Abstract
Purpose:
Assess systemic vascular endothelial growth factor-A (VEGF) levels after treatment with intravitreous aflibercept, bevacizumab or ranibizumab.
Design:
Comparative-effectiveness trial with participants randomly assigned to 2-mg aflibercept, 1.25-mg bevacizumab, or 0.3-mg ranibizumab following a retreatment algorithm.
Participants:
Participants with available plasma samples (N=436)
Methods:
Plasma samples were collected before injections at baseline, 4-week, 52-week and 104-week visits. In a pre-planned secondary analysis, systemic free-VEGF levels from an ELISA immunoassay were compared across anti-VEGF agents and correlated with systemic side effects.
Main Outcome Measures:
Changes in the natural log (ln) of plasma VEGF levels.
Results:
Baseline free-VEGF levels were similar across all 3 groups. At 4 weeks, mean ln(VEGF) changes were −0.30±0.61, −0.31±0.54, −0.02±0.44 pg/ml for the aflibercept, bevacizumab, and ranibizumab groups, respectively. The adjusted differences between treatment groups (adjusted CI; P-value) were −0.01 (−0.12, +0.10; P=0.89), −0.31 (−0.44, −0.18; P<0.001), and −0.30 (−0.43, −0.18; P<0.001) for aflibercept-bevacizumab, aflibercept-ranibizumab, and bevacizumab-ranibizumab, respectively. At 52 weeks, a difference in mean VEGF changes between bevacizumab and ranibizumab persisted (−0.23 [−0.38,−0.09]; P<0.001); the difference between aflibercept and ranibizumab was −0.12 (P=0.07) and between aflibercept and bevacizumab was +0.11 (P=0.07). Treatment group differences at 2 years were similar to 1 year. No apparent treatment differences were detected at 52 or 104 weeks in the cohort of participants not receiving injections within 1 or 2 months before plasma collection. Participants with (N=9) and without (N=251) a heart attack or stroke had VEGF levels that appeared similar.
Conclusions:
These data suggest that decreases in plasma free-VEGF levels are greater after treatment with aflibercept or bevacizumab compared with ranibizumab at 4 weeks. At 52 and 104 weeks, a greater decrease was observed in bevacizumab versus ranibizumab. Results from two subgroups of participants who did not receive injections within at least 1 month and 2 months before collection, suggest similar changes in VEGF levels after injections stop. It is unknown whether VEGF levels return to normal as the drug is cleared from the system, or whether the presence of the drug affects the assay’s ability to accurately measure free-VEGF. No significant associations between VEGF concentration and systemic factors were noted.
Introduction
Reported systemic side effects of intravenous bevacizumab, when used to treat certain cancers, include hypertension, proteinuria, and cardiovascular and gastrointestinal complications.1 One possible explanation for these events is the lowering of systemic VEGF levels following intravenous anti-vascular endothelial growth factor-A (anti-VEGF) treatment. Intravitreous injections of anti-VEGF agents are standard care for many patients around the world with choroidal or retinal neovascularization or macular edema from a variety of conditions including age-related macular degeneration, diabetic retinopathy, or retinal vascular occlusions. Low systemic drug concentrations of anti-VEGF have been detected after intravitreous injections.2–4 Therefore, there have been concerns that systemic drug levels after intravitreous injections, and their potential impact on systemic VEGF levels, could be associated with an increased risk of systemic side effects; although to date, there is no definitive evidence for an increased risk.5
The Diabetic Retinopathy Clinical Research Network (DRCR.net) performed a comparative effectiveness trial of the three anti-VEGF agents commonly used to treat diabetic macular edema (Protocol T). As part of a pre-planned ancillary study, plasma samples were collected to assess the effects of intravitreous injections of 3 separate anti-VEGF agents on systemic free-VEGF levels and the correlation of free-VEGF levels to systemic side effects.
Methods
Study Overview
The study protocol is available at www.drcr.net (date accessed: 9 June 2017). The study adhered to the tenets of the Declaration of Helsinki, and was approved by multiple institutional review boards (IRB). Written informed consent was obtained prior to enrollment. Participants (N = 660) in Protocol T were randomly assigned 1:1:1 to treatment groups receiving 0.05-mL injections of either: 2.0-mg intravitreous aflibercept, 1.25-mg intravitreous bevacizumab, or 0.3-mg intravitreous ranibizumab. All participants were at least 18 years old, had Type 1 or 2 diabetes mellitus, and had one study eye with a best corrected visual-acuity letter score of 78 through 24 (approximate Snellen equivalent, 20/32 to 20/320) and central-involved diabetic macular edema (CI-DME) on optical coherence tomography (OCT). Follow-up visits were scheduled every 4 weeks during the first year. In the second year of follow-up, protocol visits could be extended to 8 or 16 weeks depending on the course of treatment. Retreatment with the anti-VEGF was assessed at each visit based on protocol-specified criteria, using visual acuity and OCT measured central subfield thickness (CST). Fellow eyes were treated with the same agent as the study eye. The occurrence of any adverse event was recorded prospectively throughout the follow-up period. At the baseline, 4, 52, and 104-week visits, after the participant had been sitting for 10 minutes, 3 sets of systolic and diastolic blood pressure measurements were recorded using an automated blood pressure monitor (OMRON BP 10 Series). Urine samples were collected at the baseline and 52-week visits to measure albumin-creatinine ratio (ACR).
