Abstract
Cordycepin is an efficient component of Cordyceps spp, a traditional Chinese medicine widely used for healthcare in China, and has been recently acted as a strong anticancer agent for clinic. However, whether and how it may play a role in combating tuberculosis, caused by Mycobacterium tuberculosis, remains unknown. Here we report that cordycepin can kill Mycobacterium by hijacking the bacterial adenosine kinase (AdoK), a purine salvage enzyme responsible for the phosphorylation of adenosine (Ado) to adenosine monophosphate (AMP). We show that cordycepin is a poor AdoK substrate but it competitively inhibits the catalytic activity of AdoK for adenosine phosphorylation. Cordycepin does not affect the activity of the human adenosine kinase (hAdoK), whereas hAdoK phosphorylates cordycepin to produce a new monophosphate derivative. Co-use of cordycepin and deoxycoformycin, an inhibitor of adenosine deaminase (ADD), more efficiently kills M. bovis and M. tuberculosis. The add-deleted mycobacterium is more sensitive to cordycepin. This study characterized cordycepin as a new mycobactericidal compound and also uncovered a potential anti-mycobacterial mechanism.
Introduction
Tuberculosis is caused by Mycobacterium tuberculosis (Mtb) and still globally threatens human health. In 2017, there were an estimated 10.0 million people fell ill with tuberculosis (TB), of which 457 560 had multidrug-resistant TB (MDR-TB) [1]. With the emergence of multidrug-resistant (MDR) M. tuberculosis strains, new drugs are urgently needed for control of TB.
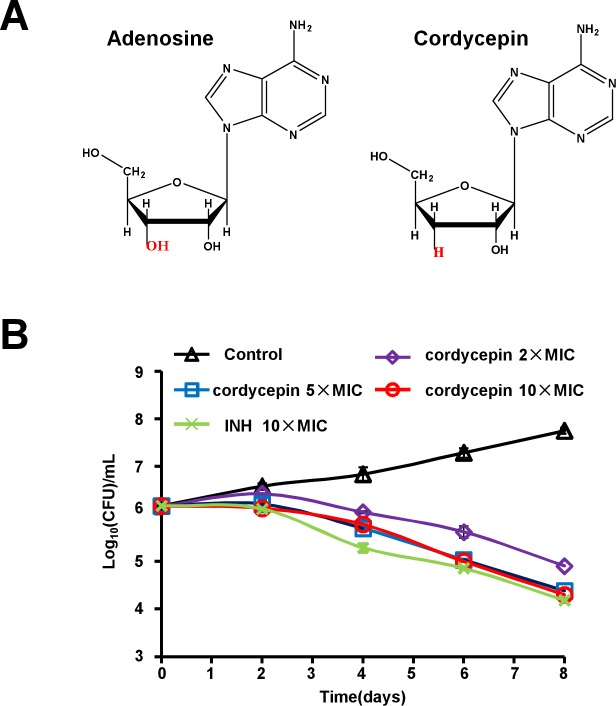
Cordycepin (3′-deoxyadenosine, 3'-dAdo) is a nucleoside and structurally similar to adenosine, but it lacks a 3′-hydroxyl group (Fig 1A). Cordycepin was first isolated from the cultures of caterpillar fungus Cordyceps militaris, a well known Traditional Chinese Medicine widely used for healthcare in China [2]. This compound has been reported to have immunological regulation, antifungal, anti-virus, anti-leukemia, anti-inflammatory and antimetastatic effects [3–10]. In the last few years, cordycepin has been reported to have anti-tumor activity in a broad spectrum of cancer types such as Mouse Melanoma and Lung Carcinoma Cells [5], human lung cancer [11], and human brain cancer [12]. Strikingly, cordycepin can inhibit the growth of several bacterial species including Mycobacterium avium and Mycobacterium bovis [13]. However, whether and how it has a killing activity on M. tuberculosis remains to be explored.
Fig 1. Cordycepin are active against Mycobacterium bovis BCG in vitro.
(A) Structures of adenosine (left panel) and cordycepin (right panel). (B) Kill kinetics of cordycepin for M. bovis BCG over a period of 8 days.
Cordycepin is a structural homolog of adenosine and can be converted into active metabolites by adenosine kinase (AdoK), adenylate kinase and pyruvate kinase in mammalian cells [14, 15]. AdoK catalyzes phosphorylation of adenosine to adenosine monophosphate (AMP) using adenosine triphosphate (ATP) as a phosphate donor and releasing adenosine diphosphate (ADP), which is an important step in the purine salvage pathway [16]. Interestingly, AdoK is rarely found in bacteria, with the single exception of Mycobacterium spp [17, 18]. The activity of AdoK, enoded by Rv2202c (MtbAdoK), has been confirmed in M. tuberculosis [19]. MtbAdoK shares low structural similarity with the well-characterized human AdoKs and behaves very differently [20, 21]. MtbAdoK has been considered as a promising target for drug development [22, 23].
In the present study, we report that cordycepin can kill Mycobacterium by hijacking the bacterial AdoK, which suggests a potential anti-mycobacterial mechanism.
Materials and methods
Strains, plasmids, enzymes and reagents
Escherichia coli (E. coli) BL21 (λ DE3) and pET28a were purchased from Novagen (Darmstadt, Germany) and used to express proteins. Restriction enzymes, T4 ligase, modification enzymes, DNA polymerase, dNTPs were obtained from TaKaRa Biotech (Shiga, Japan). PCR primers were synthesized by Invitrogen (Carlsbad, USA). Ni-NTA (Ni2+-nitrilotriacetate) agarose was purchased from Qiagen (Hilden, Germany). 7H9 and 7H10 broths were purchased from Becton, Dickinson Company (New Jersey, USA). Cordycepin (3’-deoxyadenosine, from Cordyceps militaris, C2689) was purchased from Tokyo chemical industry CO., LTD. (Tokyo, Japan).
Cloning, expression and purification of recombinant proteins
adoK (Rv2202c) from M. tuberculosis H37Rv genome and hAdoK from Homo sapiens genome were amplified by PCR using specific primers (5′-TGTAGAATTCATGTGACGATCGCGGTAACCGG-3′, and 5′- TATATATCTAGACTAGGCCAGCACGGCGACGA-3′for adoK,5′-ATGCGGCCGCAATGACGTCAGTCAGAGAAAA-3′ and 5′-ATGCGCTCTAGATCAGTGGAAGTCTGGCTTCT-3′ for hAdoK), and the mutant genes adoK-S115L and adoK-V33A were amplified by PCR using cordycepin resistant strains genome as templates. The amplified DNA fragments were cloned into the modified pET28a expression vectors to produce recombinant plasmids (S1 Table). E. coli BL21 cells were used to express the recombinant proteins. The recombinant E. coli BL21 cells were grown in 1 L Luria broth (LB) medium up to OD600 of 0.6. Protein expression was induced by the addition of 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) at 16°C for 18 h. The harvested cells were resuspended and sonicated in binding buffer (20 mM Tris-HCl, pH 8.0, 500 mM NaCl, 5 mM imidazole). The lysate was centrifuged at 10000 g for 30 min, and the supernatant was loaded onto the affinity column (Ni-NTA agarose affinity matrix). The column-bound protein was washed with a wash buffer (20 mM Tris-HCl, pH 8.0, 500 mM NaCl, 50 mM imidazole). The elution was dialyzed for 2 h and stored in buffer (20 mM Tris-HCl, pH 8.0, 100 mM NaCl, 5 mM MgCl2, 10% glycerol) at -80°C. Protein concentration was detected using the Bradford method.
Minimum inhibitory concentrations assays
MICs was determined using tube-broth dilution methods as previously described with several modifications [24]. For this assay, mycobacterial strains were grown in 7H9 broth without Tween-80. Other bacteria were grown in LB media. Mid-log phase culture was diluted to 5×106 CFU·ml-1, and 0.1 ml of the dilution was used to inoculate 2.5 ml culture media containing various concentrations (0–1.2 mM) of cordycepin. Positive control (isoniazid, 0.36 μM) and growth control (no compound) were included. Each concentration has three repetitions. Tubes were incubated while shaking at 37°C for 2 weeks for slow-growing mycobacterial strains and for 1–2 days for the other bacteria. The experiments were repeated two times.
Kill kinetic assay
Kill kinetics of cordycepin for M. bovis BCG were performed as previously described with some modifications [25]. M. bovis BCG was cultured in 7H9 broth to mid-log phase (OD600≈1.0). Bacteria were diluted to ~1.5×106 CFU ml-1. Cordycepin was added at 0 (control), 0.12, 0.3 or 0.6 mM, isoniazid at 3.6 μM. At indicated time points, samples were taken and CFU counts were performed.
