Abstract
Purpose
HCM is the most common inherited cardiomyopathy. Historically, there has been poor correlation between genotype and phenotype. However, CMR has the potential to more accurately assess disease phenotype. We characterized phenotype with CMR in a cohort of patients with confirmed HCM and high prevalence of genetic testing.
Methods
Patients with a diagnosis of HCM, who had undergone contrast-enhanced CMR were identified. Left ventricular mass index (LVMI) and volumes were measured from steady-state free precession sequences. Late gadolinium enhancement (LGE) was quantified using the full width, half maximum method. All patients were prospectively followed for the development of septal reduction therapy, arrhythmia or death.
Results
We included 273 patients, mean age 51.2 ± 15.5, 62.9% male. Of those patients 202 (74.0%) underwent genetic testing with 90 pathogenic, likely pathogenic, or rare variants and 13 variants of uncertain significance identified. Median follow-up was 1138 days. Mean LVMI was 82.7 ± 30.6 and 145 patients had late gadolinium enhancement (LGE). Patients with beta-myosin heavy chain (MYH7) mutations had higher LV ejection fraction (68.8 vs 59.1, p<0.001) than those with cardiac myosin binding protein C (MYBPC3) mutations. Patients with MYBPC3 mutations were more likely to have LVEF < 55% (29.7% vs 4.9%, p = 0.005) or receive a defibrillator than those with MYH7 mutations (54.1% vs 26.8%, p = 0.020).
Conclusions
We found that patients with MYBPC3 mutations were more likely to have impaired ventricular function and may be more prone to arrhythmic events. Larger studies using CMR phenotyping may be capable of identifying additional characteristics associated with less frequent genetic causes of HCM.
Introduction
Hypertrophic cardiomyopathy (HCM) is a common hereditary cardiac disorder with a prevalence of approximately 2 cases per 1000 persons.[1] It is caused by mutations in genes encoding sarcomere proteins, [2,3] with more than two dozen putative disease-associated genes identified. MYH7 encoding the β-myosin heavy chain and MYBPC3 encoding cardiac myosin-binding protein C are the most common genes harboring causative mutations.[4–6] HCM is a frequent cause of sudden cardiac death (SCD) in youth and a significant underlying pathology for cardiac morbidity and mortality in adults.[5] It is believed that myocardial fibrosis, a hallmark of HCM, contributes to the development of SCD, ventricular tachyarrhythmias, and congestive heart failure (CHF).[7–11]
Cardiovascular magnetic resonance imaging (CMR) has emerged as a valuable tool for assessing HCM through quantification of ventricular volumes, mass, and identification of myocardial fibrosis with late gadolinium enhancement (LGE) to assess SCD risk.[12–14] The volume and morphology of LGE have been associated with worse cardiovascular outcome, including higher incidence and recurrence of ventricular tachyarrhythmia, hospital admissions due to progressive CHF, and an independent predictor of all cause and cardiac mortality.[15–18] It has also been demonstrated that LGE is more common in patients with a positive genetic test as compared to those with a negative genetic test; however, HCM is known for marked pleiotropy for any specific phenotype. [19,20]
The purpose of this study was to determine whether CMR findings could identify specific genotype-phenotype relationships in HCM through measurement of ventricular volumes, mass and function or characterization of LGE. Furthermore, we assessed the associations between CMR characteristics, genetic diagnosis and adverse cardiovascular events.
Methods
Patient population
This study was a retrospective analysis of data acquired in consecutive patients with HCM who underwent contrast-enhanced CMR at Stanford Hospital and Clinics between December 2006 and December 2017. Patients were excluded if the diagnosis of HCM could not be confirmed (n = 83) or if CMR studies were performed at an outside institution and images were not available for interpretation (n = 3). The diagnosis of HCM was based on standard clinical criteria including all components of the history, physical examination, electrocardiography, echocardiography, and CMR.[21] Alternate diagnoses including aortic stenosis and infiltrative cardiomyopathies were excluded by experienced cardiologists with additional training in HCM. Patients with a history of myectomy or alcohol septal ablation prior to CMR were excluded. Patient demographics were collected from existing patient records.
Patients were offered genetic testing with patient consent through clinical care at the Stanford Center for Inherited Cardiovascular Diseases. Genetic testing was performed in 202 patients. Genetic testing was performed with exonic sequencing of at least 8 myofilament-encoding, HCM-susceptibility genes as part of commercially available HCM genetic tests during the period of study (Ambry Genetics, Aliso Viejo, CA; Correlagen Diagnostics, Inc., Waltham, MA; GeneDX, Gaithersburg, MD; Invitae Corp, San Francisco, CA; Laboratory for Molecular Medicine, Cambridge MA; PGx Testing, Garden Ridge, TX; Transgenomic Molecular Laboratory, Omaha, NE). Sequences were compared with the reference human genome and variants detailed by the genetic testing company. All reported variants were independently investigated by the multidisciplinary team which included dedicated genetic counsellors and scored according to the confidence with which they could be called disease-causing.[22] This was based on type of variant, position of variant, prior co-segregation data, and in the case of novel variants, tools based on conservation and constraint as described previously.[23] S1 Table summarizes the classification of genetic variants. Patients were classified as having no variant found or having either variant of uncertain significance (VUS) or disease-associated variants meeting classification types ‘likely pathogenic’ or ‘pathogenic’. Gene variants which occurred at a population frequency <1 in 10,000 were categorized as rare variants. Patients with more than one variant were classified according to the disease causing-variant as described above, if one was found. No patients had disease-causing variants identified in more than one gene. Patients without a disease-associated variant, but with rare VUS in either MYH7 or MYBPC3 were included with patients with disease-associated variants in the respective genes. Separate analyses were performed in which these patients were included with other patients having a VUS as a sensitivity analysis, results in S2 and S3 Tables. Characteristics of patients who did not undergo genetic testing are shown in S4 Table.
Image acquisition and analysis
CMRs were ordered as part of routine clinical care, and were typically performed to better delineate anatomy or to determine extent of LGE. All CMR images were acquired on a 1.5-Tesla whole-body scanner (Signa, GE Healthcare, Milwaukee, WI) with the patient in a supine position using an 8-element phased-array radiofrequency coil with breath-holding and cardiac gating. Cine images of the LV in short and long axes were acquired using a steady-state free precession sequence (SSFP, TR 2.4–3.9, TE 0.9–2.0, slice thickness 8 mm). LGE images (segmented k-space inversion recovery sequence, TR 3.4–5.0, TE 1.1–1.5, TI 150–300, slice thickness 8 mm) were acquired throughout the entire LV starting at 10 min, after administration of 0.1–0.2 mmol/kg of gadolinium diethylenetriaminepentaacetic acid (Gd-DTPA, Magnevist, Schering AG, Germany). The inversion time was set to null the signal of normal myocardium after Gd-DTPA and was adjusted during the scan as necessary.
