Abstract
Numerous G-protein-modulated ionic conductances are present in central neurons and play major roles in regulating neuronal excitability. Accordingly, endogenous factors that alter the operation of these conductances may have profound effects on neuronal function. We now report that several G-protein-modulated ionic conductances in hippocampal neurons are very much altered when Cl−is the predominant anion in the recording electrode. We used both sharp-electrode and whole-cell techniques in rat hippocampal slices to determine whether hippocampal CA1 pyramidal cell properties are altered by KCl-filled, as compared with KCH3SO3- or K-gluconate-filled, electrodes. We studied the effects of the anions on synaptically evoked GABAB responses and baclofen- and serotonin-induced currents as well as on a voltage-activated cation current, Ih. High intracellular concentrations of chloride ([Cl−]i) depressed all the responses without altering resting cell properties. Intermediate [Cl−]i reduced baclofen-induced currents as well as Ih in a dose-dependent manner. In KCH3SO3-filled cells, equimolar substitution of GTPγS for Tris-GTP results in activation of a K+ conductance that hyperpolarizes cells and lowers their input resistance. These effects of GTPγS were blocked in KCl-filled cells. In view of the tight coupling between the G-protein and activation of the GABAB-activated K+conductance, the effect of Cl− ions is likely to be exerted either on the G-protein or the K+ channel itself. We observed substantial effects of Cli− at concentrations that are believed to exist during development in the CNS as well as during pathological conditions, such as spreading depression. Thus, the results we describe must be taken into consideration during such physiological and pathological conditions as well as in experimental studies of G-protein-modulated conductances.
Keywords: GABAB, baclofen, serotonin, Ih, spreading depression, anions
We have noticed that large GABAB responses are rare in CA1 pyramidal cells when KCl is the major constituent in the recording electrode solution (compare with Fig. 1 in Pitler and Alger, 1992; Pham and Lacaille, 1996). Chloride-dependent GABAA responses are reversed and very large when intracellular chloride concentration ([Cl−]i) is high, so it appears that the GABAB response is reduced selectively. Although several explanations are conceivable, it could be that high [Cl−]i affects GABABresponses. However, no thorough study of this issue has been performed.
Intracellular recording techniques offer many advantages for the study of neuronal function. However, it has been known since the earliest studies using intracellular techniques (Coombs et al., 1955) that the ions present in the electrolyte solution in the intracellular electrode diffuse into the cell being studied and can affect cellular properties. Whole-cell voltage clamp is a very powerful and widely used technique that has many advantages over traditional intracellular recording. Access to, and control over, the internal milieu as well as improved clamp control are major advantages of large-bore patch pipettes over traditional high-resistance intracellular electrodes. However, alterations of normal cellular constituents can compromise cellular functioning drastically. Classic studies performed on the squid giant axon established early on the variable ability of different anions to restore action potential amplitude (Tasaki et al., 1965). Although often overlooked, high intracellular concentrations of anions (Cl−, F−, gluconate−, et cetera) can alter various electrophysiological characteristics of excitable cells (Baker et al., 1962; Adams and Oxford, 1983; Nakajima et al., 1992; Zhang et al., 1994).
Because the normal intracellular concentration of Cl− is ∼8 mm (McCormick, 1990) and Cl−-based patch electrode solutions often contain ∼150 mm Cl−, it is quite possible that these abnormally high concentrations could affect the cell adversely. High intracellular concentrations of KCl ([KCl]i) can modify G-proteins (Nakajima et al., 1992) and K+ channels (Adams and Oxford, 1983). Because these studies were performed on cardiac atrial cells and the squid giant axon, respectively, and used very high (≥400 mm) [Cl−]i, we wanted to determine whether KCl affected mammalian central neurons at concentrations that commonly are used in patch pipette solutions. Zhang et al. (1994) reported that certain anions attenuate the slow Ca2+-dependent K+ conductance in hippocampal neurons but that this could be explained by an effect on intracellular Ca2+ handling. If high [Cl−]i does affect G-protein-linked responses, such as those mediated by GABAB receptor activation, then conditions in which [Cl−]i is high, such as during development, and during pathological conditions, such as spreading depression (Lux et al., 1986), will affect those responses. We have undertaken the present experiments to determine whether hippocampal CA1 pyramidal cell properties are altered by KCl-filled, as compared with KCH3SO3- or K-gluconate-filled, electrodes. Our results support the hypothesis that [Cl−]i attenuates in a dose-dependent manner both GABAB- and serotonin-mediated currents in CA1 neurons as well as a voltage-activated cation current,Ih. Moreover, the [Cl−]i effects are exerted at the level of the G-protein-linked pathway.
A preliminary report of this work has appeared in abstract form (Lenz et al., 1994).
MATERIALS AND METHODS
Preparation of slices. Adult male Sprague Dawley rats (125–300 gm, 30–60 d) were anesthetized deeply with halothane and decapitated. Both hippocampi were removed and placed on agar blocks in a slicing chamber containing oxygenated, partially frozen saline. A Vibratome (Technical Products International) was used to cut transverse slices at 400 μm intervals. Slices were transferred to a holding chamber where they were maintained at the interface of physiological saline and humidified 95% O2/5% CO2atmosphere at room temperature. Slices were allowed at least 1 hr to recover before being transferred to a submerged perfusion-type chamber (Nicoll and Alger, 1981) where they were perfused with saline (29–31°C) at 0.5–1 ml/min.
Solutions. The bath solution contained the following (in mm): 124 NaCl, 25 NaHCO3, 3.5 KCl, 2.5 CaCl2, 2 MgSO4 or 2 MgCl2,1.25 NaHPO4, and 10 glucose. When monosynaptic GABAB responses were studied, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 20 μm), 2-amino-5-phosphonovaleric acid (APV; 50 μm), and bicuculline (20 μm) were present in the saline to block ionotropic glutamate- and GABAA-mediated responses, respectively. CGP 35348 (1 mm) was used in some experiments to antagonize GABAB receptors. This concentration blocked the synaptic GABAB response completely as well as that mediated by a 2 min bath application of baclofen (5 μm). Serotonin (10 μm) was bath-applied for 2 min.