Screening and characterization of cordycepin-resistant mutants
To select resistant mutants, 7H10 agar plates containing 5× and 10×MIC of cordycepin were prepared. Log phase Mycobacterium bovis (M. bovis) BCG cultures (7 ml culture, OD600≈1.0) were treated under the UV irradiation using the 15 W ultraviolet lamp (TUV15W/G15T8, PHILIPS, Poland) for 2.5 minutes and cultured in Middlebrook 7H9 media for 24 hours. Then, the cultures were spread on those cordycepin containing plates and incubated at 37°C for 4 weeks. Colonies were recovered and propagated in 7H9 broth containing corresponding level of the cordycepin. Genomic DNA was isolated using with a DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Whole-genome sequencing was performed using Illumina technology with sequencing libraries prepared following the protocol of SureSelectXT Target Enrichment System Paired-End Sequencing Library (Agilent Technologies, Inc. Santa Clara, USA) at Shanghai Biotechnology Corporation. Sequencing reads were aligned to the M. bovis BCG Pasteur 1173P2 reference genome with the Burrows-Wheeler Aligner (BWA, v0.7.10) with default [26]. The SNPs (adoK-S115L and adoK-V33A) identified by whole-genome sequencing were validated by Sanger sequencing.
Construction of the adoK and add deletion mutant of M. bovis BCG
Knockout of the adoK and add gene in M. bovis BCG was performed as described previously with some modifications [27]. A pMind derived suicide plasmid carrying a hygromycin (hyg) resistance gene was constructed and a reporter gene lacZ was inserted as a selection marker. The primers for genes knock-out were shown as follows: 5′-GCGATTAATTAAGTCGATCGATTTCGTCGA-3′ and 5′- ATATACTAGTCAC AAAATCTCCGTCCTTCG-3′ for adoK upstream 1000 bp fragment; 5′- ATAGAAG CTTATGCGATTCCGCGTCTGCT-3′ and 5′-ATATGCTAGCAGATCGTCGGTACC T CGA-3′ for adoK downstream 946 bp fragment. 5′- GCGCTTAATTAAAAATC ACGCTGCCATTGGTG-3′ and 5′- ATAACTAGTCACCAGACGATCCGATCGAC GAT-3′ for add upstream 1000 bp fragment, 5′- GCGCAAGCTTTCAGCAAGTT CTCTGGTAT-3′ and 5′-ATATGCTAGCGAAAGGTGGAGCGCCCGTA-3′ for add downstream 1000 bp fragment. Genomic DNA from allelic exchange mutants in which the adoK or add gene had been deleted was identified by PCR analysis using primers of adoK or add and the hyg gene.
Cordycepin sensitivity assays
The recombinant strains were grown in Middlebrook 7H9 media (supplemented with 10% OADC enrichment, 0.05% Tween-80, and 0.2% glycerol) containing 30 μg/ml Kan for a week. Cells were cultured to an OD600 between 1.5 and 2.0, and each culture was diluted in 100 ml fresh 7H9 broth to an OD600 of approximately 0.15 with or without the indicated concentration of cordycepin. The cultures were then grown at 37°C with shaking at 160 rpm. Aliquots were taken at the indicated times and plated on 7H10 medium (supplemented with 10% OADC enrichment and 0.2% glycerol) to determine colony-forming units [28]. The experiments were repeated three times.
Adenosine kinase activity assays
The assays for adenosine kinase activity of AdoK were performed as previously described with some modifications [19]. Ten microliter reaction mixtures contain 50 mM Tris–HCl (pH 8.0), 10 mM KCl, 10 mM MgCl2, 12.5 μCi/ml (final concentration) [γ-32P] ATP, 1.5 mM ado or various concentrations of cordycepin (0.75 mM–3 mM). The activity of human Ado kinase was determined as described above with the following changes about buffer component: 50 mM Tris–HCl (pH 7.5), 40 mM KCl, 1 mM MgCl2, 12.5 μCi/ml (final concentration) [γ-32P] ATP, 1.5 mM ado or various concentrations of cordycepin (0.75 mM–3 mM). The reaction mixtures were co-incubated for 30 min at 37°C and then stopped by the addition of 50 mM EDTA. Products were separated by thin-layer chromatography (TLC) on a PEI Cellulose F -coated strip (Merck) in 1 M formic acid and 0.5 M Lithium chloride at room temperature for 40 min [29]. The TLC plate was exposed to a storage phosphor screen for 5 h. Image was obtained using FLA-5100 Scanner, and the remaining [γ-32P] ATP was quantified by Multi Gauge software. The experiments were repeated three times.
HPLC and LC-MS analysis
Ado and cordycepin were used as substrates for adenosine kinase activity assays. The reaction products were detected by HPLC as described earlier with some modifications [30, 31]. The reaction buffer was the same as in the adenosine kinase activity assays with addition of 5 mM ATP and 0.5 mM Ado or cordycepin. Reactions proceeded at 37°C for 1 h and terminated by the addition of an equal volume of 1 M perchloric acid. Then, the samples were neutralized to pH 6.0 with 5 M K2CO3. The mixtures were centrifuged to remove the precipitated KClO4. The supernatant samples (10 μl) were injected into C-18 columns (Hypersil ODS2, 250×4.6 mm, 5μm) separated by reverse-phase HPLC (LC20AT). Buffer A (100 mM Na2HPO4, 100 mM KH2PO4, 5 mM Tetrabutylammonium bromide, pH6.0) and buffer B (methanol) were used at a 95:5 gradient at a flow rate of 1 ml/min. Nucleotides were detected at 259 nm wavelength. The new product synthesized by human adenosine kinase was analyzed using an Agilent 6540 Ultra High Definition (UHD) Accurate-Mass quadrupole time of flight (Q-TOF) LC-MS system. The samples (1 μl) were injected into a ZORBAX Eclipse Plus C18 (2.1×100 mm, 3.5 μm) analytical column(Agilent Technologies) operated at 30°C using 5% CH3OH and 95% H2O as mobile phases A and B, respectively, (5%-35% A in 11 min) at a flow rate of 0.3 ml/min. All samples were injected in duplicate. Experimental parameters were set as follows: fragmentor, 140V; Vcap, 4000V and gas temperature, 350°C. The scanning range of the Q-TOF was m/z 100 to 1000 [32, 33].
Cytotoxicity assay
Lactate dehydrogenease (LDH) cytotoxicity of cordycepin or deoxyformycin was determined as previously described with some modifications [34]. Bone marrow-derived macrophages (BMDMs) were seeded at 5×104 per well in 96-wells plate and incubated overnight in an incubator at 37°C, 5% CO2. And on the next day, cells were treated by cordycepin at the concentration of 0 (control), 0.04, 0.08, 0.32, 0.4, 0.8 and 1.6 mM or 0.4 mM deoxyformycin for 18 h. LDH activity in the media was determined using Pierce LDH Cytotoxicity Assay Kit (Thermo Scientific, USA) according to the manufacturer’s protocols.
Intracellular survival assay
Bone marrow-derived macrophages (BMDMs) were seeded at 5×105 per well in 24-wells plate and infected with M. bovis BCG at a multiplicity of infection (MOI) = 1 in DMEM/F12 medium plus 10% fetal bovine serum (FBS) as previously described with some modifications [35]. The infection was carried for 4 h, at which time the monolayer was washed three times with PBS to remove extracellular bacteria and fresh medium with inhibitor was added. After 18 h, cells were lysed with 0.05% (w/v) SDS for 10 min at room temperature and serial dilutions were plated on Middlebrook 7H10 solid medium with 10% OADC and incubated at 37°C. CFUs were enumerated after 14 days of incubation.
Results
Cordycepin has antibacterial activities to M. bovis and M. tuberculosis
To detect potential anti-mycobacterial activity of cordycepin, we determined and compared the minimum inhibitory concentration (MIC) of cordycepin on several mycobacterial and non-mycobacterial strains. Strikingly, cordycepin can effectively prevent the growth of M. bovis BCG, M. tuberculosis H37Ra, and M. marinum M. The MIC value for them is determined as 0.06 mM, 0.1 mM and 0.18 mM, respectively (Table 1). By contrast, no antibacterial activity was observed for the fast-growing M. smegmatis and non-mycobacterial species, including Escherichia coli, Staphylococcus aureus, and Bacillus thuringiensis, even the drug concentration was raised up to 12×MIC of M. tuberculosis H37Ra.
Table 1. MIC determination of cordycepin against selected microorganisms.
| Organism and genotype | Cordycepin MIC(mM) |
|---|---|
| M. bovis BCG Pasteur 1173P2 | 0.06 |
| M. tuberculosis H37Ra | 0.1 |
| M. marinum M | 0.18 |
| Cordycepin -resistant M. bovis BCG (CR01) | >1.2 |
| Cordycepin -resistant M. bovis BCG (CR02) | >1.2 |
| M. smegmatis | >1.2 |
| Escherichia coli | >1.2 |
| Staphylococcus aureus | >1.2 |
| Bacillus thuringiensis | >1.2 |
MIC, minimal inhibitory concentration. The MIC was determined by tube-broth dilution methods.
