Abstract
O-linked N-acetylglucosamine (O-GlcNAc) is a dynamic post-translational modification occurring on myriad proteins in the cell nucleus, cytoplasm, and mitochondria. The donor sugar for O-GlcNAcylation, uridine-diphosphate N-acetylglucosamine (UDP-GlcNAc), is synthesized from glucose through the hexosamine biosynthetic pathway (HBP). The recycling of O-GlcNAc on proteins is mediated by two enzymes in cells—O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA), which catalyze the addition and removal of O-GlcNAc, respectively. O-GlcNAcylation is involved in a number of important cell processes including transcription, translation, metabolism, signal transduction, and apoptosis. Deregulation of O-GlcNAcylation has been reported to be associated with various human diseases such as cancer, diabetes, neurodegenerative diseases, and cardiovascular diseases. A better understanding of the roles of O-GlcNAcylation in physiopathological processes would help to uncover novel avenues for therapeutic intervention. The aim of this review is to discuss the recent updates on the mechanisms and impacts of O-GlcNAcylation on these diseases, and its potential as a new clinical target.
Keywords: O-GlcNAcylation, Cancer, Diabetes, Neurodegenerative disease, Cardiovascular disease
1. Introduction
O-GlcNAcylation was first described by Torres and Hart on a monocyte cell surface over 30 years ago (de Jesus et al., 2018). Later studies demonstrated that, differing from traditional forms of protein glycosylation, which are stable and mostly restricted to endoplasmic reticulum and Golgi resident, O-GlcNAcylation is a dynamic and reversible modification, and mainly occurs in the cytoplasm, mitochondria, and nucleus (Issad et al., 2010). O-GlcNAcylation exhibits similar features to phosphorylation, as the modification sites are serine and/or threonine, and the sugar can be attached or removed rapidly in response to different environmental stimuli. Moreover, O-GlcNAcylation has been shown to have extensive interplay with phosphorylation through regulating the phosphorylation of adjacent residues or competing for the same serine or threonine residue (Leney et al., 2017).
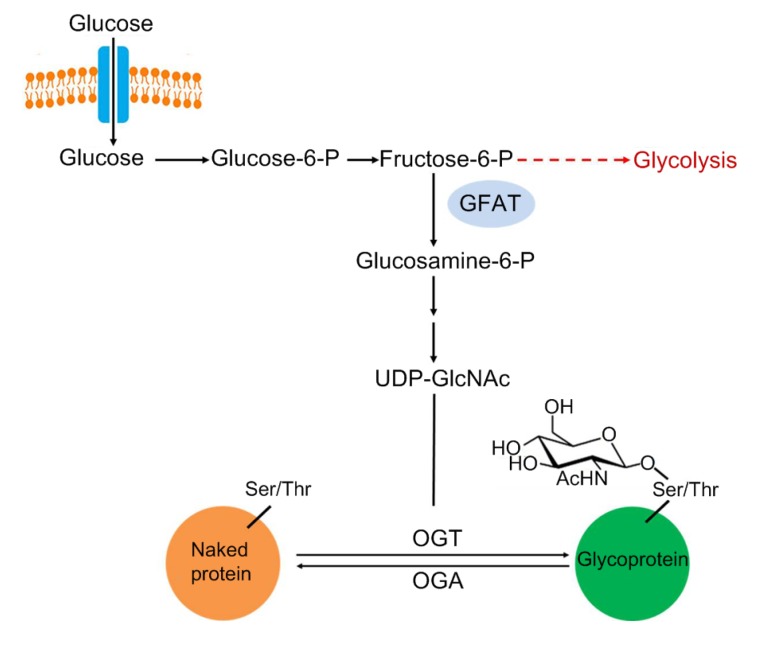
Uridine-diphosphate N-acetylglucosamine (UDP-GlcNAc), the sugar nucleotide donor for O-GlcNAcylation, is synthesized as the final product by hexosamine biosynthetic pathway (HBP) (Fig. 1). HBP diverges from glycolysis, with approximately 2%–5% of all cellular glucose funneled into the HBP. Glutamine-fructose-6-phosphate amidotransferase (GFAT), which converts fructose-6-phosphate to glucosamine-6-phosphate, is the first and rate-limiting enzyme of HBP (Teo et al., 2010). The addition and removal of the UDP-GlcNAc are mediated by two highly conserved enzymes, O-linked N-acetylglucosamine (O-GlcNAc) transferase (OGT) and O-GlcNAcase (OGA), respectively. OGT is a unique glycosyltransferase in metazoans and is expressed as three isoforms—the 116-kDa nucleocytoplasmic (ncOGT), the 103-kDa mitochondrial (mOGT), and the short 78-kDa isoform (sOGT). All OGT isoforms contain two distinct domains: an N-terminal domain containing tetraticopeptide repeat (TPR) motifs which are important for the recognition of different protein substrates, and a C-terminal domain that possesses glycosyltransferase activity (Liu et al., 2015; Joiner et al., 2019). OGA, the single enzyme responsible for removing O-GlcNAc, contains two domains—a hexosaminidase domain at the N-terminal and a histone acetyltransferase domain at the C-terminal of the protein. Two isoforms of OGA have been identified—a long one (OGA-L) of 102 kDa found in the nucleocytoplasm, and a shorter one (OGA-S) of 76 kDa mainly present in the nucleus (Liu et al., 2015; Wani et al., 2017).
Fig. 1.
Hexosamine biosynthetic pathway
Glucose is converted to UDP-GlcNAc (the sugar nucleotide donor for O-GlcNAcylation) through the hexosamine biosynthetic pathway (HBP). The rate-limiting step of the HBP is catalyzed by the GFAT (glutamine fructose-6-phosphate amidotransferase), which uses glutamine to convert fructose-6-phosphate into glucosamine-6-phosphate. Protein O-GlcNAcylation is catalyzed by O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA) for the addition to and removal from the serine and threonine residues, respectively
A number of small molecule probes have been developed to modulate cellular levels of O-GlcNAcylation. Alloxan, the first reported OGT inhibitor, completely blocks the activity of recombinant OGT in vitro at the concentration of 1 mmol/L. However, alloxan is not a specific OGT inhibitor as it also inhibits other critical enzymes, such as glucokinase and OGA (Lee et al., 2006). Benzoxazolinone (BZX), a small molecule that contains a five-heteroatom dicarbamate core, inactivates OGT by forming a carbonyl crosslink in the OGT active site. This inhibition process is irreversible, and thus BZX cannot be adopted widely due to its potential cytotoxicity (Jiang et al., 2011). Ac-5SGlcNAc, an OGT inhibitor reported recently, is taken up by cells and converted to UDP-5SGlcNAc, which binds to OGT and inhibits its activity (Cecioni and Vocadlo, 2013). On the other hand, inhibiting OGA is an effective way to increase cellular O-GlcNAcylation. PUGNAc, a 2-acetamido-2-deoxy-D-glucono-1,5-lactone (GDL) derivative, has been frequently used as an OGA inhibitor for about a decade, but it is not specific and reacts with other hexosaminidases (Macauley and Vocadlo, 2010). NButGT was originally shown to act in Cos-7 cells to increase O-GlcNAc levels dramatically. Notably, no apparent cellular toxicity was observed (Macauley and Vocadlo, 2010). Thiamet-G, another OGA inhibitor developed several years ago, showed better stability and selectivity than NButGT. It was reported as a promising agent for the treatment of Alzheimer’s disease (AD) in a few studies (Yuzwa et al., 2008).
