Abstract
Necroptosis is a tightly regulated form of necrosis that requires the activation of receptor-interacting protein (RIP) kinases RIPK1 and RIPK3, as well as the RIPK3 substrate mixed lineage kinase domain-like protein (MLKL). Because of membrane rupture, necroptotic cells release damage-associated molecular patterns (DAMPs) that evoke immune responses. Necroptosis is emerging as an important cellular response in the modulation of cancer initiation, progression, and metastasis. Necroptosis of cancer cells is considered to be an immunogenic cell death capable of activating anti-tumor immunity. Necroptosis also participates in the promotion of myeloid cell-induced adaptive immune suppression and thus contributes to oncogenesis. In addition, necroptosis of endothelial cells and tumor cells is conducive to tumor metastasis. In this review, we summarize the current knowledge of the complex role of necroptosis in cancer and discuss the potential of targeting necroptosis components for cancer therapies.
Keywords: Cell death, Necroptosis, Cancer, Mixed lineage kinase domain-like protein (MLKL), Receptor-interacting protein (RIP) kinase
1. Introduction
Cell death is an essential aspect of development and tissue homeostasis in multicellular organisms. Apoptosis is a well-defined form of programmed cell death characterized by cell shrinkage, DNA condensation, and the formation of apoptotic bodies that are surrounded by an intact plasma membrane (Kerr et al., 1972). Apoptosis is mediated by a family of cystine proteases termed caspases; the activity of these caspases is initiated by apoptotic stimuli via the activation of the death receptor pathway (“extrinsic pathway”) or the mitochondrial pathway (“intrinsic pathway”). In contrast, necrosis has traditionally been viewed as an unregulated form of cell death characterized by morphological features including cell swelling and cell membrane rupture.
More recently, however, a regulated form of necrosis known as necroptosis (also “programmed necrosis”) has been experimentally confirmed in detail. Necroptosis is controlled by receptor-interacting protein (RIP) kinases RIPK1, RIPK3, and mixed lineage kinase domain-like protein (MLKL) (Holler et al., 2000; Cho et al., 2009; He et al., 2009; Zhang et al., 2009; Sun et al., 2012; Zhao et al., 2012). Necroptosis was first considered as a backup form of cell death that can function when apoptosis is inhibited in response to the activation of death receptors (He and Wang, 2018; Yuan et al., 2019), but it is now well established that necroptosis can be initiated by various stimuli, including the ligands of death receptors, Toll-like receptors (TLRs), interferon (IFN) receptors, and certain pathogens (Fig. 1).
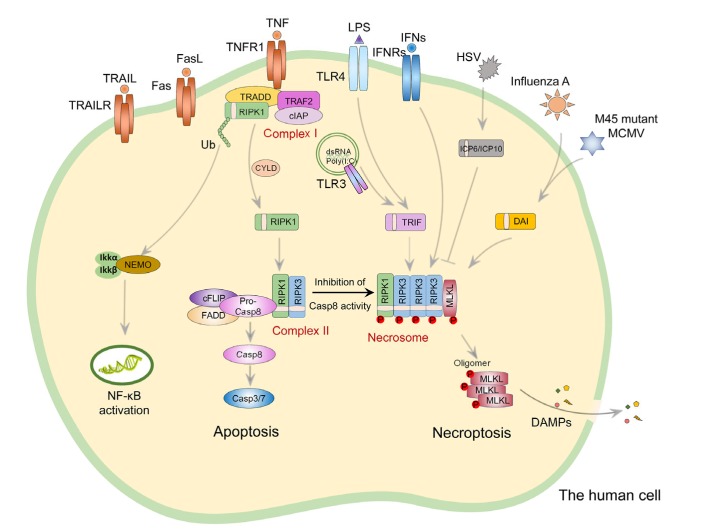
Fig. 1.
Necroptosis signaling pathway
Necroptosis can be caused by a variety of stimuli, including the ligands of the death receptor, TLRs, IFN receptors, and certain pathogens. TNF-α-activated TNFR1 forms Complex I by recruiting RIPK1, TRADD, cIAP1/2, and TRAF2. In Complex I, RIPK1 is ubiquitinated by cIAP1/2 and eventually activates NF-κB signaling pathway. In the presence of Smac mimetic, which leads to the degradation of cIAPs, RIPK1 is disassociated from Complex I and deubiquitinated by CYLD, followed by interacting with FADD and procaspase-8 to form a cytosolic protein complex (Complex II). This complex activates caspase-8 and eventually leads to apoptosis. When caspase-8 activity is inhibited, RIPK1 interacts with RIPK3 to form a protein complex (necrosome) via their respective RHIM domains, leading to activation of both RIPK1 and RIPK3. Activated RIPK3 further phosphorylates MLKL, which forms oligomerization and translocates to the plasma membrane for the execution of necroptosis. The necroptotic cells finally release cellular contents including DAMPs, which act as endogenous danger signals to elicit inflammatory responses. The RHIM-dependent interaction between RIPK3 and RIPK1 is also required for IFNα/β/γ-induced necroptosis. Furthermore, RIPK3 can be activated through interacting with other RHIM-containing proteins such as TRIF, DAI, and the viral protein ICP6. In TLR3 or TLR4-induced necroptosis, TRIF is recruited to RIPK3 through their RHIM domains. During infection of MCMV or influenza A virus, DAI activates RIPK3 to induce necroptosis. In the mouse cells infected with HSV-1/2, binding of ICP6/10 to mouse RIPK3 leads to the activation of RIPK3 and necroptosis. However, in the human natural host cells, ICP6/10 also can interact with human RIPK3, and this association results in the inactivation of RIPK3. Necroptosis can be inhibited by Nec-1 (RIPK1 inhibitor), GSK872 (RIPK3 inhibitor), and NSA (human MLKL inhibitor). Casp: caspase; cIAP1/2: cellular inhibitor of apoptosis 1/2; cFLIP: cellular Fas-associated DD-like interleukin 1β-converting enzyme-inhibitory protein; CYLD: cylindromatosis; DAI: DNA-dependent activator of interferon-regulatory factor; DAMPs: damage-associated molecular patterns; dsRNA: double-stranded RNA; FasL: Fas ligand; FADD: Fas-associating protein with a novel death domain; HSV: herpes simplex virus; IFN: interferon; IFNR: IFN receptor; ICP6: ribonucleotide reductase large subunit; LPS: lipopolysaccharide; MCMV: murine cytomegalovirus; MLKL: mixed lineage kinase domain-like protein; Nec-1: necrostatin-1; NEMO: NF-κB essential modifier; NF-κB: nuclear factor-κB; NSA: necrosulfonamide; RIPK: receptor-interacting protein (RIP) kinase; RHIM: RIP homotypic interaction motif; TLR: Toll-like receptor; TNF-α: tumor necrosis factor-α; TNFR1: TNF receptor 1; TRADD: TNFR1-associated death domain protein; TRAIL: TNF-related apoptosis-inducing ligand; TRAILR: TRAIL receptor; TRAF2: TNFR-associated factor 2; TRIF: Toll/interleukin-1 receptor (TIR)-domain-containing adapter-inducing interferon-β
Cancer is a malignant disease associated with abnormal cell proliferation and cell death. It has been established that cancer cells can evade apoptotic responses. Therefore, induction of necroptosis could be an alternative strategy for killing cancer cells. However, increasing evidence is accumulating that many cancer cells dampen necroptosis via epigenetic silencing of RIPK3 (Koo et al., 2015; Yang et al., 2017). In contrast to apoptosis, necroptosis results in the release of damage-associated molecular patterns (DAMPs), which further activate immune responses. The inflammatory implications of necroptosis enhance the immunogenicity of dying cancer cells and thereby increase anti-tumor immunity (Yatim et al., 2015; Aaes et al., 2016; Yang et al., 2016). In addition to its suppressive role for tumorigenesis, necroptosis has been implicated in oncogenesis and in cancer metastasis. Viewed collectively, it is thus clear that necroptosis functions in complex ways in cancer initiation and development. This review focuses on recent advances in the understanding of the necroptosis machinery and its role in cancer initiation, development, metastasis, and treatment.