Whole-cell patch electrodes had resistances of 3–6 MΩ and were filled with one of three solutions (in mm): (1) 150–160 KCH3SO3, 10 HEPES, 2 BAPTA, 0.2 CaCl2, 1 MgATP, 1 MgCl2, and 0.3 Tris-GTP, pH 7.25; (2) 150–160 KCl, 10 HEPES, 2 BAPTA, 0.2 CaCl2, 1 MgATP, 1 MgCl2, and 0.3 Tris-GTP, pH 7.25; (3) 150 KC6H11O7(K-gluconate), 10 KCl, 10 HEPES, 2 BAPTA, 0.2 CaCl2, 1 MgATP, and 0.3 Tris-GTP, pH 7.25. For the experiments performed with intermediate [Cl−]i (see Fig. 5), the electrode solution contained either 45 KCl and 120 KCH3SO3 or 65 KCl and 100 KCH3SO3 with 10 HEPES, 2 BAPTA, 0.2 CaCl2, 1 MgATP, 1 MgCl2, and 0.3 Tris-GTP, pH 7.25. In a few experiments, as noted, the nonhydrolyzable analog of GTP, GTPγS (0.3 mm), was substituted for Tris-GTP. Intracellular recordings also were performed with sharp electrodes having resistances of 40–100 MΩ and filled with either 3m KCl or 2 mKCH3SO3.
Fig. 5.
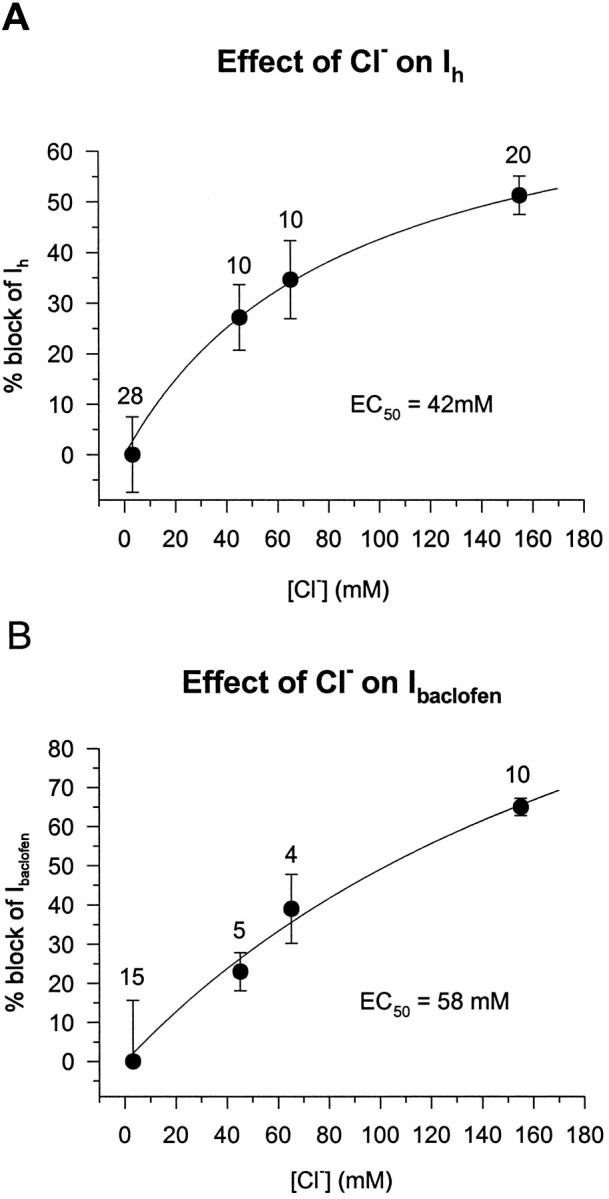
Intermediate concentrations of Cli− reduce the baclofen-induced current and Ih in a dose-dependent manner.A, Ih was elicited by a 1 sec, 20 mV hyperpolarizing voltage step from a holding potential between −55 and −58 mV. Ih was measured from cells recorded with electrodes containing 3, 45, 65, and 155 mm Cl−. The current obtained from cells filled with 3 mm Cl− was designated 0% control, and the data were normalized to this. The dose–response curve was obtained by fitting the data with a computer-generated best-fit equation of the form: % block = % max. block · [KCl]/(EC50 + [KCl]). There was a 51% block ofIh at 155 mmCl− and an EC50 of 42 mm. The numbers of cells are indicated abovethe mean values. B, A 2 min bath application of 5 μm baclofen to cells filled with the same [Cl−]i as in Aproduced a similar dose–response curve. The curve was obtained as inA. Cl− (155 mm) produced a 65% block of the baclofen response with an EC50 of 58 mm.
CNQX was purchased from Research Biochemicals International (Natick, MA), and BAPTA was purchased from Molecular Probes (Eugene, OR). CGP 35348 was a generous gift from CIBA-Geigy (Basel, Switzerland). All other drugs and chemicals were obtained from Sigma Chemical (St. Louis, MO).
Whole-cell and intracellular recordings and data analysis.CA1 pyramidal cell recordings were obtained either with conventional intracellular or the “blind” whole-cell patch-clamp recording technique (Blanton et al., 1989). Cells obtained with the whole-cell technique were voltage-clamped near their resting potential soon after break-in. Acceptable cells had resting potentials equal to or greater than −55 mV and input resistances >35 MΩ (except those cells recorded with GTPγS; see below). Series resistance was <12 MΩ at the beginning of an experiment and was compensated by 60–70%. Cells were discarded if series resistance increased to >30 MΩ during an experiment. Bipolar concentric stimulating electrodes (Rhodes Electronics) were positioned in stratum radiatum (s. radiatum) to allow orthodromic activation of CA1 pyramidal cells. Liquid junction potentials between the three intracellular solutions and the extracellular solution were measured according to the method of Neher (1992). These junction potentials were small—KCl (3 mV), KCH3SO3 (4 mV), K-gluconate (11 mV)—and were not corrected for.
An Axoclamp-2 (Axon Instruments, Foster City, CA) was used for all experiments. Evoked synaptic currents or potentials were elicited at 0.2 Hz and were filtered at 2 kHz with an eight-pole Bessel filter (Frequency Devices, Haverhill, MA) and digitized at 5 kHz by a Digidata 1200 analog-to-digital converter (Axon Instruments). Data also were stored on a VCR-based tape recorder system (Neuro-corder DR-484, Neuro Data Instruments) and played into a computer for off-line analysis with pCLAMP 6.0 software (Axon Instruments). The effects of the three intracellular solutions on various responses were assessed by one-way ANOVA, followed by unpaired Student’s t tests (SigmaStat, Jandel Scientific, Corte Madera, CA). The significance level chosen wasp < 0.05, and all data are reported as mean ± SEM.
RESULTS
When evoking synaptic responses in hippocampal CA1 neurons, we often observed that GABAB-mediated IPSPs were small or nonexistent when recorded with KCl-filled electrodes. To test the hypothesis of an interaction between high [Cl−]i and GABAB-mediated responses, we began by examining synaptically evoked GABABIPSPs under different recording conditions.