With the development of sensitive analytical techniques, O-GlcNAcylation has been detected on more than 1000 proteins, including but not limited to transcription factors, metabolic enzymes, and signaling proteins. A growing body of evidence reveals that O-GlcNAcylation plays a key role in many critical biological processes including transcription, translation, metabolism, signal transduction, and autophagy (Butkinaree et al., 2010; Ferron et al., 2018). On the other hand, deregulation of O-GlcNAcylation has been closely associated with numerous human diseases such as cancer, diabetes, neurodegenerative and cardiovascular diseases (Darley-Usmar et al., 2012; Banerjee et al., 2016; Pinho et al., 2018). However, the detailed molecular mechanisms by which O-GlcNAc signaling contributes to these diseases remain largely elusive, and have just begun to receive attention. Here we provide a brief summary on how this post-translational modification functions as a critical factor involving physiological and pathological processes. This might become a novel avenue for possible therapeutic intervention.
2. Cancer and O-GlcNAc
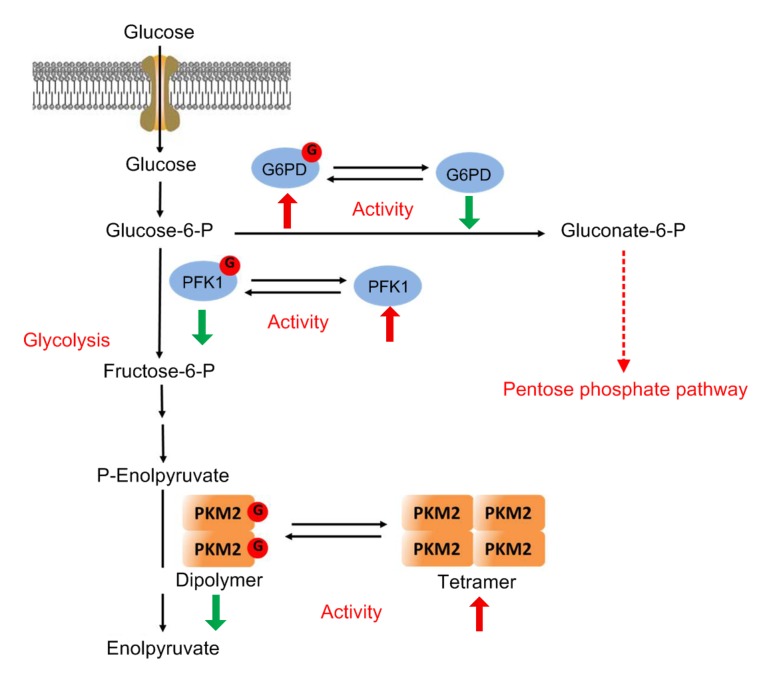
Cancer is one of the leading causes of mortality worldwide (Bray et al., 2018). Cancer cells rewire their metabolism and signaling networks to promote growth, survival, proliferation, and long-term maintenance. Rapidly proliferating cancer cells tend to produce ATP via glycolysis rather than the mitochondrial oxidative phosphorylation, even in the presence of ample oxygen. This metabolic phenomenon, discovered by Otto Warburg in the last century, is now widely observed across a wide range of cancers (Ferrer et al., 2016; Liberti and Locasale, 2016). In addition, cancer cells exhibit increased flux through HBP, and hence enhanced cellular O-GlcNAcylation (Bond and Hanover, 2015). A large number of metabolic enzymes are found to possess O-GlcNAcylation in several proteomic studies, suggesting a critical role of O-GlcNAcylation in regulating cellular metabolism (Fig. 2). The underlying mechanisms have just begun to be elucidated. Phosphofructokinase-1 (PFK1), a key regulatory glycolytic enzyme catalyzing the conversion of fructose-6-phosphate to fructose-1,6-bisphosphate, is dynamically modified by O-GlcNAc at Ser529. O-GlcNAcylation inhibits PFK1 activity and redirects glucose flux through the pentose phosphate pathway (PPP), thereby promoting cancer cell survival and proliferation (Yi et al., 2012). Glucose-6-phosphate dehydrogenase (G6PD) is the rate-limiting enzyme of the PPP. O-GlcNAcylation at Ser84 activates G6PD activity and increases glucose flux through the PPP, leading to increased precursor accumulation for nucleotide and lipid biosynthesis in human lung cancer cells (Rao et al., 2015). Pyruvate kinases (PKs) are the final rate-limiting enzymes of glycolysis. The O-GlcNAc modification of pyruvate kinase M2 isoform (PKM2) destabilizes its active tetrameric structure, and leads to nuclear translocation of PKM2, resulting in increased glucose consumption and lactate production and enhanced level of lipid and DNA synthesis in cancer cells (Wang et al., 2017). Taken together, O-GlcNAcylation plays an important role in reprogramming the metabolic network of cancer cells to promote tumor growth.
Fig. 2.
