Abstract
Respiratory distress syndrome is the leading cause of respiratory failure in preterm infants. The incidence and severity of respiratory distress syndrome are inversely related to the gestational age of the newborn. The major underlying pathophysiologic mechanisms are surfactant deficiency and anatomic, structural immaturity of the lung. Recent improvements such as antenatal steroid treatment to enhance pulmonary maturity, appropriate resuscitation facilitated by placental transfusion and immediate use of continuous positive airway pressure for alveolar recruitment, early rescue administration of surfactant, ventilation with gentler modes to minimize damage to the immature lungs, and the other supportive therapies have significantly decreased respiratory distress syndrome-related morbidity and mortality. This guideline was addressed to overview the mentioned improvements in order to standardize respiratory distress syndrome management in neonatal intensive care units in Turkey.
Keywords: Antenatal steroids, caffeine, oxygenation, respiratory distress syndrome, surfactant, ventilation
Abstract
Respiratuvar distres sendromu preterm bebeklerde solunumsal yetmezliğin en sık nedenidir. Hastalığın sıklığı ve şiddeti gebelik haftası azaldıkça artmaktadır. Respiratuvar distres sendromuna neden olan asıl fizyopatolojik mekanizma surfaktan eksikliği ile anatomik ve yapısal immatüritedir. Akciğer olgunlaşmasını hızlandırmak üzere antenatal steroid uygulanması, plasental transfüzyonunda da alındığı preterm bebeğe uygun doğum odası canlandırma politikaları, doğum salonundan itibaren sürekli pozitif havayolu basıncı uygulanması, surfaktan tedavisinin erken ve kurtarıcı olarak uygulanması, daha nazik ventilasyon yöntemlerinin kullanılması ve diğer destekleyici tedaviler ile respiratuvar distress sendromu ilişkili morbidite ve mortalite günümüzde anlamlı olarak azalmıştır. Bu kılavuzun amacı konusu geçen tedavi yöntemlerinin ayrıntılı olarak gözden geçirilmesi, respiratuvar distres sendromu tedavisi ve yönetiminin ülkemiz yenidoğan yoğun bakım birimlerinde standart olarak yapılmasını sağlayabilmektir.
Introduction
Despite recent improvements in neonatal care, respiratory distress syndrome (RDS) still remains as major cause of morbidity and mortality in preterm infants. It is mainly caused by alveolar surfactant deficiency along with structural immaturity of the lung. Surfactant, a mixture of phospholipids, reduces the surface tension and keeps the alveoli remain open. When surfactant is deficient diffuse atelectasis develops, and results with impaired gas exchange and ventilation perfusion imbalance (1).
The incidence of RDS increases as gestational age decreases (1). A recent analysis obtained from a national database conducted by Turkish Neonatal Society in 2016-2017 revealed that the incidence of RDS and surfactant treatment rates were 70.3% and 58.7 % among 3490 <32 weeks preterm infants in Turkey. These rates were 86.5% and 78.8%, respectively in 1539 preterm infants at 28 weeks and below (with the permission of Baş Ay et al., unpublished data). We think that the high rates of RDS and surfactant use in our country may be related to the low rate of antenatal steroid use (42.5%), even at tertiary perinatal centers.
The first national guideline for RDS and surfactant replacement therapy was published in 2014 and recommendations were made to minimize the RDS associated morbidity and mortality regarding neonatal care settings of our country (2). Former guideline has been recently updated by Turkish Neonatal Society.
Antenatal care
The management of RDS begins before birth. The obstetricians and pediatricians should take part in the perinatal team and act together to make decisions which may be beneficial both for the mother and the infant.
Decision for place of birth
Premature infants at high risk for RDS should be delivered in centers where appropriate skills for stabilization and respiratory support are available (non-invasive ventilation, intubation, surfactant treatment, mechanical ventilation (MV)). In-utero (maternal) transport should be considered if the conditions of the mother and the fetus are suitable. Tocolytic agents and antibiotics (in case of premature rupture of membranes) may be initiated within the indications to delay the preterm labor and to gain time for antenatal steroid treatment and transport of the infant. If the delivery is taking place under emergency conditions, first the infant should be stabilized and later on transferred to a tertiary neonatal intensive care unit in a transport incubator with ongoing respiratory support, by a transport team which has experience in the neonatal care (3-5).
Antenatal steroid treatment
Antenatal corticosteroid given to mother with anticipated preterm delivery improves survival, reduces the risk of RDS, necrotizing enterocolitis (NEC), intraventricular haemorrhage (IVH) and sepsis developing within the first 48 hours of life. Antenatal steroid is recommended for all pregnant women between the 231/7 and 346/7 weeks of gestation with risk of preterm labor. The recommended treatment regimens are 12 mg betamethasone im (total 2 doses) at 24-hour intervals, and if it is not available, 6 mg dexamethasone im (total of 4 doses) at 12-hour intervals. It has been shown that single-dose therapy has no significant short-term adverse effect on the pregnant woman or fetus. The optimal benefit of the treatment is between 24 h after the initiation upto 7 days; benefits diminishes beyond 14 days. Even the course cannot be completed, if there is no contraindication in the pregnant woman and if the birth is not inevitable, steroid treatment should be started anyway (6). There is a continuing debate as to whether steroids should be repeated 1 or 2 weeks after the first course for women with threatened preterm labour. The World Health Organization recommends a second course or a single-dose rescue steroid regimen if preterm labor repeats 1-2 weeks following the first course, but before the 34th week of gestation (7). Such repeat courses do not reduce the risk of neonatal death, but reduce RDS and other short-term health problems, although birth weight is reduced and long-term beneficial effects are lacking.