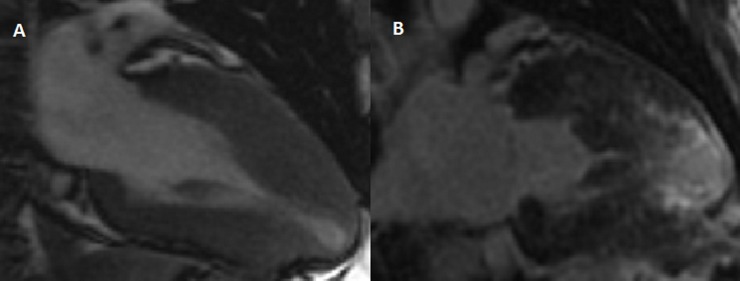
Cine images were analyzed using MASS analysis software (MASS Analysis Plus Version 6.0, Leiden University). Semi-automated contours were manually adjusted to match the endocardial and epicardial borders and exclude the papillary muscles from short-axis images at end-diastole.[24] The same contours were used to calculate left ventricular (LV) and right ventricular (RV) end-diastolic volumes (LVEDV and RVEDV), LV and RV end-systolic volumes (LVESV and RVESV), and LV and RV ejection fractions (LVEF and RVEF). Normal LV mass was defined as < 81 g/m2 for males and < 62 g/m2 for females.[24] Septal morphology and cavity contour was evaluated, in the long axis view and scored for 4 subtypes (sigmoid, reverse curvature, apical or other) as previously described.(26) Analysis of LGE was performed visually by defining the areas of hyperenhancement in all myocardial segments as seen on the long and short-axis slices and quantified using a full width, half maximum (FWHM) method. The FWHM method defines core scar as voxels that contain a signal intensity at least 50% of the maximal signal intensity.[25] Grey zone scar is defined as areas with less than 50% of the maximal intensity but greater than the peak signal intensity in remote myocardium.[25] A case example is shown in Fig 1. Two authors (RM and SH) performed image analysis. A subset of 10 patients was read by both authors for ventricular mass, volume, morphology, presence of LGE and scar quantification. Inter-rater reliability was good between readers (Pearson’s r>0.95 for all variables).
Fig 1. Cardiac magnetic resonance characterization of a patient with a MYH7 variant.
Panel A. Cine images at end systole showing apical hypertrophy with apical aneurysm. Panel B. Delayed enhancement images showing extensive LGE in the distal myocardial segments including the apex.
Outcomes
Patients were followed prospectively for cardiovascular events including: alcohol septal ablation, septal myectomy, sustained VT, appropriate implanted cardiac defibrillator (ICD) shock, SCD, and all-cause death. SCD included patients with resuscitated cardiac arrest. Sustained VT was defined as ventricular rhythms faster than 100 beats per minute and lasting for more than 30 seconds or treated with anti-tachycardia pacing on review of ambulatory ECG monitoring or implanted cardiac device logs. Appropriate ICD shock was defined as a ventricular rhythm greater than 100 beats per minute which resulted in ICD discharge (excluding anti-tachycardia pacing). Follow up was obtained during scheduled clinic visits supplemented by telephone contact with patients or their relatives to ensure more complete follow-up. All patients had at least 90 days of clinical follow-up.
Statistical analysis
Continuous variables were summarized as mean (standard deviation [SD]) if normally distributed and compared using a Student’s t-test. Continuous variables which were not normally distributed were summarized as median (interquartile range [IQR]) and compared using a Wilcoxon rank-sum test. Categorical variables are summarized as number (proportion) and compared using a Fisher’s Exact test.
We performed multivariable Cox regression analyses to assess for the association between the presence of late gadolinium enhancement and clinical outcomes as well as between genetic diagnoses and clinical outcomes. Due to low event numbers, events were combined as: septal ablation or myectomy, sustained VT or appropriate ICD shock, and all-cause mortality. Models were corrected for age and gender. All statistical tests were two-sided and a p-value <0.05 was considered significant. A sensitivity analysis with rare variants included as VUS, with results in S2 and S3 Tables. All analyses were performed using Stata/IC version 13.1 (StataCorp, College Station, Texas). The study protocol was approved by the Institutional Review Board at Stanford University.
Results
Clinical characteristics
We included 273 patients with a diagnosis of HCM who underwent CMR imaging. Patient characteristics are outlined in Table 1. The cohort was predominantly Caucasian (64.5%) men (62.9%) with a mean age 51.2 ± 15.5 (standard deviation). LGE was present in 145 (53.1%) patients. Patients with pathogenic, likely pathogenic or rare MYH7 variants were a similar mean age as those with MYBPC3 variants (45.7 vs. 45.9 years, p = 0.959). Patients with LGE were also less likely to be Caucasian (57.2% vs 72.7%, p = 0.011) and were more likely to be in New York Heart Association (NYHA) class I (65.5 vs 53.1%, p = 0.048).
Table 1. Baseline population characteristics.
