Abstract
Purpose of review
This manuscript reviews the epidemiological data linking psychosocial stress to cardiovascular disease (CVD), describes recent advances in understanding the biological pathway between them, discusses potential therapies against stress-related CVD, and identifies future research directions.
Recent findings
Metabolic activity of the amygdala (a neural center that is critically involved in the response to stress) can be measured on 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) yielding a neurobiological signal that independently predicts subsequent CVD events. Furthermore, a serial pathway from ↑amygdalar activity → ↑hematopoietic tissue activity → ↑arterial inflammation → ↑CVD events has been elucidated, providing new insights into the mechanism linking stress to CVD.
Summary
Psychosocial stress and stress conditions are independently associated with CVD in a manner that depends on the degree and duration of stress as well as the individual response to a stressor. Nevertheless, the fundamental biology remains incompletely defined, and stress is often confounded by adverse health behaviors. Thus, most clinical guidelines do not yet recognize psychosocial stress as an independent CVD risk factor or advocate for its treatment in CVD prevention. Clarification of this neurobiological pathway provides a better understanding of the underlying pathophysiology and suggests opportunities to develop novel preventive strategies and therapies.
Keywords: Psychosocial stress, Cardiovascular disease, PET imaging, Amygdalar activity
Introduction
In early psychoanalytic psychiatry, neurosis was theorized to “cathect” organs to produce physical disease [1]. However, even now, the biology underlying this association remains incompletely defined. Current science requires the discovery of pathophysiologic mechanisms linking the brain to periphery to understand the association between the mental and physical. Ultimately, the impact of an organism’s environment, including psychosocial stress, must be understood at organic, tissue, cellular, and molecular levels.
Stress, which may exist in many forms, is an unavoidable element of human existence that often leads to a maladaptive physiological response [2]. Although acute emotional stress has long been linked to acute cardiovascular events [3–7], the long-term effects of chronic stress on physical well-being, and specifically cardiovascular disease (CVD), have only recently been recognized [8–11, 12••, 13]. Nevertheless, clinical guidelines continue to lag behind this evidence.
Although some guidelines recommend stress management in patients at high risk of CVD [14], such a practice is not yet established as a method of primary prevention in the general population [15, 16]. Because the prevalence of chronic stress and stress conditions is rapidly increasing in the modern world [17], the identification of stress as an independent risk factor for CVD and the development of novel preventive strategies have emerged as public health challenges needing urgent attention.
A key next step in addressing this need is elucidating the mechanistic neurobiological pathways that link stress to CVD. It has been established that external stressors trigger a coordinated response from the two main effector limbs of the stress response, the sympathetic nervous system (SNS) and the hypothalamic-pituitary-adrenal (HPA) axis. The former mobilizes necessary resources for flight or fight, whereas the latter (among other things) protects the individual from the adverse effects of this mobilization to maintain homeostasis [18]. SNS activation increases inflammation and inflammatory cell output, which is known to drive multiple pathologies including CVD [19]. In contrast, the end product of the HPA axis, viz., corticosterone in rodents and cortisol in humans, is well known for its anti-inflammatory effects, although under certain circumstances, it may also be pro-inflammatory [20]. These two systems are variably stimulated in different individuals by different stressors over different time courses. Upstream nervous system factors modulate both the SNS and HPA axis based upon individual experience and deployment of coping strategies. The salience network of the brain, a network of connected neural centers (which prominently include the amygdala), represents one of these upstream factors and plays a critical role in governing individual autonomic and hormonal responses [21••, 22–24].
Recent research has harnessed advanced multi-system 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/ CT), and more recently magnetic resonance imaging (18F-FDG-PET/MRI), simultaneously to evaluate metabolic activity in neural centers and disparate organ systems to make important discoveries about how the environment impacts the brain and leads to downstream pathology. Specifically, it has been shown that increased resting metabolic activity in the amygdala, an important center in the emotional and physiological response to stress, on 18F-FDG-PET/CT is increased in chronic stress conditions and associates with perceived stress [21••, 25–27]. Furthermore, amygdalar activity associates downstream with increased bone marrow activity (indicative of leukopoiesis) and arterial wall activity (indicative of inflammation). Amygdalar activity, in series with bone marrow and arterial wall activity, as well as independently, predicts adverse CVD events and also independently predicts incident diabetes mellitus [21••, 28••, 29]. Characterization of this mechanism has created the opportunity to evaluate novel interventions aimed at different targets within this pathway with the hopes of attenuating the pathophysiologic consequences of stress.
In this review, we discuss existing evidence and recent insights into different types of psychosocial stress, the forms of CVD associated with psychosocial stress in epidemiological studies, the mechanisms underlying the association between stress and CVD, potential therapies targeting stress-related CVD, and future directions for further research on this topic.
Forms of psychosocial stress
Psychosocial stress may result from a variety of etiologies and circumstances. The experience of stress and its impact are often unique to an individual organism. Nevertheless, psychosocial stress ultimately activates the same stress response systems (SNS and HPA axis) regardless of its source. Psychosocial stress can occur acutely or chronically, and the duration of the stress response has an impact on both the incidence and the type of CVD consequences. As such, we have categorized psychosocial stress according to its duration as acute or chronic for the purposes of the discussion below.
Acute psychosocial stress
The potential impact of acute psychosocial stress (APS) on CVD has frequently been assessed by evaluating the aftermath of happenstance natural disasters and adverse emotional events (e.g., receiving a cancer diagnosis, gambling losses). APS is associated with an increased risk for acute cardiovascular complications such as myocardial infarction (MI), left ventricular dysfunction, and arrhythmias. Following the Northridge earthquake in 1994 in Los Angeles, CA, a 2–5-fold increase in the rate of CVD deaths was observed in and around the city, with the greatest risk in individuals with underlying coronary artery disease (CAD) [3, 4]. Other earthquake studies have shown a relative increase in SNS activity manifest in increases in heart rate, low-frequency/high-frequency heart rate variability ratio [30], and blood viscosity with risk of thromboembolism [31, 32]. In the absence of underlying CAD, APS is also being recognized as a likely precipitant of Takotsubo cardiomyopathy, an acute but usually transient heart failure syndrome, with elevated levels of circulating catecholamines in response to acute stressors playing a key role in the underlying pathophysiology [7, 33]. Similarly, an association has been reported between acute emotional stress and aortic dissection [34].
Although disasters and acute stressors provide opportunities to study the association between APS and CVD, these studies are inevitably confounded by consequent lifestyle changes, which include changes in diet and exercise, skipped medications, and exposure to toxins, among others. To control for such factors, researchers have tried to study the association between APS and CVD in the laboratory setting. This approach remains limited, as there are few ways that experimental psychological stress can be ethically implemented, and the generalizability of these methods to real-world stressors remains questionable. For example, in one such experimental study with these limitations, Dimsdale and Moss reported that epinephrine levels increased approximately threefold among resident physicians making formal presentations at conferences; the increase was even more pronounced in those whose presentations were interrupted by a sudden blowout of the projector bulb [35].
