Abstract
Collapsin-1 or semaphorin III(D) inhibits axonal outgrowth by collapsing the lamellipodial and filopodial structures of the neuronal growth cones. Because growth cone collapse is associated with actin depolymerization, we considered whether small GTP-binding proteins of the rho subfamily might participate in collapsin-1 signal transduction. Recombinant rho, rac1, and cdc42 proteins were triturated into embryonic chick (DRG) neurons. Constitutively active rac1 increases the proportion of collapsed growth cones, and dominant negative rac1 inhibits collapsin-1-induced collapse of growth cones and collapsin-1 inhibition of neurite outgrowth. DRG neurons treated with dominant negative rac1 remain sensitive to myelin-induced growth cone collapse. Similar mutants of cdc42 do not alter growth cone structure, neurite elongation, or collapsin-1 sensitivity. Whereas the addition of activated rho has no effect, the inhibition of rho withClostridium botulinum C3 transferase stimulates the outgrowth of DRG neurites. C3 transferase-treated growth cones exhibit little or no lamellipodial spreading and are minimally responsive to collapsin-1 and myelin. These data demonstrate a prominent role for rho and rac1 in modulating growth cone motility and indicate that rac1 may mediate collapsin-1 action.
Keywords: collapsin-1, semaphorin, rac1, rho, growth cone collapse, neurite outgrowth, dorsal root ganglion neuron
Neuronal growth cones possess the sensory apparatus to distinguish among innumerable potential pathways and targets during nervous system development and regeneration (for review, see Strittmatter, 1995). Extracellular signals induce changes in the actin-based cytoskeleton of the growth cone and hence the morphology and motility of the growth cone. The molecular mechanisms by which extracellular clues are transduced to cytoskeletal rearrangements are defined poorly.
The semaphorin or collapsin family of proteins has been recognized as an important negative regulator of axonal outgrowth and terminal arborization (Kolodkin et al., 1992, 1993; Luo et al., 1993). Chick collapsin-1 induces growth cone collapse and a cessation of neurite outgrowth from at least a subset of DRG neurons (Raper and Kapfhammer, 1990; Luo et al., 1993). Insect semaphorins have a demonstratedin vivo role in axonal pathfinding and synaptic terminal branching (Kolodkin et al., 1992; Matthes et al., 1995). There are at least seven vertebrate semaphorins identified, and there may be as many as 20 members of this family (Inagaki et al., 1995; Luo et al., 1995;Messersmith et al., 1995; Puschel et al., 1995; Adams et al., 1996). A decrease in actin filaments after collapsin-1 application has been documented (Fan et al., 1993). The mechanisms by which collapsin-1 binding to an unidentified transmembrane receptor triggers this depolymerization are unclear.
In non-neuronal cells, the rho subfamily of monomeric ras-related GTP-binding proteins has prominent effects on the actin-based cytoskeleton and on cell shape (Hall, 1990, 1994). In fibroblasts, rho activation has been linked to stress fiber formation and focal adhesions, rac1 activation has been linked to membrane ruffling and lamellipodia, and cdc42 activation has been linked to filopodial formation (Nobes and Hall, 1995). Single amino acid substitutions have been identified that produce constitutively active or dominant negative forms of each of these proteins. The C3 transferase fromClostridium botulinum ADP ribosylates rho specifically and inactivates the G-protein.
The contribution of this class of G-proteins to the regulation of neuronal growth cone motility has come under investigation only recently. In neuroblastoma cells, the binding of lysophosphatidic acid (LPA) or thrombin binding to heterotrimeric G-protein-coupled receptors induces rapid neurite retraction (Jalink and Moolenaar, 1992; Jalink et al., 1994). The C3 transferase from C. botulinum has been shown to block the action of LPA, indicating that rho activation mediates the LPA regulation of neurite length in these cells (Jalink et al., 1994). A downstream target of activated rho has been identified as myosin light chain phosphorylase (Kimura et al., 1996), and an inhibitor of myosin light chain kinase, KT5926, also blocks LPA-induced neurite retraction (Jalink et al., 1994).
Further evidence for the involvement of rho-related small G-proteins in the regulation of neurite outgrowth comes from studies in which activated or dominant negative forms of these proteins are expressedin vivo. Alterations of rac1 activity, and to a lesser extent of cdc42 activity, lead to a failure in axonal extension from many neurons in the fly (Luo et al., 1994). Mice expressing constitutively active rac1 in cerebellar Purkinje cells exhibit alterations in dendritic morphology (Luo et al., 1996).
The present study was designed to examine the action of rho, rac1, and cdc42 activation or inhibition on the outgrowth and the sensitivity to collapsin-1 of chick DRGs. The data indicate that both rho and rac1 are capable of modulating chick DRG neurite outgrowth in culture and that rac1 activation may mediate the inhibitory effects of collapsin-1 on neurite outgrowth.
MATERIALS AND METHODS
Preparation of proteins: G-proteins, collapsin, and myelin. Monomeric human G-proteins and C. botulinum C3 transferase were produced in bacteria as glutathioneS-transferase (GST) fusion proteins and then were treated with thrombin to remove the GST moiety (Nobes and Hall, 1995). Thrombin was removed from the samples by absorption top-aminobenzamidine-agarose. The following derivatives were produced: wild-type rhoA (rho), a constitutively active form of rhoA with Gly at position 14 mutated to Val (V14rho), wild-type rac1 (rac), a constitutively active form of rac1 with Gly at position 12 mutated to Val (V12rac), a dominant negative form of rac1 with Thr at position 17 mutated to Asn (N17rac), wild-type cdc42 (cdc42), a constitutively active form of cdc42 with Gly at position 12 mutated to Val (V12cdc42), a dominant negative form of cdc42 with Thr at position 17 mutated to Asn (N17cdc42), and the C3 exoenzyme from C. botulinum (C3). The rho and V14rho proteins contain a substitution at position 25 of Asn for Phe to enhance stability in Escherichia coli.
