Abstract
Neonatal infections are a major cause of morbidity and mortality in the first month of life, especially in developing countries. Despite advances in neonatology, neonatal infections still haves clinical importance because of nonspecific signs and symptoms, no perfect diagnostic marker, and interference with non-infectious diseases of newborns. Diagnosis is typically made by clinical and laboratory findings. Empiric antibiotic therapy should be started in a newborn with signs and symptoms of infection after cultures are taken according to the time of the signs and symptoms, risk factors, admission from community or hospital, focus of infection, and antibiotic susceptibility estimation. Treatment should be continued according to clinical findings and culture results. Intrapartum antibiotic prophylaxis, proper hand washing, aseptic techniques for invasive procedures, appropriate neonatal intensive care unit design, isolation procedures, and especially breast milk use are needed to prevent infections. The use of diagnosis and treatment protocols increases clinical success.
Keywords: Diagnosis of neonatal infections, meningitis, neonatal infections, sepsis, treatment of neonatal infections
Abstract
Yenidoğan enfeksiyonları gelişmekte olan ülkeler başta olmak üzere yaşamın ilk ayı içerisinde önemli bir morbidite ve mortalite nedenidir. Yenidoğan alanında yaşanan gelişmelere rağmen özgül belirti ve bulgularının olmaması, tanı koyacak mükemmel bir belirtecin bulunmaması ve yenidoğan dönemine ait enfeksiyon dışı klinik tablolar ile karışabilmesi nedeniyle klinik önemi devam etmektedir. Klinik ve laboratuvar bulguları birlikte değerlendirilerek tanı konulmaktadır. Enfeksiyon düşündüren belirti ve bulguların olduğu bebekte kültür örnekleri alınır alınmaz belirti ve bulguların başlama zamanı, etkenin edinildiği ortam ve varsa enfeksiyon odağına ilişkin bilgiler temelinde, olası etkenler ya da riskler ile olası antibiyotik duyarlığına göre ampirik antibiyotik tedavisi başlanmalı ve kültür sonuçları, klinik izlem ve gerektiğinde tekrarlanan laboratuvar tetkikleri ışığında tedavi değişikliği planlanmalıdır. Enfeksiyonların önlenmesi için intrapartum profilaksi, el yıkama, girişimlerde aseptik tekniklerin kullanılması, yoğun bakım biriminin uygun düzenlenmesi, izolasyon önlemleri ve özellikle anne sütü kullanımı önem taşımaktadır. Tanı ve tedavi protokollerinin kullanımı başarıyı artırmaktadır.
Introduction
Neonatal sepsis is a clinical syndrome that involves systemic signs and symptoms related to infection and growth of a specific causative agent in blood culture in the first month of life. Despite advances in the area of neonatology, neonatal infections are still an important cause of morbidity and mortality. Absence of specific findings related to sepsis and the fact that non-infectious clinical conditions in the neonatal period are manifested with similar findings make early diagnosis and initiation of treatment difficult.
The diagnosis of sepsis is made by evaluating associated clinical and laboratory findings. There is no perfect diagnostic marker. Infants with sepsis should be rapidly diagnosed and unnecessary treatment should be prevented in infants who do not have sepsis.
The objective of this guideline is to establish a common and standard opinion and care unity based on current and evidence-based medical scientific data in relation to the diagnosis, treatment, short- and long-term follow-up, and prevention for neonatal sepsis in Turkey.
General information
Epidemiology of neonatal sepsis in Turkey and in the world
The incidence of neonatal sepsis has been reported as 1-8.1 per 1000 live births; the incidence is lower in developed countries (1). The incidence of early-onset sepsis has been reported as 0.57% in infants weighing more than 2500 g and 10.96% between 401 g and 1500 g (2). The incidence of late-onset sepsis has been reported as 51.2% in infants weighing between 501 g and 750 g, 15-25% in infants below 1500 g, and 1.6% in those weighing over 2500 g (3).
In our country, the incidence of late-onset sepsis has been reported as 6.4-14.1% and the mortality rate has been reported to range between 0% and 75% (4, 5). The mortality rate has declined from 30-40% to 5-10% in recent years (6). In preterm infants, mortality may increase to higher rates.
Definitions
Proven sepsis: Patients in whom clinical and laboratory findings are compatible with sepsis and the causative agent has been shown.
Clinical sepsis: Patients in whom clinical and laboratory findings are compatible with sepsis, but a causative agent has not been shown.
Suspicious sepsis: Presence of risk factors in a infant (whether a clinical sign is present or not) or observation of findings suggesting sepsis in the follow-up.
Early-onset sepsis: Sepsis detected in the first three days of life (<72 hours).
Late-onset sepsis: Sepsis diagnosed on the 4th-30th day of life.
Very late-onset sepsis: Sepsis diagnosed in the time period between the 30th day and the day of discharge.
Risk factors for neonatal sepsis
The frequency of sepsis in preterm infants is 3-10-fold higher in preterm infants compared with term infants. In the presence of premature rupture of membranes (PROM) (≥18 hours) and chorioamnionitis, the risk of early-onset sepsis is 1-3% (a 10-fold increase) (7). The rectal, vaginal group B streptococcus (GBS) colonization rate has been reported as 15-40% in mothers in the United States of America (USA), and it was found as 2-10.6% in our country (8).
Fetal distress, low APGAR score and resuscitation, and multiple pregnancy increase the risk of early-onset sepsis; invasive interventions including frequent blood sampling, intubation, mechanical ventilation and placement of catheter/probe, insufficient breastmilk, long-term parenteral nutrition, reduction in gastric acidity, and need for surgical intervention in particular increase the risk of late-onset sepsis.
Causative agent microorganisms
The most common agents in early-onset sepsis include GBS and Escherichia coli (E. coli). GBS is the causative agent in 43-58% of cases and E. coli is the causative agent in 18-29% (9). Listeria monocytogenes (L. monocytogenes), other Gram-negative bacilli, and staphylococci are found as causative agents with a lower frequency. GBS is found more commonly in term infants (73%) and E. coli is found more commonly in preterm infants (81%) (2). In the USA, the frequency of GBS decreased after routine screening and intrapartum antibiotic prophylaxis was initiated. Some studies reported an increase in the frequency of E. coli, especially in very-low-birth-weight (VLBW) infants (10).
In late-onset sepsis, coagulase-negative staphylococci (CNS) including mainly Staphylococcus epidermidis (S. epidermidis) are observed most commonly with a rate of 53.2-77.9% in developed countries. On the other hand, there are also countries and clinics in which Gram-negative bacilli including E. coli, Klebsiella, and pseudomonas species are in the forefront (3). Staphylococcus aureus (S. aureus) and candida species are among the other agents.
