Abstract
Although many studies have examined the location and function of the mirror neuron system (MNS) in human adults, we know relatively little about its development. The current study fills this gap by using fMRI to examine for the first time the development of the brain regions implicated in action execution, action observation, and their overlap. We examined age-related differences in brain activation by contrasting a group of children (n = 21) and adults (n = 18). Surfaced-based analyses of action execution and action observation revealed that brain activity for action observation and execution in children is similar to adults, though adults displayed greater activity than children within the right superior parietal lobe during action execution and the occipital lobe during action observation compared to control. Further, within-individual measures of overlapping activation between action observation and execution revealed age-related differences, such that adults, compared to children, displayed more spatial overlap. Moreover, the extent of the overlap in activation across conditions was related to better motor skills and action representation abilities in children. These data indicate that the MNS changes between middle childhood and adulthood. The data also demonstrate the functional significance of the putative MNS to motor skills and action representation during development.
Keywords: Action execution, Action observation, Brain development, fMRI, Mirror neuron system
1. Introduction
The mirror neuron system (MNS) is a network of brain regions that are active when individuals perform an action as well as when they observe others perform the same or similar actions. Brain regions that show this pattern of activation include the superior parietal lobe, the inferior parietal lobe/intraparietal sulcus (IPL), superior temporal sulcus (STS), posterior middle temporal gyrus, dorsal premotor, and ventral premotor/inferior frontal gyrus (IFG) (Caspers et al., 2010; Molenberghs et al., 2012). Because most studies investigating the MNS in humans cannot examine the mirroring properties of specific neurons (Mukamel et al., 2010), we use the term MNS to describe the broad network of regions that display similar activity during both action execution and action observation. Since the discovery of single neurons with mirroring properties in non-human primates, the MNS is thought to integrate the performance of actions with the ability to understand others’ actions and intentions (Di Pellegrino et al., 1992; Gallese et al., 1996; Rizzolatti et al., 1996). However, the function and significance of the MNS has been widely debated (Hickok, 2009, 2014; Van Overwalle and Baetens, 2009).
A developmental approach may be particularly useful in providing insight into the function and significance of the MNS (Ferrari et al., 2013; Woodward and Gerson, 2014). The abilities to perceive and execute actions improve across childhood (Calero et al., 2013; Wang et al., 2011), and recent research suggests that these abilities are related to neural correlates of action observation and action execution (Cannon et al., 2016; van Elk et al., 2008). Investigations that capitalize on these early periods of change can shed valuable light on the function and significance of the MNS by examining (a) the extent of overlap in brain activity for action execution and action observation, (b) how this overlap may be different at later points in development, and (c) how it may be related to developing behavioral abilities to execute and mentally represent action.
Although there is a large corpus of research investigating the location and function of the MNS, most work in this area has been performed in adults and relatively little is known about its development. Recent work in infancy and early childhood suggests that the MNS is present during early development. This work primarily uses EEG mu-rhythm desynchronization over the sensorimotor areas of the scalp as a proxy for MNS activity. Like in adults, infants and children demonstrate similar brain activity (mu-rhythm desynchronization) during action observation and action execution (Fox et al., 2016; Marshall and Meltzoff, 2011). Mu desynchronization increases from infancy to adulthood (Thorpe et al., 2016). Intriguingly, mu desynchronization seems to be related to motor experience such that infants’ motor abilities relate to changes in mu desynchronization when controlling for age (Cannon et al., 2016; van Elk et al., 2008). Moreover, increasing motor experience by training infants in novel actions increases mu desynchronization associated with perception of those actions (Gerson et al., 2015; Paulus et al., 2012). Researchers have also argued that mu desynchronization may be related to infants’ representation of others’ actions (Woodward and Gerson, 2014). Indeed, 9-month-old infants display mu desynchronization specifically for goal-directed actions (Southgate et al., 2010). Another study found that 7-month-olds showed greater mu desynchronization on trials in which they subsequently imitated the goal-directed behavior of the experimenter (Filippi et al., 2016). Together, these findings suggest developmental changes in the neural system supporting action execution and action observation, and that these changes are related to both motor skills and action understanding from early in development. These findings therefore have intriguing implications for the development and function of the MNS.