| Total (n = 273) |
No LGE (n = 128) |
LGE+ (n = 145) |
p-value | |
|---|---|---|---|---|
| Age at CMR | 51.2 ± 15.5 | 50.6 ± 15.0 | 52.0 ± 16.1 | 0.474 |
| Male (%) | 173 (62.9) | 73 (57.0) | 100 (69.0) | 0.045 |
| BSA (m2) | 1.94 ± 0.25 | 1.95 ± 0.24 | 1.94 ± 0.26 | 0.859 |
| Proband | 268 (98.2) | 125 (97.7) | 143 (98.6) | 0.668 |
| Maximal LV wall thickness (mm) | 18 (16–21) | 16 (15–19) | 20 (16–24) | <0.001 |
| Ethnicity | ||||
| Caucasian | 176 (64.5) | 93 (72.7) | 83 (57.2) | 0.011 |
| Asian | 41 (15.0) | 17 (13.3) | 24 (16.6) | 0.500 |
| Latino | 24 (8.8) | 12 (9.4) | 12 (8.3) | 0.832 |
| African-American | 9 (3.3) | 2 (1.6) | 7 (4.8) | 0.180 |
| Other | 22 (8.1) | 5 (3.9) | 17 (11.7) | 0.024 |
| Congestive Heart Failure | ||||
| NYHA I | 163 (59.7) | 68 (53.1) | 95 (65.5) | 0.048 |
| NYHA II | 73 (26.7) | 37 (28.9) | 36 (24.8) | 0.494 |
| NYHA III | 34 (12.5) | 21 (16.4) | 13 (9.0) | 0.069 |
| NYHA IV | 4 (1.5) | 2 (1.6) | 2 (1.4) | 1.000 |
| Resting LVOT gradient | 2 (0–28) | 4 (0–33) | 1 (0–26) | 0.574 |
| Atrial Fibrillation | 30 (11.0) | 15 (11.7) | 15 (10.3) | 0.847 |
| Dyslipidemia | 84 (30.8) | 37 (28.9) | 47 (32.4) | 0.600 |
| Hypertension | 108 (39.6) | 57 (44.5) | 51 (35.2) | 0.137 |
| Diabetes | 19 (7.0) | 6 (4.7) | 13 (9.0) | 0.233 |
| Risk Factors for SCD | ||||
| LV wall thickness > 30 mm | 9 (3.3) | 1 (0.8) | 8 (5.5) | 0.039 |
| FH SCD | 108 (39.6) | 44 (34.4) | 64 (44.1) | 0.108 |
| Unexplained syncope | 65 (23.8) | 31 (24.2) | 34 (23.5) | 0.888 |
| h/o SCD or sustained VT | 7 (2.6) | 3 (2.3) | 4 (2.8) | 1.000 |
| Hypotension on ETT | 33 (12.1) | 16 (12.5) | 17 (11.7) | 0.855 |
| Rest gradient >30mmHg | 68 (24.9) | 34 (26.6) | 34 (23.5) | 0.577 |
| History of NSVT | 78 (28.6) | 32 (25.0) | 46 (31.7) | 0.230 |
| Medications | ||||
| Beta-blocker | 137 (50.2) | 66 (51.6) | 71 (49.0) | 0.706 |
| Anti-arrhythmic therapy | 31 (11.3) | 11 (8.6) | 20 (13.8) | 0.447 |
LV–left ventricle, LVOT–left ventricular outflow tract, NSVT–Non-sustained ventricular tachycardia, NYHA–New York Heart Association, SCD–Sudden Cardiac Death, VT–ventricular tachycardia.
Population phenotypes
CMR characteristics for patients with and without LGE are shown in Table 2. Patients with LGE had a higher maximal wall thickness compared to patients without (median 20 vs 16, p<0.001). Patients with LGE also had lower LVEF (median 61.1 vs 65.5, p<0.001). Lastly, patients with LGE were less likely to have proximal septal hypertrophy (13.8% vs 39.8%, p<0.001) and more likely to have reverse curvature (44.8% vs 27.3%, p = 0.004) morphology.
Table 2. CMR characteristics stratified by late gadolinium enhancement.
| Total (n = 273) |
No LGE (n = 128) |
LGE+ (n = 145) |
p-value | |
|---|---|---|---|---|
| LV mass indexed (g/m2) | 77.3 (62.5–96.9) | 69.1 (55.9–85.2) | 87.7 (69.6–106.8) | <0.001 |
| Maximal LV wall thickness (mm) | 18 (16–21) | 16 (15–19) | 20 (16–24) | <0.001 |
| LVEF (%) | 63.8 (57.1–70.0) | 65.5 (61.2–72.2) | 61.1 (56.6–68.0) | <0.001 |
| LVEDVI (ml/m2) | 82.0 (72.0–94.3) | 80.0 (69.1–91.4) | 83.3 (72.2–95.0) | 0.063 |
| LVESVI (ml/m2) | 29.3 (22.1–37.9) | 26.9 (20.5–36.4) | 31.9 (24.0–40.0) | 0.002 |
| RVEF (%) | 62.0 (56.6–67.7) | 61.1 (55.2–67.8) | 62.6 (58.7–67.6) | 0.062 |
| RVEDV indexed (ml/m2) | 76.5 (62.3–89.1) | 79.4 (62.7–90.9) | 75.4 (62.0–88.5) | 0.246 |
| RVESV indexed (ml/m2) | 28.0 (20.8–36.9) | 30.3 (21.5–39.1) | 25.9 (20.5–35.2) | 0.044 |
| Morphology | ||||
| Sigmoid | 71 (26.0) | 51 (39.8) | 20 (13.8) | <0.001 |
| Reverse Curvature | 100 (36.6) | 35 (27.3) | 65 (44.8) | 0.004 |
| Apical | 46 (16.9) | 18 (14.1) | 28 (19.3) | 0.262 |
| Concentric or indeterminate | 56 (20.5) | 24 (18.8) | 32 (22.1) | 0.550 |
EF–Ejection Fraction, EDV–End diastolic volume index, ESVI–End systolic volume index, LV–left ventricle, RV–right ventricle, SVI–stroke volume.
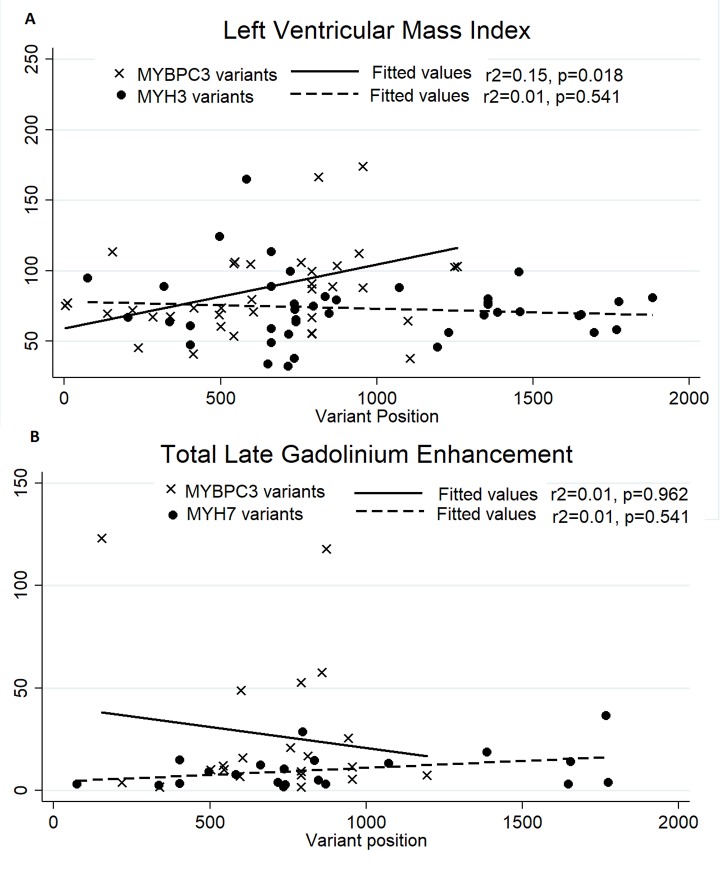
Genetic testing was performed in 202 patients. The CMR characteristics stratified by genetic testing diagnosis are shown in Table 3. Patients with pathogenic, likely pathogenic or rare MYH7 variants had higher LVEF than those with MYBPC3 variants (68.8 vs 59.1, p<0.001) and higher RVEF (67.3 vs 60.8, p = 0.018). Additionally, patients with MYBPC3 variants were more likely to have LVEF < 55% (29.7% vs 4.9%, p = 0.005). However, maximal wall thickness, LV morphology, and presence of LGE were similar. Patients without an identifiable gene variant had higher LVMI (84.4 vs 72.2, p = 0.008). There was no difference in the proportion of patients with LGE between patients with and without identified gene variants (55.3% vs 44.4% p = 0.159). However, in those patients with LGE, scar volume was higher in patients with an identified pathogenic, likely pathogenic or rare variant (total scar 9.14 g vs 4.40 g, p = 0.020) and there was a higher proportion of LV mass replaced by scar (5.05% vs 2.28%, p = 0.002). Correlation between variant position and phenotype is shown in Fig 2. MYBPC3 variant position was associated with LVMI (p = 0.018), but with poor overall correlation (r2 = 0.15).