High intracellular chloride ([Cl−]i) inhibits synaptic GABAB responses
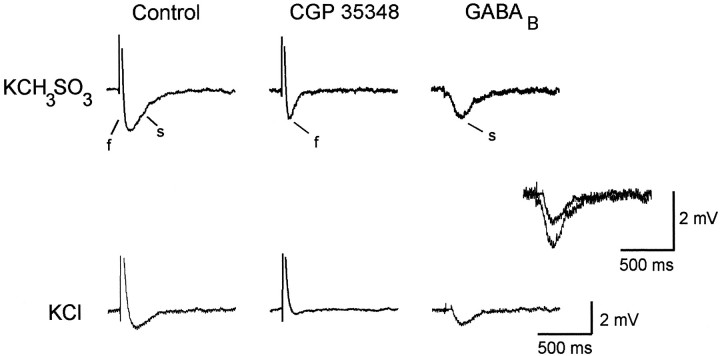
In cells recorded with KCH3SO3-filled high-resistance intracellular electrodes, a multiphasic synaptic response is reliably observable when stimulation is given in s. radiatum (Fig. 1; Davies et al., 1990). To prevent the occurrence of an afterhyperpolarization (AHP), which might contaminate the synaptic response, we used stimulus intensities that produced EPSPs that were just subthreshold for action potential initiation in the recorded cell. The initial depolarizing potential (truncated) is the CNQX-sensitive EPSP, which is followed immediately by a rapidly rising GABAA-mediated IPSP (labeledf for fast). The prolonged hyperpolarization (labeleds for slow) is mediated by the activation of GABAB receptors and can be blocked by the GABABreceptor antagonist CGP 35348 (Dutar and Nicoll, 1988; Olpe and Karlsson, 1990). The response in CGP 35348 (middle traces) consists of the EPSP, followed by the GABAA IPSP. Subtraction of the synaptic response obtained in CGP 35348 (middle traces) from the response recorded in control saline (left-hand column) reveals the GABAB-mediated IPSP in isolation (right-hand column). The slow IPSP had a latency to peak of 190 msec and was blocked by 1 mm CGP 35348 (middle trace), thereby confirming that it is a GABAB-mediated response.
Fig. 1.
Synaptically evoked GABAB responses are attenuated in cells filled with KCl. While recording intracellularly with high-resistance electrodes in CA1 pyramidal cells, we elicited synaptic responses by electrical stimulation in the CA1 s. radiatum. The synaptic response recorded from a KCH3SO3-filled cell consists of a depolarizing EPSP, followed by an IPSP with an initial rapid rise and slow phase (left trace). The initial rapid phase of the IPSP (f) is mediated by activation of GABAA receptors and can be blocked by bicuculline (not shown). The slow phase of the IPSP (s) is mediated by activation of GABAB receptors and is blocked by 1 mm CGP 35348 (middle trace). Theright trace is a subtraction of the CGP 35348 trace from the control trace and represents the GABAB-mediated slow IPSP in isolation. The EPSPs recorded during control and in the presence of CGP 35348 are 12 mV in amplitude and were truncated for display purposes. In the KCl-filled cell (bottom traces), the GABAA-mediated IPSP is depolarizing, as is the EPSP, which together result in a 10 mV depolarization in control and in the presence of CGP 35348. Subtracted traces illustrating the GABAB-mediated response are superimposed and expanded to demonstrate that the GABAB response in KCl-filled cells is smaller than in KCH3SO3-filled cells. Resting membrane potentials were −62 mV in the KCH3SO3-filled cell and −60 mV in the KCl-filled cell. Similar results were seen in three other KCH3SO3-filled and three other KCl-filled cells.
When the recording electrode contained 3 m KCl, the GABAB component of the synaptic response was attenuated (Fig. 1, bottom traces). The GABAA-mediated fast IPSP is depolarizing in KCl-filled cells because the normal inward driving force for Cl− is reversed in these cells. Addition of 1 mm CGP 35348 (middle trace) blocked the small GABAB-mediated slow IPSP. The subtracted traces obtained from both KCl-filled and KCH3SO3-filled cells are expanded and superimposed to demonstrate that the CGP-35348-sensitive component in the KCl-filled cell is clearly smaller than that recorded in the KCH3SO3-filled cell. The KCl-filled cell illustrated in Figure 1 displayed the largest GABABresponse of the four KCl-filled cells that we examined in this way.
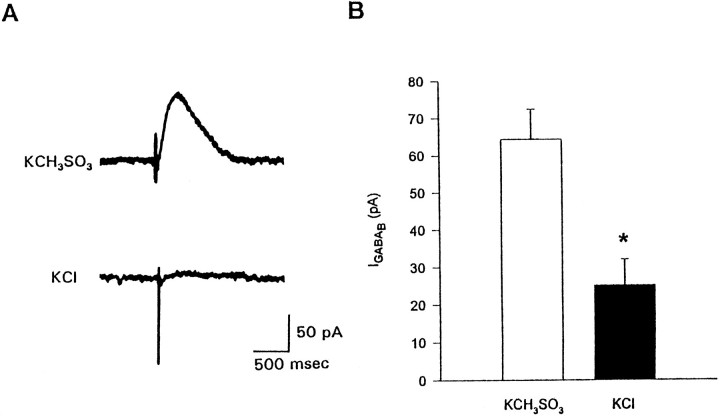
Because these initial experiments were performed with high-resistance (40–100 MΩ) intracellular electrodes and because the diffusion of small molecules from such electrodes is linearly related to the access resistance, the diffusion of the electrode solution into the cell might have been incomplete (Pusch and Neher, 1988). Moreover, cells could be voltage-clamped more effectively with low-resistance electrodes. Therefore, we used the whole-cell patch-clamp technique to insure maximal dialysis of the neuron with the electrode solution and to improve clamp control. To isolate the evoked GABABresponse, we added CNQX (20 μm), APV (50 μm), and bicuculline (20 μm) to the bathing solution to block ionotropic glutamate and GABAA receptors, respectively. Orthodromic activation of CA1 neurons was achieved by a stimulating electrode placed within s. radiatum on the CA3 side of the recording electrode, but no more than 0.5 mm from it. For each cell the maximum GABAB response was obtained by stimulating at intensities up to 800 μA for 70 μsec or until further increases in intensity did not result in an increased current. Each cell was voltage-clamped at −55 mV to minimize contributions of different driving forces to the magnitude of the synaptic current. Low-frequency (0.2 Hz) high-intensity stimulation invariably elicited a monosynaptic GABAB response when we recorded from a KCH3SO3-filled cell (Fig.2A; Davies et al., 1990). This synaptic current displayed paired-pulse depression, was occluded by baclofen application, and was blocked completely by 1 mm CGP 35348, thus indicating it was a GABAB-mediated current (data not shown). However, when KCl was the main electrolyte in the electrode solution, the monosynaptic GABAB responses were much smaller. The mean maximum monosynaptic GABAB IPSC from seven KCl-filled cells (25.3 ± 6.9 pA) was significantly less than the mean response from eight KCH3SO3-filled cells (64.4 ± 8.1 pA) (Fig. 2B; p < 0.005).