Collapsin-His6 was prepared as described (Goshima et al., 1995). Myelin fractions were prepared from bovine brain, and the proteins extracted with 2% octylglucoside were tested in growth cone collapse after the removal of detergent by dialysis (Igarashi et al., 1993).
DRG culture conditions and trituration method. The preparation of chick E7 DRG explant and dissociated neuron cultures has been described previously (Strittmatter et al., 1994b; Goshima et al., 1995). For trituration experiments, neurons were suspended in 25 mm Tris-HCl, 150 mm NaCl, 5 mmMgCl2, and 1 mm dithiothreitol, pH 7.5, with the rho subfamily proteins at 5 mg/ml or with C3 transferase at 0.1 mg/ml; then the suspension was passed 50 times through a Gilson P200 pipette tip (Strittmatter et al., 1994b; Goshima et al., 1995). After trituration, neurons were plated in 25 volumes of F 12 medium with 10% FBS and with 50 ng/ml 7 S-NGF on a glass surface precoated sequentially with 100 μg/ml poly-l-lysine and with 20 μg/ml laminin. For experiments with LPA, triturated neurons were transferred to serum-free medium (F-12 medium with 1% fatty acid-free BSA and with 50 ng/ml 7 S-NGF) for 3 hr before the growth cone collapse assay.
Neurite outgrowth and growth cone collapse. For outgrowth assays, triturated cells were plated for 1.5–2 hr, and then the agents to be tested were added to the medium. After an additional 2–3 hr of incubation, the cells were fixed, and total neurite length per neuron was measured for 75–150 cells (Strittmatter et al., 1994b; Goshima et al., 1995). The growth cone collapse assay for explant cultures has been described in detail (Raper and Kapfhammer, 1990; Strittmatter et al., 1994b; Goshima et al., 1995). For triturated cells, neurons were cultured for 4 hr before test compounds were added for 20–30 min. The fraction of collapsed growth cones was scored as described for explant cultures.
Immunohistology. Dissociated chick E7 DRG neurons were cultured for 24 hr and then were fixed for 30 min with ice-cold 4% paraformaldehyde and 20% sucrose in PBS. Samples then were incubated with 4 μg/ml anti-rac1 mouse monoclonal antibody directed against human rac1 (Upstate Biotechnology, Lake Placid, NY). In some cases, rac1 protein at 1 mg/ml was added with the antibody to the incubation to demonstrate the specificity of the staining. Bound antibody was detected by the avidin–biotin complex method (Vector Laboratories, Burlingame, CA) with horseradish peroxidase enzyme and diaminobenzidine substrate as described (Goshima et al., 1995).
RESULTS
Comparison of collapsin-1 action with LPA and thrombin action
As a first step in assessing the role of small G-proteins in collapsin-1 action, we compared the effects of readily available pharmacological agents on collapsin-1 action with their effects on LPA and thrombin action. The myosin light chain kinase inhibitor KT5926 blocks LPA-induced neurite retraction and also decreases the potency of recombinant collapsin-1 as a growth cone collapse factor (Fig.1A). A number of other agents had little or no effect on collapsin-1 action, including tyrosine kinase inhibitors, protein kinase A inhibitors, voltage-sensitive Ca2+ channel blockers, and depolarization with KCl (data not shown). The more general protein kinase inhibitor staurosporine and the protein kinase C activator phorbol 12-myristate 13-acetate both induced growth cone collapse at concentrations of <10 nm, but their action was not synergistic with collapsin-1 (data not shown).
Fig. 1.