2. Discovery of necroptosis as a form of regulated necrosis
The tumor necrosis factor (TNF) superfamily of cytokines, including TNF-α, TNF-related apoptosis-inducing ligand (TRAIL), and FasL/CD95L, is classic ligands for the activation of death receptors. Ligation of a death receptor (e.g. TNF receptor 1 (TNFR1)) by its ligand (e.g. TNF-α) triggers the extrinsic pathway of apoptosis. After the initial discovery that TNF-α is able to induce necrosis in certain cell lines in addition to apoptosis (Laster et al., 1988), it was later found that TNF-induced necrosis is aggravated by inhibition of caspases in the presence of the chemical caspase inhibitor benzyloxycarbonyl-Val-Ala-Asp (OMe)-fluoromethylketone (zVAD-fmk) and by the expression of a viral inhibitor of caspase-8, cytokine response modifier A (CrmA) (Vercammen et al., 1998). Furthermore, more evidence suggests that apoptosis induced by TNF, TRAIL, and FasL/CD95L can be switched to necrosis when caspase activity is defective or inhibited.
In 2000, the protein kinase RIPK1 was identified as an essential regulator for necrotic cell death induced by TNF-α, TRAIL, or FasL/CD95L in a kinase-activity-dependent manner (Holler et al., 2000). It thus became clear that death receptor-initiated necrosis is tightly regulated by the kinase activity of RIPK1. This form of death receptor-initiated RIPK1-dependent necrosis was referred to as “programmed necrosis” (Chan et al., 2003). In 2005, the term necroptosis was introduced to describe this regulated necrosis when necrostatin-1 (Nec-1) was identified as a specific chemical inhibitor of TNF-induced necrosis (Degterev et al., 2005). Nec-1 was further found to inhibit the kinase activity of RIPK1 (Degterev et al., 2005), and this inhibitor has been used extensively for the detection of necroptosis and for the evaluation of the in vivo basic biological significance of necroptosis (Yuan et al., 2019).
In 2009, the kinase RIPK3 was found to regulate necroptosis (Cho et al., 2009; He et al., 2009; Zhang et al., 2009). RIPK3 can associate with RIPK1 to form a protein complex (termed a necrosome) through the RIP homotypic interaction motif (RHIM) domains of both proteins (Sun et al., 2002). Formation of the necrosome activates RIPK3, and this process leads to the phosphorylation of RIPK3. In 2012, MLKL was found to be a downstream substrate of RIPK3 and its phosphorylation by RIPK3 was confirmed to be essential for necroptosis execution (Sun et al., 2012; Zhao et al., 2012). RIPK3-mediated phosphorylation of MLKL activates MLKL, leading to MLKL oligomerization and membrane translocation for the execution of necroptosis (Cai et al., 2014; Chen et al., 2014; Wang HY et al., 2014).
3. Activation and execution of necroptosis
TNF-induced necroptosis is the best characterized pathway of necroptosis. Activation of TNFR1 by TNF-α is known to activate nuclear factor-κB (NF-κB) signaling, apoptosis, and necroptosis. Upon TNF-α ligation, TNFR1 is activated by inducing trimer formation. Activated TNFR1 receptors form a membrane signaling complex (called Complex I) by recruiting RIPK1, TNFR1-associated death domain protein (TRADD), cellular inhibitors of apoptosis (cIAPs), and TNFR-associated factor 2 (TRAF2) (Micheau and Tschopp, 2003). In this complex, the E3 ubiquitin ligases, cIAP1 and cIAP2, induce RIPK1 ubiquitination (Bertrand et al., 2008; Haas et al., 2009). RIPK1 further generates binding sites for NF-κB essential modifier (NEMO), which leads to further recruitment and activation of IκB kinase (IKK) α/β and promotes activation of the NF-κB signaling pathway (Gerlach et al., 2011; O'Donnell et al., 2012).
Smac (Diabo) is a pro-apoptotic protein that can interact with inhibitor of apoptosis proteins (IAPs) to relieve their suppression of apoptosis. In the presence of Smac mimetic (a chemical compound that mimics Smac and induces the degradation of cIAPs), RIPK1 is deubiquitinated by cylindromatosis (CYLD) or A20 (Wertz et al., 2004; Wright et al., 2007; Hitomi et al., 2008). RIPK1 is disassociated from Complex I and binds to Fas-associating protein with a novel death domain (FADD) and procaspase-8 to form Complex II in the cytoplasm (Micheau and Tschopp, 2003). The precursor caspase-8 was cleaved into the active caspase-8, which further cleaves the downstream casepase-3/7, eventually leading to apoptosis. When caspase-8 activation is inhibited, RIPK1 interacts with RIPK3 to form a protein complex (necrosome) via their RHIM domains (Declercq et al., 2009), leading to activation and phosphorylation of both RIPK1 (human RIPK1 at Ser14/15 and Ser161/Ser166, and mouse RIPK1 at Lys45/Lys115) (Degterev et al., 2008; McQuade et al., 2013) and RIPK3 (human RIPK3 at Ser227 and mouse RIPK3 at Thr231/Ser232) (Sun et al., 2012; Chen et al., 2013). Activated RIPK3 further phosphorylates the substrate MLKL, which is a pseudokinase. RIPK3-mediated phosphorylation of MLKL (human MLKL at Thr357/Ser358 and mouse MLKL at Ser345/Ser347) (Sun et al., 2012; Tanzer et al., 2015; Rodriguez et al., 2016) induces a conformational change of MLKL and leads to the activation of MLKL, which further forms oligomerization and translocates to the plasma membrane for the execution of necroptosis (Cai et al., 2014; Chen et al., 2014; Wang X et al., 2014).
It has been shown that MLKL forms disulfide bond-dependent amyloid-like polymers to induce necroptosis (Liu et al., 2017). Dovey et al. (2018) identified inositol phosphate (IP) kinases inositol polyphosphate multikinase (IPMK) and inositol 1,3,4-trisphosphate 5/6-kinase (ITPK1) as critical mediators of MLKL oligomerization and membrane localization. The highly phosphorylated IP molecule produced by these IP kinases, IP6, displaces the MLKL auto-inhibitory region, suggesting the requirement of a specific IP code in coordinating MLKL conformational changes to unleash necroptosis. Prior to membrane disruption, the activation of MLKL triggers the formation of plasma membrane bubbles, which display exposed phosphatidylserine (Gong et al., 2017; Yoon et al., 2017; Zargarian et al., 2017). Endosomal sorting complex required for transport-III (ESCRT-III) components are required for the formation of these membrane bubbles and for the release of phosphor-MLKL-containing vesicles (Gong et al., 2017; Yoon et al., 2017). Inhibition of ESCRT components increased the sensitivity of cells to necroptosis. These results suggest that the ESCRT-III machinery provides protection against MLKL-mediated membrane damage by actively shedding broken membrane blebs. The necroptotic cells finally release cellular contents including DAMPs, which act as endogenous danger signals to elicit inflammatory responses.
Activation of RIPK3 is essential for the induction of necroptosis. The RHIM-dependent interaction of RIPK3 and RIPK1 is also required for IFN-induced necroptosis (Thapa et al., 2013) (Fig. 1). In addition to interacting with RIPK1, RIPK3 can be activated by binding to other RHIM-containing proteins, including Toll/interleukin-1 receptor (TIR)-domain-containing adapter-inducing interferon-β (TRIF), DNA-dependent activator of interferon-regulatory factor (DAI, also known as ZBP1 or DLM1), and the viral protein ribonucleotide reductase large subunit (ICP6) (Wang X et al., 2014; Guo et al., 2015; Huang et al., 2015) (Fig. 1). TLR3 and TLR4 are pathogen recognition receptors that can activate NF-κB activation and interferon regulatory factor 3 (IRF3)-mediated production of type I IFNs via the adaptor protein TRIF (Yamamoto et al., 2003). TLR3 can be activated by double-stranded RNA (dsRNA) molecules such as poly(I:C), while TLR4 recognizes the lipopolysaccharide (LPS), a component of Gram-negative bacteria. In TLR3-or TLR4-induced necroptosis, TRIF is recruited to RIPK3 via their respective RHIM domains (He et al., 2011; Najjar et al., 2016). Blockage of RIPK1 kinase activity by Nec-1 inhibits TLR4-induced macrophage necroptosis, while RIPK1 is not required for TLR3-induced necroptosis of mouse fibroblasts (He et al., 2011; Kaiser et al., 2013), suggesting that RIPK3 can be activated by TRIF.
