Abstract
Pain control is an important ethical issue to be considered and constitutes the basis of treatment in premature and term newborns. The inadequacy of pain control in these infants in neonatal intensive care units leads to neurodevelopmental and behavioral problems in the long term. For this reason, it is extremely important to raise awareness of the presence of pain in newborn infants, to reduce invasive procedures applied to infants as much as possible, and to minimize pain with non-pharmacologic or pharmacologic treatments when it is inevitable.
Keywords: Newborn, pain, treatment
Abstract
Ağrı kontrolü prematüre ve zamanında doğan yenidoğan bebeklerde dikkat edilmesi gereken önemli bir etik sorundur ve tedavinin temelini teşkil eder. Yenidoğan yoğun bakım birimlerinde izlenen bu bebeklerde ağrı kontrolünde yetersizlik, bebeklerde ileri dönem nörogelişimsel ve davranış ile ilgili sorunlara yol açmaktadır. Bu nedenle yenidoğan bebeklerde ağrının varlığı ile ilgili farkındalığı arttırmak, bebeklere uygulanan invaziv girişimleri mümkün olduğunca azaltmak, ağrının kaçınılmaz olduğu durumlarda ise bunu farmakolojik olmayan yollardan ya da farmakolojik tedavi ile en aza indirmek son derece önemlidir.
Introduction
In recent years, the survival rate of newborns followed up in neonatal intensive care units (NICUs), especially in 24-32–week-old babies, is increasing compared with the past. These babies are exposed to recurrent painful interventions in the NICU, during a period in which the brain develops rapidly and stress perception systems are organized. Excessive neuronal activity due to pain in the postnatal period causes somatosensory and functional changes in pain perception procedure in the long term. Therefore, inadequate pain control leads to neurodevelopmental and behavioral problems in infants.
One of the most important goals is to prevent recent and advanced morbidity during the follow-up of newborn babies in the NICU and the role of pain control should not be forgotten during this phase.
Definition and physiology of pain
The sensation of pain in newborn babies begins from the intrauterine period (1). Communication between the cortex and the thalamus begins after the prenatal 20th week (2). From birth, peripheral sensitivity to mechanical, thermal, and chemical stimuli or nociceptor’s response to primary hyperalgia develop and pain signals reach the somatosensory cortex. Babies give physiologic, behavioral, and hormonal responses to pain (3). Therefore, with the knowledge of the damage and stress caused by pain, it is both a medical and ethical responsibility to develop appropriate pain control strategies, especially in premature infants (4).
Painful interventions to which newborns are exposed
Some of the painful interventions that newborn babies face during intensive care and outpatient follow-up are heel stick, venous and arterial vascular interventions, percutaneous central venous catheter insertion, intramuscular and subcutaneous injections, nasogastric catheter insertion, postural drainage, circumcision, urinary catheter insertion, tracheal intubation, endotracheal aspiration, lumbar puncture (LP), chest tube insertion and withdrawal, dressing change, examination for retinopathy of prematurity (ROP), and laser therapy.
Heel stick in term and premature infants is more painful than venous interventions performed by an experienced nurse. Endotracheal aspiration, in which considerable pain is felt, should not be performed unless absolutely necessary (5). It is also important to control pain when pulling a chest tube, which is a very painful procedure (6).
There is no precise definition of chronic pain for the newborn. In newborns, the term long-term or persistent pain may be used. Necrotizing enterocolitis (NEC), meningitis, bone fracture, osteomyelitis, septic arthritis, tissue ischemia and necrosis, nerve lesion, and skin damage are the leading causes of long-lasting pain in newborn infants. This type of pain causes neurologic problems in the newborn, similar to acute pain. It is also critical to control postsurgical pain (7).
Evaluation of pain response in newborns
The main purpose of evaluating the pain response is to determine the cause of the painful condition, the level of pain, and the need for intervention against it. In this way, it is aimed to avoid inadequate or unnecessary treatment. The American Academy of Pediatrics and the Canadian Pediatric Association newborn pain control programs recommend both routine pain control and evaluation before and after painful interventions (7).
For all doctors and nurses involved in neonatal care, awareness of the presence of pain and treatment needs is the first step in effective pain control.
Objective evaluation of pain in newborns can be achieved by using structured methods. These structured methods should be preferred to include a number of physiologic, behavioral or other variables. The method to evaluate pain response should be appropriate for gestational age.
Physiologic variables
Physiologic changes observed during painful interventions due to activation of the sympathetic nervous system include increased heart rate, increased blood pressure, decreased oxygen saturation, increased respiratory rate and intracranial pressure, and sweating on the palms. Additional known effects are changes in respiratory pattern, skin color, and pupil size following painful stimuli (8). In some studies, electroencephalography (EEG) or electromyography (EMG) patterns have also been used to assess pain, but these methods are not yet fully considered to guide clinical practice (9).
Behavioral variables
Behavioral variables are considered important indicators of pain in newborn infants. Crying (crying forms, duration, acoustic patterns), facial expressions (scowl, blinking, nasolabial wrinkle and opening of the mouth), hand and body movements, muscle tone, behavioral status changes, ability to disconsolate are counted as the behavioral responses of newborn babies to pain (8).
Structured methods used in the assessment of pain response
There are over 40 methods in the assessment of pain response in newborns. The most commonly used methods are “Premature Infant Pain Profile” (PIPP), “Crying, Requires Oxygen Saturation, Increased Vital Signs, Expression, Sleeplessness (CRIES), Neonatal Infant Pain Scale (NIPS), Neonatal Pain Agitation and Sedation Scale” (N-PASS), “Neonatal Facing Coding System” (NFCS), “Pain Assessment Tool” (PAT), “Scale for Use in Newborns” (SUN), “Echelle de la Douleur Inconfort Nouveau-Ne” (Neonatal Pain and Discomfort Scale, EDIN) and “Bernese Pain Scale for Neonates” (BPSN) (7, 8). Some methods assess both sedation and pain response at the same time (N-PASS and COMFORT).