Clinical findings in neonatal sepsis
In early-onset sepsis, signs and symptoms occur in the first 24 hours in most infants and in the first 48 hours in almost all of cases (90%). Multiple organs or systems are generally involved in early-onset sepsis, whereas multisystem involvement or single focus involvement (pneumonia, arthritis, osteomyelitis) may be observed in late-onset sepsis (11) (Table 1).
Table 1.
Characteristics of neonatal sepsis
| Early-onset neonatal sepsis The first three days of life | Late-onset neonatal sepsis 4th-30th day | Very late-onset neonatal sepsis | |
|---|---|---|---|
| Risk factors | Frequently present | Generally absent | Variable |
| Mode of transmission | Vertical, generally through the maternal genital canal | Vertical or from the environment postnatally | From the environment |
| Clinical characteristics | Fulminant course, multiorgan involvement | Insidious or acute, Focal infection Meningitis is frequent | Insidious |
| Mortality | 5-20% | 5% | Low |
| Causative Agents | GBS | Coagulase negative | Coagulase negative |
| E. Coli | staphylococci | staphylococci | |
| Viridans streptococci Enterococci | S. Aureus, | S. Aureus | |
| Coagulase negative staphylococci | Candida | Candida | |
| S. Aureus | E. Coli | E. Coli | |
| Haemophilus influenza | Enterococci | Klebsiella | |
| Listeria monocytogenes | Klebsiella | Pseudomonas | |
| Klebsiella | Pseudomonas | ||
| GBS | |||
| L. Monocytogenes |
GBS: group B streptococci
In neonatal sepsis, system findings include apnea, groaning, increased respiratory rate, nasal flaring, retractions and cyanosis in the respiratory system; tachycardia or bradycardia, hypotension, peripheral circulation disorder and prolongation in capillary filling time in the circulatory system; feeding intolerance, vomiting, distension, diarrhea, jaundice, hepatomegaly and necrotizing enterocolitis (NEC) in the gastrointestinal system; petechia, purpura, jaundice, and bleeding in the hematologic system; pustules, abscess, omphalitis, cutis marmaratus and sclerema in the cutaneous system; and restlessness, inability to suck, hypoactivity, tendency to sleep, decreased tonus, seizure, and temperature irregularity in the central nervous system.
In the differential diagnosis, problems should be considered that are specific for the neonatal period including transient tachypnea of the newborn, apnea, meconium aspiration, aspiration pneumonia, respiratory distress syndrome (RDS), patent ductus arteriosus, NEC, hypoxic ischemic encephalopathy, intraventricular hemorrhage, congenital heart diseases, metabolic problems including hypoglycemia and hypocalcemia, and congenital metabolic diseases.
Laboratory findings in neonatal sepsis
Growth of the etiologic agent in blood culture is the gold standard for the diagnosis of sepsis. Studies are being conducted to find methods that could make the diagnosis of sepsis in a few hours, but tests with high sensitivity and specificity have not yet been found. Use of multiple tests in association is helpful in the diagnosis.
Blood culture: Most positive blood cultures (90%) are detected within the first 48 hours (11). The sensitivity of blood culture is 50-80%. A positive blood culture is diagnostic, but a negative blood culture does not exclude sepsis. The risk of contamination is high if blood culture is not obtained under appropriate conditions. A negative blood culture may be related with an insufficient amount of blood (1 mL), initiation of antibiotic before obtaining culture samples, maternal antibiotic use, low bacterial concentration in blood, and intermittent and short-term bacteremia.
Cerebrospinal fluid culture: Lumbar puncture (LP) should be performed in all infants in whom antibiotic treatment has been initiated because of symptoms of sepsis before treatment, in infants in whom a positive blood culture result has been obtained and LP has not been performed before, and in infants who do not respond to antibiotic treatment (12). LP is not recommended for infants in whom antibiotic treatment has been initiated postnatally because of risk factors, but symptoms of sepsis are absent, for infants whose general states are not appropriate for LP, and for infants who have thrombocytopenia, disrupted skin integrity on the area where LP would be performed or a meningomyelocele sac.
Urine culture: Routine urine culture is not recommended because the rate of urine culture positivity is low in infants with early-onset sepsis. In late-onset sepsis, a urine sample should be obtained by way of urethral catheterization or suprapubic bladder aspiration because the urinary system is observed as a source more commonly (13).
Tracheal aspirate culture: Tracheal aspirate culture should be obtained in patients in whom ventilator-related pneumonia is considered or when there is change in the amount and characteristics of secretion. It may be difficult to differentiate colonization and infection. Its diagnostic value is low (1).
White blood cell count: The positive predictive value of white blood cell counts for sepsis is very low. It is in the normal range in 50% of proven sepsis cases (12). Maternal fever, hypertension and preeclampsia, mode of delivery, perinatal asphyxia, meconium aspiration, intraventricular hemorrhage, pneumothorax, hemolytic disease, reticulocytosis, seizure, and even prolonged crying may lead to a change in leucocyte and neutrophil counts. It is between 6000 and 30,000/mm3 in the first 24 hours and subsequently, it is between 5000 and 20, 000/mm3. Presence of neutropenia is more valuable compared with neutrophilia. Table 2 shows variance in the neutrophil count by gestational age and postnatal age (14). The ratio of immature neutrophil count to the total neutrophil count (I/T) is the most sensitive marker for neonatal sepsis. In particular, the negative predictive value of the I/T ratio is high. Its normal value is 0.16 at birth, wheras it is reduced to 0.12 at the 60th hour; a value of ≥0.2 is significant for sepsis (14).
Table 2.
Neutrophil count by gestational week and postnatal age
| Lower limit at delivery | Lower limit at peak | |
|---|---|---|
| >36 weeks | 3500/mm3 | (after 8 hours) 7500/mm3 |
| 28–36 weeks | 1000/mm3 | (after 6 hours) 3500/mm3 |
| <28 weeks | 500/mm3 | (after 24 hours) 1500/mm3 |
Platelet count: A low platelet count is a late nonspecific marker for sepsis. The platelet count is below 100.000/mm3 in 50% of infants with bacterial infections (15).
C-reactive protein (CRP): CRP reaches measurable levels 4-18 hours after onset of infection and peaks between the 8-60th hour. Its serum half-life is 24-48 hours. As a response to treatment, it reduces to normal in 5-10 days and is used in the evaluation of treatment response. Its normal concentration in newborns is below 1 mg/dL. It may be influenced by different conditions including maternal fever, PROM, difficult labor, perinatal asphyxia, past surgery and use of steroids. This should be considered. A CRP increase detected with measurements performed with 12-24 hour intervals is helpful in the diagnosis of infection (16). The negative predictive value of normal values for early sepsis is 99.7%. If serial CRP values are negative, the diagnosis of sepsis is excluded.