Fig. 2.
Monosynaptically evoked GABABresponses recorded under whole-cell voltage clamp are greatly reduced in cells containing high intracellular [Cl−].A, Monosynaptic GABAB responses were elicited by electrical stimulation in s. radiatum in the presence of 20 μm CNQX, 50 μm APV, and 20 μmbicuculline. Traces are from two cells, one recorded with a patch electrode solution containing 155 mmKCH3SO3 (open bar) and the other with a solution containing 155 mm KCl (filled bar). B, Bar graph showing that the average peak monosynaptic GABAB response recorded from KCl-filled cells (25.3 ± 6.9 pA, n = 7) is significantly smaller than from KCH3SO3-filled cells (64.4 ± 8.1 pA, n = 8; p< 0.005).
Baclofen and serotonin responses are reduced in KCl-filled cells
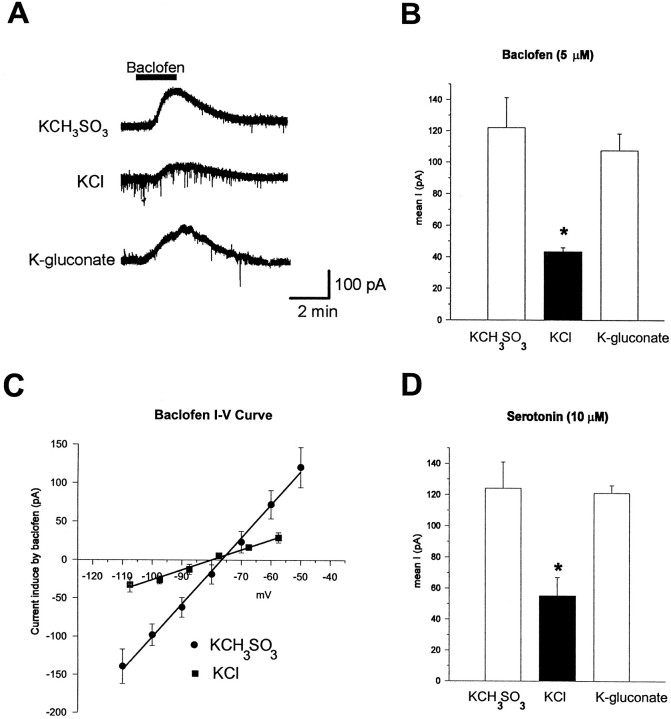
To determine whether high [Cl−]iaffected responses mediated by extrasynaptic as well as synaptic GABAB receptors and to insure that we were activating maximal numbers of GABAB receptors in all cells, we bath-applied baclofen for brief periods. Bath application of baclofen directly hyperpolarizes cells by activating GABAB receptors (Newberry and Nicoll, 1984), which activate an outward current carried by K+ ions (Gahwiler and Brown, 1985). Figure3A illustrates that a 2 min bath application of 5 μm baclofen causes a large outward current in KCH3SO3-filled cells. However, the same application of baclofen to a KCl-filled cell voltage-clamped at the same resting potential (−60 mV) results in a substantially smaller current. As is shown in Figure 3B, the mean baclofen current measured from KCl-filled cells (43 ± 2.7 pA, n = 10) is significantly less than that measured in KCH3SO3-filled cells (122 ± 19.2 pA,n = 15; p < 0.005). These results confirm that GABAB responses are smaller in KCl-filled cells. We determined the conductance of the baclofen response by a series of 200 msec voltage steps between −50 and −110 mV before and during the peak baclofen response. Subtraction of the conductance obtained during the control period from the conductance during the peak baclofen response gave the baclofen conductance. As shown in Figure3C, baclofen conductance in KCl-filled cells was significantly smaller (1.5 ± 0.18 nS, n = 4) than the baclofen conductance measured in KCH3SO3-filled cells (4.2 ± 0.49 nS,n = 5; p < 0.005). There was no significant difference in the reversal potentials of the baclofen currents of KCl-filled, as compared with KCH3SO3-filled, cells.
Fig. 3.
Baclofen and serotonin responses are reduced in KCl-filled cells. A, Traces of outward currents elicited by a 2 min bath application of 5 μm baclofen recorded under whole-cell voltage clamp. The three traces are from three separate cells recorded with intracellular solutions, based on three different salts: KCH3SO3 (155 mm), KCl (155 mm), and K-gluconate (150 mm). All cells were voltage-clamped between −58 and −60 mV. Downward deflections in the KCl trace are spontaneous IPSCs. B, Group data showing peak baclofen responses recorded with the three different intracellular solutions. Baclofen responses recorded in KCl-filled cells (filled bar) are significantly reduced, as compared with those in either KCH3SO3-filled (open bar) or K-gluconate-filled (open bar) cells (p < 0.005), whereas responses in KCH3SO3-filled and K-gluconate-filled cells were not different (p = 0.62).C, Baclofen I–V plot for four KCl-filled and five KCH3SO3-filled cells. The line is fit to the data points by linear regression analysis. Baclofen conductance was obtained by averaging the slopes of the linear portions of theI–V plots from each cell. D, Bath application of 10 μm serotonin for 2 min elicited an outward current similar in amplitude and duration to baclofen, which was greatly reduced in Cl−-filled cells. Serotonin responses from four KCl-filled cells are significantly less than those measured in five KCH3SO3-filled and six K-gluconate-filled cells (p < 0.02). Serotonin responses from KCH3SO3-filled and K-gluconate-filled cells were not different (p > 0.7).
To determine whether high [Cl−]i was responsible for the decreased GABAB-mediated currents, we repeated the baclofen application to cells filled with a K-gluconate-based intracellular solution. Baclofen responses measured from K-gluconate-filled cells (107 ± 10.8 pA, n = 7) were not statistically different from those measured from KCH3SO3-filled cells (p> 0.5). However, the mean baclofen response in KCl-filled cells was significantly smaller than the response obtained from cells filled with K-gluconate (p < 0.005). Thus it appears that the Cl− ion per se causes the decrease in GABAB-receptor-mediated responses.