In response to infection of a mutant murine cytomegalovirus (MCMV) which lacks the viral RIHM-containing protein viral inhibitor of RIP activation (vIRA) or influenza A virus, DAI activates RIPK3 and necroptosis of host cells independently of both RIPK1 and TRIF (Upton et al., 2010, 2012; Kuriakose et al., 2016; Najjar et al., 2016; Nogusa et al., 2016; Thapa et al., 2016). In mouse cells infected with human herpes simplex virus (HSV)-1, mouse RIPK3 recruits the viral protein ICP6 and activates necroptosis. Together, these studies have established RIPK3 as a central molecular node for receiving necroptosis-initiating signals from multiple pathways via RIHM-dependent interactions. Interestingly, RIPK3 can also be inactivated via RIHM-mediated interaction with the viral protein ICP6 in the natural host human cells (Huang et al., 2015; Yu et al., 2016).
4. Necroptosis in tumor suppression
4.1. Defective necroptosis response in many cancer cells
Cell death of tumor cells is generally viewed as beneficial to cancer prevention and therapy, and evasion of apoptosis is considered a critical hallmark of cancer cells. It is therefore not surprising that cancer cells often have defects in their necroptosis responses. Downregulation of RIPK3 was observed in multiple types of human cancer samples, including breast cancers (Koo et al., 2015), colorectal cancers (Feng et al., 2015), and acute myeloid leukemia (AML) cells (Nugues et al., 2014). Indeed, RIPK3 was found to be silenced in a variety of cancer cells. Treatment with a hypomethylating agent or knockdown of DNA methyltransferase 1 (DNMT1) or UHRF1 (ubiquitin-like, containing PHD and RING finger domains 1) promoted hypomethylation of the Ripk3 promoter, leading to the induction of RIPK3 expression in RIPK3-null cancer cells (Koo et al., 2015; Yang et al., 2017). These results suggest that defects in RIPK3 expression in cancer cells may result from methylation of the Ripk3 promoter. Restored expression of RIPK3 by hypomethylating agents promoted the sensitivity to chemotherapeutics (Koo et al., 2015). Hypomethylating agents have been used for clinical treatment of myelodysplastic syndrome and AML. Therefore, induction of RIPK3 expression by hypomethylating agents may provide an opportunity for the development of anti-cancer therapies for treating RIPK3-null cancer cells.
Moreover, it has been shown that low RIPK3 expression is associated with poor clinical outcomes in human colorectal cancer and that overexpression of RIPK3 can attenuate the migration and invasion of colorectal cancer cells (Feng et al., 2015). The ectopic expression of RIPK3 in RIPK3-null cancer cells inhibited tumor growth (Koo et al., 2015; Yang et al., 2017). These results suggest that the deletion or downregulation of RIPK3 in tumor cells favors cell survival and tumorigenesis. In addition, low expression of MLKL was shown to be associated with poor prognosis in patients with early-stage resected pancreatic adenocarcinoma (Colbert et al., 2013), ovarian cancer (He et al., 2013), and cervical cancer (Ruan et al., 2015); the mechanism leading to the downregulation of MLKL in these cancer samples has not been explored. These studies suggest that core necroptosis components RIPK3 and MLKL may serve as tumor suppressors.
4.2. Activation of necroptosis as a mechanism to overcome apoptosis resistance
Induction of cancer cell death is an important strategy for killing cancer cells. Cancer cells typically develop resistance to apoptosis via defective caspase activity owing to gene mutations or silencing. Since necroptosis tends to occur in the absence of caspase activation, it is conceivable that necroptosis is an alternative mode of cell death to overcome apoptosis resistance. This idea is strongly supported by the discovery that necroptosis is activated in caspase-8-deficient colorectal cancer cells in response to Smac mimetic and that necroptosis results in significant tumor regression in both a hereditary and a xenograft mouse model (He et al., 2017) (Fig. 2). The Smac mimetic birinapant, in combination with the clinical caspase-8 inhibitor emricasan/IDN-6556, was shown to effectively trigger necroptosis of acute myelogenous leukemia (AML) cells, and this was found to prolong the survival of mice bearing MLL-AF9 or MLL-ENL birinapant-resistant AML cells (Brumatti et al., 2016). Birinapant has been also shown to induce RIPK1-dependent apoptosis and necroptosis in patient-derived acute lymphoblastic leukemia (ALL) cells and to exhibit an anti-tumor effect on Smac mimetic-sensitive ALL in vivo (McComb et al., 2016). Another Smac mimetic (BV6) was able to activate TNF-dependent necroptosis in patient-derived AML cells, although these cells were resistant to apoptosis (Safferthal et al., 2017). Similarly, BV6 could trigger necroptosis in pancreatic carcinoma cells when caspase activation was blocked (Hannes et al., 2016).
Fig. 2.
Necroptosis of cancer cells in improving chemotherapy efficacy
Necroptosis can be induced for killing certain tumor cells by some chemotherapy drugs. Moreover, necroptosis of tumor cells acts as ICD, which is characterized by the release of ATP and DAMPs (e.g. HMGB1) from dying cells. This ICD promotes DC maturation and CD8+ T cell anti-tumor immunity. MTX: mitoxantrone; ICD: immunogenic cell death; ATP: adenosine triphosphate; DAMPs: damage-associated molecular patterns; HMGB1: high mobility group box 1; DC: dendritic cell
Additionally, the plant natural product shikonin was shown to induce cell death of MCF-7 that could be blocked by Nec-1 (Han et al., 2007) (Fig. 2). Further studies have shown that shikonin upregulates the expression levels of RIPK1 and RIPK3 and induces the formation of the RIPK1/RIPK3 necrosome complex in multiple cancer cells (Fu et al., 2013; Park et al., 2013; Lu et al., 2017). A reactive oxygen species (ROS) scavenger was shown to attenuate shikonin-induced necrosome formation (Park et al., 2013; Lu et al., 2017). Treatment of shikonin repressed tumor growth of both primary and metastatic osteosarcomas in vivo and resulted in increased tumor necrosis and elevated levels of RIPK1 and RIPK3 in primary tumor tissues (Fu et al., 2013) (Table 1). Therefore, triggering necroptosis of tumor cells may represent an attractive new strategy for the treatment of cancer, including apoptosis-resistant cancer.
Table 1.