Procalcitonin (PCT): PCT reaches its peak value at the postnatal 24th hour (about 1.5-2.5 ng/mL) and reduces below 0.5 ng/mL at the postnatal 48-72nd hour. Following exposure to endotoxin, it starts to increase in 2-4 hours, peaks at the 6-8th hour, and keeps this level for at least for 24 hours. A PCT level above 2-2.5 ng/mL after the 72nd hour suggests infection (17). The fact that it increases in the postnatal period and in conditions including preeclampsia, chorioamnionitis, birth asphyxia, intracranial hemorrhage, and hypoxia limits its diagnostic use.
Interleukin-6 (IL-6): Following exposure to bacterial elements, IL-6 rapidly increases before CRP and rapidly returns to normal again (generally in 24 hours) as the inflammatory response decreases with the initiation of antibiotic treatment. It has a high negative predictive value. Its threshold value has been reported to range between 10 and 500 pg/mL. Figure 1 shows temporal variations in IL-6-8, CRP, PCT, and cell-surface marker CD64 (18).
Figure 1.
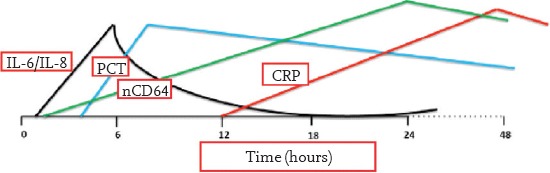
Temporal variations in IL-6, IL-8, CRP, PCT and nCD64 from the beginning of sepsis
CRP: C-reactive protein; PCT: procalcitonin
There are scoring systems that use information related to frequency, gestational week, PROM, and intrapartum antibiotic administration for the prediction of early-onset sepsis (19). Scoring systems including Töllner, and the European Medicines Agency (EMA, Table 3) in which clinical and laboratory findings are used in association are helpful for the diagnosis. The presence of leukopenia, neutropenia, a I/T neutrophil value of >0.2, a CRP value of >1.0 mg/dL, a PCT value of >2 ng/mL, and a platelet count of <100,000/mm3 in a infant is significant for the diagnosis of sepsis.
Table 3.
EMA sepsis scoring
| EMA sepsis scoring | |||
|---|---|---|---|
| Clinical findings | |||
| Body temperature: 38.5°C or lower than 36°C or temperature irregularity | Respiratory: apnea, tachypnea increased oxygen and ventilation need | ||
| Cardiovascular: | Bradycardia, tachycardia or rhythm irregularity | Gastrointestinal: | Feeding intolerance |
| Urine 1 mL/kg/hour | Poor sucking | ||
| Hypotension | Abdominal distension | ||
| Impaired peripheral perfusion | |||
| Skin and subcutaneous lesions: | Petechiae | Nonspecific: | Irritability |
| Sclerema | Lethargy | ||
| Hypotonicity | |||
| Laboratory findings | |||
| Leukocyte count: <4000/ mm3 or >20,000/ mm3 | |||
| Immature/total neutrophil ratio: ≥0.2 | |||
| Platelet count: <100,000/ mm3 | |||
| CRP >15 mg/L (1.5 mg/dL) or procalcitonin ≥2 ng/mL | |||
| Blood glucose monitoring (at least two times): | |||
| Hyperglycemia (>180 mg/dL or 10 mMol/L) or | |||
| Hypoglycemia (<45 mg/dL or 2.5 mMol/L) | |||
| Metabolic acidosis: Base excess >10 mEq/L or serum lactate >2 mMol/L | |||
| Positivity in at least two of six clinical categories and in at least two of 6 laboratory categories is considered clinical sepsis. | |||
| May be used up to the postnatal 44th week. | |||
| European Medicines Agency (EMA), Report on the Expert Meeting on Neonatal and Pediatric Sepsis, 2010 | |||
RECOMMENDATIONS
In a newborn evaluated with suspicion of sepsis because of present signs and symptoms, complete blood count, CRP, blood culture should be requested, LP should be performed, lung radiography should be obtained if there is respiratory problem, and treatment should be initiated (Figure 2).
Figure 2.

Approach to a infant with clinical signs for sepsis (for early or late sepsis)
CSF: cerebrospinal fluid; CRP: C-reactive protein; LP: lumbar puncture
Diagnostic and therapeutic approaches according to the presence of chorioamnionitis, which is one of the main risk factors for sepsis, intrapartum antibiotic prophylaxis for GBS, prematurity and PROM are shown in Figure 3. The possibility of sepsis is low if the leucocyte count, neutrophil count, I/T ratio, LP, CRP, and PCT are found to be normal, signs and symptoms suggesting sepsis regress in 24 hours, repeated acute phase reactants are negative or a non-infectious condition that explains the present signs and symptoms is detected, and cultures remain negative in a infant in whom antibiotic treatment has been initiated. Antibiotic treatment is terminated at the end of 48 hours in such infants. Antibiotic treatment should be continued for up to 7-10 days if the laboratory findings and clinical course are compatible with sepsis, and up to 14-21 days if meningitis is present (Figure 4).
Figure 3.
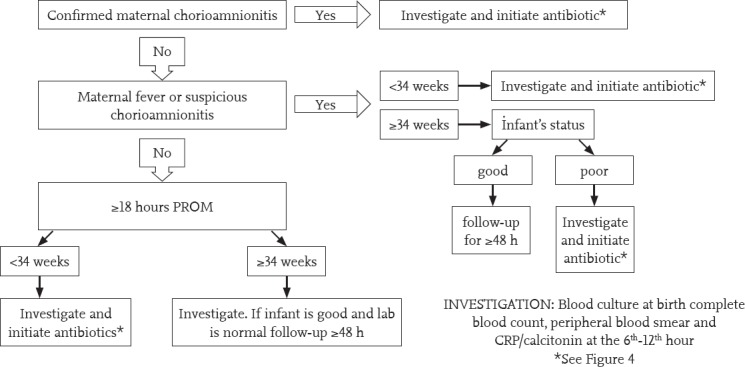
Approach to a asymptomatic infant with risk factors for early sepsis
CRP: C-reactive protein; PROM: premature rupture of membranes; PS: peripheral smear
Figure 4.
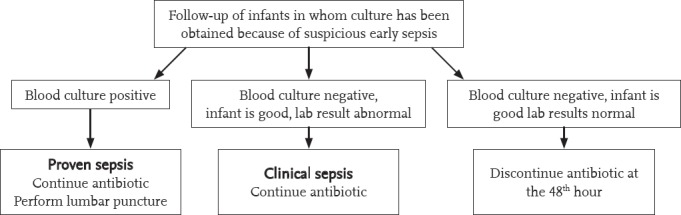
Follow-up of the asymptomatic infant with risk factors for sepsis in whom antibiotic treatment has been initiated
Treatment
Antibiotic treatment in early sepsis
In empiric treatment, ampicillin or penicillin G and an aminoglycoside (primarily gentamicin) should be used. Cefotaxime should not be used in empiric treatment because development of resistance occurs very rapidly and it has no effect on L. monocytogenes and enterococci. If meningitis is present or possible, a combination of ampicillin plus cefotaxime should be preferred because higher bactericidal serum and cerebrospinal fluid (CSF) concentrations can be obtained with cefotaxime.