Regulation of metabolism by O-GlcNAcylation in cancer cells
Phosphofructokinase-1 (PFK1), a critical regulatory glycolytic enzyme catalyzing the conversion of fructose-6-phosphate to fructose-1,6-bisphosphate, is modified by O-GlcNAc at Ser529. O-GlcNAcylation inhibits PFK1 activity and redirects glucose flux through the pentose phosphate pathway (PPP), thereby promoting cancer cell survival and proliferation. Glucose-6-phosphate dehydrogenase (G6PD), the rate-limiting enzyme of the PPP, is O-GlcNAcylated at Ser84. O-GlcNAcylation activates G6PD activity and increases glucose flux through the PPP, leading to increased precursor accumulation for nucleotide and lipid biosynthesis. Pyruvate kinases (PKs) are the final rate-limiting enzymes of glycolysis. The O-GlcNAcylation of pyruvate kinase M2 isoform (PKM2) at Thr405 and Ser406 destabilizes its active tetrameric structure, resulting in increased glucose consumption and lactate production and enhanced level of lipid and DNA synthesis in cancer cells
Cancer cells are exposed to various environmental stresses including endoplasmic reticulum stress, oxidative stress, nutrient stress, and hypoxia. However, deregulation of signal transduction in cancer cells provides characteristic approaches to respond to these stresses, thereby promoting tumorigenesis (Buono and Longo, 2018). Nuclear factor κB (NF-κB), an important transcription factor, plays a key role in cancer-related processes such as cell proliferation, apoptosis, angiogenesis, and metastasis and has been regarded as a potential therapeutic target in numerous types of cancers (Patel et al., 2018). High activation of NF-κB has been reported as an important survival mechanism of cancer and requires posttranslational modifications, such as phosphorylation and acetylation. In addition, both Thr322 and Thr352 of the NF-κB p65 unit can be modified by O-GlcNAc. O-GlcNAcylation on Thr352, but not Thr322, is critical for increased transcriptional activity of NF-κB by blocking its binding to inhibitor of NF-κB α (IκBα) under hyperglycemic conditions (Yang et al., 2008a). Another study confirmed that hyper-O-GlcNAcylation contributes to NF-κB oncogenic activation and inhibits apoptosis in pancreatic cancer (Ma et al., 2013). Recently, it has been reported that OGT promotes fatty liver-associated liver cancer through regulating palmitic acid metabolism and inducing ER stress, thereby activating the oncogenic NF-κB pathway (Xu et al., 2017). These data suggest that in cancer cells regulation of the NF-κB pathway by O-GlcNAcylation might be multifaceted. c-Myc, another important transcription factor overexpressed in various cancers, mediates cancer cell proliferation, differentiation, and apoptosis (Itkonen et al., 2013). It was reported that c-Myc is O-GlcNAcylated at Thr58, a known site of phosphorylation and mutation hot spot in human lymphomas, suggesting that this region is associated with increased tumorigenicity (Chou et al., 1995). In prostate cancer, OGT promotes cancer cell growth by maximizing c-Myc activity in cooperation with c-Myc copy number amplification (Itkonen et al., 2013). β-Catenin plays a role in intracellular adhesion and is a transcriptional co-activator of the Wnt signaling pathway. Its nuclear accumulation in response to gene mutations is directly linked to the onset of typical cancers, through which it regulates cell proliferation and metastasis (Cui et al., 2018). It has been demonstrated that β-catenin is O-GlcNAcylated at Ser23, and this modification inhibits β-catenin’s nuclear translocation and decreases its transcriptional activity, leading to a significant decrease of proliferation in prostate cancer and osteosarcoma cells (Ha et al., 2014). A recent study also reported that in osteosarcoma cells, neurotrophin receptor-interacting MAGE (melanoma-associated antigen) homolog induces O-GlcNAcylation and nuclear localization of β-catenin. However, this nuclear β-catenin fails to recruit other co-activators, leading to inhibition of the Wnt signaling (Chen et al., 2017). However, research from another lab showed that in NIH-3T3 murine fibroblasts, O-GlcNAcylation increases β-catenin expression and promotes its transcriptional activity, leading to enhanced tumor migration and development (Harosh-Davidovich and Khalaila, 2018). This discrepancy may be due to the fact that different types of cell were used. Additionally, a number of other apoptosis-and cycle-related proteins, such as CREB (cAMP response element-binding protein), P53, HIF-1α (hypoxia-inducible factor 1 α,), Id2 (inhibitor of differentiation 2), FoxO1 (forkhead box O1) and AKT (protein kinase B), were found to be modified and regulated by O-GlcNAc, through which cancer cells may ultimately escape the fate of death and proliferate endlessly (Özcan et al., 2010; Ferrer et al., 2016).
Collectively, these data suggest a positive regulatory role of O-GlcNAcylation in regulating cancer development and progression. In this regard, selective targeting of O-GlcNAcylation appears to be a promising therapeutic strategy for cancer in the future.
3. Diabetes and O-GlcNAc
Clinically, diabetes can be classified into two main types. Type 1, formally known as insulin-dependent diabetes, relates to an overall decrease or loss in insulin production, affecting about 5%–10% of diabetic patients (Chetan et al., 2019). Type 2 diabetes (T2D), found in greater than 90% of diabetic patients, is a leading cause of morbidity and mortality worldwide and has risen markedly in developing countries. One of the major features of T2D is insulin resistance, defined as the inability of insulin to trigger appropriate glucose uptake (Carpenter et al., 2019; Hurtado and Vella, 2019). The potential involvement of HBP in the development of insulin resistance in primary cultured adipocytes was first demonstrated by using a GFAT inhibitor, azaserine (Teo et al., 2010). In a subsequent study, overexpression of OGT in muscle and adipose tissues resulted in the T2D phenotype, suggesting that O-GlcNAcylation may play an important role in the development of insulin resistance (McClain et al., 2002). In addition, elevation of cellular O-GlcNAcylation by PUGNAc attenuated insulin signaling in 3T3-L1 adipocytes, suggesting that O-GlcNAcylation is the link between the HBP and insulin resistance (Vosseller et al., 2002).
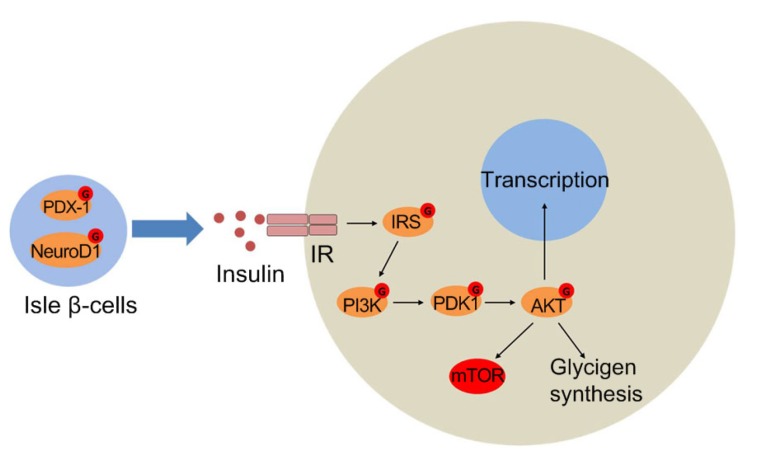
Insulin is secreted and stored by β-cells in the pancreas. The activity of the insulin gene (Ins1/2) in isle β-cells is thought to be regulated by the synergistic action of the transcription factors pancreatic/duodenal homeobox-1 protein (PDX-1) and neurogenic differentiation factor 1 (NeuroD1) (Peterson and Hart, 2016). Elevating cellular O-GlcNAcylation increased intracellular insulin levels and preserved glucose-stimulated insulin secretion in β-cells, partially because elevated O-GlcNAcylation increased the mRNA levels of Ins1/2 by elevating histone H3 transcriptional activation marks (Durning et al., 2016). In addition, the localization of NeuroD1 is regulated by O-GlcNAcylation. Under low glucose conditions, NeuroD1 is mainly in the cytosol; however, treatment with OGA inhibitor induces NeuroD1 translocation into the nucleus, leading to enhanced expression of insulin genes (Andrali et al., 2007). Two major O-GlcNAcylation sites on PDX-1 were identified in Min6 cells. Elevated glucose concentration increases PDX-1 O-GlcNAcylation, enhances DNA binding activity of PDX-1 and insulin expression (Gao et al., 2003).