Potential cancer therapy by targeting necroptosis
| Cancer model | Small molecule | Major finding | Reference |
| Osteosarcoma | Shikonin | Shikonin increased tumor necrosis and repressed tumor growth of both primary and metastatic osteosarcomas in vivo | Fu et al., 2013 |
| Lung metastasis models of B16 and LLC1 | Nec-1 | Nec-1 reduced tumor cell-induced endothelial necroptosis and inhibited tumor cell extravasation and metastasis | Strilic et al., 2016 |
| 5-(2,3-Dihydro-1H-indol-5-yl)-7H-pyrrolo[2,3-d] pyrimidin-4-amine (RIPK1 inhibitor) | This compound reduced the metastasis of B16 tumor cells in vivo | Li et al., 2018 | |
| AML | BV6 | BV6 activated TNF-dependent necroptosis in patient-derived AML blasts | Safferthal et al., 2017 |
| Nec-1 | Nec-1 in combination with IFN-γ significantly enhanced human primary AML cell differentiation and attenuated the clonogenic capacity | Xin et al., 2017 | |
| Breast cancer | NSA | NSA inhibited xenograft tumor growth of MDA-MB-231 in nude mice | Liu et al., 2016 |
| Colorectal cancer | Smac mimetic | Smac mimetic promoted the necroptosis of caspase-8-deficient colorectal cancer cells and inhibited the development of MC38 and ApcMin/+ mouse colorectal cancer | He et al., 2017 |
| GSK872 | GSK872 significantly reduced tumors in the spontaneous intestinal tumor model (ApcMin/+ mice) | Jayakumar and Bothwell, 2019 | |
| EL4, TC-1 | MTX | Cancer cell necroptosis induced by MTX exhibited the features of immunogenic cell death (ICD) and activated anticancer immune responses | Yang et al., 2016 |
| PDA | GSK'547 (RIPK1 inhibitor) | Inhibition of RIPK1 by GSK'547 induced immunogenic programming of TAMs, leading to activation of adaptive immune responses and tumor suppression. GSK'547 synergizes with PD-1 and ICOS-based Immunotherapy | Wang et al., 2018 |
LLC1: Lewis lung carcinoma line 1; Nec-1: necrostatin-1; AML: acute myeloid leukemia; TNF: tumor necrosis factor; IFN: interferon; NSA: necrosulfonamide; MTX: mitoxantrone; PDA: pancreatic ductal adenocarcinoma; RIPK: receptor-interacting protein (RIP) kinase; TAMs: tumor-associated macrophages; PD-1: checkpoint receptor; ICOS: co-stimulatory ligand
4.3. Tumor suppressive activity of necroptosis through control of apoptosis
Strong in vivo evidence for RIPK3-mediated tumor suppression has been provided by the finding that RIPK3 deletion enhances the development of hepatic tumors in a hepatocellular carcinoma (HCC) model induced by the liver parenchymal cell (LPC)-specific deletion of transforming growth factor-β (TGF-β)-activated kinase 1 (Tak1) (Vucur et al., 2013). Hepatocyte cell death is commonly involved in the progression of liver fibrosis and the HCC that is caused by chronic liver disease. LPC-specific TAK1 knockout (TAK1LPC-KO) mice display severe hepatic inflammation as well as spontaneous LPC apoptosis and necrosis (Bettermann et al., 2010). Compared to TAK1LPC-KO and TAK1/Casp-8LPC-KO (combined ablations of TAK1 and caspase-8 in LPC) mice, TAK1LPC-KO/RIPK3−/− (ablation of TAK1 in LPC together with a full knockout of Rip3) mice exhibited significantly higher inflammatory responses in the liver, suggesting that LPC apoptosis rather than necroptosis contributes to hepatic inflammation (Vucur et al., 2013). Loss of RIPK3 caused increased apoptosis and proliferation of TAK1-deficient LPC, leading to increased inflammation and carcinogenesis in TAK1LPC-KO/RIPK3−/− mice (Vucur et al., 2013). Therefore, manipulation of hepatic apoptosis and necroptosis signaling might represent a new strategy for the prevention and/or treatment of HCC. Recently, it has been shown that the expression of Ripk3 was higher in intrahepatic cholangiocarcinoma (ICC) than in HCC (Seehawer et al., 2018). The necroptosis-dependent hepatic cytokine microenvironment leads to ICC development, while suppression of necroptosis shifted cell death towards apoptosis and switched ICC to HCC (Seehawer et al., 2018). These results suggest that the tumor microenvironment determines lineage commitment in liver tumorigenesis.
4.4. Tumor suppressive activity of necroptosis through promotion of cell death and differentiation
The tumor suppressive activity of necroptosis is also supported by studies in AML. AML is maintained by a rare population of leukemia-initiating cells (LICs) characterized by inhibition of differentiation and cell death. It has been shown that RIP3 expression is significantly reduced in CD34+ cells from AML patients compared with that in CD34+ cells from healthy donors (Nugues et al., 2014). Re-expression of RIPK3 in RIP3-null leukemia cell lines induced apoptosis in a smaller proportion of cells, and also activated necroptosis in the presence of the caspase inhibitor, while expression of a kinase dead form of RIPK3 (RIPK3-D161N) in mouse DA1-3b leukemia cells triggered massive apoptosis which was shown to depend on NF-κB activity (Nugues et al., 2014). The induced expression of RIPK3-D161N but not wild-type RIPK3 prolonged the survival of mice after injection of leukemia cells (Nugues et al., 2014). These results suggest that a decrease in RIPK3 expression in AML cells may contribute to the suppression of both necroptosis and apoptosis.
Moreover, RIPK3 was found to be expressed at reduced levels in samples from patients with FMS-like tyrosine kinase 3 (FLT3)-mutated AML (Höckendorf et al., 2016) (Fig. 3). Mice lacking RIPK3 showed enhanced survival of FLT3-internal tandem duplication (FLT3-ITD)-transformed hematopoietic stem and progenitor cells (HSPCs) and increased leukemogenesis. Similarly, MLKL-deficient mice accelerated the onset of AML (Höckendorf et al., 2016). It has been suggested that TNFR-mediated RIPK1/RIPK3-dependent cell death restricts the accumulation of malignant progenitors. In addition, RIPK3-mediated inflammasome activation promoted differentiation of LICs by eliciting interleukin-1β (IL-1β) signaling (Höckendorf et al., 2016). These results suggest that RIPK3 has tumor-suppressive function(s) in FLT3-mutated AML subtypes through the promotion of necroptosis and the differentiation of LICs.
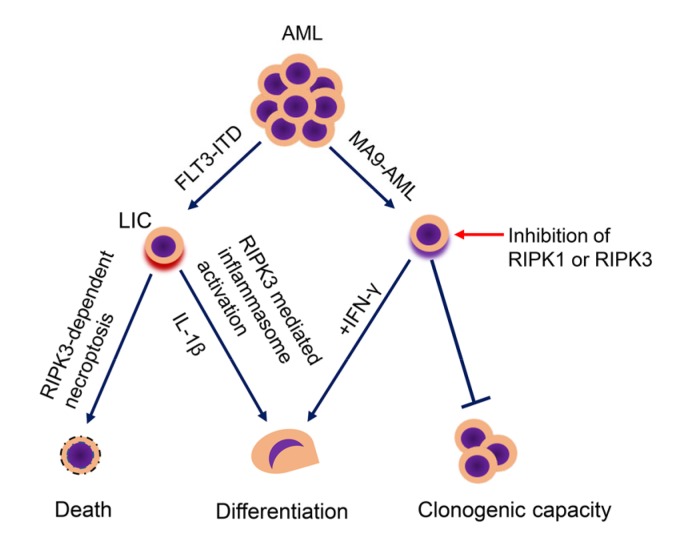
Fig. 3.
Effect of necroptosis on AML development
In the FLT3-ITD-induced AML model, RIPK3 promotes necroptosis of LIC and stimulates LIC differentiation by activating the inflammasome to release IL-1β. In the MA9-AML cells, inhibition of RIPK1 or RIPK3 sensitizes cells to IFN-γ-induced differentiation and reduces clonogenic capacity. AML: acute myeloid leukemia; FLT3: FMS-like tyrosine kinase 3; ITD: internal tandem duplication; LIC: leukemia-initiating cell; IL-1β: interleukin-1β; MA9: Mll-AF9; IFN: interferon; RIPK: receptor-interacting protein (RIP) kinase
4.5. Necroptosis in anti-tumor immunity
Immunogenic cell death (ICD) can activate host immune responses, especially to malignant tumor cells, to produce strong and persistent anti-cancer immunity (Casares et al., 2005). Therefore, the necroptosis of cancer cells is becoming increasingly important because of its roles in immunogenicity and in activating the immune system. Studies have shown that induction of cancer cell necroptosis triggers the release of DAMPs to induce dendritic cell (DC) maturation (Yatim et al., 2015; Aaes et al., 2016) (Fig. 2). It was observed that release of inflammatory mediators such as DAMPs by dying cells is not enough to cause CD8+ T cell cross-priming. Rather, robust cross-priming was found to require RIPK1 signaling and NF-κB-induced intracellular transcription of dying cells (Yatim et al., 2015). Moreover, vaccination with these necroptotic cancer cells induced cross-priming of T cells and the production of IFN-γ in response to tumor antigen stimulation, and this prevented tumor growth, thus demonstrating the immunogenic nature of necroptotic cancer cells and its association with anti-tumor immunity (Aaes et al., 2016). Additionally, chemotherapy agents like mitoxantrone (MTX) can reinstate anticancer immunosurveillance by inducing the ICD of tumor cells. Cancer cells undergoing necroptosis induced by MTX exhibited biochemical hallmarks of ICD including calreticulin (CRT) exposure and the release of adenosine triphosphate (ATP) and high mobility group box (HMGB). Deletion of RIPK3 or MLKL in cancer cells repressed chemotherapy-induced ICD and anticancer immune responses in vivo (Yang et al., 2016). These results suggest that necroptosis can contribute to ICD signaling and tumor immunogenicity.