Delivery room management
Identification of risks and availability of appropriate team
The resuscitation of a high-risk premature infant is a difficult condition that requires more than one team member to be available. The tasks of the team members must be defined by the team leader before the birth and resuscitation should be implemented accordantly (8). The healthcare provider involved in the resuscitation procedure must have an updated Neonatal Resuscitation Program certificate. Prenatally, the team should have knowledge about pregnancy, and risks must be defined in advance. Each unit should have a checklist of materials that may be required in premature infants’ delivery room stabilization or resuscitation (8).
Delayed cord clamping
ILCOR-2015 recommends delayed cord clamping at least 30 sec in all term and preterm infants who do not require resuscitation. The optimal time for cord clamping is not clear yet in those who require a resuscitation. Placental transfusion is the first step of resuscitation in a premature infant who has a high risk for RDS development. As delayed cord clamping allows a higher hematocrit level, less transfusion requirement, higher blood pressure, and lesser NEC and IVH development, infants should be placed for at least 30 sec (up to 120 sec) at or below the maternal level if it is possible (8, 9). In emergency situations such as maternal bleeding or resuscitation requirement of premature infant, milking the umbilical cord 2-4 times at a distance of 20 cm from the umbilicus level may also have similar effect. Further large sample studies are needed to clarify the effect of cord milking on preterm infants (10).
Prevention of hypothermia and hyperthermia
Hypothermia in premature infants is associated with increased morbidity and mortality. During hypothermia, oxygen consumption increases, infants fail to respond resuscitation, coagulation parameters are adversely affected, acidosis develops, and the transition from fetal circulation to neonatal circulation is delayed. All interventions to the premature infant should be performed in the delivery room at an environmental temperature of at least 26°C and under radiant warmer. The radiant warmer and the incubator in the delivery room must be appropriately preheated for 15-20 minutes. Premature infants born under the 30th gestational week should be placed in a plastic bag immediately after delivery without drying, a hat should be worn on their heads, and they should be placed under a radiant warmer or in an incubator. Heating and humidification of the gases helps to prevent heat loss. The warmer should be switched to servo-control within 10 minutes to prevent overheating for infants whose interventions continue under a radiant warmer. Transport to the neonatal intensive care unit should be done within a transport incubator ensuring heat control (8).
Monitoring oxygenation
Monitoring of saturation and heart rate by placing the pulse oximeter to the infants right wrist allows titration of oxygen consumption. In majority of very-low-birth-weight premature infants, safe transition is achieved by using 21-30% oxygen. The oxygen concentration used in the resuscitation should be controlled by an air/oxygen blender. A minimum of 5 cm-H2O continuous positive airway pressure (CPAP) applied in the delivery room with a nasal prong or mask allows safer transition in a significant portion of preterm infants who have good respiratory drive, mostly without any oxygen need (8, 11, 12).
Non-invasive respiratory support
Extremely premature infants have difficulty in initiating effective breathing after birth due to pulmonary immaturity and may need respiratory support. Early initiation of CPAP in the delivery room for spontaneously breathing premature infants with ≤32 weeks gestation stabilizes the patient and reduces the need for surfactant and MV. It is appropriate to start early CPAP application in the delivery room, which should be started with at least 5 cm-H2O pressure. The mask or binasal prongs can be preferred as interfaces. The use of a T-piece device ensures safe and controlled CPAP administration and respiratory support therapy. Positive pressure ventilation should be avoided in patients with good respiratory drive, but it can be performed in the event of apnea and/or bradycardia. Attention should be paid to low and high tidal volumes applied, which may damage the immature lung, and controlled ventilation should be used if it is necessary (8, 12).
Surfactant treatment
Exogenous surfactant treatment is known to reduce mortality and morbidity in premature infants with RDS. Studies comparing surfactant therapy with placebo demonstrated that severity of RDS, mortality rate, the risk of mid and long-term pulmonary morbidities such as BPD and air-leakage are decreased with surfactant therapy (13). When surfactant treatment is considered, questions about which preperation should be given to whom, when, and how arise.
Surfactant products
There are three natural surfactants currently available in Turkey and comparison among them is represented in Table 1. In this guideline, the recommendations regarding surfactant treatment will be made through the preparations available in our country. Natural surfactants, which have been shown to be more effective in infants with RDS, should be preferred to synthetic surfactants (14). Animal-derived (natural) surfactants have been shown to be more effective in preventing air-leakage and mortality than synthetic surfactants. Studies on new-generation synthetic surfactants, including SP-B and SP-C together, are currently underway. In the first clinical trial with CHF5633, it was reported that it was well tolerated and effective in the treatment of RDS, but the improvement in respiratory function was dose-dependent (15, 16). However, a large series of randomized controlled trials are needed to comment on the new generation of synthetic surfactants. The mortality-reducing effect of the surfactant is most pronounced in premature infants whose gestational age are less than 30 weeks or birth weights are less than 1250 g (13).
Table 1.
Surfactant products currently available in Turkey
| Surfactant | Origin | Phospolipid concentration | Protein concentration | Initial dose | Repetitive dose scheme |
|---|---|---|---|---|---|
| Poractant alpha | Minced porcine lung, lipid extraction and purification by liquid gel chromatography | 76 mg/mL | 1 mg/mL contains 0.45 mg/mL surfactant B protein and 0.55 mg/mL surfactant protein C) | 1.25-2.5 mL/kg | 1.25 mL/kg Every 12 hours, no more than 2 repeat doses, 3 doses in total. Total recommended dose together with initial dose is 5 mL/kg |
| Beractant | Minced bovine lung, lipid extraction DPPC, palmitic acid tripalmitin supplementation | 25 mg/mL | <1 mg/mL (contains surfactant protein B and C) | 4 mL/kg | 4 mL/kg, at least 6-hour intervals no more than 3 repeat doses. 4 doses in the first 48 hours of life. |
| Calfactant | Calf lung lavage, lipid extraction | 35 mg/Ml | 0.7 mg/mL (contain 0.26 mg/mL surfactant protein B and 0.44 mg/mL surfactant protein C) | 3 mL/kg | 3 mL/kg, every 12 hours, no more than 2 repeat doses total 3 doses recommended. |
DPPC: “dipalmitoylphosphatidycholine”
Although each unit reports different results for different surfactant preparations, all three natural surfactants are effective in RDS treatment, and BPD incidence and long-term neurodevelopmental outcomes have not changed with the type of surfactant.