Treatment response is monitored by the infants state and laboratory tests. Improvement of the signs and symptoms in 24-48 hours following the initiation of treatment and returning of the white cell count, I/T ratio, and CRP level to normal in 48-72 hours indicate appropriate response. Treatment of clinical sepsis is continued for 7-10 days, and treatment of proven sepsis is continued for 10 days.
RECOMMENDATIONS
In early sepsis, ampicillin/penicillin G and gentamycin should be used in empiric treatment. Use of cephalosporin should be avoided in empiric treatment. Ampicillin and cefotaxime treatment is recommended if meningitis is present or possible. Treatment of clinical sepsis is continued for 7-10 days, and treatment of proven sepsis is continued for 10 days.
Antibiotic treatment in late sepsis
In the treatment of community-acquired late-onset sepsis, treatment with ampicillin and gentamycin or third-generation cephalosporin (e.g. cefotaxime) for 7-10 days is appropriate. Empiric treatment for nosocomial late-onset sepsis should be adjusted according to the pathogens observed more commonly in units and antibiotic sensitivity results. Gentamycin (or amikacin)/third-generation cephalosporin (e.g. cefotaxime, ceftazidime) should be initiated in association with vancomycin. The treatment period is 10-14 days. In the treatment of multi-resistant Gram-negative sepsis, ceftazidime, piperacillin-tazobactam or carbapenem should be used in accompaniment of an aminoglycoside (generally amikacin) according to the antibiogram. Amphotericin B is the first-line option in the treatment of fungal infections. Linezolid may be used in the treatment of vancomycin-resistant Gram-positive microorganisms, which have been observed more commonly in recent years, and colistin may be used in treatment of carbapenem-resistant Gram-negative microorganisms including Acinetobacter baumanni and enterobacter species (20).
Improvement of signs and symptoms in 24-48 hours following the initiation of treatment and returning of the white cell count, I/T ratio, CRP, and PCT levels to normal in 48-72 hours indicate appropriate response in both early-onset and late-onset sepsis.
RECOMMENDATIONS
In the treatment of community-acquired late-onset sepsis, treatment with ampicillin and gentamycin or third-generation cephalosporin (e.g. cefotaxime) for 7-10 days is appropriate. If meningitis is present, it is recommended that cefotaxime should be added to ampicillin and gentamycin.
In nosocomial late-onset neonatal sepsis, unit characteristics should be considered. Treatment with gentamycin (or amikacin) in accompaniment of vancomycin should be initiated or third-generation cephalosporin (cefotaxime, ceftazidime) in accompaniment of vancomycin should be initiated if Gram-negative sepsis is suspected or if there is a fulminant course. The treatment period is 10-14 days in late-onset neonatal sepsis.
Neonatal meningitis
Clinical findings and diagnosis
In developed countries, the incidence of culture-confirmed meningitis has been reported to be 0.25 per 1000 live births and mortality has been reported to be 10-15% (21). In preterm infants, mortality is increased 2-fold. GBS and E. Coli are the causative agents in 70% of cases of meningitis detected in the first 72 hours. The other causative agents include Gram-negative bacilli, L. monocytogenes and enterococci. KNS, S. aureus, E. coli, and Klebsiella species are found in cases of late-onset meningitis, especially in VLBW infants (22).
In the presence of nonspecific findings including absence of sucking, hypoactivity, lethargy, apnea, seizure, bulging fontanel, vomiting, respiratory distress and body temperature changes, meningitis should be excluded because it leads to a high rate of mortality and sequelae and accompanies sepsis with a rate of 20-25%. Blood culture is negative in 15-38% of infants with meningitis (21).
CSF values in the neonatal period are different compared with infants and children. CSF laboratory characteristics in neonatal meningitis are as follows:
Increase in the white cell count (20-30 cells/mm3, sensitivity and specificity 80%); this increase is greater in Gram-negative meningitis.
Increase in protein concentration (150 mg/dL in preterms, 100 mg/dL in term infants) (23). High values are also found in parameningeal infections, congenital infections, and following intracranial hemorrhage.
Decreased glucose concentration (<20 mg/dL in preterms, <30 mg/dL in term infants or less than 70-80% of the simultaneous blood glucose value)
The frequency of traumatic lumbar puncture ranges between 13.8% and 39.5%. Pleocytosis may be evaluated through comparison with the leukocyte/erythrocyte ratio on complete blood cell count. Different formulas are currently used; a leukocyte/erythrocyte ratio of >1:100 indicates pleocytosis. One thousand erythrocytes in 1 µL increases CSF protein by 1 mg/dL; the glucose concentration does not change. LP may be repeated after 24-48 hours. Following treatment initiation, culture is expected to become negative after 48 hours in Gram-positive meningitis, and after 72 hours in Gram-negative meningitis (24).
Ventriculitis: If infection in the CSF still persists and clinical improvement does not occur or deterioration is present despite a reduced CSF cell count, and even CSF is sterile on the fourth day, ventricular inflammation should be suspected.
Treatment and prognosis
In early and late neonatal meningitis, cefotaxime in association with ampicillin or cefotaxime in association with vancomycin may be used empirically; an aminoglycoside may also be added to this treatment. Meropenem should be used if multiresistance is present for Gram-negative bacilli. The treatment period is 14 days if the causative agent is Gram-positive, 21 days if the causative agent is Gram-negative bacilli, or 14 days after a negative culture (whichever is longer). In ventriculitis and brain abscess, treatment should be completed to 6-8 weeks. The benefit of steroids as assistive treatment has not been shown.
Intrathecal-intraventricular treatment; intrathecal-intraventricular gentamycin treatment may be administered if Gram-negative bacilli persist despite sufficient bactericidal levels in the CSF. A difference in mortality and morbidity could not be found in patients who received intrathecal-intraventricular treatment in addition to parenteral treatment.
Mild deficits are found in 21-38% of surviving infants and severe neurologic sequelae (hearing deficit, mental retardation, seizures) and hydrocephaly are found in 24-29%. The predictive value of prolonged seizures, coma, use of inotropic agents, and leukopenia is high in terms of mortality and severe sequelae at the age of one year (25).
Neonatal pneumonia
PROM, maternal chorioamnionitis, prematurity, fetal tachycardia, and maternal intrapartum fever are risk factors for early-onset pneumonia; mechanical ventilation, anomaly of the airway, prolonged hospitalization, and aspiration of gastric content are risk factors for late-onset pneumonia. In developed countries, the most important causative agent of early-onset pneumonia is GBS and other symptoms of infection are added to respiratory distress that starts immediately after delivery. Group B streptococcal pneumonia causes an RDS-like appearance on lung radiography. Other causative agents include E. Coli, S. Aureus, S. pneumoniae, and Klebsiella species. In addition, herpes simplex virus and candida species may cause pneumonia (26).