Similarly, a large body of laboratory research has been performed to assess acute cardiovascular responses to mental stress. Acute mental stress has not only been shown to have adverse changes in blood pressure and heart rate variability but has also been shown to alter myocardial perfusion [30, 36]. Within this paradigm, Deanfield et al. carried out an experiment in 16 patients with stable angina, in which they compared findings on rubidium-82 PET imaging between a physical (bicycle ergometry) and a mental (serial seven subtraction) stressor. Both physical and mental stress elicited abnormal regional perfusion in 12 patients. With physical stress, abnormal perfusion correlated well with ST segment changes and anginal symptoms. In contrast, there was discordance among perfusion abnormalities, ST segment changes, and chest pain in the group subjected to mental stress [37]. However, several other studies have shown that mild acute mental stress, like mental arithmetic, can induce myocardial ischemia. Importantly, this holds true even at low heart rates and blood pressure, suggesting that the mechanism is most likely mediated by impaired local vascular endothelial function rather than increased hemodynamic demand [38–40].
In a study by Jiang et al., a series of patients with known CAD were assessed using radionuclide ventriculography to evaluate left ventricular ejection fraction (LVEF) and regional wall motion in response to mental arithmetic. They observed that 67% of CAD patients showed either wall motion abnormalities or a > 5% drop in LVEF in response to the acute mental stress; those who developed a drop in LVEF were more than twice as likely to have an adverse CVD event during 4 years of follow-up [41]. Sheps et al. similarly showed that the degree of wall motion abnormality in response to acute mental stress (public speaking) predicted all-cause mortality in CAD patients in 5 years of follow-up [11].
Chronic psychosocial stress
Chronic psychosocial stress (CPS) can arise from major life changes (e.g., job stress, marital discord, death of a spouse or loved one, burden of caregiving), adverse socioeconomic factors (e.g., income, crime, education, racial inequality), and chronic psychiatric conditions (e.g., anxiety, depression, post-traumatic stress disorder). There is a wealth of data linking CPS and CVD; the magnitude of risk for CVD events associated with CPS has been reported to be equal to that attributed to traditional CVD risk factors [9]. For example, the rate of implanted defibrillator firings was higher than expected in the months following the 2001 terrorist attack on the World Trade Center in New York, irrespective of the nature of the underlying cardiomyopathy [42]. Similarly, in a cohort study from the Nurses’ Health Study, the stress from caring for a sick spouse almost doubled the risk of CVD mortality [43].
Although many of the studies evaluating the association of CPS with CVD focus on the effects of chronic major stressors (e.g., marital dissolution, death of a spouse or a loved one), the role of chronic milder stressors in the pathogenesis of CVD has been increasingly recognized. For example, Johansen et al. showed that chronic stress related to upcoming deadlines at work was associated with a sixfold increase in MI incidence [44]. Further, in women with underlying CAD, CPS from daily marital tension was associated with a threefold increased risk of CVD events [13].
Depression and anxiety have consistently been shown to associate with CVD and adverse CVD events. In fact, the 2012 European guidelines for cardiovascular disease prevention recognize depression, anxiety, and psychosocial stressors as risk factors for incident CVD and a worsened prognosis in patients with known CVD [45]. Depression independently associates with CVD events in patients with or without pre-existent CAD [46–48] and has been shown to almost double the risk of developing new CVD [49]. Furthermore, depression is a significant predictor of both survival and subsequent adverse cardiovascular events after an index acute coronary syndrome [49, 50] and among individuals with heart failure [51]. Moreover, depressed patients with CAD and heart failure are more likely to have a poorer quality of life and greater physical limitations even after adjusting for CVD severity [52, 53].
Similarly, chronic anxiety has been found to confer an approximate threefold increased risk of CVD events [54, 55]. In a recent meta-analysis, individuals with anxiety disorder were at increased risk for incident CAD and CV death, independent of traditional CVD risk factors [56]. These results have been corroborated in large prospective cohort studies [57, 58]. Additionally, posttraumatic stress disorder (PTSD) is strongly associated with CVD (both incident and progressive). For example, World War II veterans with PTSD had significantly increased risk of CAD and hypertension [59]. Another prospective cohort study showed that women with PTSD are more than three times more likely to develop CAD, even after adjusting for depression, anxiety, and traditional CVD risk factors [60].
Specific cardiovascular diseases related to psychosocial stress
In this section, we review some of the key manifestations of CVD that have been associated with psychosocial stress in epidemiologic studies (Table 1).
Table 1.
Cardiovascular manifestations of different types of psychosocial stress
| Psychosocial stress duration | Examples of inciting stressors | Cardiovascular effects |
|---|---|---|
| Acute | Natural disasters Acute emotional stressors |
Hypertension Tachycardia Reduced heart rate variability Takotsubo syndrome Myocardial ischemia Myocardial infarction Sudden death Acute aortic dissection Pulmonary embolism |
| Chronic | Job stress Marital discord Death of a loved one Burden of caregiving Racial inequality Low socioeconomic status Psychiatric conditions (e.g., anxiety, depression, posttraumatic stress disorder) |
Hypertension Coronary artery disease Myocardial infarction Cerebrovascular disease Peripheral vascular disease Congestive heart failure Atrial fibrillation |
Coronary artery disease and myocardial infarction
In addition to the previously described data, there is a substantial body of literature linking CPS to CAD, and APS to acute coronary events, independent of other cardiovascular risk factors [61, 62]. The landmark INTERHEART study included a sample of approximately 25,000 people from more than 50 countries. It showed that individuals with chronic daily stress had more than twice the risk of developing an MI compared to those without chronic stress after adjusting for confounding factors [9]. APS more frequently incites acute MI than physical exertion, and the risk of MI immediately following an anger outburst is about twice baseline [5, 6, 63].
Cerebrovascular disease
Although individual studies of the association between psychosocial stress and stroke have yielded less consistent results, a meta-analysis by Booth et al. showed that CPS is independently associated with increased risk of stroke [64]. Likewise, depression and PTSD have been found to confer an increased stroke risk [65, 66•].
Sudden cardiac death and ventricular arrhythmias
APS has been identified as a significant precipitant of sudden cardiac death in the setting of natural disasters [4] and stressful life events. In fact, even heightened stress from viewing World Cup soccer has been associated with a twofold increased risk of an acute cardiovascular event [67]! Individuals at the highest risk appear to be those with underlying CAD. Findings from these studies and others suggest that APS likely leads to acute plaque rupture due to locally increased shear stress, or more fatal arrhythmias secondary to increased SNS activity and exaggeration of regional myocardial ischemia [30, 68, 69]. Depression is independently associated with a more than threefold increased risk of sudden cardiac death [70].
Atrial fibrillation
In a prospective multi-cohort study, CPS in the form of long work hours was independently associated with an increased risk of atrial fibrillation [71]. Similar results were published by Fransson et al. in a separate prospective cohort study and meta-analysis [72].
Heart failure
Whereas APS is known to associate with acute left ventricular dysfunction as in Takotsubo cardiomyopathy, CPS has also been shown to have adverse outcomes in established heart failure (HF) patients. Endrighi et al. showed in a prospective study that CPS was a significant predictor of cardiovascular morbidity and all-cause mortality in HF patients [73]. The association between CPS and incident HF is rather weak and has largely been reported in individuals with poor health at baseline [74]. As previously described, depression has also been shown to increase the risk of all-cause mortality and contribute to impaired quality of life and physical and social functioning in HF [65, 70, 75, 76].