Dosing and administration of surfactant
The recommended initial doses of each surfactant type available in Turkey are summarized in Table 1. Poractant alpha at an initial dose of 200 mg/kg was shown to be associated with lower mortality rate in comparison to 100 mg/kg of the same preperation or 100 mg/kg beractant. However, it is not yet clear whether this relationship depends on the applied dose or the surfactant type itself (17). The surfactant is applied with aseptic method. Positioning may aggravate the clinical severity in unstable patients, thus care should be taken in these condition. There is insufficient data on which position the surfactant application is most effective. In a study in which three different surfactant administration positions were compared, no difference was found between positions in terms of efficacy and adverse effects (18). The patient should be closely monitored during and after the procedure for endotracheal tube obstruction, apnea, desaturation, bradycardia, tachycardia, surfactant reflux, volutrauma, hyperventilation, pulmonary hemorrhage, unilateral surfactant application, and increased risk for hemodynamically significant patent ductus arteriosus (hsPDA). Tracheal aspiration should be avoided for 1-6 hours after the administration of the surfactant if the clinical condition of the patient permits. The patient’s response to treatment is assessed through reduced FiO2 requirement, decreased work of breathing, reduced clinical signs of respiratory distress, improved pulmonary mechanics, reduced respiratory support [“peak inspiratory pressure” (PIP), “positive end-expiratory pressure” (PEEP), “mean airway pressure” (MAP)], and improvement in blood gas and radiologic examinations (chest radiograph, ultrasonography).
When to treat with surfactant?
Early CPAP use in the delivery room as a non-invasive respiratory support reduces the need for advanced respiratory support, including surfactant use and intubation (13). Prophylactic surfactant administration may lead to unnecessary intubation rate and surfactant use. The need for prophylactic surfactant treatment has decreased with the widespread use of antenatal steroid treatment, stabilization of preterm infants by prophylactic CPAP in the delivery room and early rescue surfactant therapy in patients with RDS. Prophylactic surfactant is recommended for premature infants less than 26 weeks of gestation without antenatal steroid treatment or in premature babies who require intubation in the delivery room for stabilization (2). Early rescue surfactant treatment reduces the need for MV and the risk of air leakage. Immediate administration of treatment (within 1-2 hours after delivery) is the current strategy proposed for the protection of the lungs in infants in whom RDS symptoms have developed and in need of surfactant treatment (19). A threshold FiO2 for the treatment of surfactant has not been clearly determined yet in the literature due to the heterogeneous nature of the patients included in the studies, different surfactant types, and doses.
Although the FiO2 requirement >30% in infants with RDS findings is considered to be an important predictor of the severity of RDS and non-invasive ventilation (NIV) failure, considering the high RDS and surfactant treatment rates in Turkey, we recommend administering surfactant in infants who require ≥40% FiO2 (20). Respiratory support and surfactant treatment in RDS are summarized in a flowchart (Figure 1). In patients receiving non-invasive respiratory support for RDS, the application of surfactant should be considered when the mean airway pressure (MAP, PEEP) is >7 cm-H2O. Infants who do not show regression of clinical signs of RDS and who have a FiO2 requirement ≥40% may require a repeat dose of surfactant. The use of poractant alpha at an initial dose of 200 mg/kg reduces the need for repeat doses of surfactant (17). The repetition intervals of the existing surfactant preparations and the maximum recommended number of repeats are different. The timing and maximum number of repeats should be decided according to the initially administered surfactant type (Table 1).
Figure 1.
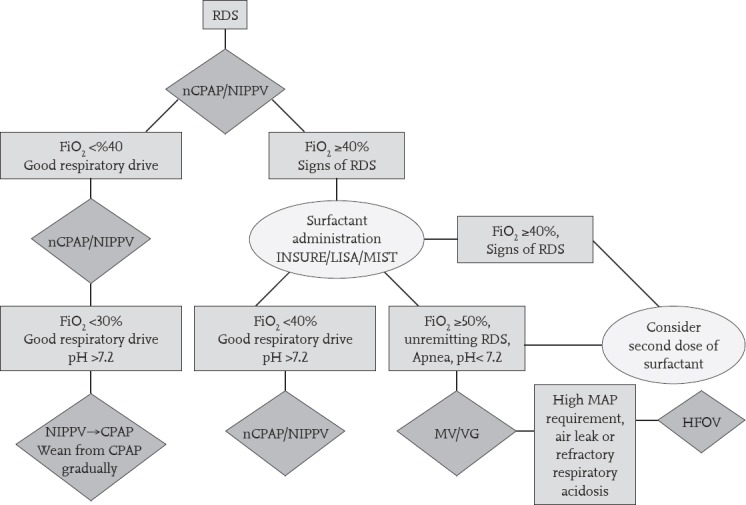
Respiratory support and surfactant therapy algorithm for respiratory distress syndrome
CPAP: continuous positive airway pressure; HFOV: high frequency oscillatory ventilation; MV: mechanical ventilation; nCPAP: nasal continuous positive airway pressure; NIPPV: nasal intermittent positive pressure ventilation; RDS: respiratory distress syndrome
Surfactant administration methods
Surfactant therapy should be performed by health care-givers who are experienced in intubation and MV of the newborn. Most premature infants can tolerate extubation after application of surfactant and continue with CPAP or nasal intermittent positive pressure ventilation (NIPPV) so INSURE (INtubate, SURfactant, Extubate) can be preferred as the surfactant application method. However, this method also requires intubation for surfactant administration and positive pressure ventilation, even for a short time which is enough to initiate significant lung injury as it was observed that even these few large artificial breaths increased the risk of BPD (21-23). Researchers have thus been looking for less invasive ways to administer surfactant without the need for intubation. As an alternative method, placing a thin catheter without using endotracheal tube and administering surfactant during spontaneous breathing is proposed. Kribs et al. (21) developed a method, called LISA (less invasive surfactant administration) in which a soft flexible catheter (feeding tube) instead of an endotracheal tube is placed in the trachea under direct laryngoscopy using Magill forceps and the surfactant is applied. The second method was developed by Dargaville et al., called MIST (minimally invasive surfactant treatment) in which a 16G semi-rigid vascular catheter is used for surfactant administration (22). In both methods, NIV continues during the surfactant application. All meta-analyses reviewing LISA methods showed that LISA/MIST reduced the need for MV in the first 72 hours of life. The incidence of BPD and death differed in studies because patients and methods were not homogeneous (24). Studies on other non-invasive methods of surfactant treatment such as pharyngeal instillation, via laryngeal mask, and aerosolization still continue (25). However, there are only scant data to recommend these methods routinely.
