Abstract
Male birds of age-limited song-learning species develop their full song repertoires in the first year of life. For this type of song learner, once song is stabilized in adulthood, it is highly stereotyped and stable over time. Traditionally, it has been believed that age-limited song learners do not depend on auditory feedback for the maintenance of adult song. A recent report, however, showed that adult song in zebra finches, age-limited learners, does change after long-term deafness. We report here that another species of age-limited learner, Bengalese finches, depends critically on auditory feedback for adult song maintenance. We surgically deafened adult males and recorded song for 12 weeks after surgery. Results show that song degraded significantly within 1 week of surgery and continued to degrade over the next 11 weeks. This represents a more rapid degradation of song than has been seen previously in age-limited species. Song deficits after deafening included a marked decrease in syllable sequence stereotypy, skewed syllable distribution within song bouts, degradation of syllable phonology, and dropped, combined, and new or unrecognizable syllables. Decreased sequence stereotypy and combined syllables appeared within 1 week of deafening and did not worsen over time. Skewed syllable distributions and syllable phonology changes appeared after 1 week and did worsen. Occurrences of dropped and new syllables appeared within 1 week and increased over time. Comparison with other species indicates that much variability exists among species in the extent to which auditory feedback is necessary for song maintenance.
Keywords: song, auditory feedback, deafening, finch, cochlear removal, song system
A significant issue in research on song control in birds has been the contribution of auditory experience to the development and maintenance of stereotyped song behavior (for review, see Marler, 1987, 1991; Konishi, 1994). Auditory experience is essential for learning and eventual production of “normal” adult song. In most song birds, a juvenile male listens to an adult sing, forms a memory or “template” of that song, and then practices his own vocalizations until they match that memory (Konishi, 1965; Dittus and Lemon, 1969; Marler and Waser, 1977; Price, 1979;Eales, 1985; Marler and Peters, 1987). Song birds fall into two different categories with respect to the capacity for change in adult song behavior. So-called open-ended learners, such as the canary, learn new songs seasonally throughout life and depend on auditory information for the production and maintenance of normal song as juveniles and adults (Nottebohm and Nottebohm, 1978; Nottebohm, 1981). Age-limited learners, such as the zebra finch and the song sparrow, learn one or more stereotyped songs during a restricted developmental period and sing only those songs in adulthood (Price, 1979; Eales, 1985; Marler, 1987).
The necessity of auditory feedback for maintenance of adult song also differs between these two categories of song birds. Canaries rely heavily on auditory feedback to maintain normal adult song; they show a rapid, marked degradation of song when deafened (Nottebohm et al., 1976). In contrast, it was believed that age-limited learners maintain stereotyped adult song indefinitely without auditory feedback. Early studies indicated that song, stereotyped by the end of the sensitive period for song learning, remained stable in adulthood and did not change after surgical deafening (Konishi, 1965; Nottebohm, 1968; Price, 1979; Bottjer and Arnold, 1984). A recent study by Nordeen and Nordeen (1992) showed somewhat different results. These authors demonstrated that stereotyped adult song in zebra finches can degrade after deafening but that this degradation does not begin in most birds until 6–8 weeks after surgery. Thus, adult zebra finch song does not remain stereotyped indefinitely without auditory feedback. This important study showed for the first time that an age-limited learner does depend in fact on auditory feedback for normal song production. Age-limited learners, however, seemed to be much less dependent on auditory input than open-ended learners, because song degraded much more slowly in the zebra finch than it did in the canary.
In this study, we report that the Bengalese finch Lonchura Striata domestica experiences very rapid song degradation after the removal of auditory feedback. This result was surprising, because the Bengalese finch is an age-limited learner, similar to the zebra finch, with respect to song learning (Dietrich, 1980; Clayton, 1987,1988, 1989) and to the acoustic characteristics of song (Immelmann, 1969). Males typically learn song during the first 70–80 d of life and do not normally incorporate new elements or notes into their songs after 120 d (Dietrich, 1980; Clayton, 1987, 1988, 1989). In adulthood, each male sings one highly stereotyped, stable song (Immelmann, 1969; Dietrich, 1980; Clayton, 1987).
MATERIALS AND METHODS
Animals. We used 12 adult male Bengalese finches. All birds were aviary-raised (Magnolia Bird Farm, Anaheim, CA) with adults of both sexes and were sent to our laboratory at 4 months of age. Birds were housed in groups of 10–15 individuals of both sexes and were maintained on a 14:10 hr light/dark cycle. All birds were 6 months of age at the beginning of this study.
Song recordings. “Undirected” (not in the presence of a female) song was recorded while each male was alone in a 20 × 12 × 14 inch wire mesh cage within a sound-isolated booth (Industrial Acoustics). A low impedance microphone (F-98; Sony, Tokyo, Japan) was placed 6 inches above the bird’s perch. The microphone was connected through a voice-activated circuit, with a 4 sec signal delay to a cassette tape recorder (PMD 201; Marantz). For each recording date, 10 singing bouts composed of several repetitions of a song were recorded. Song bouts were defined as episodes of continuous singing surrounded by 2 or more seconds of silence. The time required to collect 10 bouts from an individual on any particular date ranged between 30 min and 8 hr.
Song was recorded from each of the 12 males at the ages of 6, 7, and 8 months (8, 4, and 0 weeks before deafening, respectively). These recordings were collected to ensure that each bird was singing a stable, stereotyped song over time. After these three initial baseline recordings, 6 of the 12 birds were surgically deafened at 8 months of age by bilateral cochlear removals as described below. The other six birds served as unmanipulated controls. Song was recorded from all 12 of the birds at 1, 2, 3, 4, 5, 6, and 12 weeks after the deafening surgeries were performed.
Surgery. Birds were anesthetized with intramuscular injections of 25 mg/kg Nembutal (Abbott Labs, Irving, TX) and 80 mg/kg Ketaset (Fort Dodge Laboratories). Feathers were trimmed around the external auditory meatus, and an incision was made in the skin covering the ear canal to expose the tympanic membrane. The tympanic membrane was cut, and the columella was removed. The cochlea (basilar papilla) was removed by inserting a tungsten wire hook through the oval window, into the proximal end of the cochlea, and by withdrawing the hook with attached cochlea. The middle ear was then filled with sterile Gelfoam (Upjohn, Kalamazoo, MI), and the incision in the skin was sealed with cyanoacrylic glue (Vetbond). This procedure was repeated on the other ear. The extracted cochleas were floated in saline and examined under a dissecting microscope to ensure that the entire structure had been successfully removed bilaterally.