Moreover, several important proteins in the insulin signaling pathway were shown to be O-GlcNAcylated (Fig. 3), including insulin receptor substrate (IRS) proteins IRS1 and IRS2, phosphoinositide-dependent kinase 1 (PDK1), phosphatidylinositol-3-OH kinase (PI3K), and AKT (Teo et al., 2010). O-GlcNAcylation of IRS1 was first detected by immunological methods in insulin resistance adipocytes (Vosseller et al., 2002). Mass spectrometry analysis further identified Ser1036 as the major O-GlcNAcylation site on IRS1 (Ball et al., 2006). Increasing O-GlcNAcylation in 3T3-L1 cells inhibited Tyr608 phosphorylation on IRS1, hence down-regulating AKT activity, contributing to insulin resistance (Whelan et al., 2010). In another study, after induction with insulin, OGT was shown to be recruited by phosphatidylinositol-3,4,5-trisphosphate (PI(3,4,5)P3) from the nucleus to the plasma membrane. Where the enzyme was tyrosine phosphorylated and activated, resulting in O-GlcNAcylation of PDK1 and AKT and attenuation of the signaling pathway (Yang et al., 2008b).
Fig. 3.
O-GlcNAcylation in insulin signaling
In isle β-cells, O-GlcNAcylated PDX-1 and NeuroD1 promote transcription of the insulin gene. On binding to insulin, the auto-phosphorylated insulin receptor (IR) catalyzes tyrosine phosphorylation of insulin receptor substrate (IRS) proteins, which results in the docking and activation of phosphatidylinositol-3-OH kinase (PI3K). PI3K produces phosphatidylinositol-3,4,5-triphosphate (PIP3), which recruits phosphoinositide-dependent kinase 1 (PDK1) and protein kinase B (AKT) to the plasma membrane. AKT activated by PDK1 phosphorylates numerous substrates to mediate physiological functions. Subsequently, PIP3-binding OGT attenuates insulin signaling by O-GlcNAcylation of IRS, PDK1, and AKT
Collectively, these data suggest a critical role of O-GlcNAcylation in the development of diabetes. However, one major question that needs to be resolved in future studies is whether O-GlcNAcylation causes diabetes or is just an effect of the overall dysfunction seen in this disease. Treatment of rats and mice with NButGT, an inhibitor of OGA, dramatically increased O-GlcNAcylation in all tissues but did not alter insulin sensitivity or glucohomeostasis, indicating that O-GlcNAc may not cause diabetes but may play a dynamic role in the progression of the disease (Macauley et al., 2010). Thus, further investigations are required to elucidate the exact role of O-GlcNAcylation in diabetes.
4. Neurodegenerative diseases and O-GlcNAc
Neurodegenerative diseases are among the most common disorders encountered by the aging population, characterized by the progressive loss of the structure and function of neurons. To date, these diseases, including AD, Parkinson’s disease (PD), and Huntington’s disease (HD), are incurable despite the research effort that has gone into understanding the underlying mechanisms of the pathologies (Rowe et al., 2019). Research has demonstrated that about 40% of all neuronal proteins and 19% of synaptosome proteins are O-GlcNAcylated, indicating the involvement of O-GlcNAcylation in neurodegeneration (Wani et al., 2017).
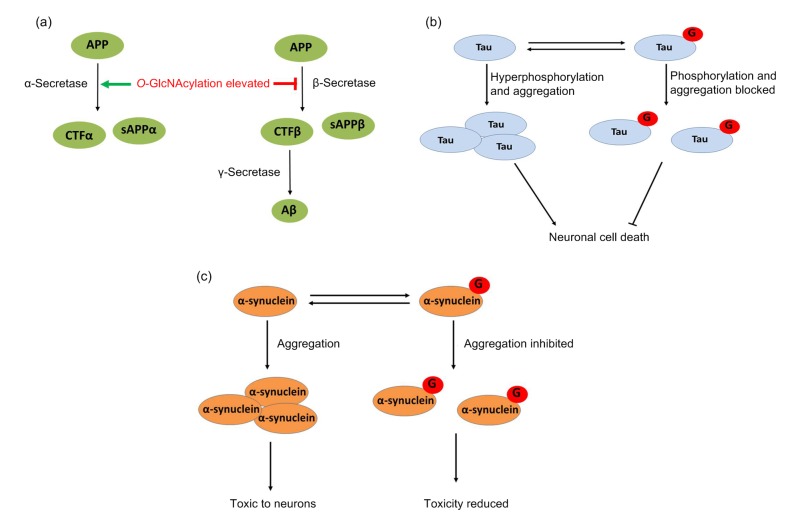
AD, the most common form of dementia, is described as a chronic neurodegenerative disease that affects memory and learning (van Giau et al., 2018). Extracellular deposits of amyloid-β (Aβ) plaques and intraneuronal neurofibrillary tangles (NFTs) are the central pathological hallmarks of AD. The involvement of glucose metabolism in AD patients has been implicated by the evidence that T2D constitutes a great risk factor for developing AD (Gudala et al., 2013; Cooper et al., 2015). However, the mechanisms by which glucose metabolism contributes to AD remain largely elusive. Aβ plaques are formed by oligomers of proteolytic fragments of the amyloid precursor protein (APP) by α-secretase. It was reported that enhancing O-GlcNAcylation activated the α-secretase process, leading to increased accumulation of soluble amyloid precursor protein α (sAPPα) fragment and inhibition of Aβ secretion and accumulation (Fig. 4a) (Borghgraef et al., 2013; Kim et al., 2013; Tan and Gleeson, 2019). Tau is a microtubule-associated protein highly enriched in neurons, and plays a role in protecting microtubules from severing and regulates synaptic function. Tau isolated from normal brain has been found to be O-GlcNAcylated on multiple sites; however, O-GlcNAcylation failed to be detected on high-molecular weight and insoluble Tau, which is the species forming the pathological aggregates in AD (Fig. 4b) (Graham et al., 2014; Zhu et al., 2014). A number of phosphorylation sites of Tau have been identified. When hyperphosphorylated, Tau proteins lose the affinity for the microtubule and are aggregated into NFTs, a key factor in the development of cognitive deficits and a characteristic of AD (Hwang and Rhim, 2018). After treatment with BZX2, an inhibitor of OGT, Tau phosphorylation increased 2-fold at Ser199 and 1.5-fold at Ser396, resulting in increased Tau aggregation. Moreover, Tau aggregation induced by BZX2 was significantly reduced by the treatment of Thiamet G, an inhibitor of OGA, suggesting an inhibitory role of O-GlcNAcylation in the aggregation of Tau (Lim et al., 2015). Nuclear magnetic resonance (NMR) and peptide analysis showed that O-GlcNAclation occurs at the Ser400 of Tau, which was blocked by the phosphorylation of neighboring residues Ser396 and Ser404. Moreover, Ser400 O-GlcNAcylation reduced Ser404 phosphorylation by cyclin-dependent protein kinase 2 (CDK2)/cyclin A3 and interrupted the glycogen synthase kinase 3b (GSK3b)-mediated sequential phosphorylation process (Smet-Nocca et al., 2011).
Fig. 4.
