Abstract
DNA double-stranded break (DSB) is one of the most catastrophic damages of genotoxic insult. Inappropriate repair of DNA DSBs results in the loss of genetic information, mutation, and the generation of harmful genomic rearrangements, which predisposes an organism to immunodeficiency, neurological damage, and cancer. The tumor repressor p53 plays a key role in DNA damage response, and has been found to be mutated in 50% of human cancer. p53, p63, and p73 are three members of the p53 gene family. Recent discoveries have shown that human p53 gene encodes at least 12 isoforms. Different p53 members and isoforms play various roles in orchestrating DNA damage response to maintain genomic integrity. This review briefly explores the functions of p53 and its isoforms in DNA DSB repair.
Keywords: p53, p53 isoform, DNA double-stranded break repair, Cell death
1. Introduction
Cells are under a wide range of DNA damage pressures from both endogenous and exogenous sources. More than 1×104 DNA lesions occur in any given cell each day. To minimize the effects of toxic insults on their DNA, cells have evolved different mechanisms for tolerating, reversing, and repairing genomic insults. The most deleterious lesion in DNA is the double-stranded break (DSB). DNA DSB can be generated in a variety of ways, such as replication, reactive oxygen species (ROS) produced during metabolic processes, normal programmed genomic rearrangements including V(D)J recombination and mitotic or meiotic recombination, ionizing radiation, and chemical drugs like camptothecin. The failure in repairing DNA DSBs can lead to loss of chromosomes. Improper repair results in mutations and chromosome rearrangement (Dudáš and Chovanec, 2004). To combat the threat of DNA DSBs, organisms have developed essentially three mechanisms for their repair, non-homologous end-joining (NHEJ), homologous recombination (HR), and single-stranded annealing (SSA) (Hakem, 2008; Hiom, 2010).
Following DSB formation, the broken ends regardless of the genetic sequence at the breaks are processed to yield appropriate substrates for direct ligation. This process is called NHEJ and is initiated by the binding of Ku70/80 with the broken ends (Hiom, 2010). Then, the Ku70/80 recruits downstream factors including DNA-dependent protein kinase catalytic subunit (DNA-PKcs) and Artemis. Finally the broken ends are ligated by the X-ray repair cross complementing 4 (XRCC4)-Ligase IV (LIG4) complex. NHEJ is the most straightforward way to repair a DSB and functions throughout the G1, S, and G2 phases of the cell cycle. However, if, as is often the case, nucleotides at the broken ends have been trimmed by nucleases or filled in by polymerases before the ligation, then small deletions and insertions are created. Therefore, NHEJ is considered to be error-prone. When cells progress through the S phase and into the G2 phase, a sister chromatid is produced by replication, one which provides a template for faithfully repairing the DNA DSBs by HR repair (Dudáš and Chovanec, 2004). To initiate HR repair, double-stranded DNA (dsDNA) ends are exonucleolytically resected to form 3' single-stranded DNA (ssDNA) tails which are bound and stabilized by the single-stranded binding protein, replication protein A (RPA). With the help of RAD52 and BRCA2, RAD51 recombinase replaces RPA to assemble a nucleoprotein filament along the ssDNA tail. This filament initiates the search for homology in the sister chromatid and invades homologous intact sequences to form heteroduplex DNA. The missing sequence at the DSB site is restored by DNA synthesis. The two sister chromatids can exchange strands to form a Holliday junction. Finally, the Holliday junction is resolved and DNA is ligated. Thus, the genetic information lost at a break in one chromatid is accurately restored by HR repair. Unlike NHEJ, HR repair is error-free. In SSA repair, the DSB site consists of direct repeats (Dudáš and Chovanec, 2004). The 3'-ssDNA tails are generated. The repeated sequences at 3'-ssDNA tails are aligned. Finally, the intervening sequences and protruding 3'-ends are removed by the endonuclease Ercc1-XPF. SSA repair does not require RAD51 but relies on RAD52, which facilitates pairing of the processed ssDNA tails before removal of the protruding 3'-ends.
The most important part of the DNA damage response is the activation of tumor repressor p53 (Levine and Oren, 2009). In response to different types and levels of stresses, p53 can be differentially activated by change in its stabilities and modifications. This results in various cellular events such as cell cycle arrest, DNA-damage repair, apoptosis and/or senescence to ensure genome integrity (Dai and Gu, 2010). Interestingly, p53 protein appears to promote only some DNA-damage repair pathways, such as base excision repair, mismatch repair, and nucleotide excision repair (Gatz and Wiesmuller, 2006; Helton and Chen, 2007; Meek, 2009), but inhibits DNA DSB repair pathways, including the HR, NHEJ, and SSA pathways (Mekeel et al., 1997; Akyüz et al., 2002; Keimling and Wiesmuller, 2009). This phenomenon seems to be contradictory to its role as a “tumor repressor.” A large number of p53 isoforms have been identified in recent years (Courtois et al., 2002; Bourdon et al., 2005; Chen et al., 2009). These p53 isoforms can either synergistically promote or antagonistically inhibit p53 functions, depending on the isoform structures and the target genes affected. This review will mainly focus on how p53 isoform Δ133p53 switches p53 signal from repressing to promoting DNA DSB repair.