In late-onset neonatal pneumonia, increased need for oxygen and ventilator adjustment, increased tracheal secretion or purulent tracheal secretion and newly-developed findings on lung radiography may be found in addition to nonspecific findings. Gram-negative bacilli, viral infections, and candida species may be causative agents in addition to S. Aureus, S. pneumoniae and S. Pyogenes (27).
Gram staining of tracheal aspirate, culture and lung radiography may be helpful in the diagnosis. A consolidated area in which air bronchograms are present on lung radiography is diagnostic. However, irregular patchy infiltrations or rarely, a normal appearance may also be found. Presence of pleural effusion is important in the differential diagnosis of early-onset pneumonia from RDS.
In treatment, an aminoglycoside or cefotaxime in association with ampicillin in the first seven days of life is appropriate. If pneumonia develops during hospitalization, an aminoglycoside or third-generation cephalosporin (cefotaxime, ceftazidime) in association with vancomycin should be used considering the characteristics of the unit. The treatment period is 10-14 days.
Neonatal urinary tract infection
The incidence of symptomatic urinary tract infection (UTI) is approximately 1%. Symptoms are generally nonspecific findings including fever, poor feeding, jaundice, vomiting, poor growth, heat instability, and lethargy. It occurs more commonly in boys. The most common causative agent is E. coli and other Gram-negative bacteria including Klebsiella, Enterobacter, and Candida species are also commonly observed (28). Routine urine culture is not recommended for newborns with nonspecific symptoms. UTI should be investigated in infants with late-onset sepsis and urinary tract anomalies.
More than 5 leukocytes per each high power field in centrifuged urine suggest infection. If bacteremia is present, ≥10 leukocytes may be found. Gemmiferous hyphae suggest systemic fungal infections. Nitrite and leukocyte esterase positivity are generally not significant. One should be careful in the interpretation of colony number in urine cultures obtained with a urine bag (the contamination rate is approximately 50%). The definite diagnosis is ideally made by growth of microorganism in a urine sample obtained by suprapubic aspiration (>1000 cfu/mL) or gentle catheterization (>10,000 cfu/mL). Renal ultrasonography (USG) should be performed in terms of urinary tract anomalies. Following treatment, voiding cystoureterography should be performed in terms of vesicoureteral reflux and DMSA should be performed in terms of development of scar.
Treatment is generally initiated with ampicillin and an aminoglycoside (e.g. gentamycin). Cephalexin, ceftriaxone or cefotaxime may also be used. The treatment period is 7-10 days and follow-up urine microscopic examination and culture are obtained at the end of treatment.
Omphalitis and funisitis
Infection of the umbilical cord (funisits) and umbilical cord stump (omphalitis) is manifested with erythema and serous or purulent discharge in this region and in the surrounding area. It generally occurs due to S. aureus or E. coli or other Gram-negative bacteria (29). Treatment should be initiated urgently because infection may spread to the portal vein and cause portal hypertension. Ampicillin and gentamycin treatment is initiated. Vancomycin and cephalosporins may be used according to the culture results. In addition, topical antibiotics including nitrafurazone and mupirocin may be used.
Osteomyelitis and septic arthritis
The incidence of osteomyelitis and septic arthritis has been reported as 0.12 per 1000 live births and 0.67 per 1000 neonatal intensive care presentations; the mortality rate has been reported as 7.3% (30). Frequently, the causative agent is S. aureus or Gram-negative bacilli.
Systemic findings including fever, absence of sucking and hypoactivity are found in addition to local findings including tenderness, swelling, erythema, pain and difficulty in moving bones and joints. Leukocytosis, elevated CRP, and growth in culture of synovial aspirate or intraoperatively obtained material (30-50%) may be found. Enlargement of joint spaces and soft tissue swelling (on the third day) are observed on direct radiography. Changes in bone can be seen after the first week. Other imaging methods include USG, scintigraphy, computed tomography (CT), and magnetic resonance imaging (MRI).
In empiric treatment, an aminoglycoside (gentamycin/amikacin) or cefotaxime in association with vancomycin should be initiated. Piperacillin-tazobactam, meropenem are used in multiresistant Gram-negative bacilli infections. The treatment period is 4-6 weeks. Surgery should be performed if pus accumulation is present. Long-term follow-up is needed in terms of bone growth and joint movements (31).
Catheter-related infections
The incidence of catheter-related infections has been reported as 2.5 per 1000 catheter days in infants below 750 g and 0.9 in those weighing over 2500 g in the USA (32). The most common causative agent is coagulase-negative staphylococci (CNS) (28%). Other common causative agents include S. aureus (19%) and Candida species (13%) (33). In infections that develop 48 hours after a catheter is placed or within 24 hours catheter removal, catheter-related infection should be considered if there is no other focus of infection. Growth of the same microorganism in cultures obtained from the catheter and peripheral vessel strengthens the diagnosis. Initiation of vancomycin and gentamycin/amikacin is recommended in empiric treatment. The treatment period is 10-14 days. Systemic treatment is not needed in patients who have catheter tip culture positivity not accompanied by clinical findings, simultaneous peripheral blood culture negativity, and phlebitis in the absence of systemic findings.
Catheter placement: Good hand hygiene in association with aseptic technique constitutes the first step in the protection against infections. All precautions related to sterility should be taken while placing a catheter (hat, mask, sterile gown, sterile gloves, and large sterile cloths). Materials should be prepared as kits. Check lists should be used. Use of antibiotics and antiseptic creams in catheter entry sites is not recommended.
Catheter removal: Catheters should be removed immediately in the event of growth of microorganisms in blood culture, except for CNS. In case of growth of CNS in blood culture, the catheter should be removed if repeated growth of CNS occurs or the patient’s clinical findings are not stable or endocarditis or metastatic infection are found. Replacement of the catheter should be considered after the 3rd-7th day of treatment and if follow-up cultures are negative.
Catheter care: Establishing a sterile area for the site of catheter, daily care of catheter entry site, cleaning with alcohol, avoiding use of multilumen catheters, and maintaining the integrity of catheter entry site are very important in terms of infection. Total parenteral fluid sets should be exchanged with 48-72-hour intervals and sets containing lipid should be exchanged every 24 hours.
Catheter time: An umbilical artery catheter dwell period longer than 7 days and a umblical vein catheter dwell period longer than 14 days increase the risk of sepsis significantly. There is no consensus in the issue of the period of use for peripheral central venous catheters; they may be used for longer than one month. There are studies suggesting that heparin administration prevents bacterial colonization and thrombosis. Covering with antibiotics or ethanol and routine use of antimicrobial-impregnated catheters and cloths with antibiotics are not recommended (34-36).