Hypertension
A growing body of research has identified CPS as a potential independent risk factor for the development of hypertension (HTN). For example, high job strain has been associated with HTN in multiple studies [77, 78]. Interestingly, racial discrimination has also been suggested as a cause of increased risk for the development of HTN and may provide one explanation for the high prevalence of HTN among African Americans living in the USA [79]. Chronic stress conditions, including PTSD, have also shown to be associated with increased risk of HTN [80].
Peripheral vascular disease
Although the relationship between psychosocial stress and peripheral artery disease (PAD) has not been studied in the same detail as other forms of CVD, a recent abstract by Thomas et al. reported increased perceived stress in patients with new onset PAD [81]. In individuals with established PAD, increased CPS independently associates with diminished walking ability and quality of life [82].
Other cardiovascular risk factors
Although the independent association between stress and CVD is increasingly apparent, it remains of tantamount importance to be aware of the contribution of adverse behavioral changes (e.g., smoking, reduced physical activity, poor dietary habits) that often accompany chronic stress [83]. Adverse health behaviors account for a substantial proportion of the excess CVD risk associated with stress [84]. Increased psychosocial stress also increases the risk for the development of metabolic diseases (e.g., obesity and type 2 diabetes mellitus), which in turn can contribute to CVD [85, 86]. Notably, however, these stress-associated health behaviors do not nearly explain the relationships between stress and CVD [87–89].
Mechanistic insights
Despite the long-hypothesized biological link between stress and physical disease [1], and the knowledge that stress triggers increased SNS, HPA axis, and systemic inflammatory activity [90, 91], the detailed mechanistic underpinnings have only recently begun to be clarified.
An organism’s response to stress is triggered by its nervous system. The hypothalamus receives afferents from several neural structures involved in the response to stress. The hypothalamus in turn activates the SNS, and (through the anterior pituitary gland) HPA axis, leading to increased cortisol levels in humans. Increased cortisol has many somatic effects, including increased insulin resistance, central redistribution of adiposity, increased blood pressure, and impaired immune response [92]. The SNS activates the adrenal medulla and the peripheral sympathetic nerves, leading to increased circulating levels of catecholamines (mainly epinephrine and norepinephrine respectively), which increase insulin resistance, blood pressure, and heart rate as well as drive systemic inflammation by increasing interleukin-6 and C-reactive protein [93, 94].
SNS and HPA axis activity can both lead to endothelial dysfunction [95, 96]. Increased inflammatory activity serves a critical role in bridging the gap between stress and atherosclerotic disease [97, 98••]. In a macaque model, increased stress due to social subordination led to alterations in the immune system, including changes in immune cell proportions, cell type-specific gene expression, and response to an immune challenge [99]. Similarly, mice under chronic stress were observed to have enhanced bone marrow activity via SNS activation of myelopoiesis, resulting in heightened production of innate immune cells and cytokines [100]. Several animal studies have taken the next step and shown that chronic stress exposure leads to increased arterial inflammation. Specifically, in one study, subordinate mice with low social status developed premature atherosclerotic lesions with high inflammatory cell burden when chronically confronted by dominant mice [101]. In a second study, mice under chronic stress exhibited worsening arterial inflammation with unstable plaque features [100].
Although the downstream biology is becoming clearer, the upstream drivers of these biological changes consequent to stress remained undefined until recently. Several neural centers in the brain’s salience network play a critical role in an organism’s response to its environment. The amygdala is one important component of this network that is involved in the emotional perception and physiological response to stressors [21••, 22, 23, 102–104]. The activity of the amygdala is relatively stable, is increased in chronic stress conditions, and associates with perceived stress [19, 21••, 105]. Furthermore, its efferents provide input to the HPA axis and the SNS [24, 106]. Accordingly, we hypothesized that increased amygdalar activity could trigger leukopoiesis in the bone marrow and, ultimately, lead to an increase in arterial inflammation and CVD events [21••, 100, 107].
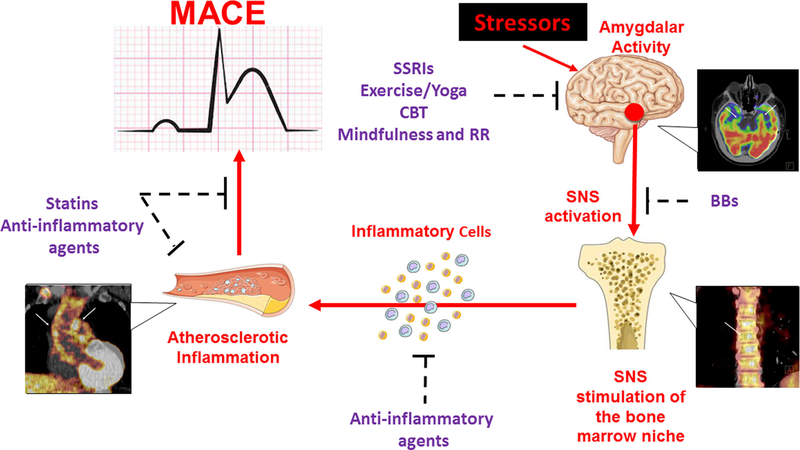
To test this hypothesis, our group employed 18F-FDG-PET/CT imaging to evaluate simultaneously the relationship between amygdalar metabolic activity, bone marrow activity, arterial inflammation, and CVD events within 5 years in a cohort of 293 patients without baseline CVD. In this study, we identified a robust association between amygdalar activity and CVD through a serial pathway of ↑amygdalar activity → ↑hematopoietic tissue activity → ↑arterial inflammation → ↑CVD event risk [21••]. This finding suggests a neurobiological mechanism that links stress to CVD with several potential targets for intervention and paves the way for further investigation (Fig. 1).
Fig. 1.
Proposed neurobiological mechanism linking psychosocial stress to cardiovascular disease, with imaging examples, and potential therapies targeting involved tissues. BBs beta blockers, CBT cognitive behavioral therapy, MACE major adverse cardiovascular events, RR relaxation response, SNS sympathetic nervous system, SSRIs selective serotonin reuptake inhibitors.
Potential therapies against cardiovascular diseases related to psychosocial stress
The recently reported pathway by which stress may lead to CVD suggests multiple targets (e.g., brain, hematopoietic tissues, arteries) with the potential to attenuate the harmful effects of stress and decrease the risk for subsequent CVD-related morbidity and mortality.
