Abstract
Neonatal encephalopathy is a major cause of neonatal mortality and morbidity. Therapeutic hypothermia is standard treatment for newborns at 35 weeks of gestation or more with intrapartum hypoxia-related neonatal encephalopathy. Term and late preterm infants with moderate-to-severe encephalopathy show improved survival and neurodevelopmental outcomes at 18 months of age after therapeutic hypothermia. Therapeutic hypothermia can increase survival without increasing major disability. Neonates with severe neonatal encephalopathy remain at risk of death or severe neurodevelopmental impairment. This guideline was prepared by the Turkish Neonatal Society to standardize the management of neonatal encephalopathy throughout the country.
Keywords: Guideline, hypoxic ischemic encephalopathy, neonatal encephalopathy, therapeutic hypothermia
Abstract
Yenidoğan ensefalopatisi yenidoğanlarda ölüm ve morbiditenin en önemli nedenlerinden biridir. Terapötik hipotermi, gebeliğin 35. haftasından sonra doğmuş yenidoğanlar için standart tedavi olup, doğum ve sonrasında gelişen hipoksi ile ilişkili yenidoğan ensefalopatisinde kullanılmaktadır. Terapötik hipotermi tedavisinden sonra, orta ve ağır ensefalopatili term ve geç preterm doğmuş bebeklerin, 18 ayda sağkalım ve nörogelişimsel sonuçlarının iyileştiği görülmektedir. Terapötik hipotermi, sakatlığı arttırmaksızın yaşam kalitesini arttırabilir. Şiddetli yenidoğan ensefalopatisi geçiren yenidoğanlar, ölüm riski altındadır ya da ciddi nörogelişimsel bozulmaya eğilimlidir. Bu rehber, Türk Neonatoloji Derneği tarafından, yenidoğan ensefalopatisi tedavisinin ülke çapında standartlaştırılması için hazırlanmıştır.
Introduction, definitions, and pathophysiology
Neonatal encephalopathy (NE) is a heterogeneous clinical syndrome that occurs in the early neonatal period in newborns born at the 35th gestational week and later. It is manifested with impaired consciousness or convulsions and characterized by respiratory depression and hypotonia (1, 2). Neonatal encephalopathy may occur in relation to numerous conditions; birth asphyxia and neonatal hypoxic ischemic encephalopathy (HIE) are responsible for most of these. NE continues to be used as a more inclusive, but a more general term because this cause-effect relationship has not been understood fully due to the natural characteristics of the neonatal brain and the complexity of the etiology (1). Although the incidence shows variance by the defined condition, it has been reported to range between 2 and 9 in 1000 live births in different series (3). The incidence of NE after the definitions were established was found as 3 per 1000 live births in one study, and hypoxic ischemic encephalopathy was observed with an incidence of 1.5 per 1000 live births (4, 5). In Turkey, 93 babies per 19,857 live births were examined under the diagnosis of HIE according to the data published by the Turkish Neonatal Society Hypoxic Ischemic Encephalopathy Study Group, and the incidence was found as 2.6 per 1000 live births and 1.2% in patients hospitalized in intensive care units (6).
The primary event in the pathophysiology of perinatal asphyxia is inadequate gas exchenge in the placenta or impaired ventilation at the pulmonary level because of postnatal events. As a result, oxygen and carbondioxide exhange is impaired and arterial hypoxemia, hypercarbia, and acidosis develop (5, 7). The changes occuring in the cerberal blood flow due to asphyxia are important. In the early phase following perinatal asphyxia, the body tries to increase cerebral blood flow by decreasing cerebral vascular resistance, adjusting cardiac output, and developing systemic hypertension. The cerebral blood flow is compensated by dilatation of the small cerebral vessels with reduced cerebral perfusion due to asphyxia. Maintenance of cerebral blood flow despite changes in the systemic blood pressure is an autoregulation mechanism. Cerebrovascular autoregulation is the system that enables the brain’s normal functional activity and uses the contraction and dilatation mechanisms of the vessles in order to keep the cerebral blood flow stable. The great cerebral blood vessels are more important in autoregulation compared with arterioles. Many chemical substances are important in the control of cerebral arterial tonus. Nitric oxide causes vascular dilatation by way of a Ca++ dependent K+ channel in the vascular endothelium and endothelin-1 and prostanoids are involved in vasoconstriction.
Hypoxia affects autoregulation negatively in association with hypercarbia and hypoglycemia. The response of the cerebral arterioles to pressure changes and CO2 concentration changes because of impaired autoregulation due to hypoxia, and this leads to changes in the cerebral blood flow independent of pressure (5). Impaired cerebrovascular autoregulation and the resulting primary and secondary energy insufficiency are responsible for the pathogenesis of cerebral injury.
Two different phases have been described at the molecular and cellular levels in cell death in the central nervous system. The first phase occurs during reperfusion and reoxygenation; the second phase starts hours later and this period may last 72 hours. In the first phase, asphyxia causes NAD to rapidly transform into NADH. When the energy requirement is not met, a shift from aerobic to anaerobic metabolism occurs and this causes an acceleration in glycolysis and the production of lactate increases. At the same time, tricarbocylic acid cycle (TCA) byproduct concentration decreases and the production of high energy phosphate decreases (5). These changes lead to a rapid reduction in phosphocreatine and reduction in cerebral adenosine triphosphate (ATP) concentration. With the reduction in adenosine triphosphate concentration, especially the Na+-K+ pump (it is critical in maintaining high intracellular K+ concentration and low intracellular Na+ concentration) cannot perform its functions. When the function of the ion pump is impaired, neuronal membrane changes. Normally, some neurons are hyperpolarized and some others are depolarized. If anoxia persists, all cells are rapidly and markedly depolarized and membrane potential disappears completely. The reduction in intracellular and extracellular pH pioneers change in membrane potentials. Hypoxia causes the production of lactate and intracellular acidosis. Extracellular acidosis develops as a result of the transfer of intracellular H+ ion and lactate to the extracellular space. With the disappearance of neuronal membrane potential, Na+, Cl- and Ca++ enter the cell and K+ exits the cell. In addition, extacellular glutamate concentrations increase with the increased glutamate secretion and decreased glutamate reuptake.