Table 3. CMR characteristics stratified by genotype.
|
MYH7 (n = 41) |
MYBPC3 (n = 37) |
Other gene variants (n = 12) | VUS (n = 13) |
No identified mutation (n = 99) |
p Value (MYH7 vs MYBPC3) | p-value (No variant vs. any variant) |
|
|---|---|---|---|---|---|---|---|
| LVMI (g/m2) | 70.4 (58.6–80.8) | 77.2 (67.3–103.3) | 68.8 (55.4–96.9) | 72.1 (61.5–83.0) | 84.4 (68.7–102.2) | 0.066 | 0.008 |
| Maximal LV thickness (mm) | 18 (15–21) | 20 (16–24) | 19 (16–23) | 18 (15–23) | 18 (16–22) | 0.075 | 0.486 |
| LVEF (%) | 68.8 (63.0–74.3) | 59.1 (54.0–67.1) | 64.0 (61.1–70.3) | 61.6 (58.4–62.5) | 66.4 (61.0–72.6) | <0.001 | 0.076 |
| LVEDVI (ml/m2) | 79.4 (69.0–94.0) | 83.5 (76.4–98.3) | 76.9 (69.5–92.1) | 82.9 (77.2–99.5) | 81.0 (70.6–94.7) | 0.206 | 0.611 |
| LVESVI (ml/m2) | 23.9 (19.5–33.3) | 32.1 (25.2–43.3) | 25.9 (23.6–35.1) | 32.3 (30.3–36.9) | 26.3 (20.5–36.4) | 0.014 | 0.092 |
| RVEF (%) | 67.3 (58.7–73.1) | 60.8 (55.7–65.2) | 61.6 (59.3–67.5) | 59.9 (54.8–62.9 | 61.9 (55.9–69.2) | 0.018 | 0.609 |
| RVEDVI (ml/m2) | 73.1 (57.2–86.9) | 78.3 (64.2–91.4) | 71.9 (62.5–96.9) | 80.0 (67.1–89.8) | 74.2 (60.5–88.9) | 0.240 | 0.677 |
| RVESVI (ml/m2) | 23.8 (18.2–31.0) | 31.4 (23.1–38.6) | 28.3 (19.4–37.5) | 33.8 (19.9–38.1) | 27.8 (19.3–36.3) | 0.022 | 0.973 |
| Morphology | |||||||
| Sigmoid | 11 (26.8) | 10 (26.8) | 3 (25.0) | 3 (23.1) | 31 (31.3) | 1.000 | 0.441 |
| Reverse Curvature | 18 (43.9) | 21 (56.8) | 4 (33.3) | 3 (23.1) | 28 (28.3) | 0.365 | 0.019 |
| Apical | 7(17.1) | 3 (8.1) | 2 (16.7) | 3 (23.1) | 24 (24.2) | 0.317 | 0.108 |
| Concentric or Indeterminate | 5 (12.2) | 3 (8.1) | 3 (25.0) | 4 (30.8) | 16 (16.2) | 0.715 | 0.846 |
| Any LGE | 22 (53.7) | 21 (56.8) | 8 (66.7) | 6 (46.2) | 44 (44.4) | 0.823 | 0.159 |
| Any sub-endocardial | 11 (26.8) | 8 (21.6) | 1 (8.3) | 2 (15.4) | 21 (21.2) | 0.610 | 1.000 |
| Any mid-myocardial | 17 (41.5) | 17 (46.0) | 8 (66.7) | 3 (23.1) | 31 (31.3) | 0.820 | 0.082 |
| Any epicardial | 6 (14.6) | 7 (18.9) | 2 (16.7) | 1 (7.7) | 8 (8.1) | 0.763 | 0.129 |
| LGE >50% wall thickness | 12 (29.3) | 7 (18.9) | 0 (0.0) | 1 (7.7) | 13 (13.1) | 0.307 | 0.257 |
| LGE Segments | 1 (0–5) | 2 (0–5) | 2 (0–4) | 0 (0–4) | 0 (0–3) | 0.725 | 0.0252 |
| Core Scar (g) | 1.77 (1.06–5.03) | 4.17 (1.92–9.14) | 2.82 (0.59–5.46) | 2.24 (0.85–7.52) | 1.34 (0.33–4.59) | 0.055 | 0.019 |
| Gray Zone Scar (g) | 6.40 (2.18–8.70) | 7.88 (4.85–22.73) | 3.08 (2.03–5.27) | 5.46 (2.68–7.74) | 2.72 (1.48–6.69) | 0.114 | 0.005 |
| Total scar (g) | 8.32 (3.00–13.95) | 11.49 (7.21–25.31) | 6.01 (2.61–11.05) | 7.69 (3.53–19.29) | 4.40 (1.88–10.54) | 0.099 | 0.020 |
| Total Scar (%LV mass) | 3.85 (2.37–10.65) | 5.80 (3.39–13.20) | 6.43 (2.08–8.90) | 3.48 (1.70–14.79) | 2.28 (1.02–6.73) | 0.308 | 0.002 |
Core scar and gray zone scar were determined using the full-width, half-maximum method. Scar quantification reflects values in patients with visual LGE. EF–Ejection Fraction, EDVI–End diastolic volume index, ESVI–End systolic volume index, LGE–late gadolinium enhancement, LV–left ventricle, LVMI–left ventricular mass index, RV–right ventricle, SV–stroke volume, VUS–variant of uncertain significance.