Potentially effective pharmacologic therapies include selective serotonin reuptake inhibitors (SSRIs), beta blockers, and anti-inflammatories. Different types of SSRIs have had mixed effects on CVD risk [108]; however, a recent meta-analysis demonstrated that SSRIs significantly decreased the risk of cardiovascular events, especially MI, among individuals with baseline depression and known CVD [109•]. Beta-adrenergic blocking agents attenuate the “fight or flight” reaction by antagonizing stress-induced catecholamine responses, both centrally (including in the amygdala) and peripherally. Such antagonism could potentially suppress the effect of noradrenaline on leukopoiesis in bone marrow and thereby inhibit cellular migration to periphery and hinder the promotion of downstream inflammatory atherosclerosis [21••, 100, 110]. Multiple randomized clinical trials have investigated the effect of anti-inflammatory therapies on vascular inflammation. Statins are the best studied agents and have been shown to significantly reduce arterial inflammation as well as the incidence of future cardiovascular events, perhaps independent of their lipid-lowering effects [97, 111, 112]. In 2018, results from the CANTOS trial (Canakinumab Anti-Inflammatory Thrombosis Outcomes Study) showed that independent of cholesterol-lowering therapies, anti-inflammatory treatment with canakinumab significantly reduced the rate of recurrent CVD via interleukin-1β inhibition [98••]. Currently, several other anti-inflammatory agents with different mechanisms or targets are under investigation. Ultimately, these and other agents may have a role for primary and secondary prevention in individuals with greater psychosocial stress.
In addition to pharmacologic therapies, non-pharmacologic interventions (e.g., mindfulness-based stress reduction, meditation, cognitive behavioral therapy) either in isolation or in combination with other therapies (e.g., pharmacological, education, exercise) have recently gained attention [113, 114•]. For example, in one study, yoga was more effective at diminishing CVD risk compared to other lifestyle modification interventions with an effect comparable to smoking cessation [115]. Mindfulness-based stress reduction has been shown to reduce blood pressure in untreated subjects with stage one essential hypertension potentially in part through effects on the modification of the transcription of key genes involved in inflammation [116•]. Furthermore, among individuals with clinical CVD who were enrolled in cardiac rehabilitation, the addition of stress-management training to cardiac rehabilitation improved stress levels and reduced CVD events beyond standard treatment in a prospective clinical trial [117]. Stress reduction strategies may impart this benefit against CVD through positive, lasting, process-specific changes in key neural centers involved in stress perception and triggering the stress response. In fact, mindfulness-based stress reduction has resulted in reductions in perceived stress and amygdalar gray matter density on MRI [118]. While these pharmacologic and non-pharmacologic therapies show promise for forestalling CVD consequent to chronic stress, additional carefully designed prospective research is required to evaluate their clinical efficacy.
Future directions
The neurobiology underlying the relationship between stress and CVD has only begun to emerge, but it already provides fertile ground for future research. Prospective studies should be performed to evaluate the roles of additional candidate mediating factors in the mechanistic pathway, including SNS activity and glucocorticoid levels, and to assess the efficacy of pharmacologic and non-pharmacologic therapies targeting components of this mechanism, taking into consideration potential confounders such as substance use, diet, and physical activity. Further, investigations into the relationship between upstream factors that contribute to psychosocial stress and amygdalar activity, such as socioeconomic status and environmental conditions, should be performed. The relationship between amygdalar activity and other downstream manifestations of CVD should be examined. Additionally, it is important to evaluate the roles of other neural centers involved in the response to stress (e.g., hippocampus, hypothalamus, prefrontal cortex, insula) in this mechanism. Given limitations in the direct measurement of perceived stress in current clinical practice, the role of amygdalar activity as a quantifiable and potentially modifiable proxy measure of perceived stress should be investigated. Finally, the impact of genetic and neurobiological factors in determining an individual’s resilience and susceptibility to disease consequent to psychosocial stress merits investigation. The answers to these important questions should further inform our understanding of the relationship between stress and CVD and provide opportunities to improve clinical care.
Conclusions
Psychosocial stress is an unavoidable consequence of daily human life that associates with an increased risk for CVD events that is on par with the risk from traditional CVD risk factors. The CVD consequences of stress ultimately depend upon its degree and duration, as well as on individual differences in responses to a stressor. Recent identification of a neurobiological mechanism in humans that bridges the gap from psychosocial stress to CVD identifies multiple novel interventions, such as anti-inflammatory medications and stress reduction with potentially high impact for preventing and treating the adverse CVD effects of stress. Furthermore, these findings take the next step towards demonstrating a causal relationship between psychosocial stress and CVD and facilitating the broad inclusion of therapies against psychosocial stress in CVD prevention and treatment guidelines.
Funding
This work is supported in part by the following grants: AHA 18CDA34110366 and National Center for Advancing Translational Sciences NIH KL2TR002542 (MTO) and NIH/NHLBI P01HL131478 (AT).
Footnotes
This article is part of the Topical Collection on Prevention
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights And Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Selye H A syndrome produced by diverse nocuous agents. 1936. J Neuropsychiatr Clin Neurosci. 1998;10(2):230–1. 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- 2.Steptoe A, Kivimaki M. Stress and cardiovascular disease. Nat Rev Cardiol. 2012;9(6):360–70. 10.1038/nrcardio.2012.45. [DOI] [PubMed] [Google Scholar]
- 3.Kloner RA, Leor J, Poole WK, Perritt R. Population-based analysis of the effect of the Northridge earthquake on cardiac death in Los Angeles County, California. J Am Coll Cardiol. 1997;30(5):1174–80. [DOI] [PubMed] [Google Scholar]
- 4.Leor J, Poole WK, Kloner RA. Sudden cardiac death triggered by an earthquake. N Engl J Med. 1996;334(7):413–9. 10.1056/NEJM199602153340701. [DOI] [PubMed] [Google Scholar]
- 5.Mittleman MA, Maclure M, Sherwood JB, Mulry RP, Tofler GH, Jacobs SC, et al. Triggering of acute myocardial infarction onset by episodes of anger. Determinants of Myocardial Infarction Onset Study Investigators. Circulation. 1995;92(7):1720–5. [DOI] [PubMed] [Google Scholar]
- 6.Strike PC, Perkins-Porras L, Whitehead DL, McEwan J, Steptoe A. Triggering of acute coronary syndromes by physical exertion and anger: clinical and sociodemographic characteristics. Heart. 2006;92(8):1035–40. 10.1136/hrt.2005.077362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watanabe H, Kodama M, Okura Y, Aizawa Y, Tanabe N, Chinushi M, et al. Impact of earthquakes on Takotsubo cardiomyopathy. JAMA. 2005;294(3):305–7. 10.1001/jama.294.3.305. [DOI] [PubMed] [Google Scholar]
- 8.Nabi H, Kivimaki M, Batty GD, Shipley MJ, Britton A, Brunner EJ, et al. Increased risk of coronary heart disease among individuals reporting adverse impact of stress on their health: the Whitehall II prospective cohort study. Eur Heart J. 2013;34(34):2697–705. 10.1093/eurheartj/eht216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):953–62. 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]
- 10.Batty GD, Russ TC, Stamatakis E, Kivimaki M. Psychological distress and risk of peripheral vascular disease, abdominal aortic aneurysm, and heart failure: pooling of sixteen cohort studies. Atherosclerosis. 2014;236(2):385–8. 10.1016/j.atherosclerosis.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 11.Sheps DS, McMahon RP, Becker L, Carney RM, Freedland KE, Cohen JD, et al. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery disease: results from the Psychophysiological Investigations of Myocardial Ischemia study. Circulation. 2002;105(15):1780–4. [DOI] [PubMed] [Google Scholar]
- 12.Stewart RAH, Colquhoun DM, Marschner SL, Kirby AC, Simes J, Nestel PJ, et al. Persistent psychological distress and mortality in patients with stable coronary artery disease. Heart. 2017;103(23):1860–6. 10.1136/heartjnl-2016-311097. [DOI] [PubMed] [Google Scholar]; •• This retrospective longitudinal study investigated the link between psychosocial stress and cardiovascular and all-cause mortality in individuals with stable CVD. The study identifies a significant association between stress and both outcomes.