Song analysis. All song records were played into a Power Macintosh 9500 and digitized at 22 kHz using the Canary 1.2 sound analysis program (Cornell Laboratory of Ornithology). Spectrographs and amplitude waveforms from the first baseline recordings made 8 weeks before deafening were used to identify each bird’s song. For experimental birds, the three initial monthly recordings at 8, 4, and 0 weeks before deafening (−8, −4, and 0) and the recordings at 1, 2, 3, 6, and 12 weeks after deafening (+1, +2, +3, +6, and +12) were chosen for analysis. Recordings at +4 and +5 weeks were eliminated because they did not seem to be different from recordings at +3 weeks. For control birds, we analyzed song from two initial monthly recordings (−4 and 0) and from the recordings at 4 and 12 weeks after the deafening surgeries were performed (+4 and 0). Of the 10 singing bouts recorded from each bird at each time point, the three longest bouts were chosen for analysis. Approximately 1000 repetitions of song from the 12 animals were analyzed.
Normal song was defined from presurgery records as follows. From presurgery baseline song recordings made 8 weeks before deafening (−8), each bird’s syllables and song were identified by determining the sequences of sounds that were sung in stable units and were repeated several times within a bout. For this species’ song, syllables were defined as discrete sets of sounds always occurring together as a unit and having silent intervals of no more than 80 msec within a sequence of vocalizations. Bengalese finch song often contains repeated sets of notes or elements that always appear together and that have silent intervals between them that are shorter and less variable than are those between entire syllables. Vocalizations we call syllables here have been referred to as “elements groups” in previous work with this species (Dietrich, 1980). The method of defining syllables used here is validated by the changes in song that occurred after deafening. Namely, those sets of sounds defined as syllables are maintained as units after deafening, although their sequence becomes disordered. Intervals of silence between 80 and 1000 msec in duration were considered intersyllable intervals. An individual’s song was defined as the sequence of syllables repeated several times within a bout. Song bouts were defined as episodes of singing initiated by introductory notes and surrounded by at least 2 sec of silence. The records used to identify each individual’s song served as a template for that individual but were then excluded from further analysis.
Hard copies of song spectrographs and amplitude waveforms were made for the three analysis bouts used for each recording time. The records from each bird were then coded and randomized so that during the analysis we did not know the recording time for each particular set of spectrographs and waveforms. Songs, syllables, and transitions between syllables were labeled on each spectrograph and waveform. Syllables were given identifying numbers in their corresponding order of appearance. For cases in which the unique elements distinguishing two similar syllables were changed or lost postoperatively, we assigned identity to a syllable based on the closest estimate of syllable order matching that of preoperative song. In such cases, this method provided the most conservative calculations of song change. Introductory notes, occurring at the initiation of a bout, were labeled but were not included in the quantitative analyses unless they were reliably produced within song repetitions. Some birds occasionally but not consistently inserted an introductory note between the syllables within songs (see, e.g., Fig. 2A). In this case, the note was considered part of a syllable if <80 msec of silence separated it from an adjacent syllable. If silent intervals between such a note and the syllables adjacent to it were >80 msec, then it was labeled as an introductory note and was omitted from further analysis. In cases in which introductory notes were reliably sung between longer syllables within songs, they were labeled as syllables and included in analyses. Any new or unrecognizable syllables that were not sung in the preoperative baseline recordings used to define a particular bird’s song were labeled with letters in the order of each syllable’s occurrence. Any combined syllables were labeled as such and were considered syllable types different from the two or more originally separate syllables composing them.
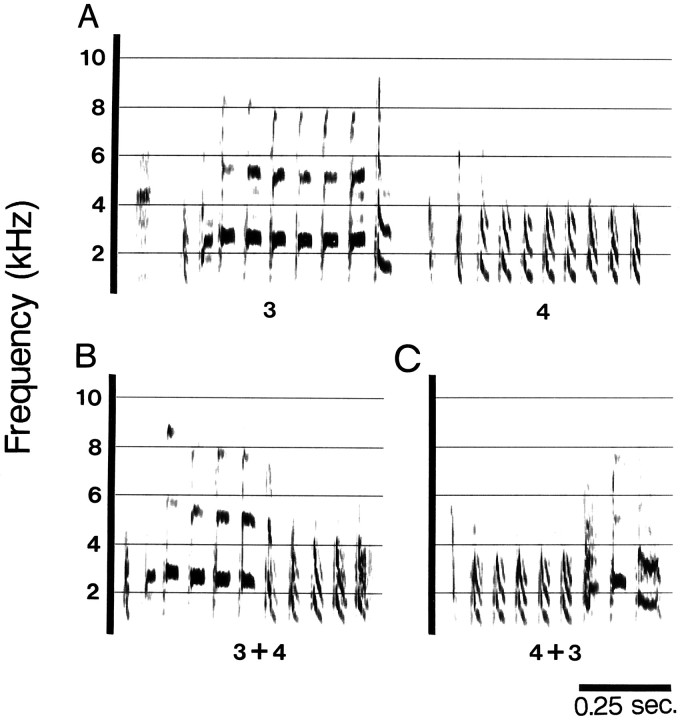
Fig. 2.
Within 1 week of deafening, the birds sang with a marked degradation of syllable sequence stereotypy. A, Sound spectrograph of two song repetitions recorded preoperatively.B, Spectrograph of the same duration from the same bird recorded in A but recorded 1 week after deafening. Syllable identities are labeled with numbers below the time axis.
The song spectrographs from each bird at each recording time were analyzed for the following properties: syllable order and sequence stereotypy; syllable distribution within a bout; syllable phonology; and instances of dropped, combined, and new or unrecognizable syllables.