Additionally, it was found that RIPK3 reduction in colorectal cancer was associated with accumulation of myeloid-derived suppressor cells (MDSCs) in the tumor microenvironment (Yan et al., 2018). Reduction of RIPK3 in MDSCs led to upregulation of NF-κB p65 and cyclooxygenase-2 (COX-2) which catalyzed the synthesis of prostaglandin E2 (PGE2). Addition of PGE2 inhibited RIPK3 expression, but enhanced the expression of p65 and COX-2 in MDSCs (Yan et al., 2018). Blockage of COX-2 or PGE2 receptors by chemical inhibitors reversed the downregulated RIPK3 and the immunosuppressive activity of MDSCs, and reduced the tumorigenesis of colorectal cancer (Yan et al., 2018). These results suggest that the RIPK3-PGE2 circuit regulates the infiltration and activity of MDSCs and colorectal cancer carcinogenesis, and thus may be a potential target for the treatment of colorectal cancer.
5. Necroptosis in tumorigenesis
5.1. Necroptosis in macrophage-mediated immune suppression
Although it is believed that the induction of necroptosis in cancer cells contributes to the death of cancer cells and the activation of anti-tumor immunity, necroptosis has been reported to promote tumorigenesis. Chronic inflammation has been recognized as one of the hallmarks of cancer and is known to be involved in tumor progression (Coussens and Werb, 2002). Given the fact that necroptosis induces the release of DAMPs and inflammatory responses, it is conceivable that necroptosis may stimulate tumor growth. Higher expression levels of RIPK1 and RIPK3 were observed in human pancreatic ductal adenocarcinoma (PDA) than in the surrounding normal pancreas tissue (Seifert et al., 2016) (Fig. 4a). Deficiency of RIPK3 in mice or inhibition of RIPK1 protected against oncogenic progression of KrasG12D-induced PDA in mice (Seifert et al., 2016). RIPK1/RIPK3 signaling-induced chemokine (C-X-C motif) ligand 1 (CXCL1) expression and ligation of Mincle by SAP130 were shown to promote macrophage-induced adaptive immune suppression and therefore accelerated oncogenesis (Seifert et al., 2016), suggesting that inhibition of RIPK1 or RIPK3 could be beneficial for treating PDA. It is noteworthy that RIPK3-deficient mice were not protected against B16 melanoma or subcutaneously implanted cancer cells with mutant Kras and p53, suggesting that the pro-tumorigenic action of RIPK3 occurs in a cell-type and context-dependent manner. Additionally, it has been shown that RIPK1 was highly expressed in tumor-associated macrophages in the PDA model and suppression of RIPK1 by a selective inhibitor GSK547-induced STAT1-dependent immunogenic reprogramming of tumor-associated macrophages (TAMs), leading to the activation of adaptive immune responses and tumor suppression (Wang et al., 2018) (Table 1). Inhibition of RIPK1 synergized with checkpoint receptor (PD-1) and co-stimulatory ligand (ICOS)-based immunotherapies (Wang et al., 2018) (Table 1). Unexpectedly, treatment of the RIPK1 inhibitor provided additive protection in RIPK3-deficient mice, suggesting that the tumor-promoting effects of RIPK1 in PDA are independent of RIPK3.
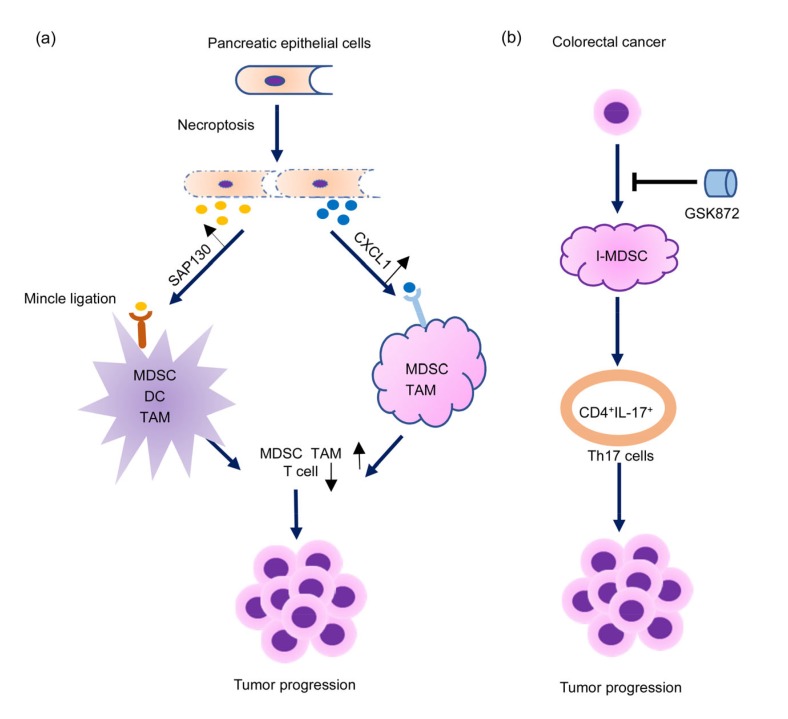
Fig. 4.
Necroptosis involved in macrophage-induced immune suppression
(a) In the PDA model, necroptosis of pancreatic epithelial cells induces CXCL1 expression and ligation of Mincle by SAP130, leading to accumulation of MDSCs and suppression of adaptive immunity. (b) In the spontaneous intestinal tumor model (ApcMin/+ mice), the RIPK3 kinase inhibitor GSK872 significantly reduces tumor growth in the small intestine by inhibiting the accumulation of I-MDSC, which mediates expansion of IL-17-producing T cells. CXCL1: chemokine (C-X-C motif) ligand 1; DC: dendritic cell; MDSC: myeloid-derived suppressor cell; I-MDSC: intermediate MDSC; TAM: tumor-associated macrophage; IL-17: interleukin-17; RIPK: receptor-interacting protein (RIP) kinase
Recently, it has been reported that inhibition of RIPK3 kinase activity by the RIPK3 kinase inhibitor small molecule GSK872 significantly reduced tumors in the small intestine of a spontaneous intestinal tumor model (ApcMin/+ mice) (Jayakumar and Bothwell, 2019) (Fig. 4b, Table 1). Intermediate MDSCs (I-MDSCs) were significantly decreased in the GSK872-treated ApcMin/+ mice, while monocytic MDSCs (M-MDSCs) and macrophages were moderately reduced. Injection of I-MDSC enhanced tumor growth and increased the number of CD4+IL-17+ cells in a mouse MC38 colon cancer model; in contrast, these phenotypes were suppressed by treatment with GSK872 (Jayakumar and Bothwell, 2019). Although these results suggest a role for RIPK3 in the promotion of intestinal tumors by regulating I-MDSCs-mediated expansion of IL-17-producing T cells, further studies will be required to dissect any necroptosis-dependent or necroptosis-independent functions of RIPK3 in the tumorigenesis of ApcMin/+ mice, for example by using genetic deletion of necroptosis components.
5.3. Other mechanisms for necroptosis-mediated tumor promotion
There was also evidence showing that deletion of RIPK1, RIPK3, or MLKL in human breast cancer MDA-MB-231 cells repressed xenograft tumor growth in nude mice (Liu et al., 2016). Similarly, deletion of RIPK3 in human 4T1 breast cancer reduced tumor growth in syngeneic Balb/C mice (Liu et al., 2016). Moreover, the MLKL inhibitor necrosulfonamide (NSA) inhibited growth of MDA-MB-231 xenograft tumors (Liu et al., 2016) (Table 1). Although previous studies have shown that necroptosis promotes cancer cell death and therefore benefits cancer treatment, the results of Liu et al. (2016) suggest the apparent tumor-promoting properties of necroptosis components in these cancer cells. p65/RelA phosphorylation, a marker indicating NF-κB activation, was reduced in RIPK3 knockout cells, while p65/RelA phosphorylation was not changed in RIPK1 or MLKL knockout cells. It is believed that aberrant activation of NF-κB is observed in most cancers and increases tumor proliferation and tumor-related inflammation. Further investigations are needed to confirm the impact of NF-κB signaling on RIPK3-mediated tumor progression and to delineate any mechanism(s) underlying tumorigenesis as driven by necroptosis components.