- 13.Orth-Gomer K, Wamala SP, Horsten M, Schenck-Gustafsson K, Schneiderman N, Mittleman MA. Marital stress worsens prognosis in women with coronary heart disease: the Stockholm Female Coronary Risk Study. JAMA. 2000;284(23):3008–14. [DOI] [PubMed] [Google Scholar]
- 14.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315–81. 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson TA, Blair SN, Daniels SR, Eckel RH, Fair JM, Fortmann SP, et al. AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update: consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. American Heart Association science advisory and coordinating committee. Circulation. 2002;106(3):388–91. [DOI] [PubMed] [Google Scholar]
- 16.Anderson TJ, Gregoire J, Pearson GJ, Barry AR, Couture P, Dawes M, et al. 2016 Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32(11):1263–82. 10.1016/j.cjca.2016.07.510. [DOI] [PubMed] [Google Scholar]
- 17.Jackson M The stress of life: a modern complaint? Lancet. 2014;383(9914):300–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338(3):171–9. 10.1056/nejm199801153380307. [DOI] [PubMed] [Google Scholar]
- 19.Brotman DJ, Golden SH, Wittstein IS. The cardiovascular toll of stress. Lancet. 2007;370(9592):1089–100. 10.1016/S0140-6736(07)61305-1. [DOI] [PubMed] [Google Scholar]
- 20.Yeager MP, Pioli PA, Guyre PM. Cortisol exerts biphasic regulation of inflammation in humans. Dose- Response. 2011;9(3):332–47. 10.2203/dose-response.10-013.Yeager. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tawakol A, Ishai A, Takx RA, Figueroa AL, Ali A, Kaiser Y, et al. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet. 2017;389(10071):834–45. 10.1016/s0140-6736(16)31714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The results of this multi-system 18F-FDG-PET/CT study provide novel mechanistic insights into the pathway linking stress to subsequent CVD. Increased metabolic activity of amygdala, a key neural center involved in the perception of stress, was found to be an independent predictor of CVD events via a pathway that involves increased bone marrow activity (an index of leukopoiesis) and arterial inflammation.
- 22.Lagraauw HM, Kuiper J, Bot I. Acute and chronic psychological stress as risk factors for cardiovascular disease: insights gained from epidemiological, clinical and experimental studies. Brain Behav Immun. 2015;50:18–30. 10.1016/j.bbi.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Wang SS, Yan XB, Hofman MA, Swaab DF, Zhou JN. Increased expression level of corticotropin-releasing hormone in the amygdala and in the hypothalamus in rats exposed to chronic unpredictable mild stress. Neurosci Bull. 2010;26(4):297–303. 10.1007/s12264-010-0329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8(7):2517–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, et al. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med. 2005;35(6):791–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox AS, Oler JA, Shelton SE, Nanda SA, Davidson RJ, Roseboom PH, et al. Central amygdala nucleus (Ce) gene expression linked to increased trait-like Ce metabolism and anxious temperament in young primates. Proc Natl Acad Sci U S A. 2012;109(44):18108–13. 10.1073/pnas.1206723109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Y, Du R, Zhu Y, Shen Y, Zhang K, Chen Y, et al. PET mapping of neurofunctional changes in a posttraumatic stress disorder model. J Nucl Med. 2016;57(9):1474–7. 10.2967/jnumed.116.173443. [DOI] [PubMed] [Google Scholar]
- 28.Osborne MT, Ishai A, Hammad B, Tung B, Wang Y, Baruch A, et al. Amygdalar activity predicts future incident diabetes independently of adiposity. Psychoneuroendocrinology. 2018;100:32–40. 10.1016/j.psyneuen.2018.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This manuscript reports that heightened amygdalar metabolic activity measured on 18F-FDG-PET/CT is associated with increased risk of incident type II diabetes independent of adiposity and other diabetes risk factors. Further analysis also demonstrated a synergistic relationship between amygdalar activity and adiposity to augment the risk of new subsequent diabetes.
- 29.Goyal A, Dey AK, Chaturvedi A, Elnabawi YA, Aberra TM, Chung JH, et al. Chronic stress-related neural activity associates with subclinical cardiovascular disease in psoriasis: a prospective cohort study. JACC Cardiovasc Imaging. 2018. 10.1016/j.jcmg.2018.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang JL, Chiou CW, Ting CT, Chen YT, Chen SA. Sudden changes in heart rate variability during the 1999 Taiwan earthquake. Am J Cardiol. 2001;87(2):245–8 A9. [DOI] [PubMed] [Google Scholar]
- 31.Matsuo T, Suzuki S, Kodama K, Kario K. Hemostatic activation and cardiac events after the 1995 Hanshin-Awaji earthquake. Int J Hematol. 1998;67(2):123–9. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe H, Kodama M, Tanabe N, Nakamura Y, Nagai T, Sato M, et al. Impact of earthquakes on risk for pulmonary embolism. Int J Cardiol. 2008;129(1):152–4. 10.1016/j.ijcard.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 33.Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352(6):539–48. 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 34.Hatzaras IS, Bible JE, Koullias GJ, Tranquilli M, Singh M, Elefteriades JA. Role of exertion or emotion as inciting events for acute aortic dissection. Am J Cardiol. 2007;100(9):1470–2. 10.1016/j.amjcard.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 35.Dimsdale JE, Moss J. Short-term catecholamine response to psychological stress. Psychosom Med. 1980;42(5):493–7. [DOI] [PubMed] [Google Scholar]
- 36.Carroll D, Ginty AT, Painter RC, Roseboom TJ, Phillips AC, de Rooij SR. Systolic blood pressure reactions to acute stress are associated with future hypertension status in the Dutch Famine Birth Cohort Study. Int J Psychophysiol. 2012;85(2):270–3. 10.1016/j.ijpsycho.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Deanfield JE, Shea M, Kensett M, Horlock P, Wilson RA, de Landsheere CM, et al. Silent myocardial ischaemia due to mental stress. Lancet. 1984;2(8410):1001–5. [DOI] [PubMed] [Google Scholar]
- 38.Burg MM, Jain D, Soufer R, Kerns RD, Zaret BL. Role of behavioral and psychological factors in mental stress-induced silent left ventricular dysfunction in coronary artery disease. J Am Coll Cardiol. 1993;22(2):440–8. [DOI] [PubMed] [Google Scholar]
- 39.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99(16):2192–217. [DOI] [PubMed] [Google Scholar]