Plasma Sample Collection and Analyses
During enrollment Protocol T participants were offered the opportunity to participate in this plasma VEGF ancillary study. Written informed consent was obtained prior to obtaining a blood sample. At the baseline, 4, 52, and 104-week visits, plasma samples were collected prior to any injection being given. Whole blood was collected in a citrate-theophylline, adenosine, dipyridamole (CTAD) tube with a solution mixture of sodium citrate, theophylline, adenosine and dipyridamole added as an anticoagulant to minimize in-vitro platelet activation. Centrifuge was used to extract the plasma. Samples were either shipped on dry ice on collection day (N = 975), immediately frozen at −80°C (N = 309) and shipped on dry ice at a later date, or shipped within 24 hours on dry ice after being either immediately frozen at −20°C (N = 265) or stored at 4°C (N = 56). All samples were shipped to the Fred Hutchinson Cancer Research Center where the samples were stored at −80°C. The Human VEGF Quantikine® ELISA immunoassay (R&D Systems, Inc., Minneapolis, MN, USA) was used to measure concentration of free-VEGF in the plasma. To minimize inter-assay variations, baseline, 4-week and 52-week samples (N = 1292) were analyzed concurrently. Unused portions of the baseline samples were separated into approximately 350μL aliquots and re-frozen at −80°C. One aliquot of each re-frozen baseline sample was analyzed concurrently with the 104-week samples (N = 313). Therefore, the 104-week samples are compared with a different measure of the baseline sample than the 4-week and 52-week samples. To evaluate the impact of the additional freeze-thaw cycle on the baseline samples, a cohort of 104-week samples (N = 34) were randomly selected to undergo an additional freeze-thaw cycle. The selected samples were then compared with the 104-week samples with only one freeze-thaw cycle.
Two duplicate free-VEGF readings were obtained from each sample and were extrapolated from the standard curve to acquire the concentration measurements. The lower limit of detection was 7.8 pg/ml. Forty-two values (3%) measuring less than 7.8 pg/ml were set to 7.8 pg/ml for analyses. The average of the two measurements and the coefficient of variation (CV) were calculated. For the main analyses, samples with a coefficient of variation ≥20% were excluded.
Statistical Methods
The free-VEGF concentration was converted to the natural log scale (ln) in order to stabilize the variance and normalize the data for regression analysis.6 The values for the mean change from baseline were truncated at 3 standard deviations from the mean, to minimize the impact of extreme outliers (N=16). Pairwise treatment group comparisons of the mean change in ln(VEGF) were conducted at each visit using analysis of covariance (ANCOVA) with adjustment for baseline ln(VEGF). Sensitivity analyses were repeated separately without data truncation on ln(VEGF) changes (N=16), excluding samples with CV ≥15% (N=61), excluding hemolyzed samples (N=48), using the measured concentration value even if below 7.8 pg/ml (N=40), or including samples regardless of whether the CV was ≥ 20% (N=16).
All P-values and confidence intervals are 2-sided. The Hochberg method was implemented for multiple treatment group comparisons to control the overall Type I error probability at 5%. SAS version 9.4 (©SAS Institute, Inc.) was used for all statistical analyses.
Results
A total of 1,605 plasma samples were collected in the ancillary study, among which only 43 samples (2.7%) were considered by the lab to have unacceptable quality or to have a CV ≥20% (eTable 1). Fourteen samples collected at the follow-up visits without baseline samples were excluded from analysis. Baseline characteristics appeared similar between participants who participated in the plasma study and who did not, although slightly more individuals with type 1 diabetes did not participate (eTable 2). Among participants with 52-week plasma samples, the median baseline free-VEGF levels were 25.00, 26.90, and 27.00 pg/ml for the aflibercept (N = 132), bevacizumab (N = 115), and ranibizumab (N = 130) groups respectively.
Change in Plasma VEGF concentrations
At 4 weeks the mean change in ln(VEGF) levels for the aflibercept, bevacizumab, and ranibizumab groups, respectively, were −0.30 ± 0.61, −0.31 ± 0.54, and −0.02 ± 0.44 pg/ml (adjusted difference [adjusted CI] for aflibercept-bevacizumab= −0.01 [−0.12 to +0.10], P=0.89; for aflibercept-ranibizumab= −0.31 [−0.44 to −0.18], P<0.001; for bevacizumab-ranibizumab= −0.30 [−0.43 to −0.18, P<0.001) (Table 1, Figure 1A). At 52 weeks the mean changes in ln(VEGF) levels for the aflibercept, bevacizumab, and ranibizumab groups, respectively, were −0.10 ± 0.49, −0.24 ± 0.60, and −0.03 ± 0.53. (Table 1, Figure 1B). The adjusted treatment group differences [adjusted CI] were: for aflibercept-bevacizumab +0.11 [−0.01 to +0.23], P=0.07; for aflibercept-ranibizumab −0.12 [−0.24 to −0.01], P=0.07; for bevacizumab-ranibizumab −0.23 [−0.38 to −0.09], P<0.001. At the 104-week visit, treatment group differences in mean changes in ln(VEGF) were similar to 52-week visit (Table 1, Figure 1C). Similar findings were observed from all sensitivity analyses (eTable 3). Although there were several different methods for storing plasma prior to shipment, the method of storage did not impact the treatment group differences for ln(VEGF) (P=0.61 at 4 weeks, 0.15 at 52 weeks, and 0.14 at 104 weeks for the interaction). To assess for potential impact of missing data, eTable 4, eTable 5, and Figure 2 show the changes in free-VEGF concentration levels for participants who had samples available for all study visits.
Table 1.
