Abstract
Introduction
Wilson disease (WD) is characterized by excessive intracellular copper accumulation in liver and brain due to defective copper biliary excretion. With highly varied phenotypes and a lack of biomarkers for the different clinical manifestations, diagnosis and treatment can be difficult.
Objective
The aim of the present study was to analyze serum metabolomics profiles of patients with Wilson disease compared to healthy subjects, with the goal of identifying differentially abundant metabolites as potential biomarkers for this condition.
Methods
Hydrophilic interaction liquid chromatography-quadrupole time of flight mass spectrometry was used to evaluate the untargeted serum metabolome of 61 patients with WD (26 hepatic and 25 neurologic subtypes, 10 preclinical) compared to 15 healthy subjects. We conducted analysis of covariance with potential confounders (body mass index, age, sex) as covariates and partial least-squares analysis.
Results
After adjusting for clinical covariates and multiple testing, we identified 99 significantly different metabolites (FDR < 0.05) between WD and healthy subjects. Subtype comparisons also revealed significantly different metabolites compared to healthy subjects: WD hepatic subtype (67), WD neurologic subtype (57), WD hepatic-neurologic combined (77), and preclinical (36). Pathway analysis revealed these metabolites are involved in amino acid metabolism, the tricarboxylic acid cycle, choline metabolism, and oxidative stress.
Conclusions
Patients with WD are characterized by a distinct metabolomics profile providing new insights into WD pathogenesis and identifying new potential diagnostic biomarkers.
Keywords: copper, metabolomics, biomarkers, phenotype
1. Introduction
Wilson disease (WD) is an autosomal recessive disorder caused by mutations in the copper-transporting, P-type ATPase gene, ATP7B (Bull et al., 1993). Disease-causing mutations lead to impaired intracellular copper trafficking and biliary excretion, deficient ceruloplasmin maturation, and consequent copper accumulation mainly in the liver and brain (Czlonkowska et al., 2018). Clinical manifestations of WD are widely variable and include hepatic, neurologic, and psychiatric signs and symptoms. Hepatic manifestations range from mild (elevated liver enzymes) to severe (acute liver failure or cirrhosis). The neurologic signs and symptoms include Parkinson-like tremors, dysarthria, and ataxia (Ala et al., 2007).
Numerous factors appear to influence the WD phenotype. Although many different ATP7B mutations have been identified, linking which variants specifically affect enzyme catalytic and transport activity has proven difficult (Huster et al., 2012) Epigenetic, environment, age, and sex-related factors may also influence the WD phenotype (Ferenci et al., 2018; Kieffer and Medici, 2017; Medici et al., 2016; Medici et al., 2014; Medici et al., 2013) further complicating diagnosis. Currently, patients with WD are identified by a combination of clinical and laboratory findings, including low ceruloplasmin, increased urinary copper excretion, elevated hepatic copper levels, and the presence of copper deposits in the cornea (Kayser–Fleischer rings); however, these tests are neither highly specific (Cabras et al., 2015) nor considered a gold standard for diagnostic purposes. Screening for ATP7B mutations is helpful though not routinely used in clinical practice due to confusion generated by the high number of potential disease-causing mutations. Also, a negative screening does not exclude a WD diagnosis. Due to these limitations, identifying new WD biomarkers is needed to improve diagnostic tools. High-throughput techniques used to identify biomarkers, such as metabolomic profiling, can deepen our understanding of disease pathogenesis and may eventually lead to new therapeutic targets (Aliasgharpour, 2015).
A small number of in vivo (Lee et al., 2011; Simpson et al., 2004; Wilmarth et al., 2012; Xu et al., 2015) and in vitro (Roelofsen et al., 2004) studies have utilized high-throughput techniques to study copper accumulation. One study used comparative proteome analyses to study asymptomatic and early-stage patients with WD; the results revealed increased serum levels of oxidative stress and inflammation-related proteins in WD (Park et al., 2009).
In the present study, untargeted metabolomics analyses were performed to characterize serum profiles of patients with hepatic, neurologic, or preclinical WD compared to healthy subjects, with the goal of identifying key metabolites and potential diagnostic biomarkers involved in the WD pathogenesis.
2. Subjects and Methods
Serum samples from patients with WD and healthy subjects were obtained from the Institute of Neurology and Psychiatry in Warsaw (Table 1). Subjects fasted for 8 hours prior to sampling. Whole venous blood was collected in 3 × 7.5 ml plastic Vacutainer tubes and allowed to clot for 45–60 min. Blood samples were centrifuged for 10 minutes at 1500 × g (4C), and serum aliquoted and stored at −80C until shipped. Serum samples were de-identified, shipped to the University of California, Davis and stored at −80°C until further analysis. Hemolytic samples were excluded. Informed written consent was obtained from each patient and healthy subject, and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Institutional Review Board at the University of California, Davis. Patients with WD were recruited at diagnosis [determined by Leipzig criteria (Ferenci et al., 2003)] and hence were not on any anti-copper treatment. According to the clinician’s experience, patients were categorized by hepatic or neurologic presentation and grouped as “symptomatic.” A subgroup of asymptomatic patients was diagnosed based on a family member’s diagnosis and defined as “preclinical.”
Table 1.
Patient characteristics
| Healthy (n=15) |
Wilson Disease (n=61) |
p-value | |||
|---|---|---|---|---|---|
| Males (n=5) |
Females (n=10) |
Malesa (n=31) |
Females (n=30) |
||
| Age (years), mean ± SD |
34.4 ± 11.4 | 37.7 ± 10.0 | 35.7 ± 10.5 | 32.3 ± 13.2 | 0.5926b 0.2408c |
| BMI (kg/m2), mean ± SD |
26.6 ± 3.9 | 22.0 ± 3.0 | 26.4 ± 6.2 | 25.0 ± 3.3 | 0.9351b 0.0139c |
| Hepatic phenotype, n (% total WD) |
N/A | N/A | 12 (20.0%) | 14 (23.3%) | N/A |
| Neurologic phenotype, n (% total WD) |
N/A | N/A | 13 (21.7%) | 9 (15.0%) | N/A |
| Preclinical, n (% total WD) |
N/A | N/A | 5 (8.3%) | 7 (11.7%) | N/A |
Phenotype not available for one patient.
males
females.
BMI, body mass index.
Untargeted metabolomics profiling was performed at the UC Davis West Coast Metabolomics Center by hydrophilic interaction liquid chromatography-quadrupole time of flight mass spectrometry (HILIC-QTOF MS).
For data analysis methods, see Supplementary Data.