One of the important conditions in neuron injury due to asphyxia is excitotoxicity. The increase in extracellular glutamate during hypoxia causes excessive stimulation of glutamate receptors and cell death. Excessive Na++ enters the cell via glutamate-dependent ion channels; additionally, Cl- and water enter the cell, which leads to osmotic lysis. The continuous increase in intracellular Ca++ stimulates a toxic cascade and this leads to necrotic cell death. In the second phase of asphyxia-realted injury and cell death, slow cell death occurs. Free radicals, inflammatory toxins, and endogeneous growth factors are considered factors that initiate apoptosis following asphyxia.
In newborns with encephalopathy, abnormal consciousness is observed (hyper-alertness, restlessness, lethargy), spontaneous movements are decreased and respiratory and feeding problems are present. The baby’s tonus and posture are impaired and their primitive reflexes may be absent. The Apgar score is frequently low in the delivery room, the baby might cry weakly or not at all. The degree and severity of neonatal encephalopathy may be classified as mild, moderate, and severe depending on the presence or absence of these clinical conditions (Table 1) (5, 7).
Table 1a.
Sarnat & Sarnat classification
| Finding | Stage 1 | Stage 2 | Stage 3 |
|---|---|---|---|
| Level of consciousness | Hyper-alert | Lethargic | Stupor, coma |
| Muscle tone | Normal | Hypotonic | Flask |
| Posture | Normal | Flexion | Decerebrated |
| Tendon reflexes/clonus | Hyperactive | Hyperactive | Absent |
| Myoclonus | Present | Present | Absent |
| Moro reflex | Live | Weak | Absent |
| Pupillae | Mydriasis | Myosis | Anisocoric |
| Seizures | Absent | Frequent | Decerebration |
| EEG findings | Normal | Ranging between low voltage to seizure activity | Burst suppression, isoelectric activity |
| Time | Less than 24 hours | 1-14 days | A few days-weeks |
| Outcome | Good | Variable | Mortality or severe sequela |
EEG: electroencephalography
The approach to patients with encephalopathy: potential causes should be investigated in order to elucidate the etiology in a newborn with encephalopathy. The most recently published scientific recommendations and consensus related to this issue have been summarized by the American College of Obstetricians and Gynecologists (ACOG) (1, 2). In this approach, history of maternal drug use, other maternal diseases, obstetric risks, intrapartum factors, and placental pathology should be investigated to demonstrate the causes.
The delivery room approach (the Turkish Neonatal Society delivery room management guideline (8)): perinatal asphyxia continues to be an important cause of mortality and morbidity worldwide. It is defined as development of hypoxemia, hypercapnia, and metabolic acidosis as a result of impaired gas exchange. As a result of this, injury may develop in the brain and other organs in the fetus and newborn.
In the delivery room, the objective is to provide sufficient perfusion in the brain and other organs in order to prevent long-term sequelae (8). Resuscitation steps are performed in accordance with the above-mentioned recommendations to provide adequate ventilation and to correct circulation urgently. In addition, the following conditions should be cared for in these babies. Hyperoxia should be avoided because it will increase reperfusion injury caused by free oxygen radicals, especially in the brain and myocardium. Reoxygenation of the tissues should be fast, but controlled. An oxygen-air mixer and pulse oximeter should be used. It is important to correct respiratory failure in babies who carry a risk for development of hypoxic ischemic encephalopathy. The PaCO2 level should be kept within the normal limits because it affects cerebral blood flow. After the initial stabilization, blood gases should be closely monitored. It is important to avoid hypothermia and hypoglycemia. In babies who are thought to have developed asphyxia and are predicted to develop NE, resuscitation should be continued by turning off radiant heaters. Volume loading frequently occurs in babies in whom multi-organ injury develops with the interruption of the placental blood flow (8). Lung compliance is reduced and pulmonary edema may develop. Volume loading is related with acute tubular necrosis and related acute renal failure or inappropriate antidiuretic hormone secretion. However, fluid loading in the delivery room also contributes to this. In these babies, volume expanders should be used carefully in the delivery room. Following resuscitation and stabilization, they should be monitored in a center where their systemic and cerebral functions can be monitored and subsequent treatments should be rapidly planned. In term or near-term babies with moderate-severe ischemic encephalopathy, therapeutic hypothermia is a treatment method with proven efficacy and its application in accordance with the recommended treatment protocols in centers with technical equipment and referral to a center where it can be applied (following initial stabilization in centers where technical equipment is not available) are important in terms of reducing mortality and morbidity (8).
Applications related to the umbilical cord (8): Indications for obtaining blood samples from the umbilical cord. Blood samples can be obtained from the umbilical cord after delivery in the following conditions: preterm delivery or meconium-stained newborn, shoulder or transverse positions with a risk of trauma in vaginal deliveries, presence of intrapartum maternal body temperature >38°C or maternal hemorrhage, and severe intrapartum cardiotocograph disorders. In addition, the American Academy of Pediatrics (AAP) Fetus-Newborn Committee recommends that cord blood sample should be obtained and blood gases should be evaluated in terms of asphyxia if the 5th minute Apgar score is ≤5 (8).
The technique for obtaining cord blood sample: the umbilical cord is clamped and separated from the placenta by cutting. The cord is clamped for the second time by leaving a 10-cm distance from the site of the first clamp and the blood sample is obtained from the umbilical artery (the artery appears more indistinct because its lumen is smaller and contains less blood compared with the vein) in the region between the two clamps into a heparinized injector (the pH and blood gas values of the blood in the clamped cord segment may remain stable for about 30 minutes at room temperature).
PaO2, PaCO2, and bicarbonate are not important in determining mortality and morbidity. The most important two parameters in the assessment are pH and base excess, which indicate metabolic acidosis. A blood gas pH value of <7.00 in the cord blood sample suggests marked fetal acidemia and a base excess value of -12 to -16 mmol/L suggests that the baby is born with hypoxia, and a base excess value of >16 mmol/L suggests that the baby has been exposed to severe hypoxia (2, 7).