Oxygen support beyond stabilization
Current best evidence suggests that it is reasonable to recommend target saturation ranges between 90 and 94%. The Neonatal Oxygenation Prospective Meta-analysis Collaboration (NeOProM) indicated that lower SpO2 target range was associated with a higher risk of death and NEC, but a lower risk of retinopathy of prematurity (ROP) treatment. However, there was no significant difference between a lower SpO2 target range compared with a higher SpO2 target range on the primary composite outcome of death or major disability at a corrected age of 18 to 24 months. There were no differences regarding physiologic bronchopulmonary dysplasia, brain injury or patent ductus arteriosus between the groups (26). In the postnatal period, significant fluctuations in oxygen saturation should be prevented and especially hyperoxemia that may occur after surfactant administration should be avoided by decreasing the FiO2 rapidly. Keeping saturation in the target range is very important for protecting premature infants against the harmful effects of hyper and hypoxemia. The servo-controlled oxygen delivery circuits developed to keep premature infants in the target saturations are promising. However, evidence is lacking on how these systems affect the short and long-term outcomes.
Non-invasive respiratory support
CPAP, NIPPV, “heated humidified high-flow nasal cannulae (HHHFNC)” and “nasal high-frequency oscillatory ventilation (nasal HFOV)” can be used as non-invasive respiratory methods to support infants with respiratory distress. Nasal CPAP should be started to all premature infants who are at risk of RDS from birth. It has been shown that initiation of CPAP from birth reduces both MV and surfactant treatment requirements. Currently, the most appropriate treatment modality for RDS is CPAP and early rescue surfactant treatment if needed. The results of SUPPORT, COIN, VON, CURPAP, and Colombian Network and Neocosur Network studies all support this modality. Recent large trials that reflect current practice demonstrated less risk of BPD or death when using early stabilization on CPAP with selective surfactant administration to infants requiring intubation, the number needed to treat was reported as 25. There seem to be no differences among devices used to deliver CPAP pressure, although the interface may be important. Although the superiority of short binasal prongs over others has been shown, recent studies showed that nasal masks can be used as effective and safe as binasal prongs (27). CPAP has been widely and successfully applied for the last 30 years as a NIV method. Even it is a non-invasive method, long-term use of CPAP treatment should also be avoided. The strategies for successfully weaning infants off NCPAP are still not well defined and there remains considerable variation between the methods. However, in recent studies gradual weaning from CPAP seems to be more successful when compared to sudden weaning (28). Bilevel CPAP is another variant of CPAP, or low-pressure NIPPV, that uses small pressure differences between inspiratory and expiratory phases. These are typically delivered through CPAP flow driver devices and generate low peak inspiratory pressures of about 9–11 cm-H2O which can be synchronized using an abdominal pressure transducer. Although it gains popularity, there is not much evidence that it confers any significant advantage over CPAP.
NIPPV is also used as first- or second-line respiratory support in many units, with conventional ventilators used to deliver peak inspiratory pressures similar to those on MV, with or without synchronization, but through nasal prongs. In most studies that showed NIPPV was superior to the other NIV methods, synchronization was used. In a Cochrane meta-analysis that compared NIPPV and CPAP in the initial treatment of RDS, NIPPV seemed to be superior to CPAP in the reduction of respiratory failure and the need for intubation (29). However, further studies are still needed to support these findings and determine the best method of delivering NIPPV. NIPPV reduces the incidence of extubation failure and the need for re-intubation within 48 hours to one week more effectively than NCPAP when used following extubation (30). The HHHFNC method is increasingly used in premature infants as an alternative NIV method. In a Cochrane meta-analysis, HHHFNC was found to be similar in terms of efficacy, BPD, and mortality rates compared with other NIV methods. When used after extubation, HHHFNC led to less nasal trauma and lower pneumothorax rates than CPAP (31). In a retrospective study including a large number of patients, HHHFNC was reported to be associated with an increased risk of BPD or death in extremely-low-birth-weight premature infants (32). In a study where HHHFNC was used for primary treatment in infants ≥28 weeks with RDS, treatment failure risk was found to be significantly higher than CPAP (33). Further studies are needed for the application of HHHFNC in extremely premature infants beacuse the majority of the studies published to date were performed in the postextubation period and in relatively bigger premature infants. Due to the successful elimination of CO2 with the HFOV method during invasive ventilation, use of this method as a NIV method (nHFOV) has been raised. Although it has been shown to be successful as a NIV method in observational case series and in in vitro studies, there are no definitive data on its mechanism of action and clinical usefulness, and randomized controlled clinical studies are needed on this subject (34).