3. Results
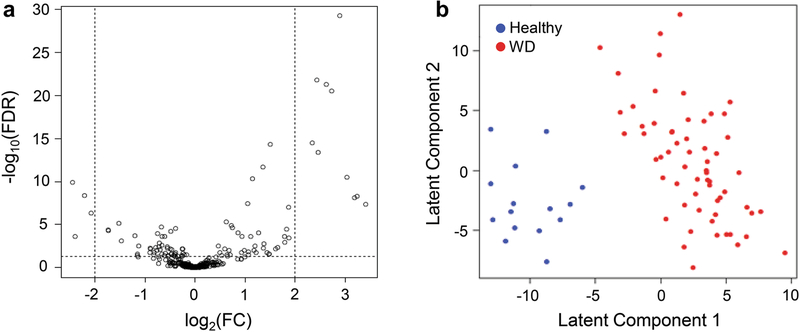
A total of 363 distinct metabolites were identified between 15 healthy and 61 patients with WD. After adjusting for clinical covariates and multiple testing, 99 metabolites (including 36 annotated) were found to be significantly different in WD compared to healthy subjects with false discovery rate (FDR) <0.05 (Fig. 1a). A partial least-squares regression with linear discriminant analysis (PLS-LDA) was able to separate WD patients from healthy controls based on their metabolomics profiles (Fig. 1b), with 97.37% classification accuracy using only the first latent component for the covariate-adjusted analysis and 100% using the first three latent components for the covariate-unadjusted analysis.
Fig. 1.
Metabolomics profiling analyses for healthy subjects vs. WD patients
1a – Volcano plot of metabolite abundance changes (covariate-adjusted). The x-axis specifies the log2 fold changes and the y-axis specifies the negative logarithm to the base 10 of the FDR values. Dotted vertical and horizontal lines reflect the filtering criteria (log2 FC=±2.0 and FDR=0.05). 1b – Score plots of the PLS-LDA analysis distinguishing WD patients (n=61) from healthy subjects (n=15) based on their metabolomics pattern. WD, Wilson disease; FC, fold change; FDR, false discovery rate, PLS-LDA, partial least-squares regression with linear discriminant analysis
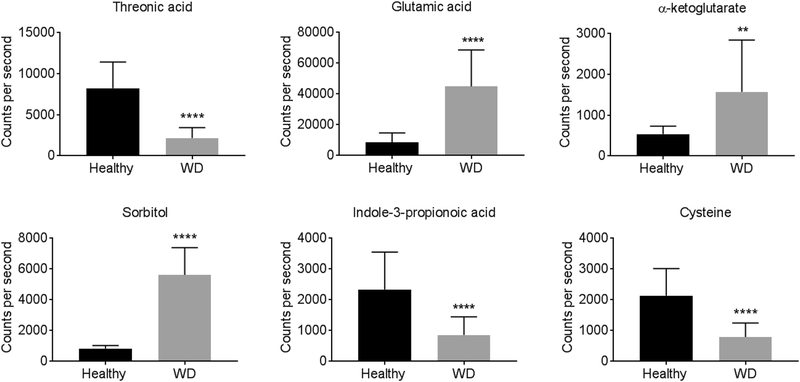
Among the 36 annotated metabolites (Table S1), 9 were more abundant and 27 were less abundant in WD. These metabolites included amino acids associated with the tricarboxylic acid (TCA) cycle (glutamic acid and α-ketoglutarate) and choline metabolism (cysteine), and metabolites related to the gut microbiota (indole-2-propionic acid, glucose, and glutamine). Glutamic acid, sorbitol, pseudo-uridine, and threonic acid exhibited changes greater than 2-fold between WD and healthy subjects. Figure 2 shows differentially abundant serum metabolites selected based on their link to copper metabolism and oxidative stress.
Fig. 2.
Comparison of select serum metabolites between healthy subjects and WD patients
Results are presented as mean ± SD; healthy subjects n=15, WD patients n=61. **p< 0.01, ****p< 0.0001 compared to healthy subjects. WD, Wilson disease
Further, we analyzed sex-specific metabolite profiles between WD and healthy subjects. Thirty-two metabolites were significantly different (FDR<0.05) in males while 56 metabolites were significant in females (Fig. S1, S2). Among them, 8 and 32 metabolites were found to be sex-specific in males and females, respectively. When comparing male and female patients with WD, no metabolites were found to differ significantly at FDR<0.05 (Fig. S3).
3.1. Metabolomics profiling analyses based on clinical manifestations
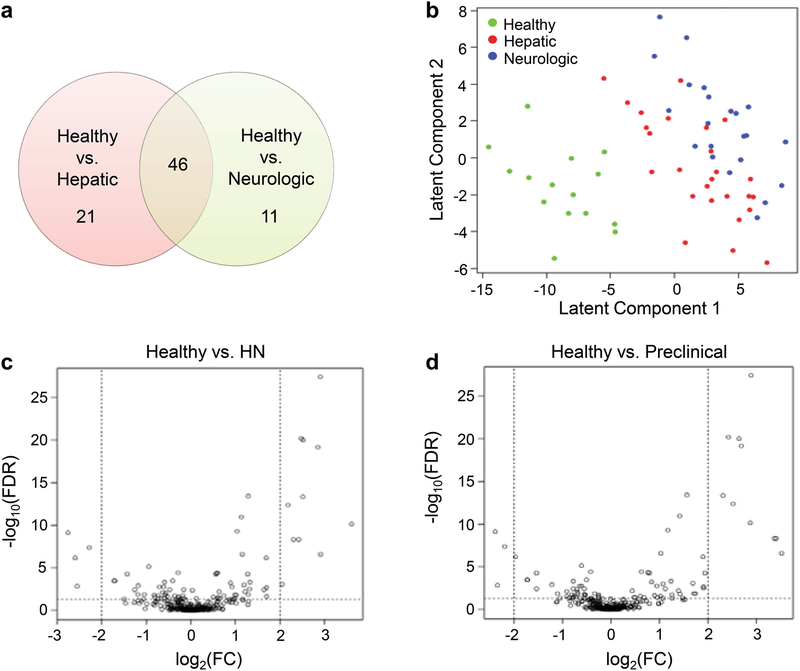
Metabolomics profiles for hepatic and neurologic presentations were analyzed in patients with WD compared to healthy subjects. Post hoc pairwise subtype comparisons, after adjusting for clinical covariates, identified 67 significantly different metabolites (FDR<0.05) between healthy and hepatic subtype, and 57 between healthy and neurologic subtype. Twenty-one of the 67 metabolites between healthy and hepatic were unique, including 2-hydroxybutanoic acid, 2-ketoisocaproic acid, α-ketoglutarate, glycerol-alpha-phosphate, oxamic acid, urea, and uridine. Eleven unique metabolites were identified between healthy and neurologic, including pyrophosphate and uric acid (Fig. 3a, Table S2). No significant metabolites were identified when directly comparing hepatic and neurologic subtypes after FDR correction. PLS‐LDA analysis could not discriminate well between hepatic and neurologic subtypes by the first two latent components, although healthy and combined WD phenotypes (neurologic and hepatic combined) showed clear separation (Fig. 3b). Volcano plots for differential analysis, comparing healthy to hepatic and healthy to neurologic, are shown in Fig. S4.
Fig. 3.
