Abstract
Recent association studies indicate several genes highly expressed by microglia influence Alzheimer’s disease (AD) risk, which suggests microglial function contributes to this disease. Here, we evaluated how one component of microglial function, cytokine release, affects AD-related phenomena. First, we used a 3-hour lipopolysaccharide (LPS) treatment to activate mouse BV2 microglial cells. Next, we removed the LPS-containing medium, added LPS-free medium, and after 6 hours collected the medium conditioned by the activated BV2 microglial cells. We then exposed human neuronal SH-SY5Y cells to the conditioned medium for 24 hours. At the end of the 24-hour exposure, we assessed amyloid precursor protein (AβPP), tau, apolipoprotein E (ApoE), and lipid status. The amount of AβPP was unaffected, although a slight decrease in soluble AβPPα suggested a subtle reduction in AβPP non-amyloidogenic processing occurred. Tau mRNA increased, but total and phosphorylated tau levels were unchanged. ApoE mRNA increased, while ApoE protein levels were lower. Per cell lipid droplet number decreased and lipid oxidation increased. These results show cytokine release by activated microglial cells can influence specific AD-relevant physiologies and pathologies.
Keywords: Alzheimer’s disease, apoe, inflammation, lipid droplets, microglia
Introduction
Brains from Alzheimer’s disease (AD) patients display prominent inflammatory changes to which microglia contribute [1, 2]. In AD brains microglia proliferate and adopt an activated state [3]. Investigators suspect beta amyloid (Aβ) aggregates, otherwise known as plaques, trigger microglial activation and that microglial activation removes Aβ to limit its toxicity [4, 5]. Based on this, some proposed leveraging microglial phagocytosis for therapeutic purposes [6, 7]. Clinical trial data from one vaccine-mediated immunotherapy intervention are indeed consistent with a microglial phagocytosis-facilitated reduction of fibrillar Aβ [8–11], although fibrillar Aβ reduction in this trial did not seem to meaningfully attenuate clinical disease progression [12, 13]. Alternatively, in AD activated microglia release inflammatory cytokines that theoretically could contribute to neurodysfunction, neurodegeneration, and disease progression [14]. This suggests interventions that reduce microglial cytokine production might also benefit AD patients [15]. Data from clinical interventions that modify or suppress the general immune system, however, are so far negative [16–18].
Relatively recent genome wide association studies (GWAS) reveal variants of genes highly expressed in microglia associate with AD risk [19, 20]. This suggests inflammatory changes in AD may not simply constitute a standard uniform reaction to other AD pathologies. Rather, more nuanced, genetically determined responses of microglia to other AD pathologies may influence whether a given individual ultimately does or does not develop clinical AD. Alternatively, associations between AD risk and single nucleotide polymorphisms (SNPs) in “microglial genes” suggest neuroinflammation in AD could potentially arise upstream of Aβ histopathology, and directly or indirectly drive Aβ histopathology.
In this study, we addressed the question of whether microglial cytokine release can influence the expression and processing of amyloid β precursor protein (AβPP), the parent protein from which Aβ derives. The effects of microglial cytokine release on tau, apolipoprotein E (ApoE), and cell lipid status were also evaluated.
Materials and Methods
Cell Culture and Treatment Conditions
BV2 mouse microglia cells and SH-SY5Y neuroblastoma cells were cultured in high glucose DMEM (Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. When not being used in experiments, cells were grown in T75 flasks at 37 °C and in humidified air containing 5% CO2.
For BV2 cell lipopolysaccharide (LPS) exposures, BV2 cells grown to 90% confluence in T75 flasks were harvested using trypsin, and transferred to 100 mm dishes. 2,500,000 cells were added to each dish. 24 hours after transferring the BV2 cells from the flasks to the dishes, we added LPS from a 1 mg/ml stock solution to each dish. The LPS stock solution was prepared by adding 1 mg of LPS (obtained from E. coli; Sigma-Aldrich) to 1 ml of sterile phosphate buffered saline (PBS), and gently swirled until the LPS fully dissolved. The final LPS concentration in the dishes was 1 μg/ml. At the completion of each LPS exposure we collected the medium. To then prepare activated BV2 cell conditioned medium, we washed the LPS-treated BV2 cells with PBS to remove any remaining LPS, and then placed the cells in fresh culture medium that did not contain LPS. At the end of the medium conditioning period, we collected the medium and filtered it using 0.2 μm membranes. The freshly prepared conditioned medium was then used to treat SH-SY5Y cells. To prepare conditioned medium from unactivated BV2 microglial cells (the control condition), all these steps were followed except we omitted the addition of the LPS.
For experiments that included SH-SY5Y cells, cells grown to 90% confluence in T75 flasks were harvested using trypsin and transferred to 60 mm dishes. 2,500,000 cells were added to each dish. 24 hours after transferring SH-SY5Y cells from flasks to dishes, the growth medium was removed and replaced with conditioned medium obtained from the LPS-activated BV2 cells, or from unactivated BV2 cells that were not treated with LPS.
BV2 Cell Enzyme-Linked Immunoabsorption Assay (ELISA)
A mouse TNFα Instant ELISA kit (eBioscience) was used to measure TNFα levels in conditioned media from BV2 cells, and an interleukin-6 (IL-6) mouse ELISA kit (Invitrogen) was used to measure IL-6 levels in conditioned media. Briefly, conditioned medium was collected and subjected to acetone precipitation by adding twice the volume of ice cold 100% acetone. The resulting mixture was incubated at −20°C for 30 minutes, and centrifuged for 10 minutes at 3,000 x g, 4°C. The pellets were washed with 70% ethanol, re-suspended in 8 M urea, and heated for 5 minutes. The resulting samples were analyzed using a 1:2 dilution. To screen for levels of the IL-1 family, we used an ELISA kit to mouse IL-1β (Abcam), in which protein from BV2-conditioned media was precipitated and resuspended using the cell extraction buffer provided with the kit.