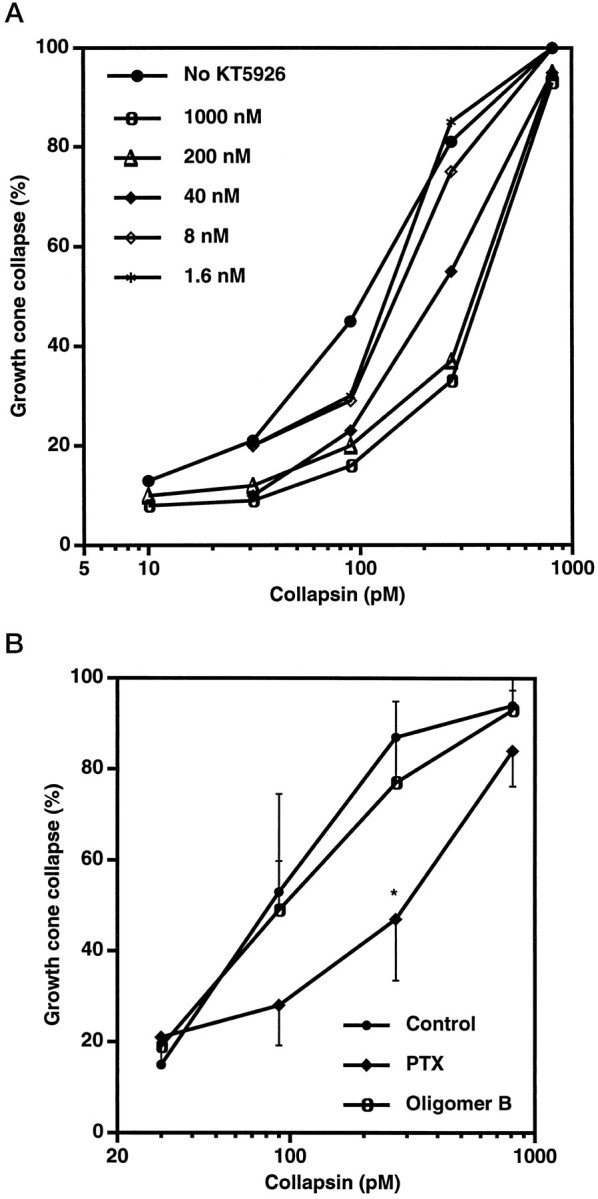
Collapsin-1-induced growth cone collapse is attenuated by KT5926 and PTX. A, Chick DRG explant cultures were preincubated for 2 hr in culture medium with KT5926 at the indicated concentrations. Then growth cone collapse was assayed. Low concentrations of KT5926 shifted the collapsin-1 dose–response curve to the right by a factor of five. KT5926 had no direct effect on growth cone collapse in the absence of collapsin-1. The means from four to six separate experiments are shown. For each point, the SEM was <10% of the value shown. B, Chick DRG explant cultures were preincubated for 3 hr in growth medium with PTX (pertussis holotoxin) at 500 ng/ml or with the oligomer B subfraction of PTX at 500 ng/ml. Then growth cone collapse was measured in the presence of the indicated concentrations of recombinant collapsin-His6. Whereas the oligomer B fraction had no effect, PTX decreased growth cone collapse at 200 pm collapsin-1 significantly (*p ≤ 0.05; Student’s two-tailed ttest). The averages from five experiments ± SEM are shown.
The actions of LPA and thrombin are mediated by receptors linked to heterotrimeric G-proteins (Jalink et al., 1994). We considered whether recombinant collapsin-1 action also involves trimeric G-protein activation. Pertussis toxin (PTX) ADP ribosylates the α subunit of heterotrimeric G-proteins of the Go or Gi class and blocks their activation by receptors. Growth cone collapse by crude whole brain membrane extracts (BMEs), which contain collapsin-1, is blocked by PTX (Igarashi et al., 1993), but this is because of the cell surface binding properties of PTX rather than the modification of G-proteins by PTX (Kindt and Lander, 1995). The isolated oligomer B fraction of PTX contains the cell surface binding domain but does not block purified recombinant collapsin-1-induced growth cone collapse (Fig. 1B). Thus, the decrease in collapsin-1 potency by intact PTX suggests that collapsin-1 action involves heterotrimeric G-protein action, strengthening the similarity with LPA and thrombin action. The failure of PTX to block at higher collapsin-1 concentrations may be attributable either to PTX-insensitive G-proteins or to non-G-protein-dependent mechanisms. Oligomer B blockade of BME action may reflect the inhibition of collapsing agents other than collapsin-1 in the crude extract.
Basal outgrowth in DRG neurons containing exogenous rho subfamily proteins
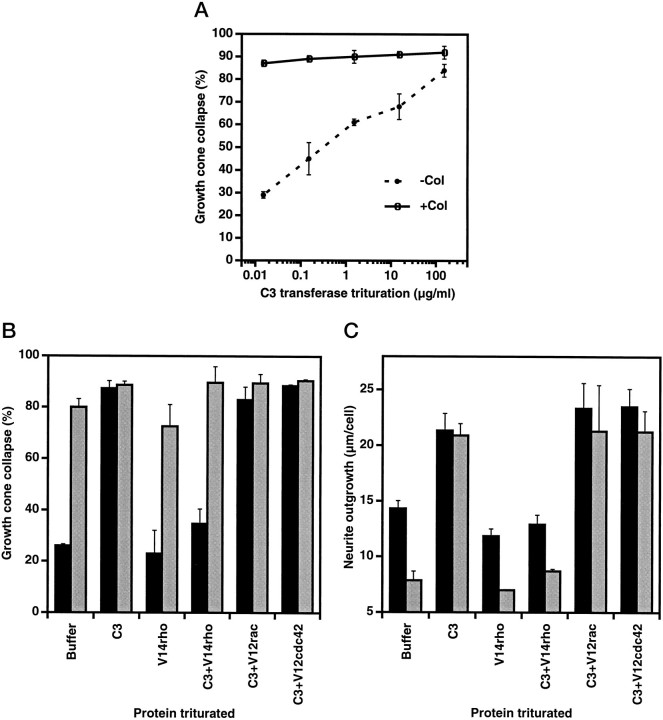
To modulate the activity of rho subfamily G-proteins in DRG neurons, purified recombinant proteins were triturated with isolated neurons. Neurons were plated immediately after trituration; neurite extension and growth cone morphology were observed 2–5 hr later (Fig.2). All of the triturated proteins were >95% pure (Fig. 2A). Four hours after plating, the neurons triturated with buffer are indistinguishable from cells that have not been triturated. None of the recombinant proteins affect the number of neurons that attach to the laminin-coated surface under these conditions. Of the proteins altering rho activity, only C3 transferase alters outgrowth. Neurite extension doubles after C3 transferase treatment (Fig. 2D), and nearly all of the growth cones exhibit greatly reduced lamellipodial spreading (Fig.2B,C). These data raise the possibility that under basal conditions a significant fraction of rho is likely to be activated. Of the rac1 proteins, the constitutively active form increases the percentage of growth cones with a collapsed appearance (Fig. 2B,C), and there is a slight trend toward decreased neurite extension that does not reach statistical significance (Fig. 2D). The weak V12rac effects mimic the action of collapsin-1. The cdc42 proteins at the same concentration do not alter growth cone appearance or neurite extension.