Metabolomics profiling analyses based on clinical manifestations
3a – Number of unique and concordant metabolites in healthy vs. hepatic and healthy vs. neurologic. 3b – Score plots of the PLS-LDA analysis for healthy subjects, hepatic subtype, and neurologic subtype. 3c, 3d – Volcano plot of metabolite abundance changes (covariate-adjusted), comparing comparing healthy vs. HN (3c) and healthy vs. preclinical (3d). The x-axis specifies the log2 fold changes and the y-axis specifies the negative logarithm to the base 10 of the FDR values. Dotted vertical and horizontal lines reflect the filtering criteria (log2 FC=± 2.0 and FDR=0.05). FC, fold change; FDR, false discovery rate; PLS-LDA, partial least-squares regression with linear discriminant analysis
3.2. Metabolomics profiling analyses based on diagnosis (preclinical and symptomatic)
Patients with WD were subdivided into either preclinical or symptomatic (HN, hepatic and neurologic combined) and compared to healthy subjects. Post hoc pairwise subtype comparisons, after adjusting for clinical covariates, resulted in 77 significant metabolites (FDR<0.05) between healthy and HN, and 36 between healthy and preclinical subtypes. Forty-three metabolites were specific to healthy vs. HN whereas only 2 metabolites were specific to healthy vs. preclinical (Table S3). No significant metabolites were found between HN and preclinical subtypes after FDR correction. Volcano plots for differential analysis, comparing healthy to HN and healthy to preclinical, are shown in Fig. 3c and 3d.
3.3. Pathways associated with differentially abundant metabolites in patients with WD compared to healthy subjects
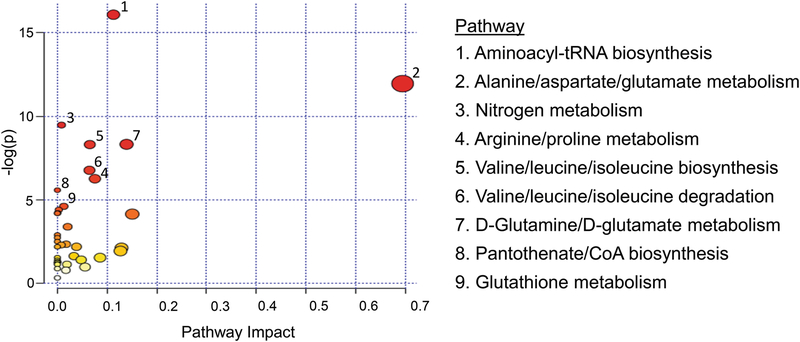
Differentially abundant metabolites in WD compared to healthy subjects were used to identify pathways and biological functions potentially impacted by WD (Fig. 4). The metabolic pathway associated with the highest number of significant metabolites was aminoacyl-tRNA biosynthesis. The most affected pathways, in general, were related to amino acid metabolism, biosynthesis, and degradation. The metabolites associated with each pathway are listed in Table S4. For our discussion, we selected metabolites involved in major biophysiological pathways such as the TCA cycle, methionine metabolism, and glutathione (GSH) biosynthesis. Importantly, these metabolites are also related to copper metabolism, oxidative stress, and WD.
Fig. 4.
Pathway analysis by MetaboAnalyst: metabolome view X-axis – pathway impact values from pathway topology analysis; y-axis – matched pathways from pathway enrichment analysis arranged by -log(p-value). Red indicates the most significant effects according to p-value and the node size is determined by pathway impact value
4. Discussion
Uncertain phenotype-genotype correlations, variability in clinical manifestations, and lack of specific diagnostic biomarkers are some of the challenges faced in WD diagnosis and treatment. Understanding WD pathogenesis and enabling access to improved diagnostic methods is of great importance. Metabolomics studies provide a useful approach to do this by identifying key metabolites with potential diagnostic and innovative therapeutic significance (Kim et al., 2016).
In this study, HILIC-QTOF MS-based serum metabolomics revealed patients with WD can be distinguished from healthy subjects based on metabolites associated with aminoacyl-tRNA biosynthesis, the TCA cycle, and choline metabolism, and metabolites related to gut microbiota. Metabolite profile comparisons could also distinguish between clinical manifestations (hepatic from neurologic) as well as healthy from preclinical subtypes. Although most of the identified metabolites were concordant between the hepatic, neurologic, and preclinical groups, we postulate a subset of differentially abundant metabolites can serve as potential WD biomarkers, providing new insights into its pathogenesis.
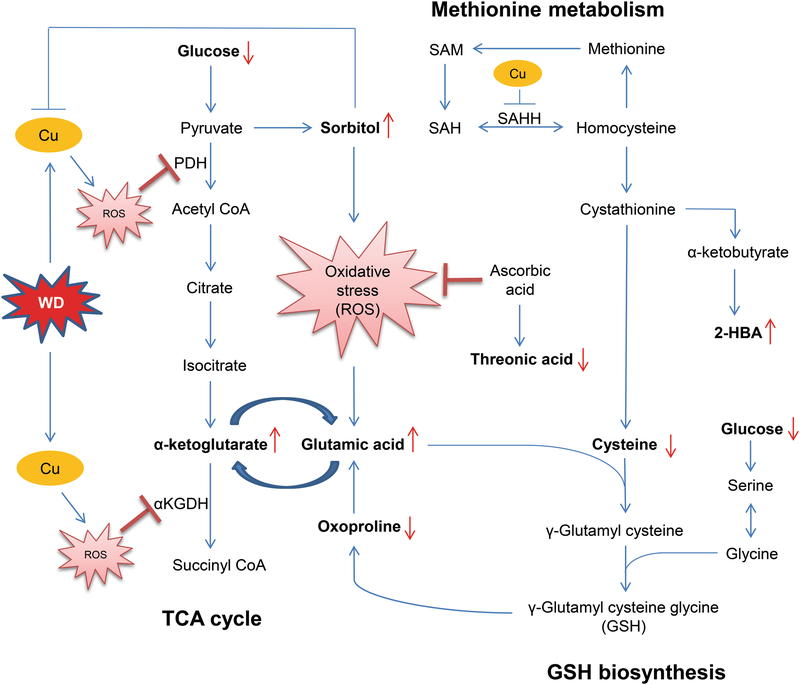
Copper-induced oxidative stress is known to contribute to the pathogenesis of WD (Kalita et al., 2014; Nagasaka et al., 2006). Our data reveal a significant alteration in metabolite levels which might be linked to copper-induced oxidative stress (Fig. 5). Among the increased annotated metabolites, sorbitol was augmented more than 3-fold in WD. Sorbitol is a sugar alcohol and plays an important role in the polyol pathway. Underlying mechanisms for the higher sorbitol are unknown, but it might be associated with glucose metabolism. Excess copper accumulation has long been reported to inhibit glycolysis and some of its enzymes (Lai and Blass, 1984), theoretically resulting in cytoplasmic accumulation of excess glucose. Removal of glucose from the cell, mediated by the polyol pathway, could cause an increased release of sorbitol into the bloodstream. Previous studies in diabetes have also indicated that impaired glucocorticoid receptor (GR) signaling induces sorbitol accumulation via polyol pathways (Eriksson et al., 1986; Gaynes and Watkins, 1989; Poulsom and Heath, 1983) and there is evidence showing a positive correlation between blood copper level and risk for developing diabetes mellitus, possibly due to augmented copper-induced oxidative stress (Zhang et al., 2017). Our analysis confirmed a previous finding in Atp7b−/− mice, a mouse model of WD, of higher plasma sorbitol levels (Wilmarth et al., 2012) . These mice also exhibited significantly reduced GR levels, which might indicate attenuated GR signaling. Furthermore, glucose is converted to pyruvate during glycolysis. Pyruvate enters the mitochondrion where it is converted into acetyl-CoA by pyruvate dehydrogenase (PDH). Since excess copper leads to copper-induced oxidative stress, which produces reactive oxygen species (ROS) known to inhibit PDH (Sheline and Choi, 2004), excess pyruvate may accumulate and be converted to sorbitol. In diabetes, sorbitol can also lead to increased production of mitochondrial superoxide anion radicals causing poly(ADP-ribose) polymerase activation which has been reported to exacerbate oxidative stress further through generation of ROS and reactive nitrogen species (Naik and Kokil, 2013; Obrosova, 2005). Sorbitol has also been shown to have a strong copper-chelating action (Briggs et al., 1981), thus high sorbitol levels in WD patients could help maintain cellular homeostasis by attempting chelation of excess copper.