Syllable order and sequence stereotypy. For the analysis of sequence stereotypy, we used a method modified from that used byScharff and Nottebohm (1991). This method is the calculation of a finalsequence stereotypy score for each singing bout by the averaging of two stereotypy ratios: a sequence linearityscore and a sequence consistency score. The maximum value for each of these scores is 1. The calculations are expressed as follows:
Sequence linearity measures in how many different ways the syllables are ordered. For example, if a bird has four syllables (e.g., 1, 2, 3, and 4) in his song, and thus also in a whole bout, he will also have at least four transition types in a bout (1 to 2, 2 to 3, 3 to 4, and 4 to 1). If he sings those four syllables in the same exact order over and over again, only four transition types will be found in a bout, and he will have a sequence linearity score of 1. If he sings those syllables in a differing order, more than four transitions types will be found in a bout, and the sequence linearity score will be <1.
Sequence consistency measures how often a particular sequence of syllables is produced. Typical transitions were defined using the same presurgery baseline recordings that were used to define each bird’s normal song mentioned above. Transitions considered typical for this ratio would be 1 to 2, 2 to 3, 3 to 4, and 4 to 1 for a bout composed of song repetitions containing four syllables. If the atypical transition 4 to 2 occurred often rather than just once in a bout, the sequence consistency score would decrease.
Sequence stereotypy scores were calculated for each of the three song bouts analyzed per recording time per bird and were averaged to give one final score for each bird at each recording time. Those scores were then averaged over all experimental birds and all control birds separately for each recording time and were statistically analyzed for changes in sequence stereotypy over time.
Additionally, to determine whether birds ever sang syllables in randomly configured sequences, a random model of syllable ordering was generated to match the actual numbers of syllable types occurring in bouts over time and the numbers of syllables sung in bouts. Sequence stereotypy scores were calculated for this random model of syllable order and were compared with the scores calculated from the actual singing bouts.
Syllable distribution. To determine whether there were changes in the frequency with which a syllable was sung within a bout, we used the Shannon information measure expressed below (Shannon, 1949;Hailman, 1977). This measure, originally designed to quantify the amount of information within a set of signals transmitted between sender and receiver, is often used to compare the relative probabilities that certain signals will be present within a set of signals. We calculated an H value for each bout, averaged all of the bout scores within a date, and compared those values across recording dates. H is calculated using the following equation:
where N equals the number of different signals (syllables) in the array (song bout), and Piequals the probability that each signal might occur as given by the relative frequency of occurrence.
For example, if a bird has four syllables in his song and he sings them in a perfectly stereotyped pattern, then each syllable has a probability of 0.25, and the H value will be 2, but if he sings syllable one one-half of the time, syllable two one-fourth of the time, and syllables three and four each one-eighth of the time, then the probability of each syllable occurring is not equal, and theH value will decrease to 1.75. A higher H value indicates that syllables one, two, three, and four are being sung equally, whereas a lower value represents an unequal distribution of syllable types within a bout. Any new syllables arising in a bird’s repertoire after deafening were not included in this analysis so thatN did not vary within an animal over time. Thus, we could directly compare H values, within a bird, for all of the recording times used throughout the study.
Syllable phonology. To measure differences in syllable phonology over time, we used spectrographic cross-correlations of three randomly selected examples of the same syllable type from each recording time. We generated cross-correlation arrays for each syllable, comparing preoperative and postoperative examples of the same syllable type within and across recording times.
Dropped, combined, and new or unrecognizable syllables. We also documented if and when syllable types were dropped from songs over time, the combining of originally distinct syllables over time, and the appearance of any new or unrecognizable syllables over time. The syllable types that could be considered dropped were limited to those that were originally defined in the normal song of each bird and that did not include new syllables appearing after deafening. Syllable types were considered dropped from a repertoire by a particular date if no example was found of that syllable in any recordings obtained on or after that date. Syllables were considered combined if the interval of silence between the two elements adjoining the syllables was less than the silent intervals between elements within each originally distinct syllable. A syllable was considered new or unrecognizable if we could not assign it an identity based on its spectrographic similarity to any syllable in that bird’s normal song.
Statistical analyses. Statistical analyses on sequence stereotypy scores, H values, and cross-correlations were performed using repeated measures, one-factor ANOVA. Post hoc comparisons were made with the Scheffé Ftest. Student’s t tests (two-tailed) were used to compare stereotypy scores and cross-correlations between control and preoperative experimental birds.
RESULTS
Normal song
Analysis of our control and preoperative song recordings indicated that song in normal adult male Bengalese finches is stereotyped and stable over time. Figure 1 shows one repetition of a song from the same bird from three different recording times that span 4 months. The similarity in syllable morphology and syllable order is clearly apparent.
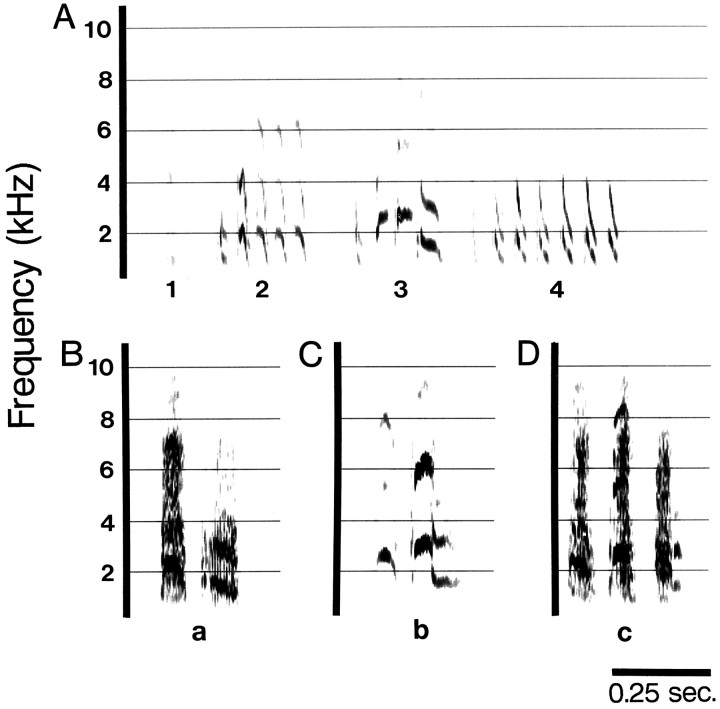
Fig. 1.

