Abstract
Exosomes and long non-coding RNAs (lncRNAs) are emerging as important elements contributing to a more comprehensive understanding of cancer development and progression. The discovery of lncRNAs in exosomes further indicates their bona fide biological functional roles in cancer development and drug resistance. In this review, we describe the biogenesis of exosomes and summarize the function of exosomal lncRNAs in the field of cancer research. These findings strikingly advance current knowledge of exosomal lncRNAs and suggest that they may be promising diagnostic biomarkers and therapeutic targets for cancer.
Keywords: Exosome, Long non-coding RNA (lncRNA), Biosynthesis, Biomarker, Drug resistance
1. Introduction
Exosomes are cup-shaped or circular nanoscale extracellular vesicles surrounded by lipid bilayers. They are commonly found in body fluids, plasma, urine, and cerebrospinal fluid. The abundance of exosomes in most body fluids suggests their potential as a prospective non-invasive fluid biopsy for cancers. They are usually formed by a process of invagination, which involves inward budding of cell membranes into an early intracellular vesicle, and subsequently this vesicle further invaginates to form an intraluminal vesicle (ILV), which gradually forms the multivesicular body (MVB), fusing with cell membranes to release exosomes. Exosomes harbor a variety of biomolecules from parent cells including proteins, peptides, lipids, mRNAs and non-coding RNAs (such as microRNAs and long non-coding RNAs (lncRNAs)). Tetrameric transmembrane proteins CD63, CD9, and CD81 found on the surface of exosomes serve as biomarkers of exosomes (Melo et al., 2015). An exosome has a diameter of usually less than 150 nm, and a density of 1.15–1.19 g/mL. Scanning electron microscopy, transmission electron microscopy, and cryoelectron microscopy are usually used to observe the morphology of exosomes. Dynamic light scattering and nanoparticle tracking analysis are applied to measure their size and concentration (Shao et al., 2018). Exosomes have characteristics that protect active substances they encapsulate from degradation during long-distance transportation in blood or other body fluids. Exosomes play a role as “transport carriers” by transporting their contents into surrounding cells, and carrying out long-distance transport of substances and exchange of signals. They participate in a variety of physiological and pathological processes, such as antigen presentation, promotion of tumor growth and migration, and regeneration from damaged tissue (Juan and Fürthauer, 2017).
Studies have consistently shown the important role of exosome-derived lncRNAs in human diseases, especially in tumors and cancers (Zhou et al., 2018). LncRNAs are a group of non-coding RNAs with a length of more than 200 nucleotides. They have a variety of secondary structures that specifically bind to a variety of proteins and nucleic acids, based on the principle of complementary base pairing. LncRNAs are distributed mainly in the nucleus but some are found in the cytoplasm. They commonly act as competitive endogenous RNAs (ceRNAs) or RNA sponges to regulate gene expression at translational and/or transcriptional levels, and participate in various physiological processes such as cell growth, proliferation, and apoptosis (Zheng et al., 2017; Sang et al., 2018). Recent studies have shown that some membrane-lipid associated lncRNAs act as mediators of many oncogenic signal pathways (Ma et al., 2018). Many exosomal lncRNAs have been shown to be abnormally expressed in several cancers, supporting their crucial role in cancer development and metastasis. Exosomal lncRNAs are potential diagnostic biomarkers for many cancers (Tan et al., 2018; Zhao et al., 2018).
