of 129 863 operations. One hundred and seventy-nine observations were removed because of misclassification. We excluded 1271 patients with unknown status in the Central Person Register, 309 patients who emigrated, and 59 patients lost to follow-up. Thus, the final study population consisted of 97 750 (97 732 first implants in Denmark) patients with a total number of 128 045 operations. Mean follow-up was 4.42 device years (DY) per device with a combined follow-up time of 566 275 DY.
Most patients (81.2%) had PM, 12.3% had ICD, and 6.5% had CRT-system with- or without ICD. The PM patients had a nearly even gender distribution (males 55.2%) and a median age of 77, while the ICD and CRT populations had predominance of males (70–80%) and were younger (Table 1).
Table 1.
Patient characteristics at first CIED implant, n = 97 732
| Variable | Pacemaker | Implantable cardioverter-defibrillator | Cardiac resynchronization therapy-pacemaker | Cardiac resynchronization therapy-defibrillator |
|---|---|---|---|---|
| n (%) | 79 318 (81.2) | 12 037 (12.3) | 2991 (3.1) | 3386 (3.4) |
| Age at implant, years, median (IQR) | 77 (69-83) | 64 (54–71) | 71 (61–78) | 68 (60–74) |
| Sex | ||||
| Female, n (%) | 35 549 (44.8) | 2383 (19.8) | 905 (30.3) | 679 (20.0) |
| Male, n (%) | 43 769 (55.2) | 9654 (80.2) | 2086 (69.7) | 2707 (80.0) |
| Indication (%) | ||||
| AV-block | 47.0 | — | 81.6 | — |
| Sinus node dysfunction | 36.5 | — | 2.5 | — |
| Atrial fibrillation | 13.4 | — | 3.9 | — |
| Primary prophylactic ICD | — | 31.7 | — | 63.2 |
| Secondary prophylactic ICD | — | 64.3 | — | 29.7 |
| Other | 3.1 | 4.0 | 12.0 | 7.1 |
Incidence
A total of 1827 systems were explanted due to DRI, coded as either ‘Systemic infection/endocarditis’ (n = 535) or ‘Local pocket infection/skin erosion’ (n = 1292). Of all, 1074 DRI occurred after first device implantation while 544 and 209 infections were observed after replacements and up-/downgrade operations, respectively. Median time to infection in de novo operations was 286 (IQR 52–932) and 415 (IQR 69–1401) days for pocket and systemic infection, respectively, vs. 269 (IQR 83–734) and 506 (IQR 112–1307) in replacement operations.
Of all, 952 patients developed a surgical site infection (infection within 12 months of operation)19 with a median time to infection of 74 (IQR 29–175) days. The 875 DRIs appearing after 12 months had a median time to infection of 1053 (IQR 643–1762) days. The proportions of DRIs categorized as surgical site infections were 51.3%, 53.7%, and 52.2% for de novo implantations, replacements-, and up-/downgrade operations, respectively.
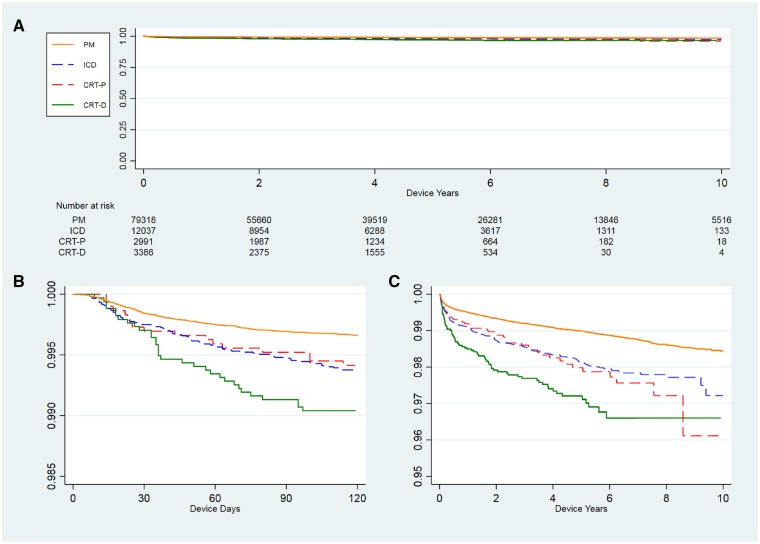
The combined incidence of infection during the device lifetime was 1.19% for PM, 1.91% for ICD, 2.18% for CRT-P, and 3.35% for CRT-D. The risk was lowest in de novo implantations (PM = 0.94%, ICD = 1.66%, CRT-P = 1.60%, and CRT-D = 2.42%) and considerably higher in all other types of operations (Table 2). The incidence of early infection ranged from 0.16% to 0.30% at 30 days and 0.31% to 0.86% at 90 days for PM and CRT-D, respectively (Figure 1).