Neuroimaging: Deep gray matter injury may be observed in total asphyxia. In a study that included 48 term babies, deep gray matter injury was observed with a rate of 74% (9). Magnetic resonance imaging (MRI) in patients with encephalopathy may be helpful in both elucidating the etiology and demonstrating the degree of involvement, and in the differential diagnosis of the pathologies including developmental brain anomaly or infarction. Therefore, MRI will be more appropriate in patients with NE. The findings on MRI can also give additional information about the prognosis in these patients. Cranial ultrasonography is also a neuroimaging method used; it is easy to perform and can be performed at the bedside, but gives limited information and depends very much on experience. Magnetic resonance spectroscopy (MRS) and diffusion-weighted imaging are also helpful techniques in the diagnosis and follow-up, similar to MRI. It is appropriate to perform MRI and diffusion weighted MRI between the postnatal 3rd and fifth days. Although studies with a high level of evidence related to the exact accurate time for performing MRI in patients with hypothermia are lacking, MRI can be performed up to the seventh day (10). Computed brain tomography has no priority or diagnostic value in these patients. Cranial ultrasonography should be performed on the first day in the early period in order to detect early perinatal events including diffuse hemorrhage and abnormal development in particular, and be subsequently repeated with appropriate intervals, because bed-side evaluation is easy (10).
Electroencephalography (EEG). EEG may be helpful in differentiating seizures from other problems and in the detection of subclinical seizures. It gives information about the severity of encephalopathy rather than its cause and may support the prediction about the prognosis. ‘Amplitude integrated’ EEG (aEEG) has also been reported to be a helpful EEG method in the diagnosis, treatment, and follow-up of NE. aEEG enables amplitude integration and recording from single channel EEG. Its advantage is that it does not require advanced education for interpretation. The emergence of a sleep-wake cycle on aEEG in the first 36 hours indicates good prognosis. The following classification scheme is recommended to define aEEG findings: continuous normal voltage; Continuous activity with the lower amplitude being approximately 7-10 µV and the upper amplitude being 10-25 µV. Discontinuous normal voltage: discontinuous background activity with the lower amplitude being always below 5 µV and the upper amplitude being above 10 µV. Burst suppression: discontinuous background with an invariable lower amplitude of 0-1 (2) µV and bursts with an amplitude of >25 µV. Continuous extremely low voltage: a background pattern with continuous very low voltage (about or below 5 µV). Flat line with no activity: inactive background below 5 µV (isoelectric line). Interpretation of the findings observed on aEEG is summarized in Figure 1. Mild disruption and normal tracks are related to better long-term outcomes, whereas patients with tracks with severe disruption have been reported to have poorer long-term outcomes (2, 5, 7).
Figure 1.
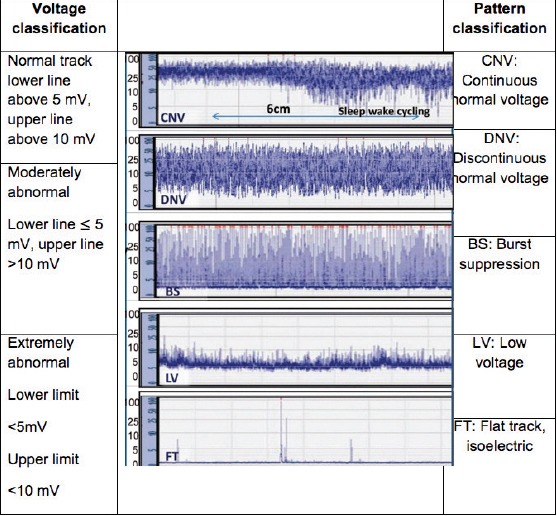
Classification of “Amplitude integrated” EEG images
Diagnosis
If the following events accompany intrapartum or peripartum hypoxic events, they increase the development of encephalopathy; these clinical conditions should be interrogated and recorded in the presence of encephalopathy.
Accompaniment of the following findings/acute events.
(1) A 5th and 10th minute Apgar score of <5; (2) A pH value of <7.00 or BE value of < -12 mmol/L in the fetal cord blood gas; (3) observation of HIE-compatible brain injury on MRI or MRS; (4) Presence of multi-organ failure or involvement.
Accompaniment of acute peripartum-intrapartum events; 1. Presence of uterine rupture, ablatio placenta, cord prolapsus, maternal hypotension, amniotic fluid emboli, maternal hypoxemia, maternal cardiovascular collapse, vasa previa or fetomaternal hemorrhage at birth; 2. Typical findings on imaging, damages to deep gray matter, cortical injury (in border zones); 3. Absence of the following conditions; abnormal fetal growth, maternal infections, fetomaternal hemorrhage, neonatal sepsis, and chronic placental lesions.
Spastic quadriplegia or dyskinetic cerebral palsy may be observed as a developmental outcome.
Some staging systems with amendments continue to be used beyond clinical findings for the diagnosis. The staging system of Sarnat and Sarnat is summarized in Table 1. In addition, Thompson’s scoring system is also widely used. Daily scoring is performed and a clinical prediction related to long-term outcomes can be provided according to the score obtained by the baby (11).
In the literature, there are many publications related to additional biomarkers for the diagnosis, but no marker has been routinely recommended to support the diagnosis.
Initiation of hypothermia treatment may be decided if at least three of the clinical findings found in the second and third columns shown in Table 2 are present.
Table 1b.
Thompson Score (Thompson CM, Puterman AS, Linley LL, et al. The value of a scoring system for hypoxic ischemic encepha¬lopathy in predicting neurodevelopmental outcome. Acta Paediatr 1997; 86: 757-61.)
| Symptom | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Tone | normal | hypertonic | hypotonic | flaccid |
| Consciousness | normal | hyper-alert | lethargic | comatose |
| Fits | none | <3 per day | >2 per day | |
| Posture | normal | fisting, cycling | distal flexion | decerebrated |
| Moro | normal | partial | absent | |
| Grasp | normal | poor | absent | |
| Suck | normal | poor | absent | |
| Respiration | normal | hyperventilation | apnea | respiratory support |
| Fontanel | normal | full | tense | |
| Date | ||||
| Time | ||||
| Tone | ||||
| Conscious-ness | ||||
| Fits | ||||
| Posture | ||||
| Moro | ||||
| Grasp | ||||
| Suck | ||||
| Respiration | ||||
| Fontanel | ||||
| TOTAL |
Here, the actual problem is the patients who are considered as stage 1. Although there is insufficient scientific evidence in the issue of initiating hypothermia treatment in this patient group, it should be kept in mind that neurologic injury develops in the future in some of these patients.