To test the effect of cordycepin on bacterial viability, we assessed the killkinetics of cordycepin at concentrations of 2×, 5× and 10× the MIC with the BCG strain. The 5× MIC of cordycepin killed ~2 log10 colony forming units (CFUs) by day 8 (Fig 1B).
These results suggest that cordycepin has a specific bactericidal activity to the slow-growing mycobacterial species such as M. bovis and M. tuberculosis.
AdoK gene mutations attribute mycobacterial resistance to cordycepin
Utilizing M. bovis BCG as an experimental strain, we further screened and identified the gene involved in the mycobacterial sensitivity to cordycepin. After UV irradiation, M. bovis BCG colonies resistant to cordycepin were selected by growing the BCG strain on 7H10 agar plates containing 10×MIC of cordycepin. We finally obtained two colonies, designated as CR01 and CR02, which can propagate when inoculated into 7H9 media containing 20×MIC of cordycepin (Table 1), suggesting that the two colonies would contain gene mutations responsible for cordycepin resistance in the mycobacterium.
We then sequenced the genomes of CR01and CR02 as well as the parental M. bovis BCG strain using Illumina sequencing technology (S2 Table). Point mutations that conferred cordycepin resistance were thereafter identified by comparing the genome sequences of the susceptible wildtype M. bovis BCG strain with two resistant mutants. Interestingly, compared to the parental strain, the only affected gene commonly in both CR01 and CR02 mutants encodes an adenosine kinase, that is AdoK which catalyzes the phosphorylation of adenosine to AMP. The point mutation was identified as S115L in CR01 and V33A in CR02 (Table 2). This SNP could be further validated by Sanger sequencing.
Table 2. Single nucleotide polymorphisms identified in the Mycobacterium bovis BCG isolates.
| Sample Name | SNP description | Locus tag | Gene name | SNP class | Codon_Change | AA change | Gene length |
|---|---|---|---|---|---|---|---|
| CR01 | G2446328A | BCG_2218c/Rv2202c | adok | Missense | tCg/tTg | S115L | 0.975kb |
| CR01 | C3234672G | BCG_2962c/Rv2940c | mas | Missense | cGc/cCc | R678P | 6.336kb |
| CR01 | G3427684A | BCG_3130c/Rv3105c | prfB | Missense | aCc/aTc | T236I | 1.137kb |
| CR02 | C2263939G | BCG_2052c/Rv2033c | - | Missense | Gtg/Ctg | V266L | 0.843kb |
| CR02 | A2446574G | BCG_2218c/Rv2202c | adok | Missense | gTg/gCg | V33A | 0.975kb |
Further complementation experiments confirmed this observation. As shown in Fig 2A and S1 Fig, when two mutant strains CR01 and CR02 were transformed with a vector expressing wildtype adoK gene, the recombinant strains could re-obtain a sensitivity to cordycepin, which is very similar to the phenotype of wild-type M. bovis BCG. To further validate the conclusion that the gene mutation of adoK is responsible for mycobacterial resistance to cordycepin, we constructed adoK-deleted M. bovis BCG (S2 Fig) and various complemented strains. As shown in Fig 2B, adoK-deleted BCG strain grew well even in 10×MIC concentration (0.64 mM) of cordycepin, whereas the adoK-complemented strain re-obtained sensitivity to 0.16 mM cordycepin. Strikingly, either adoK-S115L or adoK-V33A mutant gene did not affect the resistant phenotype of adoK-deleted M. bovis BCG (S2B Fig).
Fig 2. AdoK is responsible for mycobacterial sensitivity to cordycepin.
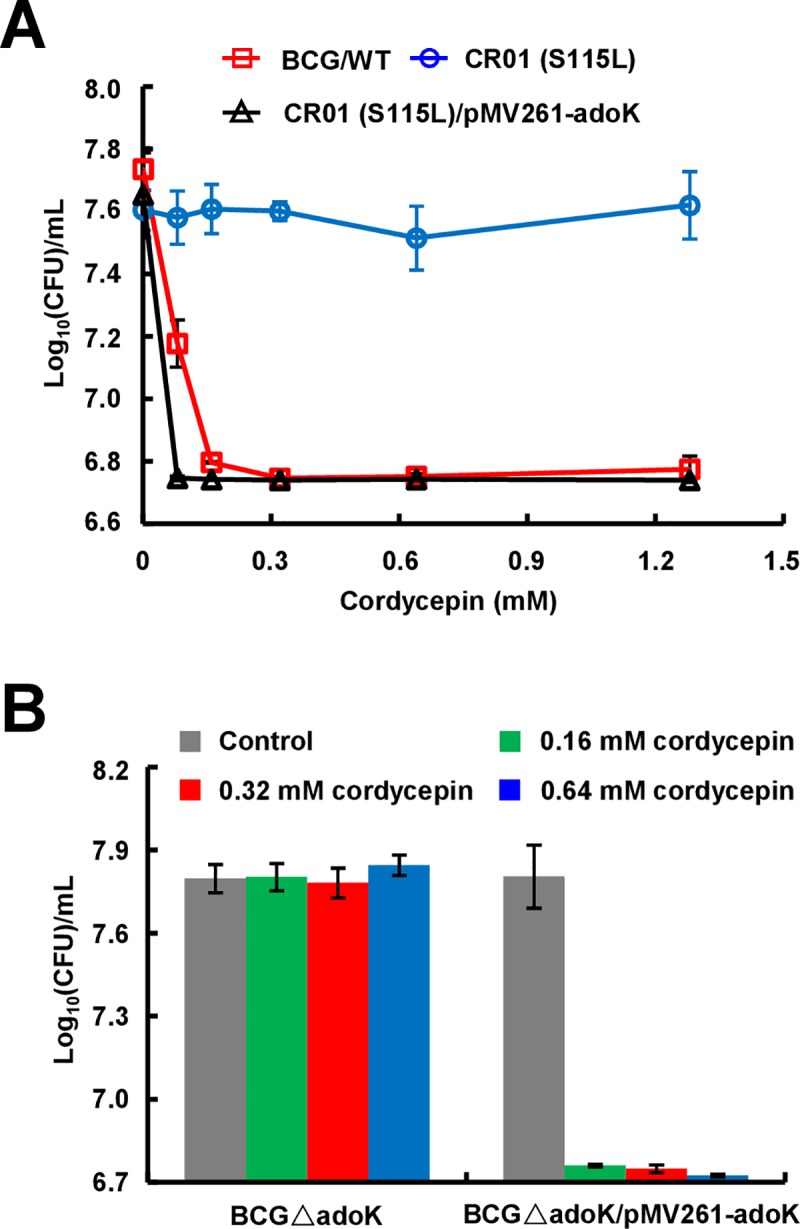
(A) Mutant strain CR01-S115L is resistant to cordycepin. Mycobacterial strains were grown in 7H9 medium containing 0, 0.08, 0.16, 0.32, 0.64, or 1.28 mM cordycepin at 37°C for 9 days. Their CFUs were then determined and indicated in the figure. Both wildtype BCG/WT and the complemented strain CR01-S115L/pMV261-adoK are sensitive to cordycepin. (B) Assays for the sensitivities of adoK-deleted and its complemented strains to cordycepin. The mycobacterial strains were grown in 7H9 medium containing 0 (control), 0.16, 0.32, and 0.64 mM cordycepin at 37°C for 9 days, and CFUs were measured. All error bars in the figures represent the standard deviations (SD) of the data derived from three biological replicates.
These results suggest that the adoK gene mutation led to the mycobacterial resistance to cordycepin.