Mechanical ventilation strategies
Despite all efforts to manage preterm infants by a non-invasive respiratory support to protect their lungs, approximately half of extremely preterm infants with RDS fail and need endotracheal intubation and MV. The criteria for NIV failure and MV requirement are defined as follow: respiratory acidosis (arterial pH <7.2 and PaCO2 >60-65 mm Hg) under NIV therapy, persistent hypoxemia (PaO2 <50 mm Hg) despite oxygen therapy, and recurrent apnea. The aim of MV is to provide ‘acceptable’ blood gas exchange whilst minimizing the risk of lung injury, hypocarbia and circulatory disturbance. Optimum ventilation is achieved when the alveoli are kept open during the entire respiratory cycle using PEEP/CPAP. In conventional ventilation, FiO2 and CO2 levels should be closely monitored for each change in ventilation settings to decide the optimum pressure level. Lung compliance is very dynamic during the management of RDS, particularly following surfactant therapy, and for an individual infant rapid changes in ventilator settings may be required. Tidal volume-targeted synchronized ventilation should be preferred because it enables clinicians to ventilate with less variable tidal volumes and real-time weaning of pressure as lung compliance improves. Volume targated ventilation has been shown to reduce the duration of invasive respiratory support, decrease the incidence of pneumothorax, hypocarbia, severe IVH, mortality, and BPD (35). The initial tidal volume should be set to 4-6 mL/kg. As soon as satisfactory gas exchange is achieved in the presence of spontaneous breathing efforts, the weaning process from the ventilator should be initiated. As the lung compliance improves, the PIP value will be reduced; with a tidal volume-targeted ventilation mode, the ventilator weaning process is started automatically. When required MAP is 7-8 cm H20 in conventional MV or 8-9 cm-H20 in HFOV, extubation should be considered. Following extubation, patient should be switched to non-invasive respiratory support through the nares with one of the mentioned NIV methods. When high pressures are needed to achieve adequate lung inflation during conventional ventilation switching to HFOV as a rescue treatment is reasonable. A meta-analysis of randomized controlled trials comparing HFOV with conventional MV shows a small uneven reduction in BPD in the HFOV group; however, this benefit is neutralized by increased air leaks in those on HFOV (36). In a randomized trial involving children who had been born extremely prematurely, those who had undergone HFOV, as compared with those who had received conventional ventilation, had superior lung function at 11 to 14 years of age with no evidence of poorer functional outcomes.
Additional strategies to reduce duration of respiratory support
Several strategies have been used specifically to improve the success of non-invasive ventilation and shorten the duration of MV including caffeine therapy, permissive hypercarbia and postnatal steroid treatment.
Permissive hypercapnia: Both hypercarbia and hypocarbia should be avoided during MV since both conditions are associated with increased risk of BPD, periventricular leukomalacia (PVL), and IVH. Systems that allow continuous and non-invasive monitoring of PaCO2 levels are recommended to maintain normocarbia. In further analysis of the SUPPORT trial, it has been shown that high target PaCO2 level was associated with increased death, IVH, BPD rates, and poor neurodevelopmental outcomes (37). The PHELBI study, in which <29 weeks and <1000 g preterm infants requiring intubation during the first 24 hours of life and 14 days of ventilation were randomized into two different target pCO2 levels, it was shown that high target pCO2 levels did not decrease the incidence of BPD (38). Mortality, IVH, and ROP rates were similar between the groups. The long-term results of the same study have been recently published and no significant differences were found between the groups in terms of neurodevelopmental outcomes (39). It is recommended to optimize pCO2 levels to prevent possible short-term adverse effects (pCO2 45-60 mm Hg).
Caffeine therapy: It is known that caffeine prevents extubation failure and BPD, decreases the need for PDA treatment, and improves survival without neurologic sequelae at corrected age of 18 months. However, these significant differences disappear at 5 years of age (40). There are randomized controlled trials showing that early initiation of caffeine treatment decreases the incidence of BPD more than late-onset treatment. In the absence of randomized trials and good evidence of safety, it seems reasonable to recommend caffeine routinely as part of a strategy to minimize the need for MV for premature infants with birth weight <1250 g. The recommended loading dose is 20 mg/kg with 5-10 mg/kg daily maintenance. Few studies suggest that doubling these doses may further reduce the risk of extubation failure, although tachycardia is more frequent (41).
Postnatal steroids: Ventilator dependent infants for more than 2 weeks who have a high risk of BPD, short-term low-dose dexamethasone therapy may be considered to facilitate extubation. Other morbidities that may cause MV dependency such as PDA, sepsis, pneumonia, additional anomalies, etc. should be excluded before considering the steroid treatment. The risks and benefits of steroid treatment should be weighed by the primary physician and informed consent should be obtained from the parents (also see Turkish Neonatal Society Guideline on the Prevention and Treatment of Bronchopulmonary Dysplasia).
Inhaled budesonide is still under investigation as an alternative therapy to systemic steroid therapy (42). Intratracheal administration of budesonide together with surfactant is a promising practice because it may reduce lung inflammation and the need for MV, however it requires further confirmation by randomized multicentre trials (43).
Other medical and supportive treatments
Antibiotics
Prophylactic antibiotics can be started until sepsis is excluded in RDS. The first choice for prophylactic antibiotics should be regimens combining penicillin or ampicillin with aminoglycoside. Preterm infants with low risk of sepsis should not be prescribed antibiotics as much as possible. As soon as sepsis is excluded, antibiotics must be discontinued. Prophylactic antifungal therapy (fluconazole/nystatin) has been shown to be effective in units where invasive fungal infections are common (over 5%) but new guidelines on this subject should be followed (44).