2. Biogenesis of exosomes
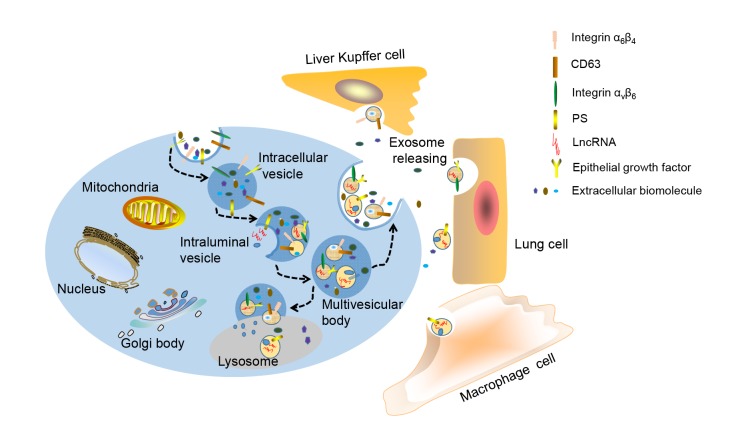
Exosomes are nanoscale extracellular vesicles containing proteins, polypeptides, and nucleic acids surrounded by lipid bilayers. They are widely found in various organisms and body fluids. Acting as cell vesicles, they share a formation mechanism similar to that of other vesicles in cells, wherein they follow the same principles of topology and require the machinery of protein assembly. However, unlike other extracellular vesicle families, exosome biogenesis is the result of fusion of an MVB with the plasma cell membrane rather than direct shedding. The biogenesis process of exosomes is roughly described as the invagination of budding from the limited membrane of late endosomes (Théry et al., 2002; Hessvik and Llorente, 2018). In detail, firstly, inward budding of the cellular plasma membrane forms early intracellular vesicles. The inner membrane of the early intracellular vesicles further invaginates to form an ILV. This mature ILV gradually forms the MVB. This process also involves the incorporation and invagination of contents on the cell membrane and specific receptors involved in lysosome fusion (Théry et al., 2002). Subsequently, the MVB is directed to fuse with lysosomes or with cell membranes upon ISGylation induction (Villarroya-Beltri et al., 2016), leading to digestion of the contents of the MVB by lysosomes (Piper and Katzmann, 2007). Alternatively, the MVB releases exosomes into the extracellular space. Although some evidence argues for unspecific targeting of exosomes, most studies support that exosomes of different cellular origins preferentially target special recipient cells after they are released into the extracellular space. For instance, exosomes carrying different compositions of integrins selectively target different recipient cells. Exosomes containing α6β4 and α6β1 are likely to target lung cells, whereas those containing αvβ6 are more likely to be delivered to Kupffer cells in the liver (Hoshino et al., 2015). Macrophages can recognize the phosphatidylserine-derived negative surface charges on the membranes of exosomes (Matsumoto et al., 2017) (Fig. 1). Both endosomal sorting complex required for transport (ESCRT)-and ceramide-dependent mechanisms have been implicated in exosome biosynthesis. Previous studies found that certain components of ESCRT complexes, such as ESCRT-0 and ESCRT-I, are capable of interacting with ubiquitinated proteins to form supercomplexes on the endosomal membrane, thereby contributing to the biosynthesis and secretion of exosomes (Tamai et al., 2010). Ceramide regulates the release of MVBs (Trajkovic et al., 2008) and decreases the cytosol level of β-catenin by inducing cell membrane curvature to form β-catenin-enriched exosomes (Chairoungdua et al., 2010). In the process of exosome biosynthesis and release, separation and fusion of vesicles from the plasma membrane are also involved. This requires the participation of small molecule GTPase monomeric protein Rabs and soluble soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) (Ohya et al., 2009; Ostrowski et al., 2010; Zeigerer et al., 2012). Rab acts as a GTPase to coordinate the assembly and disassembly of vesicles as well as vesicle trafficking along the cytoskeleton of cells. At least five Rab family proteins have been reported to be involved in the biosynthesis of exosomes. Among them, Rab27a is responsible for controlling the size of multivesicular endosomes (MVEs), and Rab27b is involved in the redistribution of MVEs around the nucleus (Ostrowski et al., 2010).
Fig. 1.
Biogenesis of exosomes and the process of their release to different target cells
Inward budding of the cellular plasma membrane forms an early intracellular vesicle. Invagination of the early intracellular vesicle forms an intraluminal vesicle (ILV). This mature ILV gradually forms the multivesicular body (MVB). The MVB fuses with lysosomes, leading to digestion of its contents, or fuses with the cell membrane, releasing the exosomes out into the extracellular space. Exosomes containing integrins α6β4 and α6β1 are likely to target lung cells, while those containing integrin αvβ6 are more likely to be delivered to Kupffer liver cells. Macrophages recognize the exosomes containing phosphatidylserine (PS)
In spite of these findings, the exact mechanisms of exosomal biosynthesis and release have not been fully elucidated. Future studies are expected to investigate the physicochemical properties of exosomes and define the precise mechanisms of exosomal biogenesis.