Cordycepin inhibits the adenosine kinase activity of AdoK
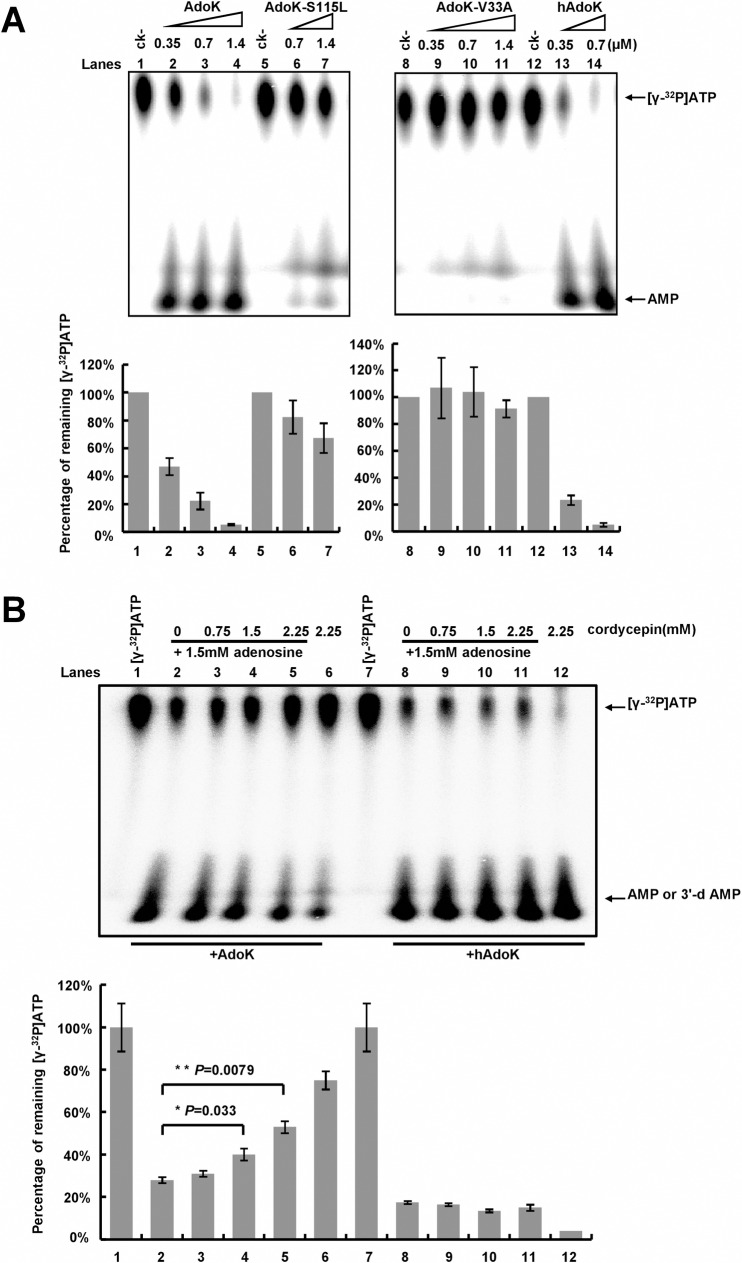
Next, we confirmed that both AdoK-S115L and AdoK-V33A mutant proteins lost most of their kinase activities. Using a Thin-layer chromatography assay and [γ32P]labeled-ATP, we observed that the AMP products gradually increased with addition of increasing amounts of AdoK proteins into the reactions, meanwhile, the radioactive ATP substrates correspondingly decreased, indicating that AdoK had adenosine kinase activity (Fig 3A, lanes 1–4). Meanwhile, we could also detect a good adenosine kinase activity for human AdoK (Fig 3A, lanes 12–14). By contrast, both AdoK-S115L and AdoK-V33A mutant proteins exhibited little activity under similar conditions (Fig 3A, lanes 5–11).
Fig 3. Thin-layer chromatography assays for the adenosine kinase activity and the inhibitory effect of cordycepin.
(A) Assays for the kinase activities of wildtype and mutant AdoK proteins. Radioactive-labeled ATP and AMP are indicated by arrows on the right of the figure. The protein concentrations are indicated on top of the panels. Quantification assays for the percentage of remaining radioactive ATP in the reactions (upper panels) were performed and correspondingly shown as the lower panels. (B) Competitive TLC assays for the inhibitory effect of cordycepin on the activity of AdoK. An increasing concentration of cordycepin gradually inhibited the activity of AdoK, but did not for hAdoK, and the remaining ATP correspondingly increased. Relative percentages of remaining [γ-32P] ATP in the reaction mixtures were quantified, and the mean values of three independent experiments along with error bars (SD) are shown. The P-values were calculated by unpaired two-tailed Student’s t-test using GraphPad Prism 5. The P-values are indicated on top of the columns.
We further examined if cordycepin can act as a substrate for AdoK, or if cordycepin inhibits its adenosine kinase activity. As shown in the Fig 3B, when using cordycepin to replace adenosine as a reaction substrate, a very weak catalytic activity for AdoK was observed. By contrast, cordycepin can competitively inhibit the activity of AdoK (Fig 3B), implying that cordycepin inhibits the mycobacterial growth by interfering with the kinase activity of AdoK.
Cordycepin is a good substrate of human AdoK kinase
Further, we examined if cordycepin also similarly affected the kinase enzyme of human AdoK. In comparison, cordycepin did not inhibit the activity of hAdoK, whereas hAdoK can efficiently catalyze phosphorylation of cordycepin, indicating that cordycepin is a substrate of hAdoK (Fig 3B). We further isolated and identified the new phosphorylated products of cordycepin by hAdoK through HPLC and LC/MS assays. As shown in S3 Fig, However, when cordycepin was used as a substrate, no new product could be detected in the reactions under similar conditions (S3B Fig). This observation is consistent with above results shown in Fig 3. By contrast, when cordycepin was co-incubated with hAdoK and ATP, a new product nearing the ADP peak could be clearly observed (Fig 4A, lower panel). Using a liquid chromatography in combination with mass spectrometry (LC-/MS) assays, we further identified the new product as 3′-deoxyadenosine monophosphate (3′-dAMP) (Fig 4B).
Fig 4. Assay for the new compound produced from cordycepin by human kinase.
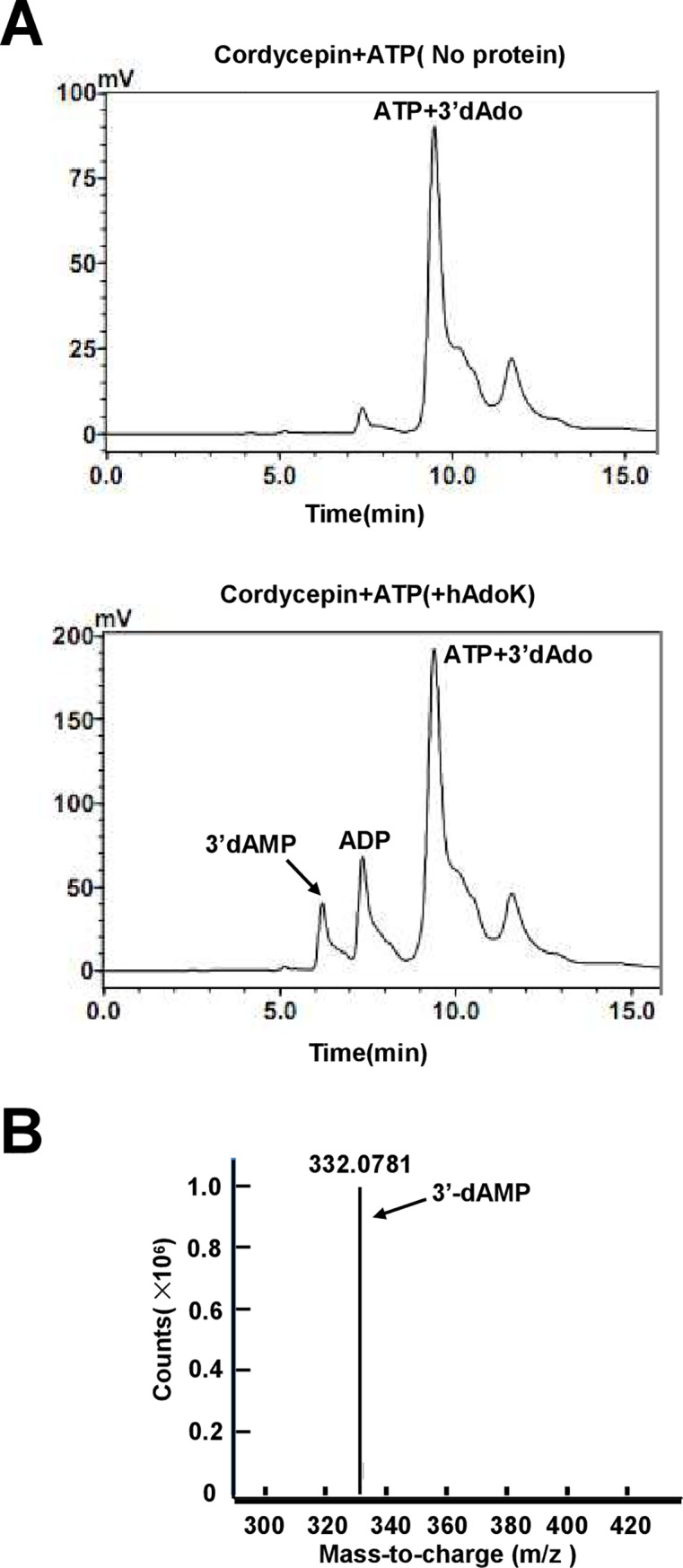
(A) HPLC separation of the new products from cordycepin. ATP and cordycepin were co-incubated with (lower panel) or without (upper panel) hAdoK. A new peak for the product on the left of ADP peak is indicated by an arrow. (B) LC-MS characterization of the new product. The mass spectra peak at m/z 332.0781 corresponds to 3′-deoxyadenosine monophosphate (3′-dAMP) in methanol and is indicated.