Change in Plasma VEGF Concentrations (pg/ml) from Baseline by Visit and Treatment Assignment
| Observed Data | Treatment Group
Comparisons: Differences in Mean Log Change Adjusted CI and Adjusted P-value b |
|||||
|---|---|---|---|---|---|---|
| Aflibercept | Bevacizumab | Ranibizumab | Aflibercept vs Bevacizumab |
Aflibercept vs Ranibizumab |
Bevacizumab vs Ranibizumab |
|
| Baseline (in 4-week cohort), N a | 139 | 130 | 141 | |||
| Mean ± SD | 28.50 ± 13.26 | 31.45 ± 22.38 | 37.55 ± 70.43 | |||
| Median (Q1, Q3) | 25.50 (19.90, 33.00) | 27.05 (19.20, 37.00) | 26.10 (19.40, 34.30) | |||
| 4 weeks | ||||||
| Mean ± SD | 23.44 ± 21.90 | 24.06 ± 24.51 | 30.43 ± 18.46 | |||
| Median (Q1, Q3) | 18.50 (13.30, 27.60) | 19.05 (13.90, 25.10) | 25.40 (19.30, 34.80) | |||
| Change at 4 weeks | ||||||
| Mean ± SD | −5.06 ± 24.10 | −7.39 ± 29.97 | −7.12 ± 70.40 | |||
| Median (Q1, Q3) | −7.10 (−17.40, +1.70) | −5.55 (−13.40, −0.90) | +0.20 (−5.00, +4.90) | |||
| Mean ± SD (ln) c | −0.30 ± 0.61 | −0.31 ± 0.54 | −0.02 ± 0.44 | −0.01 (−0.12, +0.10) P=0.89 |
−0.31 (−0.44, −0.18) P<0.001 |
−0.30 (−0.43, −0.18) P<0.001 |
| Median (Q1, Q3) (ln) c | −0.35 (−0.74, +0.06) | −0.28 (−0.54, −0.04) | +0.01 (−0.20, +0.21) | |||
| Baseline (in 52-week cohort), N a | 132 | 115 | 130 | |||
| Mean ± SD | 28.10 ± 13.44 | 31.69 ± 23.59 | 33.47 ± 26.13 | |||
| Median (Q1, Q3) | 25.00 (19.85, 34.00) | 26.90 (18.80, 37.00) | 27.00 (20.10, 35.80) | |||
| 52 weeks | ||||||
| Mean ± SD | 26.40 ± 16.82 | 27.05 ± 35.36 | 34.89 ± 56.30 | |||
| Median (Q1, Q3) | 22.60 (16.40, 31.05) | 20.10 (13.70, 29.10) | 26.45 (19.90, 33.90) | |||
| Change at 52 weeks | ||||||
| Mean ± SD | −1.70 ± 14.05 | −4.63 ± 39.39 | +1.43 ± 55.56 | |||
| Median (Q1, Q3) | −3.10 (−9.55, +7.10) | −3.90 (−11.60, +2.90) | +0.20 (−5.20, +3.80) | |||
| Mean ± SD (ln) c | −0.10 ± 0.49 | −0.24 ± 0.60 | −0.03 ± 0.53 | +0.11 (−0.01, +0.23) P=0.07 |
−0.12 (−0.24, −0.01) P=0.07 |
−0.23 (−0.38, −0.09) P<0.001 |
| Median (Q1, Q3) (ln) c | −0.13 (−0.41, +0.25) | −0.17 (−0.61, +0.15) | +0.01 (−0.21, +0.16) | |||
| Baseline (in 104-week cohort), N a | 98 | 84 | 78 | |||
| Mean ± SD | 24.61 ± 12.11 | 31.26 ± 31.33 | 32.10 ± 27.03 | |||
| Median (Q1, Q3) | 21.20 (17.05, 31.01) | 23.23 (17.39, 34.17) | 24.40 (17.04, 33.31) | |||
| 104 weeks | ||||||
| Mean ± SD | 24.51 ± 16.81 | 22.57 ± 19.58 | 38.61 ± 74.94 | |||
| Median (Q1, Q3) | 19.65 (15.45, 27.65) | 18.29 (12.63, 26.19) | 23.77 (18.20, 28.27) | |||
| Change at 104 weeks | ||||||
| Mean ± SD | −0.11 ± 15.90 | −8.69 ± 36.99 | +6.51 ± 77.27 | |||
| Median (Q1, Q3) | −0.50 (−8.07, +4.80) | −4.35 (−13.19, +3.91) | +0.14 (−9.54, +5.45) | |||
| Mean ± SD (ln) c | −0.04 ± 0.50 | −0.27 ± 0.72 | −0.03 ± 0.61 | +0.15 (−0.01, +0.31) P=0.07 |
−0.11 (−0.25, +0.03) P=0.13 |
−0.26 (−0.44, −0.08) P=0.002 |
| Median (Q1, Q3) (ln) c | −0.02 (−0.35, +0.25) | −0.20 (−0.58, +0.22) | +0.01 (−0.35, +0.24) | |||
The analyses only include samples that had acceptable quality with a CV less than 20% at both baseline and the corresponding protocol visit. All VEGF concentrations below 7.8 pg/ml were truncated at 7.8 pg/ml: 17 samples were truncated in 4-week cohort (5 at baseline and 12 at 4 weeks), 13 samples were truncated in 52-week cohort (5 at baseline, 1 both, and 7 at 52 weeks), and 10 samples were truncated in 104-week cohort (5 at baseline and 5 at 104 weeks).
The change in ln(VEGF) concentration was the difference between baseline level and follow-up level (both on natural logarithm scale), and was truncated at 3 standard deviations from the mean at [−1.97, +1.66] pg/ml. A total of 16 values were truncated: 5 at 4 weeks (1 in aflibercept, 2 in bevacizumab and, 2 in ranibizumab); 5 at 52 weeks (3 in bevacizumab and 2 in ranibizumab); and 6 at 104 weeks (3 in bevacizumab and 3 in ranibizumab).
The pairwise treatment group comparisons of the change in ln(VEGF) concentration were performed using ANCOVA, with the adjustment for baseline VEGF concentration (natural log scale). Reported P-values have been adjusted for multiple treatment-group comparisons to account for an overall type I error rate of 0.05, and corresponding [1-(ɑ÷i)]×100% confidence intervals are reported, where i is the rank (1, 2, 3) of the Hochberg-adjusted P-value from among the descending ordered raw pairwise P-values.
Figure 1. Change in Plasma ln(VEGF) Concentrations (pg/ml) by Treatment Group Assignment and Visit.