3. Exosomal lncRNAs and drug resistance
Chemotherapy is one of the most common treatments for cancer, but drug resistance poses a key challenge for the effectiveness of cancer chemotherapy. Moreover, the clinical applicability of cancer chemotherapy is greatly affected by late diagnosis. Interactions between malignant and non-transformed cells shape the tumor microenvironment (TME) (Balkwill et al., 2012). The TME is composed mainly of cancer-associated fibroblasts (CAFs), extracellular matrix, soluble factors (such as cytokines, hormones, growth factors, and enzymes), and some physical factors such as pH and oxygen concentration (Soysal et al., 2015). Exosomes are an important component of the TME and carry many functional molecules, depending on their cellular origins. For example, exosome-delivered integrins α6β4 and α6β1 have been reported to be associated with lung metastasis, (Hoshino et al., 2015). Moreover, lncRNAs from tumor-derived exosomes can mediate drug resistance in cancer cells. For example, lncRNA-ROR in exosomes released by hepatocellular carcinoma (HCC) cancer cells can induce chemotherapy resistance, such as with sorafenib treatment (Takahashi et al., 2014a). Similarly, lncRNA RP11-838N2.4 derived from exosomes reduced the expression of apoptosis-associated proteins caspase-3 and cleaved poly(ADP-ribose) polymerase (PARP), thereby enhancing erlotinib resistance in non-small cell lung cancer (NSCLC) cells (Zhang et al., 2018). ATP-binding cassette transporters have been recognized as central to the mediation of drug resistance and the most studied are the transporting P-glycoproteins. A study reported that treatment of HCC cells with chemotherapeutic drugs induced enhanced release of exosomes containing lincRNA-VLDLR, which in turn promoted drug resistance by upregulating the expression of ATP-binding cassette, sub-family G member 2 (ABCG2), a member of the ATP-biding cassette transporter family known to function in multidrug resistance (Takahashi et al., 2014b).
LncRNA also mediates cell-nonautonomous drug resistance. Qu et al. (2016) reported that lncRNA-ARSR (lncRNA activated in RCC (renal cell carcinoma) with sunitinib resistance) could be incorporated into exosomes and transmitted to sensitive cells, resulting in sunitinib resistance. Aberrant expression of B-cell lymphoma 2 (Bcl-2) has been implicated in chemotherapeutic resistance in tumor cells. A recent study found that LncRNA-PART1 facilitated drug resistance in human epidermal growth factor receptor 2-positive (HER2+) breast cancer by acting as a molecular sponge for miR-129 to regulate the expression of Bcl-2. LncRNA-SNGH14 was found to mediate resistance to chemotherapy drugs in esophageal squamous cell carcinoma by regulating the expression of Bcl-2 (Dong et al., 2018; Kang et al., 2018). Resistance to chemotherapeutic drugs is a major factor contributing to poor prognosis of many cancers. A promising approach to reverse this adverse phenomenon is to enhance drug-induced apoptosis. As such, future studies to determine the possible role of tumor-derived exosomal lncRNAs in drug-induced apoptosis and fully elucidate the underlying mechanisms are needed for better management of chemotherapeutic drugs and to improve the survival of cancer patients.
4. Exosomal lncRNAs as biomarkers for diagnosis
Exosomes are an attractive source of diagnostic biomarkers because they harbour abundant biomolecules, including varieties of proteins and genetic materials reflecting their cellular origins, and can be obtained in a non-invasive manner. The easy-to-access feature of exosomes makes it possible to perform repeated measurements and obtain longitudinal data for dynamic monitoring of tumor cells.