Table 2.
Incidence and incidence rate of device-related infection in the Danish CIED population 1982–2018
| Variable | Devices | Device years | Events | Overall incidence |
Incidence rate /1000 DY |
||||
|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | ||||||||
| Total | 128 045 | 566 275 | 1827 | 1.43% | 1.36 | 1.49 | 3.23 | 3.08 | 3.38 |
| Sex | |||||||||
| Female | 51 484 | 241 524 | 505 | 0.98% | 0.90 | 1.07 | 2.09 | 1.92 | 2.28 |
| Male | 76 561 | 324 750 | 1322 | 1.73% | 1.64 | 1.82 | 4.07 | 3.86 | 4.30 |
| Operation & Device Type | |||||||||
| Pacemaker | |||||||||
| Total | 100 374 | 460 196 | 1194 | 1.19% | 1.12 | 1.26 | 2.59 | 2.45 | 2.75 |
| First | 79 318 | 364 744 | 744 | 0.94% | 0.87 | 1.01 | 2.04 | 1.90 | 2.19 |
| Replacement | 17 265 | 77 742 | 359 | 2.08% | 1.87 | 2.30 | 4.62 | 4.16 | 5.12 |
| Up-/downgrade | 3791 | 17 708 | 91 | 2.40% | 1.94 | 2.94 | 5.14 | 4.18 | 6.31 |
| ICD | |||||||||
| Total | 16 718 | 69 766 | 320 | 1.91% | 1.71 | 2.13 | 4.59 | 4.11 | 5.12 |
| First | 12 037 | 52 040 | 200 | 1.66% | 1.44 | 1.91 | 3.84 | 3.35 | 4.41 |
| Replacement | 3959 | 15 015 | 92 | 2.32% | 1.88 | 2.84 | 6.13 | 4.99 | 7.52 |
| Up-/downgrade | 722 | 2711 | 28 | 3.88% | 2.59 | 5.56 | 10.33 | 7.13 | 14.96 |
| CRT-P | |||||||||
| Total | 4630 | 15 848 | 101 | 2.18% | 1.78 | 2.64 | 6.37 | 5.24 | 7.75 |
| First | 2991 | 10 965 | 48 | 1.60% | 1.19 | 2.12 | 4.38 | 3.30 | 5.81 |
| Replacement | 769 | 2249 | 26 | 3.38% | 2.22 | 4.91 | 11.56 | 7.87 | 16.97 |
| Up-/downgrade | 870 | 2632 | 27 | 3.10% | 2.05 | 4.48 | 10.26 | 7.03 | 14.96 |
| CRT-D | |||||||||
| Total | 6323 | 20 464 | 212 | 3.35% | 2.92 | 3.83 | 10.36 | 9.05 | 11.85 |
| First | 3386 | 12 131 | 82 | 2.42% | 1.93 | 3.00 | 6.76 | 5.44 | 8.39 |
| Replacement | 1339 | 3537 | 67 | 5.00% | 3.90 | 6.31 | 18.94 | 14.91 | 24.07 |
| Up-/downgrade | 1598 | 4795 | 63 | 3.94% | 3.04 | 5.02 | 13.14 | 10.26 | 16.82 |
Figure 1.