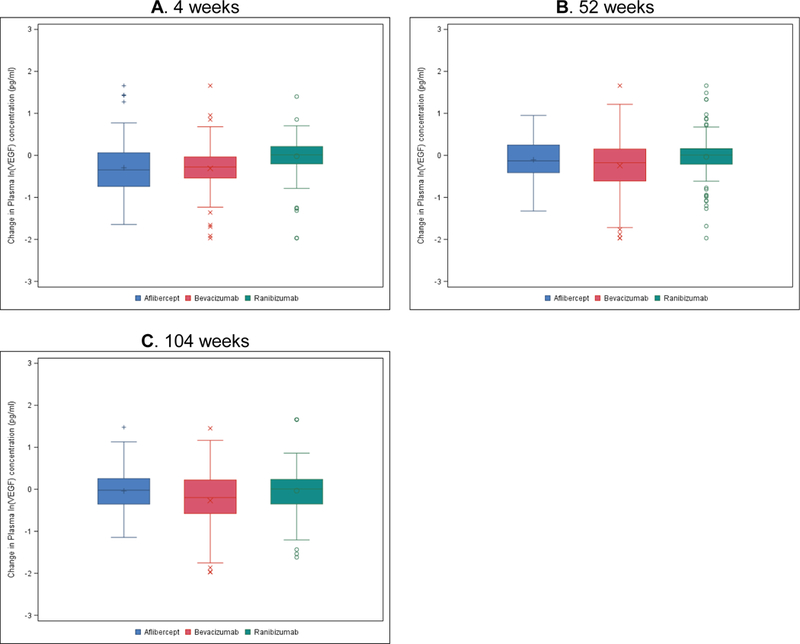
A) All participants at 4 weeks. N=139/ 130/ 141 for aflibercept/ bevacizumab/ ranibizumab group. P=0.89/ <0.001/ <0.001 for aflibercept vs. bevacizumab/ aflibercept vs. ranibizumab/ bevacizumab vs. ranibizumab. B) All participants at 52 weeks. N=132/ 115/ 130 for aflibercept/ bevacizumab/ ranibizumab group. P=0.07/ 0.07/ <0.001 for aflibercept vs. bevacizumab/ aflibercept vs. ranibizumab/ bevacizumab vs. ranibizumab. C) All participants at 104 weeks. N=98/ 84/ 78 for aflibercept/ bevacizumab/ ranibizumab group. P=0.07/ 0.13/ 0.002 for aflibercept vs. bevacizumab/ aflibercept vs. ranibizumab/ bevacizumab vs. ranibizumab.
Figure 2. Mean Change in Plasma VEGF Concentrations (pg/ml) by Treatment Group Assignment in Participants with Complete Data through 52 and 104 Weeks.
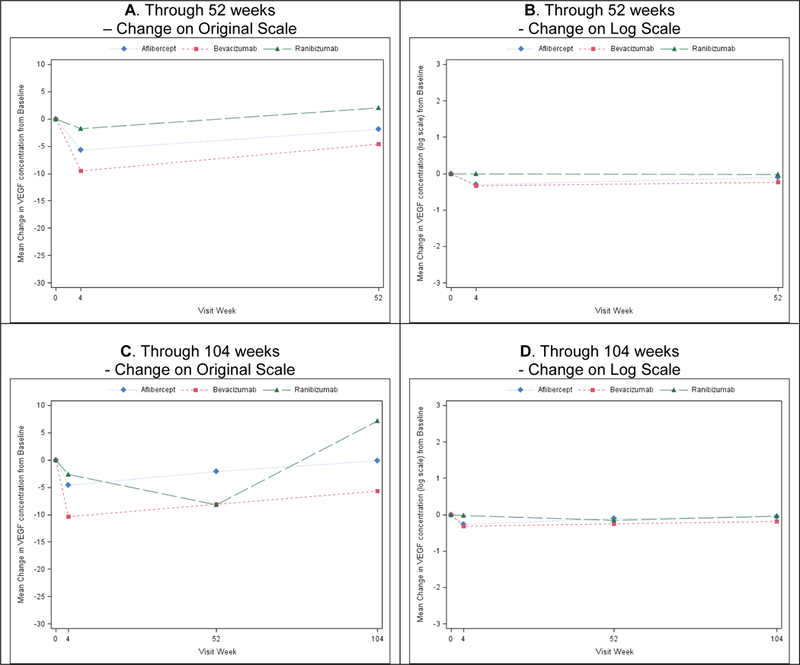
A) Through 52 weeks on original scale. B) Through 52 weeks on log scale. N=122/ 111/ 121 for aflibercept/ bevacizumab/ ranibizumab group. Pairwise comparisons for aflibercept vs. bevacizumab/ aflibercept vs. ranibizumab/ bevacizumab vs. ranibizumab: P=0.88/ <0.0001/ <0.0001 at 4 weeks; and P=0.11/ 0.09 / 0.001 at 52 weeks. C) Through 104 weeks on original scale. D) Through 104 weeks on log scale. N=87/ 71/ 71 for aflibercept/ bevacizumab/ ranibizumab group. Pairwise comparisons for aflibercept vs. bevacizumab/ aflibercept vs. ranibizumab/ bevacizumab vs. ranibizumab: P=0.90/ <0.0001/ <0.0001 at 4 weeks; P=0.35/ 0.69 / 0.28 at 52 weeks; and P=0.23/ 0.23 / 0.03 at 104 weeks.