Patients who do not fully meet the diagnostic criteria (babies with a gestational age of 34-35 weeks, babies who are within the first 6-12 hours, patients with a cord blood gas pH above 7 and a BE of between -12 and -16) and those whose criteria for initiation of hypothermia treatment are not clear are discussed with the consultant physician and the physician or academic member who is in charge of intensive care unit, the patient’s profit/loss balance, success of treatment, long-term follow-up, and legal dimensions are evaluated, and the final decision for initiating treatment is made after informing the family.
According to the results of large, randomized, controlled studies, the diagnosis is made and treatment decision can be made considering the following criteria (7, 8, 12):
Treatment criteria: (1) babies with a gestational age of ≥36 weeks and aged below ≤6 hours; (2) a pH value of ≤7.00 or BE value of ≤ -16 mmol/L in the cord blood gas or in the blood sample obtained in the first hour after delivery; (3) a tenth minute Apgar score of <5 or continuing need for resuscitation; (4) presence of moderate or severe encephalopathy findings on clinical assessment.
a. Some studies also add aEEG findings. A center in which hypothermia treatment is applied should use aEEG because it is necessary both diagnostically and in the follow-up.
b. In babies whose pH or BE values are inappropriate, it is appropriate to initiate treatment when two additional findings are positive (a low Apgar score and presence of encephalopathy) (12).
c. There is evidence indicating that application of hypothermia might increase the mortality in newborns with a history of maternal inflammation and chorioamnionitis. Although this is not clear, one should be careful in this aspect. It means that there may be patients for whom initiation of treatment is not appropriate, though the diagnostic criteria are met.
Exclusion criteria for treatment: (1) babies who are aged over six hours (may change with the consultant’s decision); (2) babies under the gestational age of 34 weeks; (3) babies weighing less than 2000 g (the border value is 1800 g for CoolCap®); (4) conditions where one is not sure of the diagnosis or documentation of other conditions that might lead to NE, congenital metabolic diseases, energy deficit diagnosed in the family with sibling history (index case) and other diseases characterized by early encephalopathy (may change with the consultant’s decision); (5) babies for whom it is thought that treatment would not be beneficial, e.g., those with very severe or diffuse parenchymal cranial hemorrhages, very severe life-threatening coagulopathy; (6) maternal chorioamnionitis, trisomies, 13, 18 or multi-organ anomalies (7, 8, 12).
Treatment
Numerous randomized controlled studies have shown that therapeutic hypothermia leads to a reduction in the rates of mortality and neurologic sequela in newborns with moderate and severe hypoxic ischemic encephalopathy and has become a standard treatment method. In a meta-analysis in which 11 randomized, controlled studies were examined, these conclusions were proven in 1505 term and late preterm babies (13). According to these results, in Cochrane reviews, the proportion of mortality and neurodevelopmental disorder after 18 months was found as 46% (312/678) in the hypothermia group and 61% in the control group in eight of these 11 studies. Accordingly, the typical risk ratio was found as 0.75 (95% CI: 0.68-0.83), and the ‘number needed to treat’ (NNT) was found as 7. When the same comparison was made for mortality, the mortality rate was found as 25% in the hypothermia group and 34% in the control group. The risk ratio was 0.75 (95% CI: 0.64-0.88) and the NNT was calculated as 11. As a result of neurodevelopmental evaluation of 917 babies in eight studies, the proportion of neurodevelopmental disorders was found as 26% (130/495) in the hypothermia group and 39% (166/422) in the control group; the relative risk was 0.77 (95% CI: 0.63-0.94) and the NNT was calculated as 8 (13).
Clinical approach: The clinical approach generally consists of supportive treatment. Hypothermia and prevention strategies should be applied for babies whose diagnostic criteria are met. In babies who need resuscitation, CPAP or intubation and sometimes chest compression, and rarely adrenaline may be required. Cord blood gas values should be obtained in babies in whom resuscitation has been performed. Cord blood gas values may also be obtained from the placental cord on the maternal side for up to 45 minutes according to some authors; one should not ignore sampling thinking that it is too late. They are important in terms of being helpful for the diagnosis. After babies are born and transferred to the ward, blood gas values should be obtained in the first few hours in patients, if necessary.
Family-centered evaluation: The patient’s diagnosis will lead to stress in the family. The family should be informed and their consent should be obtained for treatment and further interventions. The interventions and therapies to be applied should be explained and the family should be adequately informed about the long-term outcomes of the babies who have and have not been treated and about the adverse effects of the procedures to be performed. According to the results obtained from hypothermia studies in patients with hypoxic ischemic encephalopathy, the benefit of hypoxia treatment is scientifically clear, unquestionable, and leaving the treatment decision to families and not applying hypothermia treatment brings legal responsibility. If the patient has been diagnosed as having hypoxic ischemic encephalopathy and a hypothermia device is not available in the center, passive hypothermia should be initiated, heaters should be turned off, and the patient should be cooled using gel cooling pads or materials with similar function until they are transferred. However, the patient’s body temperature must be closely monitored in the meantime. Some studies have shown that passive hypothermia is as efficient as therapeutic hypothermia (14). Passive hypothermia may be applied in places with insufficient infrastructure and in cases where the patient cannot be transferred.
Therapeutic hypothermia: Treatment performed by using the neuroprotective effect of cooling the brain tissue by keeping the body temperature within a specific range for a certain period is called therapeutic hypothermia. Cooling the patient with the assistance of gel cooling pads and some assistive devices including cooled frozen models and similar batteries or using devices including incubator radiant heaters and open beds with a heating feature by turning them off is generally called passive hypothermia. Applying this procedure for treatment using a device designed for this objective is called ‘therapeutic hypothermia,’ or ‘active cooling’ in the medical literature. In some literature, the term cooling is used, hypothermia is used in others (7, 15). If the device can operate by changing its own temperature settings according to the baby’s body temperature, this is called ‘servo-controlled’ cooling therapy.