Therefore, cordycepin does not inhibit hAdoK but acts as its substrate, and hAdoK can phosphorylate cordycepin to produce a new deoxyadenosine derivative, 3′-dAMP.
Deoxycoformycin enhances the bactericidal activity of cordycepin in vitro
Cordycepin was previously shown to be hydrolyzed by adenosine deaminase (ADD) [36]. In M. tuberculosis, ADD is encoded by Rv3313c. To further dissect the bactericidal mechanism of cordycepin, we constructed add-deleted M. bovis BCG strain (S2A Fig) and its complemented strain (Δadd/pMV261-add), and then compared their differential cordycepin-sensitivities. As shown in Fig 5, the growth of add-deleted strain was more significantly inhibited by 0.08 mM cordycepin than that of wild type strain (Fig 5B, P = 0.0025 on day 3). This inhibition phenotype can be effectively rescued when the add gene was complemented into the add-deleted strain (Fig 5B). No significant growth difference was observed among the add-deleted (Δadd/pMV261), complemented strain and the wild type strain in the absence of drugs (Fig 5A). These results implied that the deaminase could hydrolyze cordycepin and relieved its inhibitory effect on the mycobacterium. To further test this theory, we determined if an inhibitor of adenosine deaminase, deoxycoformycin, enhances the mycobactericidal effect of cordycepin. As shown in Fig 6, if compared to either cordycepin (0.06 mM) or deoxycoformycin (0.15mM) alone provided into the medium, co-use of these two drugs more effectively inhibited the growth of both M. bovis BCG (Fig 6A, P = 0.0004 on day 6) and M. tuberculosis H37Ra (Fig 6B, P = 0.0046 on day 10).
Fig 5. Effects of adenosine deaminase shunt on the mycobactericidal activity of cordycepin.
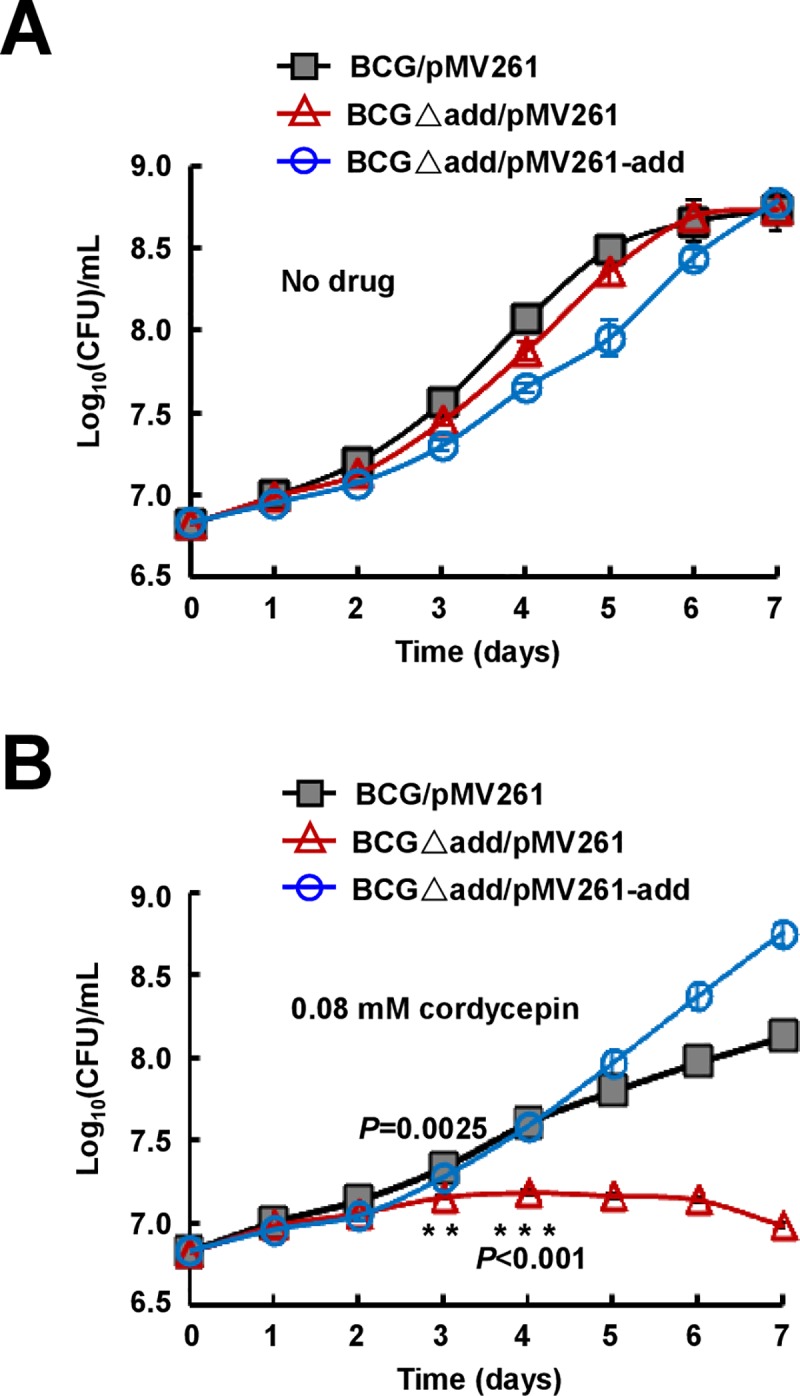
Mycobacterial strains were grown in 7H9 medium without (A) or with 0.08 mM cordycepin (B). The add-deleted BCG strain is more sensitive to cordycepin than wildtype strain. Asterisks (*) denote a significant difference between the add-deleted and wildtype strain, and the P-values are indicated. The P-values were calculated by unpaired two-tailed Student’s t-test using GraphPad Prism 5.
Fig 6. Effect of deoxycoformycin on the bactericidal role of cordycepin.
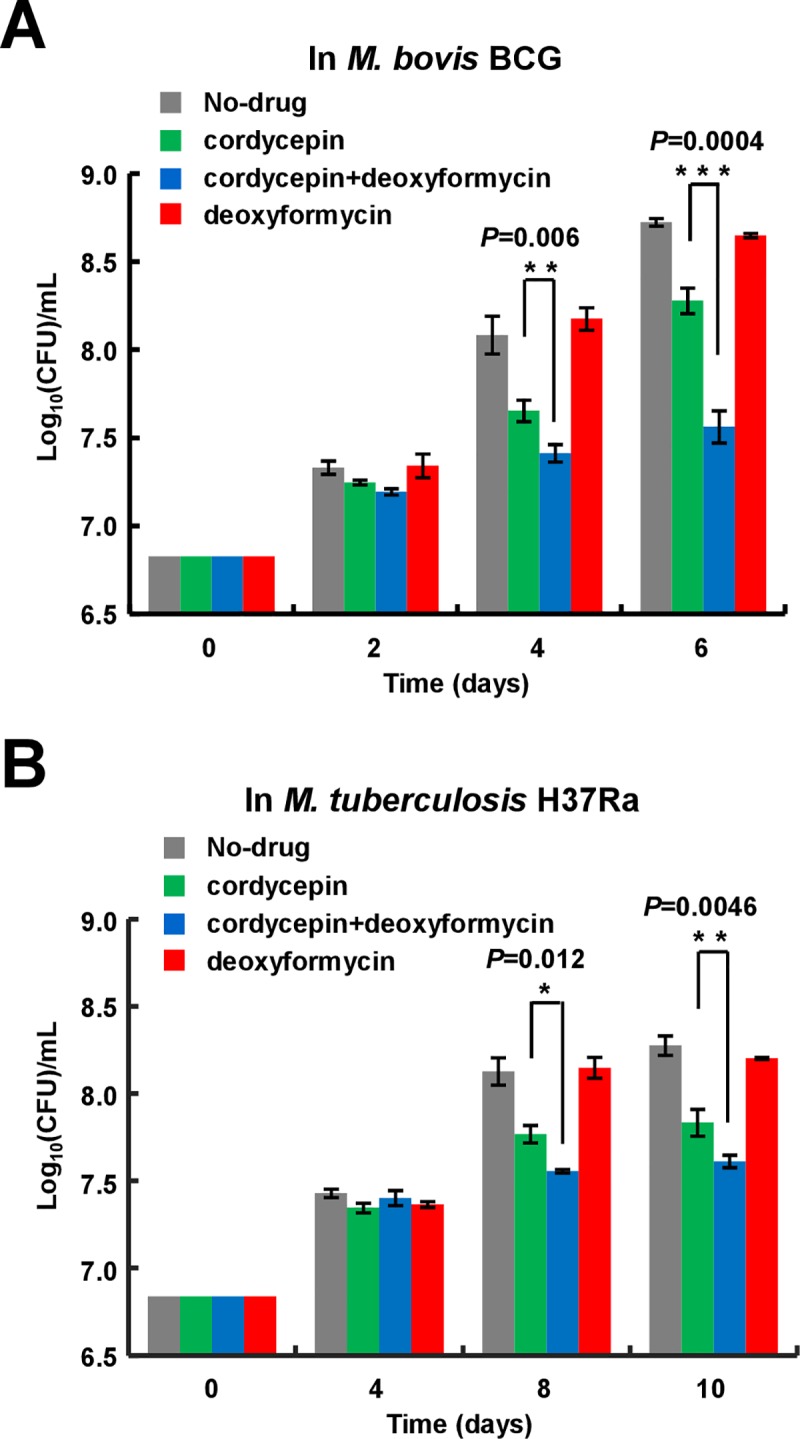
M. bovis BCG (A) and M. tuberculosis H37Ra (B) strains were grown in 7H9 medium in the presence of either cordycepin or deoxycoformycin, or both drugs, and the CFUs were measured, respectively. All experiments were repeated three times. Error bars represent the SD in the figure. Asterisks indicate P-values (*, P<0.05; **, P<0.01; ***, P<0.001) from the unpaired two-tailed Student’s t-test using GraphPad Prism 5.