Relationships between change in VEGF concentrations and study drug injections
The minimum number of days between an injection in either eye and the 52-week plasma collection was 7; the maximum was 283 (median 35 days). There were 228 (60%) participants who did not have an injection within 1 month (30 days) of the 52-week visit and 135 (36%) who did not have an injection within 2 months (56 days) of the 52-week visit. Table 2 and eTable 6 include the distribution of timing between the annual visit and the last injection prior to that visit. No treatment group differences were observed for the mean changes in ln(VEGF) levels at 52 weeks among participants who did not receive injections within 1 month prior to the 52-week visit (N = 228): +0.03 ± 0.44 in the aflibercept group, −0.07 ± 0.52 in the bevacizumab group, −0.04 ± 0.55 in the ranibizumab group (P=0.78/ 0.95/ 0.78 for aflibercept-bevacizumab/ aflibercept-ranibizumab/ bevacizumab-ranibizumab, respectively) (Table 2, Figure 3). Similarly no treatment group differences were observed at 52 weeks among participants who did not receive injections within 2 months prior to the visit (N = 135): +0.08 ± 0.42 in the aflibercept group, −0.06 ± 0.48 in the bevacizumab group, +0.05 ± 0.54 in the ranibizumab group (P=0.43/ 0.79/ 0.44 for aflibercept-bevacizumab/ aflibercept-ranibizumab/ bevacizumab-ranibizumab, respectively) (Table 2, Figure 3). Participants who received an aflibercept or bevacizumab injection within 1 or 2 months before the 52-week visit showed a significantly greater mean decrease in ln(VEGF) than participants who received ranibizumab (Table 2, Figure 3); 104-week results were similar (eTable 6, eFigure 1). The adjusted mean change (decrease) in ln(VEGF) in the group of participants who did not receive an injection within 2 months of the 52-week visit was significantly smaller than in the group who did (−0.01 vs. −0.20, estimated difference = 0.21 [95% CI, 0.11 to 0.31], P<0.001).
Table 2.
Change in Plasma VEGF Concentrations (pg/ml) at 52 Weeks from Baseline by Treatment Assignment and Injection Status
| Observed Data | Treatment Group
Comparisons: Differences in Mean Log Change Adjusted CI and Adjusted P-value b |
|||||
|---|---|---|---|---|---|---|
| Aflibercept | Bevacizumab | Ranibizumab | Aflibercept vs Bevacizumab | Aflibercept vs Ranibizumab | Bevacizumab vs Ranibizumab | |
| VEGF concentration available at both baseline and 52-week visits, N a | 132 | 115 | 130 | |||
| Days between last injection and 52-week plasma collection, Median (Q1, Q3) | 34 (28, 68) | 35 (28, 90) | 35 (28, 101) | |||
| Within 1 month prior to 52-week plasma collection | ||||||
| No injections, N (%) | 76 (58%) | 70 (61%) | 82 (63%) | |||
| Baseline | ||||||
| Mean ± SD | 27.49 ± 13.54 | 29.97 ± 16.50 | 33.66 ± 28.22 | |||
| Median (Q1, Q3) | 24.95 (19.90, 32.60) | 27.65 (20.10, 34.80) | 26.70 (20.80, 36.80) | |||
| 52 weeks | ||||||
| Mean ± SD | 28.60 ± 17.78 | 32.44 ± 43.78 | 37.22 ± 69.32 | |||
| Median (Q1, Q3) | 24.30 (19.35, 31.70) | 23.50 (16.10, 33.60) | 26.45 (20.20, 32.30) | |||
| Change at 52 weeks | ||||||
| Mean ± SD | +1.11 ± 13.40 | +2.47 ± 42.55 | +3.55 ± 68.15 | |||
| Median (Q1, Q3) | +0.35 (−6.40, +8.60) | −1.00 (−6.50, +5.40) | +0.55 (−4.80, +3.90) | |||
| Mean ± SD (ln) c | +0.03 ± 0.44 | −0.07 ± 0.52 | −0.04 ± 0.55 | +0.07 (−0.10, +0.24) P=0.78 |
+0.00 (−0.14, +0.15) P=0.95 |
−0.06 (−0.23, +0.10) P=0.78 |
| Median (Q1, Q3) (ln) c | +0.02 (−0.27, +0.33) | −0.05 (−0.28, +0.23) | +0.03 (−0.16, +0.17) | |||
| Received injections, N (%) | 56 (42%) | 45 (39%) | 48 (37%) | |||
| Baseline | ||||||
| Mean ± SD | 28.94 ± 13.38 | 34.36 ± 31.67 | 33.14 ± 22.41 | |||
| Median (Q1, Q3) | 25.15 (19.45, 34.60) | 26.40 (18.60, 38.50) | 27.10 (19.35, 34.20) | |||
| 52 weeks | ||||||
| Mean ± SD | 23.43 ± 15.08 | 18.68 ± 10.79 | 30.93 ± 19.78 | |||
| Median (Q1, Q3) | 18.85 (12.90, 30.50) | 15.00 (11.40, 22.50) | 26.55 (18.35, 33.95) | |||
| Change at 52 weeks | ||||||
| Mean ± SD | −5.52 ± 14.14 | −15.68 ± 31.22 | −2.21 ± 21.14 | |||