Choosing the method for pain assessment
The method chosen to assess the pain response in newborns should be suitable for the type of pain to be evaluated.
Acute pain: Refers to painful experiences that occur immediately after interventions that cause disruption of skin integrity or tissue damage for diagnostic or therapeutic purposes. It is the most common type of pain in the NICU.
Postoperative pain: This is the type of pain observed in the first 24-48 hours in newborn infants after surgery. N-PASS and COMFORT are recently developed scoring systems that have been used for evaluating postoperative pain response by providing the possibility of evaluating sedation with pain (10, 11).
Prolonged / persistent / chronic pain: the term of “chronic pain” is not suitable for the neonatal period because the concept of chronic pain in children and adults covers a period of three months. For this reason, it may be preferable to use the terms ‘prolonged’ or ‘persistent’ pain in newborn infants. ‘Prolonged’ or ‘stubborn’ pain in newborn infants may be due to necrotizing enterocolitis, peritonitis, bone fractures, meningitis, as well as procedures such as mechanical ventilation and chest tube insertion. N-PASS, COMFORT neo and EDIN scores are available for pain assessment in these cases (8).
Among the pain scoring systems that can be used in the NICU, one or two methods should be selected according to the patient profile, then the doctor, and especially the nurses who will score on these methods, should be trained.
Pain management in the newborn
Pain management in the neonatal period is very important because its permanent effects have been shown. There are two management modalities, pharmacologic and non-pharmacologic. What is important, however, is to reduce and eliminate painful stimuli as much as possible, rather than to treat them.
Reduction of painful interventions
The first way to reduce the painful event is to reduce interventions in the newborn. What can be done in this regard are blood sampling from catheters, planning of all samples to be taken at one time in non-catheterized patients, and transcutaneous monitoring of blood gases, if possible. In particular, the placement of percutaneous central catheters may prevent patients from undergoing frequent insertions of peripheral catheters. Tracheal aspiration, which is a very painful procedure in premature infants followed in a mechanical ventilator, should not be made unnecessarily.
Environmental and behavioral precautions
The non-painful care, treatment and examination procedures should be grouped as much as possible (7). Intervals in which babies can sleep comfortably without being touched should be created in their routine procedure. Therefore, it is recommended that there should be an interval of at least 2 hours between invasive procedures (12).
The behavioral status of newborn babies is also important in the perception of pain. For non-urgent interventions, a baby should not be awakened from sleep; calm vigilance should be preferred (12). Although no studies have investigated the effects of sound and light levels on pain responses in preterm infants, clinical experience shows that light and sound levels are kept as low as possible during painful interventions.
Using a mechanical lancet during heel stick reduces pain. According to the amount of blood to be taken, less painful venous blood sampling can be preferred in term infants. To reduce the pain observed during the removal of plasters, it is recommended to avoid large plaster surfaces as much as possible, and use either mineral oils or silicone-based special adhesive removers for their removal (13).
Non-pharmacologic methods
Breastfeeding and breast milk, pacifier, skin-to-skin contact, positioning the baby, touching, performing massage, providing painless sensory stimuli such as sound and smell can effectively reduce discomfort and pain in preterm and term babies (14).
Breastfeeding or expressed breast milk
Breast milk is preferred because it is physiologic and has no potential adverse effects when compared with other options recommended in the treatment of pain in the newborn. It has been emphasized that the pain relief effect of milk can be caused by fat and protein components as well as taste. Breastfeeding has similar efficacy to sugary solutions (15). However, it is not possible to apply to premature infants who are intubated and not yet able to feed orally.
When the effects of breastfeeding – except for contact factors – were investigated, 2 mL of breast milk given 2 minutes before receiving venous blood sample in term babies caused a significant decrease in pain responses, but less than with a 25% glucose solution (16). In a study conducted in preterm infants, 5 mL of expressed breast milk resulted in a significant decrease in pain scores (17). Finally, the analgesic efficacy of giving expressed breast milk during ROP examinations has been demonstrated (18).
In light of the studies conducted, 2-5 mL of breast milk – preferably final milk – might be given prior to interventions leading to mild-to-moderate pain.
Non-nutritive sucking
Non-nutritive sucking (pacifier use) is the most widely studied non-pharmacologic method in the treatment of neonatal pain. It is effective in reducing pain-related stress in both preterm and term babies (14). Pain control in infants can be improved by giving pacifiers soaked in sugary solutions.
Positioning
During painful interventions, holding babies in the midline flexion posture can ease their calmness. Loosely swaddling the baby during interventions was found to be effective on physiologic and behavioral pain responses (19).
Other non-pharmacologic methods
There are studies showing that touching, performing massage, speaking, playing music and in-utero sounds, visual stimulation by moving toys has an effect on reducing pain (13). There are some other effective methods such as giving individualized developmental care, smelling vanilla, and hand swaddling by parents prior to interventions.
Pharmacologic treatment
Sugary solutions
The oral administration of sugary solutions such as sucrose, glucose, saccharin, and fructose has analgesic efficacy in newborns (20). Giving the sugary solutions together with the pacifier increases their effectiveness.
Sugary solutions may be used prior to minor painful procedures (such as heel stick, venous blood sampling, venous catheterization, arterial blood sampling, urinary catheter insertion, intramuscular or subcutaneous injection, nasogastric tube insertion, dressing change, removal of adhesive tapes). Application of sucrose with topical anesthetics was found to be effective in the treatment of pain during ROP examinations (21).