Normal adult Bengalese finch song was recorded from control birds 10 times over 4 months. Song was stereotyped and stable over time. A, One song repetition from recordings made from a control bird 4 weeks before the experimental birds were deafened. B, Repetition of a song from the same bird from recordings made 8 weeks later. C, Recording from the same bird 12 weeks after the experimental birds were deafened (16 weeks after the recording shown in A). Syllable identities are labeled with numbers below the time axis.
Normal songs are composed of two to seven syllables (mean = 4) and are an average of 2.1 sec in duration. Syllables are generally composed of repeated elements that feature harmonics or sequences of fast frequency modulated sweeps (Fig. 1, syllables 2, 4,respectively). Frequency components range between 0.5 and 10 kHz. Some syllables are shorter and more simple call-like acoustic units that are repeated a variable number of times within any one repetition of a syllable (Fig. 1, syllable 3). Spectrographs of normal songs (see Figs. 1, 2A, 8A) and spectrographs of normal syllables (see Figs. 1, 2A,5A,B, 7A,8A) are shown.
Fig. 8.
After deafening, the birds sang new or unrecognizable syllables. A, Sound spectrograph of one song repetition recorded preoperatively. B,C, D, Examples of new or unrecognizable syllables sung by the same bird recorded in A but 3 weeks after deafening. Identities of original syllables are labeled with numbers. New or unrecognizable syllables are labeled with lower case letters.
Fig. 5.
A, B, Sound spectrographs showing two different syllables from two different birds recorded preoperatively. C–H, Spectrographs of the same syllables from the same birds recorded in A andB but recorded (C, D) 2 weeks after deafening, (E, F) 6 weeks after deafening, and (G, H) 12 weeks after deafening. Black bars indicate the portion of the spectrograph containing the syllable.
Fig. 7.
Within 1 week of deafening, some originally distinct syllables were truncated and combined. A, Sound spectrograph of two different syllables from one experimental bird’s song recorded preoperatively. B, An example of the combination of the two originally distinct syllables shown inA recorded 1 week after deafening. C, An example of the same combination, also recorded 1 week after deafening, in which the order of syllables has been reversed. Syllable identities are labeled with numbers below the time axis.
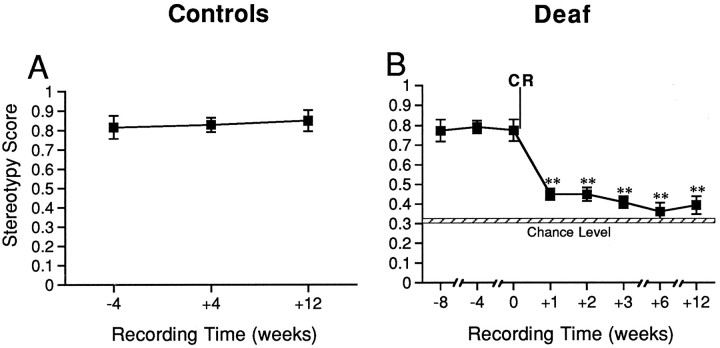
Sequence stereotypy scores for normal song bouts indicate that stereotypy in syllable order was high and stable over several months in normal birds; overall scores (mean ± SEM) for control and preoperative birds were 0.830 ± 0.050 (see Fig. 3A) and 0.780 ± 0.048 (see Fig. 3B, before CR). Sequence stereotypy did not change over the 3 months of initial preoperative recordings, and control and preoperative scores did not differ. Across all control and experimental birds, normal song was composed of relatively equal presentations (mean ± SEM;H value = 1.98 ± 0.045) of each syllable type in a song bout (see Fig. 4).
Fig. 3.
Sequence stereotypy scores at each recording time were averaged for control and experimental birds. A, Scores for controls were similarly high and consistent over time.B, Scores for experimental birds were similarly high over time before deafening by cochlear removal (CR), decreased significantly by 1 week after deafening, and were similarly low between 1 and 12 weeks after deafening. The hatched bar gives the mean score (±SEM) of the random model and indicates the score for a chance configuration of syllables. By 6 weeks after deafening, stereotypy scores were not statistically different from random. Numbers below the xaxis indicate the number of weeks before or after the experimental birds were deafened. Error bars represent ± SEM; **p < 0.001, compared with predeafening scores.
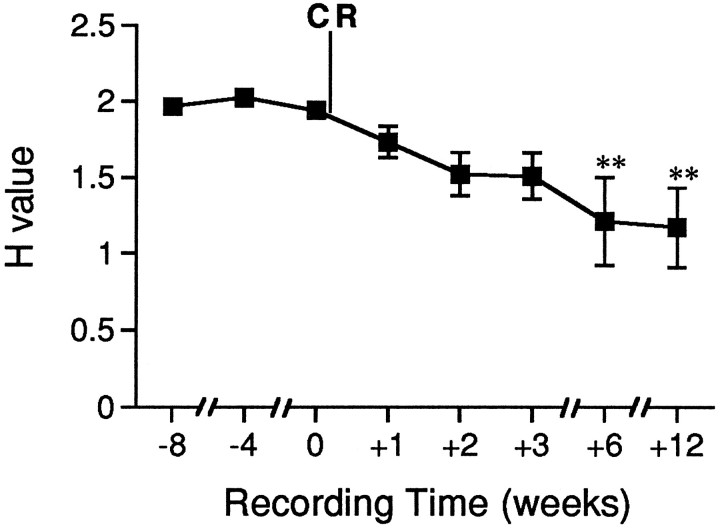
Fig. 4.
Syllable distribution was significantly more skewed and variable in deafened birds. Mean H values are plotted for preoperative and postoperative recordings from experimental birds. H values decreased significantly between preoperative recordings and those made 6 weeks after deafening by cochlear removal (CR). Numbers below thex axis indicate the number of weeks before or after deafening of the birds. Error bars represent ± SEM; **p < 0.001, compared with predeafening scores.