Fluid, temperature, and nutrition management
A premature infant’s body temperature should be between 36.5 and 37.5°C. Plastic/polyurethane wrap/bags can be used to prevent heat loss, especially those under 30 weeks of gestational age. Servo-controlled incubators that adjusting skin temperature to 36.5°C reduce neonatal mortality. To maintain minimal evaporative heat loss, it is better to ensure that environmental humidity is 60-90% depending on gestational age. Starting intravenous fluid treatment with 70-80 mL/kg/day is enough for most premature infants in humidified incubators. Extremely immature infants may have greater fluid requirements initially. The fluid volume should be individually adjusted for each infant according to serum electrolyte concentrations and body weight. In the first days of life, sodium intake should be restricted, and sodium intake should be initiated only after controlling fluid balance and electrolyte levels when diuresis begins. Parenteral nutrition should be initiated from the first day of life in order to prevent growth retardation. Amino acids are started at 3 g/kg/day and can be rapidly increased to 4 g/kg/day. Lipids can be started at 1 g/kg/day and increased to 3 g/kg/day. Minimal enteral nutrition should be started from birth in all premature infants if there is no contraindication. The best nutrients for premature infants is their own mother’s breastmilk. Breastmilk may be started as 10-20 ml/kg/day in clinically stable infants. 20-30 mL/kg/day increases can be done according to the clinical situation in following days. (also see Turkish Neonatal Society Guideline on Enteral/Parenteral Nutrition of the Preterm Infant).
Blood pressure, perfusion, and patent ductus arteriosus management
Arterial hypotension should be treated with inotropes only in infants who exhibits signs of impaired tissue perfusion such as oliguria, acidosis, prolonged capillary refill time. Dopamine is the drug of choice in hypotensive infants with low systemic vascular resistance, whereas dobutamine is the first-line for patients with low cardiac output. Epinephrine and hydrocortisone may also be used in the treatment of cases with resistant hypotension (45). There is still no accepted international consensus on the treatment of hypotension. Hemoglobin levels of premature infants on respiratory support should be kept within normal limits. Transfusion thresholds in infants with ongoing respiratory support are defined as 12 g/dL in the first week, 11 g/dL in the second week, 10 g/dL in the third week of life, and 9 g/dL in beyond. In patients with little or no respiratory support, transfusion thresholds can be 1.5-2 g/dL lower in each defined week (46). The results of the studies “Transfusion of Prematures (TOP-NCT01702805)” and “Effects of transfusion thresholds on neurocognitive outcome of extremely-low-birth-weight infants (ETNNO NCT01393496)” are waited in order to delineate the optimum erythrocyte transfusion limits in premature infants.
Conservative treatment, cyclooxygenase (COX) inhibitors, and surgical treatment are options of treatment in patent ductus arteriosus. It is still controversial when and how to treat hsPDA. Conservative treatment is an acceptable strategy provided if the infant is thriving, tolerating feeds and on minimal respiratory support. Cyclo-oxygenase inhibitors such as indomethacin or ibuprofen promote ductal closure, ibuprofen has fewer side effects so it can be the first choice in hsPDA requiring medical treatment. More recently paracetamol has been shown to promote ductal closure however should be preferred in patients who do not respond to COX inhibitors or if they are contraindicated. Surgical ligation of PDA is associated with worse long-term neurodevelopmental outcome, and although it is not clear whether this is due to the PDA or its treatment, surgery should only be considered after medical therapy has failed (47).
Pain and sedation should not be neglected in the treatment of RDS. However, sedation is not required in clinically stable ventilated patients. In premature infants who are treated for RDS, as far as possible, the number of painful interventions should be reduced, they should be monitorized with pain scales, a non-pharmacologic intervention such as pacifiers or sucrose solutions should be employed during painful procedures. Paracetamol and opioid analgesics can be used to maximize comfort with the physician’s discretion when they are necessary (47).
Other recommendations for management of a patient with respiratory distress syndrome
Inhaled nitric oxide (iNO) is not recommended in the treatment of premature infants with RDS (48). However, it can be used in severe hypoxemia caused by pulmonary hypertension. Early initiation of iNO in infants with RDS has no benefit in preventing neurologic damage and BPD. Surfactant use may be considered in cases with secondary secondary surfactant inactivation, such as pulmonary hemorrhage, meconium aspiration syndrome, and pneumonia, but there is still insufficient evidence to support this treatment (49).
Footnotes
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
Çıkar Çatısması: Yazarlar çıkar çatışması bildirmemişlerdir.
Mali Destek: Yazarlar bu çalışma için mali destek almadıklarını beyan etmişlerdir.