Fig. 2.
Growth cone collapse and neurite outgrowth in DRG neurons triturated with the rho subfamily proteins. A, The protein preparations used for trituration were separated by SDS-PAGE and were stained with Coomassie blue. The migration of 45, 36, 25, and 21 kDa Mr standards is shown on theright. B, DRG neurons were triturated with the indicated proteins at 5 mg/ml for the rho family proteins and at 0.1 mg/ml for C3 transferase. After 4 hr of culture, growth cone collapse was assessed with (gray bars) or without (solid bars) a 20 min exposure to 200 pmcollapsin-His6. The data are averages ± SEM for three to nine separate experiments. The values marked with anasterisk are significantly different (p ≤ 0.05; Student’s two-tailedt test) from the values for buffer-triturated cells under the same conditions. C, DRG neurons were triturated with the indicated proteins and were exposed to collapsin-His6 as described in B. Actin was visualized by staining formalin-fixed cells with rhodamine-phalloidin. Magnification, 500×. D, DRG neurons were triturated with the indicated proteins at 5 mg/ml for the rho family proteins and at 0.1 mg/ml for C3 transferase. After 2 hr of culture, neurons were exposed to 0 (solid bars) or 200 pm(gray bars) collapsin-His6 for an additional 3 hr, and then the average total neurite outgrowth per cell was determined (Goshima et al., 1995). The data are averages ± SEM for three to nine separate experiments. The values marked with anasterisk are significantly different (p ≤ 0.05; Student’s two-tailedt test) from the values for buffer-triturated cells under the same conditions.
Collapsin-1 sensitivity in DRG neurons containing rho subfamily proteins
Neurons triturated with rho family members were exposed to collapsin-1, and then growth cone morphology and neurite extension were examined. In control cultures, exposure to collapsin-1 for 20 min increases the percentage of collapsed growth cones from 15 to 70% (Fig. 2B,C). Exposure to collapsin-1 during the interval from 2 to 5 hr after plating decreases the extent of outgrowth by 50% (Fig. 2D). Collapsin-1-induced changes in growth cone collapse and in neurite outgrowth are attenuated markedly in neurons treated with dominant negative N17rac (Fig. 2B–D). In contrast, constitutively active V12rac-treated and wild-type rac-treated cells exhibit essentially normal responsiveness to collapsin-1. Trituration with cdc42 proteins or buffer does not alter collapsin-1 sensitivity. Similarly, wild-type and activated rho do not alter collapsin-1 action. However, the C3 transferase-treated neurons displaying increased neurite outgrowth are minimally sensitive to the inhibitory effects of collapsin-1 (Fig. 2D). The decreased lamellipodial morphology of growth cones in C3 transferase-treated cultures is only slightly enhanced by collapsin-1 (Fig.2B,C).
Characterization of rac1 effects in DRG neurons
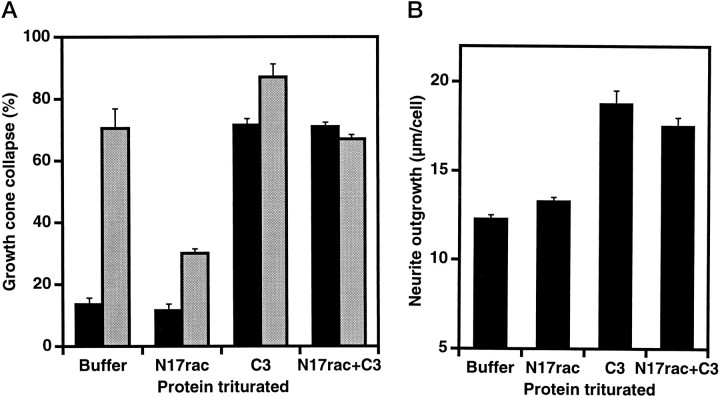
The effect of dominant negative N17rac trituration is dependent on the dose of rac1 protein present during the trituration; concentrations in excess of 1 mg of protein/ml are required to achieve >50% inhibition of collapsin-1-induced growth cone collapse (Fig.3A). The specificity of N17rac action for endogenous rac1 pathways is suggested by the inactivity of dominant negative N17cdc42 (Fig.2B,D). Furthermore, the cotrituration of constitutively active V12rac, but not constitutively active V14rho or V12cdc42, reverses partially the N17rac inhibition of collapsin-1-induced growth cone collapse (Fig. 3B).
Fig. 3.