Repeated measurement overcomes the drawbacks of individual differences by comparing data from the same individuals. Compared to circulating tumor cells and nucleic acids directly exposed in body fluids, tumor-derived exosomes are more stable and their concentration is higher. It was reported that integrin CD47 on the surface of exosomes can protect exosomes from phagocytosis by monocytes and macrophages (Matsumoto et al., 2017). At the same time, exosomes are easier to penetrate into various body fluids because of their small sizes. Exosomes mediate the exchange of signals and materials between donor and recipient cells, reflecting the real-time status of a live body. All of those properties make exosomes an ideal choice of liquid biopsy. Many studies have demonstrated an obvious asymmetrical distribution of RNAs between the original cells and their exosomes (Matsumoto et al., 2017). RNAs have been reported to be selectively sorted into exosomes (Valadi et al., 2007; Matsumoto et al., 2017). The findings of lncRNAs differently expressed in exosomes in many cancers suggest that exosomal lncRNAs could reflect the physiological and pathological status of their parental cells. Thus, it is plausible that tumor-derived exosomes containing specific tumor-associated lncRNAs may be employed as biomarkers to signal tumor development. Expression profiles of exosomal lncRNAs show great differences between healthy people and cancer patients. The abnormal expression of exosomal lncRNAs in cancer patients and at different stages of the disease compared with healthy subjects makes them a potential biomarker for cancer diagnosis. A recent study associated poor survival with increased lncRNA HOTTIP expression in gastric cancer (GC) patients. Moreover, exosomal lncRNA HOTTIP in serum had a better diagnostic accuracy for GC than the commonly used tumor markers carcino-embryonic antigen (CEA) and carbohydrate antigens19-9 and 72-4 (CA19-9 and CA72-4), supporting the potential use of exosomal lncRNA HOTTIP as a diagnostic and prognostic biomarker of GC (Zhao et al., 2018). Another study used an lncRNA microarray to screen for differential expression of lncRNAs and found that three lncRNAs were superior to α-fetoprotein (AFP) as diagnostic biomarkers for HCC (Tang et al., 2015). In a recent study using a combined Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway and Gene Ontology (GO) analysis approach, nine lncRNAs were identified as the most significantly differential lncRNAs in tumor tissues from colorectal cancer (CRC) patients. Among those nine lncRNAs, lncRNA-BLACAT1 was determined to be significant as a biomarker for CRC diagnosis (Dai et al., 2017). Precise liquid biopsy is a promising approach to improve early diagnosis and cancer prognosis. Circulating exosomes can be obtained by non-invasive methods and deliver differentially expressed lncRNAs. The abundance of exosomes in body fluids can increase the sensitivity of tumor diagnosis and the effectiveness of liquid biopsy. Longitudinal monitoring of differential expression of lncRNAs in tumor-derived exosomes can provide a more accurate and real-time view of tumor development, making precision medicine more promising.
5. Conclusions
Increasing evidence highlights the regulatory role of exosomes and lncRNAs in cancer progression and prognosis. A comprehensive understanding of the functions of exosomes and their components in cancer development is essential for cancer diagnosis. Exosomes carry specific nucleic acids and keep them stable in body fluids. Abnormal expression of tumor-derived exosomal lncRNAs has been associated with cancers of different stages. These findings show the potential of using exosomal lncRNAs for liquid biopsy for cancer diagnosis. The most studied exosomal lncRNAs in cancers are summarized in Table 1. Exosomes were considered to be garbage when they were first discovered. Later on, studies revealed that they are small and non-toxic natural liposomes widely distributed in body fluids and can be effectively and selectively absorbed by recipient cells (Bunggulawa et al., 2018). Of critical importance, exosomes are capable of targeting specific tissues and cells rather than interacting with cells at random in the intercellular microenvironment (Hoshino et al., 2015). These advantages make them ideal biomarkers for disease diagnosis and prognosis, and a promising choice as drug delivery vehicles. For example, serum-derived exosomes have been used to deliver tyrosinase-related protein-2 (TRP2) peptides to lymph nodes in preclinical trials (Park et al., 2018). However, the application of exosomes in clinical settings remains challenging because they are currently expensive to isolate, difficult to detect and classify due to heterogeneity, and show discrepancies between in vitro and in vivo experimental systems (Li Q et al., 2018). In addition, whether exosomal lncRNAs can serve as disease-specific biomarkers for cancer diagnosis and prognosis needs further study, because many of them have been detected in multiple tumors (Wang et al., 2014; Li Q et al., 2018; Tan et al., 2018).