Taken together, our results suggest that deoxycoformycin, an inhibitor of adenosine deaminase, can enhance the bactericidal role of cordycepin.
Deoxycoformycin enhances the bactericidal activity of cordycepin in host cell
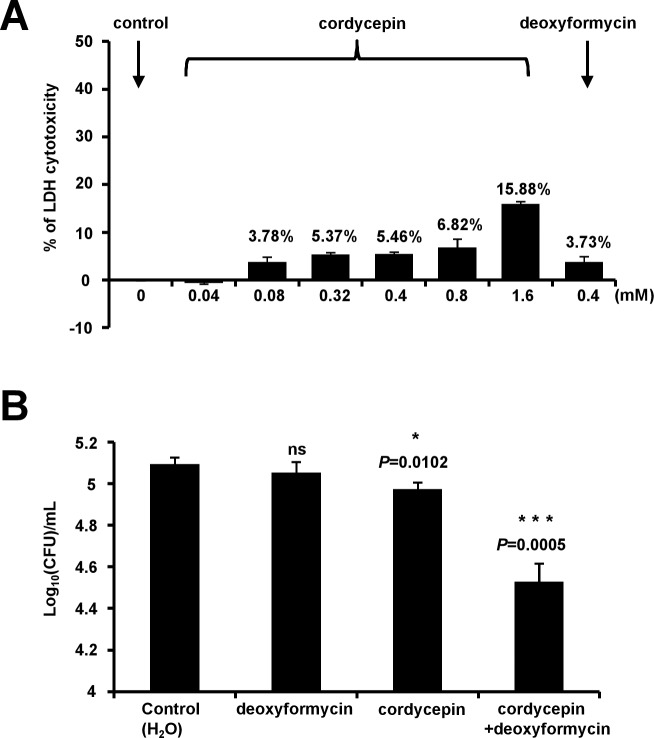
As cordycepin exhibited a dose-dependent mycobactericidal effect in vitro, and deoxyformycin can enhance the bactericidal role of cordycepin, we analysed the cytotoxicity of cordycepin and deoxyformycin to bone marrow-derived macrophages (BMDMs). As shown in Fig 7A, cordycepin shows no obvious cytotoxicity to BMDMs even the concentration was raised up to 0.8 mM (13×MIC of M. bovis BCG). And the deoxycoformycin also showed no obvious cytotoxicity to BMDMs when the concentration was 0.4 mM. Further, we determined bactericidal activity of cordycepin or/ and deoxycoformycin in host cell. As shown in Fig 7B, cordycepin (0.4 mM) can effectively inhibit the growth of intracellular Mycobacterium bovis BCG (P<0.05). By contrast, deoxycoformycin (0.4 mM) has no obvious effect on the growth of intracellular Mycobacterium. Interestingly, co-use of these two drugs more effectively inhibited the growth of the intracellular Mycobacterium bovis BCG (P<0.001).
Fig 7. Effects of cordycepin and deoxyformycin on host cell and intracellular Mycobacterium bovis BCG.
(A) Determination of LDH cytotoxicity of cordycepin or deoxyformycin in BMDMs. BMDMs (5×104 cells per well) were plated in a 96-well plate and incubated overnight in an incubator at 37°C, 5% CO2. And on the next day, different concentrations of cordycepin or deoxyformycin were added to the culture media and incubated for 18 hours. LDH cytotoxicity was measured using the Pierce LDH Cytotoxicity Assay Kit. All experiments were repeated three times. Error bars are standard deviations. (B) BMDMs were infected with Mycobacterium bovis BCG (MOI = 1) and treated with 0.4 mM cordycepin or/and 0.4 mM deoxyformycin for 18 h, and the mycobacterial survival was assayed by determining colony-forming units (CFUs). All experiments were repeated three times. Error bars represent the SD in the figure. Asterisks indicate P-values (*, P<0.05; ***, P<0.001) from the unpaired two-tailed Student’s t-test using GraphPad Prism 5.
Therefore, our results indicated that deoxycoformycin enhances the bactericidal activity of cordycepin in host cell.
Discussion
Cordycepin is an efficient component of a traditional Chinese medicine, Cordyceps spp. As a nucleoside analogue, it has been confirmed to have a broad spectrum of biological activity [3–4, 6–8, 13, 37–38]. In the last few years, the anti-tumor activity and mechanism of cordycepin have been extensively studied [5, 10–12]. However, there is no document on its anti-bacterial mechanism to date. In the present study, we report that cordycepin has a good killing activity on slow-growth mycobacteria such as M. tuberculosis H37Ra and M. bovis BCG. An active AdoK kinase in mycobacteria is required for the bacterial sensitivity to cordycepin. We showed that cordycepin is an effective substrate of human AdoK kinase, whereas it competitively inhibited the activity of mycobacterial AdoK. Our study uncovered a potential killing mechanism of cordycepin on mycobacteria.
AdoK is a purine salvage enzyme that catalyzes the phosphorylation of adenosine to AMP [19]. The purine salvage pathway is one of the promising pathways for developing novel nucleoside analogs with anti-tuberculosis activity [18]. For example, 2-Methyladenosine (methyl-Ado) is a previously reported nucleoside analogue that selectively inhibits the growth of M. tuberculosis [22] and it is primarily converted to methyl-AMP by adenosine kinase in vivo. However, methyl-Ado is a better substrate for M. tuberculosis AdoK than for its homolog in human [19]. The anti-tuberculosis activity of the compound attributes to the differential substrate preference of their adenosine kinases in mycobacteria and human. In the present study, we found that cordycepin is a poor substrate of AdoK and it can competitively inhibit the catalytic activity of AdoK. Using a cordycepin-resistant mutant strain screening in combination with whole-genome sequencing method, we confirmed that either adoK -V33A or adoK-S115L mutation in M. bovis BCG resulted in loss of the mycobacterial sensitivity to cordycepin. Further, we found that both AdoK-V33A and AdoK-S115L mutant proteins almost lost their adenosine kinase activities (Fig 3A). And either adoK-S115L or adoK-V33A mutant gene could not affect the resistant phenotype of adoK-deleted M. bovis BCG to cordycepin. Therefore, the current study implied that an active AdoK kinase is required for the mycobacterial sensitivity to cordycepin, and AdoK is a potential target of cordycepin in M. tuberculosis and M. bovis BCG. Strikingly, an insoluble macromolecular structure could be clearly observed when a higher concentration of cordycepin was mixed with AdoK in vitro (S4 Fig), thereby preventing the use of a traditional Isothermal Titration Calorimetry assay to evaluate the physical interaction between cordycepin and AdoK. Although mycobacteria have additional purine nucleoside metabolism shunts such as adeenosine deaminase pathway [39, 40], it is proposed that mycobacteria preferentially use AdoK to directly produce AMP for further DNA or RNA synthesis [18]. Nevertheless, in the absence of adoK gene, mycobacteria can alternatively use adenosine deaminase or other pathway for survival. This is why adoK-deleted BCG is resistant to cordycepin. Our findings, together with previous observations, support a model, in which cordycepin selectively hijacks mycobacterial AdoK to form an uncharacterized lethal intermediate structure, which finally kills the mycobacteria. In this regard, an active AdoK kinase in mycobacteria is required for the mycobactericidal mechanism of cordycepin. By contrast, the human AdoK can efficiently catalyze phosphorylation of cordycepin to produce intoxic 3′-dAMP and, therefore, does not have a side effect.