In order to observe the effects of sugary solutions, it should be given 2 minutes before the intervention onto the anterior part of the tongue where the majority of the taste buds are located. It is thought that the sucrose with a sugary taste causes endogenous opioid release within the 2 min interval before the painful intervention is performed.
Sucrose solutions
The most widely used and investigated sugary solutions worldwide are sucrose solutions, which are known to be effective in newborns with gestational ages of 25-42 weeks (22). The amount of sucrose shown to be effective in the treatment of neonatal pain is between 0.012 and 0.12 g (0.05 - 0.5 mL 24% sucrose solution) (22). In preterm infants 0.2-0.3 mL, in term infants, 1-2 mL of a 12-24% concentration sucrose solution can be given. Accessibility is low in our country. Dose adjustment is recommended with consideration to the postconceptional age (22).
Glucose solutions
Glucose solutions, which can be reached more easily in our country, can be considered as an alternative to sucrose among sugary solutions. The clinically analgesic efficacy of 2 mL of 30% glucose given before the venous blood collection in term babies has been demonstrated. However, the efficacy of 30% glucose is lower than the sucrose solution at the same concentration. The administration of 2 mL of 20% glucose solution prior to heel stick effectively suppresses increased heart rate and behavioral pain responses in preterm infants. In patients with heel stick or venous blood sampling, analgesic efficacy can be observed at concentrations of more than 20% (23).
It was thought that 20-30% glucose might be an alternative to sucrose for minor painful interventions (24).
Glucose solutions may be given as 1-2 mL at 20-30% concentrations in babies with lower gestational age (12).
Adverse effects of sugary solutions
Careful monitoring of vital signs, coughing, and gag reflex is required when delivering sugary solutions. Administration of very frequent and high doses have potential adverse effects such as hyperglycemia, fluid overload, and necrotizing enterocolitis. Concern for neurologic limitation in infants with low gestational ages continues. When the neuromotor development of infants younger than 31 gestational weeks given either sucrose or water prior to each painful interventions within the first seven days of life were compared, motor development scores in babies receiving sucrose (>10 times per day) were lower at 36-40 postconceptional age, which was correlated with sucrose dose (25). In another study that evaluated the efficacy and safety of administering sucrose and pacifier for pain control in the first 28 days of life in babies older than 26 weeks gestational age, no adverse effects or neurologic risks were observed (26). Although Holsti et al. reported no serious adverse effects in the short term (27), they highlighted the negative effects on attention and motor development that were found in the only study evaluating neurodevelopmental effects. The suggested mechanisms that may lie beneath these possible adverse effects are that chronic exposure to sugar can lead to the development of alternative pathways associated with attention and motor development in the brain.
Topical anesthetics
Topical anesthetics can be used prior to venous interventions, lumbar puncture, and intravenous catheter insertion. It should be applied half an hour before the procedure in the newborn. It is not appropriate to use topical medication during heel stick because pain is more likely to be caused by squeezing. It is known that topical lidocaine and prilocain preparations causes methemoglobinemia in some cases. EMLA cream has been shown to be effective (28). EMLA cream is recommended to be used carefully in preterm infants. Among other topical analgesics, tetracaine could be used (29). The complications of topical creams might be methemoglobinemia and skin rash (30).
Lidocaine
Lidocaine inhibits axonal conduction by blocking sodium ion channels and is used for circumcision procedures.
Opiates (morphine, fentanyl, remifentanil, alfentanil, sufentanil)
In the treatment of moderate pain, opiates provide very effective treatment. Morphine and fentanyl are the most commonly used agents for analgesia and sedation. In some NICUs, the more potent sufentanil or the shorter duration alfentanil, remifentanil or mixed opiates (tramadol) are also recommended for use (31-34). Morphine is the most commonly used agent in newborn anesthesia, but routine use in ventilated infants is not recommended due to the occurrence of hypotension, prolongation of mechanical ventilation, and delay of enteral feeding (35).
Fentanyl has less hemodynamic effects and provides faster analgesia. Fentanyl can be used when a fast opiate effect is needed and in postoperative pain. Its significant adverse effects are bradycardia, hypotension, laryngospasm, and chest rigidity (36). In newborns with hypotension and in premature babies of 21-26 weeks of gestational age, hypotension, bradycardia, intraventricular hemorrhage, impaired intestinal motility, and neurodevelopmental disorders should be considered when fentanyl is being used.
The chemical structure of remifentanil is similar to fentanyl but its analgesic effect is three times higher and reaches the highest effect in 3-15 minutes. It can be used for intubation or short-term procedures such as central catheter insertion. Alfentanil’s duration of effect is 20-30 min. In patients with opioid-induced chest wall rigidity, opioid antagonist naloxone is used. If there is no naloxone, muscle rigidity can be solved by applying muscle paralysis.
When the opiates are used for a long time, instead of stopping suddenly, they should be tapered off in order to prevent the occurrence of withdrawal symptoms. Although there are different approaches in various clinics, an example of a morphine reduction protocol considering duration of morphine use and Finnegan score is given in Figure 1 (37).
Figure 1.