The reduced GABAB response recorded from cells with high [Cl−]i could be produced by Cl− acting at any one of several sites within the cell. The chloride ions could interact with the GABABreceptor specifically, which could result in a decreased ability of an agonist to activate the receptor, or they could interact with the G-protein or the K+ channel to which the receptor is coupled. To address the possibility that high [Cl−]i interacts specifically with the GABAB receptor, we briefly bath-applied (2 min) 10 μm serotonin (5-HT) to cells filled with the different electrode solutions. GABAB and 5-HT1a receptors appear to be coupled via G-proteins to the same K+channel (Andrade et al., 1986). If the effects of Cl− are specific to the GABAB-receptor-mediated response, then the outward current elicited by activation of serotonin receptors should be of similar magnitude irrespective of the electrode solution. As illustrated in Figure 3D, this is not the case. Serotonin produced a similar current in both KCH3SO3-filled (121 ± 4.8 pA, n = 6) and K-gluconate-filled (124 ± 16.9 pA, n = 5; p > 0.5) cells, whereas in KCl-filled cells the mean 5-HT response was reduced significantly (55 ± 11.9 pA, n = 4;p < 0.05). These data support the idea that high [Cl−]i mediates its effects, not via specific interaction with the GABAB receptor per se, but rather via interaction either with the G-protein involved in coupling the receptors to the K+ channel or with the K+ channel itself.
Ih is greatly reduced in Cl−-filled cells
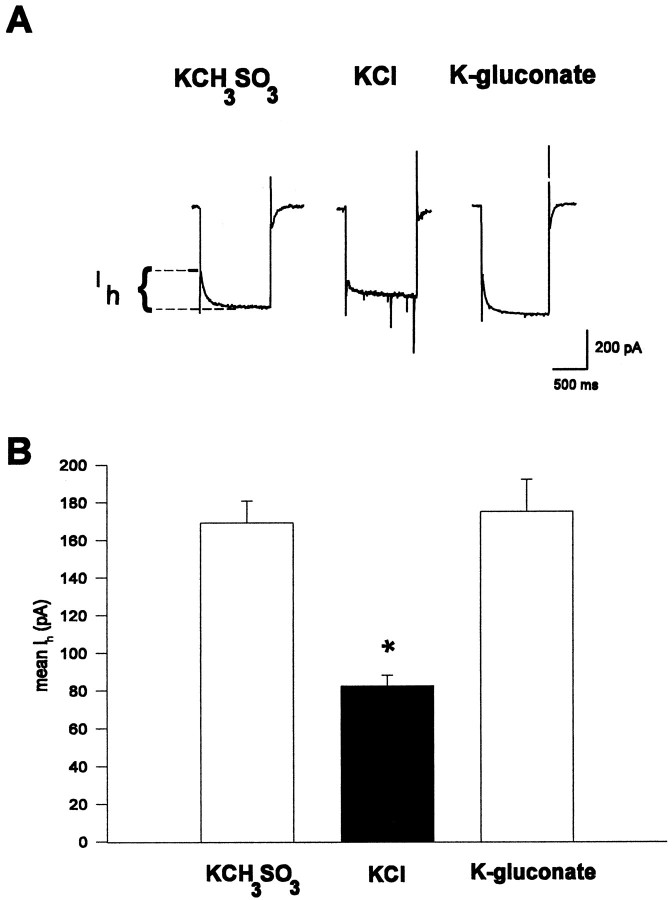
To determine whether high [Cl−]iaffects currents other than those mediated by neurotransmitter receptors, such as GABAB and 5-HT, we investigated the effects of various intracellular solutions on the hippocampalIh. The Ih is a hyperpolarization-activated inward cationic current found in hippocampal CA1 pyramidal cells (Halliwell and Adams, 1982; Maccaferri et al., 1993). This slowly activating current is thought to be mediated by a nonspecific, monovalent cationic conductance and is highly regulated by numerous neurotransmitters that act via G-proteins (Bobker and Williams, 1989; Jiang et al., 1993; Maccaferri and McBain, 1996). Figure4A illustrates that a 20 mV, 1 sec hyperpolarizing voltage step from −60 mV given ∼5 min after break-in produces an inwardly relaxing current associated with a membrane conductance increase, Ih, that is greatly reduced in cells with high [Cl−]i. The group data in Figure4B demonstrate that the Ihmeasured in KCH3SO3- and K-gluconate-filled cells did not differ (KCH3SO3: 169.2 ± 11.2 pA, n = 28; K-gluconate: 175.0 ± 17.1 pA,n = 9; p > 0.7), whereas theIh from KCl-filled cells was significantly smaller than either (82.3 ± 5.7 pA, n = 20;p < 0.001).
Fig. 4.
Ih is reduced in cells with high [Cl−]i. A,Ih was elicited by giving a 1 sec, 20 mV hyperpolarizing voltage step from rest shortly after breaking into the cell. Magnitudes of Ih recorded from three different cells with whole-cell patch electrodes filled with three different solutions are displayed as the slowly activating inward current. Ih from the illustrated traces are KCH3SO3, 160 pA; KCl, 60 pA; and K-gluconate, 180 pA. B, Group data showing the meanIh from cells filled with KCH3SO3 (n = 28), KCl (n = 20), and K-gluconate (n = 9). The Ih measured in Cl−-filled cells is significantly smaller than that measured in either the KCH3SO3-filled or K-gluconate-filled cells (p < 0.0001). All cells were voltage-clamped between −55 and −58 mV.
To determine whether high [Cl−]ireduced the maximal Ih or whether it shifted the voltage dependence of activation to more negative potentials, we maximally activated Ih by giving a series of 2 sec hyperpolarizing voltage steps from −57 to −117 mV in 10 mV increments (data not shown). The conductance of theIh between −117 and −67 mV was determined from linear regression of the slope of the linear portion of the current versus voltage (I–V) plot. The conductance ofIh measured from KCl-filled cells was significantly smaller (5.89 ± 0.84 nS, n = 7) than that measured in KCH3SO3-filled cells (17.2 ± 1.5 nS, n = 7; p < 0.0005). Furthermore, linear extrapolation of the averaged data in theI–V plot from both KCl-filled and KCH3SO3-filled cells intersected the ordinate at the same voltage, indicating that the voltage dependence of activation was not changed. Together these indicate that high [Cl−]i reduced the maximalIh.
Because it was apparent that high concentrations of Cl− were quite effective at reducing both GABAB-mediated current and the G-protein-modulatedIh, we wanted to determine the effects of intermediate [Cl−]i on these currents. Cells filled with 45 or 65 mm KCl displayed reduced baclofen-induced currents and Ih, as compared with KCH3SO3-filled cells. Figure5 is a graphical representation of these data, which were fit by a computer-generated hyperbolic equation of the form % block = (% max. block)([KCl])/(EC50 + [KCl]). Assuming that maximal block occurred at 155 mmCl− and that no block was present with 0 mm Cl−, the EC50 was 42 mm for block of Ih and was 58 mm Cl− for block of the baclofen-induced current. Thus, [Cl−]i reduces GABAB-mediated currents as well as the voltage-activated, G-protein-modulated Ih in a dose-dependent manner.