SH-SY5Y Cell and Medium Immunochemistry
To generate SH-SY5Y whole cell lysates, cells from the 60 mm dishes were rinsed in PBS, scraped until detached, and lysed in ice-cold RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1% NP-40, pH 7.4) that contained a protease inhibitor cocktail (Thermofisher). The resulting lysates were centrifuged at 18,000 x g for 2 minutes, and the pellets discarded. A BCA protein assay kit (Thermo Scientific/Pierce) was used to measure protein concentrations.
We also quantified the amount of soluble AβPPα (sAPPα) that was present in the medium 24 hours after we added it to the SH-SY5Y cells. To do this, we collected the conditioned medium from the SH-SY5Y cells, and added two volumes of ice cold 100% acetone to precipitate the medium proteins. The resulting mixture was incubated at −20°C for 30 minutes, and centrifuged for 10 minutes at 3,000 x g, 4°C. The pellets were washed with 70% ethanol, resuspended in 8 M urea, and heated for 5 minutes. A BCA protein assay kit (Thermo Scientific/Pierce) was used to measure the protein concentration for each replicate.
Equal amounts of protein were resolved through SDS-PAGE using 4–15% Criterion TGX Tris-glycine polyacrylamide gels (Bio-Rad). Gel proteins were transferred to polyvinylidene fluoride (PVDF) membranes (Invitrogen).
Membranes were blocked in 5% bovine serum albumin (BSA) with PBS-Tween 20 (PBST) at room temperature while rocking, and incubated in primary antibody in 5% BSA with PBST overnight at 4°C while rocking. Table 1 indicates the antibodies used and their dilutions. Membranes were washed 3 times with PBST and placed in secondary antibody at 1:4000 dilutions in 5% skimmed milk with PBST for 1 hour at room temperature. Membranes were washed 3 times with PBST and incubated with Supersignal West Femto chemiluminesence reagent (Thermo-Pierce). Images were captured using a Chemidoc imaging station (Bio-Rad). For the sAβPP PVDF membrane, following Western blotting Amido Black (Sigma) total protein staining of the PVDF membrane was completed and densitometry analysis of the Amido Black-stained membrane was performed using a Chemidoc imaging station. The Amido Black densitometry data were then used to accomplish normalization of the sAβPP Western blot bands.
Table 1.
Antibodies used.
| Antibody | Dilution | Company/Provided By |
|---|---|---|
| Anti-β-Amyloid 6E10 | 1:1000 | Biolegend |
| Tau12 | 1:100,000 | Millipore |
| PHF1 | 1:250 | Dr. Peter Davies |
| Apolipoprotein E | 1:2000 | Abcam |
| Soluble AβPP α | 1:500 | Immuno-Biological Laboratories |
| β-Actin | 1:4000 | Cell Signaling |
| β-Tubulin | 1:2000 | Cell Signaling |
| Horseradish Peroxidase Conjugated Goat anti-Mouse | 1:4000 | Thermofisher |
| Horseradish Peroxidase Conjugated Goat anti-Rabbit | 1:4000 | Thermofisher |
Quantitative PCR
SH-SY5Y cells obtained from the 60 mm dishes were placed in a solution containing 1.5 ml of Trizol and 0.5 ml of chloroform. The suspension was centrifuged at 12,000 x g for 15 minutes at 4°C. The colorless upper-aqueous phase was collected, added to 0.6 ml of 100% isopropanol, and centrifuged at 12,000 x g for 10 minutes at 4°C. The supernatant was discarded, and the RNA pellet was washed in 75% ethanol and re-suspended in RNAase-free water.
RNA to cDNA reverse transcription was performed on 1 μg of total RNA using iScript Reverse Transcription Supermix (Bio-Rad). Quantitative real-time PCR (qPCR) was performed using an Applied Biosystems 7300 Real Time PCR System. To determine cycle threshold (CT) values for TNFα, AßPP, ApoE, IL-1β, and ß-Actin (ACTB), TaqMan Universal Master Mix (Applied Biosystems) and TaqMan primers (Thermofisher) were used. To determine CT values for tau and 36B4, we used SYBR Green Supermix (Bio-Rad) and primers to tau (forward primer 5’-CGAAGTGATGGAAGATCACG-3’; reverse primer 5’-GCTTCTTCAGCTTTCAGGCC-3’) and 36B4 (forward primer 5’-CAGCAAGTGGGAAGGTGTAATCC-3’; reverse primer 5’-CCCATTCTATCATCAACGGGTACAA-3’). TNFα, AßPP, IL-1β, and ApoE cycle threshold (CT) values were normalized to the ACTB CT value, and the tau CT value was normalized to the 36B4 CT value using the 2−ΔΔCT method.
Lipid Analyses
We used BODIPY dyes to determine per-cell lipid droplet counts from both living and fixed SH-SY5Y cells. In both cases, SH-SY5Y cells were plated at 20,000 cells per well in black-sided, clear-bottomed 96 well microplates. For the living cell assessments, at the end of the 24-hour conditioned medium treatment we stained the SH-SY5Y cells with BODIPY 500/510 (4,4-Difluoro-5-Methyl-4-Bora-3a,4a-Diaza-s-Indacene-3-Dodecanoic Acid; Thermofisher) according to the manufacturer’s instructions, along with Hoechst 33342, for 30 minutes at 37 °C, in humidified air containing 5% CO2. For fixed cell BODIPY staining, at the end of the 24-hour conditioned medium treatment we washed twice with PBS and fixed the cells for 15 minutes with 4% paraformaldehyde (PFA) at room temperature. The cells were then washed twice with PBS, and stained for 30 minutes with BODIPY 493/503 (4,4-Difluoro-1,3,5,7,8-Pentamethyl-4-Bora-3a,4a-Diaza-s-Indacene; Thermofisher) according to the manufacturer’s instructions, along with Hoechst 33342, at 37 °C. Wells containing either living or fixed stained cells were visualized with an Olympus IX71 fluorescence microscope equipped with a DP71 digital camera. Three to four image fields were taken for each well, and Image J software was used to determine the per cell number of lipid vesicles for the cells in each field.