An important limitation of the MNS research in infancy and early childhood is that the most commonly used measure—EEG mu-rhythm desynchronization—lacks the spatial resolution to evaluate whether the same neural sources are active during action execution and action observation. As such, it is crucial to investigate the development of the MNS using methods that provide greater spatial resolution such as fMRI. To our knowledge, there are no studies examining the normative development of the neural regions that are active during both action observation and action execution. The inclusion of separable action observation and action execution conditions is necessary for drawing conclusions about MNS activity, given this system is identified by similar activity during action observation and action execution. There are a few fMRI studies examining action observation and/or action execution in children (Biagi et al., 2016; Ohnishi et al., 2004; Reynolds et al., 2019, 2015; Wadsworth et al., 2018, 2017), but even here, there are few studies examining developmental change (Shaw et al., 2011, 2012).
In general, studies of the neural correlates of action observation find that children and adults activate similar areas when observing object-directed actions (Biagi et al., 2016; Ohnishi et al., 2004; Shaw et al., 2011, 2012). The areas activated are commonly discussed as the action observation network. The MNS and the action observation network are largely overlapping, but the latter extends further to occipitotemporal areas involved in processing visual information and actions (e.g., biological motion) that are not active during action execution. For instance, similar to studies with adults, children (7–13 years) displayed activation in the dorsal premotor, IPL, STS, fusiform gyrus, and visual association areas when observing object-related hand actions (Ohnishi et al., 2004). Likewise, the only study examining developmental changes between children (7–15; mean = 11 years) and adults (20–38; mean = 31 years) in the action observation network found that children and adults displayed a similar activation network. However, the children’s network was less lateralized to the left hemisphere than adults (Biagi et al., 2016).
The few studies that included action execution and action observation conditions within the same children also find similar networks to the ones in adults, involving IPL, IFG, dorsal premotor as well as occipital areas (Reynolds et al., 2019, 2015; Wadsworth et al., 2017). Importantly, none of these studies involving action execution (with or without action observation) in childhood examined age-related differences in the MNS. Moreover, none of these studies tested for differences in mirroring activity by measuring the extent of the overlap between action observation and action execution conditions. Finally, to our knowledge, no fMRI study with children has examined the functional relevance of the MNS by evaluating the relations between overlapping brain activity for action observation and action execution, and motor skills and/or children’s ability to understand or represent other’s actions. Thus, the function of the MNS in development is still unclear.
In the present study, we examine, for the first time, the neural areas implicated in action execution and action observation as well as their overlap in children and adults. We employed a novel task in which participants perform and observe the same reach-grasp action, while controlling for overt shifts in attention across conditions, in order to better isolate the brain activity associated with observing and executing actions beyond that associated with domain general attentional demands. By using a surface-based conjunction analysis, we examined which areas display significant activation during both execution and observation. Examining the conjunction on the surface—as opposed to the volume—provides several advantages such as restricting the activation to gray matter, and improving inter-subject alignment and spatial specificity (Oosterhof et al., 2011). This analysis allowed us to identify the shared cortical regions between action observation and action execution as well as to test for age differences in those regions, thus facilitating better examination of MNS activity and development. We expected greater activation in regions identified as part of the MNS for adults compared to children. Moreover, in order to investigate the functional significance of the MNS in children, we evaluated the relation between the amount of overlap in activation between action execution and observation and indexes of children’s motor skills, action representation ability, and age. Based on the infant and adult literature, we hypothesized that the extent of execution-observation overlap would be associated with better motor skills, better action representation abilities, and that the extent of overlap would increase with age.