Mechanism of action: Each 1°C reduction in body temperature causes a 6-10% reduction in brain metabolism. The efficiency of hypothermia treatment may vary according to the severity of brain injury and the patient’s genetic characteristics. The following four factors should be considered for successful treatment (15): (1) rapid initiation of treatment and initiating treatment as soon as damage develops are very important, ‘time is brain’. (2) Cooling period; cooling for a certain time period and in the same range is essential for the efficiency of treatment. (3) Warming; it is very important to apply warming slowly and gradually. (4) Management of adverse effects and prevention.
Effects of apoptosis and mitochondrial dysfunction: ischemia and reperfusion injury may lead to cellular necrosis and apoptosis, also known as programmed cell death. The development of apoptosis occurs in association with a few events, one of these is mitochondrial dysfunction. Subsequently, ‘caspase’ enzymes are activated and other problems related to cellular energy metabolism occur. It is known that hypothermia treatment is efficient from the very beginning of these steps; it prevents the initiation of these steps or those that have been initiated. Apoptosis begins later compared with the other mechanisms and continues for the long term. Therefore, it is important to initiate hypothermia treatment in the therapeutic window. It has been experimentally shown that hypothermia corrects dysfunction in the ion pumps, reduces the amount of calcium entering into the cell, and thus decreases neurotoxicity (15, 16).
Inflammation: Ischemia reperfusion injury initiates the formation of inflammation in the brain and this secondarily causes brain injury. Ischemia- reperfusion leads to an increase in pro-inflammatory cytokines including interleukin (IL)-1β, tumor necrosis factor (TNF)-α, monocyte chemotactic protein (MCP1), which is a chemokine, and proinflammatory mediators in the microglia and circulating leukocytes (16).
Disruption in the blood-brain barrier:
After brain injury, vascular permeability increases and subsequently edema occurs. Hypothermia corrects these changes by reducing the activity of matrix metalloproteinases.
Formation of free radicals. Inflammation triggered by ischemia and reperfusion is generally associated with formation of free oxygen radicals. These include superoxide, peroxynitrite, hydrogen peroxide and hydroxyl radicals. As a result of these events, peroxidation of lipids, proteins, and nucleic acids occurs. Hypothermia by itself cannot clean all free oxygen radicals in the damaged cells, but may reduce them to levels at which endogenous antioxidant mechanisms can handle the situation (16).
Adverse effects: Sinus bradycardia may be observed in 5% of patients. One of the most common adverse effects is thrombocytopenia, which is observed in 31% of patients. According to the results of Cochrane reviews, therapeutic hypothermia does not cause an increase in major cardiac arrhythmias, severe hypotension (MAP <40 mm Hg), and hypotension requiring use of inotropic agents (13).
Time to initiate hypothermia: Active cooling should be initiated as soon as possible. It would be a correct approach to turn off the heater in the delivery room when it is recognized that the patient needs resuscitation. Studies have shown that the sooner hypothermia is initiated, the better the efficiency (17). As soon as the decision for hypothermia is made, the relevant intensive care unit should be called, the patient’s place should be kept available, and the necessary materials for monitorization and aEEG should be prepared. The patent’s rectal body temperature should be monitored and aEEG should also be persistently monitored. The aEEG trace obtained in the first half hour should be examined and the evaluation should be performed accordingly; aEEG monitoring should be continued throughout the hypothermia period, if possible. Hypothermia should not be delayed for any intervention (placement of umbilical catheter, placement of aEEG monitor probe, obtaining blood sample). One of the most important problems is newborns with a suspicion of intrapartum hypoxia who are subsequently evaluated as normal. Some newborns can overcome this troublesome process and enter a more stable period, and some may even be asymptomatic in the first hours. Subsequently, they may have a seizure. This process may occur much later after the first six hours, which is the period of initiation of hypothermia.
There is still no consensus on how these babies should be approached and studies continue. Studies related to the issue of evaluating these babies with markers immediately after delivery and making a diagnosis in the early period are ongoing. Even though there is insufficient evidence, the tendency to giving treatment to such patients gradually increases.
Intensive care conditions: Therapeutic hypothermia should be performed in a level 3 neonatal intensive care unit. These babies need mechanical ventilation, rectal temperature monitoring, and very close monitoring of the vital signs. In addition, they should be followed up in a center where hematologic monitoring, neuroimaging (MRI), aEEG, neurology consultation, and long-term neurodevelopmental evaluation can be performed. The multidisciplinary problems of these babies persist in the long-term.
The management, system assessments, and points to be addressed are summarized in Table 3. The first-line drug to be preferred should be dobutamine in patients with HIE when hypotension develops; it may be administered at a dosage of 5-20 mcg/kg/min. The risk of NEC related to the gastrointestinal system is increased in these babies. The most accurate approach in the issue of nutrition and delaying feeding is to initiate feeding to a small extent and preferring breastmilk. Antibiotic treatment may be initiated after sepsis assessment related to infections and antibiotics is performed. Also, considering local preferences, penicillin G (25,000-50,000 units/kg) or ampicillin (50 mg/kg/day 2-3 X iv) and gentamycin (4-5 mg/kg) combination may be recommended. The coagulation profile should be followed up in cases of immunodeficiency and infection. Hypoxic ischemic encephalopathy will lead to disruption in coagulation parameters, disseminated intravascular coagulation may develop in this patient group, and one should also be careful in this regard. In some articles, it has been emphasized that hypothermia might also have an influence on these coagulation factors (18). There are scientific data indicating that hypothermia has positive effects in retinopathy and that it protects the retina (19, 20).
Table 2.