(A) Kaplan–Meier curve of the time to infection after de novo implant in different types of devices in the Danish CIED population, 1982–2018, n = 97 732. (B) The first 120 days after implantation. (C) Enlarged portion of (A).
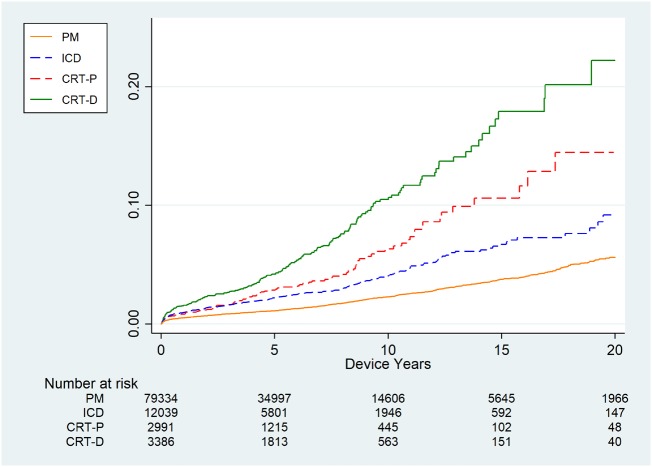
The incidence rate of DRI after first implantations was 2.04, 3.84, 4.38, and 6.76/1000 DY for PM, ICD, CRT-P, and CRT-D, respectively. The incidence rate was considerable higher in reoperations with rates at 4.62–5.14/1000 DY for PM replacement and up-/downgrade operations and as high as 18.94/1000 DY for CRT-D replacement operations (Table 2 and Supplementary material online, Table S2). The lifetime risk of infection for a patient living with a device through several years is illustrated in Take home figure.
Risk factors
The multiple proportional hazard analysis showed that device type was associated with a higher risk of infection with uni- and multivariable HR of 1.83, 2.48, and 4.19 and 1.26, 1.67, and 2.22 for ICD, CRT-P, and CRT-D systems, respectively as compared to PM. Any kind of reoperation was correlated with a substantial but similar increase in risk of infection with HR of 5.95 and 6.91 for each replacement and up-/downgrade operations. These findings persisted in the multivariable analysis with HR of 4.93 (CI 4.17–85.84) and 4.39 (CI 3.65–5.29), respectively. We did not find any significant difference between upgrades and replacement operations, whatever the type of device, but the HR increased with each operation summing up in HR of 61.35 for five or more operations as compared to de novo implantations.
When we examined device indication, we found that PM and CRT-P patients that received their device due to AV-block (HR 1.47, CI 1.31–1.67) had the highest risk of DRI, while the risk was highest in those ICD and CRT-D patients that received a primary prophylactic defibrillator unit (HR 1.48, CI 1.23–1.79) (Supplementary material online, Table S3). In univariate analysis, risk of DRI was significantly higher in ICD-centres, but in the multivariable analysis, the risk was significantly lower in ICD-centres.
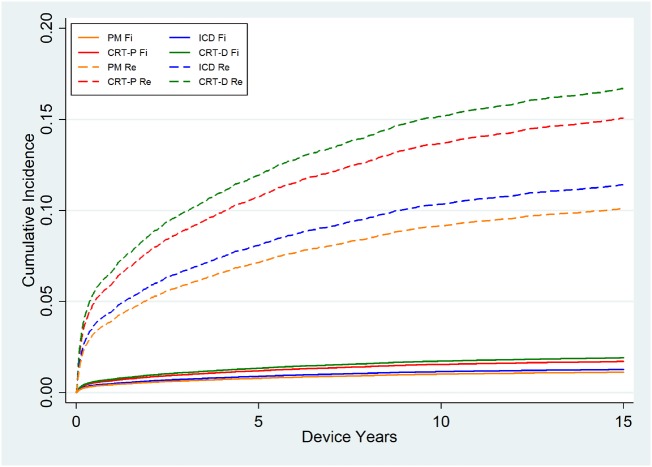
Male sex, young age, prior DRI, longer operation time, and higher complexity of the operation as well as BMI > 30 were all associated with higher risk of DRI in univariate analysis; however, BMI and operation time were not significantly associated with higher risk of DRI in the multivariable analysis (Table 3 and Supplementary material online, Table S3). Cumulative incidence function curves of the time to infection with death as competing risk were calculated and graphically illustrated in Figure 2.