O-GlcNAcylation and neurodegeneration
(a) Amyloid precursor protein (APP) can be cleaved by either α-secretase or β-secretase. When APP is cleaved by α-secretase, a soluble N-terminal fragment (sAPPα) and a C-terminal fragment (CTFα) are produced. This pathway is non-amyloidogenic. In contrast, when APP is cleaved by β-secretase, a soluble N-terminal fragment (sAPPβ) and a membrane-bound C-terminal fragment (CTFβ) are produced. CTFβ is then further processed by γ-secretase, producing a soluble N-terminal fragment (amyloid-β or Aβ). Accumulation of Aβ in the extracellular space results in its aggregation to produce amyloid plaques. Amyloid plaques interfere with normal neuronal and synaptic functions, ultimately leading to neuronal cell death. Increased O-GlcNAcylation has been shown to enhance the non-amyloidogenic pathway and inhibit the amyloidogenic pathway, thus providing neuroprotection. (b) Tau O-GlcNAcylation is decreased with concomitant increase in its phosphorylation. In the hyperphosphorylated state, Tau is prone to aggregation, leading to neuronal cell death. Hyper-O-GlcNAcylation decreases Tau phosphorylation, increases its glycosylation, and makes Tau less susceptible to aggregation, thereby providing neuroprotection. (c) O-GlcNAcylation of α-synuclein. O-GlcNAcylation Thr72 on α-synuclein decreases the aggregation of the protein and renders it less toxic to neuronal cells
PD is a chronic, intensifying neurodegenerative disorder that mainly affects the motor system, characterized by dopaminergic neuronal cell death and the presence of ubiquitin and α-synuclein (α-Syn)-positive protein aggregates, known as Lewy bodies, in the substantial nigra pars compacta (Lin et al., 2019). α-Syn is O-GlcNAcylated at Thr72. A peptide-based aggregation acceleration assay indicated that O-GlcNAcylation is critical for inhibiting α-Syn aggregation and potentially plays a protective role in synucleinopathies (Marotta et al., 2012). More direct evidence showed that Thr72 O-GlcNAcylation on full-length α-Syn protein has a notable and substoichiometric inhibitory effect on α-Syn aggregation and blocks the toxicity of α-Syn to neurons (Fig. 4c) (Marotta et al., 2015).
Above all, these data indicate that deregulation of O-GlcNAc may contribute to the development and progression of neurodegenerative diseases. Much of the research in this area has focused on the regulation of aberrant protein aggregation by the interplay with phosphorylation. However, many other factors including kinase signaling, transcription, and proteasomal degradation are equally important. A comprehensive understanding of the underlying mechanisms by which O-GlcNAcylation regulates neurodegeneration would provide novel approaches for targeted intervention and therapies against neurodegenerative disorders.
5. Cardiovascular diseases and O-GlcNAc
Cardiovascular diseases, including coronary heart disease, angina, cerebrovascular disease, and rheumatic heart disease, are disorders that involve the heart and blood vessels. Eighty percent of cardiovascular disease deaths are due to heart attacks and strokes. The cardiovascular system is uniquely sensitive to metabolic changes. Individuals at risk of cardiovascular diseases may demonstrate raised blood pressure, glucose, and lipids as well as being overweight and having obesity. In addition, diabetes presents a primary, independent risk factor for cardiovascular disease. However, to date the underlying mechanisms which connect metabolic disorders with cardiac dysfunction are still underexplored. Recent studies indicate that O-GlcNAcylation might serve as a key player in the primary pathophysiology of cardiovascular diseases (Dassanayaka and Jones, 2014; Zachou et al., 2019).
The first evidence of the potential beneficial effect of O-GlcNAcylation is that glucosamine addition protected neonatal rat ventricular myocytes (NRVMs) against acute ischemia/reperfusion (I/R) injury (Champattanachai et al., 2007). Treatment with alloxan (an OGT inhibitor) or azaserine (a GFAT inhibitor) significantly reduced intracellular O-GlcNAcylation levels and blocked the protective effect (Champattanachai et al., 2007). Moreover, elevating O-GlcNAcylation by PUGNAc increased cell viability and reduced apoptosis and oxidative stress in response to I/R injury (Ngoh et al., 2009). In addition to in vitro experiments, it has been reported that when mice hearts were subjected to ischemic preconditioning, pharmacological augmentation of O-GlcNAcylation was sufficient to reduce myocardial infarct size and improve cardiac myocyte survival (Jones et al., 2008). Inhibition of mitochondrial permeability transition pore (mPTP) opening at reperfusion is critical for cell survival. The central element of mPTP, voltage-dependent anion channels (VDACs), possesses O-GlcNAcylation. Researchers hypothesized that O-GlcNAcylation of VDACs might affect the formation of mPTP and thus protect the cardiomyocytes (Mailleux et al., 2016).
Several studies have demonstrated a protective role of O-GlcNAcylation in the context of trauma hemorrhage. In the rat model, treatment with glucosamine or PUGNAc, which increased protein O-GlcNAcylation, improved recovery of organ perfusion and function, and reduced the levels of circulating interleukin 6 (IL-6) and tumor necrosis factor α (TNF-α) after trauma hemorrhage (Yang et al., 2006; Zou et al., 2007). A subsequent study revealed that the inflammatory process occurring during trauma hemorrhage was associated with an increase in NF-κB-DNA binding activity in the heart. Treatment with glucosamine attenuated the activation of the NF-κB pathway. This improved cardiac function following hemorrhagic shock (Zou et al., 2009).
Heart failure happens when the heart fails to pump sufficiently to supply blood flow to tissues, and is associated with symptoms of pulmonary edema, dyspnea, and fatigue (Snipelisky et al., 2019). It has been confirmed that a failing heart is subject to elevated metabolic demands. In the mouse model, ablation of O-GlcNAcylation did not seem to be harmful for normal cardiac function. However, ablation significantly exacerbated cardiac dysfunction in samples with infarct-induced heart failure, leading to increased apoptosis and decreased survival rates (Watson et al., 2010). Troponin T (TnT) is a subunit of the troponin complex, which binds to tropomyosin and helps contraction of skeletal and heart muscles. A recent report demonstrated that heart failure increased TnT O-GlcNAcylation at Ser190 through both increased OGT and decreased OGA activity. However, it is still unclear whether TnT O-GlcNAcylation is an adaptive or maladaptive mechanism in ischemic heart failure (Dubois-Deruy et al., 2015).
Diabetes is a major risk factor for ischemic heart disease. Chronically increased O-GlcNAcylation in the hearts of diabetic animals has been implicated in glucose toxicity and associated with multiple facets of diabetic cardiac dysfunction (Marsh et al., 2014). Cardiac-type sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA2a) plays a major role in cardiac muscle contractility and is regulated by phospholamban (PLN). PLN is phosphorylated at Ser16 and this modification is critical for the SERCA2a regulation. It has been reported that PLN is also O-GlcNAcylated at Ser16. In diabetic conditions, O-GlcNAcylation inhibited PLN phosphorylation and decreased its association with SERCA2a, leading to the deterioration of cardiac function (Mailleux et al., 2016). A recent study indicates that diabetic cardiac dysfunction may be partially due to the mis-localization of OGT and OGA. Delocalization of OGT and OGA might change O-GlcNAcylation patterns of substrate proteins, thereby altering calcium handling and other normal cardiac functions (Ramirez-Correa et al., 2015).