Fig. 5.
Network of key metabolites and their associated pathways relevant to copper and oxidative stress in WD Differential metabolites are indicated in bold text. Red arrows represent decreased or increased levels in the serum of WD patients. 2-HBA, 2-hydroxybutanoic acid; αKGDH, α-ketoglutarate dehydrogenase; Cu, copper; GSH, glutathione; PDH, pyruvate dehydrogenase; ROS, reactive oxygen species; SAH, S-adenosylhomocysteine; SAHH, S-adenosylhomocysteine hydrolase; SAM, S-adenosylmethionine
We observed significant changes in metabolites related to the TCA cycle in patients with WD compared to healthy subjects. In particular, glutamic acid and α-ketoglutarate were both found to be increased in WD. α-ketoglutarate is an intermediate in the TCA cycle interconvertible with glutamic acid by transamination; therefore, glutamic acid can directly impact energy metabolism via the TCA cycle (Maus and Peters, 2017; Zheng et al., 2014). Copper-induced ROS was shown to block the TCA cycle by inhibiting α-ketoglutarate dehydrogenase, which converts α-ketoglutarate to succinyl CoA (Sheline and Choi, 2004), with a consequent increase in α-ketoglutarate levels. The elevated glutamic acid levels observed in WD patients are likely due to conversion from the increased α-ketoglutarate. Our data showing high glutamic acid levels is consistent with previously published data indicating that copper-induced oxidative stress might be responsible for elevated glutamic acid and cytokines (Kalita et al., 2014). Copper, then, may have duo impact on the TCA cycle through oxidative stress affecting both α-ketoglutarate dehydrogenase and glutamic acid.
Metabolites in GSH biosynthesis and methionine metabolism e.g., cysteine, 2-hydroxybutanoic acid, and oxoproline, were significantly different in WD. Cysteine was reduced more than 2-fold in patients with WD. Cysteine functions as an antioxidant; clinical studies have shown cysteine supplementation improves skeletal muscle and immune system functions by reducing plasma levels of tumor necrosis factor α, decreasing body fat vs. lean body mass, and increasing plasma albumin levels (Droge, 2005). N-acetyl-cysteine, a derivative of cysteine, is used in the treatment of alcoholic hepatitis and acetaminophen poisoning (Whyte et al., 2007). Cysteine is also a primary component in GSH production, a critical antioxidant against oxidative stress, particularly in the liver. In a mouse model with a hepatocyte-specific deletion in the glutamate-cysteine ligase catalytic subunit gene, causing impaired hepatic GSH production, mice became moribund by about 4 weeks of age (Chen et al., 2007). They exhibited highly elevated liver enzymes, steatosis, abnormal rough endoplasmic reticulum, and multiple abnormal hepatic mitochondria morphologies leading to compromised membrane potential and respiration. Previous studies by us and others showed copper accumulation is associated with reduced S-adenosyl homocysteine hydrolase expression and activity (Li et al., 2007; Medici et al., 2013), possibly leading to reduced cysteine and GSH levels with consequent compromised antioxidant defense. This may explain the reduced plasma cysteine observed in this study and previous reports of significantly low hepatic GSH in WD patients (Summer and Eisenburg, 1985). Orally administered cysteine can also act as a copper chelator by forming a copper-cysteine complex, thereby inhibiting copper absorption in the gut (Baker and Czarnecki-Maulden, 1987). In untreated patients with WD, high plasma copper levels were shown to inhibit L-cysteine/L-cystine uptake in erythrocytes (Mandal et al., 2016) and GSH concentration was found to be reduced and significantly dependent on the L-cysteine/L-cystine uptake. Excess copper, then, can reduce cysteine bioavailability which may lead to increased cysteine demand (Robbins and Baker, 1980).
2-hydroxybutanoic acid (2-HBA) is derived from α-ketobutyrate as a byproduct of GSH anabolism when cystathionine is converted to cysteine. Elevated levels of 2-HBA serves as an early indicator for insulin resistance and compromised glucose regulation, and seems to be derived via increased lipid oxidation and oxidative stress (Gall et al., 2010). 2-HBA is primarily produced in the liver upon excessive demand for GSH under increased oxidative stress. In the WD hepatic phenotype, therefore, copper-induced oxidative stress and reduced cysteine levels might be possible underlying mechanisms involved in up-regulation of 2-HBA.
Oxoproline, reduced 1.5-fold in WD, is another metabolite involved with GSH biosynthesis and is converted to glutamate by 5-oxoprolinase. Previous studies have suggested 5-oxoproline levels indicate the availability of glycine and/or cysteine (Metges, 2000). Low levels of oxoproline may be a consequence of reduced cysteine levels in WD.
An intermediate likely contributing to many of the above altered metabolites is the iron sulfur cluster (ISC). ISCs play key roles in biochemical processes such as energy production, metabolic conversions, DNA maintenance, gene expression regulation, protein translation, and the anti-viral response (Braymer and Lill, 2017). They are required for functional enzymes involved in the TCA cycle e.g., succinate dehydrogenase and aconitase, and respiratory chain complexes I–III (Rouault, 2012) as well as for amino acid biosynthesis, including cysteine and glutamate (Rocha and Dancis, 2016).