Table 3.
Risk factors for device-related infections
| Variable | Univariate |
Multivariable |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Devices | HR | 95% CI | P-value | HR | 95% CI | P-value | ||||
| Sex | ||||||||||
| Female | 51 484 | 1 | <0.001 | 1 | ||||||
| Male | 76 561 | 1.94 | 1.75 | 2.14 | 1.55 | 1.40 | 1.72 | <0.001 | ||
| Operation type | ||||||||||
| First implant | 97 732 | 1 | <0.001 | 1 | ||||||
| Replacement | 23 332 | 5.95 | 5.09 | 6.95 | 4.93 | 4.17 | 5.84 | <0.001 | ||
| Up-/downgrade | 6981 | 6.91 | 5.82 | 8.20 | 4.39 | 3.65 | 5.29 | <0.001 | ||
| Device type | ||||||||||
| PM | 100 374 | 1 | <0.001 | 1 | ||||||
| ICD | 16 718 | 1.83 | 1.62 | 2.07 | 1.26 | 1.09 | 1.47 | 0.002 | ||
| CRT-P | 4630 | 2.48 | 2.02 | 3.04 | 1.68 | 1.34 | 2.11 | <0.001 | ||
| CRT-D | 6323 | 4.19 | 3.62 | 4.85 | 2.22 | 1.83 | 2.70 | <0.001 | ||
| Age | ||||||||||
| 0–20 | 1171 | 1.62 | 1.16 | 2.25 | 1.70 | 1.22 | 2.37 | 0.002 | ||
| 21–50 | 7976 | 1.36 | 1.16 | 1.58 | 1.40 | 1.20 | 1.64 | <0.001 | ||
| 51–60 | 11 847 | 1.21 | 1.05 | 1.40 | 1.20 | 1.04 | 1.39 | 0.013 | ||
| 61–70 | 26 714 | 1 | <0.001 | 1 | ||||||
| 71–80 | 42 355 | 0.75 | 0.66 | 0.84 | 0.81 | 0.72 | 0.92 | 0.001 | ||
| 81–90 | 32 476 | 0.43 | 0.37 | 0.51 | 0.53 | 0.45 | 0.62 | <0.001 | ||
| 91+ | 5506 | 0.30 | 0.18 | 0.48 | 0.38 | 0.23 | 0.61 | <0.001 | ||
| Year of implantation | ||||||||||
| 1979–89 | 8871 | 1.40 | 1.13 | 1.72 | 1.84 | 1.48 | 2.29 | <0.001 | ||
| 1990–99 | 20 070 | 1 | <0.001 | 1 | ||||||
| 2000–09 | 43 007 | 1.44 | 1.23 | 1.68 | 1.23 | 1.05 | 1.44 | 0.011 | ||
| 2010–18 | 56 097 | 2.06 | 1.77 | 2.40 | 1.42 | 1.20 | 1.67 | <0.001 | ||
| Total number of operations | ||||||||||
| 1 | 97 750 | 1 | <0.001 | NA | ||||||
| 2 | 22 851 | 5.54 | 4.78 | 6.43 | ||||||
| 3 | 5452 | 16.08 | 12.98 | 19.91 | ||||||
| 4 | 1401 | 33.60 | 24.84 | 45.43 | ||||||
| 5+ | 591 | 61.35 | 41.20 | 91.36 | ||||||
| Prior infection | ||||||||||
| No | 125 998 | 1 | <0.001 | 1 | ||||||
| Yes | 2047 | 4.36 | 3.57 | 5.34 | 1.65 | 1.34 | 2.04 | <0.001 | ||
| Centre | ||||||||||
| PM-centre | 43 649 | 1 | <0.001 | 1 | ||||||
| ICD-centre | 84 396 | 1.36 | 1.23 | 1.50 | 0.89 | 0.79 | 0.995 | 0.041 | ||
| Complexity | ||||||||||
| 1–2 | 63 297 | 1 | <0.001 | 1 | ||||||
| 3 | 53 009 | 1.01 | 0.91 | 1.12 | 1.25 | 1.10 | 1.42 | 0.001 | ||
| 4+ | 11 739 | 2.61 | 2.30 | 2.96 | 1.20 | 1.03 | 1.40 | 0.019 | ||
Results from the univariate and multivariable proportional hazards analysis.