2. p53 and its isoforms
The full-length p53 protein is a transcription factor and consists of a transactivation domain (TAD), a proline-rich domain (PRD), a DNA-binding domain (DBD), a nuclear localization signal (NLS), an oligomerization domain (OD), and a C-terminal regulatory domain (CTD) (Okorokov and Orlova, 2009), as shown in Fig. 1. Under normal conditions, p53 protein remains at low levels. However, it can be activated by a wide variety of stress signals such as DNA damage, starvation, hypoxia, heat/cold shock, oncogene activation, developmental stress, etc. (Vogelstein et al., 2000; Levine et al., 2006). In response to a stress signal, the activation of p53 can be achieved by a series of post-translational modifications that include phosphorylation, acetylation, methylation, sumoylation, neddylation, and non-proteolytic mono-ubiquitylation (Pietsch et al., 2008). Activated p53 binds to p53 responsive elements (REs) in the promoter regions to induce or repress the expression of its target genes. Usually, the p53 REs are found within a few thousand base pairs from the transcriptional start site of its target genes. There are two types of p53 REs. The first is composed of two pairs (half-sites) of pentamers arranged head-to-head, 5'-RRRC(A/T)(A/T)GYYY-3' (R: purine; Y: pyrimidine), separated by 0–38 nucleotides in the promoters of activated downstream genes, and the second type contains two pairs of pentamers arranged end-to-head, 5'-RRRC(A/T)(N)RRRC(A/T)-3' or 5'-(A/T)GYYY (N)(A/T)GYYY-3', separated by 0–13 nucleotides in the promoters of repressed downstream genes (Hoh et al., 2002; Amson et al., 2011). The biological roles of p53 downstream genes can be divided into three major groups: the negative regulators of p53 (mouse double minute protein 2 (MDM2), constitutive photomorphogenetic 1 (COP1), and p53-induced-RING-H2 (PIRH-2), etc.), the negative regulators of the cell cycle (p21, 14-3-3δ, and growth arrest and DNA damage 45 (GADD-45), etc.), and the apoptotic factors (BAX, p53-upregulated modulator of apoptosis (PUMA), NOXA, and DR, etc.). Activation of p53 leads to two cellular events, either cell cycle arrest to allow the repair of the damage or apoptosis in cells where the damage cannot be fixed. In addition to its transcription activity, p53 can also function in cytoplasm by directly interacting with anti-apoptotic proteins such as Bcl-2 and Bcl-XL in mitochondria, acting in a way similar to the so-called BH3 only protein (PUMA) (Chipuk et al., 2005; Moll et al., 2005).
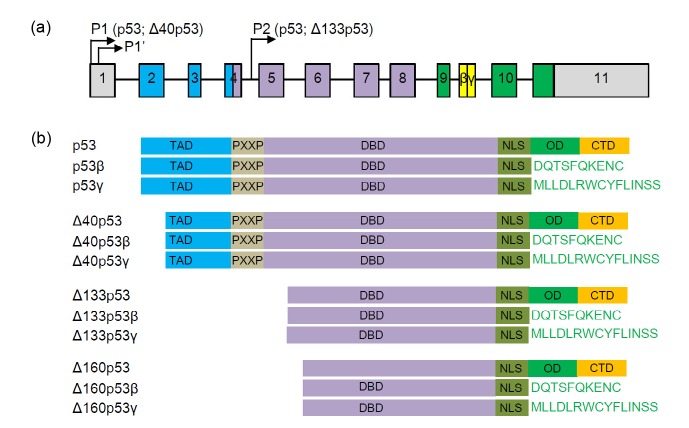
Fig. 1.
Human p53 and its isoforms
(a) Diagram of human p53 gene structure. Alternative promoters (P1, P1', and P2) and alternative splicing sites (β, γ) are indicated. (b) p53 protein isoforms. TAD: transactivation domain; PXXP: proline-rich domain; DBD: DNA-binding domain; NLS: nuclear localization signal; OD: oligomerization domain; CTD: C-terminal regulatory domain
The MDM2 and the protein p14ARF are the main players of the p53 pathway (de Oca Luna et al., 1995; Langheinrich et al., 2002). The MDM2 is an E3 ligase which ubiquitylates p53 and targets it for proteasomal degradation, whereas p14ARF inhibits MDM2 and raises p53 levels.
Human p53 gene has 11 exons and can create at least 12 different isoform proteins (Fig. 1) using multiple promoters, alternative splicing, and the internal ribosome entry site (IRES) (Joruiz and Bourdon, 2016; Vieler and Sanyal, 2018). The usage of the promoter (P1) leads to the production of p53 and Δ40p53 isoforms, whereas the internal promoter regulates the expression of Δ133p53 and Δ160p53 isoforms. The C-terminal isoforms β and γ can be produced by the alternative splicing of the intron 9 (Fig. 1a).
2.1. p53β and p53γ
There are three C-terminal isoforms α, β, and γ. The α-isoform denotes full-length p53 of C-terminal and α is usually omitted. The β and γ isoforms lack the OD and CTD (Fig. 1b). However, p53β can form a complex with full-length p53 and selectively enhance p53 transcriptional activity at the BAX promoter, but not at the p21 promoter, thus driving the cell fate preferentially to apoptosis in response to stress conditions (Bourdon et al., 2005). In normal culture or physiological conditions, p53β can also coordinate with p53 to accelerate replicative cellular senescence through upregulating the expression of microRNA-34a (miR-34a) (Fujita et al., 2009; Mondal et al., 2013). p53γ protein is unstable and localizes in either the nucleus of most cells or the cytoplasm of some cells. It has been observed that both β and γ isoforms were abnormally distributed in different cancer tissues (Anensen et al., 2006).