Rac1 in collapsin-1 regulation of growth cone motility. A–C, DRG neurons were triturated with buffer or with various concentrations of the indicated G-proteins. Growth cone collapse with or without a 20 min exposure to collapsin-His6 was determined as described in Figure 2. The data are averages ± SEM for two to four separate experiments.A, Growth cone collapse after trituration of DRG neurons with various concentrations of N17rac protein was determined with (○) or without (•) exposure to 200 pm collapsin-His6. B, Growth cone collapse after trituration of DRG neurons with N17rac at 0 or 2.5 mg/ml and with the indicated constitutively active G-proteins at 0 or 5 mg/ml was determined in the absence (solid bars) or in the presence (gray bars) of 200 pmcollapsin-His6. Note that V12rac partially reverses the N17rac inhibition of collapsin-1-induced growth cone collapse.C, Growth cone collapse after trituration with buffer, with constitutively active V12rac, or with dominant negative N17rac was determined for DRG neurons exposed to the indicated concentrations of collapsin-His6. D, DRG neurons were stained with 4 μg/ml monoclonal anti-rac1 antibody as described in Materials and Methods. Note the staining of rac1 in growth cone structures (top, bright-field). The addition of 1 mg/ml recombinant rac1 protein to the primary antibody solution abolished all staining (bottom,bright-field). The bottom region contains three growth cones detectable by differential interference contrast observation (data not shown). Scale bar, 25 μm.
After trituration with dominant negative N17rac, the collapsin-1 dose–response curve for DRG growth cone collapse is shifted to the right by a factor of 15 (EC50 from 60 pm to 2 nm, Fig. 3C). The residual weak effect of collapsin-1 as a growth cone collapse factor in N17rac-triturated cells may be caused by an incomplete rac1 blockade achieved by the trituration method or by nonrac1 dependent collapsin-1-induced growth cone collapse mechanisms. As described above, trituration with constitutively active V12rac induces collapse of 20% of the growth cones (Fig. 2B). The dose–response curve for collapsin-1-induced growth cone collapse is shifted to the left by a factor of two after trituration with constitutively active V12rac (EC50 from 60 to 30 pm, Fig.3C).
If rac1 is an endogenous modulator of collapsin-1-induced growth cone collapse, it must be present in the growth cone. Histological staining for rac1 demonstrates that the protein is found in growth cones and is present in filopodial structures at the very tip of the growth cone (Fig. 3D). Thus, the protein is in a position to mediate collapsin-1 action.
C3 transferase action in DRG neurons
The ability of the C3 exoenzyme to ADP-ribosylate specifically rho in mammalian cells, including neuroblastoma cells, has been demonstrated previously (Jalink et al., 1994). The action of C3 transferase in DRG neurons depends on the dose of C3 exoenzyme present during the trituration, with as little as 1 μg/ml causing >50% of the DRG growth cones to collapse (Fig.4A). Although constitutively active V14rho does not alter basal growth cone collapse or outgrowth (Fig. 2B,D), trituration with this protein reverses the C3 transferase effects on growth cone collapse and outgrowth (Fig.4B,C). Neither constitutively active V12rac nor V12cdc42 reverses C3 transferase action. Taken together, these data support the specificity of C3 transferase for rho inhibition after trituration into DRG neurons.
Fig. 4.
C3 transferase action in DRG neurons. DRG neurons were triturated, cultured, and assayed as described in Figure 2. The data are averages ± SEM for two to four separate experiments.A, Growth cone collapse after the indicated concentrations of C3 transferase were present during the trituration of DRG neurons was determined in the presence and absence of 200 pm collapsin-His6 (Col).B, Growth cone collapse was determined after the trituration of DRG neurons with buffer, 4 μg/ml C3 transferase, 5 mg/ml V14rho, or both proteins and after a subsequent exposure of the neurons to 0 (gray bars) or 200 pm(solid bars) collapsin-His6. Results for trituration with both C3 transferase and either V12rac or V12cdc42 are also given. C, Average total neurite outgrowth per cell for neurons triturated as described in B was determined after plating with (gray bars) or without (solid bars) 200 pmcollapsin-His6.
Dominant negative rac1 does not block the effects of rho inactivation
The decrease in growth cone area caused by C3 transferase treatment is associated with increased neurite extension, whereas that caused by collapsin-1 is associated with decreased extension. We considered whether dominant negative rac1 could block the effects of rho inhibition by C3 transferase as it blocks collapsin-1 action. When C3 transferase and N17rac are cotriturated, DRG neurites resemble C3 transferase-triturated neurites (Fig. 5). Thus, modulation of neurite extension by rho is not mediated primarily through rac1. Rho may act in separate pathway(s) and/or function downstream of rac1 to regulate growth cone morphology and neurite extension.
Fig. 5.
The effects of C3 transferase are not blocked by N17rac. DRG neurons were triturated with buffer, 5 mg/ml N17rac, 0.1 mg/ml C3 transferase, or both proteins. The data are averages ± SEM for three to five separate experiments. A, Neurons were cultured for 4 hr with the indicated proteins, and then growth cone collapse was assessed with (gray bars) or without (solid bars) a 20 min exposure to 200 pm collapsin-His6. B, Average total neurite outgrowth per cell for neurons triturated with the indicated proteins was determined 4 hr after plating.