Clinical findings in neonatal encephalopathy. Hypothermia should be initiated, if at least three parameters from the 2nd and 3rd columns are present
| Circle each parameter if it is present in the patient | |||
|---|---|---|---|
| Hypothermia should be initiated, if at least three parameters from the 2nd and 3rd columns are present | |||
| 1 | 2 | 3 | |
| Fits | None | Mostly focal or multifocal. Note: If the patient is in the first 6 hours, if the gestational week, birth weight blood gases are appropriate and if seizure has been observed, hypothermia should be initiated anyway independent of this list | Rare (excluding decerebrated status) or frequent seizures |
| Level of consciousness | Normal Hyper-alert | Lethargic, reduced activity | Stupor/coma Unresponsive to external stimuli |
| Spontaneous activity while awake | Active, moving, does not stand in the same position | Not active, weak | No activity |
| Posture | Moving, does not stand at a point | Distal flexion, full extension or frog positon | Decerebrated posture whether stimulus is present or not present (all extremities are extended) |
| Tone | Normal, resistant to passive movement Hypertonic jitteriness | Hypotonic, flaccid, local or generalized | Wholly flaccid, hypotonic |
| Primitive reflexes | Suck: sucks finger or tube actively Moro: normal Extremity flexion and then extension occur with stimulus | Suck: poor Moro: deficient, insufficient | Suck: disappeared fully Moro: absent fully |
| Autonomous system | Pupillae: Normal, response to light: (+) Cardiac rate: >100/min Respiration: Regular spontaneous respiration | Pupillae: reduced <3 mm, but response to light (+) Cardiac rate: bradycardic, varies between <100/min and 120/min. Respiration: Periodic respiration, irregular respiratory effort | Pupillae: Fixed dilated, response to light (-) Cardiac rate: may be bradycardic, irregular rate pattern, variable Respiration: Apneic, need for ventilation |
Which patients are not routinely given hypothermia treatment? Therapeutic hypothermia is contraindicated in patients with severe head trauma and diffuse intracranial hemorrhage (severe stroke). Although there is insufficient evidence to indicate that hypothermia treatment would be beneficial for babies with a gestational age below 36 weeks and for babies who have passed the postnatal sixth hour, these babies should also be evaluated as if they will be given hypothermia treatment; it would be appropriate to consult authorized individuals on this issue if the patient could benefit from this treatment and the treatment decision should be made after discussion.
Which method is superior? Selective head cooling or whole-body cooling? Although clinical studies have found similar results, there are no randomized-controlled studies comparing the two different methods. There may be some differences during practical use. In head cooling, only the fontanelle is cooled to 30°C and one tries to keep the rectal temperature at 34°±0.5. In this method, placement of electroencephalogram probes may be more difficult. In whole- body cooling, skin complications may be observed more frequently (17, 21-23). One study showed that 65% of centers used whole-body cooling, 25% used selective head cooling, and 10% used both methods. Servo-controlled systems may be a little more expensive, but they are more successful in maintaining the targeted temperature range. Whole body cooling is preferred with a higher rate and there is more experience with this method. Although superiority of one method over another has not been demonstrated, whole-body cooling may be recommended practically. Which body temperature range should be targeted? Studies have indicated a range between 32.5°C and 35°C. The optimal rectal or esophageal temperature is 34±0.5°C. In the guide, rectal temperature was accepted as central body temperature.
Treatment period. The optimal treatment period is 72 hours. Treatment of babies who have seizures while warming should be prolonged up to 96 hours (additional 24 hours).
Warming period. The rectal temperature should not be elevated with a rate of more than 0.5°C per hour, and the final body temperature should not be elevated above 37°C (±0.2). The baby should be closely monitored in the subsequent 24 hours in terms of hyperthermia. If the patient has a seizure during warming, cooling should be continued for a further 24 hours.
Anti-edema treatment. Steroids or mannitol should not be given to babies who receive cooling treatment. In babies who develop hypotension, steroids may be given, if necessary, in the algorithm of hypotension treatment. In case of brain edema, Na and Mg should be kept at the upper level of normal.
Control and treatment of seizures: Seizures that occur in the early period, have insufficient response to treatment, and last for a long periods are associated with poor prognosis. Treatment principles of neonatal convulsions are valid. Prophylactic anticonvulsants are not routinely recommended. Metabolic causes should be primarily treated in a newborn with HIE; hypoglycemia, hypocalcemia, and hypomagnesemia should be corrected. One hundred milligrams pyridoxine may be given intravenously for pyridoxine-dependent seizures and the daily dosage may be increased up to 500 mg. Meanwhile, EEG should be closely monitored. In patients who do not respond to pyridoxine, folinic acid may also be tried (leucovorin 2.5 mg iv). In hypoxic ischemic encephalopathy, up-to-date neonatal convulsion treatment applies for treatment of seizures and the first-line drug is phenobarbital. The loading dose is 20-30 mg/kg (the daily dose can be increased up to 50 mg/kg) and treatment can be continued at a dose of 5 mg/kg. If seizures cannot be controlled, a loading dose of phenytoin (20-30 mg/kg) can be added and treatment can be continued with a maintenance dose of 5 mg/kg. If there is no response and seizures persist, midazolam (0.15 mg/kg IV bolus) and levetiracetam (40 mg/kg IV bolus divided into three doses) can be administered. Finally, lidocaine can be administered at a bolus dose of 2 mg/kg and as a four-hour infusion (7 mg/kg/hour), and subsequently, treatment can be continued with half of the dose (3.5 mg/kg in 12 hours and 1.75 mg/kg in the other 12 hours). Treatment is then continued with phenobarbital. This approach is summarized in Figure 2. When the decision is made for babies to be discharged, it is recommended to apply Amiel Tison scoring because it is a simple and easily applicable method to predict long-term prognosis (24). According to this examination method, which is scored as mild, moderate, and severe, babies are classified as normal, moderately abnormal or severely abnormal, and this scoring method is applied before the baby is discharged. In babies who are being followed up in an outpatient setting for the long term, Bayley II and Bayley III motor and psychosocial developmental assessment markers are used. The pharmacodynamics of some drugs given to patients during hypothermia may be affected and this should be considered (16). A summary of these drugs is presented in Table 4.
Figure 2.
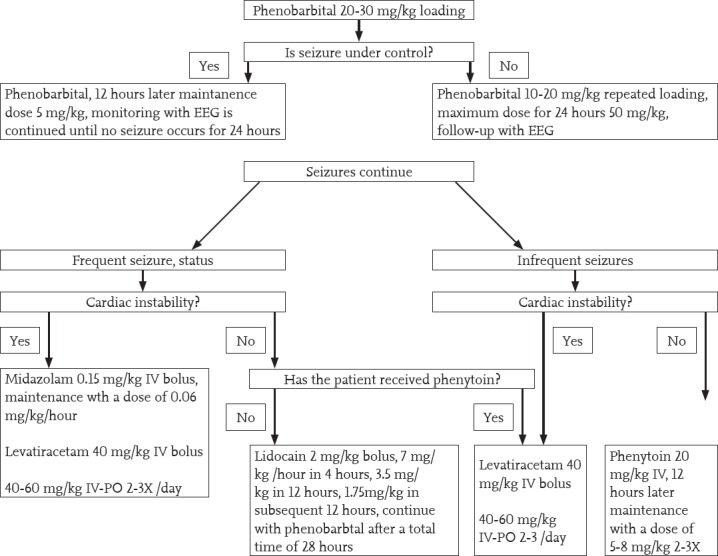
Diagram for management of seizures in hypoxic ischemic encephalopathy
EEG: electroencephalograph
Table 3.