| Median (Q1, Q3) | −6.90 (−12.95, +0.20) | −8.80 (−22.80, −2.70) | −0.90 (−6.50, +3.35) | |||
| Mean ± SD (ln) c | −0.28 ± 0.50 | −0.51 ± 0.63 | −0.03 ± 0.48 | +0.20 (+0.02, +0.38) P=0.03 |
−0.29 (−0.49, −0.09) P=0.003 |
−0.49 (−0.72, −0.26) P<0.001 |
| Median (Q1, Q3) (ln) c | −0.28 (−0.61, +0.02) | −0.42 (−0.89, −0.12) | −0.03 (−0.26, +0.12) | |||
| Within 2 month prior to 52-week plasma collection | ||||||
| No injections, N (%) | 43 (33%) | 42 (37%) | 50 (38%) | |||
| Baseline | ||||||
| Mean ± SD | 28.57 ± 15.53 | 29.45 ± 17.84 | 30.25 ± 16.00 | |||
| Median (Q1, Q3) | 23.20 (19.90, 34.10) | 25.35 (18.00, 32.40) | 26.35 (20.60, 37.80) | |||
| 52 weeks | ||||||
| Mean ± SD | 31.78 ± 21.14 | 28.03 ± 18.95 | 43.53 ± 88.27 | |||
| Median (Q1, Q3) | 26.30 (21.70, 35.10) | 23.50 (16.90, 32.90) | 26.50 (19.00, 30.40) | |||
| Change at 52 weeks | ||||||
| Mean ± SD | +3.21 ± 14.05 | −1.42 ± 21.61 | +13.27 ± 79.91 | |||
| Median (Q1, Q3) | +4.40 (−5.20, 10.20) | +0.65 (−4.90, +5.40) | +1.20 (−3.20, +3.80) | |||
| Mean ± SD (ln) c | +0.09 ± 0.42 | −0.06 ± 0.48 | +0.05 ± 0.54 | +0.15 (−0.09, +0.39) P=0.43 |
+0.03 (−0.16, +0.22) P=0.79 |
−0.12 (−0.33, +0.10) P=0.44 |
| Median (Q1, Q3) (ln) c | +0.15 (−0.18, +0.38) | +0.02 (−0.19, +0.21) | +0.05 (−0.13, +0.17) | |||
| Received injections, N (%) | 89 (67%) | 73 (63%) | 80 (62%) | |||
| Baseline | ||||||
| Mean ± SD | 27.88 ± 12.39 | 32.98 ± 26.37 | 35.48 ± 30.76 | |||
| Median (Q1, Q3) | 25.10 (19.80, 33.90) | 27.60 (19.20, 38.50) | 27.15 (19.95, 34.65) | |||
| 52 weeks | ||||||
| Mean ± SD | 23.81 ± 13.68 | 26.49 ± 42.13 | 29.50 ± 16.29 | |||
| Median (Q1, Q3) | 20.70 (14.60, 29.60) | 18.10 (12.60, 26.30) | 26.35 (19.90, 34.10) | |||
| Change at 52 weeks | ||||||
| Mean ± SD | −4.07 ± 13.50 | −6.48 ± 46.70 | −5.98 ± 30.58 | |||
| Median (Q1, Q3) | −4.60 (−10.60, +0.90) | −6.50 (−13.70, 0.00) | −0.65 (−7.70, +3.70) | |||
| Mean ± SD (ln) c | −0.20 ± 0.50 | −0.35 ± 0.65 | −0.09 ± 0.51 | +0.11 (−0.04, +0.25) P=0.15 |
−0.19 (−0.35, −0.03) P=0.02 |
−0.30 (−0.48, −0.12) P<0.001 |
| Median (Q1, Q3) (ln) c | −0.22 (−0.47, +0.05) | −0.29 (−0.68, 0.00) | −0.02 (−0.31, +0.13) | |||
All VEGF concentration values below 7.8 pg/ml were truncated at 7.8 pg/ml. The analyses include only participants whose samples had acceptable quality and a CV less than 20% at both baseline and 52 weeks.
The change in ln(VEGF) concentration was the difference between baseline level and follow-up level (both on natural logarithm scale), and was truncated at 3 standard deviations from the mean at [−1.97, +1.66] pg/ml.
The pairwise treatment group comparisons of the change in ln(VEGF) concentration were performed using ANCOVA, with the adjustment for baseline VEGF concentration (on natural log scale). Reported P-values have been adjusted for multiple treatment-group comparisons to account for an overall type I error rate of 0.05, and corresponding [1-(ɑ÷i)]×100% confidence intervals are reported, where i is the rank (1, 2, 3) of the Hochberg-adjusted P-value from among the descending ordered raw pairwise P-values.
Figure 3. Change in Plasma ln(VEGF) Concentrations (pg/ml) at 52 weeks by Treatment Group Assignment and Injection Status.
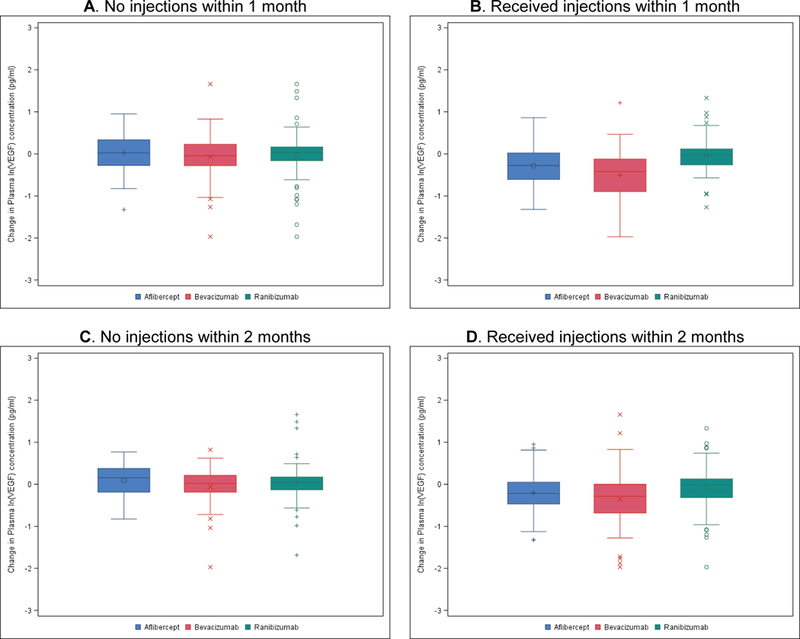
A) No injections within 1 month. N=76/ 70/ 82 for aflibercept/ bevacizumab/ ranibizumab group. P=0.78/ 0.95/ 0.78 for aflibercept vs. bevacizumab/ aflibercept vs. ranibizumab/ bevacizumab vs. ranibizumab. B) Received injections within 1 month. N=56/ 45/ 48 for aflibercept/ bevacizumab/ ranibizumab group. P=0.03/ 0.003/ <0.001 for aflibercept vs. bevacizumab/ aflibercept vs. ranibizumab/ bevacizumab vs. ranibizumab. C) No injections within 2 months. N=43/ 42/ 50 for aflibercept/ bevacizumab/ ranibizumab group. P=0.43/ 0.79/ 0.44 for aflibercept vs. bevacizumab/ aflibercept vs. ranibizumab/ bevacizumab vs. ranibizumab. D) Received injections within 2 months. N=89/ 73/ 80 for aflibercept/ bevacizumab/ ranibizumab group. P=0.15/ 0.02/ <0.001 for aflibercept vs. bevacizumab/aflibercept vs. ranibizumab/ bevacizumab vs. ranibizumab.