Cl− effects on GTPγS
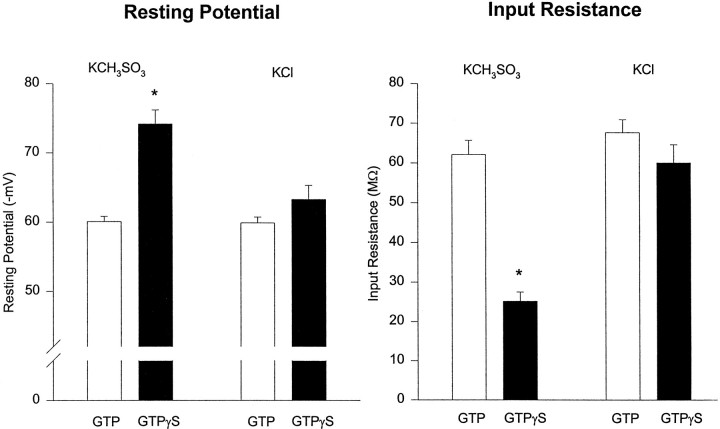
Because the depressant effects of high [Cl−] i were not restricted to a single G-protein-linked neurotransmitter receptor or ion channel type, we considered the possibility that high [Cl−]i might interfere with the G-protein pathway more directly. To do so, we investigated cells to which the hydrolysis-resistant analog of GTP (guanosine 5′-O-13-thiotriphosphate, GTPγS), an activator of G-proteins, was applied internally. It has been suggested that GTPγS activates the same K+ channels that are activated by both baclofen and 5-HT (Andrade et al., 1986). Indeed, we found that application of either baclofen or 5-HT had no additional effect on cells recorded with GTPγS-filled electrodes (n = 2; data not shown), as expected if the neurotransmitter-linked channels already had been opened by the GTP analog. In agreement with previous reports (Andrade et al., 1986), we observed that, in KCH3SO3-filled cells, equimolar substitution of GTPγS for Tris-GTP resulted in a significantly more negative resting potential (GTP: −60 ± 0.8 mV, n = 21; GTPγS: −74 ± 2.0 mV, n = 5; p < 0.0001) and low input resistance (GTP: 62 ± 3.6 MΩ,n = 24; GTPγS: 25 ± 2.3 MΩ, n= 5; p < 0.0001) (Fig.6). However, we found that, in KCl-filled cells, substitution of GTPγS for Tris-GTP did not result in significant differences in either membrane potential (GTP: −60 ± 0.9 mV, n = 13; GTPγS: −63 ± 2.0 mV,n = 8; p > 0.08) or input resistance (GTP: 67 ± 3.2 MΩ, n = 20; GTPγS: 60 ± 4.6 MΩ, n = 8; p > 0.2). Application of baclofen to KCl-filled cells containing GTPγS produced only a small outward current (30 ± 7.6 pA, n = 3), which decayed approximately three times more slowly than that in Tris-GTP-containing cells. Thus, high [Cl−]i blocks the effects of GTPγS on input resistance and resting membrane potential.
Fig. 6.
High [Cl−]iblocks the effects of GTPγS on input resistance and resting membrane potential. Substituting 300 μm GTPγS for 300 μm Tris-GTP in the whole-cell recording electrode reduces input resistance in, and hyperpolarizes significantly, cells recorded with KCH3SO3 electrodes by activating a K+ conductance (p < 0.0001). Contrariwise, in cells filled with KCl there was no significant difference in either input resistance or resting membrane potential when equimolar GTPγS was substituted for GTP. Additionally, there was no difference in input resistance or resting membrane potential between KCl-filled and KCH3SO3-filled cells recorded with 300 μm Tris-GTP (p > 0.2).
DISCUSSION
The results of this study show that high [Cl−]i significantly reduces G-protein-modulated currents in CA1 neurons. We found that monosynaptic GABAB currents in KCl-filled cells are greatly reduced, as compared with those in KCH3SO3-filled cells. Furthermore, the responses to brief applications of both baclofen and 5-HT were smaller in cells filled with high [Cl−]. Interestingly, the effects of Cl− ions were not limited to neurotransmitter-activated K+ currents. The voltage-dependent, nonspecific cation current,Ih, was reduced as well. Finally, high [Cl−]i blocked the effects of GTPγS on resting membrane potential and input resistance that normally are seen in KCH3SO3-filled cells. We conclude that high [Cl−]i affects cellular properties by interacting with G-protein-modulated ionic conductances.
It is difficult to determine which intracellular site(s) the Cl− ions affect. Because the currents elicited by both baclofen and 5-HT were similarly reduced in KCl-filled cells, as compared with those in KCH3SO3-filled or K-gluconate-filled cells, it is unlikely that Cl−ions interact directly with the GABAB receptor or a unique GABAB-receptor-linked pathway. The observations thatIh was reduced in KCl-filled cells and that high [Cl−]i blocked the effects of GTPγS on a K+ conductance further argue against a unique interaction with the GABAB receptor. This is an important point, because the G-protein activated by the GABABreceptor is coupled very tightly (Andrade et al., 1986) to the inwardly rectifying K+ channel that mediates the GABAB response (Gahwiler and Brown, 1985). The model is that these channels are gated directly by the activated G-protein. If indeed the effects of [Cl−]i occur at a site downstream of the receptor, then it would seem that there are few possible sites of action. Two equally tenable, nonexclusive explanations are that high [Cl−]iinterferes with the normal functioning of either the G-proteins or the membrane channels themselves.
There is precedent for both of these possibilities. Anions affect G-proteins (Higashijima et al., 1987) and G-protein-mediated activation of K+ channels (Nakajima et al., 1992), and in both cases Cl− was the most potent anion tested. Another possibility is that Cl− ions do not affect the G-protein but, rather, interact directly with monovalent cation channels. The possibility that high [Cl−]i can modulate cation channels in the squid axon has been suggested. Adelman et al. (1966) found that sodium currents in squid axons progressively decline when the axon is perfused with high concentrations of KCl, and Cl−ions suppress the amplitude and activation rate of delayed rectifier K+ currents in these axons (Adams and Oxford, 1983). Our results would support an interaction with two separate channels: (1) the K+ channel activated by GTPγS as well as by GABAB and 5-HT receptor activation, and (2) the nonselective cation channel mediating Ih.Velumian et al. (1996) reported that Ih was greatly reduced when internal CH3SO4− was replaced with Cl− or gluconate−. Our findings primarily agree with theirs, although we did not find that K-gluconate depressed Ih. This difference can be explained most easily as a difference in Ca2+ buffering, because Velumian et al. (1996) found that addition of 1–3 mm BAPTA to their internal recording solution could “rehabilitate” the attenuated Ih obtained from K-gluconate-filled cells. Zhang et al. (1994) reported that high [Cl−]i appeared to inhibit the slow voltage-independent, Ca2+-activated K+ AHP in hippocampal cells, although they also suggested that Cl− ions might act simply by disrupting Ca2+ homeostasis.