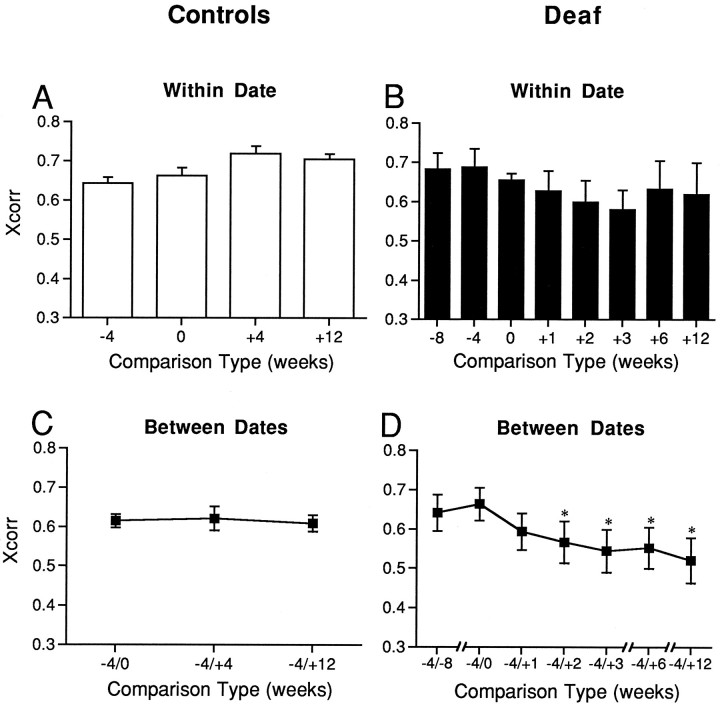
Analysis of the changes in syllable phonology by cross-correlation showed that syllables were very similar in phonology over time in control and in presurgery experimental birds. For all syllable types and all animals combined, cross-correlations (X/corr) of syllables sung by control animals were moderate (mean ± SEM; X/corr = 0.680 ± 0.017) and did not change within a recording session. Correlations were also moderate and stable (mean ± SEM; X/corr = 0.616 ± 0.011) for comparisons of the same syllable type sung by control birds during recordings that spanned 4 months (see Fig. 6C). Comparison of within-date cross-correlations with between-date cross-correlations showed no significant difference (p > 0.1).
Fig. 6.
Syllables were cross-correlated within a recording date and across recording dates. Syllables sung 2 weeks after deafening were significantly different from the same syllables sung preoperatively. A, Controls, Mean correlations for repetitions of the same syllable within recording dates. B, Deaf, Mean correlations for repetitions of the same syllable within recording dates.C, Controls, Cross-correlations of syllables from recordings made 4 weeks before and 4 and 12 weeks after the experimental birds were deafened (8 weeks and 16 weeks apart).D, Cross-correlations of the same syllables sung 4 weeks before and 1, 2, 3, 6, and 12 weeks after deafening. Numbers below thex axis indicate the number of weeks before or after the experimental birds were deafened. Error bars represent ± SEM; *p < 0.05; repeated measures ANOVA.
No occurrences of dropped or new or unrecognizable syllables were observed in control and preoperative song recordings (see Table 1). In preoperative recordings from one experimental bird, we found two instances of combined syllables. These were the only observations of syllable combinations in normal song.
Table 1.
Numbers of dropped, combined, and new or unrecognizable syllables for the six experimental birds over the entire recording period
| Recording time | No. of syllables dropped | No. of syllable combinations | No. of combinations | No. of new syllables | No. of new occurrences |
|---|---|---|---|---|---|
| Preop month 1 | 0 | 1 | 2 | 0 | 0 |
| Preop month 2 | 0 | 0 | 0 | 0 | 0 |
| Preop month 3 | 0 | 0 | 0 | 0 | 0 |
| Deaf—week 1 | 2 | 6 | 25 | 9 | 21 |
| Deaf—week 2 | 3 | 7 | 32 | 11 | 45 |
| Deaf—week 3 | 3 | 6 | 18 | 17 | 61 |
| Deaf—week 6 | 7 | 7 | 30 | 12 | 103 |
| Deaf—week 12 | 7 | 6 | 19 | 14 | 80 |
Numbers of dropped, combined, and new or unrecognizable syllables were counted for song bouts recorded during three preoperative (preop) months and 1, 2, 3, 6, and 12 weeks after deafening. Number of syllable combinations refers to the overall number of types of combinations. Number of combinations refers to the number of times a combination of any type occurred. Number of new syllables refers to the number of new syllable types. Number of new occurrences refers to the number of times any new syllable occurred.
Effects of deafening on syllable order
Postoperative song recordings showed that deafening resulted in a rapid and marked degradation of song stereotypy, which began within 1 week of surgery in the first postoperative recordings. Although we began systematically recording song from all birds at 1 week, we did observe qualitative changes in song before 1 week; we could detect changes such as the repetition of syllable types and incomplete songs.
The first song change observed quantitatively was a decrease in sequence stereotypy. For example, the likelihood of syllable 2 following syllable 1 decreased significantly between sequences of syllables sung during the preoperative months and those sung 1 week after deafening. In deafened birds, the ordering of syllables was often so unpredictable that we were no longer able to define a song repetition. Interestingly, the occurrence of syllable 1 (as defined in preoperative recordings) being sung first in a bout was still relatively consistent. Common changes made in singing bouts recorded 1 week after deafening included singing one syllable several times consecutively and skipping syllables. An example is shown in Figure2. Figure 2A shows a sequence of syllables comprising two repetitions of song sung before deafening, whereas B shows a sequence of approximately the same duration sung by the same bird 1 week after deafening.
Quantitative analysis of song spectrographs showed that sequence stereotypy scores decreased from 0.780 ± 0.048 (mean ± SEM) before deafening to 0.412 ± 0.037 over the 12 weeks after deafening (Fig. 3B). At each postoperative time point, sequence stereotypy scores were significantly different from preoperative scores (p < 0.001); postoperative scores did not differ significantly among the recordings made 1, 2, 3, 6, and 12 weeks after deafening.
According to our random model for the probability of one particular syllable following another by chance alone, a sequence stereotypy score of 0.315 ± 0.018 (mean ± SEM) would be expected. This value represents the averaged scores for syllable strings (bouts) in which the syllables were randomly ordered. These model syllable strings exactly matched the actual number of syllable types and the overall numbers of syllables sung in analyzed bouts of deafened birds. Comparison of this value with averaged scores for the actual analyzed song bouts indicated that sequence stereotypy in deafened birds gradually degraded over time from less stereotyped than normal to not significantly different from random. The random model value was significantly below the scores for birds singing 1, 2, and 3 weeks after deafening but was not different from the scores for birds singing 6 and 12 weeks after deafening (Fig. 3B).