- 40.Arrighi JA, Burg M, Cohen IS, Kao AH, Pfau S, Caulin-Glaser T, et al. Myocardial blood-flow response during mental stress in patients with coronary artery disease. Lancet. 2000;356(9226):310–1. 10.1016/s0140-6736(00)02510-1. [DOI] [PubMed] [Google Scholar]
- 41.Jiang W, Babyak M, Krantz DS, Waugh RA, Coleman RE, Hanson MM, et al. Mental stress—induced myocardial ischemia and cardiac events. JAMA. 1996;275(21):1651–6. [DOI] [PubMed] [Google Scholar]
- 42.Steinberg JS, Arshad A, Kowalski M, Kukar A, Suma V, Vloka M, et al. Increased incidence of life-threatening ventricular arrhythmias in implantable defibrillator patients after the World Trade Center attack. J Am Coll Cardiol. 2004;44(6):1261–4. 10.1016/j.jacc.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 43.Lee S, Colditz GA, Berkman LF, Kawachi I. Caregiving and risk of coronary heart disease in U.S. women: a prospective study. Am J Prev Med. 2003;24(2):113–9. [DOI] [PubMed] [Google Scholar]
- 44.Johansen C, Feychting M, Moller M, Arnsbo P, Ahlbom A, Olsen JH. Risk of severe cardiac arrhythmia in male utility workers: a nationwide Danish cohort study. Am J Epidemiol. 2002;156(9):857–61. [DOI] [PubMed] [Google Scholar]
- 45.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33(13):1635–701. 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 46.Rudisch B, Nemeroff CB. Epidemiology of comorbid coronary artery disease and depression. Biol Psychiatry. 2003;54(3):227–40. [DOI] [PubMed] [Google Scholar]
- 47.Carney RM, Freedland KE. Depression, mortality, and medical morbidity in patients with coronary heart disease. Biol Psychiatry. 2003;54(3):241–7. [DOI] [PubMed] [Google Scholar]
- 48.Rugulies R Depression as a predictor for coronary heart disease. A review and meta-analysis. Am J Prev Med. 2002;23(1):51–61. [DOI] [PubMed] [Google Scholar]
- 49.Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27(23):2763–74. 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- 50.Meijer A, Conradi HJ, Bos EH, Anselmino M, Carney RM, Denollet J, et al. Adjusted prognostic association of depression following myocardial infarction with mortality and cardiovascular events: individual patient data meta-analysis. Br J Psychiatry. 2013;203(2):90–102. 10.1192/bjp.bp.112.111195. [DOI] [PubMed] [Google Scholar]
- 51.Jiang W, Alexander J, Christopher E, Kuchibhatla M, Gaulden LH, Cuffe MS, et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med. 2001;161(15):1849–56. [DOI] [PubMed] [Google Scholar]
- 52.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290(2):215–21. 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gottlieb SS, Kop WJ, Ellis SJ, Binkley P, Howlett J, O’Connor C, et al. Relation of depression to severity of illness in heart failure (from Heart Failure And a Controlled Trial Investigating Outcomes of Exercise Training [HF-ACTION]). Am J Cardiol. 2009;103(9):1285–9. 10.1016/j.amjcard.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kubzansky LD, Kawachi I, Weiss ST, Sparrow D. Anxiety and coronary heart disease: a synthesis of epidemiological, psychological, and experimental evidence. Ann Behav Med. 1998;20(2):47–58. 10.1007/bf02884448. [DOI] [PubMed] [Google Scholar]
- 55.Albert CM, Chae CU, Rexrode KM, Manson JE, Kawachi I. Phobic anxiety and risk of coronary heart disease and sudden cardiac death among women. Circulation. 2005;111(4):480–7. 10.1161/01.Cir.0000153813.64165.5d. [DOI] [PubMed] [Google Scholar]
- 56.Roest AM, Martens EJ, de Jonge P, Denollet J. Anxiety and risk of incident coronary heart disease: a meta-analysis. J Am Coll Cardiol. 2010;56(1):38–46. 10.1016/j.jacc.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 57.Janszky I, Ahnve S, Lundberg I, Hemmingsson T. Early-onset depression, anxiety, and risk of subsequent coronary heart disease: 37-year follow-up of 49,321 young Swedish men. J Am Coll Cardiol. 2010;56(1):31–7. 10.1016/j.jacc.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 58.Nabi H, Hall M, Koskenvuo M, Singh-Manoux A, Oksanen T, Suominen S, et al. Psychological and somatic symptoms of anxiety and risk of coronary heart disease: the health and social support prospective cohort study. Biol Psychiatry. 2010;67(4):378–85. 10.1016/j.biopsych.2009.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kang HK, Bullman TA, Taylor JW. Risk of selected cardiovascular diseases and posttraumatic stress disorder among former World War II prisoners of war. Ann Epidemiol. 2006;16(5):381–6. 10.1016/j.annepidem.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 60.Kubzansky LD, Koenen KC, Jones C, Eaton WW. A prospective study of posttraumatic stress disorder symptoms and coronary heart disease in women. Health Psychol. 2009;28(1):125–30. 10.1037/0278-6133.28.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stansfeld SA, Fuhrer R, Shipley MJ, Marmot MG. Psychological distress as a risk factor for coronary heart disease in the Whitehall II study. Int J Epidemiol. 2002;31(1):248–55. [DOI] [PubMed] [Google Scholar]
- 62.Hemingway H, Marmot M. Evidence based cardiology: psychosocial factors in the aetiology and prognosis of coronary heart disease. Systematic review of prospective cohort studies. Bmj. 1999;318(7196):1460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tofler GH, Stone PH, Maclure M, Edelman E, Davis VG, Robertson T, et al. Analysis of possible triggers of acute myocardial infarction (the MILIS study). Am J Cardiol. 1990;66(1):22–7. [DOI] [PubMed] [Google Scholar]
- 64.Booth J, Connelly L, Lawrence M, Chalmers C, Joice S, Becker C, et al. Evidence of perceived psychosocial stress as a risk factor for stroke in adults: a meta-analysis. BMC Neurol. 2015;15:233 10.1186/s12883-015-0456-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pan A, Sun Q, Okereke OI, Rexrode KM, Hu FB. Depression and risk of stroke morbidity and mortality: a meta-analysis and systematic review. Jama. 2011;306(11):1241–9. 10.1001/jama.2011.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Remch M, Laskaris Z, Flory J, Mora-McLaughlin C, Morabia A. Post-traumatic stress disorder and cardiovascular diseases: a cohort study of men and women involved in cleaning the debris of the World Trade Center Complex. Circ Cardiovasc Qual Outcomes. 2018;11(7):e004572 10.1161/circoutcomes.117.004572. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This observational prospective cohort study reports an independent association between PTSD and CVD events in first responders who participated in cleaning of the debris of the World Trade Center. There was a higher incidence of MI or stroke during 4-year follow-up among these workers.