Our results cannot be explained by secondary effects on Ca2+, because our buffering conditions always included 2 mm BAPTA, and the data constitute good evidence that indeed Cl− can affect G-protein-linked conductances more directly. In view of the number of disparate channel types influenced by Cl−, it is tempting to speculate that Cl− affects some common intermediary, such as the G-protein itself.
GABAB receptor activation underlies many important physiological phenomena, such as synaptic inhibition and paired-pulse depression (Davies et al., 1990; Pitler and Alger, 1994), and it has been shown that GABAB receptor antagonists block LTP induction by certain stimulation protocols (Olpe and Karlsson, 1990;Davies et al., 1991). Ih and variousIh-like currents have been characterized widely in several mammalian nerve preparations (Mayer and Westbrook, 1983;Maccaferri et al., 1993) as well as in cardiac atrial cells [referred to there as If (DiFrancesco et al., 1986)]. This current plays an integral role in the slow rhythmic burst-firing properties of thalamic relay neurons (McCormick and Pape, 1990), in pacemaking the action potential characteristics of O-A interneurons in the hippocampus (Maccaferri and McBain, 1996), and in the pacemaker current of sino–atrial myocytes. Therefore, disruption of these physiological properties by introduction of high [Cl−]i may seriously alter the normal functioning of the cell and obscure correct interpretation of the electrophysiological recordings.
Indeed, there are many examples in which high [Cl−]i is correlated with reduced or absent GABAB responses. Using perforated patch to study the developmental change in the GABAA receptor reversal potential in embryonic and early postnatal rat neocortical cells, Owens et al. (1996) reported that [Cl−]i is high (27–37 mm) at young ages and decreases with development. Luhmann and Prince (1991) found that baclofen-induced responses essentially were absent from newborn rat cortical neurons. Interestingly, both somatic and dendritic GABAB responses matured during the second and third postnatal week, simultaneous with a shift of EGABAA to more hyperpolarized potentials because of decreasing [Cl−]i. Misgeld et al. (1984) found that baclofen produced only slight hyperpolarizations and small conductance increases in granule cells, whereas it elicited large hyperpolarizations accompanied by large conductance increases in CA3 cells. EGABAA was depolarized significantly more in the granule cells than in the CA3 cells, thus implying a higher [Cl−]i in granule cells. It is also possible that the apparent difficulty in observing GABAB-mediated miniature IPSCs (Alger and Nicoll, 1980;Otis and Mody, 1992) is related in part to the use of Cl−-based solutions in these experiments. Furthermore, it is interesting to note that investigators have had difficulty obtaining functional expression of GABABreceptors in Xenopus oocytes, which have high resting [Cl−]i (∼35 mm).
In light of our finding that [Cl−]i ∼40 mm can reduce GABAB and Ih currents significantly, it appears that there are many instances (such as during development) when Cl− can reach concentrations that will interfere with these currents and potentially compromise normal cellular functioning. These results may be particularly relevant to the understanding of pathophysiological phenomena, such as spreading depression, thought to involve massive influx of Cl− (Lux et al., 1986). Our results suggest that some of the K+ conductances potentially available for repolarizing strongly depolarized cells and limiting the extent of pathological activity would, in fact, be compromised by high [Cl−]i. Reducing GABABconductance in particular should contribute to more pronounced epileptiform activity (Traub et al., 1993). Our data support the growing recognition that internal anions may have important influences on cellular excitability in the CNS.
Footnotes
This work was supported by United States Public Health Service Grants NS30219 and NS22010 (B.E.A.). R.A.L. was supported by National Institutes of Health Neurosciences Training Grant NS07375. We thank Drs. F. Le Beau and W. Morishita, as well as L. Martin, S. Mason, and N. Varma, for their comments on a draft of this manuscript. This manuscript will comprise part of a thesis submitted in partial fulfillment of the Ph.D. degree requirements of R.A.L. We thank E. Elizabeth for expert word processing and editorial assistance.
Correspondence should be addressed to Dr. B. E. Alger, Department of Physiology, University of Maryland School of Medicine, 665 West Baltimore Street, Baltimore, MD 21201.
Dr. Pitler’s present address: Neurogen Corporation, 35 Northeast Industrial Road, Branford, CT 06405.
REFERENCES
- 1.Adams DJ, Oxford GS. Interaction of internal anions with potassium channels of the squid giant axon. J Gen Physiol. 1983;82:429–448. doi: 10.1085/jgp.82.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adelman WJ, Jr, Dyro FM, Senft JP. Internally perfused axons: effects of two different anions on ionic conductance. Science. 1966;151:1392–1394. doi: 10.1126/science.151.3716.1392. [DOI] [PubMed] [Google Scholar]
- 3.Alger BE, Nicoll RA. Spontaneous inhibitory postsynaptic potentials in hippocampus: mechanism for tonic inhibition. Brain Res. 1980;200:195–200. doi: 10.1016/0006-8993(80)91108-7. [DOI] [PubMed] [Google Scholar]
- 4.Andrade R, Malenka RC, Nicoll RA. A G-protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science. 1986;234:1261–1265. doi: 10.1126/science.2430334. [DOI] [PubMed] [Google Scholar]
- 5.Baker PF, Hodgkin AL, Shaw TI. The effects of changes in internal ionic concentrations of the electrical properties of perfused giant axons. J Physiol (Lond) 1962;164:355–374. doi: 10.1113/jphysiol.1962.sp007026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanton MG, Lo Turco JJ, Kriegstein AR. Whole-cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989;30:203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- 7.Bobker DH, Williams JT. Serotonin augments the cationic current Ih in central neurons. Neuron. 1989;2:1535–1540. doi: 10.1016/0896-6273(89)90041-x. [DOI] [PubMed] [Google Scholar]
- 8.Coombs JS, Eccles JC, Fatt P. The specific ionic conductances and the ionic movements across the motoneuronal membrane that produce the inhibitory postsynaptic potential. J Physiol (Lond) 1955;130:326–373. doi: 10.1113/jphysiol.1955.sp005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies CH, Davies SN, Collingridge GL. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol (Lond) 1990;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies CH, Starkey SJ, Pozza MF, Collingridge GL. GABAB autoreceptors regulate the induction of LTP. Nature. 1991;349:609–611. doi: 10.1038/349609a0. [DOI] [PubMed] [Google Scholar]