Sensitivity analyses performed for all above subgroups suggested similar conclusions to the analyses limiting cohort by injection status within 1 and 2 months prior to either annual visit (data not shown).
Association between VEGF concentrations and systemic factors
Among the total 436 participants in the plasma ancillary study, 6 participants died from potential vascular or unknown causes prior to 52 weeks, and 7 after 52 weeks. Anti-Platelet Trialists’ Collaboration (APTC) events of either non-fatal stroke or non-fatal myocardial infarction occurred in 2% of participants (N=7) prior to 52 weeks and 3% over the course of 2 years (N=9). The ln(VEGF) concentrations at baseline and annual visits are shown in eFigure 2, stratified by the occurrence of non-fatal stroke, non-fatal myocardial infarction, or no APTC event. In(VEGF) values in the group of participants who had an APTC event were similar to participants without an event.
There were no significant associations between mean arterial pressures and In(VEGF) at each study visit (eTable 7, eFigure 3); or between the changes in mean arterial pressure and In(VEGF) (eFigure 4). Approximately one-third of participants in each treatment group had microalbuminuria at baseline, and another 30% of the participants in each group had macroalbuminuria (eTable 8). Average ln(VEGF) levels were similar within albumin-creatinine ratio subgroups at baseline (P=0.83) and 52 weeks (P=0.09) (eFigure 5). Similarly, there were no associations identified between changes in albumin-creatinine ratio and changes in ln(VEGF) levels (eFigure 6).
Freeze-thaw experiment and comparison of baseline measurements
The mean ln(VEGF) concentration for the baseline measurements (N = 263) that had one freeze-thaw cycle was larger (3.31 ± 0.53) compared with mean of the second set, which had two freeze-thaw cycles (3.18 ± 0.55) (P<0.001). The mean difference between the matched pairs was −0.14 ± 0.19 with 95% limits of agreement of [−0.51, +0.24] (eFigure 7). In a sensitivity analysis of the 104-week data comparing mean changes of ln(VEGF), results were similar regardless of which baseline measurement was used (eTable 3). Furthermore, in the freeze-thaw experiment from the 104-week samples, no differences were observed for the mean ln(VEGF) levels between measurements taken after one freeze-thaw cycle (N = 34) and after two cycles (P=0.80). The 95% limits of agreement were [−0.60, +0.57] (eFigure 8).
Discussion
The administration of an anti-VEGF medication into the vitreous results in small but measurable systemic levels of the drug.2–4 It remains uncertain whether this systemic exposure after administration of intravitreous anti-VEGF agents results in systemic complications.5 To our knowledge, studies to date suggest that there is no adverse definitive association of reduced systemic VEGF levels after intravitreous anti-VEGF injection and systemic complications. In fact, the only evidence to date is from one trial which actually suggested that lower VEGF levels in patients treated with intravitreous anti-VEGF injections were associated with a reduced risk for APTC events.7 Even in retinopathy of prematurity, where it is hypothesized that patients may be at particularly high risk for complications, there is no clear relationship.8
Prior studies suggest that the clearance of ranibizumab from the blood is more rapid than aflibercept or bevacizumab.2–4 Evidence suggests that after an intravitreous ranibizumab injection, this monoclonal antibody fragment is largely cleared from the blood within a week, compared with the monoclonal antibody bevacizumab or fusion protein aflibercept, which persist longer.5 Thus, the systemic exposure to ranibizumab appears to be less than aflibercept or bevacizumab. Previous studies have shown a decrease in serum or plasma VEGF levels after intravitreous injection of anti-VEGF agents.9–15 Although there is little evidence linking plasma or serum VEGF levels after an intravitreous injection with complications including APTC events,7 the current study was designed to assess whether administration of the 3 different anti-VEGF medications for DME resulted in differences in plasma VEGF levels.
The median plasma VEGF levels at baseline were similar in all 3 treatment groups and within the normal expected range.4 At 4 weeks, the decrease in VEGF levels was larger with aflibercept and bevacizumab compared with ranibizumab; no statistically significant differences between aflibercept and bevacizumab were identified. At the 52- and 104-week visit, ln(VEGF) decrease in levels for bevacizumab, but not aflibercept, remained, statistically, significantly greater than those noted with ranibizumab.
We are uncertain whether VEGF concentrations can be measured reliably in the presence of a competing VEGF binding agent (i.e. aflibercept, bevacizumab, or ranibizumab), or whether the impact of the anti-VEGF agents with different binding affinities will alter the assay results differently for the three agents. In an attempt to eliminate any effect that the presence of anti-VEGF drug in the plasma might have on the assay used to measure VEGF, the DRCR.net investigators pre-specified that the results should be presented both for the entire cohort (as above) and for patients who did not receive an injection for at least 1 or 2 months prior to the annual visit. Based on prior work, the investigators hypothesized that ranibizumab would be cleared from the blood at 1 month, whereas the other 2 drugs might be present. It was not certain whether any remaining levels of aflibercept or bevacizumab at these time points would be high enough to have a measurable effect on the assay. Ultimately at the 52 and 104-week visits, there were no treatment group differences identified among the cohort of participants who did not receive an injection within 1 or 2 months of plasma collection, but among the cohort of participants who received an injection, there were significant differences between treatment in each group.