Approaches in some clinical conditions in neonatal encephalopathy
| System | Approach |
|---|---|
| Respiratory system | Ventilator support may be required, Avoid hyperoxia, hyperoxia is a risk factor for poor long-term outcomes in patients who receive therapeutic hypothermia. Excessive venti- lation and subsequent hypocarbia may lead to severe brain hypoperfusion, cellular alkalosis and poor neurodevelopmental outcomes. |
| Cardiovascular system | Hypotension may cause shock, cardiomegaly, arrhythmias, heart failure and cardiac ischemia. Keep the mean blood pressure above 35-40 mm Hg. Pay attention to fluid loading if you are not sure about hypovolemia. Inotropes may be needed sometimes (dobutamine 5-20 mcg/kg/min should be preferred). Prevent iatrogenic hypertension. Performing echocardiography is appropriate, hypovolemia, poor myocardial contractility, low flows, etc. |
| Neurological system | In moderate and severe HIE, maintain continuous aEEG up to 96 hours, video EEG may be beneficial for monitoring clinical and subclinical seizures. In this way, you will have an idea about long-term effects and prognosis. |
| Kidney | Oliguria, hematuria, proteinuria, myoglobinuria, polyuria and renal failure may develop. Urea and creatine levels should be measured. One may start fluid treatment with 10% dextrose at a dose of 40-50 ml/kg. Fluid balance, intake, output should be monitored, body weight should be measured every 12 hours. In babies who receive hypothermia treatment, one should pay attention to nephrotoxic agents; for example, gentamycin may be administered with longer intervals (every 36 hours). If oliguria/anuria develops and if you think that the patient is hypovolemic, load 0.9% NaCl; urinary catheter should be placed, dopamine or one of the other inotropic agents may be initiated. |
| Metabolic | Hypo/hyperglycemia, hypocalcemia hyponatremia, hypomagnesemia, lactic acidosis may be observed, blood glucose, calcium, magnesium, lactate, electrolytes, blood and urine osmolarity should be measured. |
| Hematology | Thrombocytopenia, thrombosis, an increase in normoblasts may be observed; complete blood count and peripheral smear should be obtained. DIC may develop, liver function tests should be performed. Coagulation profile should be obtained. Administration of additional vitamin K and FFP may be needed. |
HIE: hypoxic ischemic encephalopathy; aEEG: Amplitude-integrated EEG; EEG: electroencephalography; NaCl: sodium chloride; DIC: disseminated intravascular coagulation; FFP: fresh frozen plasma
Table 4.
Some drugs used during hypothermia and interaction states (16)
| Class | Drug | Metabolism | Effect of hypothermia | Recommended dose |
|---|---|---|---|---|
| Anticonvulsants | Phenobarbital | CYP2C19 | Half-life increases 2-fold | Loading two times maximally, no need for change maintenance |
| Phenytoin | CYP2C9, CYP2C19, renal, bile, UGT glucuronidation | Clearance reduces to 67% The drug’s stay time in the body is prolonged by 180% | Check the level before loading a second dose | |
| Midazolam | CYP3A | Clearance reduces by 11% for each 1°C | Be careful with repeated doses and infusion | |
| Sedatives | Fentanyl | CYP3A | Increase in plasma levels by 25% | Accumulation occurs with repeated doses and infusions, one should be careful |
| Antibiotics | Gentamycin | Renal | Watch for renal failure | |
| Ampicillin | Renal | It is thought that there is no effect | Watch for renal failure | |
| Inotropic agents | Dobutamine | COMT | Adjust the dose by clinical need | |
| Dopamine | COMT/MAO | Accumulation may occur | Hypothermia may decrease response to inotropes | |
| Noradrenaline | COMT/MAO | α adrenergic action is in the forefront throughout hypothermia |
COMT: catechol-o-methyl transferase; MAO: monoamine oxidase
Analgesics should be used because of the pain effect of hypothermia. Morphine at a dose of 100 mcg/kg (or fentanyl 5 mcg/kg) may be used. A summary of all approaches is shown in Figure 3.
Figure 3.
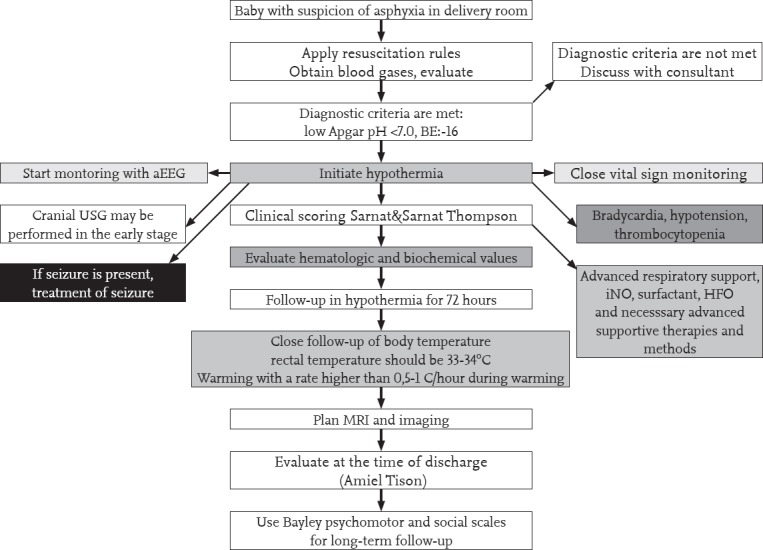
Summarized algorithm of approach in hypoxic ischemic encephalopathy
aEEG: amplitude-integrated EEG; EEG: electroencephalography; INO: inhaled nitric oxide; HFO: high-frequency ventilation; MRI: magnetic resonance imaging; USG: ultrasonography
Footnotes
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
Çıkar Çatısması: Yazarlar çıkar çatışması bildirmemişlerdir.