In addition, it has been shown that RIPK3 is more highly expressed in the M4 and M5 subtypes of AML (Xin et al., 2017) (Fig. 3). Knockout of RIPK1 or RIPK3 in MA9-AML cells sensitized cells to IFN-γ-induced differentiation and repressed leukemia development (Xin et al., 2017). Treatment with the RIPK1 inhibitor Nec-1 combined with IFN-γ significantly enhanced human primary AML cell differentiation and attenuated clonogenic capacity, suggesting that blockage of RIPK1/RIPK3 signaling may be a new approach for treatment of several subtypes of AML in combination with IFN-γ (Xin et al., 2017) (Table 1). However, such a negative role for RIPK1/RIPK3 in AML cell differentiation is inconsistent with the previous observation that RIPK3 signaling promotes LIC cell death and differentiation in FLT3-IDT-induced AML (Höckendorf et al., 2016). The role of RIPK1/RIPK3 signaling in AML pathogenesis thus appears to vary among the different subtypes of AML, so the therapeutic potential of strategies targeting necroptosis must be carefully evaluated in relevant mouse models and in large and diverse patient cohorts.
6. Necroptosis in the promotion of metastasis
Metastasis is the major cause of cancer-related death and is understood to be a complex process resulting in the spread of cancer cells to distant parts of the body through the circulatory system. It has been shown that in vitro co-culture of tumor cells with endothelial cells induces endothelial cell death via RIPK1-RIPK3-MLKL-dependent necroptosis (Strilic et al., 2016). In lung metastasis models of B16 and LLC1 in vivo, endothelium-specific caspase 8 deficiency could inhibit apoptosis and further promote necroptosis, which eventually aggravated lung metastasis (Strilic et al., 2016). Endothelial cell-specific deletion of RIPK3, the complete loss of MLKL, or necrosatin-1 treatment was each shown to reduce tumor cell-induced endothelial necroptosis as well as tumor cell extravasation and metastasis. This endothelial necroptosis was induced via the activation of death receptor 6 (DR6) in endothelial cells upon exposure to amyloid precursor protein (APP) in tumor cells. These results suggest a role for necroptosis in the disruption of vascular endothelial barriers for promoting metastasis (Strilic et al., 2016) (Fig. 5a). Recently, it has been reported that endothelial TGF-β-activated kinase 1 (TAK1) acts as a negative regulator of endothelial necroptosis and metastasis (Yang et al., 2019). TAK1 deletion resulted in increased expression of RIPK3 in endothelial cells and enhanced both necroptosis of endothelial cells and metastasis of tumor cells (Yang et al., 2019). Deficiency of RIPK3 effectively inhibited tumor metastasis in epithelium-specific TAK1 knockout mice (Yang et al., 2019). These results support the idea that sustained TAK1 signaling is required for controlling the low levels of necroptosis components in endothelial cells.
Fig. 5.
Necroptosis of endothelial cells in the promotion of tumor metastasis
(a) Necroptosis of endothelial cells is induced via the activation of DR6 in endothelial cells upon exposure to APP in tumor cells. This process destroys vascular endothelial barriers to promote metastasis. (b) Activation of RIPK1/RIPK3 signaling promotes VEGF-induced vascular permeability to mediate tumor cell extravasation independent of necroptosis. (c) Necroptosis of tumor cells promotes tumor metastasis. APP: amyloid precursor protein; DR6: death receptor 6; RIPK: receptor-interacting protein (RIP) kinase; VEGF: vascular endothelial growth factor; VEGFR: VEGF receptor
Nevertheless, there is also evidence showing that loss of RIPK3 kinase activity or loss of MLKL had no significant effect on metastasis, although the complete loss of RIPK3 or loss of RIPK1 kinase activity was confirmed to suppress tumor cell extravasation and metastasis (Hänggi et al., 2017). Cell death in primary endothelial cells upon co-incubation of tumor cells was shown to be independent of RIPK3 or MLKL. It was found that RIPK1 kinase activity and RIPK3 promote vascular endothelial growth factor (VEGF)-dependent activation of p38/HSP27 mitogen-activated protein (MAP) kinase signaling axis to facilitate vascular permeability and vessel sprouting (Hänggi et al., 2017) (Fig. 5b). These results suggest a necroptosis-independent function for RIPK1/RIPK3 in vascular permeability that may contribute to tumor metastasis. Although further studies are needed to elucidate the precise molecular mechanism through which RIPK1 and RIPK3 may mediate tumor cell extravasation, these observations reveal that RIPK1 and RIPK3 can be viewed as potential targets for developing therapeutic interventions against metastasis. Indeed, studies showed that blockage of RIPK1 kinase activity by Nec-1 or a different RIPK1 inhibitor significantly reduced tumor metastasis in vivo (Strilic et al., 2016; Li et al., 2018) (Table 1).
In addition to endothelial cell necroptosis, necroptosis of tumor cells was recently found to promote tumor metastasis (Jiao et al., 2018). The phosphorylation of MLKL, the marker of necroptosis activation, was detected in areas of tumor necrosis in both human breast cancer samples and mouse breast cancer models, including a spontaneous breast cancer model (MMTV-PyMT mice) and syngeneic breast cancer model (Jiao et al., 2018). Deletion of MLKL in mouse MVT-1 breast cancer cells greatly reduced breast cancer metastasis to the lung (Jiao et al., 2018). These results provide strong evidence suggesting that tumor cell necroptosis occurs during tumor development and participates in the pathogenesis of cancer metastasis (Fig. 5c).
7. Concluding remarks
Necroptosis is now recognized as a major form of regulated cell death. It is mediated by RIPK1/RIPK3 activation, followed by MLKL phosphorylation, oligomerization, and membrane translocation, and finally membrane disruption. Necroptosis is becoming increasingly attractive as a field of study given its implications in the pathogenesis of inflammatory diseases, degenerative diseases, and cancer. As a highly inflammatory form of regulated cell death, necroptosis has very complicated roles in cancer initiation, development, and metastasis. Although the necroptosis field has developed rapidly in recent years, it also faces many challenges. For example, it remains unclear how the action of MLKL links to membrane leakage. Given that RIPK3 expression is restricted in most tumor cells, we do not yet understand the action of RIPK3 in other cells including immune cells and stromal cells in the tumor microenvironment. RIP kinase necroptosis components are now understood to mount inflammatory responses by causing necroptosis or directly regulating inflammatory signaling pathways, but it is not yet known if RIPK1 and/or RIPK3 activate the necroptosis pathway or/and the immune responses to affect tumorigenesis. What are the regulatory mechanisms of necroptosis in different types of cancer? In addition, it is not clear whether there is a regulatory mechanism that controls metastasis through necroptosis at different stages of cancer progression. Further precise characterization of necroptosis, using specific biomarkers in animal models and human pathological samples, will be critical for the validation of the significance of necroptosis in different types of cancer. Exploring these questions will almost certainly contribute to a deeper basic biological understanding of the mechanisms of necroptosis and its action in the regulation of tumorigenesis and should provide major actionable insights to guide the development of novel therapeutic strategies for cancer that target necroptosis components.
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 31671436, 31600133, 31771533, and 31830051), the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Natural Science Foundation of Jiangsu Province (No. BK20160314), the Fok Ying Tung Education Foundation for Young Teachers (No. 151020), and the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (Nos. 2017NL31002 and 2017NL31004)
Contributors: Fang ZHU and Su-dan HE wrote and edited the manuscript. Wei ZHANG and Tao YANG contributed to preparation of the figures and proof reading. All authors read and approved the final manuscript.