We used BODIPY 581/591 (Thermofisher) to quantify the extent of SH-SY5Y cell lipid peroxidation. BODIPY 581/591, when exposed to peroxyl radicals, shifts its fluorescence emission peak from 590 nm (red) to 510 nm (green). This fluorescence shift is detectable by flow cytometry using a fluorescence-activated cell sorting (FACS) instrument. For these experiments, 2,000,000–4,000,000 SH-SY5Y cells were harvested and resuspended in 2 ml HBSS supplemented with Ca2+ and Mg2+. The cells were stained with BODIPY 581/591 (1:1000) for 30 minutes at 37 °C, in humidified air containing 5% CO2. Next, the SH-SY5Y cells were washed twice with HBSS and analyzed by FACS. The extent of oxidation was calculated using the following formula: (green fluorescence intensity) / (green fluorescence intensity + red fluorescence intensity).
We further used fluorescence microscopy to visualize the BODIPY 581/591 stained cells Briefly, SH-SY5Y cells were stained with BODIPY 581/591 and Hoechst 33342. Green, red, blue fluorescence signals were separately recorded using an Olympus IX71 fluorescence microscope equipped with a DP71 digital camera.
Statistical Analyses
Unless otherwise specifically indicated, six separate experiments were performed for each assay. Data were summarized by means and standard errors (standard error of the mean; SEM). A one-way ANOVA test, followed by Fisher’s least significant difference (LSD) post hoc testing, was used to compare groups of three or more. Independent t-tests were used for two-group comparisons. P values less than 0.05 were considered statistically significant. All statistical analyses were conducted using SPSS 24.0 (SPSS Inc).
Results
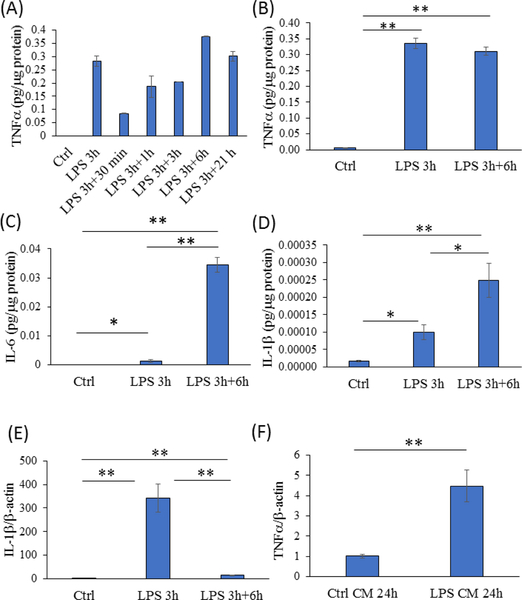
Per existing reports [21, 22], we used a 1 μg/ml LPS concentration to activate BV2 microglia cell cytokine production. In preliminary experiments, we observed 3, 6, and 12-hour 1 μg/ml LPS exposures produced medium TNFα levels that exceeded those from shorter 1-hour and longer 24-hour treatment durations (data not shown). We further found that after removing the LPS, the 3-hour treated cells showed the most sustained TNFα production (data not shown), with medium levels plateauing after 6 hours at an amount which approximated that induced by the direct treatment (Figure 1a, b). Six hours after being removed from a 3-hour LPS treatment, IL-6 showed a robust increase over baseline, and indeed this increase exceeded the level that was present after 3 hours of direct LPS exposure (Figure 1c). After 3 hours, medium IL-1β was also elevated over baseline, and during the 6-hour medium conditioning period that followed removal of the LPS, IL-1β also continued to accumulate (Figure 1d). Although IL-1β production clearly continued following LPS removal, IL-1β mRNA expression reverted towards its baseline level following the LPS removal (Figure 1e).
Figure 1. Validation of the experimental protocol.
(A) For the BV2 cells, medium TNFα levels were essentially undetectable without LPS and markedly increased after a 3-hour, 1 μg/ml LPS exposure. After removal of LPS, the BV2 cells continued to release TNFα, with medium levels plateauing after 6 hours. n=2 for each parameter. (B) For the activated BV2 cells, medium TNFα levels in the 6-hour conditioned medium from previously LPS-treated cells approximated those induced by a direct, 3-hour 1 μg/ml LPS treatment. n=6 for each parameter. (C) Medium IL-β levels increased after the 3-hour LPS exposure and continued to accumulate during the 6- hour medium conditioning period. n=6 for each parameter. (D) Medium IL-ip levels increased after the 3-hour LPS exposure and continued to accumulate during the 6-hour medium conditioning period. n=3 for each parameter. (E) BV2 cell IL-1β mRNA expression rose sharply during the 3-hour LPS exposure, and approached but did not reach the baseline level 6 hours after the LPS was removed. n=3 for each parameter. (F) SH-SY5Y cells maintained in the 6- hour conditioned medium from activated BV2 cells for 24 hours showed an approximate 4.5-fold increase in their endogenous TNFα mRNA expression. n=5 for the control, n=6 for the cells in conditioned medium. *p<0.05 versus control, **p<0.01 versus control. Ctrl=control, CM=conditioned medium. Error bars are SEM.
We then established that a 24-hour exposure to this conditioned medium physiologically affected SH-SY5Y cells, by demonstrating an approximate 4.5-fold increase in their TNFα mRNA expression (Figure 1f). These preliminary experiments defined a protocol in which BV2 cells were treated with 1 μg/ml LPS for 3 hours, the LPS-containing medium was removed and replaced with LPS-free medium, the LPS-free medium was conditioned by the activated BV2 cells for 6 hours, and the activated BV2 cell conditioned medium was added to SH-SY5Y cells for 24 hours.