2.2. Δ40p53
Δ40p53 isoforms (also named p47 or ΔNp53) can be produced from P1 or P1' promoters. The Δ40p53 protein is an N-terminal truncated protein with a partial deletion in the TAD due to alternative splicing of exon 2 and/or alternative initiation of translation at ATG-40 (Fig. 1b). The deleted part of the TAD contains major activating phosphorylation sites and the MDM2-interacting motif. Because of the absence of MDM2-binding motif, Δ40p53 protein does not interact with MDM2 and therefore escapes the rapid degradation mechanism that regulates the full-length p53 level. Δ40p53 can regulate the function of p53 by oligomerizing with p53 in vivo (Courtois et al., 2002). Unlike p53, as Δ40p53 has a truncated TAD, it is capable of inducing a different set of p53-responsive genes. It can induce many apoptosis-associated genes such as p53-induced protein with a death domain (PIDD), but repress cell cycle arrest genes such as p21 (Ohki et al., 2007). In addition, Δ40p53 has a stronger tendency to form oligomers than p53 (Powell et al., 2008), which results in dosage effects of Δ40p53 expression. Low levels of Δ40p53 can synergistically promote the transactivation activity of p53, whereas high levels of Δ40p53 induce a different set of genes (Powell et al., 2008).
2.3. Δ133p53
The Δ133p53 is transcribed from intron 4 (Fig. 1a) and conserved in zebrafish, Drosophila, and the mouse (Bourdon et al., 2005; Chen et al., 2005). This evolutionary conservation indicates the importance of the isoforms in the p53 signal pathway. The isoform Δ133p53 lacks 132 amino acids from the N-terminal of p53, which contains both the MDM2-interacting motif and the TAD and part of the DBD (Fig. 1b). Therefore, Δ133p53 imposes a more drastic effect on the p53 signal pathway. In response to both developmental and DNA damage stresses, Δ133p53 is directly transactivated by full-length p53 (Chen et al., 2009; Marcel et al., 2010b; Aoubala et al., 2011). In turn, Δ133p53 inhibits p53-dependent apoptosis and G1 arrest without inhibiting p53-dependent G2 arrest by differentially modulating the expression of p53 target genes. Δ133p53 can form a complex with p53 both in vitro and in vivo. The anti-apoptotic activity of zebrafish Δ113p53, a Δ133p53 orthologue, is dependent on interacting with p53 (Ou et al., 2014). Basal Δ133p53 expression can inhibit p53-mediated replicative senescence in normal human fibroblasts, T-lymphocytes and astrocytes by repressing miR-34a expression (Fujita et al., 2009; Mondal et al., 2013). Δ133p53 has been found to be overexpressed in some cancer cells. This promotes angiogenesis and tumor progression (Bernard et al., 2013). Interestingly, under conditions of sub-toxic ROS stress, Δ133p53 does not antagonize the activity of full-length p53, but coordinates with p53 to promote cell survival by promoting antioxidant gene expression (Gong et al., 2016). Δ133p53β can promote cancer stem cell potential by stimulating the expression of the key pluripotency factors SOX2, OCT3/4, and NANOG (Arsic et al., 2015).
2.4. Δ160p53
Like Δ133p53, Δ160p53 isoform is also translated from Δ133p53 transcript through the alternative translation start codon at Met160 (Fig. 1b) (Marcel et al., 2010a). Δ160p53 may have a function similar to mutant p53s due to the lack of a major part of the DNA-binding domain. It has been reported that cells carrying p53 mutations, such as R175H or R248Q that are frequently found in human cancers, overexpress Δ160p53 isoform (Candeias et al., 2016). Downregulation of Δ160p53 impairs the “gain-of-function” cancer phenotypes induced by mutant p53, suggesting that the Δ160p53 isoform possesses pro-oncogenic potential.
3. p53 family members
The p53 family has three members, p53, p63, and p73. All encode a number of isoforms and share a high level of protein sequence similarity, particularly in the DNA-binding domain. Thus, p63 and p73 can transcribe p53 target genes causing cell cycle arrest and apoptosis (Bourdon, 2007). However, p53, p63, and p73 are not entirely functionally redundant, as they exhibit promoter selectivity and have a number of unique target genes. The distinct functions of three genes have been demonstrated by studies on knockout mice. p53 knockout mice are viable and develop normally, but grow a tumor at a later stage (Donehower et al., 1992). p63 null mice die a few days after birth and display severe developmental defects, including failure to develop limbs, skin, and other epithelial tissues (Mills et al., 1999). Knockout of p73 results in infertility, spontaneous and carcinogen-induced tumorigenesis, as well as hippocampal dysgenesis (Yang et al., 2000; Tomasini et al., 2008; Wilhelm et al., 2010). These results suggest that p53 mainly functions in DNA damage response and p63 plays a key role in development, whereas p73 is involved in both processes of DNA damage response and development.