In eukaryotic cells, mitochondrial ISC assembly proteins function in an environment known to contain a copper(I) pool. With a stronger binding affinity, it has been shown in vitro that copper can preferentially displace iron in the ISC assembly (Brancaccio et al., 2017). It is also established that ISCs are highly vulnerable to ROS damage (Vernis et al., 2017). Moreover, previous studies in E. coli revealed copper overload inactivated ISC enzymes such as isopropyl malate dehydratase, fumarase A, and 6-phospho-gluconate dehydratase (Macomber and Imlay, 2009). ISC assembly processes are highly conserved among yeast, bacteria, and humans (Rouault, 2012); therefore, within the scope of our data, it is possible the impact of excess copper on ISCs is two-fold – directly inhibiting ISC synthesis or damaging existing ISCs by iron displacement and indirectly damaging existing ISCs by ROS generation with subsequent ROS-induced damage. This could lead to a deleterious cascade in which cysteine and glutamic acid levels and subsequent glutathione levels in WD patients are decreased. Since cysteine is also a required component of assembly proteins that form ISCs (Vallieres et al., 2017), reduced cysteine levels can further compound ISC damage as glutathione is required for maturation of cytosolic ISC proteins (Sipos et al., 2002) thus creating a continuously detrimental cycle of ISC/amino acid/glutathione damage and reduction.
Our study revealed threonic acid was reduced more than 3-fold in serum of patients with WD. Threonic acid is a breakdown product of ascorbic acid (AA) metabolism. This metabolite is linked with the insufficiency or deprivation of AA which scavenges free radicals such as oxygen, superoxide, and hydroxyl radicals (Gao et al., 2012; Thomas and Hughes, 1983). Previous reports described significant AA reduction and compromised antioxidant defense in untreated WD patients (Attri et al., 2006; Ogihara et al., 1995), and linked copper-mediated oxidative stress to mitochondrial dysfunction (Rossi et al., 2004). AA accumulates in mitochondria as a protective agent where most free radicals are produced. If increased oxidative stress in WD mitochondria is a valid inference, increased AA utilization should initially elevate threonic acid until AA levels are depleted, at which point the threonic acid level would decrease.
Uric acid levels were reduced in WD neurologic vs. healthy subjects. Low plasma uric acid levels at presentation with hepatic or neurologic manifestations in patients with WD have been previously described and may be due to renal tubular failure and reduced uric acid synthesis in the liver (Nussinson et al., 2013; Roberts et al., 2008; Umeki et al., 1986; Wang et al., 2015). Reduced levels of uric acid were also associated with neurodegenerative diseases like Parkinson’s disease (Winquist et al., 2010). Uric acid acts as a natural antioxidant (Ames et al., 1981) and low serum uric acid likely indicates, again, reduced antioxidant defense in WD.
We found reduced uridine in WD hepatic compared to healthy subjects. Uridine plays many important roles in hepatic metabolism, including liver detoxification, immune homeostasis (Chong et al., 1999), enhancement of mitochondrial function (Banasch et al., 2006), and suppression of fat accumulation (Le et al., 2013); however, the exact mechanisms regulating its protective roles are unknown. As liver controls the formation and degradation of uridine (Gasser et al., 1981), hepatic copper-induced liver damage in WD patients might be causatively associated with low uridine levels.
An additional novel finding is reduced indole-3-proprionic acid (IPA) levels in our patients with WD. In the human gastrointestinal microbiome, IPA is endogenously produced and detected in vivo only in the presence of Clostridium sporogenes (Wikoff et al., 2009). By using tryptophan, C. sporogenes synthesizes indole and, subsequently, IPA at detectable levels in the host plasma. IPA plays a role in fibrosis and inflammatory-related gene suppression in proximal tubular cells (Yisireyili et al., 2017) and protects hepatic microsomal membranes from iron-induced oxidative damage in cancer (Karbownik et al., 2001). Human hepatocytes cultured with IPA also demonstrated attenuated copper- and arsenic-mediated mitochondrial and cellular DNA damage (Chinnasamy et al., 2016). Considering its multiple protective roles, low IPA levels indicate yet another facet of compromised cellular defense in WD.
Microbiomics has become a very dynamic field with recently published data supporting the importance of microbiome-associated bacteria and their products for liver homeostasis (Mazagova et al., 2015) and copper chelation in hepatocyte mitochondria (Lichtmannegger et al., 2016), and dietary copper impact on microbial activity (Song et al., 2018). Peripherally, the copper-regulatory role of ATP7B in intestinal epithelial cells has also been reported (Pierson et al., 2018). Other metabolites from our analyses are also associated with microbial activity, but merit a separate space for adequate discussion. As such, we suspect microbiome status has a significant impact on WD copper metabolism, warranting further microbiomic analyses.
Metabolomics profiling has been studied in conditions that are included in the differential diagnosis of WD. One of the most extensive studies on metabolomics in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis (NASH) included the serum and liver metabolomics profiles in more than 500 patients (Alonso et al., 2017). The profile of different subtypes of NASH patients included different levels of amino acids (methionine, glutamic acid, taurine, aspartic acid, and glutamine), bile acids, fatty acids, triglycerides, glycerophospholipids, and sphingomyelins. The metabolic profile of autoimmune hepatitis was explored in 2 main studies. One of the studies described two bile acids, three long-chain acylcarnitines, seven glycerophospholipids, a bilirubin, and a retinyl ester as being associated with early autoimmune hepatitis (Zhou et al., 2016). The second study showed that autoimmune hepatitis was associated with changes in metabolites typically associated with changes in energy metabolism, including pyruvate, lactate, acetate, acetoacetate, and glucose (Wang et al., 2014). Therefore, even though we cannot make a direct comparison, it appears a WD metabolomics profile has distinct features that could be implemented in the diagnostic process of this condition.
In summary, we investigated the serum metabolomics profiles of a group of patients with WD compared to healthy subjects, identifying differentially abundant metabolites that may contribute to understanding WD pathogenesis and could lead to novel diagnostic biomarker development. Study limitations include the lack of significant findings in subgroup comparisons after correction for multiple testing at FDR<0.05 due to the relatively small number of subjects in subgroups. Untargeted metabolomics profiling also has the disadvantage of detecting high numbers of unannotated compounds, which was the case in our analysis. Many of the unannotated metabolites could have biological implications relevant to WD but could not be studied. Future validation studies in gene/gene expression analysis should be conducted to confirm the present findings and determine the impact of any altered enzymatic activity, providing more mechanistic explanations.
Supplementary Material
Acknowledgements
We would like to acknowledge Triston Mosbacher (Department of Public Health Sciences, Division of Biostatistics, University of California, Davis) for his contribution to the analysis.
Funding:This research was supported by the National Institutes of Health/NIDDK through grant number R01DK104770 (to V.M.).
List of abbreviations:
- WD
Wilson disease
- HILIC-QTOF MS
hydrophilic interaction liquid chromatography-quadrupole time of flight mass spectrometry
- PLS-LDA
partial least-squares regression with linear discriminant analysis
- FDR
false discovery rate
- TCA
tricarboxylic acid
- HN
hepatic-neurologic manifestations combined
- GSH
glutathione
- GR
glucocorticoid receptor
- PDH
pyruvate dehydrogenase
- ROS
reactive oxygen species
- 2-HBA
2-hydroxybutanoic acid
- AA
ascorbic acid
- IPA
indole-3-proprionic acid
Footnotes
Conflict of Interest: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors who participated in this study declared they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Compliance with ethical standards
This study was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Institutional Review Board at the University of California, Davis.