The multivariable model includes sex, operation type, and device type, and age, year of implantation, prior infection, centre, and complexity (number of hardware pieces implanted and removed during the same operation).
Figure 2.
Cumulative incidence function with death as competing risk of the time to first infection in different device types after first implants (Fi) and replacement (Re) operations. The competing risk analysis is adjusted for sex, age, year of implantation, centre, and complexity (number of hardware pieces implanted and removed during the same operation).
Take home figure.
Cumulative lifetime risk of device-related infection in different types of devices in the Danish CIED population, 1982–2018, n = 97 750.
Discussion
We found lowest incidence of DRI in PM patients and considerable higher in all other types of CIEDs. The risk was lowest in de novo implantations and several folds higher in all types of reoperations. In multivariable analysis, both ICD and CRT systems were statistically significant independent risk factors as compared to pacemakers. Other independent risk factors were young age, male sex, and prior DRI.
Incidence
We observed a combined incidence of DRI of 1.19% for PM patients with an incidence rate of 2.59/1000 DY. The incidence was lowest in de novo implantation and considerable higher in all types of reoperations. These findings are in line with our previous study10 but lower than other studies.20 In ICD patients, the combined incidence of DRI was 1.91% during the device lifetime. A recent study13 of 200 909 North American ICD and CRT-D patients reported a combined incidence of 1.7% for ICD and CRT-D patients within 6 months of follow-up. Although the risk is highest within the first 12 months after implantation, these numbers are higher than ours, likely because this study included ICD and CRT-D systems. Our incidence also is lower than the 2.2% after 3 months reported in an earlier retrospective study.21 However, this study included patients who were not operated by specialized electrophysiologist. In our population, all ICD implantations are performed in six centres by specialized electrophysiologist with a high annual number of operations which may add to explain the low incidence of DRI.
The risk of DRI in CRT patients has been reported sparsely in the literature and often as subgroups in mixed device populations. Other studies15,16 reported combined DRI incidence of about 4.2–4.8% in CRT patients. These studies report few infections, a short follow-up and no stratification for operation type. In our dataset with lifelong follow-up, we observed lower DRI risk with a combined incidence of 2.18% and 3.35% for CRT-P and CRT-D, respectively and even lower after de novo implantation.
Selected CRT patients have been upgraded from a non-CRT device, and the longevity of a CRT-device is considerably shorter than for a PM. Therefore, CRT patients experience more reoperations and a higher total number of operations when compared to PM and ICD patients. These factors might partly explain the increased incidence of DRI, however the incidence is still higher in de novo implantations as compared to pacemakers. This indicates that device type is an independent risk factor, likely because CRT-implantations are more technical challenging and time-consuming. Other risk factors related to comorbidity, which have not been examined in this study, may also be important.
Risk factors
Implantable cardioverter-defibrillator patients had a slightly higher risk of DRI as compared to PM patients, whereas CRT-P and CRT-D systems were associated with considerably higher risk of DRI. De novo implantations had lower risk for DRI in all device types, while any reoperation was associated with a significantly increased risk of DRI, independent of the type of device and the type of reoperation.
It is thus evident that patients undergoing reoperations are high-risk patients, and whenever surgery is performed, it is important to take appropriate actions to prevent infections. Currently, we do not have evidence of the best way to reduce DRI risk in these patients, but a recently published randomized trial22 has demonstrated that an antibiotic envelope can reduce pocket infections in high-risk patients. Our findings emphasize the importance of considering choice of device type thoroughly when planning CIED therapy in order to minimize risk of early reoperation. Furthermore, the device industry must focus on increasing device longevity. Even though all CIED operations in Denmark are performed by specialized cardiologist, we still find a significant lower risk in ICD-centres. This emphasizes the importance of operators keeping a high annual number of operations.