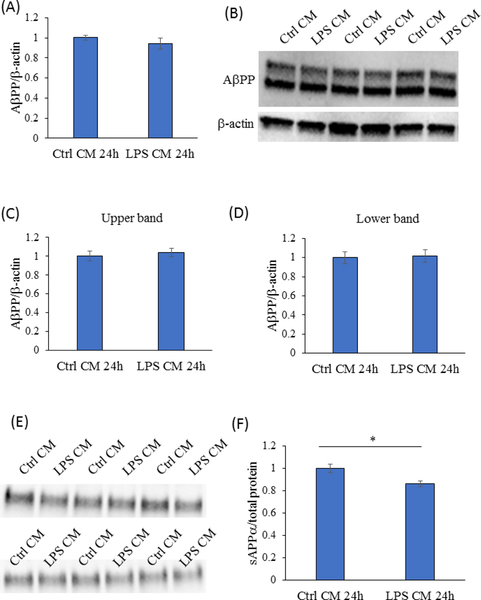
Amyloid plaques represent a classic AD histopathology feature. The plaques largely contain Aβ peptides, which derive from a larger protein, AβPP. AβPP undergoes secretase-mediated cleavage through an amyloidogenic pathway, which generates Aβ, or a non-amyloidogenic pathway, which does not. The 24-hour exposure to the activated BV2 cell-conditioned medium did not change SH-SY5Y cell AβPP mRNA or protein levels (Figure 2a–d). However, the amount of sAβPPα, which is generated through the non-amyloidogenic pathway, fell by 13.5% (p<0.05) (Figure 2e, f). Preserved AβPP, in conjunction with reduced sAβPPα, suggests the activated BV2 cell-conditioned medium diverted AβPP processing from the non-amyloidogenic pathway.
Figure 2. 24-hour exposure to the activated BV2 cell conditioned medium did not alter SH- SY5Y cell AβPP levels but reduced sAβPPα.
(A) AβPP mRNA levels from SH-SY5Y cells maintained for 24 hours in conditioned media from activated and unactivated BV2 cells were comparable. n=6 for both groups. (B) Representative Western blot for ApPP revealed a double band pattern. (C) Densitometry analysis for both the upper and lower ApPP Western blot bands revealed equivalent AβPP protein levels in SH-SY5Y cells maintained in conditioned media from activated and unactivated BV2 cells. n=6 for both groups. (E) Western blot for sAβPPα. (F) After normalizing to the Amido Black density from each lane, densitometry analysis of the sAβPPα Western blot bands revealed sAβPPα levels were lower in the medium from SH-SY5Y cells maintained in activated BV2 cell-conditioned medium than they were in the medium from SH-SY5Y cells maintained in unactivated BV2 cell-conditioned medium. n=6 for both groups. *p<0.05. Ctrl=control, CM=conditioned medium. Error bars are SEM.
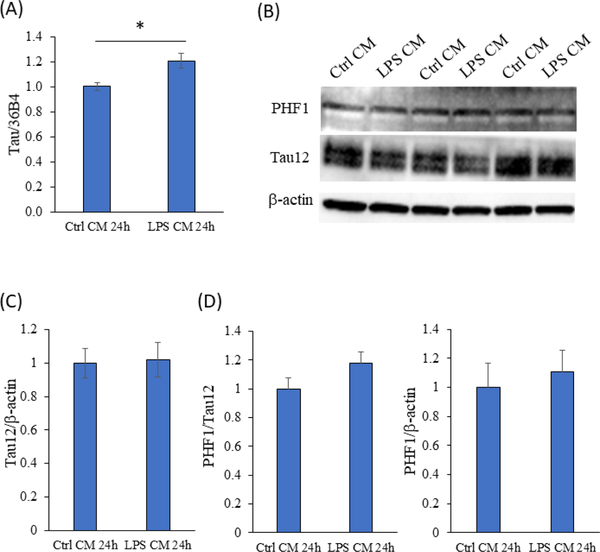
Neurofibrillary tangles constitute another classic AD histopathology. The tangles consist of tau protein. Tau mRNA levels did increase by 21% in the cells maintained in the activated BV2 cell conditioned medium (p<0.05), but levels of total tau protein, detected using the Tau12 antibody, and phosphorylated tau, detected using the paired helical filamentous 1 (PHF1) antibody that detects serine 396/404 phosphorylation, remained equivalent (Figure 3).
Figure 3. 24-hour exposure to the activated BV2 cell conditioned medium increased SH- SY5Y cell tau mRNA but did not alter total or phospho-tau levels.
(A) Tau mRNA increased by 21% in the SH-SY5Y cells treated with conditioned medium from the activated BV2 cells. n=6 per group, *p=0.01. (B) Representative Western blot showing immunostaining with the PHF1 antibody to phospho-tau, and the Tau12 antibody to total tau. (C) Densitometry analysis shows comparable total tau protein levels. (D) Tau phosphorylation levels, when normalized to either total tau or actin, were comparable. n=6 for both groups. Ctrl=control; CM=conditioned medium. Error bars are SEM.
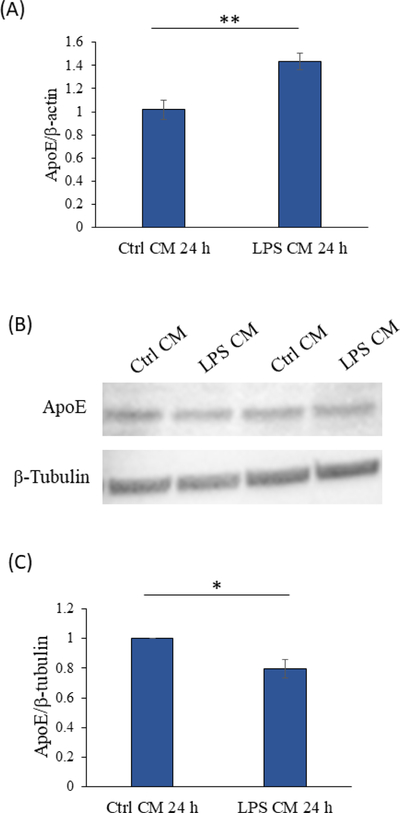
More recently, linkage analysis [23, 24] followed by association studies [25, 26] implicated a role for ApoE, the protein product of the APOE gene, in sporadic AD. Relative to SH-SY5Y cells maintained in the unactivated BV2 cell-conditioned control medium, cells maintained in the conditioned medium from the LPS-activated BV2 cells showed a 43% increase in their ApoE mRNA level (p<0.005). However, the ApoE protein level was 20% lower in lysates prepared from SH-SY5Y cells maintained in conditioned medium from activated BV2 cells than it was in lysates prepared from SH-SY5Y cells maintained in control medium (p<0.05) (Figure 4).