Data Availability Statement
The metabolomics and metadata reported in this paper are available via Metabolomics Workbench http://www.metabolomicsworkbench.org/, and study can be found under ST001118.
Informed consent
Written informed consent was obtained from each patient and healthy subject in this study.
References
- Ala A, Walker AP, Ashkan K, Dooley JS and Schilsky ML (2007) Wilson’s disease. The Lancet 369, 397–408. [DOI] [PubMed] [Google Scholar]
- Aliasgharpour M (2015) A review on copper, ceruloplasmin and wilson’s disease.
- Alonso C, Fernandez-Ramos D, Varela-Rey M, Martinez-Arranz I, Navasa N, Van Liempd SM, Lavin Trueba JL, Mayo R, Ilisso CP, de Juan VG, Iruarrizaga-Lejarreta M, delaCruz-Villar L, Minchole I, Robinson A, Crespo J, Martin-Duce A, Romero-Gomez M, Sann H, Platon J, Van Eyk J, Aspichueta P, Noureddin M, Falcon-Perez JM, Anguita J, Aransay AM, Martinez-Chantar ML, Lu SC and Mato JM (2017) Metabolomic Identification of Subtypes of Nonalcoholic Steatohepatitis. Gastroenterology 152, 1449–1461 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames BN, Cathcart R, Schwiers E and Hochstein P (1981) Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A 78, 6858–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attri S, Sharma N, Jahagirdar S, Thapa BR and Prasad R (2006) Erythrocyte metabolism and antioxidant status of patients with Wilson disease with hemolytic anemia. Pediatr Res 59, 593–7. [DOI] [PubMed] [Google Scholar]
- Baker DH and Czarnecki-Maulden GL (1987) Pharmacologic role of cysteine in ameliorating or exacerbating mineral toxicities. J Nutr 117, 1003–10. [DOI] [PubMed] [Google Scholar]
- Banasch M, Goetze O, Knyhala K, Potthoff A, Schlottmann R, Kwiatek MA, Bulut K, Schmitz F, Schmidt WE and Brockmeyer NH (2006) Uridine supplementation enhances hepatic mitochondrial function in thymidine-analogue treated HIV-infected patients. AIDS 20, 1554–6. [DOI] [PubMed] [Google Scholar]
- Brancaccio D, Gallo A, Piccioli M, Novellino E, Ciofi-Baffoni S and Banci L (2017) [4Fe-4S] Cluster Assembly in Mitochondria and Its Impairment by Copper. J Am Chem Soc 139, 719–730. [DOI] [PubMed] [Google Scholar]
- Braymer JJ and Lill R (2017) Iron-sulfur cluster biogenesis and trafficking in mitochondria. J Biol Chem 292, 12754–12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs J, Finch P, Matulewicz MC and Weigel H (1981) Complexes of copper(II), calcium, and other metal ions with carbohydrates: Thin-layer ligand-exchange chromatography and determination of relative stabilities of complexes. Carbohydrate Research 97, 181–188. [Google Scholar]
- Bull PC, Thomas GR, Rommens JM, Forbes JR and Cox DW (1993) The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet 5, 327–37. [DOI] [PubMed] [Google Scholar]
- Cabras T, Sanna M, Manconi B, Fanni D, Demelia L, Sorbello O, Iavarone F, Castagnola M, Faa G and Messana I (2015) Proteomic investigation of whole saliva in Wilson’s disease. Journal of Proteomics 128, 154–163. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yang Y, Miller ML, Shen D, Shertzer HG, Stringer KF, Wang B, Schneider SN, Nebert DW and Dalton TP (2007) Hepatocyte-specific Gclc deletion leads to rapid onset of steatosis with mitochondrial injury and liver failure. Hepatology 45, 1118–28. [DOI] [PubMed] [Google Scholar]
- Chinnasamy T, Sharma Y, Gupta P and Gupta S (2016) Arsenic (As) and Copper (Cu) Serve as Cofactors to Produce Hepatotoxicity With Mitochondrial Damage, Double Strand DNA Breaks and Disruption of ATM Signaling. The FASEB Journal 30, 1026.3–1026.3. [Google Scholar]
- Chong AS, Huang W, Liu W, Luo J, Shen J, Xu W, Ma L, Blinder L, Xiao F, Xu X, Clardy C, Foster P and Williams JA (1999) In vivo activity of leflunomide: pharmacokinetic analyses and mechanism of immunosuppression. Transplantation 68, 100–9. [DOI] [PubMed] [Google Scholar]
- Czlonkowska A, Litwin T, Dusek P, Ferenci P, Lutsenko S, Medici V, Rybakowski JK, Weiss KH and Schilsky ML (2018) Wilson disease. Nat Rev Dis Primers 4, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droge W (2005) Oxidative stress and ageing: is ageing a cysteine deficiency syndrome? Philos Trans R Soc Lond B Biol Sci 360, 2355–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson UJ, Naeser P and Brolin SE (1986) Increased accumulation of sorbitol in offspring of manifest diabetic rats. Diabetes 35, 1356–63. [DOI] [PubMed] [Google Scholar]
- Ferenci P, Caca K, Loudianos G, Mieli-Vergani G, Tanner S, Sternlieb I, Schilsky M, Cox D and Berr F (2003) Diagnosis and phenotypic classification of Wilson disease. Liver Int 23, 139–42. [DOI] [PubMed] [Google Scholar]
- Ferenci P, Stremmel W, Czlonkowska A, Szalay F, Viveiros A, Stattermayer AF, Bruha R, Houwen R, Pop T, Stauber R, Gschwantler M, Pfeiffenberger J, Yurdaydin C, Aigner E, Steindl-Munda P, Dienes HP, Zoller H and Weiss KH (2018) Age,sex, but not ATP7B genotype effectively influences the clinical phenotype of Wilson disease. Hepatology. [DOI] [PubMed] [Google Scholar]
- Gall WE, Beebe K, Lawton KA, Adam KP, Mitchell MW, Nakhle PJ, Ryals JA, Milburn MV, Nannipieri M, Camastra S, Natali A, Ferrannini E and Group RS (2010) alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS One 5, e10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Chen W, Li R, Wang M, Chen C, Zeng R and Deng Y (2012) Systematic variations associated with renal disease uncovered by parallel metabolomics of urine and serum. BMC Syst Biol 6Suppl 1, S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser T, Moyer JD and Handschumacher RE (1981) Novel single-pass exchange of circulating uridine in rat liver. Science 213, 777–8. [DOI] [PubMed] [Google Scholar]
- Gaynes BI and Watkins JB 3rd (1989) Comparison of glucose, sorbitol and fructose accumulation in lens and liver of diabetic and insulin-treated rats and mice. Comp Biochem Physiol B 92, 685–90. [DOI] [PubMed] [Google Scholar]