4. p53 in DNA DSB repair
An abundance of evidence has demonstrated that full-length p53 counteracts DNA DSB repair. Using three enhanced green fluorescent protein (EGFP)-based DNA DSB repair test systems, two studies showed that p53 plays a role in restraining three DNA DSB repair pathways including HR, NHEJ, and SSA (Akyüz et al., 2002; Keimling and Wiesmuller, 2009). More evidence revealed that p53 has direct regulatory activity in DNA DSB repair, as mutations in p53 that impair or even abolish its transcriptional activity and cell cycle regulatory capacity, and do not significantly affect its inhibition of HR (Willers et al., 2000; Boehden et al., 2003; Linke et al., 2003). Protein association studies showed that p53 can form complexes with a number of HR components such as RAD51 (Buchhop et al., 1997; Linke et al., 2003), RPA (Romanova et al., 2004), RAD54 (Linke et al., 2003), BRCA1 (Zhang et al., 1998), BRCA2 (Marmorstein et al., 1998), BLM (Wang et al., 2001), and WRN (Blander et al., 1999). The interaction between p53 and either RAD51 or RAD54 inhibits HR repair by interrupting RAD51 polymerization and RAD51-mediated strand exchange (Yoon et al., 2004). p53 modulates the helicase activities of BLM and WRN to counteract Holliday junction unwinding. The interaction of p53 with RPA mediates suppression of HR repair. p53 can also regulate HR repair at the transcription level through repressing the expression of RAD51, RECQ4, and WRN (Arias-Lopez et al., 2006; Gatz and Wiesmuller, 2006).
p53 protein rapidly accumulates to a high level in response to DNA DSB stress at an early stage. However, activated p53 also activates the expression of MDM2, an E3 ligase that mediates the degradation of p53 in the later stage. Therefore, it is of great interest to understand the role of p53 signal pathway in response to DNA DSB stress in the later stage.
5. Δ133p53 and p73 in DNA DSB repair
The restraining role of p53 in DNA DSB repair had remained elusive for many years until our recent discoveries demonstrated that Δ113p53/Δ133p53 functions to promote DNA DSB repair in 2015 and 2018 (Gong L et al., 2015; Gong HJ et al., 2018). In 2005, Δ133p53 and its zebrafish orthologous Δ113p53 were first identified in two reports, one on analysis of 5'-rapid amplification of cDNA end (RACE) polymerase chain reaction (PCR) products of p53 transcripts in human normal tissues (Bourdon et al., 2005) and the other done by us on analysis of a zebrafish genetic mutant def hi429 (Chen et al., 2005). Our following investigation discovered that Δ113p53 was strongly induced in response to DNA damage such as γ-irradiation and DNA damage drugs, camptothecin and roscovitine. More importantly, we found that Δ113p53 is a p53 target gene and functions to antagonize p53-mediated apoptosis (Chen et al., 2009).
As Δ113p53 is a p53 target gene, we were wondering what functions of Δ113p53 are in other stress conditions. To our surprise, both human Δ133p53 and zebrafish Δ113p53 were strongly induced by γ-irradiation, but not by ultraviolet (UV) irradiation or heat shock treatment; in contrast, full-length p53 was activated by all three treatments (Gong et al., 2015). Protein analysis from both human cells and zebrafish showed that full-length p53 protein accumulated to the highest level as early as 4 h post irradiation (hpi), whereas Δ113p53 protein peaked later, at 24 hpi upon γ-irradiation. The result suggested that Δ113p53/Δ133p53 may play a role in DNA DSB repair. Using three EGFP-repairing-aided visual-cum-quantitative analysis reporter systems, we found that Δ113p53/Δ133p53 promoted all three DNA DSB repair pathways, including HR, NHEJ, and SSA, in a p53-independent manner. The positive role of Δ113p53/Δ133p53 on the DNA DSB repair was confirmed by analysis on DNA damage extents and repair foci of genomic DNA upon γ-irradiation. Interestingly, apoptotic activity was correlated positively with the level of p53 protein and negatively with the level of Δ113p53 protein, whereas the DNA damage repair corresponded to the level of Δ113p53 protein in γ-irradiated zebrafish embryos. Through deleting one of p53 REs in the P2 promoter, we generated a zebrafish Δ113p53 knockout mutant (Δ113p53M/M). The expression of Δ113p53 was completely blocked in the mutant, but the activation of full-length p53 was not affected. In normal conditions, the mutant did not show any effects. However, the Δ113p53M/M mutant embryos were more sensitive to γ-irradiation due to accumulating higher level of DNA damage extents and apoptotic cells. The significance of Δ113p53 in DNA DSB repair was further evaluated by blocking apoptosis with the overexpression of bcl2L (anti-apoptotic protein) in the irradiated mutant embryos. Blocking apoptosis with bcl2L significantly increased embryo viabilities in both wild type (WT) and Δ113p53M/M upon γ-irradiation, whereas the difference of viability between WT and Δ113p53M/M embryos still remained. The data from human cells also showed that knockdown of Δ133p53 coupled with γ-irradiation increased DNA damage and resulted in cell growth arrest at the G2 phase that in turn enhanced cell senescence at the later stage. Therefore, we demonstrated that both functions of Δ113p53/Δ133p53 in antagonizing apoptosis and promoting DNA DSB repair are important for its pro-survival characters upon DNA damage stress (Gong et al., 2015).
Unlike full-length p53, Δ113p53 does not form a complex with either Rad51 or Rpa. It promotes DNA DSB repair at the transcription level by enhancing the expression of the DNA DSB repair genes rad51, lig4, and rad52, which is independent of full-length p53. Using promoter function analysis, gel shift and chromatin immunoprecipitation (ChIP) assays, we showed that Δ113p53 bound to a novel type of p53 RE in the promoters of three repair genes. Taken together, Δ113p53/Δ133p53 promotes DNA DSB repair via upregulating the expression of DNA DSB repair genes (Gong et al., 2015). This finding raised a question on how Δ133p53, lacking the TAD, induces the transcription of these repair genes in a p53-independent manner.