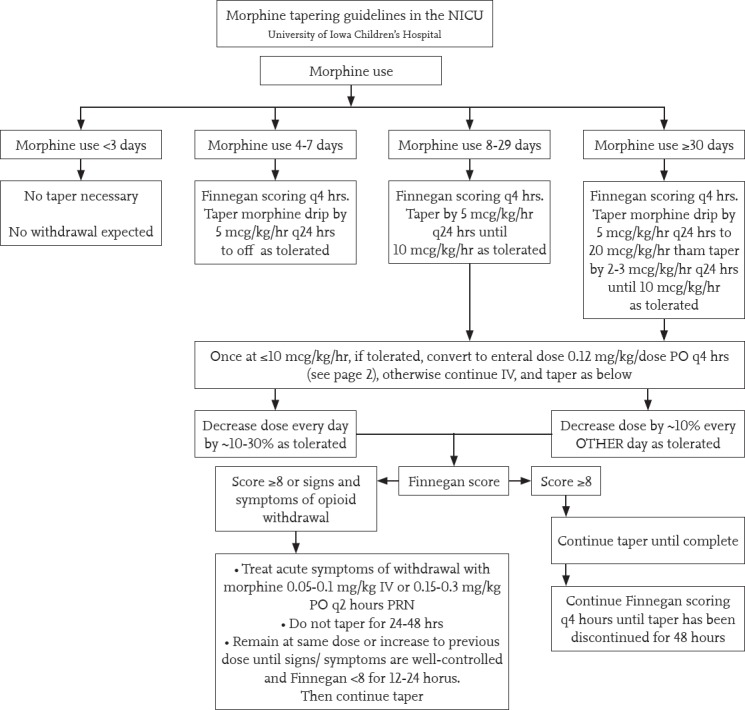
Morphine tapering guideline
Written: 06/15/09 Sarah Tierney Pharm D, Julie Lindower MD, MPH &Stephanie Stewart RN, MSN; Updated: 11/17/09, 3/12/2012 Jonathan Klein, MD and Sarah Tierney, Pharm D, University of Iowa Children’s Hospital, NeonatologyTextbook
Non-opioid pain treatments
Dexmedetomidine
Dexmedetomidine is a selective alpha 2 adrenergic receptor agonist. It has a strong sedative and analgesic effect, with weak respiratory depression. There are studies reporting that it may cause bradycardia and hypothermia in neonates (38). In a pharmacokinetic study performed in newborns whose gestational age ranged from 28 to 40 weeks, it was reported that analgesia and sedation were provided with the maintenance dose given at 0.05-0.2 mcg/kg/st dose following a loading dose of 0.05-0.2 mcg/kg (39).
Acetaminophen (Paracetamol)
Acetaminophen inhibits cyclooxygenase (COX)-2 and reduces postsurgical opiate use. The most important toxicity is on the liver. However, it is safe and effective at the appropriate doses. Dosages according to gestational age are as follows:
20-30 mg/kg/day between 24-30 gestational weeks
35-50 mg/kg/day between 31-36 gestational weeks
50-60 mg/kg/day between 37 and 42 gestational weeks
60-75 mg/kg/day in postnatal 1-3 months (40).
In the recently published Cochrane analysis, it was reported that paracetamol does not reduce acute pain, should not be used during painful procedures, and the effect of reducing morphine dose should be investigated (41).
Non-steroidal anti-inflammatory drugs (NSAID)
NSAIDs are mostly used for ductus closure in newborns. They have an analgesic, antipyretic, and antiinflammatory effect by inhibiting the effect of COX-1 and COX-2. There is not enough information about the analgesic effects in neonates. The adverse effects are renal dysfunction, platelet dysfunction, and pulmonary hypertension (42). It has been shown to have positive effects on human cerebral circulation.
Ketamine
Ketamine provides analgesia, amnesia, and sedation. Studies on the newborn are limited. Ketamine increases blood pressure, heart rate, and respiratory rate, and causes bronchodilation. It is a good option for intubation or extracorporeal membrane oxygenation cannulation in the hypotensive neonate because it does not affect cerebral blood flow (43). In one study, 2 mg/kg was used during endotracheal aspiration (44). It may have neurotoxic effects on the developing brain (45).
Other sedatives
Phenobarbital
This can be used for sedation together with opiates. It has no analgesic effect.
Propofol
Although it has widespread use in young children, there is not enough information about its use in the newborn. It is recommended to be used with caution because of its potential neurotoxic effects, which are inversely proportional to the postconceptional and neonatal ages. It may cause hypotension, and decrease heart rate and oxygen saturation (46).
Chloral hydrate
Chloral hydrate can be used mostly for sedation, its analgesic effect is not very strong. In one study, an increased incidence of apnea and desaturation in term and preterm infants was observed (47). If chloral hydrate is used in outpatient and inpatient settings, a physician to resuscitate and the necessary medical equipment should be present nearby. Patients treated with chloral hydrate should be supervised until complete alertness is achieved.
Benzodiazepines
Benzodiazepines activate gamma aminobutyric acid (GABA) receptors, but do not have an analgesic effect. These drugs provide sedation and myorelaxation. Its adverse effects are myoclonic jerks, respiratory depression, and hypotension. Midazolam is the most commonly used benzodiazepine. It has been reported that doubts about the use of midazolam have increased gradually and the reliability has decreased in the NICU (48). It may cause slowing in bilirubin metabolism, especially in neonatal asphyxia and premature infants. Routine use is not recommended.
Analgesia for mechanical ventilation
Mechanical ventilation is the most common cause of chronic pain in the NICU. However, despite investigation in various studies, the ideal analgesia method could not be determined in patients who were mechanically ventilated. In one of the studies on the use of continuous analgesics or sedatives in mechanically ventilated infants, it was observed that the use of morphine for seven days or less had no effect on the neurologic results of the patients (49). In another study, it was found that morphine used up to 14 days prolonged the duration of mechanical ventilation (50). Fentanyl treatment has also been shown to prolong the duration of mechanical ventilation. Remifentanil may be used, especially when short-term intubation is required (33). In spite of this, the NEOPAIN study showed that neurodevelopment at school age was better in patients who received morphine (50).
Recommendations for interventions
Here, control methods for painful interventions that are frequently applied to newborn babies are presented according to international recommendations (51).