Table 1.
Exosomal lncRNAs associated with human diseases
| LncRNA | Disease type | Biological function | Reference | |
| LincRNA-VLDLR | Hepatocellular carcinoma | Promotes drug resistance by upregulating the expression of ATP-binding cassette, sub-family G member 2 (ABCG2) | Takahashi et al., 2014b | |
| LncRNA-ARSR | Renal cell carcinoma | Acts as a competitive endogenous RNAs (ceRNAs) for miR-34 and miR-449, and promotes sunitinib resistance in renal cell carcinoma | Qu et al., 2016 | |
| LncRNA-BLACAT1 | Colorectal cancer | Diagnosis biomarker | Dai et al., 2017 | |
| LncRNA FAL1 | Hepatocellular carcinoma | Acts as a ceRNA to promote cell proliferation and migration | Li BG et al., 2018 | |
| LncRNA HOTAIR | Bladder cancer/laryngeal squamous cell carcinoma/glioblastoma multiform | Facilitates tumor initiation and progression, promotes tumor migration and invasion, and serves as a biomarker | Berrondo et al., 2016; Tan et al., 2018 | |
| LncRNA HOTTIP | Gastric cancer | Diagnostic and prognostic biomarker | Zhao et al., 2018 | |
| LncRNA Camk-A | Pancreatic cancer/breast cancer | Involved in regulating CaM kinase and inducing the remodeling of tumor microenvironment through nuclear factor-κB (NF-κB) signaling | Sang et al., 2018 | |
| LncRNA BCAR4 | Breast cancer | Involved in Yes-associated protein (YAP)-dependent glycolysis, thereby reprogramming glucose metabolism | Zheng et al., 2017 | |
| LncRNA-ROR | Hepatocellular carcinoma | Enhances sorafenib or doxorubicin resistance in hepatocellular carcinoma (HCC) cells | Takahashi et al., 2014a | |
| LncRNA RP11-838N2.4 | Non-small cell lung cancer | Enhances erlotinib resistance of non-small cell lung cancer (NSCLC) cells | Zhang et al., 2018 | |
| LncRNA-SNHG14 | Breast cancer | Promotes trastuzumab chemoresistance | Dong et al., 2018 | |
In summary, exosomes are pluripotent carriers involved in tumorigenesis, progression, and drug resistance. Creating specific modifications in lncRNAs to target certain biomolecules is a promising approach for therapeutical intervention of tumors and cancers. Furthermore, given the roles of exosomes in physiological and pathological conditions, strategies that interfere with their release and direct exosome-mediated intercellular communication may be used in the future. Since exosomal lncRNAs demonstrate multiple effects on cancer development, it is important to further explore the process of exosome biosynthesis and the mechanisms involved in the sorting and trafficking of lncRNAs in exosomes. Elucidating the roles of exosomal lncRNAs in tumorigenesis, metastasis, and drug resistance, and evaluating their impacts on the TME are areas of considerable interest for cancer-targeted therapy.