A previous ChIP-based analysis showed that p73 bound to the promoters of some DNA DSB repair genes, such as RAD51, MRE11, and BRCA2. However, the overexpression of p73 did not greatly increase the expression of these genes (Lin et al., 2009). These studies indicate that p73 plays a role in DNA DSB repair, which promotes us to investigate whether Δ133p53 coordinates with p73 to promote DNA DSB repair. As expected, we found that the expression of both p73 and Δ133p53 proteins increased to the highest level at 24 hpi and p73 formed a complex with Δ133p53, but not p53, after γ-irradiation (Gong et al., 2018). Furthermore, through various methods, such as EGFP-repairing-aided visual-plus-quantitative analysis reporter systems, comet assays, and repair focal analyses, we demonstrated that Δ133p53 and p73 are interdependent in promoting all three DNA DSB repair pathways. ChIP assay showed that Δ133p53 promoted the binding of p73 to the promoters of the DNA DSB repair genes RAD51, RAD52, and LIG4. This was required for p73 to transcribe the expression of the repair genes. Thus, p73 and Δ133p53 work together to ensure genomic integrity upon DNA DSBs (Gong et al., 2018).
In addition to the accumulation of p73 protein at 24 hpi, p73 also peaked as early as 4 hpi after γ-irradiation. Upon DNA damage, there are two mechanisms for p73 activation, one from protein phosphorylation by c-Ab1 tyrosine kinase (Gong et al., 1999), and the other from upregulation of its messenger RNA (mRNA) expression by the transcription factor E2F1 (Irwin et al., 2000; Gonzalez et al., 2003). Our quantitative reverse-transcription PCR (qRT-PCR) results showed that p73 mRNA was upregulated at 12 hpi, but not before 4 hpi. The knockdown of c-Abl kinase resulted in a decrease of p73 protein at 4 hpi, but not at 24 hpi. More interestingly, when p73 was depleted upon γ-irradiation, apoptotic activity decreased only in the early stage (before 6 hpi), but not in the later stage (24 hpi), whereas DNA DSB accumulation was increased at 2 and 5 dpi (Gong et al., 2018). These data illustrate that the first p73 protein peak results from protein stabilization with the aim of promoting apoptosis, whereas the second p73 protein peak is translated from increased mRNA to promote DNA damage repair. The finding suggests that both p73 functions in promoting apoptosis and DNA DSB repair are important for its tumor repressing role, which may explain the spontaneous and carcinogen-induced tumorigenesis observed in p73 knockout mice.
A number of reports showed that the expression of Δ133p53 was elevated in different cancer tissues such as breast cancer, renal cell carcinoma, ovarian cancer, intrahepatic cholangiocarcinoma, colon cancer, and lung carcinoma (Bourdon et al., 2005; Fujita et al., 2009; Moore et al., 2010; Nutthasirikul et al., 2013; Kim and An, 2016; Fragou et al., 2017). This indicated that the upregulated Δ133p53 might be correlated with tumorigenesis because of its anti-apoptotic function or its role in promoting angiogenesis. There are a large number of somatic mutations in the promoter region of Δ133p53 in the COSMIC (Catalogue of Somatic Mutations in Cancer) cancer database. One of these mutated residues locates in exon 4. Interestingly, the mutations in this residue do not change the codon of full-length p53 protein. However, we found that the mutations attenuated the activation of Δ133p53 in response to the DNA damage (Gong et al., 2018). Therefore, the loss of Δ133p53 function might be also associated with tumorigenesis because of its role in DNA damage repair.
6. Model of p53 signal in DNA DSB repair
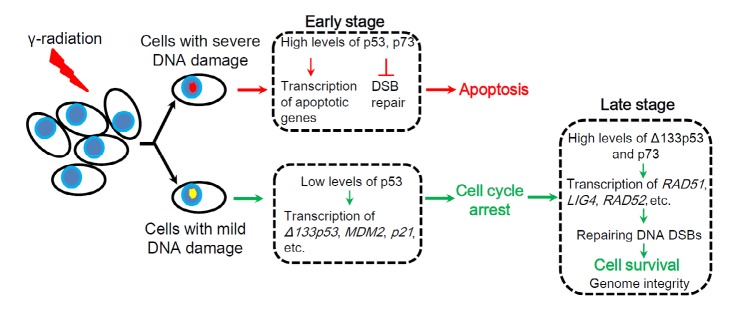
In summary, we propose a model to illustrate how the p53 signal protects genomic integrity upon ionizing irradiation (Fig. 2). At the early stage after irradiation, in cells with severe DNA damage, full-length p53 and p73 proteins are quickly stabilized to a high level, which inhibits DNA DSB repair and induces the expression of apoptotic genes, in turn to guide cells to undergo apoptosis. However, in cells with less and fixable DNA damage, p53 protein is activated at a relatively low level to transcribe its target genes such as MDM2, p21, and Δ133p53, etc., while p73 mRNA is also transcribed by E2F1. The expression of MDM2 targets p53 protein for degradation, but not Δ133p53, due to Δ133p53 without MDM2-interacting motif. The cell cycle is arrested by the expression of negative regulators such as p21. Therefore, in the later stage, both p73 and Δ133p53 proteins can accumulate to higher levels, while p53 protein decreases to the basal level. The expression of Δ113p53 not only protects cells from death by its anti-apoptotic function, but also coordinates with p73 to transcribe repair genes to promote DNA DSB repair. Finally, genomic integrity is restored in survival cells.