Young age and male sex were independent patient-related risk factors associated with higher DRI risk. These findings have been described by others in the field of cardiology23,24 as well as in prosthetic joint infections.25 There are no obvious reason for this diversity amongst gender; however, bacterial skin colonization differs among genders26 which may reflect differences in host response to bacterial exposure. Risk of infection in the oldest patients was low compared to younger patients. As we only included DRI with hardware removal, patients, with unrecognized DRI, who died before removal or were conservatively treated, were not included. In the early part of the study period, there was a higher risk related to device removal procedures, and a proportion of elderly fragile patients may not have been considered for device removal procedures. This should however not be an issue within the most recent decade. It is also plausible that a small proportion of elderly patients had unrecognized DRI, since such patients are more often admitted to non-cardiology departments where the infection might remain undiagnosed. Still it is very likely from these data that the DRI risk is considerable higher in younger patients. The reason remains unclear and should be investigated further.
Device indication as a marker of underlying heart disease also seemed to play a role but could not be included in the multivariable analysis due to collinearity. However, this might indicate that comorbidity influences risk of DRI, in line with a recent published meta-analysis.27 Obesity often lead to surgical complications28 and BMI > 30 correlated to a higher risk of DRI in our data, but not statistically significant in the multivariable model. Long duration of surgery was also expected associated with increased DRI risk, but this could not be confirmed, probably due to collinearity with device type.
We observed the highest risk of infection in the early period (Table 3), although rate of infection has increased in recent years. This increase may be due to a higher number of CRT systems and reoperations. Nevertheless, these findings persisted in the multivariable model, suggesting that there are more patient-related risk factors, yet to be revealed.
Strengths and limitations
This study is a retrospective study and therefore conclusions about causality cannot be drawn. Data were entered prospectively in DPIR, and the patients were followed prospectively. With more than 35 years of reporting and follow-up it would be unlikely that the entered data are flawed by any systematic bias. All registers are prone to underreporting of complications as well as misclassifications, but this register is audited regularly and considered complete with respect to changes in hardware with a high validity and quality in registration of complications.17
The observed incidence of DRI represents the lowest estimate since DRI was counted only for patients who had their system removed due to infection. However, the large number of consecutive patients in a nationwide setting with lifelong follow-up is likely to provide reliable minimal estimates for the absolute risk of DRI.
Conclusion
In this nationwide cohort of the Danish CIED population with a lifelong follow-up, we found the lowest incidence and incidence rate of DRI in de novo PM implantations. The risk was slightly higher in ICD systems and considerable higher in CRT systems. Majority of risk factors for DRI were patient- and operation-related such as total number of operations, type of operation, young age, and male sex.
It is important to be aware of these risk factors and consider the device indication and the choice of device type thoroughly when planning CIED therapy in order to modify adjustable risk factors and minimize risk of early reoperation. Furthermore, the device industry should focus on increasing device longevity to reduce the number of replacement operations.
Funding
The Danish Heart Association and the Region of Southern Denmark (15-R99-A5950-22895, 16/36792).
Conflict of interest: Dr J.C.N. received grant from the Novo Nordisk Foundation (NNF16OC0018658).