Mali Destek: Yazarlar bu çalışma için mali destek almadıklarını beyan etmişlerdir.
References
- 1.Executive summary: Neonatal encephalopathy and neurologic outcome, second edition. Report of the American college of obstetricians and gynecologists'task force on neonatal encephalopathy. Obstet Gynecol. 2014;123:896–901. doi: 10.1097/01.AOG.0000445580.65983.d2. [DOI] [PubMed] [Google Scholar]
- 2.Martinello K, Hart AR, Yap S, Mitra S, Robertson NJ. Management and investigation of neonatal encephalopathy: 2017 update. Arch Dis Child Fetal Neonatal Ed. 2017;102:F346–58. doi: 10.1136/archdischild-2015-309639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee AC, Kozuki N, Blencowe H, et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr Res. 2013;74:50–72. doi: 10.1038/pr.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86:329–38. doi: 10.1016/j.earlhumdev.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 5. www.uptodate.com/contents/etiology and pathogenesis of neonatal encephalopathy . Erişim tarihi: 07.2017.
- 6.Türk Neonatoloji Derneği Hipoksik İskemik Ensefalopati Çalışma Grubu. Türkiye'de yenidoğan yoğun bakım ünitelerinde izlenen hipoksik iskemik ensefalopatili olgular, risk faktörleri, insidans ve kısa dönem prognozları. Çocuk Sağlığıve HastalıklarıDergisi. 2008;51:123–9. [Google Scholar]
- 7. wwwa.uptodate.com/contents/clinical-features-diagnosisand- treatment-of-neonatal-encephalopathy.07.2017 .
- 8. http://www.neonatology.org.tr/wpcontent/uploads/2016/12/dogum_odasi_yonetimi.pdf. erişim tarihi: 07.2017.
- 9.Okereafor A, Allsop J, Counsell SJ, et al. Patterns of brain injury in neonates exposed to perinatal sentinel events. Pediatrics. 2008;121:906–14. doi: 10.1542/peds.2007-0770. [DOI] [PubMed] [Google Scholar]
- 10.Vann C, Poskitt KJ, Miller SP. “Advanced neuroimaging techniques for the term newborn with encephalopathy.”. Pediatr Neurol. 2009;40:181–8. doi: 10.1016/j.pediatrneurol.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Thompson CM, Puterman AS, Linley LL, et al. The value of a scoring system for hypoxic ischaemic encepha¬lopathy in predicting neurodevelopmental outcome. Acta Paediatr. 1997;86:757–61. doi: 10.1111/j.1651-2227.1997.tb08581.x. [DOI] [PubMed] [Google Scholar]
- 12.Committee on Fetus and Newborn. Papile LA, Baley JE, et al. Hypothermia and neonatal encephalopathy. Pediatrics. 2014;133:46. doi: 10.1542/peds.2014-0899. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs S.E, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;1:CD003311. doi: 10.1002/14651858.CD003311.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramos G, Brotschi B, Latal B, Bernet V, Wagner B, Hagmann C Swiss Neonatal Network. Therapeutic hypothermia in term infants after perinatal encephalopathy the last 5 years in Switzerland. Early Hum Dev. 2013;89:159–64. doi: 10.1016/j.earlhumdev.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 15.Lang TR, Hartman TK, Hintz SR, Colby CE. “Hypothermia for the treatment of neonatal ischemic encephalopathy: is the genie out of the bottle?.”. Am J Perinatol. 2007;24:27–31. doi: 10.1055/s-2006-958157. [DOI] [PubMed] [Google Scholar]
- 16.Wood T, Thoresen M. Physiological responses to hypothermia. Semin Fetal Neonatal Med. 2015;20:87–96. doi: 10.1016/j.siny.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Thoresen M, Tooley J, Liu X, et al. Time is brain: starting therapeutic hypothermia within three hours after birth improves motor outcome in asphyxiated newborns. Neonatology. 2013;104:228–33. doi: 10.1159/000353948. [DOI] [PubMed] [Google Scholar]
- 18.Oncel MY, Erdeve O, Calisici E, et al. The effect of wholebody cooling on hematological and coagulation parameters in asphyxic newborns. Pediatr Hematol Oncol. 2013;30:246–52. doi: 10.3109/08880018.2013.771240. [DOI] [PubMed] [Google Scholar]
- 19.Reinhard K, Mutter M, Gustafsson E, et al. Hypothermia promotes survival of ischemic retinal ganglion cells. Invest Ophthalmol Vis Sci. 2016;57:658–63. doi: 10.1167/iovs.15-17751. [DOI] [PubMed] [Google Scholar]
- 20.Salido EM, Dorfman D, Bordone M, et al. Global and ocular hypothermic preconditioning protect the rat retina from ischemic damage. PLoS One. 2013;8:e61656. doi: 10.1371/journal.pone.0061656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunn AJ, Gluckman PD, Gunn TR. Selective head cooling in newborn infants after perinatal asphyxia: a safety study. Pediatrics. 1998;102:885–92. doi: 10.1542/peds.102.4.885. [DOI] [PubMed] [Google Scholar]
- 22.Celik Y, Atıcı A, Gulası S, Okuyaz C, Makharoblıdze K, Sungur MA. Comparison of selective head cooling versus whole-body cooling. Pediatr Int. 2016;58:27–33. doi: 10.1111/ped.12747. [DOI] [PubMed] [Google Scholar]
- 23.Akisu M, Huseyinov A, Yalaz M, et al. Selective head cooling with hypothermia suppresses the generation of platelet- activating factor in cerebrospinal fluid of newborn infants with perinatal asphyxia. Prostaglandins Leukot Essent Fatty Acids. 2003;69:45–50. doi: 10.1016/s0952-3278(03)00055-3. [DOI] [PubMed] [Google Scholar]
- 24.Amiel-Tison C. Update of the Amiel-Tison neurologic assessment for the term neonate or at 40 weeks corrected age. Pediatr Neurol. 2002;27:196–212. doi: 10.1016/s0887-8994(02)00436-8. [DOI] [PubMed] [Google Scholar]