Fig. 2.
A model for the p53 signal to protect genomic integrity upon ionizing irradiation
DSB: double-stranded break
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 31571511 and 31871500)
Contributors: Yu-xi ZHANG and Wen-ya PAN drew the figures, and edited the manuscript and the format of reference list. Jun CHEN wrote the manuscript. All authors read and approved the final manuscript.
Compliance with ethics guidelines: Yu-xi ZHANG, Wen-ya PAN, and Jun CHEN declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Akyüz N, Boehden GS, Süsse S, et al. DNA substrate dependence of p53-mediated regulation of double-strand break repair. Mol Cell Biol. 2002;22(17):6306–6317. doi: 10.1128/MCB.22.17.6306-6317.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amson R, Pece S, Lespagnol A, et al. Reciprocal repression between p53 and TCTP. Nat Med. 2011;18(1):91–99. doi: 10.1038/nm.2546. [DOI] [PubMed] [Google Scholar]
- 3.Anensen N, Oyan AM, Bourdon JC, et al. A distinct p53 protein isoform signature reflects the onset of induction chemotherapy for acute myeloid leukemia. Clin Cancer Res. 2006;12(13):3985–3992. doi: 10.1158/1078-0432.CCR-05-1970. [DOI] [PubMed] [Google Scholar]
- 4.Aoubala M, Murray-Zmijewski F, Khoury MP, et al. p53 directly transactivates Δ133p53α, regulating cell fate outcome in response to DNA damage. C. ell Death Differ. 2011;18(2):248–258. doi: 10.1038/cdd.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arias-Lopez C, Lazaro-Trueba I, Kerr P, et al. p53 modulates homologous recombination by transcriptional regulation of the RAD51 gene. EMBO Rep. 2006;7(2):219–224. doi: 10.1038/sj.embor.7400587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arsic N, Gadea G, Lagerqvist EL, et al. The p53 isoform Δ133p53β promotes cancer stem cell potential. Stem Cell Rep. 2015;4(4):531–540. doi: 10.1016/j.stemcr.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernard H, Garmy-Susini B, Ainaoui N, et al. The p53 isoform, Δ133p53α, stimulates angiogenesis and tumour progression. Oncogene. 2013;32(17):2150–2160. doi: 10.1038/onc.2012.242. [DOI] [PubMed] [Google Scholar]
- 8.Blander G, Kipnis J, Leal JFM, et al. Physical and functional interaction between p53 and the Werner’s syndrome protein. J Biol Chem. 1999;274(41):29463–29469. doi: 10.1074/jbc.274.41.29463. [DOI] [PubMed] [Google Scholar]
- 9.Boehden GS, Akyüz N, Roemer K, et al. p53 mutated in the transactivation domain retains regulatory functions in homology-directed double-strand break repair. Oncogene. 2003;22(26):4111–4117. doi: 10.1038/sj.onc.1206632. [DOI] [PubMed] [Google Scholar]
- 10.Bourdon JC. p53 family isoforms. Curr Pharm Biotechnol. 2007;8(6):332–336. doi: 10.2174/138920107783018444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourdon JC, Fernandes K, Murray-Zmijewski F, et al. p53 isoforms can regulate p53 transcriptional activity. Genes Dev. 2005;19(18):2122–2137. doi: 10.1101/gad.1339905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchhop S, Gibson MK, Wang XW, et al. Interaction of p53 with the human Rad51 protein. Nucleic Acids Res. 1997;25(19):3868–3874. doi: 10.1093/nar/25.19.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Candeias MM, Hagiwara M, Matsuda M. Cancer-specific mutations in p53 induce the translation of Δ160p53 promoting tumorigenesis. EMBO Rep. 2016;17(11):1542–1551. doi: 10.15252/embr.201541956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Ruan H, Ng SM, et al. Loss of function of def selectively up-regulates Δ113p53 expression to arrest expansion growth of digestive organs in zebrafish. Genes Dev. 2005;19(23):2900–2911. doi: 10.1101/gad.1366405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Ng SM, Chang CQ, et al. p53 isoform Δ113p53 is a p53 target gene that antagonizes p53 apoptotic activity via BclxL activation in zebrafish. Genes Dev. 2009;23(3):278–290. doi: 10.1101/gad.1761609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chipuk JE, Bouchier-Hayes L, Kuwana T, et al. PUMA couples the nuclear and cytoplasmic proapoptotic function of p53. Science. 2005;309(5741):1732–1735. doi: 10.1126/science.1114297. [DOI] [PubMed] [Google Scholar]
- 17.Courtois S, Verhaegh G, North S, et al. ΔN-p53, a natural isoform of p53 lacking the first transactivation domain, counteracts growth suppression by wild-type p53. Oncogene. 2002;21(44):6722–6728. doi: 10.1038/sj.onc.1205874. [DOI] [PubMed] [Google Scholar]
- 18.Dai C, Gu W. p53 post-translational modification: deregulated in tumorigenesis. Trends Mol Med. 2010;16(11):528–536. doi: 10.1016/j.molmed.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Oca Luna RM, Wagner DS, Lozano G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53 . Nature. 1995;378(6553):203–206. doi: 10.1038/378203a0. [DOI] [PubMed] [Google Scholar]