Fig 2. Correlation between variant position and phenotype.
Panel A shows the correlation between variant position and left ventricular mass index. Variant position was associated with LVMI in MYBPC3 variants (p = 0.018). Panel B shows the lack of correlation between variant position of total late gadolinium enhancement.
Clinical outcomes
Patients were followed clinically with median duration of follow-up of 1138 days (Interquartile range 230–1971). Clinical events during follow-up are shown in Table 4. Patients with LGE were more likely to have an ICD implanted (34.5 vs 19.5%, p = 0.007). However, there was no difference in the number of patients with an appropriate ICD shock (14% vs 16%, p = 1.000). Patients with LGE were more likely to have sustained VT or appropriate ICD shock (31.0 vs 19.5%, p = 0.037). Summary of multivariable Cox proportional Hazards analyses for the presence of LGE, adjusted for age and gender, are shown in S1 Fig. Presence of LGE was associated with increase in sustained VT or appropriate ICD shock (adjusted HR 1.94, 95% CI 1.16–3.24). However, LGE was not associated with a need for septal reduction therapy (adjusted HR 0.84, 95% CI 0.49–1.44, p = 0.53) or death (adjusted HR 1.12, 95% CI 0.33–3.86, p = 0.86). Similarly total scar volume was associated with an increase in sustained VT or appropriate ICD shock (adjusted HR 1.02 per g, 95% CI 1.00–1.03, p = 0.018), but not septal reduction therapy (adjusted HR 0.99, 95% CI 0.97–1.02) or death (adjusted HR 1.01, 95% CI 0.99–1.04, p = 0.343).
Table 4. Clinical outcomes stratified by presence of LGE.
| Total (n = 273) |
No LGE (n = 128) |
LGE+ (n = 145) |
p-value | |
|---|---|---|---|---|
| Myectomy/ Septal Ablation | 56 (20.5) | 31 (24.2) | 25 (17.2) | 0.177 |
| ICD Implanted | 75 (27.5) | 25 (19.5) | 50 (34.5) | 0.007 |
| Appropriate ICD Shock | 11 of 75 (14.7) | 4 of 25 (16.0) |
7 of 50 (14.0) |
1.000 |
| Sustained VT or Appropriate ICD shock | 70 (25.6) | 45 (31.0) | 25 (19.5) | 0.037 |
| Sudden Cardiac Death | 6 (2.2) | 4 (3.1) | 2 (1.4) | 0.424 |
| All-cause mortality | 11 (4.0) | 6 (4.7) | 5 (3.5) | 0.760 |
ICD–implantable cardioverter defibrillator, VT–ventricular tachycardia.
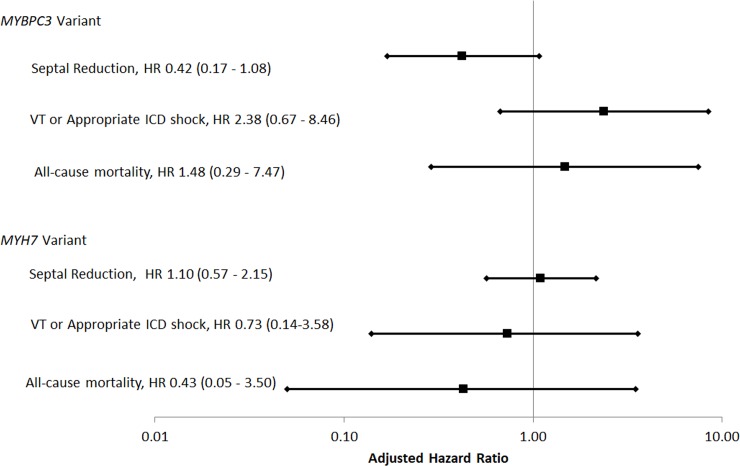
Clinical events stratified by genetic testing findings are shown in Table 5. Patients with MYBPC3 variants were more likely to have an ICD implanted than those with MYH7 variants (54.1% vs 26.8%, p = 0.020) without a difference in appropriate ICD shock (4/20 [20.0%] vs 2 of 11 [18.2%], p = 1.00). There was a trend towards an increase in sustained VT or appropriate ICD shock in patients with MYBPC3 variants (27.0% vs 12.2%, p = 0.150) Summary of multivariable Cox proportional hazards analyses, adjusted for age and gender, are shown in Fig 3. There was no difference in time to septal reduction therapy in patients with MYBPC3 (adjusted HR 0.42, 95% CI 0.17–1.08, p = 0.071) or MYH7 variants (adjusted HR 1.10, 95% CI 0.57–2.15, p = 0.768). Similarly, there was no difference in time to sustained VT or appropriate ICD shock with MYBPC3 (adjusted HR 2.38, 95% CI 0.67–8.46, p = 0.1829) or MYH7 gene variants (adjusted HR 0.73, 95% CI 0.14–3.58, p = 0.699). There was no increased risk of death with MYBPC3 (1.48, 95% CI 0.29–7.47, p = 0.6329) or MYH7 variants (0.43, 95% CI 0.05–3.50, p = 0.428).
Table 5. Clinical outcomes by genetic testing result.
|
MYH7 (n = 41) |
MYBPC3 (n = 37) |
Other gene variants (n = 12) | VUS (n = 13) |
No identified mutation (n = 99) |
p Value (MYH7 vs MYBPC3) | p-value (No variant vs. any variant) |
|
|---|---|---|---|---|---|---|---|
| Myectomy/ Septal Ablation | 12 (29.3) | 5 (13.5) | 1 (8.3) | 2 (15.4) | 30 (30.3) | 0.108 | 0.102 |
| ICD Implanted | 11 (26.8) | 20 (54.1) | 5 (41.7) | 4 (30.8) | 26 (26.3) | 0.020 | 0.072 |
| Appropriate ICD Shock | 2 of 11 (18.2) |
4 of 20 (20.0) |
0 of 5 (0.0) |
0 of 4 (0.0) |
5 of 26 (19.2) |
1.000 | 0.741 |
| Sustained VT or Appropriate ICD shock | 5 (12.2) | 10 (27.0) | 1 (8.3) | 3 (23.1) | 27 (27.3) | 0.150 | 0.179 |
| Sudden Cardiac Death | 0 (0.0) | 2 (5.4) | 0 (0.0) | 1 (7.7) | 2 (2.0) | 0.222 | 1.000 |
| All-cause Mortality | 1 (2.4) | 2 (5.4) | 0 (0.0) | 0 (0.0) | 6 (6.1) | 0.601 | 0.324 |
ICD–implantable cardioverter defibrillator, VT–ventricular tachycardia.