It is unknown whether low systemic VEGF levels in the blood result in an increased risk of systemic complications; or if there is an effect, what threshold is needed to have clinical relevance. To our knowledge, the relationship between VEGF blood levels and VEGF tissue levels is uncertain and even the effect of tissue levels is unknown. This trial was not designed to assess the relationship between APTC events and VEGF levels. VEGF levels were not obtained at the time of the APTC events, impacting the ability to assess relationship. Based on the information collected, there are no data from this trial to suggest that patients with lower VEGF blood levels were at an increased risk of complications. However, given the small numbers of events, even if a relationship exists, it would have been difficult to detect. We did not detect any associations between VEGF levels and blood pressure or albuminuria (eFigure 3–6).
There are shortcomings in the current analysis. There was a slightly smaller number of samples available at 104 weeks than at other visits. We have no way to know what the VEGF levels were between the 4 time points of collection. In addition, for the participants who died during the study, we do not have a measurement near the time of death. Another limitation is the wide range between time of last injection and time of data collection at the annual visits. Moreover, this study was not powered to examine the relationship between plasma VEGF and systemic events. Finally, although a sensitivity analysis shows little impact, the variability in site shipping processes also might have impacted the results.
In summary, for the cohort as a whole at 4 weeks, decreases in the plasma free-VEGF levels were significantly greater in the aflibercept and bevacizumab groups compared with the ranibizumab group. At the 52- and 104-week visits in the overall cohort the statistically significant difference only persisted for ranibizumab compared with bevacizumab.. Analysis of participants at the 52- and 104-week visits who had not received intravitreous injections within 1 or 2 months prior to the visit revealed no differences in the change in VEGF levels among the 3 treatment groups. Significant differences between treatment groups were observed among the smaller cohort of participants who had received injections in this time period where decrease in VEGF level with ranibizumab was less than with aflibercept or bevacizumab. We are uncertain whether the presence of anti-VEGF drug in the blood affects the plasma free-VEGF measurements and accounts for some or all of the differences identified between changes in VEGF levels. The investigators believe that the clinical importance of these findings remains unknown.
Supplementary Material
Acknowledgments
Financial Support: Supported through a cooperative agreement from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland (grants: EY14231, EY23207, and EY18817). Regeneron Pharmaceutical provided the afiibercept and Genentech provided the ranibizumab for the study. Genentech also provided funding for the plasma collection from this ancillary study. As per the DRCR.net Industry Collaboration Guidelines (available at www.drcr.net), the DRCR.net had complete control over the design of the protocol, ownership of the data, and all editorial content of presentations and publications related to the protocol. The funding organization (National Institutes of Health) participated in oversight of the conduct of the study and review of the manuscript but not directly in the conduct of the study, nor in the collection, management, or analysis of the data. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Financial Disclosures: A complete list of all DRCR.net investigator financial disclosures can be found at www.drcr.net
References
- 1.Ranpura V, Hapani S, Chuang J, et al. Risk of cardiac ischemia and arterial thromboembolic events with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis of randomized controlled trials. Acta Oncol 2010;49:287–97. [DOI] [PubMed] [Google Scholar]
- 2.Avery RL, Castellarin AA, Steinle NC, et al. Systemic Pharmacokinetics and Pharmacodynamics of Intravitreal Aflibercept, Bevacizumab, and Ranibizumab. Retina 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kong L, Bhatt AR, Demny AB, et al. Pharmacokinetics of bevacizumab and its effects on serum VEGF and IGF-1 in infants with retinopathy of prematurity. Invest Ophthalmol Vis Sci 2015;56:956–61. [DOI] [PubMed] [Google Scholar]
- 4.Avery RL, Castellarin AA, Steinle NC, et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol 2014;98:1636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avery RL, Gordon GM. Systemic Safety of Prolonged Monthly Anti-Vascular Endothelial Growth Factor Therapy for Diabetic Macular Edema: A Systematic Review and Meta-analysis. JAMA Ophthalmol 2016;134:21–9. [DOI] [PubMed] [Google Scholar]
- 6.Igarashi T The rationale for using logarithmic transformation of concentration data in toxicokinetic studies. J Toxicol Sci 1995;20:67–72. [DOI] [PubMed] [Google Scholar]
- 7.Rogers C, Scott L, Reeves B, et al. Serum vascular endothelial growth factor levels in the IVAN Trial; Relationships with drug, dosing, and systemic serious adverse events. Ophthal Retina 2017;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.VanderVeen DK, Melia M, Yang MB, et al. Anti-Vascular Endothelial Growth Factor Therapy for Primary Treatment of Type 1 Retinopathy of Prematurity: A Report by the American Academy of Ophthalmology. Ophthalmology 2017;124:619–33. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Sawada T, Sawada O, et al. Serum and plasma vascular endothelial growth factor concentrations before and after intravitreal injection of aflibercept or ranibizumab for age-related macular degeneration. Am J Ophthalmol 2014;158:738–44 e1. [DOI] [PubMed] [Google Scholar]
- 10.Zehetner C, Kirchmair R, Huber S, et al. Plasma levels of vascular endothelial growth factor before and after intravitreal injection of bevacizumab, ranibizumab and pegaptanib in patients with age-related macular degeneration, and in patients with diabetic macular oedema. Br J Ophthalmol 2013;97:454–9. [DOI] [PubMed] [Google Scholar]
- 11.Wu WC, Lien R, Liao PJ, et al. Serum levels of vascular endothelial growth factor and related factors after intravitreous bevacizumab injection for retinopathy of prematurity. JAMA Ophthalmol 2015;133:391–7. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Yao Z, Kaila N, et al. Pharmacokinetics of ranibizumab after intravitreal administration in patients with retinal vein occlusion or diabetic macular edema. Ophthalmology 2014;121:2237–46. [DOI] [PubMed] [Google Scholar]
- 13.Bakri SJ, Snyder MR, Reid JM, et al. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology 2007;114:855–9. [DOI] [PubMed] [Google Scholar]
- 14.Bakri SJ, Snyder MR, Reid JM, et al. Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology 2007;114:2179–82. [DOI] [PubMed] [Google Scholar]
- 15.Investigators IS, Chakravarthy U, Harding SP, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology 2012;119:1399–411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