- 67.Wilbert-Lampen U, Leistner D, Greven S, Pohl T, Sper S, Volker C, et al. Cardiovascular events during World Cup soccer. N Engl J Med. 2008;358(5):475–83. 10.1056/NEJMoa0707427. [DOI] [PubMed] [Google Scholar]
- 68.Kario K, Matsuo T, Kobayashi H, Yamamoto K, Shimada K. Earthquake-induced potentiation of acute risk factors in hypertensive elderly patients: possible triggering of cardiovascular events after a major earthquake. J Am Coll Cardiol. 1997;29(5):926–33. [DOI] [PubMed] [Google Scholar]
- 69.Yamabe H, Hanaoka J, Funakoshi T, Iwahashi M, Takeuchi M, Saito K, et al. Deep negative T waves and abnormal cardiac sympathetic image (123I-MIBG) after the Great Hanshin Earthquake of 1995. Am J Med Sci. 1996;311(5):221–4. [DOI] [PubMed] [Google Scholar]
- 70.Lahtinen M, Kiviniemi AM, Junttila MJ, Kaariainen M, Huikuri HV, Tulppo MP. Depressive symptoms and risk for sudden cardiac death in stable coronary artery disease. Am J Cardiol. 2018;122(5):749–55. 10.1016/j.amjcard.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 71.Kivimaki M, Nyberg ST, Batty GD, Kawachi I, Jokela M, Alfredsson L, et al. Long working hours as a risk factor for atrial fibrillation: a multi-cohort study. Eur Heart J. 2017;38(34):2621–8. 10.1093/eurheartj/ehx324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fransson EI, Nordin M, Magnusson Hanson LL, Westerlund H. Job strain and atrial fibrillation—results from the Swedish Longitudinal Occupational Survey of Health and meta-analysis of three studies. Eur J Prev Cardiol. 2018;25(11):1142–9. 10.1177/2047487318777387. [DOI] [PubMed] [Google Scholar]
- 73.Endrighi R, Waters AJ, Gottlieb SS, Harris KM, Wawrzyniak AJ, Bekkouche NS, et al. Psychological stress and short-term hospitalisations or death in patients with heart failure. Heart. 2016;102(22):1820–5. 10.1136/heartjnl-2015-309154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ogilvie RP, Everson-Rose SA, Longstreth WT Jr, Rodriguez CJ, Diez-Roux AV, Lutsey PL. Psychosocial factors and risk of incident heart failure: the multiethnic study of atherosclerosis. Circ Heart Fail. 2016;9(1):e002243 10.1161/circheartfailure.115.002243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adelborg K, Schmidt M, Sundboll J, Pedersen L, Videbech P, Botker HE et al. Mortality risk among heart failure patients with depression: a nationwide population-based cohort study. J Am Heart Assoc. 2016;5(9). doi: 10.1161/jaha.116.004137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rumsfeld JS, Havranek E, Masoudi FA, Peterson ED, Jones P, Tooley JF, et al. Depressive symptoms are the strongest predictors of short-term declines in health status in patients with heart failure. J Am Coll Cardiol. 2003;42(10):1811–7. [DOI] [PubMed] [Google Scholar]
- 77.Markovitz JH, Matthews KA, Whooley M, Lewis CE, Greenlund KJ. Increases in job strain are associated with incident hypertension in the CARDIA study. Ann Behav Med. 2004;28(1):4–9. 10.1207/s15324796abm2801_2. [DOI] [PubMed] [Google Scholar]
- 78.Guimont C, Brisson C, Dagenais GR, Milot A, Vezina M, Masse B, et al. Effects of job strain on blood pressure: a prospective study of male and female white-collar workers. Am J Public Health. 2006;96(8):1436–43. 10.2105/ajph.2004.057679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brondolo E, Rieppi R, Kelly KP, Gerin W. Perceived racism and blood pressure: a review of the literature and conceptual and methodological critique. Ann Behav Med. 2003;25(1):55–65. 10.1207/s15324796abm2501_08. [DOI] [PubMed] [Google Scholar]
- 80.Kibler JL, Joshi K, Ma M. Hypertension in relation to posttraumatic stress disorder and depression in the US National Comorbidity Survey. Behav Med. 2009;34(4):125–32. 10.3200/bmed.34.4.125-132. [DOI] [PubMed] [Google Scholar]
- 81.Merrill Thomas DMB, Krishna K, Patel KG, Smolderen K. Mental health concerns in patients presenting with new or an exacerbation of peripheral arterial disease symptoms: insights from the international portrait registry. J Am Coll Cardiol 71(11). doi: 10.1016/S0735-1097(18)32581-6. [DOI] [Google Scholar]
- 82.Aquarius AE, De Vries J, Henegouwen DP, Hamming JF. Clinical indicators and psychosocial aspects in peripheral arterial disease. Arch Surg. 2006;141(2):161–6; discussion 6. 10.1001/archsurg.141.2.161. [DOI] [PubMed] [Google Scholar]
- 83.Lallukka T, Lahelma E, Rahkonen O, Roos E, Laaksonen E, Martikainen P, et al. Associations of job strain and working overtime with adverse health behaviors and obesity: evidence from the Whitehall II Study, Helsinki Health Study, and the Japanese Civil Servants Study. Soc Sci Med. 2008;66(8):1681–98. 10.1016/j.socscimed.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 84.Hamer M, Molloy GJ, Stamatakis E. Psychological distress as a risk factor for cardiovascular events: pathophysiological and behavioral mechanisms. J Am Coll Cardiol. 2008;52(25):2156–62. 10.1016/j.jacc.2008.08.057. [DOI] [PubMed] [Google Scholar]
- 85.Isasi CR, Parrinello CM, Jung MM, Carnethon MR, Birnbaum-Weitzman O, Espinoza RA, et al. Psychosocial stress is associated with obesity and diet quality in Hispanic/Latino adults. Ann Epidemiol. 2015;25(2):84–9. 10.1016/j.annepidem.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kumari M, Head J, Marmot M. Prospective study of social and other risk factors for incidence of type 2 diabetes in the Whitehall II study. Arch Intern Med. 2004;164(17):1873–80. 10.1001/archinte.164.17.1873. [DOI] [PubMed] [Google Scholar]
- 87.Stewart-Knox B,E Duffy M, Bunting B, Parr H, Vas de Almeida MD, Gibney M Associations between obesity (BMI and waist circumference) and socio-demographic factors, physical activity, dietary habits, life events, resilience, mood, perceived stress and hopelessness in healthy older Europeans. BMC Public Health. 2012;12:424 10.1186/1471-2458-12-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aschbacher K, Kornfeld S, Picard M, Puterman E, Havel PJ, Stanhope K, et al. Chronic stress increases vulnerability to diet-related abdominal fat, oxidative stress, and metabolic risk. Psychoneuroendocrinology. 2014;46:14–22. 10.1016/j.psyneuen.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mooy JM, de Vries H, Grootenhuis PA, Bouter LM, Heine RJ. Major stressful life events in relation to prevalence of undetected type 2 diabetes: the Hoorn study. Diabetes Care. 2000;23(2):197–201. [DOI] [PubMed] [Google Scholar]
- 90.McCanlies EC, Araia SK, Joseph PN, Mnatsakanova A, Andrew ME, Burchfiel CM, et al. C-reactive protein, interleukin-6, and posttraumatic stress disorder symptomology in urban police officers. Cytokine. 2011;55(1):74–8. 10.1016/j.cyto.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 91.Lampert R, Tuit K, Hong KI, Donovan T, Lee F, Sinha R. Cumulative stress and autonomic dysregulation in a community sample. Stress. 2016;19(3):269–79. 10.1080/10253890.2016.1174847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Girod JP, Brotman DJ. Does altered glucocorticoid homeostasis increase cardiovascular risk? Cardiovasc Res. 2004;64(2):217–26. 10.1016/j.cardiores.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 93.Huang QH, Takaki A, Arimura A. Central noradrenergic system modulates plasma interleukin-6 production by peripheral interleukin-1. Am J Phys. 1997;273(2 Pt 2):R731–8. 10.1152/ajpregu.1997.273.2.R731. [DOI] [PubMed] [Google Scholar]