Collectively, O-GlcNAcylation might play different roles in the progression of the cardiovascular disease. O-GlcNAcylation seems to be protective against acute ischemia-reperfusion injury and trauma hemorrhage in the short term; however, this modification appears to potentiate the harmful effect for cardiac function within a chronic disease such as diabetic cardiomyopathy.
6. Conclusions and perspectives
In the past 30 years, it has become increasingly clear that protein O-GlcNAcylation regulates a number of critical cellular processes. However, the potential importance of protein O-GlcNAcylation in mediating the pathological and physiological processes of many human diseases, such as cancer, diabetes, neurodegenerative and cardiovascular diseases, has only been reported recently and remains largely unexplored. These chronic diseases present major alterations in cellular metabolism, which results in O-GlcNAcylation deregulation. The deregulation of O-GlcNAcylation further disrupts cellular signal transduction pathways and potentially promotes disease progression. Uncovering the molecular mechanisms of O-GlcNAc signaling involved in relevant human diseases will provide opportunities for improved treatment. In this regard, development of highly specific inhibitors of OGT and OGA, which are still insufficient, would lead to potential new approaches for further investigation of O-GlcNAcylation function and serve as potential drugs.
In addition, although O-GlcNAcylation appears to be as abundant as phosphorylation, so far our knowledge of the fundamental mechanisms involved in regulating O-GlcNAcylation is still very limited. One of the major problems is the lack of reliable detection tools, particularly site-specific antibodies, which are already generally taken for granted in the investigation of many other protein modifications. Although a chemoenzymatic labelling approach had been available (Yi et al., 2012), the experimental processes of this approach were time-consuming and complicated. It is expected that there will be an increasing number of researchers driven to establish a set of simpler and more reliable O-GlcNAc detection methods.
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 91753125, 31270865, 31322019, and 31570804), the National Key Research and Development Program of China (No. 2016YFA0100303), and the Zhejiang Provincial Natural Science Foundation of China (No. LR15C050001)
Contributors: Wen YI designed and directed the project. Hao NIE and Wen YI wrote the paper.
Compliance with ethics guidelines: Hao NIE and Wen YI declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Andrali SS, Qian QW, Özcan S. Glucose mediates the translocation of neurod1 by O-linked glycosylation. J Biol Chem. 2007;282(21):15589–15596. doi: 10.1074/jbc.M701762200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ball LE, Berkaw MN, Buse MG. Identification of the major site of O-linked β-N-acetylglucosamine modification in the C terminus of insulin receptor substrate-1. Mol Cell Proteom. 2006;5(2):313–323. doi: 10.1074/mcp.M500314-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerjee PS, Lagerlöf O, Hart GW. Roles of O-GlcNAc in chronic diseases of aging. Mol Aspects Med. 2016;51:1–15. doi: 10.1016/j.mam.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Bond MR, Hanover JA. A little sugar goes a long way: the cell biology of O-GlcNAc. J Cell Biol. 2015;208(7):869–880. doi: 10.1083/jcb.201501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borghgraef P, Menuet C, Theunis C, et al. Increasing brain protein O-GlcNAc-ylation mitigates breathing defects and mortality of Tau.P301l mice. PLoS ONE. 2013;8(12):e84442. doi: 10.1371/journal.pone.0084442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 7.Buono R, Longo VD. Starvation, stress resistance, and cancer. Trends Endocrinol Metab. 2018;29(4):271–280. doi: 10.1016/j.tem.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butkinaree C, Park K, Hart GW. O-linked β-N-acetylglucosamine (O-GlcNAc): extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress. Biochim Biophys Acta. 2010;1800(2):96–106. doi: 10.1016/j.bbagen.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpenter R, DiChiacchio T, Barker K. Interventions for self-management of type 2 diabetes: an integrative review. Int J Nurs Sci. 2019;6(1):70–91. doi: 10.1016/j.ijnss.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cecioni S, Vocadlo DJ. Tools for probing and perturbing O-GlcNAc in cells and in vivo . Curr Opin Chem Biol. 2013;17(5):719–728. doi: 10.1016/j.cbpa.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 11.Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein-associated O-GlcNAc. Am J Physiol Cell Physiol. 2007;292(1):C178–C187. doi: 10.1152/ajpcell.00162.2006. [DOI] [PubMed] [Google Scholar]
- 12.Chen YX, Jin L, Xue B, et al. Nrage induces β-catenin/Arm O-GlcNAcylation and negatively regulates Wnt signaling. Biochem Biophys Res Commun. 2017;487(2):433–437. doi: 10.1016/j.bbrc.2017.04.080. [DOI] [PubMed] [Google Scholar]
- 13.Chetan MR, Thrower SL, Narendran P. What is type 1 diabetes? Medicine. 2019;47(1):5–9. doi: 10.1016/j.mpmed.2018.10.006. [DOI] [Google Scholar]
- 14.Chou TY, Hart GW, Dang CV. c-Myc is glycosylated at threonine 58, a known phosphorylation site and a mutational hot spot in lymphomas. J Biol Chem. 1995;270(32):18961–18965. doi: 10.1074/jbc.270.32.18961. [DOI] [PubMed] [Google Scholar]
- 15.Cooper C, Sommerlad A, Lyketsos CG, et al. Modifiable predictors of dementia in mild cognitive impairment: a systematic review and meta-analysis. Am J Psychiatry. 2015;172(4):323–334. doi: 10.1176/appi.ajp.2014.14070878. [DOI] [PubMed] [Google Scholar]
- 16.Cui C, Zhou XL, Zhang WD, et al. Is β-catenin a druggable target for cancer therapy? Trends Biochem Sci. 2018;43(8):623–634. doi: 10.1016/j.tibs.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Darley-Usmar VM, Ball LE, Chatham JC. Protein O-linked β-N-acetylglucosamine: a novel effector of cardiomyocyte metabolism and function. J Mol Cell Cardiol. 2012;52(3):538–549. doi: 10.1016/j.yjmcc.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dassanayaka S, Jones SP. O-GlcNAc and the cardiovascular system. Pharmacol Ther. 2014;142(1):62–71. doi: 10.1016/j.pharmthera.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Jesus T, Shukla S, Ramakrishnan P. Too sweet to resist: control of immune cell function by O-GlcNAcylation. Cell Immunol. 2018;333:85–92. doi: 10.1016/j.cellimm.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubois-Deruy E, Belliard A, Mulder P, et al. Interplay between troponin T phosphorylation and O-N-acetylglucosaminylation in ischaemic heart failure. Cardiovasc Res. 2015;107(1):56–65. doi: 10.1093/cvr/cvv136. [DOI] [PubMed] [Google Scholar]