Figure 4. 24-hour exposure to the activated BV2 cell conditioned medium increased SH- SY5Y cell ApoE mRNA but decreased ApoE protein levels.
(A) ApoE mRNA levels. n=6 per group. (B) Representative Western blot of ApoE protein. (C) ApoE protein levels. n=4 per group. *p<0.05, **p<0.005. Ctrl=control, CM=conditioned medium. Error bars are SEM.
ApoE plays a role in brain lipid transport and is therefore relevant to lipid homeostasis. Because ApoE mRNA and ApoE protein levels were altered by the conditioned medium from the LPS-activated BV2 microglia, we used BODIPY dyes to screen the lipid status of the conditioned media-treated SH-SY5Y cells. In one experiment, BODIPY 493/503 staining was performed on fixed cells. In the other, BODIPY 500/510 staining was performed on living cells. In both experiments we observed trends towards reduced numbers of lipid droplets in the SH-SY5Y cells treated with conditioned medium from activated BV2 cells (11% lower mean in the fixed cells, p=0.17; 13% lower in the living cells, p=0.10). When the data from both experiments were combined, there was a significant (p<0.05) 11% reduction in the mean number of lipid droplets in the cells maintained for 24 hours in activated BV2 cell-conditioned medium (Figure 5a–e). We also used another BODIPY dye, BODIPY 581/591, to assess lipid peroxidation. Relative to cells maintained in the conditioned medium from unactivated BV2 microglia, SH-SY5Y cells maintained for 24 hours in the conditioned medium from the activated BV2 microglia showed a 9.5% increase in lipid peroxidation (p<0.001) (Figure 5f, g).
Figure 5. In SH-SY5Y cells, 24-hour exposure to the activated BV2 cell conditioned medium decreased the per cell number of lipid droplets and increased lipid peroxidation.
(A) Representative fluorescence microscopy image of fixed SH-SY5Y cells stained with BODIPY 493/503 and Hoeschst 33342. (B) Representative fluorescence microscopy image of living SH-SY5Y cells stained with BODIPY 500/510 and Hoeschst 33342. (C) For the fixed cells, the mean number of lipid droplets per cell, normalized to the controls placed in conditioned medium from unactivated BV2 cells, is shown. n=35 images analyzed for the controls, and n=38 images analyzed for the active condition. (D) For the living cells, the mean number of lipid droplets per cell, normalized to the controls placed in conditioned medium from unactivated BV2 cells, is shown. n=21 images analyzed for the controls, n=20 images analyzed for the active condition. (E) When the data from the fixed and living cells are combined, the mean number of lipid droplets in the SH-SY5Y cells maintained in the conditioned medium from the activated BV2 cells was 11% lower than it was in the medium from the unactivated BV2 control cells. (F) Representative fluorescence microscopy image of live SH-SY5Y cells stained with BODIPY 581/591 and Hoeschst 33342. (G) As determined by FACS, BODIPY 581/591 staining revealed lipid peroxidation levels were 9.5% higher in SH-SY5Y cells maintained in the activated BV2 cell-conditioned medium. *p<0.05, **p<0.001. Ctrl=control; CM=conditioned medium. Error bars are SEM.
Discussion
Recent genetic studies indicate genes with relatively high microglial expression influence AD risk [19, 20, 27–29]. This suggests microglia are mechanistically relevant to AD. Here, we considered whether microglial activation could influence AD-relevant pathologies and phenomena. In support of this possibility, we found that under our specific experimental protocol conditioned medium from LPS-activated microglia affected AβPP processing, increased tau transcription, increased APOE transcription but decreased intracellular ApoE protein levels, and altered lipid homeostasis in a neuronal cell line.
One popular AD mechanistic hypothesis, the amyloid cascade hypothesis [30], postulates Aβ directly or indirectly induces other AD pathologies or phenomena. Consistent with this view, experimental studies do reveal Aβ can initiate inflammatory changes [31, 32]. GWAS data that argue microglia play a role in AD are not necessarily inconsistent with the amyloid cascade hypothesis, as microglia with different genetic backgrounds may simply respond differently to Aβ, and through this variably modify AD risk. Our experiments were not designed to confirm or refute whether Aβ initiates inflammatory changes in AD. The GWAS data, though, do raise the question of whether microglial contributions to AD are independent or perhaps even upstream of Aβ. We considered this latter possibility, and probed this proposition by designing an experiment in which microglial activation, rather than Aβ exposure, was the initiating experimental manipulation.
We are not the first to consider such an experiment, and it is important to discuss our findings within the context of the current literature. Arguably, the most relevant related study is that of Lee et al. [33], who used LPS and interferon γ to activate human microglia, collected the conditioned medium, and added the conditioned medium to differentiated SH-SY5Y and N-tera2/D1 cells. Like us, they found cells treated with conditioned medium had more tau mRNA than untreated cells, and demonstrated decreased non-amyloidogenic AβPP processing; they further showed evidence of increased amyloidogenic AβPP processing. Unlike us, they also observed increased tau protein and increased AβPP. They did not evaluate ApoE or lipid homeostasis. Key methodologic differences between our study and Lee et al. include the use of differentiated vs undifferentiated neuronal cells, the derivation of the microglial cells (human temporal lobectomies vs the mouse BV2 line), the microglial activation protocol, whether LPS was present (Lee et al.) or absent (our study) from the conditioned medium, the duration of medium conditioning, and the duration of neuronal cell exposure to the conditioned medium. Another relevant study reported intraperitoneal injection of LPS in mice induced changes in cerebellar (although not cerebral) AβPP expression [34].
In terms of AβPP processing and tau mRNA levels, our findings appear to validate those of Lee et al. [33]. Regarding our divergent findings, due to the methodologic differences stated above we would not conclude one report is more correct than the other. The Lee et al. study may have used a more potent stimulation protocol. We cannot conclude that one study was more physiologically relevant than the other. Both studies, though, find activated microglia produce and release chemicals, compounds, or substrates that can influence AD-related phenomena.