Heel stick: Heating, acetaminophen or local anesthetics (EMLA cream) prior to heel stick are ineffective in pain control. The use of mechanical lancets is less painful. Visual-auditory stimuli that distract the attention of the baby before the intervention can be applied by the mother or nurse. Skin contact with the mother, sugary solutions or expressed breast milk with a pacifier if the mother is not present during the intervention and other non-drug methods suitable for the baby’s clinical condition can be used.
Venous, arterial interventions and peripherally inserted central catheterization: Before the intervention, the baby should be gently positioned. If possible, thin intravenous cannulas (24-G, 26-G) should be preferred. Non-drug methods such as sugary solutions suitable for the baby’s clinical condition, breast milk and pacifier must be applied. If there is sufficient time, local anesthetic cream may be applied to the place where intervention is performed. Peripheral insertion of the central catheter is more painful than ordinary venous access because of the need for greater venous cannulas. Systemic opioid administration may be considered in mechanically ventilated infants. Ketamine may be considered in selected cases. Arterial procedures are more painful than venous procedures. Local anesthetic may be applied prior to arterial interventions (arterial blood sampling or peripheral arterial line insertion) and percutaneously inserted central venous catheters.
Umbilical artery and vein catheterization: There is no neural network in the umbilical cord. If sutures are used to fix the catheters, they should be stitched to the cord tissue instead of the skin. Non-pharmacologic methods should be applied.
Central catheter insertion: Low-dose opioid or deep analgesia/sedation may be applied in accordance with the patient’s clinical condition.
Intramuscular or subcutaneous injection: If possible, medications should be administered via the intravenous route. If there is an obligatory intramuscular or subcutaneous administration of medications, extra-fine needles should be used. Non-pharmacologic methods or pre-intervention local anesthetic cream may be used.
Tracheal intubation and extubation: Appropriate pain control and sedation during elective tracheal intubation may facilitate the procedure. Intubation performed with less effort in shorter time while reducing physiologic fluctuations and pain sensations in the baby. Prior to elective tracheal intubation, fentanyl (1-3 mcg/kg) or morphine (10-30 mcg/kg) and midazolam (50-100 mcg/kg) may be administered. In selected cases ketamine 1 mg/kg might be preferred. Low-dose ketamine (0.5 mg/kg) or dexmedetomidine (0.5 mcg/kg) administration is recommended because the respiratory effort can be suppressed by analgesia and sedation if short-term intubation is planned (e.g. for the administration of surfactant) acetaminophen. Atropine (0.02 mg/kg, a minimum of 0.1 mg/kg) should be given, especially before ketamine and dexmedetomidine. In a recent study comparing the combinations of different analgesic and sedative drugs used during elective tracheal intubation, only the combination of fentanyl and midazolam was reported to improve clinical pain scores. When nasal intubation is performed, a low-dose (0.3 mL/kg) 2% lidocaine gel facilitates the progression of the tube. When extubation is performed, non-pharmacologic methods for pain control should be applied after the adhesive tapes are loosened appropriately.
Tracheal aspiration (endotracheal tube suctioning is possibly more appropriate daha uygun olabilir): Unless necessary, endotracheal tube suctioning should be avoided in infants on mechanical ventilation. Suctioning should be performed quickly and the aspiration catheter should not exceed the tip of the endotracheal tube. Non-pharmacologic methods (e.g. giving the baby’s hands and feet flexion posture) might be applied. Low-dose (0.3 mcg/kg) fentanyl may be given systemically.
Chest tube insertion: In addition to non-pharmacologic methods, local anesthetic cream should be applied in non-emergency conditions, subcutaneous lidocaine injection should be performed in emergency cases. If the patient is already intubated and mechanically ventilated, systemic opioids can be given; ketamine may be considered in unintubated newborns. Systemic analgesia is recommended when chest physiotherapy is applied. Pain may also occur during chest tube withdrawal, therefore non-pharmacologic methods, local anesthetics, and systemic analgesia may be given when necessary.
Gastric tube insertion: Non-pharmacologic methods, local anesthetics, and gel application may be considered.
Lumbar puncture: Before the intervention, non-pharmacologic pain control methods (such as sugary solutions, pacifier, breast milk) should be applied according to the patients’ condition. Local anesthetic cream should be applied if there is sufficient time. There are also centers where subcutaneous lidocaine injection is performed.
Suprapubic bladder aspiration: Non-pharmacologic methods, local anesthetic creams, and subcutaneous lidocaine may be given. Intravenous fentanyl (0.5-1.0 mcg/kg) is also one of the options for pain control.
Circumcision: In addition to providing non-pharmacologic methods, local anesthetic creams, lidocaine and regional anesthesia, it is recommended to administer intravenous or oral acetaminophen before and after the procedure.
Wound care and dressing: Non-pharmacologic methods, local anesthetic creams according to the surface area when required, systemic opioids or deep sedation may be considered.
Examination for retinopathy of prematurity: Environmental and behavioral precautions must be taken. In addition, non-nutritive suction, breast milk, and sugary solutions could be given. Local anesthesia can be achieved with 0.4% oxybuprocaine or 1% tetracaine, and intravenous opioids or ketamine may be used if required during RetCam screening.
Turkish Neonatal Society Recommendations
Pain modulation in premature and term babies cannot be performed like in older children. Therefore, they feel more pain.
Newborns with untreated recurrent painful interventions may experience permanent neurologic and behavioral problems in the future; pain perceptions and neuroendocrine stress responses might be impaired.
Pain is a vital sign that should be continuously monitored in the NICU.
The main purpose of monitoring the pain response is determining the painful condition of the baby, determining the level of pain, and the need for intervention against it.