- 94.Goebel MU, Mills PJ, Irwin MR, Ziegler MG. Interleukin-6 and tumor necrosis factor-alpha production after acute psychological stress, exercise, and infused isoproterenol: differential effects and pathways. Psychosom Med. 2000;62(4):591–8. [DOI] [PubMed] [Google Scholar]
- 95.Mangos GJ, Walker BR, Kelly JJ, Lawson JA, Webb DJ, Whitworth JA. Cortisol inhibits cholinergic vasodilation in the human forearm. Am J Hypertens. 2000;13(11):1155–60. [DOI] [PubMed] [Google Scholar]
- 96.Eisenach JH, Clark ES, Charkoudian N, Dinenno FA, Atkinson JL, Fealey RD, et al. Effects of chronic sympathectomy on vascular function in the human forearm. J Appl Physiol (1985). 2002;92(5):2019–25. 10.1152/japplphysiol.01025.2001. [DOI] [PubMed] [Google Scholar]
- 97.Singh P, Emami H, Subramanian S, Maurovich-Horvat P, Marincheva-Savcheva G, Medina HM et al. Coronary plaque morphology and the anti-inflammatory impact of atorvastatin: a multicenter 18F-fluorodeoxyglucose positron emission tomographic/computed tomographic study. Circ Cardiovasc Imaging. 2016;9(12). doi: 10.1161/circimaging.115.004195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12)):1119–31. 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]; •• In this randomized trial, comparing canakinumab with placebo among patients with a history of myocardial infarction and elevated high-sensitivity C-reactive protein, it was shown that direct modulation of an inflammatory pathway (i.e., IL-1β blockade) was associated with reduced adverse cardiovascular events independent of lipid reduction. This study reiterates the role of inflammation in cardiovascular diseases.
- 99.Snyder-Mackler N, Sanz J, Kohn JN, Brinkworth JF, Morrow S, Shaver AO, et al. Social status alters immune regulation and response to infection in macaques. Science. 2016;354(6315):1041–5. 10.1126/science.aah3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20(7):754–8. 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Razzoli M, Nyuyki-Dufe K, Gurney A, Erickson C, McCallum J, Spielman N et al. Social stress shortens lifespan in mice. Aging Cell. 2018:e12778. doi: 10.1111/acel.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Muscatell KA, Dedovic K, Slavich GM, Jarcho MR, Breen EC, Bower JE, et al. Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain Behav Immun. 2015;43:46–53. 10.1016/j.bbi.2014.06.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gray TS, Bingaman EW. The amygdala: corticotropin-releasing factor, steroids, and stress. Crit Rev Neurobiol. 1996;10(2):155–68. [DOI] [PubMed] [Google Scholar]
- 104.Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24(24):5506–15. 10.1523/jneurosci.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schaefer SM, Abercrombie HC, Lindgren KA, Larson CL, Ward RT, Oakes TR, et al. Six-month test-retest reliability of MRI-defined PET measures of regional cerebral glucose metabolic rate in selected subcortical structures. Hum Brain Mapp. 2000;10(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacol Biochem Behav. 2002;71(3):431–47. [DOI] [PubMed] [Google Scholar]
- 107.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487(7407):325–9. 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nezafati MH, Eshraghi A, Vojdanparast M, Abtahi S, Nezafati P. Selective serotonin reuptake inhibitors and cardiovascular events: a systematic review. J Res Med Sci. 2016;21:66 10.4103/1735-1995.189647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim Y, Lee YS, Kim MG, Song YK, Kim Y, Jang H, et al. The effect of selective serotonin reuptake inhibitors on major adverse cardiovascular events: a meta-analysis of randomized-controlled studies in depression. Int Clin Psychopharmacol. 2018. 10.1097/YIC.0000000000000238. [DOI] [PubMed] [Google Scholar]; • This meta-analysis of ten randomized controlled trials showed that the use of selective serotonin reuptake inhibitors in patients with depression and prior cardiovascular events significantly decreased the risk of future myocardial infarctions and major adverse cardiovascular events overall.
- 110.Nahrendorf M, Swirski FK. Lifestyle effects on hematopoiesis and atherosclerosis. Circ Res. 2015;116(5):884–94. 10.1161/circresaha.116.303550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–81. 10.1016/s0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tawakol A, Fayad ZA, Mogg R, Alon A, Klimas MT, Dansky H, et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol. 2013;62(10):909–17. 10.1016/j.jacc.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 113.Tan MP, Morgan K. Psychological interventions in cardiovascular disease: an update. Curr Opin Psychiatry. 2015;28(5):371–7. 10.1097/yco.0000000000000181. [DOI] [PubMed] [Google Scholar]
- 114.Levine GN, Lange RA, Bairey-Merz CN, Davidson RJ, Jamerson K, Mehta PK et al. Meditation and cardio-vascular risk reduction: a scientific statement from the American Heart Association. J Am Heart Assoc. 2017;6(10). doi: 10.1161/jaha.117.002218. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This consensus statement from the American Heart Association describes the existing evidence for meditation in the prevention of cardiovascular disease. It concludes that meditation may serve as a potentially effective adjunct to guideline-directed therapy for CVD risk reduction and prevention and recommends design and implementation of further longitudinal prospective studies to investigate the effect of meditation on cardiovascular disease.
- 115.Chu P, Pandya A, Salomon JA, Goldie SJ, Hunink MG. Comparative effectiveness of personalized life-style management strategies for cardiovascular disease risk reduction. J Am Heart Assoc. 2016;5(3):e002737 10.1161/jaha.115.002737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bhasin MK, Denninger JW, Huffman JC, Joseph MG, Niles H, Chad-Friedman E, et al. Specific transcriptome changes associated with blood pressure reduction in hypertensive patients after relaxation response training. J Altern Complement Med. 2018;24(5):486–504. 10.1089/acm.2017.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]; • In this single-arm prospective trial, authors provided the first insights into the molecular mechanisms underlying the beneficial effects of the relaxation response (RR) on hypertension. It was shown that the RR-induced reduction of blood pressure was associated with differential expression of genes in a select set of biological pathways.
- 117.Blumenthal JA, Sherwood A, Smith PJ, Watkins L, Mabe S, Kraus WE, et al. Enhancing cardiac rehabilitation with stress management training: a randomized, Clinical Efficacy Trial. Circulation. 2016;133(14):1341–50. 10.1161/CIRCULATIONAHA.115.018926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Holzel BK, Carmody J, Evans KC, Hoge EA, Dusek JA, Morgan L, et al. Stress reduction correlates with structural changes in the amygdala. Soc Cogn Affect Neurosci. 2010;5(1):11–7. 10.1093/scan/nsp034. [DOI] [PMC free article] [PubMed] [Google Scholar]