Fig 3. Summary of associations between clinical outcomes and genetic diagnosis.
Note logarithmic scale.
Discussion
Classically HCM has been characterized by poor correlation between genotype and phenotype. We sought to establish a correlation using CMR to characterize morphology which has potential benefits over echocardiography for this purpose. The high temporal and spatial resolution of CMR with superior intrinsic contrast allows more precise evaluation of myocardial morphology and reproducible quantitative assessment of ventricular volumes and function. [26–29] We found that patients with MYBPC3 variants were more likely to have impaired ventricular function compared to patients with MYH7 variants and had a trend towards an increase in arrhythmic events, with a higher proportion of patients receiving ICDs. Finally, we found that LGE burden was higher in patients with identifiable gene variants. Our findings, in a small population, suggest that there is correlation between genotype and phenotype, however the impact on clinical outcomes is less clear.
Phenotype in patients with genetic variants
Genetic testing for HCM is used clinically in the form of targeted exonic sequencing of known disease-causing genes.[23] Echocardiography has traditionally been used for genotype-phenotype correlation in HCM, and prior studies have shown that the reverse curvature septal morphological subtype was a predictor of positive genetic testing.[30] Studies using CMR to help characterize HCM genotype-phenotype relationships have also found that more patients with any genetic mutation had reverse curvature HCM in comparison to sigmoidal HCM or apical HCM, indicating that CMR may be useful in genotype-phenotype analysis.[19] In the same study, it was noted that LGE was more common in those with a positive genetic test in comparison to those with a negative test. In our study, we found no association between presence of LGE and genetic diagnosis but did find larger volumes of LGE associated with the presence of an identifiable variant. Additionally, we did not see an association between genetic testing result and overall morphology. We used contemporary genetic testing data and found a larger proportion of patients with abnormal genetic testing compared to previous studies.[30,31]
Differences between MYH7 and MYBPC3
In our cohort, the most common variants occurred in MYH7 and MYBPC3. Our analysis revealed that the presence of a MYBPC3 variant was associated with a lower LVEF and a higher prevalence of low LVEF. Interestingly, Weissler-Snir et al. found a similar trend in a cohort of HCM patients characterized with CMR.[31] No other mutation groups were sufficiently prevalent to allow further characterization. MYBPC3 is a key component of myocardial thick filaments and variants have been associated with dilated cardiomyopathy.[32] Additionally, Additionally, MYBPC3 variants have been associated with impaired ventricular function in patients with coronary artery disease.[33] However, it is not clear why ventricular function is less impaired in patients with MYH7 variants since an interaction between the two genes seems to be necessary to maintain systolic function.[34] There was also a trend towards increased LGE burden in patients with MYBPC3 variants, which itself was associated with an increase in sustained VT or ICD shock. While we did not see these phenotypic differences translate into clinical outcomes, differences may be seen in larger populations. Data from the sarcomeric human cardiomyopathy (SHARE) registry showed that overall clinical outcomes may be worse in patients with MYH7 variants.[35] However, they found that the incidence of cardiac arrest was higher in patients with MYBPC3 variants.[35] Interestingly, variant position was significantly associated with LVMI in patients with MYBPC3 variants, although with poor overall correlation. Our findings suggest that wider use of CMR as well as genetic testing may help to characterize the phenotypes of other disease-associated genes.
Correlation between CMR characteristics and clinical outcomes
One of the major benefits of CMR characterization in patients with HCM is to quantify LGE which has significant prognostic ability.[16,17] Our findings were consistent with previously published data that demonstrated increased LGE in HCM particularly in areas of increased wall thickness,[16]. and that reverse curvature septal morphology was associated with LGE as previously described.[19,36] We found ICD implantation to be more common, likely representing this increased incidence as well as the use of LGE as a risk stratification tool.[37] ICD shocks were not more common in this group compared to patients without LGE. However, given the low incidence of events in this population and the importance of LGE in other larger cohorts it is likely that our study was not sufficiently powered to demonstrate an association.[38] [39,40]
Limitations
Our study has several important limitations. We had a relatively small patient sample and were not able to assess phenotypic features seen in less frequent gene variants. Since the presence of LGE was based on presence on two orthogonal views, it’s possible that small foci of LGE were not included. Additionally, variation in gadolinium dosing may have impacted LGE identification and quantification. Some of our negative findings, particularly with respect to LGE, may be due to lack of statistical power. However, we were able to demonstrate differences in morphology between the most common variants. We did not assess RV mass, RV wall thickness, or atrial morphology and these may also be associated with genotype. The small patient sample, with limited follow-up duration in some patients, may have impaired our ability to assess for differences in clinical outcomes between groups. However, our data may provide mechanistic insights into data from larger studies such as the SHARE registry. Finally, genetic testing was not complete in our cohort which may have influenced some of our findings. However, our study is one of the largest published cohorts with comprehensive CMR and genetic characterization to date.
Conclusions
CMR may be useful to characterize genotype-phenotype relationships in HCM. We found that patients with MYBPC3 mutations were more likely to have impaired ventricular function and may be more prone to arrhythmic events. Larger studies using CMR phenotyping may be capable of identifying additional characteristics associated with less frequent genetic causes of HCM.
Supporting information
HR–hazard ratio.
(TIF)
Several genetic variants were present in more than one patient and several patients had more than one genetic variant.
(DOCX)
* indicates statistically significant difference between VUS and other groups. EF–Ejection Fraction, EDV–End diastolic volume, ESV–End systolic volume, LV–left ventricle, LVMI–Left ventricular mass index, RV–right ventricle, SV–stroke volume.
(DOCX)
ICD–implantable cardioverter defibrillator, VT–ventricular tachycardia.
(DOCX)
LV–left ventricle, LVOT–left ventricular outflow tract, NSVT–Non-sustained ventricular tachycardia, NYHA–New York Heart Association, SCD–Sudden Cardiac Death, VT–ventricular tachycardia.
(DOCX)
Acknowledgments
We would like to thank David Hsu for his assistance with data collection.