For pain monitoring a structured and multivariable method should be used. N-PASS scale can be used in our country (for N-PASS and other pain scales check “Newborn and Pain Management Guideline 2015”).
There may be limitations in monitoring pain response in newborns with neuromuscular blockade, on a mechanical ventilator or with neurologic deficits. In these cases, physiologic pain responses (sympathetic system stimulation symptoms such as heart rate variability, pupil dilatation) should be closely monitored.
-
The most effective approach in pain control is to reduce painful interventions. Therefore, the following approaches are recommended for adoption:
- Use of catheters for blood sampling
- Taking a blood sample for all tests at once
- Use of non-invasive monitoring parameters
- Use of peripherally inserted central catheters
- Avoiding routine endotracheal suctioning in babies on mechanical ventilation
Postoperative pain: this pain is observed in the first 24-48 hours in newborn infants after surgery. The development of postoperative pain is already expected by healthcare providers and therefore better monitored. N-PASS could be used for monitoring pain and sedation in postoperative infants starting from the 23rd gestational week till the first 100 days of life.
-
Environmental and behavioral precautions that can be used are:
- Grouping and performing non-painful interventions at one time
- No painful interventions for at least 2 hours after a painful procedure
- Choosing a calm state of alertness for non-urgent interventions
- Ensure that the baby feels safe before starting the intervention
- Keeping light and sound as low as possible during painful interventions
- Using mechanical lancets for heel stick
- Choosing venous blood sampling in term babies – depending on the amount of blood
- Removal of plasters in such a way that the baby does not feel pain (use of silicone adhesive removers or wetting of the plasters before removal)
- Performing interventions by an experienced health caregiver
-
For mild-to-moderate painful interventions, in accordance with the clinical condition of the baby, primarily non-pharmacologic methods, if necessary, a few of pharmacologic agents should be applied together by targeting synergistic effects.
- The most physiologic method is breastfeeding of the infant. If the mother can be close to the baby during the intervention, skin contact should be ensured even if breastfeeding is not possible.
- If breastfeeding of the baby is not possible, 2-5 mL of expressed breast milk, preferably last milk should be given.
- The use of pacifiers should be ensured during painful interventions in all babies who can suck.
-
The amount and frequency of administration of sugary solutions should not be too high in infants with a low gestational week. They can be given to the anterior of the tongue with pacifiers, if possible, 2 minutes before the painful interventions.i. Sucrose: 0.2-0.3 mL in preterm infants, 1-2 ml in term infants at 12-24% concentrations (glucose may be preferred in our country because sucrose is expensive and usually unavailable)ii. Glucose: 1-2 mL, 20-30% concentrations
- Other methods: Facilitated positioning, touching, performing massage, speaking, eye contact, playing music or in-utero sounds, visual stimulation by moving toys, application of individualized developmental care principles.
Footnotes
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
Çıkar Çatısması: Yazarlar çıkar çatışması bildirmemişlerdir.
Mali Destek: Yazarlar bu çalışma için mali destek almadıklarını beyan etmişlerdir.
References
- 1.Anand KJS, Hicley PR. Pain and its effects in the human neonate and fetus. NEJM. 1987;317:1321–9. doi: 10.1056/NEJM198711193172105. [DOI] [PubMed] [Google Scholar]
- 2.Hardman MP, Manning N, Hall RW, Anand KJ, Clancy B. Neurodevelopmental changes of fetal pain. Semin Perinatol. 2007;31:275–82. doi: 10.1053/j.semperi.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Giannakoulopoulos X, Sepulveda W, Kourtis P, Glover V, Fisk NM. Fetal plasma cortisol and beta-endorphin response to intrauterine needling. Lancet. 1994;344:77–81. doi: 10.1016/s0140-6736(94)91279-3. [DOI] [PubMed] [Google Scholar]
- 4.Van Howe RS, Svoboda JS. Neonatal pain relief and the Helsinki Declaration. J Law Med Ethics. 2008;36:803–23. doi: 10.1111/j.1748-720X.2008.00339.x. [DOI] [PubMed] [Google Scholar]
- 5.Alinejad-Naeini M, Mohagheghi P, Peyrovi H, Mehran A. The effect of facilitated tucking during endotracheal suctioning on procedural pain in preterm neonates: a randomized controlled crossover study. Glob J Health Sci. 2014;6:278. doi: 10.5539/gjhs.v6n4p278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruce E, Franck L, Howard RF. The efficacy of morphine and Entonox analgesia during chest drain removal in children. Paediatr Anaesth. 2006;16:302–8. doi: 10.1111/j.1460-9592.2005.01751.x. [DOI] [PubMed] [Google Scholar]
- 7.American Academy of Pediatrics Committee on Fetus and Newborn, American Academy of Pediatrics Section on Surgery, Canadian Paediatric Society Fetus and Newborn Committee. Batton DG, Barrington KJ, Wallman C. Prevention and management of pain in the neonate: an update. Pediatrics. 2006;118:2231. doi: 10.1542/peds.2006-2277. [DOI] [PubMed] [Google Scholar]