Compliance with ethics guidelines: Fang ZHU, Wei ZHANG, Tao YANG, and Su-dan HE declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Aaes TL, Kaczmarek A, Delvaeye T, et al. Vaccination with necroptotic cancer cells induces efficient anti-tumor immunity. Cell Rep. 2016;15(2):274–287. doi: 10.1016/j.celrep.2016.03.037. [DOI] [PubMed] [Google Scholar]
- 2.Bertrand MJM, Milutinovic S, Dickson KM, et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30(6):689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Bettermann K, Vucur M, Haybaeck J, et al. TAK1 suppresses a NEMO-dependent but NF-κB-independent pathway to liver cancer. Cancer Cell. 2010;17(5):481–496. doi: 10.1016/j.ccr.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Brumatti G, Ma CY, Lalaoui N, et al. The caspase-8 inhibitor emricasan combines with the SMAC mimetic birinapant to induce necroptosis and treat acute myeloid leukemia. Sci Trans Med. 2016;8(339):339ra69. doi: 10.1126/scitranslmed.aad3099. [DOI] [PubMed] [Google Scholar]
- 5.Cai ZY, Jitkaew S, Zhao J, et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 2014;16(1):55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casares N, Pequignot MO, Tesniere A, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202(12):1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan FKM, Shisler J, Bixby JG, et al. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem. 2003;278(51):51613–51621. doi: 10.1074/jbc.M305633200. [DOI] [PubMed] [Google Scholar]
- 8.Chen WZ, Zhou ZR, Li LS, et al. Diverse sequence determinants control human and mouse receptor interacting protein 3 (RIP3) and mixed lineage kinase domain-like (MLKL) interaction in necroptotic signaling. J Biol Chem. 2013;288(23):16247–16261. doi: 10.1074/jbc.M112.435545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Li WJ, Ren JM, et al. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 2014;24(1):105–121. doi: 10.1038/cr.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho YS, Challa S, Moquin D, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137(6):1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colbert LE, Fisher SB, Hardy CW, et al. Pronecrotic mixed lineage kinase domain-like protein expression is a prognostic biomarker in patients with early-stage resected pancreatic adenocarcinoma. Cancer. 2013;119(17):3148–3155. doi: 10.1002/cncr.28144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Declercq W, Vanden Berghe T, Vandenabeele P. RIP kinases at the crossroads of cell death and survival. Cell. 2009;138(2):229–232. doi: 10.1016/j.cell.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Degterev A, Huang ZH, Boyce M, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1(2):112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 15.Degterev A, Hitomi J, Germscheid M, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4(5):313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dovey CM, Diep J, Clarke BP, et al. MLKL requires the inositol phosphate code to execute necroptosis. Mol Cell. 2018;70(5):936–948e7. doi: 10.1016/j.molcel.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng X, Song Q, Yu A, et al. Receptor-interacting protein kinase 3 is a predictor of survival and plays a tumor suppressive role in colorectal cancer. Neoplasma. 2015;62(4):592–601. doi: 10.4149/neo_2015_071. [DOI] [PubMed] [Google Scholar]
- 18.Fu ZZ, Deng BY, Liao YX, et al. The anti-tumor effect of shikonin on osteosarcoma by inducing RIP1 and RIP3 dependent necroptosis. BMC Cancer, 13:580. 2013 doi: 10.1186/1471-2407-13-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerlach B, Cordier SM, Schmukle AC, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471(7340):591–596. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- 20.Gong YN, Guy C, Olauson H, et al. ESCRT-III acts downstream of MLKL to regulate necroptotic cell death and its consequences. Cell. 2017;169(2):286–300e16. doi: 10.1016/j.cell.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo H, Pang K, Wei Y, et al. Herpes virus entry mediator in human corneal epithelial cells modulates the production of inflammatory cytokines in response to HSV type 1 challenge. Ophthalmic Res. 2015;54(3):128–134. doi: 10.1159/000437209. [DOI] [PubMed] [Google Scholar]
- 22.Haas TL, Emmerich CH, Gerlach B, et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol Cell. 2009;36(5):831–844. doi: 10.1016/j.molcel.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Han WD, Li L, Qiu S, et al. Shikonin circumvents cancer drug resistance by induction of a necroptotic death. Mol Cancer Ther. 2007;6(5):1641–1649. doi: 10.1158/1535-7163.MCT-06-0511. [DOI] [PubMed] [Google Scholar]
- 24.Hänggi K, Vasilikos L, Valls AF, et al. RIPK1/RIPK3 promotes vascular permeability to allow tumor cell extravasation independent of its necroptotic function. Cell Death Dis. 2017;8(2):e2588. doi: 10.1038/cddis.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hannes S, Abhari BA, Fulda S. Smac mimetic triggers necroptosis in pancreatic carcinoma cells when caspase activation is blocked. Cancer Lett. 2016;380(1):31–38. doi: 10.1016/j.canlet.2016.05.036. [DOI] [PubMed] [Google Scholar]
- 26.He GW, Günther C, Thonn V, et al. Regression of apoptosis-resistant colorectal tumors by induction of necroptosis in mice. J Exp Med. 2017;214(6):1655–1662. doi: 10.1084/jem.20160442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He L, Peng K, Liu Y, et al. Low expression of mixed lineage kinase domain-like protein is associated with poor prognosis in ovarian cancer patients. Onco Targets Ther. 2013;6:1539–1543. doi: 10.2147/OTT.S52805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He SD, Wang XD. RIP kinases as modulators of inflammation and immunity. Nat Immunol. 2018;19(9):912–922. doi: 10.1038/s41590-018-0188-x. [DOI] [PubMed] [Google Scholar]
- 29.He SD, Wang L, Miao L, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell. 2009;137(6):1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 30.He SD, Liang YQ, Shao F, et al. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci USA. 2011;108(50):20054–20059. doi: 10.1073/pnas.1116302108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hitomi J, Christofferson DE, Ng A, et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135(7):1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Höckendorf U, Yabal M, Herold T, et al. RIPK3 restricts myeloid leukemogenesis by promoting cell death and differentiation of leukemia initiating cells. Cancer Cell. 2016;30(1):75–91. doi: 10.1016/j.ccell.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Holler N, Zaru R, Micheau O, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1(6):489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 34.Huang Z, Wu SQ, Liang YJ, et al. RIP1/RIP3 binding to HSV-1 ICP6 initiates necroptosis to restrict virus propagation in mice. Cell Host Microbe. 2015;17(2):229–242. doi: 10.1016/j.chom.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Jayakumar A, Bothwell ALM. RIPK3-induced inflammation by I-MDSCs promotes intestinal tumors. Cancer Res. 2019;79(7):1587–1599. doi: 10.1158/0008-5472.CAN-18-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiao DL, Cai ZY, Choksi S, et al. Necroptosis of tumor cells leads to tumor necrosis and promotes tumor metastasis. Cell Res. 2018;28(8):868–870. doi: 10.1038/s41422-018-0058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaiser WJ, Sridharan H, Huang CZ, et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288(43):31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wideranging implications in tissue kinetics. Br J Cancer. 1972;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koo GB, Morgan MJ, Lee DG, et al. Methylation-dependent loss of RIP3 expression in cancer represses programmed necrosis in response to chemotherapeutics. Cell Res. 2015;25(6):707–725. doi: 10.1038/cr.2015.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuriakose T, Man SM, Malireddi RKS, et al. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci Immunol. 2016;1(2):aag2045. doi: 10.1126/sciimmunol.aag2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laster SM, Wood JG, Gooding LR. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol. 1988;141(8):2629–2634. [PubMed] [Google Scholar]
- 42.Li Y, Xiong Y, Zhang G, et al. Identification of 5-(2,3-dihydro-1H-indol-5-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine derivatives as a new class of receptor-interacting protein kinase 1 (RIPK1) inhibitors, which showed potent activity in a tumor metastasis model. J Med Chem. 2018;61(24):11398–11414. doi: 10.1021/acs.jmedchem.8b01652. [DOI] [PubMed] [Google Scholar]
- 43.Liu SZ, Liu H, Johnston A, et al. MLKL forms disulfide bond-dependent amyloid-like polymers to induce necroptosis. Proc Natl Acad Sci USA. 2017;114(36):E7450–E7459. doi: 10.1073/pnas.1707531114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu XJ, Zhou M, Mei L, et al. Key roles of necroptotic factors in promoting tumor growth. Oncotarget. 2016;7(16):22219–22233. doi: 10.18632/oncotarget.7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu B, Gong X, Wang ZQ, et al. Shikonin induces glioma cell necroptosis in vitro by ROS overproduction and promoting RIP1/RIP3 necrosome formation. Acta Pharmacol Sin. 2017;38(11):1543–1553. doi: 10.1038/aps.2017.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McComb S, Aguadé-Gorgorió J, Harder L, et al. Activation of concurrent apoptosis and necroptosis by SMAC mimetics for the treatment of refractory and relapsed ALL. Sci Transl Med. 2016;8(339):339ra70. doi: 10.1126/scitranslmed.aad2986. [DOI] [PubMed] [Google Scholar]