- 21.Durning SP, Flanagan-Steet H, Prasad N, et al. O-linked β-N-acetylglucosamine (O-GlcNAc) acts as a glucose sensor to epigenetically regulate the insulin gene in pancreatic beta cells. J Biol Chem. 2016;291(5):2107–2118. doi: 10.1074/jbc.M115.693580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrer CM, Sodi VL, Reginato MJ. O-GlcNAcylation in cancer biology: linking metabolism and signaling. J Mol Biol. 2016;428(16):3282–3294. doi: 10.1016/j.jmb.2016.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferron M, Denis M, Persello A, et al. Protein O-GlcNAcylation in cardiac pathologies: past, present, future. Front Endocrinol (Lausanne), 9:819. 2018 doi: 10.3389/fendo.2018.00819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao Y, Miyazaki JI, Hart GW. The transcription factor PDX-1 is post-translationally modified by O-linked N-acetylglucosamine and this modification is correlated with its DNA binding activity and insulin secretion in Min6 β-cells. Arch Biochem Biophys. 2003;415(2):155–163. doi: 10.1016/s0003-9861(03)00234-0. [DOI] [PubMed] [Google Scholar]
- 25.Graham DL, Gray AJ, Joyce JA, et al. Increased O-GlcNAcylation reduces pathological tau without affecting its normal phosphorylation in a mouse model of tauopathy. Neuropharmacology. 2014;79:307–313. doi: 10.1016/j.neuropharm.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 26.Gudala K, Bansal D, Schifano F, et al. Diabetes mellitus and risk of dementia: a meta-analysis of prospective observational studies. J Diabetes Investig. 2013;4(6):640–650. doi: 10.1111/jdi.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ha JR, Hao L, Venkateswaran G, et al. β-Catenin is O-GlcNAc glycosylated at serine 23: implications for β-catenin’s subcellular localization and transactivator function. Exp Cell Res. 2014;321(2):153–166. doi: 10.1016/j.yexcr.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 28.Harosh-Davidovich SB, Khalaila I. O-GlcNAcylation affects β-catenin and E-cadherin expression, cell motility and tumorigenicity of colorectal cancer. Exp Cell Res. 2018;364(1):42–49. doi: 10.1016/j.yexcr.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 29.Hurtado MD, Vella A. What is type 2 diabetes? Medicine. 2019;47(1):10–15. doi: 10.1016/j.mpmed.2018.10.010. [DOI] [Google Scholar]
- 30.Hwang H, Rhim H. Functional significance of O-GlcNAc modification in regulating neuronal properties. Pharmacol Res. 2018;129:295–307. doi: 10.1016/j.phrs.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Issad T, Masson E, Pagesy P. O-GlcNAc modification, insulin signaling and diabetic complications. Diabetes Metab. 2010;36(6):423–435. doi: 10.1016/j.diabet.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Itkonen HM, Minner S, Guldvik IJ, et al. O-GlcNAc transferase integrates metabolic pathways to regulate the stability of c-MYC in human prostate cancer cells. Cancer Res. 2013;73(16):5277–5287. doi: 10.1158/0008-5472.CAN-13-0549. [DOI] [PubMed] [Google Scholar]
- 33.Jiang JY, Lazarus MB, Pasquina L, et al. A neutral diphosphate mimic crosslinks the active site of human O-GlcNAc transferase. Nat Chem Biol. 2011;8(1):72–77. doi: 10.1038/nchembio.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joiner CM, Li H, Jiang JY, et al. Structural characterization of the O-GlcNAc cycling enzymes: insights into substrate recognition and catalytic mechanisms. Curr Opin Struct Biol. 2019;56:97–106. doi: 10.1016/j.sbi.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones SP, Zachara NE, Ngoh GA, et al. Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation. 2008;117(9):1172–1182. doi: 10.1161/CIRCULATIONAHA.107.730515. [DOI] [PubMed] [Google Scholar]
- 36.Kim C, Nam DW, Park SY, et al. O-linked β-N-acetylglucosaminidase inhibitor attenuates β-amyloid plaque and rescues memory impairment. Neurobiol Aging. 2013;34(1):275–285. doi: 10.1016/j.neurobiolaging.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Lee TN, Alborn WE, Knierman MD, et al. Alloxan is an inhibitor of O-GlcNAc-selective N-acetyl-β-D-glucosaminidase. Biochem Biophys Res Commun. 2006;350(4):1038–1043. doi: 10.1016/j.bbrc.2006.09.155. [DOI] [PubMed] [Google Scholar]
- 38.Leney AC, el Atmioui D, Wu W, et al. Elucidating crosstalk mechanisms between phosphorylation and O-GlcNAcylation. Proc Natl Acad Sci USA. 2017;114(35):E7255–E7261. doi: 10.1073/pnas.1620529114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41(3):211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim S, Haque MM, Nam G, et al. Monitoring of intracellular tau aggregation regulated by OGA/OGT inhibitors. Int J Mol Sci. 2015;16(9):20212–20224. doi: 10.3390/ijms160920212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin G, Wang LP, Marcogliese PC, et al. Sphingolipids in the pathogenesis of Parkinson’s disease and Parkinsonism. Trends Endocrinol Metab. 2019;30(2):106–117. doi: 10.1016/j.tem.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Dai SJ, Xing LJ, et al. O-linked β-N-acetylglucosamine modification and its biological functions. Sci Bull. 2015;60(12):1055–1061. doi: 10.1007/s11434-015-0816-x. [DOI] [Google Scholar]
- 43.Ma ZY, Vocadlo DJ, Vosseller K. Hyper-O-GlcNAcylation is anti-apoptotic and maintains constitutive NF-κB activity in pancreatic cancer cells. J Biol Chem. 2013;288(21):15121–15130. doi: 10.1074/jbc.M113.470047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Macauley MS, Vocadlo DJ. Increasing O-GlcNAc levels: an overview of small-molecule inhibitors of O-GlcNAcase. Biochim Biophys Acta. 2010;1800(2):107–121. doi: 10.1016/j.bbagen.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 45.Macauley MS, Shan XY, Yuzwa SA, et al. Elevation of global O-GlcNAc in rodents using a selective O-GlcNAcase inhibitor does not cause insulin resistance or perturb glucohomeostasis. Chem Biol. 2010;17(9):949–958. doi: 10.1016/j.chembiol.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mailleux F, Gélinas R, Beauloye C, et al. O-GlcNAcylation, enemy or ally during cardiac hypertrophy development? Biochim Biophys Acta. 2016;1862(12):2232–2243. doi: 10.1016/j.bbadis.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 47.Marotta NP, Cherwien CA, Abeywardana T, et al. O-GlcNAc modification prevents peptide-dependent acceleration of α-synuclein aggregation. Chembiochem. 2012;13(18):2665–2670. doi: 10.1002/cbic.201200478. [DOI] [PubMed] [Google Scholar]