- 20.Donehower LA, Harvey M, Slagle BL, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356(6366):215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 21.Dudáš A, Chovanec M. DNA double-strand break repair by homologous recombination. Mutat Res. 2004;566(2):131–167. doi: 10.1016/j.mrrev.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Fragou A, Tzimagiorgis G, Karageorgopoulos C, et al. Increased Δ133p53 mRNA in lung carcinoma corresponds with reduction of p21 expression. Mol Med Rep. 2017;15(4):1455–1460. doi: 10.3892/mmr.2017.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujita K, Mondal AM, Horikawa I, et al. p53 isoforms Δ133p53 and p53β are endogenous regulators of replicative cellular senescence. Nat Cell Biol. 2009;11(9):1135–1142. doi: 10.1038/ncb1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gatz SA, Wiesmuller L. p53 in recombination and repair. Cell Death Differ. 2006;13(6):1003–1016. doi: 10.1038/sj.cdd.4401903. [DOI] [PubMed] [Google Scholar]
- 25.Gong HJ, Zhang YX, Jiang KP, et al. p73 coordinates with Δ133p53 to promote DNA double-strand break repair. Cell Death Differ. 2018;25(6):1063–1079. doi: 10.1038/s41418-018-0085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gong JG, Costanzo A, Yang HQ, et al. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature. 1999;399(6738):806–809. doi: 10.1038/21690. [DOI] [PubMed] [Google Scholar]
- 27.Gong L, Gong HJ, Pan X, et al. p53 isoform Δ113p53/Δ133p53 promotes DNA double-strand break repair to protect cell from death and senescence in response to DNA damage. Cell Res. 2015;25(3):351–369. doi: 10.1038/cr.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gong L, Pan X, Yuan ZM, et al. p53 coordinates with Δ133p53 isoform to promote cell survival under low-level oxidative stress. J Mol Cell Biol. 2016;8(1):88–90. doi: 10.1093/jmcb/mjv069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez S, Prives C, Cordon-Cardo C. p73α regulation by Chk1 in response to DNA damage. Mol Cell Biol. 2003;23(22):8161–8171. doi: 10.1128/MCB.23.22.8161-8171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Hakem R. DNA-damage repair; the good, the bad, and the ugly. EMBO J. 2008;27(4):589–605. doi: 10.1038/emboj.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helton ES, Chen XB. p53 modulation of the DNA damage response. J Cell Biochem. 2007;100(4):883–896. doi: 10.1002/jcb.21091. [DOI] [PubMed] [Google Scholar]
- 32.Hiom K. Coping with DNA double strand breaks. DNA Repair. 2010;9(12):1256–1263. doi: 10.1016/j.dnarep.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 33.Hoh J, Jin S, Parrado T, et al. The p53MH algorithm and its application in detecting p53-responsive genes. Proc Natl Acad Sci USA. 2002;99(13):8467–8472. doi: 10.1073/pnas.132268899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irwin M, Marin MC, Phillips AC, et al. Role for the p53 homologue p73 in E2F-1-induced apoptosis. Nature. 2000;407(6804):645–648. doi: 10.1038/35036614. [DOI] [PubMed] [Google Scholar]
- 35.Joruiz SM, Bourdon JC. p53 isoforms: key regulators of the cell fate decision. Cold Spring Harb Perspect Med. 2016;6(8):a026039. doi: 10.1101/cshperspect.a026039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keimling M, Wiesmuller L. DNA double-strand break repair activities in mammary epithelial cells–influence of endogenous p53 variants. Carcinogenesis. 2009;30(7):1260–1268. doi: 10.1093/carcin/bgp117. [DOI] [PubMed] [Google Scholar]
- 37.Kim S, An SS. Role of p53 isoforms and aggregations in cancer. Medicine (Baltimore) 2016;95(26):e3993. doi: 10.1097/MD.0000000000003993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langheinrich U, Hennen E, Stott G, et al. Zebrafish as a model organism for the identification and characterization of drugs and genes affecting p53 signaling. Curr Biol. 2002;12(23):2023–2028. doi: 10.1016/S0960-9822(02)01319-2. [DOI] [PubMed] [Google Scholar]
- 39.Levine AJ, Oren M. The first 30 years of p53: growing ever more complex. Nat Rev Cancer. 2009;9(10):749–758. doi: 10.1038/nrc2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levine AJ, Hu W, Feng Z. The p53 pathway: what questions remain to be explored? Cell Death Differ. 2006;13(6):1027–1036. doi: 10.1038/sj.cdd.4401910. [DOI] [PubMed] [Google Scholar]
- 41.Lin YL, Sengupta S, Gurdziel K, et al. p63 and p73 transcriptionally regulate genes involved in DNA repair. PLoS Genet. 2009;5(10):e1000680. doi: 10.1371/journal.pgen.1000680. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Linke SP, Sengupta S, Khabie N, et al. p53 interacts with hRAD51 and hRAD54, and directly modulates homologous recombination. Cancer Res. 2003;63(10):2596–2605. [PubMed] [Google Scholar]
- 43.Marcel V, Perrier S, Aoubala M, et al. Δ160p53 is a novel N-terminal p53 isoform encoded by Δ133p53 transcript. FEBS Lett. 2010;584(21):4463–4468. doi: 10.1016/j.febslet.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Marcel V, Vijayakumar V, Fernández-Cuesta L, et al. p53 regulates the transcription of its delta133p53 isoform through specific response elements contained within the TP53 P2 internal promoter. Oncogene. 2010;29(18):2691–2700. doi: 10.1038/onc.2010.26. [DOI] [PubMed] [Google Scholar]