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 81672791 and 81872300), the Foundation of the Department of Education of Zhejiang Province, China (No. Y201224954), the Zhejiang Provincial Natural Science Foundation of China (No. LY14H040007), and the Zhejiang Provincial Natural Science Fund for Distinguished Young Scholars of China (No. LR18C060002)
Contributors: Ai-fu LIN and Zhi-jian CAI contributed to the study design and data analysis, and edited the manuscript. Jie LUO wrote the manuscript. Lei QU and Xiao FAN contributed to the figure and table design. Yan XIONG, Pei-fen FU, and En-chun LI edited the manuscript. All authors read and approved the final manuscript and, therefore, had full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines: Jie LUO, Yan XIONG, Pei-fen FU, En-chun LI, Lei QU, Xiao FAN, Zhi-jian CAI, and Ai-fu LIN declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012;125(23):5591–5596. doi: 10.1242/jcs.116392. [DOI] [PubMed] [Google Scholar]
- 2.Berrondo C, Flax J, Kucherov V, et al. Expression of the long non-coding RNA HOTAIR correlates with disease progression in bladder cancer and is contained in bladder cancer patient urinary exosomes. PLoS ONE. 2016;11(1):e0147236. doi: 10.1371/journal.pone.0147236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunggulawa EJ, Wang W, Yin TY, et al. Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnol. 2018;16(1):81. doi: 10.1186/s12951-018-0403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chairoungdua A, Smith DL, Pochard P, et al. Exosome release of β-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol. 2010;190(6):1079–1091. doi: 10.1083/jcb.201002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai MY, Chen XL, Mo SY, et al. Meta-signature lncRNAs serve as novel biomarkers for colorectal cancer: integrated bioinformatics analysis, experimental validation and diagnostic evaluation. Sci Rep, 7:46572. 2017 doi: 10.1038/srep46572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong HY, Wang W, Chen R, et al. Exosome-mediated transfer of lncRNA-SNHG14 promotes trastuzumab chemoresistance in breast cancer. Int J Oncol. 2018;53(3):1013–1026. doi: 10.3892/ijo.2018.4467. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75(2):193–208. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoshino A, Costa-Silva B, Shen TL, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juan T, Fürthauer M. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin Cell Dev Biol. 2017;74:66–77. doi: 10.1016/j.semcdb.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 10.Kang M, Ren MP, Li Y, et al. Exosome-mediated transfer of lncRNA PART1 induces gefitinib resistance in esophageal squamous cell carcinoma via functioning as a competing endogenous RNA. J Exp Clin Cancer Res, 37: 171. 2018 doi: 10.1186/s13046-018-0845-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Li BG, Mao R, Liu CF, et al. LncRNA FAL1 promotes cell proliferation and migration by acting as a ceRNA of miR-1236 in hepatocellular carcinoma cells. Life Sci. 2018;197:122–129. doi: 10.1016/j.lfs.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Li Q, Huang QP, Wang YL, et al. Extracellular vesicle-mediated bone metabolism in the bone microenvironment. J Bone Miner Metab. 2018;36(1):1–11. doi: 10.1007/s00774-017-0860-5. [DOI] [PubMed] [Google Scholar]
- 13.Ma YX, Zhang JM, Wen LX, et al. Membrane-lipid associated lncRNA: a new regulator in cancer signaling. Cancer Lett. 2018;419:27–29. doi: 10.1016/j.canlet.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto A, Takahashi Y, Nishikawa M, et al. Role of phosphatidylserine-derived negative surface charges in the recognition and uptake of intravenously injected B16BL6-derived exosomes by macrophages. J Pharm Sci. 2017;106(1):168–175. doi: 10.1016/j.xphs.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 15.Melo SA, Luecke LB, Kahlert C, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523(7559):177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohya T, Miaczynska M, Coskun Ü, et al. Reconstitution of Rab-and SNARE-dependent membrane fusion by synthetic endosomes. Nature. 2009;459(7250):1091–1097. doi: 10.1038/nature08107. [DOI] [PubMed] [Google Scholar]