Inhibitory effects of myelin are not mediated by rho family members
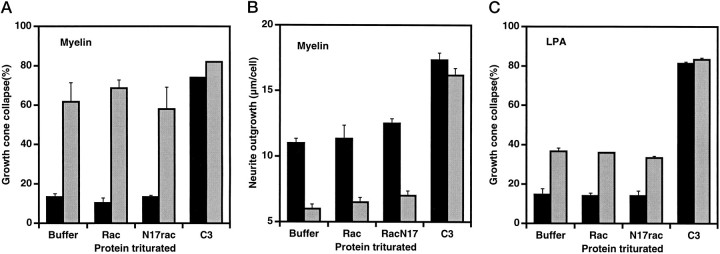
Components of CNS myelin have inhibitory influences on neurite regeneration and alter cultured DRG neuron morphology in a manner similar to that of collapsin-1 (Bandtlow et al., 1993). Growth cone collapse after exposure to CNS myelin extract is not altered by trituration with N17rac (Fig.6A,B). This indicates that the Ca2+i dependent pathway used by inhibitory components of myelin (Bandtlow et al., 1993) is distinct from the rac1 dependent pathway used by collapsin-1. The rapidly growing, small growth cones present in C3 transferase-treated cultures are insensitive to myelin (Fig.6A,B). LPA induces collapse of a small fraction of the DRG growth cones (Fig. 6C). This fraction is not altered by N17rac, implying that LPA-induced collapse proceeds via a different pathway than collapsin-1-induced collapse.
Fig. 6.
Growth cone collapse by myelin or LPA is not blocked by N17rac. DRG neurons were triturated with the indicated proteins as described in Figure 2. The data are averages ± SEM for three separate experiments. A, Neurons were cultured for 4 hr with the indicated proteins, and growth cone collapse was assessed after a 30 min exposure to buffer (solid bars) or to CNS myelin extract at 5 μg protein/ml (gray bars). B, After 2 hr of culture, neurons were cultured for an additional 2 hr with (gray bars) or without (solid bars) CNS myelin extract at 5 μg protein/ml. The average total neurite outgrowth per cell was determined after 4 hr. C, Neurons were cultured for 4 hr with the indicated proteins, and growth cone collapse was assessed after a 30 min exposure to buffer (solid bars) or to 1 μm LPA (gray bars).
DISCUSSION
Rac1 mediates collapsin-1 action
Several lines of data from this study support the hypothesis that rac1 mediates collapsin-1 action in DRG neurons. Trituration of dominant negative N17rac nearly abolishes growth cone collapse by collapsin-1 and greatly reduces neurite outgrowth inhibition by collapsin-1. Other rho subfamily members do not have these effects. The presence of rac1 in the growth cone is consistent with a role for this protein in collapsin-1 signaling. Constitutively active V12rac weakly mimics collapsin-1 action. The small magnitude of V12rac action may be caused by (1) the contribution of nonrac1 dependent mechanisms in collapsin-1-induced collapse, (2) the inefficiency of the trituration method, or (3) desensitizing mechanisms occurring during the 3–5 hr after trituration. Although collapsin-1 action is inhibited by N17rac, the effects of other extracellular proteins that induce the same morphological changes are not blocked by trituration with N17rac. This indicates that rac1 is specifically involved in collapsin-1 action and that the Ca2+-mediated growth cone collapse induced by components of CNS myelin does not use this monomeric G-protein.
Rho regulates neurite outgrowth, but the effects of rho are not altered by collapsin-1
The inhibition of rho with C3 transferase also alters the morphology of DRG neurons. This implies a significant level of rho activation in DRG growth cones under basal conditions. Furthermore, the data suggest that rho activation may decrease outgrowth but leads to greater growth cone spreading. In DRG neurons treated with a low dose of C3 transferase to reduce rho activity, constitutively active V14rho does increase growth cone spreading and decrease neurite outgrowth. The decreased growth cone spreading and increased outgrowth rate of rho-inhibited neurons are modulated only minimally by collapsin-1. These effects distinguish rho action from rac1 activation and collapsin-1 addition. Although it seems that rho exerts effects different from those exerted by rac1 and collapsin-1, growth cone morphology and motility may reflect additive rho and rac1 regulation. While rho activation is downstream of rac1 activation in 3T3 fibroblasts (Nobes and Hall, 1995), this does not seem to be the case in DRG growth cones. Rho does not seem to be the primary mediator of collapsin-1 effects, but it may be a target for other DRG growth cone regulators, as suggested for LPA and thrombin (Jalink et al., 1994). The myosin light chain kinase inhibitor KT5926 may counteract myosin light chain phosphorylase regulation by rho (Kimura et al., 1996). In so doing, KT5926 partially reproduces the C3 transferase effect and decreases collapsin-1 sensitivity.
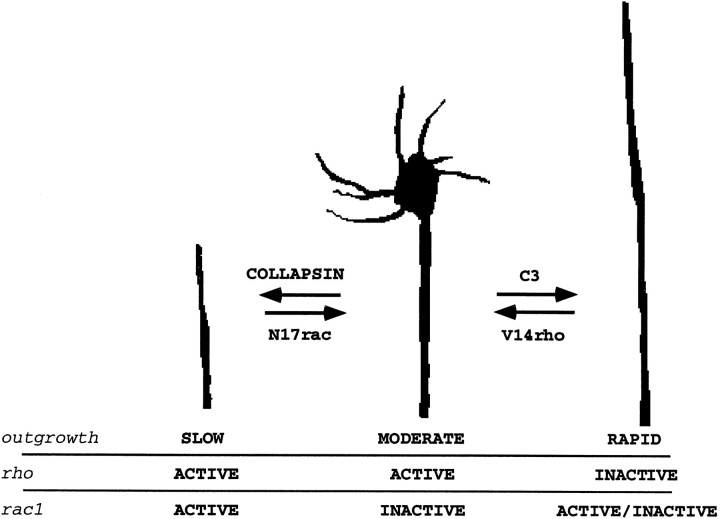
Correlation of rho and rac1 activation with three states of DRG growth cone motility
The present study identifies three alternative states for DRG growth cones in culture (Fig. 7). Under basal conditions, growth cones spread and advance at a moderate rate. Collapsin-1 decreases outgrowth rates and collapses growth cone lamellipodia and filopodia. Collapsin-1-induced alterations in growth cone behavior may be mediated by rac1 activation and are blunted by the presence of dominant negative N17rac. In contrast, the inhibition of rho function by C3 transferase increases outgrowth rate and decreases growth cone area. The basal state seems to be correlated with rho activation and rac1 inactivity. Confirmation of this model will require methods to monitor the activation state of both rac1 and rho within DRG growth cones.