References
- 1.Wiingreen R, Greisen G, Ebbesen F, et al. Surfactant need by gestation for very preterm babies initiated on earlynasal CPAP: A Danish observational multicentre study of 6,628 infants born 2000-2013. Neonatology. 2017;111:331–6. doi: 10.1159/000451021. [DOI] [PubMed] [Google Scholar]
- 2.Özkan H, Erdeve Ö, Karadağ A. Respiratory distress syndrome and surfactant replacement therapy guideline. 2014:1–24. [Google Scholar]
- 3.Saugstad OD. Delivery room management of term and preterm newly born infants. Neonatology. 2015;107:365–71. doi: 10.1159/000381159. [DOI] [PubMed] [Google Scholar]
- 4.Stock SJ, Bricker L, Norman JE, West HM. Immediate versus deferred delivery of the preterm baby with suspected fetal compromise for improving outcomes. Cochrane Database Syst Rev. 2016;7:CD0089688. doi: 10.1002/14651858.CD008968.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eke AC, Chalaan T, Shukr G, Eleje GU, Okafor CI. A systematic review and meta-analysis of progestogen use for maintenance tocolysis after preterm labor in women with intact membranes. Int J Gynaecol Obstet. 2016;132:11–6. doi: 10.1016/j.ijgo.2015.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2017;3:CD004454. doi: 10.1002/14651858.CD004454.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crowther CA, McKinlay CJ, Middleton P, Harding JE. Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes. Cochrane Database Syst Rev. 2015;7:CD003935. doi: 10.1002/14651858.CD003935.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perlman JM, Wyllie J, Kattwinkel J, et al. Resuscitation chapter collaborators. Part 7: Neonatal resuscitation: 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2015;132:S204–41. doi: 10.1161/CIR.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 9.Tarnow-Mordi W, Morris J, Kirby A, et al. Australian placental transfusion study collaborative group. Delayed versus immediate cord clamping in preterm infants. N Engl J Med. 2017 Oct 29; doi: 10.1056/NEJMoa1711281. doi: 10.1056/NEJMoa1711281. [DOI] [PubMed] [Google Scholar]
- 10.Alan S, Arsan S, Okulu E, et al. Effects of umbilical cord milking on the need for packed red blood cell transfusions and early neonatal hemodynamic adaptation in preterm infants born ≤1500 g: A prospective, randomized, controlled trial. J Pediatr Hematol Oncol. 2014;36:e493–8. doi: 10.1097/MPH.0000000000000143. [DOI] [PubMed] [Google Scholar]
- 11.Oei JL, Vento M, Rabi Y, et al. Higher or lower oxygen for delivery room resuscitation of preterm infants below 28 completed weeks gestation: A meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2017;102:F24–30. doi: 10.1136/archdischild-2016-310435. [DOI] [PubMed] [Google Scholar]
- 12.Subramaniam P, Ho JJ, Davis PG. Prophylactic nasal continuous positive airway pressure for preventing morbidity and mortality in very preterm infants. Cochrane Database Syst Rev. 2016;6:CD001243. doi: 10.1002/14651858.CD001243.pub3. [DOI] [PubMed] [Google Scholar]
- 13.Rojas-Reyes MX, Morley CJ, Soll R. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2012:CD000510. doi: 10.1002/14651858.CD000510.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ardell S, Pfister RH, Soll R. Animal derived surfactant extract versus protein free synthetic surfactant for the prevention and treatment of respiratory distress syndrome. Cochrane Database Syst Rev. 2015:CD000144. doi: 10.1002/14651858.CD000144.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Sweet DG, Turner MA, Straňák Z, et al. A first-in-human clinical study of a new SP-B and SP-C enriched synthetic surfactant (CHF5633) in preterm babies with respiratory distress syndrome. Arch Dis Child Fetal Neonatal Ed. 2017;102:F497–503. doi: 10.1136/archdischild-2017-312722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ricci F, Murgia X, Razzetti R, Pelizzi N, Salomone F. In vitro and in vivo comparison between poractant alfa and the new generation synthetic surfactant CHF5633. Pediatr Res. 2017;81:369–75. doi: 10.1038/pr.2016.231. [DOI] [PubMed] [Google Scholar]
- 17.Singh N, Halliday HL, Stevens TP, et al. Comparison of animal-derived surfactants for the prevention and treatment of respiratory distress syndrome in preterm infants. Cochrane Database Syst Rev. 2015:CD010249. doi: 10.1002/14651858.CD010249.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karadag A, Ozdemir R, Degirmencioglu H, et al. comparison of three different administration positions for intratracheal beractant in preterm newborns with respiratory distress syndrome. Pediatr Neonatol. 2016;57:105–12. doi: 10.1016/j.pedneo.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Bahadue FL, Soll R. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2012;11:CD001456. doi: 10.1002/14651858.CD001456.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dargaville PA, Gerber A, Johansson S, et al. Incidence and outcome of cpap failure in preterm infants. Pediatrics. 2016;138:pii: e20153985. doi: 10.1542/peds.2015-3985. doi: 10.1542/peds.2015-3985. [DOI] [PubMed] [Google Scholar]
- 21.Göpel W, Kribs A, Ziegler A, et al. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open-label, randomised, controlled trial. Lancet. 2011;378:1627–34. doi: 10.1016/S0140-6736(11)60986-0. [DOI] [PubMed] [Google Scholar]
- 22.Dargaville PA, Aiyappan A, Cornelius A, et al. Preliminary evaluation of a new technique of minimally invasive surfactant therapy. Arch Dis Child Fetal Neonatal Ed. 2011;96:F243. doi: 10.1136/adc.2010.192518. [DOI] [PubMed] [Google Scholar]
- 23.Kanmaz HG, Erdeve O, Canpolat FE, et al. Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial. Pediatrics. 2013;131:e502. doi: 10.1542/peds.2012-0603. [DOI] [PubMed] [Google Scholar]
- 24.Aldana-Aguirre JC, Pinto M, Featherstone RM, Kumar M. Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2017;102:F17–23. doi: 10.1136/archdischild-2015-310299. [DOI] [PubMed] [Google Scholar]
- 25.Sardesai S, Biniwale M, Wertheimer F, et al. Evolution of surfactant therapy for respiratory distress syndrome: past, present, and future. Pediatr Res. 2017;81:240–8. doi: 10.1038/pr.2016.203. doi: 10.1038/pr.2016.203. [DOI] [PubMed] [Google Scholar]