- 17.Ostrowski M, Carmo NB, Krumeich S, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 18.Park O, Choi ES, Yu GH, et al. Efficient delivery of tyrosinase related protein-2 (TRP2) peptides to lymph nodes using serum-derived exosomes. Macromol Biosci. 2018;18(12):1800301. doi: 10.1002/mabi.201800301. [DOI] [PubMed] [Google Scholar]
- 19.Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol. 2007;23:519–547. doi: 10.1146/annurev.cellbio.23.090506.123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qu L, Ding J, Chen C, et al. Exosome-transmitted lncARSR promotes sunitinib resistance in renal cancer by acting as a competing endogenous RNA. Cancer Cell. 2016;29(5):653–668. doi: 10.1016/j.ccell.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Sang LJ, Ju HQ, Liu GP, et al. LncRNA CamK-A regulates Ca2+-signaling-mediated tumor microenvironment remodeling. Mol Cell. 2018;72(1):71–83e7. doi: 10.1016/j.molcel.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Shao HL, Im H, Castro CM, et al. New technologies for analysis of extracellular vesicles. Chem Rev. 2018;118(4):1917–1950. doi: 10.1021/acs.chemrev.7b00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soysal SD, Tzankov A, Muenst SE. Role of the tumor microenvironment in breast cancer. Pathobiology. 2015;82(3-4):142–152. doi: 10.1159/000430499. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi K, Yan IK, Kogure T, et al. Extracellular vesicle-mediated transfer of long non-coding RNA ROR modulates chemosensitivity in human hepatocellular cancer. FEBS Open Bio. 2014;4:458–467. doi: 10.1016/j.fob.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi K, Yan IK, Wood J, et al. Involvement of extracellular vesicle long noncoding RNA (linc-VLDLR) in tumor cell responses to chemotherapy. Mol Cancer Res. 2014;12(10):1377–1387. doi: 10.1158/1541-7786.MCR-13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamai K, Tanaka N, Nakano T, et al. Exosome secretion of dendritic cells is regulated by Hrs, an ESCRT-0 protein. Biochem Biophys Res Commun. 2010;399(3):384–390. doi: 10.1016/j.bbrc.2010.07.083. [DOI] [PubMed] [Google Scholar]
- 27.Tan SK, Pastori C, Penas C, et al. Serum long noncoding RNA HOTAIR as a novel diagnostic and prognostic biomarker in glioblastoma multiforme. Mol Cancer, 17:74. 2018 doi: 10.1186/s12943-018-0822-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang JW, Jiang RQ, Deng L, et al. Circulation long non-coding RNAs act as biomarkers for predicting tumorigenesis and metastasis in hepatocellular carcinoma. Oncotarget. 2015;6(6):4505–4515. doi: 10.18632/oncotarget.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 30.Trajkovic K, Hsu C, Chiantia S, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 31.Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 32.Villarroya-Beltri C, Baixauli F, Mittelbrunn M, et al. ISGylation controls exosome secretion by promoting lysosomal degradation of MVB proteins. Nat Commun, 7: 13588. 2016 doi: 10.1038/ncomms13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang JT, Zhou YD, Lu JG, et al. Combined detection of serum exosomal miR-21 and HOTAIR as diagnostic and prognostic biomarkers for laryngeal squamous cell carcinoma. Med Oncol. 2014;31(9):148. doi: 10.1007/s12032-014-0148-8. [DOI] [PubMed] [Google Scholar]
- 34.Zeigerer A, Gilleron J, Bogorad RL, et al. Rab5 is necessary for the biogenesis of the endolysosomal system in vivo . Nature. 2012;485(7399):465–470. doi: 10.1038/nature11133. [DOI] [PubMed] [Google Scholar]
- 35.Zhang W, Cai XR, Yu J, et al. Exosome-mediated transfer of lncRNA RP11-838N2.4 promotes erlotinib resistance in non-small cell lung cancer. Int J Oncol. 2018;53(2):527–538. doi: 10.3892/ijo.2018.4412. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Zhao R, Zhang YL, Zhang X, et al. Exosomal long noncoding RNA HOTTIP as potential novel diagnostic and prognostic biomarker test for gastric cancer. Mol Cancer. 2018;17(1):68. doi: 10.1186/s12943-018-0817-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng X, Han H, Liu GP, et al. LncRNA wires up Hippo and Hedgehog signaling to reprogramme glucose metabolism. EMBO J. 2017;36(22):3325–3335. doi: 10.15252/embj.201797609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou RH, Chen KK, Zhang JT, et al. The decade of exosomal long RNA species: an emerging cancer antagonist. Mol Cancer, 17:75. 2018 doi: 10.1186/s12943-018-0823-z. [DOI] [PMC free article] [PubMed] [Google Scholar]