Fig. 7.
Model for rho and rac1 regulation of DRG growth cone function. Three states for DRG growth cones are classified by morphological appearance, neurite outgrowth rate, rho activation state, and rac1 activation. See Discussion for details.
Mechanism of rac1 activation: dbl proteins, G-protein cascade, and CRMP
The mechanism by which rac1 in neurons might be activated by extracellular collapsin-1 is unclear. In other cell types, proteins with domains homologous to the human dbl protein act upstream of rac1 as guanine nucleotide exchange factors (Boguski and McCormick, 1993), but the presence of these proteins in neuronal growth cones has not been studied. Receptors of several classes seem to be capable of activating rac1 in other cells, including receptor tyrosine kinases, serpentine receptors coupled to heterotrimeric G-proteins, and cytokine receptors of the tumor necrosis factor class. A central role for heterotrimeric G-proteins in growth cone signal transduction is supported by a number of studies (Strittmatter et al., 1990, 1993,1994a, 1995). Data presented here indicate that heterotrimeric G-proteins (Fig. 1B) may be involved in collapsin-1 signaling. We have identified an intracellular family of neuronal proteins, CRMPs, that are required for collapsin action, but their interaction with other members of this signaling pathway is not established (Goshima et al., 1995; Wang and Strittmatter, 1996). There are no data indicating that intracellular Ca2+levels are likely to mediate collapsin action. Identification of a collapsin-binding receptor will facilitate greatly further delineation of this pathway.
Rac1 effectors in DRG neurons
Rac1 is capable of reorganizing the actin-based cytoskeleton in non-neuronal cells and of activating a number of protein kinases (Hall, 1994; Coso et al., 1995; Minden et al., 1995; Nobes and Hall, 1995). Collapsin-1-induced changes in cell shape may be mediated by protein kinases such as PAK (Manser et al., 1994). After activation by rac1, such kinases are hypothesized to modulate cytoskeletal function. The recent advances in the understanding of the effects of rho on non-neuronal cell shape (Kimura et al., 1996) predict that similar experiments will be feasible for rac1 in developing neurons.
Footnotes
This work was supported by grants to S.M.S. from National Institutes of Health and from the Spinal Cord Research Fund of the Paralyzed Veterans of America. S.M.S. is a John Merck Scholar in the Biology of Developmental Disorders in Children. We thank A. Hall for the G-protein expression plasmids.
Correspondence should be addressed to Dr. Stephen M. Strittmatter, Departments of Neurology and Neurobiology, Yale University School of Medicine, P.O. Box 208018, New Haven, CT 06520.
REFERENCES
- 1.Adams RH, Betz H, Puschel AW. A novel class of murine semaphorins with homology to thrombospondin is differentially expressed during early embryogenesis. Mech Dev. 1996;57:33–45. doi: 10.1016/0925-4773(96)00525-4. [DOI] [PubMed] [Google Scholar]
- 2.Bandtlow CE, Schimdt MF, Hassinger TD, Schwab ME, Kater SB. Role of intracellular calcium in NI-35-evoked collapse of neuronla growth cones. Science. 1993;259:80–83. doi: 10.1126/science.8418499. [DOI] [PubMed] [Google Scholar]
- 3.Boguski MS, McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366:643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- 4.Coso OA, Chiariello M, Yu JC, Teramoto H, Crespo P, Xu N, Miki T, Gutkind JS. The small GTP-binding proteins rac1 and cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 5.Fan J, Mansfield SG, Redmond T, Gordon-Weeks PR, Raper JA. The organization of F-actin and microtubules in growth cones exposed to a brain-derived collapsing factor. J Cell Biol. 1993;121:867–878. doi: 10.1083/jcb.121.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goshima Y, Nakamura F, Strittmatter P, Strittmatter SM. Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature. 1995;376:509–514. doi: 10.1038/376509a0. [DOI] [PubMed] [Google Scholar]
- 7.Hall A. The cellular functions of small GTP-binding proteins. Science. 1990;249:635–640. doi: 10.1126/science.2116664. [DOI] [PubMed] [Google Scholar]
- 8.Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- 9.Igarashi M, Strittmatter SM, Vartanian T, Fishman MC. G Protein mediation of signals that cause growth cone collapse. Science. 1993;259:77–79. doi: 10.1126/science.8418498. [DOI] [PubMed] [Google Scholar]
- 10.Inagaki S, Furuyama T, Iwahashi Y. Identification of a member of the mouse semaphorin family. FEBS Lett. 1995;370:269–272. doi: 10.1016/0014-5793(95)00850-9. [DOI] [PubMed] [Google Scholar]