Effects of deafening on syllable distribution within song bouts
In deafened birds, some syllables were sung more frequently than others. For most deafened birds (five of six), the favored syllable (the one sung most often) was the first syllable in a song. In four of six birds, this syllable was less complex acoustically, and shorter in duration, than the other syllables. In deafened birds, Hvalues decreased with each successive recording time, and the variability among H values for bouts scored within a session increased over time. The decline in H values indicates that the appearance of different syllables within a bout changed from being near equal in normal birds to clearly favoring some syllables over others in deafened birds. The lower H values for song bouts in deafened birds represented the combined effects of the repeated singing of one syllable type and the dropping of other syllable types from song repetitions. These effects are shown in Figure4. By 6 weeks after deafening,H values had decreased significantly from those for preoperative recordings (p < 0.001).H values decreased from 1.98 ± 0.045 (mean ± SEM) preoperatively to 1.21 ± 0.189 by 6 weeks after deafening.
Effects of deafening on syllable phonology
In the second week after deafening, breakdowns in the phonology of some syllables occurred in all six birds, although other syllables seemed to remain normal. For many of the longer syllables, the fundamental frequencies and harmonics seemed to increase or decrease. In other instances, the starting or ending elements of syllables were missing. Figure 5 shows examples of changes in syllable phonology in two birds between preoperative recordings and 2, 6, and 12 weeks after deafening. Each syllable type exhibiting one or more of these deficits seemed to exhibit them consistently within and across recording times, but with variations in the degree of deficit. These forms of song degradation worsened dramatically over time.
Cross-correlations for individual syllables in control birds within any recording session (Fig.6A) were relatively stable. The mean ± SEM was 0.680 ± 0.017, with no significant changes over 4 months. Cross-correlations of syllables from recording sessions separated by 1 to 4 months were 0.616 ± 0.011 and did not differ as a function of the separation period (Fig.6C). In deafened birds, the cross-correlations for between-date comparisons decreased significantly over the 12 weeks after deafening (Fig. 6B,D). In contrast to controls, deafened birds sang syllables with cross-correlations that were significantly higher within a recording date than between recording dates (p < 0.05). Cross-correlations within any recording date tended to be lower and more variable after deafening than before deafening (Figs.6A,B). For example, over 12 weeks, the mean ± SEM decreased from 0.674 ± 0.035 to 0.619 ± 0.080. These changes were not significantly different, however.
Cross-correlations of syllables before and after deafening (Fig.6D) were significantly lower than either predeafening or control cross-correlations. The mean ± SEM of preoperative cross-correlations was 0.650 ± 0.044. By 2 weeks after deafening, cross-correlations with the same syllables at 4 weeks before surgery had dropped to 0.566 ± 0.053 and by 12 weeks were only 0.520 ± 0.058. Changes in cross-correlations between preoperative and postoperative syllables, however, were surprisingly small compared with the marked changes we observed when qualitatively examining syllable spectrographs (see examples in Fig. 5). This suggests that the spectrograph cross-correlation is not a particularly sensitive measure of changes in syllable phonology.
Dropped, combined, and new or unrecognizable syllables
For each song bout analyzed by the measures described above, we also documented any syllables that a bird dropped from his repertoire, any syllables that were combined, and any new or unrecognizable syllables that appeared. These data are shown in Table1. No instances of dropped syllables were observed in either control or preoperative birds. By 1 week after deafening, two of six birds had deleted one syllable type. The number of syllables dropped continued to increase until the final recording sessions. By 12 weeks after deafening, 7 of the total of 26 syllable types in experimental birds were no longer present in song bouts. At this time, five of the six birds had deleted at least one syllable type.
No combined syllables were found in recordings from control birds. Four of the six experimental birds combined previously distinct syllables by 1 week after deafening. These syllables were usually sung consecutively in the normal recordings. They were truncated and combined in song from deafened birds (Fig. 7). We counted six combined syllable types in the recordings made 1 week after deafening and 25 total repetitions of these (Table 1). This deficit did not worsen over time; there was no overall increase in the number of combined syllables or in the number of times syllable combinations occurred over the postoperative 12 week period.
New or unrecognizable syllables first appeared 1 week after deafening and increased in overall number as well as in frequency of occurrence over time (Table 1). No new syllables were found in recordings from control birds. The generation of new or unrecognizable syllables was highly variable among individual experimental birds. One bird never sang new syllables, whereas another bird eventually developed six new syllables. Twelve weeks after deafening, five of the six birds sang new syllable types, but the number of types per bird was variable. In general, these syllables were shorter in duration and less complex acoustically than were most normal syllables (Fig.8). Some new syllables also seemed to be transient. It was common to identify a new syllable in one set of recordings from a particular week and not to find that syllable in recordings made in subsequent weeks.
DISCUSSION
Our results demonstrate that adult Bengalese finches, which normally sing stereotyped and stable song, show various and profound deficits by only 1 week after deafening. Traditionally, it has been thought that only open-ended learners depend heavily on auditory feedback for the maintenance of adult song. Furthermore, it has been suggested that the strong dependence of open-ended learners on auditory feedback is related to their ability to learn new songs in adulthood. Our results show that in a species that does not normally show adult changes in song, the neural circuitry responsible for relaying auditory feedback plays a very important role in adult song maintenance. This finding is consistent with preliminary observations in the same species of changes in adult song after auditory deprivation (Okanoya et al., 1991). In this way, Bengalese finches behave more like open-ended learners than age-limited learners.
Characteristics of song degradation: implications for differential roles of auditory feedback
The removal of auditory feedback in Bengalese finches did not result in a complete loss of the original song. Instead, it produced deficits that significantly altered the song. These deficits were consistent across birds. Characteristics of degraded song that reflect changes in the temporal patterning of song, such as sequence stereotypy and combined syllables, appeared in the first week and were maintained but did not significantly worsen over 12 weeks. Syllable phonology, the characteristic that most directly reflects vocal control, did not begin to change until after 1 week and worsened dramatically over the following weeks. This difference in the timing of degradation of the temporal pattern versus phonology of syllables suggests that the circuitry responsible for songs as temporal patterns, and syllables as separate units, may depend on auditory feedback differently.