Fungal infections
Fungi are the third most common causative agent responsible of late-onset neonatal infections. They affect 4-8% of VLBW infants and the mortality rate is 30% (37). Candida albicans (C. albicans) is responsible of 45-55% of cases and non-albicans Candida species including C. parapsilosis, C. tropicalis, C. krusei, and C. glabrata are the other causative agents (38). Thrombocytopenia, glucose intolerance, leukopenia or leukocytosis and CRP and PCT elevations may be found in addition to feeding intolerance, lethargy, apnea and fever. Meningitis, meningoencephalitis, endocarditis, endophthalmitis and renal involvement may be observed.
In the treatment of systemic candidiasis, amphotericin B deoxylate is the first-line treatment choice (39). If renal failure, hypocalemia, bone marrow depression develop, treatment should be continued with liposomal amphotericin B or amphotericin B lipid complex. In recent years, use of micafungin among the echinocandins with its broad spectrum and tolerability has been included in treatment choices in the treatment of systemic candidiasis. In cases of central nervous system involvement, flucytosine should be added to amphotericin B, or fluconazole if it is not available. Lipid formulations of amphotericin B with poor renal penetration should not be used in the treatment of renal candidiasis. Although there is no consensus on the optimal treatment duration, treatment is continued at least for 14 days after culture becomes negative in most units. However, amphotericin B treatment is continued until the cumulative dose is 20-25 mg, especially in VLBW infants in some units. In cases of central nervous system involvement, treatment should be continued until CSF tests become normal, besides obtaining a negative culture result. If endocarditis is present, treatment should be continued for at least for 6 weeks.
Supportive treatments in neonatal infections
Vital signs, fluid and electrolyte balance, intake and output, blood glucose, blood gases, and renal and liver functions should be closely monitored in newborns with sepsis. Enteral or parenteral nutrition should be continued. Electrolyte and glucose levels should be kept within normal limits, appropriate fluid-electrolyte treatment should be administered, acidosis and hypovolemia should be prevented, and shock should be recognized early and inotropic agents in addition to fluid treatment should be administered. Hypoxia should be corrected and a ventilator should be used if respiratory failure develops. Anticonvulsive treatment should be administered if convulsions occur. Fresh frozen plasma, platelet or erythrocyte transfusions should be administered if disseminated intravascular coagulation is present. Corticosteroids should only be used in the presence of adrenal failure.
Intravenous immunoglobulin (IVIG) treatment: Studies have shown that use of IVIG and immunoglobulin M-rich IVIG in the treatment of confirmed or clinical sepsis does not decrease the mortality rate, the mortality rate at the age of two years or major disability rates. Therefore, use of IVIG is not recommended and new studies are not required (40).
Hematopoetic colony-stimulating factors and granulocyte transfusion treatment: Use of hematopoetic colony-stimulating factors is not recommended because a clear benefit of use of these factors for granulocytes and granulocyte-macrophages has not been described (41).
Pentoxifylline treatment: A meta-analysis of a small number of studies in which pentoxifylline, which is a phosphodiesterase inhibitor, was used as an immunomodulator showed that it might be used as supportive treatment in addition to antibiotic treatment in confirmed sepsis and Gram-negative sepsis in preterm infants. However, studies with a greater number of patients should be conducted (42).
Prophylaxis for fungal infections
Intravenous fluconazole, oral nystatin, and IV amphotericin B have been used with the objective of prophylaxis in many studies. Fluconazole prophylaxis is one of the best choices in reducing the frequency of Candida infections (43). Therefore, administration of fluconazole at a dosage of 3 mg/kg two times a week is recommended in extremely low birth weight infants (<1000 g) in units in which the incidence of Candida is high (≥10%). Fluconazole prophylaxis administration is recommended every three days in the first two weeks, every other day between the 2nd and 4th weeks, and daily between the 4th and 6th weeks. It has been found that oral nystatin treatment decreases the frequency of invasive fungal infections (44). Prophylaxis is continued for six week at a maximum or until requirement for intravenous access disappears.
Prevention of infections
Precautions to be taken in delivery room
Hand washing must be performed meticulously.
Gloves should be used during vaginal examination, placement of head electrodes, and instrumental delivery.
Risk factors for group B streptococci should be evaluated and intrapartum antibiotic treatment should be initiated.
Precautions to be taken in neonatal unit
The key to decreasing infections related to healthcare is meticulous hand washing. All employees and visitors should wash their hands carefully before entering neonatal unit. Hands should be washed or alcohol-based disinfectant agents should be used each time before/after the infant is handled or after touching the surrounding working area.
Respirator equipment should be cleaned and humidifying sterile water should be frequently exchanged.
A different stethoscope should be available for each infant.
Aseptic surgical techniques should be used during procedures including intubation, umbilical catheterization, peripheral central catheter placement, intravenous cannulation, intercostal catheter placement, and LP.
Chlorhexidine is more efficient as an antiseptic agent compared with iodine.
Fungal prophylaxis with fluconazole or nystatin may be used in ELBW infants in units in which the frequency of candidemia is high.
Isolation techniques should be performed for infected infants.
Overcrowding should be prevented and the unit’s traffic should be minimized.
Use of breastmilk should be increased because of the immunologic properties of breastmilk.
There is evidence indicating that the use of lactoferrin and probiotics prevents the development of sepsis and reduces mortality (45, 46).
Rational antibiotic use in neonatal sepsis
It has been reported that antibiotic resistance is the cause of deaths related to neonatal sepsis with a rate of approximately 30% worldwide, with a higher rate in low and moderate-income countries. In India, mortality related to antibiotic-resistant bacterial infections is the leading cause of neonatal deaths (58,000 neonatal deaths in 2013). The fact that antibiotic resistance is in the front row in neonatal deaths is a great problem, especially in developing countries for the time being. However, it is also an expected misfortune for developed countries. Antibiotics are the most commonly used drugs in neonatal intensive care units. Increased antibiotic resistance as a result of inappropriate antibiotic use is an inevitable outcome (47).
As a result of antibiotic resistance, untreatable infections, increased morbidity and mortality, prolongation in hospitalization duration, spread of infections with multidrug resistance, and cost increase occur. An “Antibiotic Stewardship” program prepared by the Infectious Diseases Society of America, Pediatric Infectious Diseases Society, and the Society for Healthcare Epidemiology of America and accepted by APA was published in 2007 to reduce antibiotic resistance, to prevent spread of infections with multidrug resistance, and to reach a better outcome for patients (48). However, there are very few publications related to the results of the application of this progam in newborns in whom antibiotics are used most commonly.
In all infants in whom antibiotic treatment has been initiated, rational antibiotic use should be fulfilled, especially by way of using accurate indications, appropriate antibiotics, appropriate dose and duration, and appropriate mode of administration. In a retrospective study, a 20% increase was found in the rate of NEC for each day of antibiotic use and a 3-fold increase was found when antibiotic treatment was used for longer than 10 days. In a study conducted with 365 VLBW infants in whom sepsis was not considered initially and antibiotic treatment was initiated because of the presence of risk factors, use of antibiotic treatment for longer than 5 days was found to be associated with an increased rate of late-onset sepsis, NEC, and mortality (49). It should be kept in mind that antibiotic use influences the development of intestinal flora negatively and increases the risk of immune dysregulation, atopy, and asthma (50).