Another interesting finding from the present study is that deoxycoformycin, an inhibitor of adenosine deaminase, can significantly enhance the bactericidal role of cordycepin. Adenosine deaminase widely exists in plants, mammals and bacteria, including M. tuberculosis [39, 41]. In mammalian cells, cordycepin was shown to be deaminated by their adenosine deaminases to form inactive metabolite 3′- deoxyinosine [41]. In the present study, we show that the add-deleted BCG strain is more sensitive to cordycepin than wild type strain (Fig 5), implying that cordycepin can also be hydrolyzed by ADD and partially lost its bactericidal activity in M. bovis BCG and M. tuberculosis. Consistently, an inhibitor of adenosine deaminase, deoxycoformycin, can significantly increase the mycobactericidal effect of cordycepin in vitro and in host cell (Figs 6 and 7). This finding supports well our model in which cordycepin kills mycobacterium by hijacking its AdoK, a major purine salvage enzyme utilized by M. tuberculosis.
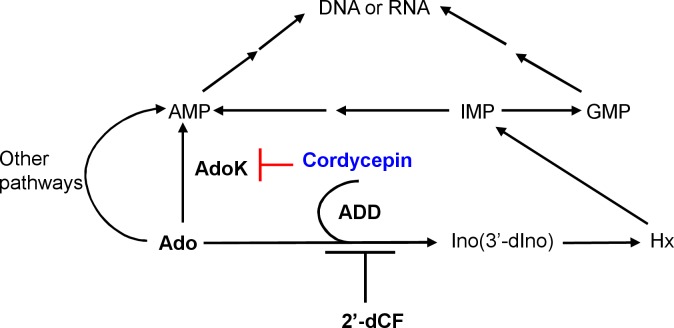
Taken together, this study characterized cordycepin as a new mycobactericidal compound, and it can target at least two different enzymes in mycobacteria as shown in Fig 8, which results in totally different consequence. On the one hand, cordycepin can arrest adenosine kinase to produce potentially toxic intermediate product, which is lethal to M. bovis and M. tuberculosis. On the other hand, cordycepin can be hydrolyzed by adenosine deaminase to become inactive metabolite, and this pathway can be cut off by deoxycoformycin.
Fig 8. Metabolic pathways of Ado in M. tuberculosis and mycobactericidal mechanism of cordycepin.
Ado is converted into AMP by adenosine kinase (AdoK) or inosine (Ino) by adenosine deaminase (ADD) in M. tuberculosis. Cordycepin targets AdoK to form a lethal intermediate structure. Deoxycoformycin (2′-dCF) inhibits the activity of ADD to enhance the mycobactericidal role of cordycepin.
Supporting information
Mutant CR02-V33Aselected on cordycepin was grown in 7H9 medium containing 0, 0.16, or 0.64 mM cordycepin at 37°C for 9 days. Samples were taken and the CFUs were measured. All experiments were repeated three times. Error bars are standard deviations.
(DOC)
(A)PCR verification of adoK and add knockout strain PCR amplification was performed using the genome of knock out strain as a template. Lane 1, adoK (WT/BCG genome); Lane 2, hyg (WT/BCG genome); Lane 3, adoK(adok-deleted BCG genome); Lane 4, hyg (adok-deleted BCG genome); Lane 5, add (WT/BCG genome); Lane 6, hyg(WT/BCG genome); Lane 7, add (add-deleted BCG genome); Lane 8, hyg(add-deleted BCG genome). The lengths of genes were shown as follows: adok, 975bp; hyg, 999bp; add, 1098bp. (B) Assays for the sensitivities of two complemented strains to cordycepin. S115L-, V33A- complemented BCG strains were grown in 7H9 medium containing 0, 0.16, 0.32, and 0.64 mM cordycepin at 37°C for 9 days. Samples were taken and the CFUs were measured. All experiments were repeated three times. Error bars are standard deviations.
(DOC)
Ado or cordycepin was used as a substrate and ATP as a phosphate donor Ado (A) or cordycepin (B) and ATP were co-incubated with (the lower panel) or without AdoK (the upper panel).
(DOC)
25 μM AdoK was mixed with 250 μM cordycepin and the visible white insoluble macromolecular can be observed. The macromolecular complex was assayed and magnified at 4×(overall panel) through Olympus optical microscope.
(DOC)
(DOC)
(DOC)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by the National Key R&D Program of China (2017YFD0500300), the National Natural Science Foundation of China (31730005 and 31300633), the Fundamental Research Funds for the Central Universities (2662016PY090), and Chang Jiang Scholars Program (To He Z-G). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. Global Tuberculosis Report 2018. WHO; 2017. [Google Scholar]
- 2.Cunningham KG, Manson W, Spring FS, Hutchinson SA. Cordycepin, a metabolic product isolated from cultures of Cordyceps militaris (Linn.) Link. Nature 1950;166:949. [DOI] [PubMed] [Google Scholar]
- 3.Zhou XX, Meyer CU, Schmidtke P, Zepp F. Effect of cordycepin on interleukin-10 production of human peripheral blood mononuclear cells. Eur J Pharmacol 2002;453:309–317. [DOI] [PubMed] [Google Scholar]
- 4.Sugar AM, McCaffrey RP. Antifungal activity of 3’-deoxyadenosine (cordycepin). Antimicrob Agents Ch 1998;42:1424–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura K, Yoshikawa N, Yamaguchi Y, Kagota S, Shinozuka K, Kunitomo M. Antitumor effect of cordycepin (3’-deoxyadenosine) on mouse melanoma and lung carcinoma cells involves adenosine A3 receptor stimulation. Anticancer Res 2006;26:43–47. [PubMed] [Google Scholar]
- 6.Wu WC, Hsiao JR, Lian YY, Lin CY, Huang BM. The apoptotic effect of cordycepin on human OEC-M1 oral cancer cell line. Cancer Chemoth Pharm 2007;60:103–111. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto K, Simizu B. Effect of cordycepin on the replication of western equine encephalitis virus. Arch Virol 1976;52:341–345. [DOI] [PubMed] [Google Scholar]
- 8.Thomadaki H, Tsiapalis CM, Scorilas A. The effect of the polyadenylation inhibitor cordycepin on human Molt-4 and Daudi leukaemia and lymphoma cell lines. Cancer Chemoth Pharm 2008;61:703–711. [DOI] [PubMed] [Google Scholar]
- 9.Kim HG, Shrestha B, Lim SY, Yoon DH, Chang WC, Shin DJ, et al. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NFkappaB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur J Pharmacol 2006;545:192–199. 10.1016/j.ejphar.2006.06.047 [DOI] [PubMed] [Google Scholar]
- 10.Lee EJ, Kim WJ, Moon SK. Cordycepin suppresses TNF-alpha-induced invasion, migration and matrix metalloproteinase-9 expression in human bladder cancer cells. Phytother Res 2010;24:1755–1761. 10.1002/ptr.3132 [DOI] [PubMed] [Google Scholar]
- 11.Hwang JH, Park SJ, Ko WG, Kang SM, Lee DB, Bang J, et al. Cordycepin induces human lung cancer cell apoptosis by inhibiting nitric oxide mediated ERK/Slug signaling pathway. Am J Cancer Res 2017;7:417–432. [PMC free article] [PubMed] [Google Scholar]
- 12.Chaicharoenaudomrung N, Jaroonwitchawan T, Noisa P. Cordycepin induces apoptotic cell death of human brain cancer through the modulation of autophagy. Toxicol In Vitro 2018;46:113–121. 10.1016/j.tiv.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 13.Cunningham KG, Hutchinson SA, Manson W, Spring FS. Cordycepin, a metabolic product from cultures of Cordyceps militaris Link. Part I. Isolation and characterization. J Chem Soc 1951;2:2299–3000. [Google Scholar]
- 14.Klenow H. Formation of themono-, di- and triphosphate of cordycepin in Ehrlich ascites- 4Q5310 tumor cells in vitro. Biochim Biophys Acta 1963;76:347–353. [PubMed] [Google Scholar]
- 15.Overgaard-Hansen K, Klenow H. Different relationships between cellular adenosine or 3'-deoxyadenosine phosphorylation and cellular adenine ribonucleotide catabolism may be obtained. J Cell Physiol 1993;154:71–79. 10.1002/jcp.1041540110 [DOI] [PubMed] [Google Scholar]
- 16.Spychala J, Datta NS, Takabayashi K, Datta M, Fox IH, Gribbin T, et al. Cloning of human adenosine kinase cDNA: sequence similarity to microbial ribokinases and fructokinases. Proc Natl Acad Sci USA 1996;93:1232–1237. 10.1073/pnas.93.3.1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long MC, Shaddix SC, Moukha-Chafiq O, Maddry JA, Nagy L, Parker WB. Structure-activity relationship for adenosine kinase from Mycobacterium tuberculosis II. Modifications to the ribofuranosyl moiety. Biochem Pharmacol 2008;75:1588–1600. 10.1016/j.bcp.2008.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy MC, Palaninathan SK, Shetty ND, Owen JL, Watson MD, Sacchettini JC. High resolution crystal structures of Mycobacterium tuberculosis adenosine kinase: insights into the mechanism and specificity of this novel prokaryotic enzyme. J Biol Chem 2007;282:27334–27342. 10.1074/jbc.M703290200 [DOI] [PubMed] [Google Scholar]