- 8.Maxwell LG, Malavolta CP, Fraga MV. Assessment of pain in the neonate. Clin Perinatol. 2013;40:457–69. doi: 10.1016/j.clp.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Fabrizi L, Slater R, Worley A, et al. A shift in sensory processing that enables the developing human brain to discriminate touch from pain. Curr Biol. 2011;21:1552–8. doi: 10.1016/j.cub.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hummel P, Puchalski M, Creech SD, Weiss MG. Clinical reliability and validity of the N-PASS: neonatal pain, agitation and sedation scale with prolonged pain. J Perinatol. 2007;28:55–60. doi: 10.1038/sj.jp.7211861. [DOI] [PubMed] [Google Scholar]
- 11.Valkenburg AJ, Boerlage AA, Ista E, Duivenvoorden HJ, Tibboel D, van Dijk M. The COMFORT-behavior scale is useful to assess pain and distress in 0- to 3-year-old children with Down syndrome. Pain. 2011;152:2059–64. doi: 10.1016/j.pain.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Lago P, Garetti E, Merazzi D, Pieragostini L, Ancora G, Pirelli A, Bellieni CV Pain Study Group of the Italian Society of Neonatology. Guidelines for procedural pain in the newborn. Acta Paediatr. 2009;98:932–9. doi: 10.1111/j.1651-2227.2009.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franck LS, Lawhon G. Environmental and behavioral strategies to prevent and manage neonatal pain. Semin Perinatol. 1998;22:434–43. doi: 10.1016/s0146-0005(98)80059-1. [DOI] [PubMed] [Google Scholar]
- 14.Pillai Riddell RR, Racine NM, Turcotte K, et al. Nonpharmacological management of infant and young child procedural pain. Cochrane Database Syst Rev. 2011:CD006275. doi: 10.1002/14651858.CD006275.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Shah PS, Herbozo C, Aliwalas LL, Shah VS. Breastfeeding or breast milk for procedural pain in neonates. Cochrane Database Syst Rev. 2012;12:CD004950. doi: 10.1002/14651858.CD004950.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahoo JP, Rao S, Nesargi S, Ranjit T, Ashok C, Bhat S. Expressed breast milk vs 25% dextrose in procedural pain in neonates, a double blind randomized controlled trial. Indian Pediatr. 2013;50:203–7. doi: 10.1007/s13312-013-0067-3. [DOI] [PubMed] [Google Scholar]
- 17.Ou-Yang MC, Chen IL, Chen CC, Chung MY, Chen FS, Huang HC. Expressed breast milk for procedural pain in preterm neonates: a randomized, double-blind, placebocontrolled trial. Acta Paediatr. 2013;102:15–21. doi: 10.1111/apa.12045. [DOI] [PubMed] [Google Scholar]
- 18.Rosali L, Nesargi S, Mathew S, Vasu U, Rao SP, Bhat S. Efficacy of expressed breast milk in reducing pain during ROP screening--a randomized controlled trial. J Trop Pediatr. 2015;61:135–8. doi: 10.1093/tropej/fmu073. [DOI] [PubMed] [Google Scholar]
- 19.McNair C, Campbell Yeo M, Johnston C, Taddio A. Nonpharmacological management of pain during common needle puncture procedures in infants: current research evidence and practical considerations. Clin Perinatol. 2013;40:493–508. doi: 10.1016/j.clp.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Harrison D, Bueno M, Yamada J, Adams-Webber T, Stevens B. Analgesic effects of sweet-tasting solutions for infants: current state of equipoise. Pediatrics. 2010;126:894–902. doi: 10.1542/peds.2010-1593. [DOI] [PubMed] [Google Scholar]
- 21.Gal P, Kissling GE, Young WO, et al. Efficacy of sucrose to reduce pain in premature infants during eye examinations for retinopathy of prematurity. Ann Pharmacother. 2005;39:1029–33. doi: 10.1345/aph.1E477. [DOI] [PubMed] [Google Scholar]
- 22.Stevens B, Yamada J, Lee GY, Ohlsson A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst Rev. 2013;1:CD001069. doi: 10.1002/14651858.CD001069.pub4. [DOI] [PubMed] [Google Scholar]
- 23.Bellieni CV, Stazzoni G, Tei M, et al. How painful is a heelprick or a venipuncture in a newborn? J Matern Fetal Neonatal Med. 2016;29:202–6. doi: 10.3109/14767058.2014.992334. [DOI] [PubMed] [Google Scholar]
- 24.Bueno M, Yamada J, Harrison D, et al. A systematic review and meta-analyses of nonsucrose sweet solutions for pain relief in neonates. Pain Res Manag. 2013;18:153–61. doi: 10.1155/2013/956549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnston CC, Filion F, Snider L, et al. Routine sucrose analgesia during the first week of life in neonates younger than 31 weeks'postconceptional age. Pediatrics. 2002;110:523–8. doi: 10.1542/peds.110.3.523. [DOI] [PubMed] [Google Scholar]
- 26.Stevens B, Yamada J, Beyene J, et al. Consistent management of repeated procedural pain with sucrose in preterm neonates: Is it effective and safe for repeated use over time? Clin J Pain. 2005;21:543–8. doi: 10.1097/01.ajp.0000149802.46864.e2. [DOI] [PubMed] [Google Scholar]
- 27.Holsti L, Grunau RE. Considerations for using sucrose to reduce procedural pain in preterm infants. Pediatrics. 2010;125:1042–7. doi: 10.1542/peds.2009-2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hui-Chen F, Hsiu-Lin C, Shun-Line C, et al. The effect of EMLA cream on minimizing pain during venipuncture in premature infants. J Trop Pediatr. 2013;59:72–3. doi: 10.1093/tropej/fms040. [DOI] [PubMed] [Google Scholar]