- Huster D, Kuhne A, Bhattacharjee A, Raines L, Jantsch V, Noe J, Schirrmeister W, Sommerer I, Sabri O, Berr F, Mossner J, Stieger B, Caca K and Lutsenko S (2012) Diverse functional properties of Wilson disease ATP7B ariants. Gastroenterology 142, 947–956 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalita J, Kumar V, Misra UK, Ranjan A, Khan H and Konwar R (2014) A study of oxidative stress, cytokines and glutamate in Wilson disease and their asymptomatic siblings. J Neuroimmunol 274, 141–8. [DOI] [PubMed] [Google Scholar]
- Karbownik M, Reiter RJ, Garcia JJ, Cabrera J, Burkhardt S, Osuna C and Lewinski A (2001) Indole-3-propionic acid, a melatonin-related molecule, protects hepatic microsomal membranes from iron-induced oxidative damage: relevance to cancer reduction. J Cell Biochem 81, 507–13. [PubMed] [Google Scholar]
- Kieffer DA and Medici V (2017) Wilson disease: At the crossroads between genetics and epigenetics-A review of the evidence. Liver Res 1, 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Kim SH, Kim JH, Hwang S and Yoo HJ (2016) Understanding Metabolomics in Biomedical Research. Endocrinol Metab (Seoul) 31, 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JC and Blass JP (1984) Neurotoxic effects of copper: inhibition of glycolysis and glycolytic enzymes. Neurochem Res 9, 1699–710. [DOI] [PubMed] [Google Scholar]
- Le TT, Ziemba A, Urasaki Y, Hayes E, Brotman S and Pizzorno G (2013) Disruption of uridine homeostasis links liver pyrimidine metabolism to lipid accumulation. J Lipid Res 54, 1044–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Kim JM, Heo SH, Mun JH, Kim J, Kim JH, Jin HY, Kim GH, Choi JH and Yoo HW (2011) Proteomic analysis of the hepatic tissue of Long-Evans Cinnamon (LEC) rats according to the natural course of Wilson disease. Proteomics 11, 3698–705. [DOI] [PubMed] [Google Scholar]
- Li M, Li Y, Chen J, Wei W, Pan X, Liu J, Liu Q, Leu W, Zhang L, Yang X, Lu J and Wang K (2007) Copper ions inhibit S-adenosylhomocysteine hydrolase by causing dissociation of NAD+ cofactor. Biochemistry 46, 11451–8. [DOI] [PubMed] [Google Scholar]
- Lichtmannegger J, Leitzinger C, Wimmer R, Schmitt S, Schulz S, Kabiri Y, Eberhagen C, Rieder T, Janik D, Neff F, Straub BK, Schirmacher P, DiSpirito AA, Bandow N, Baral BS, Flatley A, Kremmer E, Denk G, Reiter FP, Hohenester S, Eckardt-Schupp F, Dencher NA, Adamski J, Sauer V, Niemietz C, Schmidt HH, Merle U, Gotthardt DN, Kroemer G, Weiss KH and Zischka H (2016) Methanobactin reverses acute liver failure in a rat model of Wilson disease. J Clin Invest 126, 2721–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macomber L and Imlay JA (2009) The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci U S A 106, 8344–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal N, Bhattacharjee D, Rout JK, Dasgupta A, Bhattacharya G, Sarkar C and Gangopadhyaya PK (2016) Effect of Copper on l-Cysteine/l-Cystine Influx in Normal Human Erythrocytes and Erythrocytes of Wilson’s Disease. Indian J Clin Biochem 31, 468–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maus A and Peters GJ (2017) Glutamate and alpha-ketoglutarate: key players in glioma metabolism. Amino Acids 49, 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazagova M, Wang L, Anfora AT, Wissmueller M, Lesley SA, Miyamoto Y, Eckmann L, Dhungana S, Pathmasiri W, Sumner S, Westwater C, Brenner DA and Schnabl B (2015) Commensal microbiota is hepatoprotective and prevents liver fibrosis in mice. FASEB J 29, 1043–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici V, Kieffer DA, Shibata NM, Chima H, Kim K, Canovas A, Medrano JF, Islas-Trejo AD, Kharbanda KK, Olson K, Su RJ, Islam MS, Syed R, Keen CL, Miller AY, Rutledge JC, Halsted CH and LaSalle JM (2016) Wilson Disease: Epigenetic effects of choline supplementation on phenotype and clinical course in a mouse model. Epigenetics 11, 804–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici V, Shibata NM, Kharbanda KK, Islam MS, Keen CL, Kim K, Tillman B, French SW, Halsted CH and LaSalle JM (2014) Maternal choline modifies fetal liver copper, gene expression, DNA methylation, and neonatal growth in the tx-j mouse model of Wilson disease. Epigenetics 9, 286–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici V, Shibata NM, Kharbanda KK, LaSalle JM, Woods R, Liu S, Engelberg JA, Devaraj S, Torok NJ, Jiang JX, Havel PJ, Lonnerdal B, Kim K and Halsted CH (2013) Wilson’s disease: changes in methionine metabolism and inflammation affect global DNA methylation in early liver disease. Hepatology 57, 555–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metges CC (2000) Contribution of Microbial Amino Acids to Amino Acid Homeostasis of the Host. The Journal of Nutrition 130, 1857S–1864S. [DOI] [PubMed] [Google Scholar]
- Nagasaka H, Inoue I, Inui A, Komatsu H, Sogo T, Murayama K, Murakami T, Yorifuji T, Asayama K, Katayama S, Uemoto S, Kobayashi K, Takayanagi M, Fujisawa T and Tsukahara H (2006) Relationship between oxidative stress and antioxidant systems in the liver of patients with Wilson disease: hepatic manifestation in Wilson disease as a consequence of augmented oxidative stress. Pediatr Res 60, 472–7. [DOI] [PubMed] [Google Scholar]
- Naik SR and Kokil GR (2013) Chapter 12 - Development and Discovery Avenues in Bioactive Natural Products for Glycemic Novel Therapeutics in Atta ur, R (Ed), Studies in Natural Products Chemistry, Elsevier; 431–466. [Google Scholar]
- Nussinson E, Shahbari A, Shibli F, Chervinsky E, Trougouboff P and Markel A (2013) Diagnostic challenges of Wilson’s disease presenting as acute pancreatitis, cholangitis, and jaundice. World J Hepatol 5, 649–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrosova IG (2005) Increased sorbitol pathway activity generates oxidative stress in tissue sites for diabetic complications. Antioxid Redox Signal 7, 1543–52. [DOI] [PubMed] [Google Scholar]
- Ogihara H, Ogihara T, Miki M, Yasuda H and Mino M (1995) Plasma copper and antioxidant status in Wilson’s disease. Pediatr Res 37, 219–26. [DOI] [PubMed] [Google Scholar]