Supplementary Material
References
- 1. Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M.. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med 1996;335:1933–1940. [DOI] [PubMed] [Google Scholar]
- 2. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH.. Amiodarone or an implantable cardioverter–defibrillator for congestive heart failure. N Engl J Med 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 3. Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas PE, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Kirchhof P, Blomstrom-Lundqvist C, Badano LP, Aliyev F, Bänsch D, Baumgartner H, Bsata W, Buser P, Charron P, Daubert JC, Dobreanu D, Faerestrand S, Hasdai D, Hoes AW, Le Heuzey JY, Mavrakis H, McDonagh T, Merino JL, Nawar MM, Nielsen JC, Pieske B, Poposka L, Ruschitzka F, Tendera M, Van Gelder IC, Wilson CM. ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 2013;34:2281–2329. [DOI] [PubMed] [Google Scholar]
- 4. Raatikainen MJP, Arnar DO, Merkely B, Nielsen JC, Hindricks G, Heidbuchel H, Camm J.. A decade of information on the use of cardiac implantable electronic devices and interventional electrophysiological procedures in the European Society of Cardiology countries: 2017 report from the European Heart Rhythm Association. Europace 2017;19:ii1–ii90. [DOI] [PubMed] [Google Scholar]
- 5. Sohail MR, Henrikson CA, Braid-Forbes MJ, Forbes KF, Lerner DJ.. Mortality and cost associated with cardiovascular implantable electronic device infections. Arch Intern Med 2011;171:1821–1828. [DOI] [PubMed] [Google Scholar]
- 6. Baman TS, Gupta SK, Valle JA, Yamada E.. Risk factors for mortality in patients with cardiac device-related infection. Circ Arrhythm Electrophysiol 2009;2:129–134. [DOI] [PubMed] [Google Scholar]
- 7. Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta J-P, Del Zotti F, Dulgheru R, El Khoury G, Erba PA, Iung B, Miro JM, Mulder BJ, Plonska-Gosciniak E, Price S, Roos-Hesselink J, Snygg-Martin U, Thuny F, Tornos Mas P, Vilacosta I, Zamorano JL, Erol Ç NP, Aboyans V, Agewall S, Athanassopoulos G, Aytekin S, Benzer W, Bueno H, Broekhuizen L, Carerj S, Cosyns B, Backer JD, Bonis MD, Dimopoulos K, Donal E, Drexel H, Flachskampf FA, Hall R, Halvorsen S, Hoen B, Kirchhof P, Lainscak M, Leite-Moreira AF, Lip GYH, Mestres CA, Piepoli MF, Punjabi PP, Rapezzi C, Rosenhek R, Siebens K, Tamargo J, Walker DM. 2015 ESC Guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC) endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36:3075–3128. [DOI] [PubMed] [Google Scholar]
- 8. Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT, Pavri BB, Kurtz SM.. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States: 1993 to 2008. J Am Coll Cardiol 2011;58:1001–1006. [DOI] [PubMed] [Google Scholar]
- 9. Voigt A, Shalaby A, Saba S.. Rising rates of cardiac rhythm management device infections in the United States: 1996 through 2003. J Am Coll Cardiol 2006;48:590–591. [DOI] [PubMed] [Google Scholar]
- 10. Johansen JB, Jørgensen OD, Møller M, Arnsbo P, Mortensen PT, Nielsen JC.. Infection after pacemaker implantation: infection rates and risk factors associated with infection in a population-based cohort study of 46299 consecutive patients. Eur Heart J 2011;32:991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klug D, Balde M, Pavin D, Hidden-Lucet F, Clementy J, Sadoul N, Rey JL, Lande G, Lazarus A, Victor J, Barnay C, Grandbastien B, Kacet S.. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators. Circulation 2007;116:1349–1355. [DOI] [PubMed] [Google Scholar]
- 12. Kirkfeldt RE, Johansen JB, Nohr EA, Jorgensen OD, Nielsen JC.. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J 2014;35:1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Prutkin JM, Reynolds MR, Bao H, Curtis JP, Al-Khatib SM, Aggarwal S, Uslan DZ.. Rates of and factors associated with infection in 200 909 Medicare implantable cardioverter-defibrillator implants. Circulation 2014;130:1037–1043. [DOI] [PubMed] [Google Scholar]