- 29.Lemyre B, Hogan DL, Gaboury I, et al. How effective is tetracaine 4% gel, before a venipuncture, in reducing procedural pain in infants: a randomized doubleblind placebo controlled trial. BMC Pediatr. 2007;7:7. doi: 10.1186/1471-2431-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taddio A, Lee CM, Parvez B, et al. Contact dermatitis and bradycardia in a preterm infant given tetracaine 4% gel. Ther Drug Monit. 2006;28:291–4. doi: 10.1097/01.ftd.0000195615.92591.9c. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt B, Adelmann C, Stutzer H, et al. Comparison of sufentanil versus fentanyl in ventilated term neonates. Klin Padiatr. 2010;222:62–6. doi: 10.1055/s-0029-1225348. [DOI] [PubMed] [Google Scholar]
- 32.Saarenmaa E, Huttunen P, Leppaluoto J, et al. Alfentanil as procedural pain relief in newborn infants. Arch Dis Child Fetal Neonatal Ed. 1996;75:F103–7. doi: 10.1136/fn.75.2.f103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silva YP, Gomez RS, Marcatto Jde O, et al. Early awakening and extubation with remifentanil in ventilated premature neonates. Paediatr Anaesth. 2008;18:176–83. doi: 10.1111/j.1460-9592.2007.02378.x. [DOI] [PubMed] [Google Scholar]
- 34.Alencar AJ, Sanudo A, Sampaio VM, et al. Efficacy of tramadol versus fentanyl for postoperative analgesia in neonates. Arch Dis Child Fetal Neonatal Ed. 2012;97:F24–9. doi: 10.1136/adc.2010.203851. [DOI] [PubMed] [Google Scholar]
- 35.Menon G, Boyle EM, Bergqvist LL, et al. Morphine analgesia and gastrointestinal morbidity in preterm infants: secondary results from the NEOPAIN trial. Arch Dis Child Fetal Neonatal Ed. 2008;93:F362–7. doi: 10.1136/adc.2007.119297. [DOI] [PubMed] [Google Scholar]
- 36.Fahnenstich H, Steffan J, Kau N, et al. Fentanyl-induced chest wall rigidity and laryngospasm in preterm and term infants. Crit Care Med. 2000;28:836–9. doi: 10.1097/00003246-200003000-00037. [DOI] [PubMed] [Google Scholar]
- 37.Finnegan LP. Neonatal abstinence syndrome: assessment and pharmacotherapy. In: Nelson N, editor. Current therapy in neonatal-perinatal medicine. 2 ed. Ontario: BC Decker; 1990. [Google Scholar]
- 38.Berkenbosch JW, Tobias JD. Development of bradycardia during sedation with dexmedetomidine in an infant concurrently receiving digoxin. Pediatr Crit Care Med. 2003;4:203–5. doi: 10.1097/01.PCC.0000059737.86673.28. [DOI] [PubMed] [Google Scholar]
- 39.Chrysostomou C, Schulman SR, Herrera Castellanos M, et al. A phase II/III, multicenter, safety, efficacy, and pharmacokinetic study of dexmedetomidine in preterm and term neonates. J Pediatr. 2014;164:276–82. doi: 10.1016/j.jpeds.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 40.van den Anker JN, Tibboel D. Pain relief in neonates: when to use intravenous paracetamol. Arch Dis Child. 2011;96:573–4. doi: 10.1136/adc.2011.211060. [DOI] [PubMed] [Google Scholar]
- 41.Ohlsson A, Shah PS. Paracetamol (acetaminophen) for prevention or treatment of pain in newborns. Cochrane Database Syst Rev. 2015;6:CD011219. doi: 10.1002/14651858.CD011219.pub2. [DOI] [PubMed] [Google Scholar]
- 42.Allegaert K, Vanhole C, de Hoon J, et al. Nonselective cyclo-oxygenase inhibitors and glomerular filtration rate in preterm neonates. Pediatr Nephrol. 2005;20:1557–61. doi: 10.1007/s00467-005-1998-2. [DOI] [PubMed] [Google Scholar]
- 43.Betremieux P, Carre P, Pladys P, et al. Doppler ultrasound assessment of the effects of ketamine on neonatal cerebral circulation. Dev Pharmacol Ther. 1993;20:9–13. doi: 10.1159/000457535. [DOI] [PubMed] [Google Scholar]
- 44.Saarenmaa E, Neuvonen PJ, Huttunen P, et al. Ketamine for procedural pain relief in newborn infants. Arch Dis Child Fetal Neonatal Ed. 2001;85:F53–6. doi: 10.1136/fn.85.1.F53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan J, Jiang H. Dual effects of ketamine: neurotoxicity versus neuroprotection in anesthesia for the developing brain. J Neurosurg Anesthesiol. 2014;26:155–60. doi: 10.1097/ANA.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 46.Welzing L, Kribs A, Eifinger F, et al. Propofol as an induction agent for endotracheal intubation can cause significant arterial hypotension in preterm neonates. Paediatr Anaesth. 2010;20:605–11. doi: 10.1111/j.1460-9592.2010.03330.x. [DOI] [PubMed] [Google Scholar]
- 47.Litman RS, Soin K, Salam A. Chloral hydrate sedation in term and preterm infants: an analysis of efficacy and complications. Anesth Analg. 2010;110:739–46. doi: 10.1213/ANE.0b013e3181ca12a8. [DOI] [PubMed] [Google Scholar]
- 48.Ng E, Taddio A, Ohlsson A. Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit. Cochrane Database Syst Rev. 2012:CD002052. doi: 10.1002/14651858.CD002052.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simons SH, van Dijk M, van Lingen RA, et al. Routine morphine infusion in preterm newborns who received ventilatory support: a randomized controlled trial. JAMA. 2003;290:2419–27. doi: 10.1001/jama.290.18.2419. [DOI] [PubMed] [Google Scholar]
- 50.Anand KJ, Hall RW, Desai N, et al. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomized trial. Lancet. 2004;363:1673–82. doi: 10.1016/S0140-6736(04)16251-X. [DOI] [PubMed] [Google Scholar]
- 51.Hall RW, Anand KJ. Pain management in newborns. Clin Perinatol. 2014;41:895–924. doi: 10.1016/j.clp.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