If AβPP non-amyloidogenic processing decreased, as our experiments suggest, it is possible that AβPP amyloidogenic processing increased. Increased amyloidogenic processing would presumably increase Aβ production. We did not directly test this. We also did not experimentally exclude the possibility that the sAβPPα decrease solely arose during the 6-hour period in which the BV2 cells were conditioning the medium. This seems extremely unlikely, though, because the BV2 line is a murine line and our antibody to human sAβPPα should not detect murine AβPP derivatives.
To our knowledge, our observations that conditioned medium from LPS-activated BV2 microglia alters SH-SY5Y neuronal cell ApoE mRNA levels, ApoE protein levels, and lipid homeostasis are quite novel. In this case we did not experimentally manipulate Aβ, so Aβ did not initiate these changes, although we cannot rule out a role for Aβ in mediating these changes. It is certainly possible, though, if not likely, that in our experimental system and under our experimental conditions, microglia-conditioned medium altered ApoE and lipid homeostasis parameters independent of Aβ.
ApoE mRNA and cell lysate protein levels changed in opposite directions. Our interpretation in this case is that increased ApoE mRNA represents either a secondary attempt to reverse a reduction in ApoE protein, or else part of a primary but not fully compensatory cell stress response. In perhaps greater support of the latter possibility, it is reported that ApoE production by neuronal cells, when unstressed, is typically limited, and under stress conditions neuronal ApoE production typically increases [35]. In our experiments cytokines in the conditioned medium would presumably stress the SH-SY5Y cells [36], which in turn could lead to increased APOE transcription. If correct, the observed ApoE protein reduction might reflect increased release or consumption of the protein as part of the stress response.
Regardless, the disproportionality of the opposing changes, with the magnitude of the 43% ApoE mRNA expression increase approximately doubling the 20% cell lysate ApoE protein decrease, indicates ApoE secretion or degradation exceeds the cells’ effort or ability to synthesize it. Whether this mismatch reflects a simple inability to keep up with ApoE loss, or a compromised ability to translate ApoE mRNA to protein, is worth considering. Indeed, with some stress responses, particularly those that feature proteostatic stress, cells can strategically ramp up the expression of some proteins while limiting the translation of others [37]. It is hard to envision, though, a situation in which a stressed cell would actively retard translation of a protein it presumably needs to facilitate a particular stress response.
Cells store lipids as lipid droplets, and stress responses induce lipid mobilization [38, 39]. An observed reduction in the per cell number of lipid droplets is consistent with a conditioned medium-induced state of SH-SY5Y cell stress. Part of this stress was potentially oxidative in nature, as BODIPY 581/591 showed a clear increase in lipid peroxidation. An increase in lipid peroxidation would presumably increase membrane turnover, with a subsequent mobilization of fatty acid and cholesterol reserves.
Links between inflammation and lipid handling/homeostasis are well-recognized. In some models, inflammation appears to mobilize lipids [40, 41], which may alleviate energy stress [42]. As ApoE represents the major central nervous system apolipoprotein [43], it is perhaps not surprising that neuroinflammation would also affect the APOE gene and ApoE protein. In support of this, links between inflammation and ApoE are reported [44, 45]. We cannot say from our experiments whether lipid homeostasis changed because ApoE dynamics changed, ApoE dynamics changed because lipid homeostasis changed, or that these are independent events.
Regardless of how inflammation-induced ApoE and lipid changes relate to each other, we speculate that elucidating this relationship could inform perceptions of AD pathology and molecular dysfunction. Despite decades of research, the AD field is still unsure about why APOE isoforms strongly associate with AD risk, or how the different ApoE protein variants defined by the common APOE polymorphisms functionally account for changes in risk. Some hypothesize direct interactions between ApoE and Aβ mediate the effect [46], while others hypothesize ApoE’s moderation of AD does not depend on Aβ interactions [47, 48]. Our data do not strongly inform this debate, but at face value are perhaps more consistent with the latter perspective.
It is important to note our study did not specifically attempt to model AD. We simply used a standard activator of microglia, LPS, to initiate microglial cytokine release from an immortalized murine microglial cell line; exposed a standard human neuronal cell line to the microglial cell conditioned medium; and assessed for specific phenomena based on the association of those phenomena with AD. Thus, it is best to interpret our findings within a very narrow context, which is that our data suggest microglia-derived products or components can influence at least some aspects of AβPP biology, tau, ApoE and lipid handling, and lipid integrity in a cell line.
We studied the effects of an acute microglial activation, which may poorly reflect the chronic state of microglial activation that exists in human AD. Due to the nature of our approach, we were unable to address whether activated microglia might also influence AD-related phenomena through phagocytosis. Interpretations are limited to the finding that products derived from microglia can influence neuronal cells. We presume that in the activated microglia cell conditioned medium, cytokines constituted the driving component. To this end we used TNFα, IL-1β, and IL-6 as markers of cytokine release, and to document the conditioned medium from the activated microglia was an “inflammatory” medium. However, we did not specifically measure levels of other cytokines, and we cannot assume or conclude any single cytokine mediated the observed SH-SY5Y cell changes.
TNFα, IL-1, and IL-6 are pro-inflammatory cytokines, produced by microglia, whose levels increase in AD [49]. Aβ is consistently found to induce the production of these cytokines [49], but conversely these cytokines can alter Aβ and other relevant histopathology, including tau. In addition to mediating inflammation responses by activating inflammatory cells, in AD transgenic mice TNFα amplifies Aβ and tau histopathology [50]. IL-1, which figures prominently in neuroinflammation events [51], was similarly shown in mice to increase tau expression, phosphorylation, and aggregation [52]. Conversely, IL-1 may promote the removal of Aβ plaques or molecular patterns that can otherwise stress a local tissue [53]. IL-6 has protean effects [54], and reportedly can facilitate or mitigate AD histopathology [55, 56]. Our study only evaluated the ability of cytokines to influence AD histopathology, and did not address the potential role of Aβ in initiating cytokine production. Clearly, the sequence of events one observes in an AD-related study depends on the nature of the model used by the study.