- 19.Long MC, Escuyer V, Parker WB. Identification and characterization of a unique adenosine kinase from Mycobacterium tuberculosis. J Bacteriol 2003;185:6548–6555. 10.1128/JB.185.22.6548-6555.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parker WB, Long MC. Purine Metabolism in Mycobacterium tuberculosis as a target for drug development. Curr Pharmac Des 2007;13:599–608. [DOI] [PubMed] [Google Scholar]
- 21.Muchmore SW, Smith RA, Stewart AO, Cowart MD, Gomtsyan A, Matulenko MA, et al. Crystal structures of human adenosine kinase inhibitor complexes reveal two distinct binding modes. J Med Chem 2006;49:6726–6731. 10.1021/jm060189a [DOI] [PubMed] [Google Scholar]
- 22.Barrow EW, Westbrook L, Bansal N, Suling WJ, Maddry JA, Parker WB, et al. Antimycobacterial activity of 2-methyl-adenosine. J Antimicrob Chemother 2003;52:801–808. 10.1093/jac/dkg444 [DOI] [PubMed] [Google Scholar]
- 23.Long MC, Allan PW, Luo MZ, Liu MC, Sartorelli AC, Parker WB. Evaluation of 3-deaza-adenosine analogues as ligands for adenosine kinase and inhibitors of Mycobacterium tuberculosis growth. J Antimicrob Chemother 2007;59:118–121. 10.1093/jac/dkl448 [DOI] [PubMed] [Google Scholar]
- 24.Lun S, Guo H, Onajole OK, Pieroni M, Gunosewoyo H, Chen G, et al. Indoleamides are active against drug-resistant Mycobacterium tuberculosis. Nat Commun 2013;4:2907 10.1038/ncomms3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koul A, Vranckx L, Dhar N, Göhlmann HW, Özdemir E, Neefs JM, et al. Delayed bactericidal response of Mycobacterium tuberculosis to bedaquiline involves remodelling of bacterial metabolism. Nat Commun 2014;5:3369 10.1038/ncomms4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan Q, Ku W, Zhang C, Heyilimu P, Tian Y, Ke Y, et al. Mutation analysis of the EBV-lymphoblastoid cell line cautions their use as antigen-presenting cells. Immunol Cell Biol 2018;96:204–211. 10.1111/imcb.1030 [DOI] [PubMed] [Google Scholar]
- 27.Yang M, Gao C, Cui T, An J, He ZG. A TetR-like regulator broadly affects the expressions of diverse genes in Mycobacterium smegmatis. Nucleic Acids Res 2012;40:1009–1020. 10.1093/nar/gkr830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang F, He ZG. Characterization of a conserved interaction between DNA glycosylase and ParA in Mycobacterium smegmatis and M. tuberculosis. PLoS One 2012;7:e38276 10.1371/journal.pone.0038276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H, Zhang Z, Yang J, He ZG. Functional characterization of DnaB helicase and its modulation by single-stranded DNA binding protein in Mycobacterium tuberculosis. FEBS J 2014;281:1256–1266. 10.1111/febs.12703 [DOI] [PubMed] [Google Scholar]
- 30.Long MC, Allan PW, Luo MZ, Liu MC, Sartorelli AC, Parker WB. Evaluation of 3-deaza-adenosine analogues as ligands for adenosine kinase and inhibitors of Mycobacterium tuberculosis growth. J Antimicrob Chemother 2007; 59:118–121. 10.1093/jac/dkl448 [DOI] [PubMed] [Google Scholar]
- 31.Li W, Cui T, Hu L, Wang Z, Li Z, He ZG. Cyclic diguanylate monophosphate directly binds to human siderocalin and inhibits its antibacterial activity. Nat Commun 2015;6:8330 10.1038/ncomms9330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xin B, Zheng J, Xu Z, Li C, Ruan L, Peng D, et al. Three Novel Lantibiotics, Ticins A1, A3, and A4, Have Extremely Stable Properties and Are Promising Food Biopreservatives. Appl Environ Microbiol 2015;81:6964–6972. 10.1128/AEM.01851-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rybniker J, Vocat A, Sala C, Busso P, Pojer F, Benjak A, et al. Lansoprazole is an antituberculous prodrug targeting cytochrome bc1. Nat Commun 2015;6: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee HJ, Ko HJ, Jung YJ. Insufficient Generation of Mycobactericidal Mediators and Inadequate Level of Phagosomal Maturation Are Related with Susceptibility to Virulent Mycobacterium tuberculosis. Infection in Mouse Macrophages. Front Microbiol 2016;7:541 10.3389/fmicb.2016.00541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan S, Zhen J, Li Y, Zhang C, Stojkoska A, Lambert N, et al. Mce-associated protein Rv0177 alters the cell wall structure of Mycobacterium smegmatis and promotes macrophage apoptosis via regulating the cytokines. Int Immunopharmacol 2019;66:205–214. 10.1016/j.intimp.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 36.Frederiksen S, Malling H, Klenow H. Isolation of 3’-deoxyadenosine (cordycepin) from the liquid medium of Cordyceps militaris. Biochim Biophys Acta 1965;95:189–193. [DOI] [PubMed] [Google Scholar]
- 37.Jagger DV, Kredich NM, Guarino AJ. Inhibition of ehrlich mouse ascites tumor growth by cordycepin. Cancer Res 1961;21:216–220. [PubMed] [Google Scholar]
- 38.Chen LS, Stellrecht CM, Gandhi V. RNA-directed agent, cordycepin, induces cell death in multiple myeloma cells. Br J Haematol 2008;140:682–691. 10.1111/j.1365-2141.2007.06955.x [DOI] [PubMed] [Google Scholar]
- 39.Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 1998;393:537–544. 10.1038/31159 [DOI] [PubMed] [Google Scholar]
- 40.Basso LA, Santos DS, Shi W, Furneaux RH, Tyler PC, Schramm VL, et al. Purine nucleoside phosphorylase from Mycobacterium tuberculosis. Analysis of inhibition by a transition-state analogue and dissection by parts. Biochemistry 2001;40:8196–8203. [DOI] [PubMed] [Google Scholar]
- 41.Cristalli G, Costanzi S, Lambertucci C, Lupidi G, Vittori S, Volpini R, et al. Adenosine deaminase: functional implications and different classes of inhibitors. Med Res Rev 2001;21:105–128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mutant CR02-V33Aselected on cordycepin was grown in 7H9 medium containing 0, 0.16, or 0.64 mM cordycepin at 37°C for 9 days. Samples were taken and the CFUs were measured. All experiments were repeated three times. Error bars are standard deviations.
(DOC)
(A)PCR verification of adoK and add knockout strain PCR amplification was performed using the genome of knock out strain as a template. Lane 1, adoK (WT/BCG genome); Lane 2, hyg (WT/BCG genome); Lane 3, adoK(adok-deleted BCG genome); Lane 4, hyg (adok-deleted BCG genome); Lane 5, add (WT/BCG genome); Lane 6, hyg(WT/BCG genome); Lane 7, add (add-deleted BCG genome); Lane 8, hyg(add-deleted BCG genome). The lengths of genes were shown as follows: adok, 975bp; hyg, 999bp; add, 1098bp. (B) Assays for the sensitivities of two complemented strains to cordycepin. S115L-, V33A- complemented BCG strains were grown in 7H9 medium containing 0, 0.16, 0.32, and 0.64 mM cordycepin at 37°C for 9 days. Samples were taken and the CFUs were measured. All experiments were repeated three times. Error bars are standard deviations.
(DOC)
Ado or cordycepin was used as a substrate and ATP as a phosphate donor Ado (A) or cordycepin (B) and ATP were co-incubated with (the lower panel) or without AdoK (the upper panel).
(DOC)
25 μM AdoK was mixed with 250 μM cordycepin and the visible white insoluble macromolecular can be observed. The macromolecular complex was assayed and magnified at 4×(overall panel) through Olympus optical microscope.
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.