- 11.DiFrancesco D, Ferroni A, Mazzanti M, Tromba C. Properties of the hyperpolarizing-activated current (If) in cells isolated from the rabbit sino–atrial node. J Physiol (Lond) 1986;377:61–88. doi: 10.1113/jphysiol.1986.sp016177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dutar P, Nicoll RA. A physiological role for GABAB receptors in the central nervous system. Nature. 1988;332:156–158. doi: 10.1038/332156a0. [DOI] [PubMed] [Google Scholar]
- 13.Gahwiler BH, Brown DA. GABAB receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proc Natl Acad Sci USA. 1985;82:1558–1562. doi: 10.1073/pnas.82.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halliwell JV, Adams PR. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982;250:71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- 15.Higashijima T, Ferguson KM, Sternweis PC. Regulation of hormone-sensitive GTP-dependent regulatory proteins by chloride. J Biol Chem. 1987;262:3597–3602. [PubMed] [Google Scholar]
- 16.Jiang Z-G, Pessia M, North RA. Dopamine and baclofen inhibit the hyperpolarization-activated cation current in rat ventral tegmental neurones. J Physiol (Lond) 1993;462:753–764. doi: 10.1113/jphysiol.1993.sp019580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenz RA, Pitler TA, Yarowsky PJ, Alger BE. Intracellular Cl− diminishes G-protein-activated K+ conductances in rat hippocampal neurons. Soc Neurosci Abstr. 1994;20:1519. [Google Scholar]
- 18.Luhmann HJ, Prince DA. Postnatal maturation of the GABAergic system in rat neocortex. J Neurophysiol. 1991;65:247–263. doi: 10.1152/jn.1991.65.2.247. [DOI] [PubMed] [Google Scholar]
- 19.Lux HD, Heinemann U, Dietzel I. Ionic changes and alterations in the size of the extracellular space during epileptic activity. In: Delgado-Escueta AV, Ward AA Jr, Woodbury DM, Porter RJ, editors. Advances in neurology, Vol 44, Basic mechanisms of the epilepsies. Molecular and cellular approaches. Raven; New York: 1986. pp. 619–639. [PubMed] [Google Scholar]
- 20.Maccaferri G, McBain CJ. The hyperpolarization-activated current (Ih) and its contribution to pacemaker activity in rat CA1 hippocampal stratum oriens-alveus interneurones. J Physiol (Lond) 1996;497:119–130. doi: 10.1113/jphysiol.1996.sp021754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maccaferri G, Mangoni M, Lazzari A, DiFrancesco D (1993) Properties of the hyperpolarization-activated current in rat hippocampal CA1 pyramidal cells. J Neurophysiol 2129–2136. [DOI] [PubMed]
- 22.Mayer ML, Westbrook GL. A voltage-clamp analysis of inward (anomalous) rectification in mouse spinal sensory ganglion neurones. J Physiol (Lond) 1983;340:19–45. doi: 10.1113/jphysiol.1983.sp014747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCormick DA. Membrane properties and neurotransmitter actions. In: Shepherd GM, editor. The synaptic organization of the brain. Oxford UP; New York: 1990. pp. 32–66. [Google Scholar]
- 24.McCormick DA, Pape H-C. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol (Lond) 1990;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Misgeld U, Klee MR, Zeise ML. Differences in baclofen sensitivity between CA3 neurons and granule cells of the guinea pig hippocampus in vitro. Neurosci Lett. 1984;47:307–311. doi: 10.1016/0304-3940(84)90531-7. [DOI] [PubMed] [Google Scholar]
- 26.Nakajima T, Sugimoto T, Kurachi Y. Effects of anions on the G-protein-mediated activation of the muscarinic K+ channel in the cardiac atrial cell membrane. Intracellular chloride inhibition of the GTPase activity of GK. J Gen Physiol. 1992;99:665–682. doi: 10.1085/jgp.99.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neher E. Correction for liquid junction potentials in patch-clamp experiments. Methods Enzymol. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- 28.Newberry NR, Nicoll RA. Direct hyperpolarizing action of baclofen on hippocampal pyramidal cells. Nature. 1984;308:450–452. doi: 10.1038/308450a0. [DOI] [PubMed] [Google Scholar]
- 29.Nicoll RA, Alger BE. A simple chamber for recording from submerged brain slices. J Neurosci Methods. 1981;4:153–156. doi: 10.1016/0165-0270(81)90049-2. [DOI] [PubMed] [Google Scholar]
- 30.Olpe H-R, Karlsson G. The effects of baclofen and two GABAB receptor antagonists on long-term potentiation. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:194–197. doi: 10.1007/BF00166964. [DOI] [PubMed] [Google Scholar]
- 31.Otis TS, Mody I. Differential activation of GABAA and GABAB receptors by spontaneously released transmitter. J Neurophysiol. 1992;67:227–235. doi: 10.1152/jn.1992.67.1.227. [DOI] [PubMed] [Google Scholar]
- 32.Owens DF, Boyce LH, Davis MBE, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated patch recordings and calcium imaging. J Neurosci. 1996;16:6414–6423. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pham TM, Lacaille J-C. Multiple postsynaptic actions of GABA via GABAB receptors on CA1 pyramidal cells of rat hippocampal slices. J Neurophysiol. 1996;76:69–80. doi: 10.1152/jn.1996.76.1.69. [DOI] [PubMed] [Google Scholar]
- 34.Pitler TA, Alger BE. Cholinergic excitation of GABAergic interneurons in the rat hippocampal slice. J Physiol (Lond) 1992;450:127–142. doi: 10.1113/jphysiol.1992.sp019119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pitler TA, Alger BE. Differences between presynaptic and postsynaptic GABAB mechanisms in rat hippocampal pyramidal cells. J Neurophysiol. 1994;72:2317–2327. doi: 10.1152/jn.1994.72.5.2317. [DOI] [PubMed] [Google Scholar]
- 36.Pusch M, Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflügers Arch. 1988;411:204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- 37.Tasaki I, Singer I, Takenaka T. Effects of internal and external ionic environment on excitability of squid giant axon. J Gen Physiol. 1965;48:1095–1123. doi: 10.1085/jgp.48.6.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Traub RD, Miles R, Jefferys JGR. Synaptic and intrinsic conductances shape picrotoxin-induced synchronized after-discharges in the guinea-pig hippocampal slice. J Physiol (Lond) 1993;461:525–547. doi: 10.1113/jphysiol.1993.sp019527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Velumian AA, Zhang L, Pennefather P, Carlen PL. Reversible inhibition of IK, IAHP, Ih, and ICa currents by internally applied gluconate in rat hippocampal pyramidal neurones. Pflügers Arch. 1996;433:343–350. doi: 10.1007/s004240050286. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Weiner JL, Valiante TA, Velumian AA, Watson PL, Jahromi SS, Schertzer S, Pennefather P, Carlen PL. Whole-cell recording of the Ca2+-dependent slow afterhyperpolarization in hippocampal neurones: effects of internally applied anions. Pflügers Arch. 1994;426:247–253. doi: 10.1007/BF00374778. [DOI] [PubMed] [Google Scholar]