- 48.Marotta NP, Lin YH, Lewis YE, et al. O-GlcNAc modification blocks the aggregation and toxicity of the protein α-synuclein associated with Parkinson’s disease. Nat Chem. 2015;7(11):913–920. doi: 10.1038/nchem.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marsh SA, Collins HE, Chatham JC. Protein O-GlcNAcylation and cardiovascular (patho)physiology. J Biol Chem. 2014;289(50):34449–34456. doi: 10.1074/jbc.R114.585984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McClain DA, Lubas WA, Cooksey RC, et al. Altered glycan-dependent signaling induces insulin resistance and hyperleptinemia. Proc Natl Acad Sci USA. 2002;99(16):10695–10699. doi: 10.1073/pnas.152346899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ngoh GA, Hamid T, Prabhu SD, et al. O-GlcNAc signaling attenuates ER stress-induced cardiomyocyte death. Am J Physiol Heart Circ Physiol. 2009;297(5):H1711–H1719. doi: 10.1152/ajpheart.00553.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Özcan S, Andrali SS, Cantrell JEL. Modulation of transcription factor function by O-GlcNAc modification. Biochim Biophys Acta. 2010;1799(5-6):353–364. doi: 10.1016/j.bbagrm.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patel M, Horgan PG, McMillan DC, et al. NF-κB pathways in the development and progression of colorectal cancer. Transl Res. 2018;197:43–56. doi: 10.1016/j.trsl.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 54.Peterson SB, Hart GW. New insights: a role for O-GlcNAcylation in diabetic complications. Crit Rev Biochem Mol Biol. 2016;51(3):150–161. doi: 10.3109/10409238.2015.1135102. [DOI] [PubMed] [Google Scholar]
- 55.Pinho TS, Verde DM, Correia SC, et al. O-GlcNAcylation and neuronal energy status: implications for Alzheimer’s disease. Ageing Res Rev. 2018;46:32–41. doi: 10.1016/j.arr.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 56.Ramirez-Correa GA, Ma JF, Slawson C, et al. Removal of abnormal myofilament O-GlcNAcylation restores Ca2+ sensitivity in diabetic cardiac muscle. Diabetes. 2015;64(10):3573–3587. doi: 10.2337/db14-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rao XJ, Duan XT, Mao WM, et al. O-GlcNAcylation of G6PD promotes the pentose phosphate pathway and tumor growth. Nat Commun, 6:8468. 2015 doi: 10.1038/ncomms9468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rowe EM, Xing V, Biggar KK. Lysine methylation: implications in neurodegenerative disease. Brain Res. 2019;1707:164–171. doi: 10.1016/j.brainres.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 59.Smet-Nocca C, Broncel M, Wieruszeski JM, et al. Identification of O-GlcNAc sites within peptides of the Tau protein and their impact on phosphorylation. Mol Biosyst. 2011;7(5):1420–1429. doi: 10.1039/c0mb00337a. [DOI] [PubMed] [Google Scholar]
- 60.Snipelisky D, Chaudhry SP, Stewart GC. The many faces of heart failure. Card Electrophysiol Clin. 2019;11(1):11–20. doi: 10.1016/j.ccep.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 61.Tan JZA, Gleeson PA. The role of membrane trafficking in the processing of amyloid precursor protein and production of amyloid peptides in Alzheimer’s disease. Biochim Biophys Acta. 2019;1861(4):697–712. doi: 10.1016/j.bbamem.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 62.Teo CF, Wollaston-Hayden EE, Wells L. Hexosamine flux, the O-GlcNAc modification, and the development of insulin resistance in adipocytes. Mol Cell Endocrinol. 2010;318(1-2):44–53. doi: 10.1016/j.mce.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Giau V, An SSA, Hulme JP. Mitochondrial therapeutic interventions in Alzheimer’s disease. J Neurol Sci. 2018;395:62–70. doi: 10.1016/j.jns.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 64.Vosseller K, Wells L, Lane MD, et al. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in AKT activation in 3T3-L1 adipocytes. Proc Natl Acad Sci USA. 2002;99(8):5313–5318. doi: 10.1073/pnas.072072399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y, Liu J, Jin X, et al. O-GlcNAcylation destabilizes the active tetrameric PKM2 to promote the Warburg effect. Proc Natl Acad Sci USA. 2017;114(52):13732–13737. doi: 10.1073/pnas.1704145115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wani WY, Chatham JC, Darley-Usmar V, et al. O-GlcNAcylation and neurodegeneration. Brain Res Bull. 2017;133:80–87. doi: 10.1016/j.brainresbull.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watson LJ, Facundo HT, Ngoh GA, et al. O-linked β-N-acetylglucosamine transferase is indispensable in the failing heart. Proc Natl Acad Sci USA. 2010;107(41):17797–17802. doi: 10.1073/pnas.1001907107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Whelan SA, Dias WB, Thiruneelakantapillai L, et al. Regulation of insulin receptor substrate 1 (IRS-1)/AKT kinase-mediated insulin signaling by O-linked β-N-acetylglucosamine in 3T3-L1 adipocytes. J Biol Chem. 2010;285(8):5204–5211. doi: 10.1074/jbc.M109.077818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu WQ, Zhang X, Wu JL, et al. O-GlcNAc transferase promotes fatty liver-associated liver cancer through inducing palmitic acid and activating endoplasmic reticulum stress. J Hepatol. 2017;67(2):310–320. doi: 10.1016/j.jhep.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 70.Yang SL, Zou LY, Bounelis P, et al. Glucosamine administration during resuscitation improves organ function after trauma hemorrhage. Shock. 2006;25(6):600–607. doi: 10.1097/01.shk.0000209563.07693.db. [DOI] [PubMed] [Google Scholar]
- 71.Yang WH, Park SY, Nam HW, et al. NFκB activation is associated with its O-GlcNAcylation state under hyperglycemic conditions. Proc Natl Acad Sci USA. 2008;105(45):17345–17350. doi: 10.1073/pnas.0806198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang XY, Ongusaha PP, Miles PD, et al. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008;451(7181):964–969. doi: 10.1038/nature06668. [DOI] [PubMed] [Google Scholar]
- 73.Yi W, Clark PM, Mason DE, et al. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science. 2012;337(6097):975–980. doi: 10.1126/science.1222278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yuzwa SA, Macauley MS, Heinonen JE, et al. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo . Nat Chem Biol. 2008;4(8):483–490. doi: 10.1038/nchembio.96. [DOI] [PubMed] [Google Scholar]
- 75.Zachou G, Armeni E, Lambrinoudaki I. Lactation and maternal cardiovascular disease risk in later life. Maturitas. 2019;122:73–79. doi: 10.1016/j.maturitas.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 76.Zhu YP, Shan XY, Yuzwa SA, et al. 2014. [Google Scholar]
- 77.Zou LY, Yang SL, Hu SH, et al. The protective effects of PUGNAc on cardiac function after trauma-hemorrhage are mediated via increased protein O-GlcNAc levels. Shock. 2007;27(4):402–408. doi: 10.1097/01.shk.0000245031.31859.29. [DOI] [PubMed] [Google Scholar]
- 78.Zou LY, Yang SL, Champattanachai V, et al. Glucosamine improves cardiac function following trauma-hemorrhage by increased protein O-GlcNAcylation and attenuation of NF-κB signaling. Am J Physiol Heart Circ Physiol. 2009;296(2):H515–H523. doi: 10.1152/ajpheart.01025.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]