- 47.McQuade T, Cho Y, Chan FKM. Positive and negative phosphorylation regulates RIP1-and RIP3-induced programmed necrosis. Biochem J. 2013;456(3):409–415. doi: 10.1042/BJ20130860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114(2):181–190. doi: 10.1016/S0092-8674(03)00521-X. [DOI] [PubMed] [Google Scholar]
- 49.Najjar M, Saleh D, Zelic M, et al. RIPK1 and RIPK3 kinases promote cell-death-independent inflammation by Toll-like receptor 4. Immunity. 2016;45(1):46–59. doi: 10.1016/j.immuni.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nogusa S, Slifker MJ, Ingram JP, et al. RIPK3 is largely dispensable for RIG-I-like receptor-and type I interferon-driven transcriptional responses to influenza A virus in murine fibroblasts. PLoS ONE. 2016;11(7):e0158774. doi: 10.1371/journal.pone.0158774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nugues AL, el Bouazzati H, Hétuin D, et al. RIP3 is downregulated in human myeloid leukemia cells and modulates apoptosis and caspase-mediated p65/RelA cleavage. Cell Death Dis. 2014;5(8):e1384. doi: 10.1038/cddis.2014.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Donnell MA, Hase H, Legarda D, et al. NEMO inhibits programmed necrosis in an NFκB-independent manner by restraining RIP1. PLoS ONE. 2012;7(7):e41238. doi: 10.1371/journal.pone.0041238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park S, Shin H, Cho Y. Shikonin induces programmed necrosis-like cell death through the formation of receptor interacting protein 1 and 3 complex. Food Chem Toxicol. 2013;55:36–41. doi: 10.1016/j.fct.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez DA, Weinlich R, Brown S, et al. Characterization of RIPK3-mediated phosphorylation of the activation loop of MLKL during necroptosis. Cell Death Differ. 2016;23(1):76–88. doi: 10.1038/cdd.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruan JY, Mei L, Zhu Q, et al. Mixed lineage kinase domain-like protein is a prognostic biomarker for cervical squamous cell cancer. Int J Clin Exp Pathol. 2015;8(11):15035–15038. [PMC free article] [PubMed] [Google Scholar]
- 56.Safferthal C, Rohde K, Fulda S. Therapeutic targeting of necroptosis by Smac mimetic bypasses apoptosis resistance in acute myeloid leukemia cells. Oncogene. 2017;36(11):1487–1502. doi: 10.1038/onc.2016.310. [DOI] [PubMed] [Google Scholar]
- 57.Seehawer M, Heinzmann F, D'Artista L, et al. Necroptosis microenvironment directs lineage commitment in liver cancer. Nature. 2018;562(7725):69–75. doi: 10.1038/s41586-018-0519-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seifert L, Werba G, Tiwari S, et al. The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression. Nature. 2016;532(7598):245–249. doi: 10.1038/nature17403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strilic B, Yang LD, Albarrán-Juárez J, et al. Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature. 2016;536(7615):215–218. doi: 10.1038/nature19076. [DOI] [PubMed] [Google Scholar]
- 60.Sun LM, Wang HY, Wang ZG, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148(1-2):213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 61.Sun XQ, Yin JP, Starovasnik MA, et al. Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J Biol Chem. 2002;277(11):9505–9511. doi: 10.1074/jbc.M109488200. [DOI] [PubMed] [Google Scholar]
- 62.Tanzer MC, Tripaydonis A, Webb AI, et al. Necroptosis signalling is tuned by phosphorylation of MLKL residues outside the pseudokinase domain activation loop. Biochem J. 2015;471(2):255–265. doi: 10.1042/BJ20150678. [DOI] [PubMed] [Google Scholar]
- 63.Thapa RJ, Chen PR, Cheung M, et al. NF-κB inhibition by bortezomib permits IFN-γ-activated RIP1 kinase-dependent necrosis in renal cell carcinoma. Mol Cancer Ther. 2013;12(8):1568–1578. doi: 10.1158/1535-7163.MCT-12-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thapa RJ, Ingram JP, Ragan KB, et al. DAI senses influenza A virus genomic RNA and activates RIPK3-dependent cell death. Cell Host Microbe. 2016;20(5):674–681. doi: 10.1016/j.chom.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7(4):302–313. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe. 2012;11(3):290–297. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vercammen D, Beyaert R, Denecker G, et al. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998;187(9):1477–1485. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vucur M, Reisinger F, Gautheron J, et al. RIP3 inhibits inflammatory hepatocarcinogenesis but promotes cholestasis by controlling caspase-8-and JNK-dependent compensatory cell proliferation. Cell Rep. 2013;4(4):776–790. doi: 10.1016/j.celrep.2013.07.035. [DOI] [PubMed] [Google Scholar]
- 69.Wang HY, Sun LM, Su LJ, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014;54(1):133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 70.Wang W, Marinis JM, Beal AM, et al. RIP1 kinase drives macrophage-mediated adaptive immune tolerance in pancreatic cancer. Cancer Cell. 2018;34(5):757–774e7. doi: 10.1016/j.ccell.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang X, Li Y, Liu S, et al. Direct activation of RIP3/MLKL-dependent necrosis by herpes simplex virus 1 (HSV-1) protein ICP6 triggers host antiviral defense. Proc Natl Acad Sci USA. 2014;111(43):15438–15443. doi: 10.1073/pnas.1412767111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wertz IE, O'Rourke KM, Zhou HL, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature. 2004;430(7000):694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 73.Wright A, Reiley WW, Chang M, et al. Regulation of early wave of germ cell apoptosis and spermatogenesis by deubiquitinating enzyme CYLD. Dev Cell. 2007;13(5):705–716. doi: 10.1016/j.devcel.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 74.Xin J, You D, Breslin P, et al. Sensitizing acute myeloid leukemia cells to induced differentiation by inhibiting the RIP1/RIP3 pathway. Leukemia. 2017;31(5):1154–1165. doi: 10.1038/leu.2016.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamamoto M, Sato S, Hemmi H, et al. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science. 2003;301(5633):640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 76.Yan GF, Zhao HK, Zhang Q, et al. A RIPK3-PGE2 circuit mediates myeloid-derived suppressor cell-potentiated colorectal carcinogenesis. Cancer Res. 2018;78(19):5586–5599. doi: 10.1158/0008-5472.CAN-17-3962. [DOI] [PubMed] [Google Scholar]
- 77.Yang CK, Li J, Yu L, et al. Regulation of RIP3 by the transcription factor Sp1 and the epigenetic regulator UHRF1 modulates cancer cell necroptosis. Cell Death Dis. 2017;8(10):e3084. doi: 10.1038/cddis.2017.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang H, Ma YT, Chen G, et al. Contribution of RIP3 and MLKL to immunogenic cell death signaling in cancer chemotherapy. Oncoimmunology. 2016;5(6):e1149673. doi: 10.1080/2162402X.2016.1149673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang LD, Joseph S, Sun TL, et al. TAK1 regulates endothelial cell necroptosis and tumor metastasis. Cell Death Differ, Epub ahead of print. 2019 doi: 10.1038/s41418-018-0271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yatim N, Jusforgues-Saklani H, Orozco S, et al. RIPK1 and NF-κB signaling in dying cells determines cross-priming of CD8+ T cells. Science. 2015;350(6258):328–334. doi: 10.1126/science.aad0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yoon S, Kovalenko A, Bogdanov K, et al. MLKL, the protein that mediates necroptosis, also regulates endosomal trafficking and extracellular vesicle generation. Immunity. 2017;47(1):51–65e7. doi: 10.1016/j.immuni.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 82.Yu XL, Li Y, Chen Q, et al. Herpes simplex virus 1 (HSV-1) and HSV-2 mediate species-specific modulations of programmed necrosis through the viral ribonucleotide reductase large subunit R1. J Virol. 2016;90(2):1088–1095. doi: 10.1128/JVI.02446-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yuan JY, Amin P, Ofengeim D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat Rev Neurosci. 2019;20(1):19–33. doi: 10.1038/s41583-018-0093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zargarian S, Shlomovitz I, Erlich Z, et al. Phosphatidylserine externalization, “necroptotic bodies” release, and phagocytosis during necroptosis. PLoS Biol. 2017;15(6):e2002711. doi: 10.1371/journal.pbio.2002711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang DW, Shao J, Lin J, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325(5938):332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 86.Zhao J, Jitkaew S, Cai ZY, et al. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci USA. 2012;109(14):5322–5327. doi: 10.1073/pnas.1200012109. [DOI] [PMC free article] [PubMed] [Google Scholar]