- 14. Nery PB, Fernandes R, Nair GM, Sumner GL, Ribas CS, Menon SMD, Wang X, Krahn AD, Morillo CA, Connolly SJ, Healey JS.. Device-related infection among patients with pacemakers and implantable defibrillators: incidence, risk factors, and consequences. J Cardiovasc Electrophysiol 2010;21:786–790. [DOI] [PubMed] [Google Scholar]
- 15. Romeyer-Bouchard C, Da Costa A, Dauphinot V, Messier M, Bisch L, Samuel B, Lafond P, Ricci P, Isaaz K.. Prevalence and risk factors related to infections of cardiac resynchronization therapy devices. Eur Heart J 2010;31:203–210. [DOI] [PubMed] [Google Scholar]
- 16. Unsworth JD, Zaidi A, Hargreaves MR.. Increased late complex device infections are determined by cardiac resynchronization therapy-defibrillator infection. Europace 2015;17:1708–1711. [DOI] [PubMed] [Google Scholar]
- 17. Møller M, Arnsbo P, Asklund M, Christensen PD, Gadsbøll N, Svendsen JH, Klarholt E, Kleist KE, Mortensen PT, Pietersen A, Simonsen EH, Thomsen PEB, Vesterlund T, Wiggers P.. Quality assessment of pacemaker implantations in Denmark. Europace 2002;4:107–112. [DOI] [PubMed] [Google Scholar]
- 18. Pedersen CB. The Danish Civil Registration System. Scand J Public Health 2011;39:22–25. [DOI] [PubMed] [Google Scholar]
- 19. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR.. Guideline for prevention of surgical site infection, 1999. Am J Infect Control 1999;27:97–134. [PubMed] [Google Scholar]
- 20. Hercé B, Nazeyrollas P, Lesaffre F, Sandras R, Chabert J-P, Martin A, Tassan-Mangina S, Bui HT, Metz D.. Risk factors for infection of implantable cardiac devices: data from a registry of 2496 patients. Europace 2013;15:66–70. [DOI] [PubMed] [Google Scholar]
- 21. Al-Khatib SM, Greiner MA, Peterson ED, Hernandez AF, Schulman KA, Curtis LH.. Patient and implanting physician factors associated with mortality and complications following implantable cardioverter-defibrillator implantation, 2002–2005: Al-Khatib—patient and physician factors and ICD outcomes. Circ Arrhythm Electrophysiol 2008;1:240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tarakji KG, Mittal S, Kennergren C, Corey R, Poole JE, Schloss E, Gallastegui J, Pickett RA, Evonich R, Philippon F, McComb JM, Roark SF, Sorrentino D, Sholevar D, Cronin E, Berman B, Riggio D, Biffi M, Khan H, Silver MT, Collier J, Eldadah Z, Wright DJ, Lande JD, Lexcen DR, Cheng A, Wilkoff BL.. Antibacterial envelope to prevent cardiac implantable device infection. N Engl J Med 2019;doi: 10.1056/NEJMoa1901111 [Epub head of print]. [DOI] [PubMed] [Google Scholar]
- 23. Joy PS, Kumar G, Poole JE, London B, Olshansky B.. Cardiac implantable electronic device infections: who is at greatest risk? Heart Rhythm 2017;14:839–845. [DOI] [PubMed] [Google Scholar]
- 24. Pavlovic NV, Randell T, Madeira T, Hsu S, Zinoviev R, Abshire M.. Risk of left ventricular assist device driveline infection: a systematic literature review. Heart Lung 2019;48:90–104. [DOI] [PubMed] [Google Scholar]
- 25. Kong L, Cao J, Zhang Y, Ding W, Shen Y.. Risk factors for periprosthetic joint infection following primary total hip or knee arthroplasty: a meta-analysis. Int Wound J 2017;14:529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fierer N, Hamady M, Lauber CL, Knight R.. The influence of sex, handedness, and washing on the diversity of hand surface bacteria. Proc Natl Acad Sci USA 2008;105:17994–17999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Polyzos KA, Konstantelias AA, Falagas ME.. Risk factors for cardiac implantable electronic device infection: a systematic review and meta-analysis. Europace 2015;17:767–777. [DOI] [PubMed] [Google Scholar]
- 28. Tjeertes E, Hoeks SSE, Beks S, Valentijn TTM, Hoofwijk A, Stolker R.. Obesity—a risk factor for postoperative complications in general surgery? BMC Anesthesiol 2015;15:112.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.