Abbreviations
- CHF
congestive heart failure
- CMR
cardiac magnetic resonance
- HCM
hypertrophic cardiomyopathy
- ICD
Implanted cardiac defibrillator
- IQR
interquartile range
- LV
left ventricle
- LVEDV
Left ventricular end-diastolic volume
- LVEF
left ventricular ejection fraction
- LVESV
Left ventricular end-systolic volume
- LVMI
left ventricular mass index
- LGE
late gadolinium enhancement
- MYH7
beta-myosin heavy chain
- MYBPC3
cardiac myosin binding protein C
- NYHA
New York Heart Association
- RVEF
right ventricular ejection fraction
- RVEDV
right ventricular end-diastolic volume
- RVESV
right ventricular end-systolic volume
- SCD
sudden cardiac death
- SD
standard deviation
- VUS
variant of uncertain significance
Data Availability
Anonymized data are held in a public repository. All files are available at: https://figshare.com/articles/hcm_data_csv/8081657.
Funding Statement
RJHM is supported by the Arthur J E Child fellowship grant. AP was supported by the Breetwor Foundation. AS was supported by a research grant to EAA from MyoKardia. EAA is a consultant and receives researching funding with Myokardia. MTW has ownership interest and is a stockholder in Personalis and has ownership interest, research funding and consulting with MyoKardia. The specific roles of these authors are articulated in the ‘author contributions’ section. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation. 1995;92(4):785–9. [DOI] [PubMed] [Google Scholar]
- 2.Seidman JG, Seidman C. The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell. 2001. February 23;104(4):557–67. [DOI] [PubMed] [Google Scholar]
- 3.Marsiglia JD, Credidio FL, de Oliveira TG, Reis RF, Antunes MO, de Araujo AQ, et al. Clinical predictors of a positive genetic test in hypertrophic cardiomyopathy in the Brazilian population. BMC Cardiovasc Disord. 2014;14:36 10.1186/1471-2261-14-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marian AJ. Update on Hypertrophic Cardiomyopathy. Tex Heart Inst J. 2010;37(3):322–3. [PMC free article] [PubMed] [Google Scholar]
- 5.Marian AJ. Hypertrophic cardiomyopathy: from genetics to treatment. Eur J Clin Invest. 2010;40(4):360–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bos JM, Towbin JA, Ackerman MJ. Diagnostic, prognostic, and therapeutic implications of genetic testing for hypertrophic cardiomyopathy. J Am Coll Cardiol. 2009;54(3):201–11. 10.1016/j.jacc.2009.02.075 [DOI] [PubMed] [Google Scholar]
- 7.Basso C, Thiene G, Corrado D, Buja G, Melacini P, Nava A. Hypertrophic cardiomyopathy and sudden death in the young: pathologic evidence of myocardial ischemia. Hum Pathol. 2000;31(8):988–98. 10.1053/hupa.2000.16659 [DOI] [PubMed] [Google Scholar]
- 8.Shirani J, Pick R, Roberts WC, Maron BJ. Morphology and significance of the left ventricular collagen network in young patients with hypertrophic cardiomyopathy and sudden cardiac death. J Am Coll Cardiol. 2000;35(1):36–44. [DOI] [PubMed] [Google Scholar]
- 9.Choudhury L, Mahrholdt H, Wagner A, Choi KM, Elliott MD, Klocke FJ, et al. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;40(12):2156–64. [DOI] [PubMed] [Google Scholar]
- 10.Varnava AM, Elliott PM, Mahon N, Davies MJ, McKenna WJ. Relation between myocyte disarray and outcome in hypertrophic cardiomyopathy. Am J Cardiol. 2001. August 1;88(3):275–9. [DOI] [PubMed] [Google Scholar]
- 11.Varnava AM, Elliott PM, Sharma S, McKenna WJ, Davies MJ. Hypertrophic cardiomyopathy: the interrelation of disarray, fibrosis, and small vessel disease. Heart. 2000;84(5):476–82. 10.1136/heart.84.5.476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moon JC, McKenna WJ, McCrohon JA, Elliott PM, Smith GC, Pennell DJ. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol. 2003;41(9):1561–7. [DOI] [PubMed] [Google Scholar]
- 13.Maron MS. The current and emerging role of cardiovascular magnetic resonance imaging in hypertrophic cardiomyopathy. J Cardiovasc Transl Res. 2009;2(4):415–25. 10.1007/s12265-009-9136-3 [DOI] [PubMed] [Google Scholar]
- 14.Kwon DH, Setser RM, Thamilarasan M, Popovic ZV, Smedira NG, Schoenhagen P, et al. Abnormal papillary muscle morphology is independently associated with increased left ventricular outflow tract obstruction in hypertrophic cardiomyopathy. Heart. 2008;94(10):1295–301. 10.1136/hrt.2007.118018 [DOI] [PubMed] [Google Scholar]
- 15.Adabag AS, Maron BJ, Appelbaum E, Harrigan CJ, Buros JL, Gibson CM, et al. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51(14):1369–74. 10.1016/j.jacc.2007.11.071 [DOI] [PubMed] [Google Scholar]
- 16.Bruder O, Wagner A, Jensen CJ, Schneider S, Ong P, Kispert EM, et al. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56(11):875–87. 10.1016/j.jacc.2010.05.007 [DOI] [PubMed] [Google Scholar]
- 17.O'Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56(11):867–74. 10.1016/j.jacc.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 18.Kwon DH, Smedira NG, Rodriguez ER, Tan C, Setser R, Thamilarasan M, et al. Cardiac magnetic resonance detection of myocardial scarring in hypertrophic cardiomyopathy: correlation with histopathology and prevalence of ventricular tachycardia. J Am Coll Cardiol. 2009;54(3):242–9. 10.1016/j.jacc.2009.04.026 [DOI] [PubMed] [Google Scholar]
- 19.Rubinshtein R, Glockner JF, Ommen SR, Araoz PA, Ackerman MJ, Sorajja P, et al. Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circ Heart Fail. 2010;3(1):51–8. 10.1161/CIRCHEARTFAILURE.109.854026 [DOI] [PubMed] [Google Scholar]
- 20.Spirito P, Autore C. Management of hypertrophic cardiomyopathy. BMJ. 2006;332(7552):1251–5. 10.1136/bmj.332.7552.1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, et al. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58(25):e212–60. 10.1016/j.jacc.2011.06.011 [DOI] [PubMed] [Google Scholar]