In deafened Bengalese finches, the first syllable in a bout was most often unchanged, i.e., the same as it had been with hearing intact. By 6 weeks after deafening, however, the remaining syllables in a song bout were sung in nearly random configurations. This finding suggests that for a Bengalese finch, hearing itself sing one syllable could be a cue for “remembering” which syllable to sing next. In this scheme, the removal of auditory feedback would result in the sudden loss of syllable sequence stereotypy that we observed. The more gradual degradation in syllable phonology demonstrates a more classical role of auditory feedback. In our deafened birds, syllables only became poorly modulated and noisy over time; repetitions of the same elements or notes within syllables increased and decreased in fundamental frequency. Such deficits are commonly reported in human vocalizations when hearing is impaired and are thought to be indicative of vocal control loss (Waldstein, 1990; Cowie, 1992). Thus, it seems that auditory feedback is also used to maintain stable syllables by continually correcting and refining vocal output to match previous iterations of the same syllable or to match a “memory” of what that syllable should be.
Auditory and proprioceptive feedback
Proprioceptive input from the vocal organ is another potential means by which sensory feedback can affect song production. Zebra finches undergo marked changes in song behavior after peripheral nerve injury and regeneration (Williams and McKibben, 1992). After the tracheosyringeal nerve that innervates the syrinx (nXIIts) is injured, song degrades immediately by showing changes in syllable phonology, although timing and syllable sequence are maintained. Over subsequent weeks, finches with nerve injury recover syllable phonology but show other deficits similar to those of our deafened birds. Williams and McKibben (1992) reported that syllables were dropped and birds sang combined and new syllables postoperatively. The results of this study also resemble our results in that degradation of syllable phonology and degradation of song temporal patterning are separated with respect to the timing of onset. In this way, both studies indicate that different feedback processes may be involved in adult song maintenance or that different behavioral processes may differentially depend on feedback.
Some deficits resulting from nXIIts injury did not improve after nerve regeneration. Williams and McKibben (1992) reported that the temporal patterning changes observed over 100 d after nerve injury stabilized in a permanently altered pattern. It should be noted, however, that these results are somewhat difficult to interpret because tracheosyringeal nerve injury most likely damages both sensory and motor fibers. Stabilization of an altered song after the removal of proprioceptive feedback and after nerve regeneration suggests that zebra finches may not possess a stored memory for song that can be accessed and used to recapitulate song with restored proprioceptive feedback. In light of this, it would be interesting to determine the effects on song in Bengalese finches of removing and then restoring auditory feedback.
Comparative effects of deafening on song
We observed changes in Bengalese finch song by 1 week after deafening. Nottebohm et al. (1976) reported changes in one adult male canary’s song also by 1 week after deafening. Although each of these species seems to show rapid changes in song after deafening, the characteristics of song changes are different. Canaries seem to maintain the order of syllables after deafening but show rapid breakdowns in the phonology of syllables. Over 13 months after deafening, syllable phonology changed drastically. Bengalese finches, on the other hand, show significant changes in syllable order by 1 week after deafening but do not show significant changes in syllable phonology until at least 2 weeks after deafening. Thus, it seems that the only two shared features of song degradation between Bengalese finches and canaries are the timing of onset and the continual degradation of syllable phonology after deafening.
Our results agree in part with those of an earlier study showing that another age-limited learner, the zebra finch, requires auditory feedback for the long-term maintenance of adult song (Nordeen and Nordeen, 1992). Between 6 and 8 weeks after deafening, zebra finches begin to lose the ability to sing stable, stereotyped song. Bengalese and zebra finches are two species of estrildid finch so similar with respect to song learning that they can be cross-tutored (Clayton, 1987,1988, 1989). Considering this similarity, it might be reasonable to assume that the neural circuitry for song is very similar between them. It is striking, however, that these two species differ in the timing and characteristics of song degradation after surgical deafening. First, song degradation in zebra finches is not consistently present until 6–8 weeks after surgery. Bengalese finches show marked deficits only 1 week after surgery. Thus, Bengalese finches seem to depend more heavily on hearing to maintain stereotyped song than do zebra finches. Second, the specific characteristics of song degradation seem to differ between the two species. The major deafening-induced deficits in zebra finch song are changes in syllable phonology (Nordeen and Nordeen, 1992). By 16 weeks after cochlear removal, only 10.9% of syllables were judged the same as, and 30.2% were judged highly similar to, preoperative syllables. In contrast to zebra finches, the earliest and most obvious deficit for Bengalese finches is a loss of stereotypy in syllable sequences. This direct comparison, however, is somewhat difficult to make because many Bengalese finch syllables are sets of elements as opposed to the shorter zebra finch syllables. We also observed major changes in syllable phonology that appeared later than did syllable sequence deficits and worsened over the 12 weeks. In both species, dropped and combined syllables as well as the appearance of new syllables were observed, but these characteristics, again, appeared later postoperatively in zebra finches than in Bengalese finches. Our results, together with those of Nordeen and Nordeen (1992), present convincing evidence that not all age-limited learners possess fixed motor circuitry for the continued production of stereotyped song. Some age-limited song-learning species do not change song significantly after deafening. For example, Konishi (1965) deafened one adult male white-crowned sparrow and found that its song had changed very little 18 months after deafening. There seems to be wide variation among avian species with respect to dependence on auditory feedback for adult song maintenance. It will be of interest to assess song maintenance in other age-limited species.
Conclusions
These results document rapid changes in adult song in an age-limited song-learning species after deafening. These findings indicate that the Bengalese finch could become an advantageous model for studying the influence of auditory feedback on song. Differences between Bengalese finches and closely related zebra finches are surprising considering how similar these birds are in other respects. Direct comparisons of species that show similar song-learning patterns but that seem to differ in the neural mechanisms used to maintain adult song could be helpful in determining the forebrain regions in which sensory information influences motor control and memory of stereotyped song. Additionally, our work indicates that the general principles by which we classify types of song learning may change with further investigation of the experiential and neural mechanisms for song in more species. We suggest, as have others (Brenowitz and Kroodsma, 1996), that an understanding of the neural basis of song in birds will benefit greatly from increasing the number of the species studied. Examining song system circuitry in various species that exhibit different behavioral characteristics could help elucidate which brain regions are important for specific aspects of song learning and maintenance.