- 11.Jalink K, Moolenaar WH. Thrombin receptor activation causes rapid neural cell rounding and neurite retraction independent of classic second messengers. J Cell Biol. 1992;118:411–419. doi: 10.1083/jcb.118.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jalink K, van Corven EJ, Hengeveld T, Morii N, Narumiya S, Moolenaar WH. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein rho. J Cell Biol. 1994;126:801–810. doi: 10.1083/jcb.126.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimura K, Ito M, Amano M, Chicharo K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Naano T, Okawa K, Iwamatsu A, Kaibichi K. Regulation of myosin phosphatase by rho and rho-associated kinase (rho-kinase). Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 14.Kindt RM, Lander AD. Pertussis toxin specifically inhibits growth cone guidance by a mechanism independent of direct G protein inactivation. Neuron. 1995;15:79–88. doi: 10.1016/0896-6273(95)90066-7. [DOI] [PubMed] [Google Scholar]
- 15.Kolodkin AL, Matthes DJ, O’Connor TP, Patel NH, Admon A, Bentley D, Goodman CS. Fasciclin IV: sequence, expression, and function during growth cone guidance in the grasshopper embryo. Neuron. 1992;9:831–845. doi: 10.1016/0896-6273(92)90237-8. [DOI] [PubMed] [Google Scholar]
- 16.Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75:1389–1399. doi: 10.1016/0092-8674(93)90625-z. [DOI] [PubMed] [Google Scholar]
- 17.Luo L, Liao YJ, Jan LY, Jan YN. Distinct morphogenetic functions of similar small GTPases: Drosophila drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 1994;8:1787–1802. doi: 10.1101/gad.8.15.1787. [DOI] [PubMed] [Google Scholar]
- 18.Luo L, Hensch TK, Ackerman L, Barbel S, Jan LY, Jan YN. Differential effects of the rac1 GTPase on Purkinje cell axons and dendritic trunks and spines. Nature. 1996;379:837–840. doi: 10.1038/379837a0. [DOI] [PubMed] [Google Scholar]
- 19.Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75:217–227. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- 20.Luo Y, Shepherd I, Li J, Renzi MJ, Chang S, Raper JA. A family of molecules related to collapsin in the embryonic chick nervous system. Neuron. 1995;14:1131–1140. doi: 10.1016/0896-6273(95)90261-9. [DOI] [PubMed] [Google Scholar]
- 21.Manser E, Leung T, Salihuddin H, Zhao Z, Lim L. A brain serine–threonine protein kinase activated by cdc42 and rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 22.Matthes DJ, Sink H, Kolodkin AL, Goodman CS. Semaphorin II can function as a selective inhibitor of specific synaptic arborizations. Cell. 1995;81:631–639. doi: 10.1016/0092-8674(95)90084-5. [DOI] [PubMed] [Google Scholar]
- 23.Messersmith EK, Leonardo ED, Shatz CJ, Tessier-Lavigne M, Goodman CS, Kolodkin AL. Semaphorin III can function as a selective chemorepellent to pattern projections in the spinal cord. Neuron. 1995;14:949–959. doi: 10.1016/0896-6273(95)90333-x. [DOI] [PubMed] [Google Scholar]
- 24.Minden A, Lin A, Claret FX, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases rac1 and cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 25.Nobes CD, Hall A. Rho, rac1, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 26.Puschel AW, Adams RH, Betz H. Murine semaphorin D/collapsin is a member of a diverse gene family and creates domains inhibitory for axonal extension. Neuron. 1995;14:941–948. doi: 10.1016/0896-6273(95)90332-1. [DOI] [PubMed] [Google Scholar]
- 27.Raper JA, Kapfhammer P. The enrichment of a neuronal growth cone collapsing activity from embryonic chick brain. Neuron. 1990;2:21–29. doi: 10.1016/0896-6273(90)90440-q. [DOI] [PubMed] [Google Scholar]
- 28.Strittmatter SM. Neuronal guidance molecules: inhibitory and soluble factors. Neuroscientist. 1995;1:255–258. [Google Scholar]
- 29.Strittmatter SM, Valenzuela D, Kennedy TE, Neer EJ, Fishman MC. Go is a major growth cone protein subject to regulation by GAP-43. Nature. 1990;344:836–841. doi: 10.1038/344836a0. [DOI] [PubMed] [Google Scholar]
- 30.Strittmatter SM, Cannon SC, Ross EM, Higashijima T, Fishman MC. GAP-43 augments G protein-coupled receptor transduction in X. laevis oocytes. Proc Natl Acad Sci USA. 1993;90:5327–5331. doi: 10.1073/pnas.90.11.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strittmatter SM, Fishman MC, Zhu X-P. Activated mutants of the α subunit of Go promote an increased number of neurites per cell. J Neurosci. 1994a;14:2327–2338. doi: 10.1523/JNEUROSCI.14-04-02327.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strittmatter SM, Igarashi M, Fishman MC. GAP-43 peptides modulate growth cone morphology and neurite outgrowth. J Neurosci. 1994b;14:5501–5513. doi: 10.1523/JNEUROSCI.14-09-05503.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strittmatter SM, Frankhauser C, Huang PL, Mashimo H, Fishman MC. Neuronal pathfinding is abnormal in mice lacking the neuronal growth cone protein GAP-43. Cell. 1995;80:445–452. doi: 10.1016/0092-8674(95)90495-6. [DOI] [PubMed] [Google Scholar]
- 34.Wang LH, Strittmatter SM. A family of rat CRMP genes is differentially expressed in the nervous system. J Neurosci. 1996;16:6197–6207. doi: 10.1523/JNEUROSCI.16-19-06197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]