- 22.Bland A, Harrington EA, Dunn K, Pariani M, Platt JCK, Grove ME, et al. Clinically impactful differences in variant interpretation between clinicians and testing laboratories: a single-center experience. Genet Med. 2018;20(3):369–73. 10.1038/gim.2017.212 [DOI] [PubMed] [Google Scholar]
- 23.Wheeler M, Pavlovic A, DeGoma E, Salisbury H, Brown C, Ashley EA. A new era in clinical genetic testing for hypertrophic cardiomyopathy. J Cardiovasc Transl Res. 2009;2(4):381–91. 10.1007/s12265-009-9139-0 [DOI] [PubMed] [Google Scholar]
- 24.Olivotto I, Maron MS, Autore C, Lesser JR, Rega L, Casolo G, et al. Assessment and significance of left ventricular mass by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2008;52(7):559–66. 10.1016/j.jacc.2008.04.047 [DOI] [PubMed] [Google Scholar]
- 25.Amado LC, Gerber BL, Gupta SN, Rettmann DW, Szarf G, Schock R, et al. Accurate and objective infarct sizing by contrast-enhanced magnetic resonance imaging in a canine myocardial infarction model. J Am Coll Cardiol. 2004;44(12):2383–9. 10.1016/j.jacc.2004.09.020 [DOI] [PubMed] [Google Scholar]
- 26.Desai MY, Dhillon A, To AC. Cardiac magnetic resonance in hypertrophic cardiomyopathy. Curr Cardiol Rep. 2011;13(1):67–76. 10.1007/s11886-010-0149-y [DOI] [PubMed] [Google Scholar]
- 27.Rickers C, Wilke NM, Jerosch-Herold M, Casey SA, Panse P, Panse N, et al. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation. 2005;112(6):855–61. 10.1161/CIRCULATIONAHA.104.507723 [DOI] [PubMed] [Google Scholar]
- 28.Moon JC, Fisher NG, McKenna WJ, Pennell DJ. Detection of apical hypertrophic cardiomyopathy by cardiovascular magnetic resonance in patients with non-diagnostic echocardiography. Heart. 2004;90(6):645–9. 10.1136/hrt.2003.014969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ommen SR. Hypertrophic cardiomyopathy. Curr Prob Cardiol. 2011;36(11):409–53. [DOI] [PubMed] [Google Scholar]
- 30.Binder J, Ommen SR, Gersh BJ, Van Driest SL, Tajik AJ, Nishimura RA, et al. Echocardiography-guided genetic testing in hypertrophic cardiomyopathy: septal morphological features predict the presence of myofilament mutations. Mayo Clin Proc. 2006. April;81(4):459–67. 10.4065/81.4.459 [DOI] [PubMed] [Google Scholar]
- 31.Weissler-Snir A, Hindieh W, Gruner C, Fourey D, Appelbaum E, Rowin E, et al. Lack of Phenotypic Differences by Cardiovascular Magnetic Resonance Imaging in MYH7 (β-Myosin Heavy Chain)- Versus MYBPC3 (Myosin-Binding Protein C)-Related Hypertrophic Cardiomyopathy. Circ Cardiovasc Imaging. 2017;10(2). [DOI] [PubMed] [Google Scholar]
- 32.Hitomi N, Kubo T, Kitaoka H, Hirota T, Hamada T, Hoshikawa E, et al. A frameshift deletion mutation in the cardiac myosin-binding protein C gene associated with dilated phase of hypertrophic cardiomyopathy and dilated cardiomyopathy. J Cardiol. 2010. September;56(2):189–96. 10.1016/j.jjcc.2010.04.003 [DOI] [PubMed] [Google Scholar]
- 33.Srivastava A, Garg N, Mittal T, Khanna R, Gupta S, Seth PK, et al. Association of 25 bp deletion in MYBPC3 gene with left ventricle dysfunction in coronary artery disease patients. PloS one. 2011;6(9):e24123 10.1371/journal.pone.0024123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang K-C, Breitbart A, De Lange WJ, Hofsteen P, Futakuchi-Tsuchida A, Xu J, et al. Novel Adult-Onset Systolic Cardiomyopathy Due to MYH7 E848G Mutation in Patient-Derived Induced Pluripotent Stem Cells. JACC: Basic Transl Sci. 2018;3(6):728–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho CY, Day SM, Ashley EA, Michels M, Pereira AC, Jacoby D, et al. Genotype and Lifetime Burden of Disease in Hypertrophic Cardiomyopathy: Insights from the Sarcomeric Human Cardiomyopathy Registry (SHaRe). Circulation. 2018; 138914):1387–98. 10.1161/CIRCULATIONAHA.117.033200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwon DH, Setser RM, Popovic ZB, Thamilarasan M, Sola S, Schoenhagen P, et al. Association of myocardial fibrosis, electrocardiography and ventricular tachyarrhythmia in hypertrophic cardiomyopathy: a delayed contrast enhanced MRI study. Int J Cardiovasc Imaging. 2008;24(6):617–25. 10.1007/s10554-008-9292-6 [DOI] [PubMed] [Google Scholar]
- 37.Fluechter S, Kuschyk J, Wolpert C, Doesch C, Veltmann C, Haghi D, et al. Extent of late gadolinium enhancement detected by cardiovascular magnetic resonance correlates with the inducibility of ventricular tachyarrhythmia in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson. 2010;12:30 10.1186/1532-429X-12-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mentias A, Raeisi-Giglou P, Smedira NG, Feng K, Sato K, Wazni O, et al. Late Gadolinium Enhancement in Patients With Hypertrophic Cardiomyopathy and Preserved Systolic Function. J Am Coll Cardiol. 2018;72(8):857–70. 10.1016/j.jacc.2018.05.060 [DOI] [PubMed] [Google Scholar]
- 39.Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014;130(6):484–95. 10.1161/CIRCULATIONAHA.113.007094 [DOI] [PubMed] [Google Scholar]
- 40.Weng Z, Yao J, Chan RH, He J, Yang X, Zhou Y, et al. Prognostic Value of LGE-CMR in HCM: A Meta-Analysis. JACC Cardiovasc Imaging. 2016;9(12):1392–402. 10.1016/j.jcmg.2016.02.031 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HR–hazard ratio.
(TIF)
Several genetic variants were present in more than one patient and several patients had more than one genetic variant.
(DOCX)
* indicates statistically significant difference between VUS and other groups. EF–Ejection Fraction, EDV–End diastolic volume, ESV–End systolic volume, LV–left ventricle, LVMI–Left ventricular mass index, RV–right ventricle, SV–stroke volume.
(DOCX)
ICD–implantable cardioverter defibrillator, VT–ventricular tachycardia.
(DOCX)
LV–left ventricle, LVOT–left ventricular outflow tract, NSVT–Non-sustained ventricular tachycardia, NYHA–New York Heart Association, SCD–Sudden Cardiac Death, VT–ventricular tachycardia.
(DOCX)
Data Availability Statement
Anonymized data are held in a public repository. All files are available at: https://figshare.com/articles/hcm_data_csv/8081657.