Aβ’s physiologic roles, should such exist, are currently not well defined. Connections between APP processing, Aβ, and inflammation could provide clues. Recent reports claim Aβ may function as an antimicrobial peptide [57]. If correct, it would make sense for Aβ to both promote and be promoted by immune responses. Here, we considered the possibility that changes in microglial cytokine production could alter neuronal cell APP processing. To more reliably inform this possibility, we ensured LPS was not present in the microglia-conditioned medium. In our experiments, therefore, LPS should not account for SH-SY5Y cell responses.
Despite its limitations, our study does support the emerging concept that microglial function influences AD histopathology and that microglia-derived inflammatory changes may initiate some AD-relevant phenomena. In addition to establishing a role for microglial function in AD molecular pathology, GWAS analyses also indicate a role for lipid metabolism [20, 58]. Such a role was previously hinted at by the fact that ApoE is critical to central nervous system lipid transport [43]. Given this perspective, the primary implications of this current study are that neuroinflammation, ApoE, and lipid homeostasis may all interact, potentially independent of Aβ, to affect AD risk and mediate AD progression.
Acknowledgments
This study was supported by the University of Kansas Alzheimer’s Disease Center (P30AG035982), and by the First Affiliated Hospital of Zhengzhou University.
References
- [1].Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, et al. (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14, 388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wyss-Coray T, Rogers J (2012) Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med 2, a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].McGeer PL, Akiyama H, Itagaki S, McGeer EG (1989) Immune system response in Alzheimer’s disease. Can J Neurol Sci 16, 516–527. [DOI] [PubMed] [Google Scholar]
- [4].Guillot-Sestier MV, Doty KR, Town T (2015) Innate Immunity Fights Alzheimer’s Disease. Trends Neurosci 38, 674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sarlus H, Heneka MT (2017) Microglia in Alzheimer’s disease. J Clin Invest 127, 3240–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, et al. (1999) Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400, 173–177. [DOI] [PubMed] [Google Scholar]
- [7].Bard F, Cannon C, Barbour R, Burke RL, Games D, et al. (2000) Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med 6, 916–919. [DOI] [PubMed] [Google Scholar]
- [8].Boche D, Denham N, Holmes C, Nicoll JA (2010) Neuropathology after active Abeta42 immunotherapy: implications for Alzheimer’s disease pathogenesis. Acta Neuropathol 120, 369–384. [DOI] [PubMed] [Google Scholar]
- [9].Nicoll JA, Barton E, Boche D, Neal JW, Ferrer I, et al. (2006) Abeta species removal after abeta42 immunization. J Neuropathol Exp Neurol 65, 1040–1048. [DOI] [PubMed] [Google Scholar]
- [10].Town T, Nikolic V, Tan J (2005) The microglial “activation” continuum: from innate to adaptive responses. J Neuroinflammation 2, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, et al. (2003) Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med 9, 448–452. [DOI] [PubMed] [Google Scholar]
- [12].Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, et al. (2005) Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 64, 1553–1562. [DOI] [PubMed] [Google Scholar]
- [13].Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, et al. (2008) Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet 372, 216–223. [DOI] [PubMed] [Google Scholar]
- [14].Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, et al. (2000) Inflammation and Alzheimer’s disease. Neurobiol Aging 21, 383–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Meraz-Rios MA, Toral-Rios D, Franco-Bocanegra D, Villeda-Hernandez J, Campos-Pena V (2013) Inflammatory process in Alzheimer’s Disease. Front Integr Neurosci 7, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Soininen H, West C, Robbins J, Niculescu L (2007) Long-term efficacy and safety of celecoxib in Alzheimer’s disease. Dement Geriatr Cogn Disord 23, 8–21. [DOI] [PubMed] [Google Scholar]
- [17].Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, et al. (2003) Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. Jama 289, 2819–2826. [DOI] [PubMed] [Google Scholar]
- [18].Aisen PS, Davis KL, Berg JD, Schafer K, Campbell K, et al. (2000) A randomized controlled trial of prednisone in Alzheimer’s disease. Alzheimer’s Disease Cooperative Study. Neurology 54, 588–593. [DOI] [PubMed] [Google Scholar]
- [19].Villegas-Llerena C, Phillips A, Garcia-Reitboeck P, Hardy J, Pocock JM (2016) Microglial genes regulating neuroinflammation in the progression of Alzheimer’s disease. Curr Opin Neurobiol 36, 74–81. [DOI] [PubMed] [Google Scholar]
- [20].Karch CM, Goate AM (2015) Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry 77, 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yoon CS, Kim DC, Quang TH, Seo J, Kang DG, et al. (2016) A Prenylated Xanthone, Cudratricusxanthone A, Isolated from Cudrania tricuspidata Inhibits Lipopolysaccharide-Induced Neuroinflammation through Inhibition of NF-kappaB and p38 MAPK Pathways in BV2 Microglia. Molecules 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lee KW, Jung SY, Choi SM, Yang EJ (2012) Effects of ginsenoside Re on LPS-induced inflammatory mediators in BV2 microglial cells. BMC Complement Altern Med 12, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schellenberg GD, Deeb SS, Boehnke M, Bryant EM, Martin GM, et al. (1987) Association of an apolipoprotein CII allele with familial dementia of the Alzheimer type. J Neurogenet 4, 97–108. [PubMed] [Google Scholar]
- [24].Pericak-Vance MA, Bebout JL, Gaskell PC Jr, Yamaoka LH, Hung WY, et al. (1991) Linkage studies in familial Alzheimer disease: evidence for chromosome 19 linkage. Am J Hum Genet 48, 1034–1050. [PMC free article] [PubMed] [Google Scholar]
- [25].Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, et al. (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261, 921–923. [DOI] [PubMed] [Google Scholar]