- 26.Saugstad OD, Aune D. Optimal oxygenation of extremely low birth weight infants: A meta-analysis and systematic review of the oxygen saturation target studies. Neonatology. 2014;105:55–63. doi: 10.1159/000356561. [DOI] [PubMed] [Google Scholar]
- 27.Say B, Kanmaz Kutman HG, Oguz SS, et al. Binasal prong versus nasal mask for applying CPAP to preterm infants: A randomized controlled trial. Neonatology. 2016;109:258–64. doi: 10.1159/000443263. [DOI] [PubMed] [Google Scholar]
- 28.Amatya S, Macomber M, Bhutada A, Rastogi D, Rastogi S. Sudden versus gradual pressure wean from nasal CPAP in preterm infants: A randomized controlled trial. J Perinatol. 2017;37:662–7. doi: 10.1038/jp.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemyre B, Laughon M, Bose C, Davis PG. Early nasal intermittent positive pressure ventilation (NIPPV) versus early nasal continuous positive airway pressure (NCPAP) for preterm infants. Cochrane Database Syst Rev. 2016;12:CD005384. doi: 10.1002/14651858.CD005384.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemyre B, Davis PG, De Paoli AG, Kirpalani H. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database Syst Rev. 2014:CD003212. doi: 10.1002/14651858.CD003212.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Wilkinson D, Andersen C, O'Donnell CP, De Paoli AG, Manley BJ. High flow nasal cannula for respiratory support in preterm infants. Cochrane Database Syst Rev. 2016;2:CD006405. doi: 10.1002/14651858.CD006405.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taha DK, Kornhauser M, Greenspan JS, Dysart KC, Aghai ZH. High flow nasal cannula use is associated with increased morbidity and length of hospitalization in extremely low birth weight infants. J Pediatr. 2016;173:50–5.e1. doi: 10.1016/j.jpeds.2016.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts CT, Owen LS, Manley BJ, et al. Nasal high-flow therapy for primary respiratory support in preterm infants. N Engl J Med. 2016;375:1142–51. doi: 10.1056/NEJMoa1603694. [DOI] [PubMed] [Google Scholar]
- 33.Mukerji A, Dunn M. High-frequency ventilation as a mode of noninvasive respiratory support. Clin Perinatol. 2016;43:725–40. doi: 10.1016/j.clp.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Klingenberg C, Wheeler KI, McCallion N, Morley CJ, Davis PG. Volume-targeted versus pressure-limited ventilation in neonates. Cochrane Database Syst Rev. 2017;10:CD003666. doi: 10.1002/14651858.CD003666.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cools F, Offringa M, Askie LM. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database Syst Rev. 2015;3:CD000104. doi: 10.1002/14651858.CD000104.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ambalavanan N, Carlo WA, Wrage LA, et al. PaCO2 in surfactant, positive pressure, and oxygenation randomised trial (SUPPORT) Arch Dis Child Fetal Neonatal Ed. 2015;100:F145. doi: 10.1136/archdischild-2014-306802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thome UH, Genzel-Boroviczeny O, Bohnhorst B, et al. Permissive hypercapnia in extremely low birthweight infants (PHELBI): A randomised controlled multicentre trial. Lancet Respir Med. 2015;3:534–43. doi: 10.1016/S2213-2600(15)00204-0. [DOI] [PubMed] [Google Scholar]
- 38.Thome UH, Genzel-Boroviczeny O, Bohnhorst B, et al. Neurodevelopmental outcomes of extremely low birthweight infants randomised to different PCO2 targets: the PHELBI follow-up study. Arch Dis Child Fetal Neonatal Ed. 2017;102:F376. doi: 10.1136/archdischild-2016-311581. [DOI] [PubMed] [Google Scholar]
- 39.Henderson-Smart DJ, De Paoli AG. Prophylactic methylxanthine for prevention of apnoea in preterm infants. Cochrane Database Syst Rev. 2010;12:CD000432. doi: 10.1002/14651858.CD000432.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt B, Anderson PJ, Doyle LW, et al. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA. 2012;307:275–82. doi: 10.1001/jama.2011.2024. [DOI] [PubMed] [Google Scholar]
- 41.Shah SS, Ohlsson A, Halliday HL, Shah VS. Inhaled versus systemic corticosteroids for the treatment of bronchopulmonary dysplasia in ventilated very low birth weight preterm infants. Cochrane Database Syst Rev. 2017;10:CD002057. doi: 10.1002/14651858.CD002057.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Venkataraman R, Kamaluddeen M, Hasan SU, Robertson HL, Lodha A. Intratracheal administration of budesonide-surfactant in prevention of bronchopulmonary dysplasia in very low birth weight infants: A systematic review and meta-analysis. Pediatr Pulmonol. 2017;52:968–75. doi: 10.1002/ppul.23680. [DOI] [PubMed] [Google Scholar]
- 43.Leonart LP, Tonin FS, Ferreira VL, et al. Fluconazole doses used for prophylaxis of invasive fungal infection in neonatal intensive care units: A network meta-analysis. J Pediatr. 2017;185:129–35. doi: 10.1016/j.jpeds.2017.02.039. [DOI] [PubMed] [Google Scholar]
- 44.Rabe H, Rojas-Anaya H. Inotropes for preterm babies during the transition period after birth: friend or foe? Arch Dis Child Fetal Neonatal Ed. 2017;102:F547–50. doi: 10.1136/archdischild-2016-311709. [DOI] [PubMed] [Google Scholar]
- 45.Nickel RS, Josephson CD. Neonatal transfusion medicine: Five major unanswered research questions for the twenty-first century. Clin Perinatol. 2015;42:499–513. doi: 10.1016/j.clp.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 46.Oncel MY, Erdeve O. Oral medications regarding their safety and efficacy in the management of patent ductus arteriosus. World J Clin Pediatr. 2016;5:75–81. doi: 10.5409/wjcp.v5.i1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barrington KJ, Finer N, Pennaforte T. Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane Database Syst Rev. 2017;1:CD000509. doi: 10.1002/14651858.CD000509.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jasani B, Kabra N, Nanavati R. Surfactant replacement therapy beyond respiratory distress syndrome in neonates. Indian Pediatrics. 2016;53:229–34. doi: 10.1007/s13312-016-0826-z. [DOI] [PubMed] [Google Scholar]