Footnotes
This work was supported by National Institutes of Health Grants DC00520 and GM07108. We thank Michael Beecher, Sarah Bottjer, Eliot Brenowitz, and Mark Konishi for their valuable advice and contributions to this work.
Correspondence should be addressed to Dr. Edwin W Rubel, Neurobiology and Behavior Program and Virginia Merrill Bloedel Hearing Research Center, Box 357923, University of Washington, Seattle, WA 98195.
REFERENCES
- 1.Bottjer SW, Arnold AP. The role of feedback from the vocal organ. I. Maintenance of stereotypical vocalizations by adult zebra finches. J Neurosci. 1984;4:2387–2396. doi: 10.1523/JNEUROSCI.04-09-02387.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenowitz EA, Kroodsma DE. The neuroethology of bird song. In: Kroodsma D, Miller E, editors. Ecology and evolution of acoustic communication in birds. Comstock; Ithaca, NY: 1996. pp. 285–304. [Google Scholar]
- 3.Clayton NS. Song learning in Bengalese finches: a comparison with zebra finches. Ethology. 1987;76:247–255. [Google Scholar]
- 4.Clayton NS. Song tutor choice in zebra finches and Bengalese finches: the relative importance of visual and vocal cues. Behaviour. 1988;104:281–299. [Google Scholar]
- 5.Clayton NS. The effects of cross-fostering on selective song learning in estrildid finches. Behaviour. 1989;109:163–175. [Google Scholar]
- 6.Cowie R. Postlingually acquired deafness: speech deterioration and the wider consequences. Mouton de Gruyter; New York: 1992. [Google Scholar]
- 7.Dietrich K. Model choice in the song development of young male Bengalese finches. Z Tierpsychol. 1980;52:57–76. [Google Scholar]
- 8.Dittus WP, Lemon RE. Effects of song tutoring and acoustic isolation on the song repertoires of cardinals. Anim Behav. 1969;17:523–533. [Google Scholar]
- 9.Eales LA. Song learning in zebra finches: some effects of song model availability on what is learnt and when. Anim Behav. 1985;33:1293–1300. [Google Scholar]
- 10.Hailman JP. Optical signals: animal communication and light. Indiana UP; Bloomington, IN: 1977. [Google Scholar]
- 11.Immelmann K. Song development in the zebra finch and other estrildid finches. In: Hinde RA, editor. Bird vocalizations. Cambridge UP; Cambridge, UK: 1969. pp. 61–77. [Google Scholar]
- 12.Konishi M. The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Z Tierpsychol. 1965;22:770–783. [PubMed] [Google Scholar]
- 13.Konishi M. An outline of recent advances in birdsong neurobiology. Brain Behav Evol. 1994;44:279–285. doi: 10.1159/000113582. [DOI] [PubMed] [Google Scholar]
- 14.Marler P. Sensitive periods and the roles of specific and general sensory stimulation in birdsong learning. In: Rauschecker JP, Marler P, editors. Imprinting and cortical plasticity: comparative aspects of sensitive periods. Wiley; New York: 1987. pp. 99–135. [Google Scholar]
- 15.Marler P. Song-learning behavior: the interface with neuroethology. Trends Neurosci. 1991;14:199–206. doi: 10.1016/0166-2236(91)90106-5. [DOI] [PubMed] [Google Scholar]
- 16.Marler P, Peters S. Selective vocal learning in a sparrow. Science. 1977;198:519–521. doi: 10.1126/science.198.4316.519. [DOI] [PubMed] [Google Scholar]
- 17.Marler P, Peters S. A sensitive period for song acquisition in the song sparrow, Melospiza melodia: a case of age-limited learning. Ethology. 1987;76:89–100. [Google Scholar]
- 18.Marler P, Waser MS. Role of auditory feedback in canary song development. J Comp Physiol Psychol. 1977;91:8–16. doi: 10.1037/h0077303. [DOI] [PubMed] [Google Scholar]
- 19.Nordeen KW, Nordeen EJ. Auditory feedback is necessary for the maintenance of stereotyped song in adult zebra finches. Behav Neural Biol. 1992;57:58–66. doi: 10.1016/0163-1047(92)90757-u. [DOI] [PubMed] [Google Scholar]
- 20.Nottebohm F. Auditory experience and song development in the chaffinch (Fringilla coelebs). Ibis. 1968;110:549–568. [Google Scholar]
- 21.Nottebohm F. A brain for all seasons: cyclical anatomical changes in song control nuclei of the canary brain. Science. 1981;214:1368–1370. doi: 10.1126/science.7313697. [DOI] [PubMed] [Google Scholar]
- 22.Nottebohm F, Nottebohm ME. Relationship between song repertoire and age in the canary, Serinus canarius. Z Tierpsychol. 1978;46:298–305. [Google Scholar]
- 23.Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary (Serinus canarius). J Comp Neurol. 1976;165:457–468. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- 24.Okanoya K, Yomeda T, Yamaguchi A, Kimura T (1991) Acoustic communication and auditory feedback in Bengalese finches. Denshi Joho Tsushin Gakkai (in Japanese) SP 90–100.
- 25.Price PH. Developmental determinants of structure in zebra finch song. J Comp Physiol Psychol. 1979;93:268–277. [Google Scholar]
- 26.Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shannon CE. The mathematical theory of communication. Illinois UP; Urbana, IL: 1949. [PubMed] [Google Scholar]
- 28.Waldstein RS. Effects of postlingual deafness on speech production: implications for the role of auditory feedback. J Acoust Soc Am. 1990;88:2099–2114. doi: 10.1121/1.400107. [DOI] [PubMed] [Google Scholar]
- 29.Williams H, McKibben JR. Changes in stereotyped central motor patterns controlling vocalization are induced by peripheral nerve injury. Behav Neural Biol. 1992;57:67–78. doi: 10.1016/0163-1047(92)90768-y. [DOI] [PubMed] [Google Scholar]