- [26].Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, et al. (1993) Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A 90, 1977–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, et al. (2013) TREM2 variants in Alzheimer’s disease. N Engl J Med 368, 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, et al. (2013) Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med 368, 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sims R, van der Lee SJ, Naj AC, Bellenguez C, Badarinarayan N, et al. (2017) Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nat Genet 49, 1373–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hardy JA, Higgins GA (1992) Alzheimer’s disease: the amyloid cascade hypothesis. Science 256, 184–185. [DOI] [PubMed] [Google Scholar]
- [31].McLarnon JG, Ryu JK (2008) Relevance of abeta1–42 intrahippocampal injection as an animal model of inflamed Alzheimer’s disease brain. Curr Alzheimer Res 5, 475–480. [DOI] [PubMed] [Google Scholar]
- [32].Rogers J, Cooper NR, Webster S, Schultz J, McGeer PL, et al. (1992) Complement activation by beta-amyloid in Alzheimer disease. Proc Natl Acad Sci U S A 89, 10016–10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lee M, McGeer E, McGeer PL (2015) Activated human microglia stimulate neuroblastoma cells to upregulate production of beta amyloid protein and tau: implications for Alzheimer’s disease pathogenesis. Neurobiol Aging 36, 42–52. [DOI] [PubMed] [Google Scholar]
- [34].Brugg B, Dubreuil YL, Huber G, Wollman EE, Delhaye-Bouchaud N, et al. (1995) Inflammatory processes induce beta-amyloid precursor protein changes in mouse brain. Proc Natl Acad Sci U S A 92, 3032–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mahley RW, Huang Y (2012) Apolipoprotein e sets the stage: response to injury triggers neuropathology. Neuron 76, 871–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, et al. (2003) Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A 100, 8514–8519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pakos-Zebrucka K, Koryga I, Mnich K, Ljujic M, Samali A, et al. (2016) The integrated stress response. EMBO Rep 17, 1374–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Thiam AR, Farese RV Jr., Walther TC (2013) The biophysics and cell biology of lipid droplets. Nat Rev Mol Cell Biol 14, 775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Welte MA (2015) Expanding roles for lipid droplets. Curr Biol 25, R470–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Coppack SW (2001) Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc 60, 349–356. [DOI] [PubMed] [Google Scholar]
- [41].Ogawa H, Nielsen S, Kawakami M (1989) Cachectin/tumor necrosis factor and interleukin-1 show different modes of combined effect on lipoprotein lipase activity and intracellular lipolysis in 3T3-L1 cells. Biochim Biophys Acta 1003, 131–135. [DOI] [PubMed] [Google Scholar]
- [42].Klosinski LP, Yao J, Yin F, Fonteh AN, Harrington MG, et al. (2015) White Matter Lipids as a Ketogenic Fuel Supply in Aging Female Brain: Implications for Alzheimer’s Disease. EBioMedicine 2, 1888–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mahley RW (2016) Central Nervous System Lipoproteins: ApoE and Regulation of Cholesterol Metabolism. Arterioscler Thromb Vasc Biol 36, 1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Aleong R, Blain JF, Poirier J (2008) Pro-inflammatory cytokines modulate glial apolipoprotein E secretion. Curr Alzheimer Res 5, 33–37. [DOI] [PubMed] [Google Scholar]
- [45].Rebeck GW (2017) The role of APOE on lipid homeostasis and inflammation in normal brains. J Lipid Res 58, 1493–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Castellano JM, Kim J, Stewart FR, Jiang H, Demattos RB, et al. (2011) Human apoE Isoforms Differentially Regulate Brain Amyloid-{beta} Peptide Clearance. Sci Transl Med 3, 89ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Chang S, ran Ma T, Miranda RD, Balestra ME, Mahley RW, et al. (2005) Lipid- and receptor-binding regions of apolipoprotein E4 fragments act in concert to cause mitochondrial dysfunction and neurotoxicity. Proc Natl Acad Sci U S A 102, 18694–18699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chen HK, Ji ZS, Dodson SE, Miranda RD, Rosenblum CI, et al. (2011) Apolipoprotein E4 domain interaction mediates detrimental effects on mitochondria and is a potential therapeutic target for Alzheimer disease. J Biol Chem 286, 5215–5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wang WY, Tan MS, Yu JT, Tan L (2015) Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med 3, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Janelsins MC, Mastrangelo MA, Park KM, Sudol KL, Narrow WC, et al. (2008) Chronic neuron-specific tumor necrosis factor-alpha expression enhances the local inflammatory environment ultimately leading to neuronal death in 3xTg-AD mice. Am J Pathol 173, 1768–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Shaftel SS, Griffin WS, O’Banion MK (2008) The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J Neuroinflammation 5, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Sheng JG, Zhu SG, Jones RA, Griffin WS, Mrak RE (2000) Interleukin-1 promotes expression and phosphorylation of neurofilament and tau proteins in vivo. Exp Neurol 163, 388–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Shaftel SS, Kyrkanides S, Olschowka JA, Miller JN, Johnson RE, et al. (2007) Sustained hippocampal IL-1 beta overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J Clin Invest 117, 1595–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Spooren A, Kolmus K, Laureys G, Clinckers R, De Keyser J, et al. (2011) Interleukin-6, a mental cytokine. Brain Res Rev 67, 157–183. [DOI] [PubMed] [Google Scholar]
- [55].Chakrabarty P, Jansen-West K, Beccard A, Ceballos-Diaz C, Levites Y, et al. (2010) Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: evidence against inflammation as a driving force for amyloid deposition. Faseb j 24, 548–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Quintanilla RA, Orellana DI, Gonzalez-Billault C, Maccioni RB (2004) Interleukin-6 induces Alzheimer-type phosphorylation of tau protein by deregulating the cdk5/p35 pathway. Exp Cell Res 295, 245–257. [DOI] [PubMed] [Google Scholar]
- [57].Kumar DK, Choi SH, Washicosky KJ, Eimer WA, Tucker S, et al. (2016) Amyloid-beta peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci Transl Med 8, 340ra372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Jones L, Harold D, Williams J (2010) Genetic evidence for the involvement of lipid metabolism in Alzheimer’s disease. Biochim Biophys Acta 1801, 754–761. [DOI] [PubMed] [Google Scholar]