Conclusively, pediatricians and neonatologists should be able to answer the following questions with scientific accuracy each time they initiate antibiotic treatment:
Why am I initiating antibiotic treatment? Is it necessary?
Which drug, what for?
Which dose, and how long?
Footnotes
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
Çıkar Çatısması: Yazarlar çıkar çatışması bildirmemişlerdir.
Mali Destek: Yazarlar bu çalışma için mali destek almadıklarını beyan etmişlerdir.
References
- 1.Nizet V, Klein J. Bacterial sepsis and meningitis. In: Wilson C, Nizet V, Maldonado Y, Remington J, Klein J, editors. Infectious diseases of the fetus and newborn infant. 8 ed. Philadelphia: Elsevier Saunders; 2016. pp. 217–71. [Google Scholar]
- 2.Stoll BJ, Hansen NI, Sanchez PJ, et al. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics. 2011;127:817–26. doi: 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong Y, Speer CP. Late-onset neonatal sepsis: recent developments. Arch Dis Child Fetal Neonatal Ed. 2015;100:F257–263. doi: 10.1136/archdischild-2014-306213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turkish Neonatal S Nosocomial Infections Study G. Nosocomial infections in neonatal units in Turkey: epidemiology, problems, unit policies and opinions of healthcare workers. Turk J Pediatr. 2010;52:50–7. [PubMed] [Google Scholar]
- 5.Yapicioglu H, Satar M, Ozcan K, et al. A 6-year prospective surveillance of healthcare-associated infections in a neonatal intensive care unit from southern part of Turkey. J Paediatr Child Health. 2010;46:337–42. doi: 10.1111/j.1440-1754.2010.01718.x. [DOI] [PubMed] [Google Scholar]
- 6.Cantey JB, Milstone AM. Bloodstream infections: epidemiology and resistance. Clin Perinatol. 2015;42:1–16. doi: 10.1016/j.clp.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Ericson JE, Laughon MM. Chorioamnionitis: implications for the neonate. Clin Perinatol. 2015;42:155–65. doi: 10.1016/j.clp.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.KarakuşM Karaca, Derici Y, Günçiner Ş. Gebelerde grup B streptokok kolonizasyonu ve antimikrobiyal dirençpaterni. Ege J Med. 2007;46:151–4. [Google Scholar]
- 9.Bedford Russell AR, Kumar R. Early onset neonatal sepsis: diagnostic dilemmas and practical management. Arch Dis Child Fetal Neonatal Ed. 2015;100:F350–354. doi: 10.1136/archdischild-2014-306193. [DOI] [PubMed] [Google Scholar]
- 10.Bizzarro MJ, Dembry LM, Baltimore RS, Gallagher PG. Changing patterns in neonatal Escherichia coli sepsis and ampicillin resistance in the era of intrapartum antibiotic prophylaxis. Pediatrics. 2008;121:689–96. doi: 10.1542/peds.2007-2171. [DOI] [PubMed] [Google Scholar]
- 11.Satar M, Özlü F. Neonatal sepsis: A continous disease burden. Turk J Pediatr. 2012;54:449–57. [PubMed] [Google Scholar]
- 12.Polin RA Committee on Fetus and Newborn. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics. 2012;129:1006–15. doi: 10.1542/peds.2012-0541. [DOI] [PubMed] [Google Scholar]
- 13.Ferrieri P, Wallen L. Neonatal bacterial sepsis. In: Gleason C, Devaskar S, editors. Avery's diseases of the newborn. 9 ed. Vol. 9. Philedelphia: Elsevier Saunders; 2012. pp. 538–51. [Google Scholar]
- 14.Schmutz N, Henry E, Jopling J, Christensen RD. Expected ranges for blood neutrophil concentrations of neonates: the Manroe and Mouzinho charts revisited. J Perinatol. 2008;28:275–81. doi: 10.1038/sj.jp.7211916. [DOI] [PubMed] [Google Scholar]
- 15.Manzoni P. Hematologic aspects of early and late-onset sepsis in preterm infants. Clin Perinatol. 2015;42:587–95. doi: 10.1016/j.clp.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Delanghe JR, Speeckaert MM. Translational research and biomarkers in neonatal sepsis. Clin Chim Acta. 2015;451:46–64. doi: 10.1016/j.cca.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 17.Pontrelli G, De Crescenzo F, Buzzetti R, et al. Accuracy of serum procalcitonin for the diagnosis of sepsis in neonates and children with systemic inflammatory syndrome: a meta-analysis. BMC Infect Dis. 2017;17:302. doi: 10.1186/s12879-017-2396-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilfillan M, Bhandari V. Biomarkers for the diagnosis of neonatal sepsis and necrotizing enterocolitis: Clinical practice guidelines. Early Hum Dev. 2017;105:25–33. doi: 10.1016/j.earlhumdev.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Probability of Neonatal Early-Onset Infection. [Accessed Nov 8 2017]. Available from: http://newbornsepsiscalculator.org/
- 20.Tzialla C, Borghesi A, Pozzi M, Stronati M. Neonatal infections due to multi-resistant strains: Epidemiology, current treatment, emerging therapeutic approaches and prevention. Clin Chim Acta. 2015;451:71–7. doi: 10.1016/j.cca.2015.02.038. [DOI] [PubMed] [Google Scholar]
- 21.Ku LC, Boggess KA, Cohen-Wolkowiez M. Bacterial meningitis in infants. Clin Perinatol. 2015;42:29–45. doi: 10.1016/j.clp.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barichello T, Fagundes GD, Generoso JS, Elias SG, Simoes LR, Teixeira AL. Pathophysiology of neonatal acute bacterial meningitis. J Med Microbiol. 2013;62:1781–9. doi: 10.1099/jmm.0.059840-0. [DOI] [PubMed] [Google Scholar]
- 23.Nascimento-Carvalho CM, Moreno-Carvalho OA. Normal cerebrospinal fluid values in full-term gestation and premature neonates. Arq Neuropsiquiatr. 1998;56:375–80. doi: 10.1590/s0004-282x1998000300005. [DOI] [PubMed] [Google Scholar]
- 24.Heath PT, Okike IO, Oeser C. Neonatal meningitis: can we do better? Adv Exp Med Biol. 2011;719:11–24. doi: 10.1007/978-1-4614-0204-6_2. [DOI] [PubMed] [Google Scholar]
- 25.Klinger G, Chin CN, Beyene J, Perlman M. Predicting the outcome of neonatal bacterial meningitis. Pediatrics. 2000;106:477–82. doi: 10.1542/peds.106.3.477. [DOI] [PubMed] [Google Scholar]
- 26.Barnett E, Klein J. Bacterial infections of the respiratory tract. In: Wilson C, Nizet V, Maldonado Y, Remington J, Klein J, editors. Infectious dseases of the fetus and newborn infant. 8 ed. Philadelphia: Elsevier Saunders; 2015. pp. 278–96. [Google Scholar]