- 45.Marmorstein LY, Ouchi T, Aaronson SA. The BRCA2 gene product functionally interacts with p53 and RAD51. Proc Natl Acad Sci USA. 1998;95(23):13869–13874. doi: 10.1073/pnas.95.23.13869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meek DW. Tumour suppression by p53: a role for the DNA damage response? Nat Rev Cancer. 2009;9(10):714–723. doi: 10.1038/nrc2716. [DOI] [PubMed] [Google Scholar]
- 47.Mekeel KL, Tang W, Kachnic LA, et al. Inactivation of p53 results in high rates of homologous recombination. Oncogene. 1997;14(15):1847–1857. doi: 10.1038/sj.onc.1201143. [DOI] [PubMed] [Google Scholar]
- 48.Mills AA, Zheng BH, Wang XJ, et al. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398(6729):708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- 49.Moll UM, Wolff S, Speidel D, et al. Transcription-independent pro-apoptotic functions of p53. Curr Opin Cell Biol. 2005;17(6):631–636. doi: 10.1016/j.ceb.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Mondal AM, Horikawa I, Pine SR, et al. p53 isoforms regulate aging-and tumor-associated replicative senescence in T lymphocytes. J Clin Invest. 2013;123(12):5247–5257. doi: 10.1172/JCI70355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moore HC, Jordan LB, Bray SE, et al. The RNA helicase p68 modulates expression and function of the Δ133 isoform(s) of p53, and is inversely associated with Δ133p53 expression in breast cancer. Oncogene. 2010;29(49):6475–6484. doi: 10.1038/onc.2010.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nutthasirikul N, Limpaiboon T, Leelayuwat C, et al. Ratio disruption of the Δ133p53 and TAp53 isoform equilibrium correlates with poor clinical outcome in intrahepatic cholangiocarcinoma. Int J Oncol. 2013;42(4):1181–1188. doi: 10.3892/ijo.2013.1818. [DOI] [PubMed] [Google Scholar]
- 53.Ohki R, Kawase T, Ohta T, et al. Dissecting functional roles of p53 N-terminal transactivation domains by microarray expression analysis. Cancer Sci. 2007;98(2):189–200. doi: 10.1111/j.1349-7006.2006.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Okorokov AL, Orlova EV. Structural biology of the p53 tumour suppressor. Curr Opin Struct Biol. 2009;19(2):197–202. doi: 10.1016/j.sbi.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Ou Z, Yin L, Chang CQ, et al. Protein interaction between p53 and Δ113p53 is required for the anti-apoptotic function of Δ113p53. J Genet Genomics. 2014;41(2):53–62. doi: 10.1016/j.jgg.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Pietsch EC, Sykes SM, McMahon SB, et al. The p53 family and programmed cell death. Oncogene. 2008;27(50):6507–6521. doi: 10.1038/onc.2008.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Powell DJ, Hrstka R, Candeias M, et al. Stress-dependent changes in the properties of p53 complexes by the alternative translation product p53/47. Cell Cycle. 2008;7(7):950–959. doi: 10.4161/cc.7.7.5626. [DOI] [PubMed] [Google Scholar]
- 58.Romanova LY, Willers H, Blagosklonny MV, et al. The interaction of p53 with replication protein a mediates suppression of homologous recombination. Oncogene. 2004;23(56):9025–9033. doi: 10.1038/sj.onc.1207982. [DOI] [PubMed] [Google Scholar]
- 59.Tomasini R, Tsuchihara K, Wilhelm M, et al. TAp73 knockout shows genomic instability with infertility and tumor suppressor functions. Genes Dev. 2008;22(19):2677–2691. doi: 10.1101/gad.1695308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vieler M, Sanyal S. p53 isoforms and their implications in cancer. Cancers. 2018;10(9):288. doi: 10.3390/cancers10090288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408(6810):307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 62.Wang XW, Tseng A, Ellis NA, et al. Functional interaction of p53 and BLM DNA helicase in apoptosis. J Biol Chem. 2001;276(35):32948–32955. doi: 10.1074/jbc.M103298200. [DOI] [PubMed] [Google Scholar]
- 63.Wilhelm MT, Rufini A, Wetzel MK, et al. Isoform-specific p73 knockout mice reveal a novel role for ΔNp73 in the DNA damage response pathway. Genes Dev. 2010;24(6):549–560. doi: 10.1101/gad.1873910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Willers H, McCarthy EE, Wu B, et al. Dissociation of p53-mediated suppression of homologous recombination from G1/S cell cycle checkpoint control. Oncogene. 2000;19(5):632–639. doi: 10.1038/sj.onc.1203142. [DOI] [PubMed] [Google Scholar]
- 65.Yang AN, Walker N, Bronson R, et al. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature. 2000;404(6773):99–103. doi: 10.1038/35003607. [DOI] [PubMed] [Google Scholar]
- 66.Yoon D, Wang YZ, Stapleford K, et al. p53 inhibits strand exchange and replication fork regression promoted by human Rad51. J Mol Biol. 2004;336(3):639–654. doi: 10.1016/j.jmb.2003.12.050. [DOI] [PubMed] [Google Scholar]
- 67.Zhang HB, Somasundaram K, Peng Y, et al. BRCA1 physically associates with p53 and stimulates its transcriptional activity. Oncogene. 1998;16(13):1713–1721. doi: 10.1038/sj.onc.1201932. [DOI] [PubMed] [Google Scholar]