- Park JY, Mun JH, Lee BH, Heo SH, Kim GH and Yoo HW (2009) Proteomic analysis of sera of asymptomatic, early-stage patients with Wilson’s disease. Proteomics Clin Appl 3, 1185–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson H, Muchenditsi A, Kim B-E, Ralle M, Zachos N, Huster D and Lutsenko S (2018) The Function of ATPase Copper Transporter ATP7B in Intestine.Gastroenterology 154, 168–180.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsom R and Heath H (1983) Inhibition of aldose reductase in five tissues of the streptozotocin-diabetic rat. Biochem Pharmacol 32, 1495–9. [DOI] [PubMed] [Google Scholar]
- Robbins KR and Baker DH (1980) Effect of high-level copper feeding on the sulfur amino acid need of chicks fed corn-soybean meal and purified crystalline amino acid diets. Poult Sci 59, 1099–108. [DOI] [PubMed] [Google Scholar]
- Roberts EA, Schilsky ML and American Association for Study of Liver, D. (2008) Diagnosis and treatment of Wilson disease: an update. Hepatology 47, 2089–111. [DOI] [PubMed] [Google Scholar]
- Rocha AG and Dancis A (2016) Life without Fe-S clusters. Mol Microbiol 99, 821–6. [DOI] [PubMed] [Google Scholar]
- Roelofsen H, Balgobind R and Vonk RJ (2004) Proteomic analyzes of copper metabolism in an in vitro model of Wilson disease using surface enhanced laser desorption/ionization-time of flight-mass spectrometry. J Cell Biochem 93, 732–40. [DOI] [PubMed] [Google Scholar]
- Rossi L, Lombardo MF, Ciriolo MR and Rotilio G (2004) Mitochondrial dysfunction in neurodegenerative diseases associated with copper imbalance. Neurochem Res 29, 493–504. [DOI] [PubMed] [Google Scholar]
- Rouault TA (2012) Biogenesis of iron-sulfur clusters in mammalian cells: new insights and relevance to human disease. Dis Model Mech 5, 155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline CT and Choi DW (2004) Cu2+ toxicity inhibition of mitochondrial dehydrogenases in vitro and in vivo. Ann Neurol 55, 645–53. [DOI] [PubMed] [Google Scholar]
- Simpson DM, Beynon RJ, Robertson DH, Loughran MJ and Haywood S (2004) Copper-associated liver disease: a proteomics study of copper challenge in a sheep model. Proteomics 4, 524–36. [DOI] [PubMed] [Google Scholar]
- Sipos K, Lange H, Fekete Z, Ullmann P, Lill R and Kispal G (2002) Maturation of cytosolic iron-sulfur proteins requires glutathione. J Biol Chem 277, 26944–9. [DOI] [PubMed] [Google Scholar]
- Song M, Li X, Zhang X, Shi H, Vos MB, Wei X, Wang Y, Gao H, Rouchka EC, Yin X, Zhou Z, Prough RA, Cave MC and McClain CJ (2018) Dietary copper-fructose interactions alter gut microbial activity in male rats. Am J Physiol Gastrointest Liver Physiol 314, G119–G130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summer KH and Eisenburg J (1985) Low content of hepatic reduced glutathione in patients with Wilson’s disease. Biochem Med 34, 107–11. [DOI] [PubMed] [Google Scholar]
- Thomas M and Hughes RE (1983) A relationship between ascorbic acid and threonic acid in guinea-pigs. Food Chem Toxicol 21, 449–52. [DOI] [PubMed] [Google Scholar]
- Umeki S, Ohga R, Konishi Y, Yasuda T, Morimoto K and Terao A (1986) Oral zinc therapy normalizes serum uric acid level in Wilson’s disease patients. Am J Med Sci 292, 289–92. [DOI] [PubMed] [Google Scholar]
- Vallieres C, Holland SL and Avery SV (2017) Mitochondrial Ferredoxin Determines Vulnerability of Cells to Copper Excess. Cell Chem Biol 24, 1228–1237 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernis L, El Banna N, Baille D, Hatem E, Heneman A and Huang ME (2017) Fe-S Clusters Emerging as Targets of Therapeutic Drugs. Oxid Med Cell Longev 2017, 3647657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhou Z, Hu J, Han Y, Wang X, Cheng N, Wu Y and Yang R (2015) Renal impairment in different phenotypes of Wilson disease. Neurol Sci 36, 2111–5. [DOI] [PubMed] [Google Scholar]
- Wang JB, Pu SB, Sun Y, Li ZF, Niu M, Yan XZ, Zhao YL, Wang LF, Qin XM, Ma ZJ, Zhang YM, Li BS, Luo SQ, Gong M, Sun YQ, Zou ZS and Xiao XH (2014) Metabolomic Profiling of Autoimmune Hepatitis: The Diagnostic Utility of Nuclear Magnetic Resonance Spectroscopy. J Proteome Res. [DOI] [PubMed] [Google Scholar]
- Whyte IM, Francis B and Dawson AH (2007) Safety and efficacy of intravenous N-acetylcysteine for acetaminophen overdose: analysis of the Hunter Area Toxicology Service (HATS) database. Curr Med Res Opin 23, 2359–68. [DOI] [PubMed] [Google Scholar]
- Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC and Siuzdak G (2009) Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A 106, 3698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmarth PA, Short KK, Fiehn O, Lutsenko S, David LL and Burkhead JL (2012) A systems approach implicates nuclear receptor targeting in the Atp7b(−/−) mouse model of Wilson’s disease. Metallomics 4, 660–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winquist A, Steenland K and Shankar A (2010) Higher serum uric acid associated with decreased Parkinson’s disease prevalence in a large community-based survey. Mov Disord 25, 932–6. [DOI] [PubMed] [Google Scholar]
- Xu J, Jiang H, Li J, Cheng KK, Dong J and Chen Z (2015) 1H NMR-based metabolomics investigation of copper-laden rat: a model of Wilson’s disease. PLoS One 10, e0119654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yisireyili M, Takeshita K, Saito S, Murohara T and Niwa T (2017) Indole-3-propionic acid suppresses indoxyl sulfate-induced expression of fibrotic and inflammatory genes in proximal tubular cells. Nagoya J Med Sci 79, 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yan C, Yang Z, Zhang W, Niu Y, Li X, Qin L and Su Q (2017) Alterations of serum trace elements in patients with type 2 diabetes. J Trace Elem Med Biol 40, 91–96. [DOI] [PubMed] [Google Scholar]
- Zheng T, Liu L, Shi J, Yu X, Xiao W, Sun R, Zhou Y, Aa J and Wang G (2014) The metabolic impact of methamphetamine on the systemic metabolism of rats and potential markers of methamphetamine abuse. Mol Biosyst 10, 1968–77. [DOI] [PubMed] [Google Scholar]
- Zhou C, Jia HM, Liu YT, Yu M, Chang X, Ba YM and Zou ZM (2016) Metabolism of glycerophospholipid, bile acid and retinol is correlated with the early outcomes of autoimmune hepatitis. Mol Biosyst 12, 1574–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.