- 27.Apisarnthanarak A, Holzmann-Pazgal G, Hamvas A, Olsen MA, Fraser VJ. Ventilator-associated pneumonia in extremely preterm neonates in a neonatal intensive care unit: characteristics, risk factors, and outcomes. Pediatrics. 2003;112:1283–9. doi: 10.1542/peds.112.6.1283. [DOI] [PubMed] [Google Scholar]
- 28.Arshad M, Seed PC. Urinary tract infections in the infant. Clin Perinatol. 2015;42:17–28. doi: 10.1016/j.clp.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Overturf G, Muller M, Nizet V. Focal Bacterial Infections. In: Wilson C, Nizet V, Maldonado Y, Remington J, Klein J, editors. Infectious diseases of the fetus and newborn infant. 8 ed. Philadelphia: Elsevier Saunders; 2015. pp. 325–55. [Google Scholar]
- 30.White K, Goldberg M. Common neonatal orthopedic ailments. In: Gleason C, Devaskar S, editors. Avery's diseases of the newborn. 9 ed. Philedelphia: Elsevier Saunders; 2012. pp. 1351–61. [Google Scholar]
- 31.Muller M, Overturf G. Bacterial infections of the bones and joints. In: Wilson C, Nizet V, Maldonado Y, Remington J, Klein J, editors. Infectious diseases of the fetus and infant. 8 ed. Philadelphia: Elsevier Saunders; 2015. pp. 291–305. [Google Scholar]
- 32.Dudeck MA, Horan TC, Peterson KD, et al. National Healthcare Safety Network report, data summary for 2011, device-associated module. Am J Infect Control. 2013;41:286–300. doi: 10.1016/j.ajic.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hocevar SN, Edwards JR, Horan TC, Morrell GC, Iwamoto M, Lessa FC. Device-associated infections among neonatal intensive care unit patients: incidence and associated pathogens reported to the National Healthcare Safety Network 2006-2008. Infect Control Hosp Epidemiol. 2012;33:1200–6. doi: 10.1086/668425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor JE, Tan K, Lai NM, McDonald SJ. Antibiotic lock for the prevention of catheter-related infection in neonates. Cochrane Database Syst Rev. 2015:CD010336. doi: 10.1002/14651858.CD010336.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balain M, Oddie SJ, McGuire W. Antimicrobial-impregnated central venous catheters for prevention of catheter-related bloodstream infection in newborn infants. Cochrane Database Syst Rev. 2015:CD011078. doi: 10.1002/14651858.CD011078.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai NM, Taylor JE, Tan K, Choo YM, Ahmad Kamar A, Muhamad NA. Antimicrobial dressings for the prevention of catheter-related infections in newborn infants with central venous catheters. Cochrane Database Syst Rev. 2016;3:CD011082. doi: 10.1002/14651858.CD011082.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly MS, Benjamin DK, Jr, Smith PB. The epidemiology and diagnosis of invasive candidiasis among premature infants. Clin Perinatol. 2015;42:105–17. doi: 10.1016/j.clp.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steinbach WJ, Roilides E, Berman D, et al. Results from a prospective, international, epidemiologic study of invasive candidiasis in children and neonates. Pediatr Infect Dis J. 2012;31:1252–7. doi: 10.1097/INF.0b013e3182737427. [DOI] [PubMed] [Google Scholar]
- 39.Bendel CM. Candidiasis. In: Wilson C, Nizet V, Maldonado Y, Remington J, Klein J, editors. Infectious diesease of the newborn and fetus. 9 ed. Philadelphia: Elsevier Saunders; 2015. pp. 1058–79. [Google Scholar]
- 40.Ohlsson A, Lacy JB. Intravenous immunoglobulin for suspected or proven infection in neonates. Cochrane Database Syst Rev. 2015:CD001239. doi: 10.1002/14651858.CD001239.pub5. [DOI] [PubMed] [Google Scholar]
- 41.Carr R, Modi N, Dore C. G-CSF and GM-CSF for treating or preventing neonatal infections. Cochrane Database Syst Rev. 2003:CD003066. doi: 10.1002/14651858.CD003066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pammi M, Haque KN. Pentoxifylline for treatment of sepsis and necrotizing enterocolitis in neonates. Cochrane Database Syst Rev. 2015:CD004205. doi: 10.1002/14651858.CD004205.pub3. [DOI] [PubMed] [Google Scholar]
- 43.Cleminson J, Austin N, McGuire W. Prophylactic systemic antifungal agents to prevent mortality and morbidity in very low birth weight infants. Cochrane Database Syst Rev. 2015:CD003850. doi: 10.1002/14651858.CD003850.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aydemir C, Oguz SS, Dizdar EA, et al. Randomised controlled trial of prophylactic fluconazole versus nystatin for the prevention of fungal colonisation and invasive fungal infection in very low birth weight infants. Arch Dis Child Fetal Neonatal Ed. 2011;96:F164–8. doi: 10.1136/adc.2009.178996. [DOI] [PubMed] [Google Scholar]
- 45.Sharma D, Shastri S, Sharma P. Role of lactoferrin in neonatal care: a systematic review. J Matern Fetal Neonatal Med. 2017;30:1920–32. doi: 10.1080/14767058.2016.1232384. [DOI] [PubMed] [Google Scholar]
- 46.Rao SC, Athalye-Jape GK, Deshpande GC, Simmer KN, Patole SK. Probiotic Supplementation and Late-Onset Sepsis in Preterm Infants: A Meta-analysis. Pediatrics. 2016;137:e20153684. doi: 10.1542/peds.2015-3684. [DOI] [PubMed] [Google Scholar]
- 47.Soll RF, Edwards WH. Antibiotic use in neonatal intensive care. Pediatrics. 2015;135:928–9. doi: 10.1542/peds.2015-0707. [DOI] [PubMed] [Google Scholar]
- 48.Ramasethu J, Kawakita T. Antibiotic stewardship in perinatal and neonatal care. Semin Fetal Neonatal Med. 2017;22:278–83. doi: 10.1016/j.siny.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Kuppala VS, Meinzen-Derr J, Morrow AL, Schibler KR. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. 2011;159:720–5. doi: 10.1016/j.jpeds.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bedford Russell AR, Murch SH. Could peripartum antibiotics have delayed health consequences for the infant? BJOG. 2006;113:758–65. doi: 10.1111/j.1471-0528.2006.00952.x. [DOI] [PubMed] [Google Scholar]