2. Methods
2.1. Participants
Twenty-one children (14 females; Meanage = 8.38; SDage = 0.91; Rangeage = 7.19–9.99 years; 71.4% Caucasian) and 18 adults (14 females; Meanage = 22.93; SDage = 2.58; Rangeage = 18.97–29.07 years; 77.8% Caucasian) provided fMRI data for this study. Four other children (2 females; Meanage = 7.65; SDage = 0.29; Rangeage = 7.34–7.94 years; 75% Caucasian) were scanned, but excluded because of excessive motion. Participants were part of a larger study that also included examination of EEG activity associated with the same reach-grasp task. Most participants (81.4%) had their EEG recorded during a prior laboratory visit (mean time between tasks = 5.65; SD = 22.09 days) and were selected for an fMRI visit based on successful task completion and usable (artifact-free) EEG data. The reason for participants completing the fMRI visit before the EEG visit was due to scheduling conflicts (i.e., scanner availability or rescheduling). During the EEG visit, participants’ behavioral assessment of motor skill and action representation abilities took place. Here, we report on only the fMRI and behavioral data for the sample of children who completed the fMRI assessment. All participants were right handed and free of any neurological or psychiatric disorder. The institutional review board of the University of Maryland approved of all experimental procedures.
2.2. MRI data collection
Imaging data were collected on a 3 T Siemens Tim Trio scanner using a 32-channel head coil. For anatomical localization, high-resolution structural images were collected using a T1-weighted sequence with TR = 1900 ms, TE = 2.32 ms, T1 = 900 ms, voxels of 0.9 mm × .45 mm × 0.45 mm, FoV 230 mm, covering the whole head. For functional data, T2*-weighted gradient echo planar images (EPI) were collected (TR = 2000 ms; TE = 24 ms, voxel size 3 mm isotropic, 36 slices, flip angle 90 and 117 volumes per run1).
2.3. fMRI task
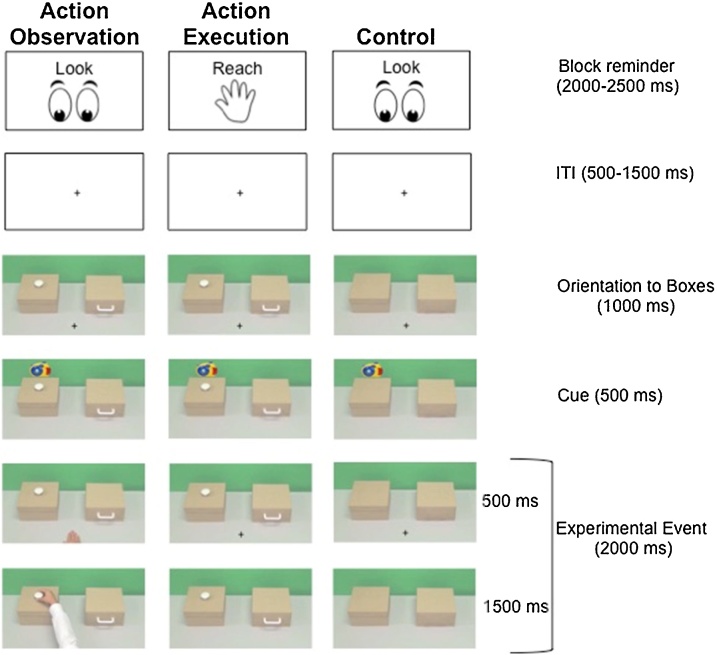
We utilized a novel experimental task depicted in Fig. 1, in which participants performed three conditions: Action Observation, Action Execution, and Scene Observation (Control). For a graphical representation of the experimental setup in the scanner, see Fig. S1. Participants had an MRI-safe table with two boxes on top of the table. This table was situated across their lap such that they could reach for the appropriate boxes without having to look at the box or move their head. Note the table was only used in the Action Execution conditions. For all conditions participants viewed images on a screen projected to a mirror above the participants’ head (as is standard in fMRI set-ups). These images supplied the visual stimulus for all conditions, including the condition in which participants executed their reach/grasp with the real boxes in front of them. Participants had extensive practice with all conditions of the task both in a mock scanner and in the real scanner bore before the data collection began, with careful attention to mastering reaching/grasping while looking at the screen and not the boxes in front of them during the Action Execution condition. In this way, the visual stimulus could be controlled across conditions.
Fig. 1.
Graphical representation of the three task conditions: Action Observation, Action Execution, and Control. Each condition was presented 8 times as a block of 5 consecutive trials for a total of 40 trials per condition or 8 blocks.
For Action Observation, participants viewed a video of an actor’s hand executing object-directed grasps of handles and knobs on boxes presented on a screen to the participants. For Action Execution, participants viewed the same boxes, handles, and knobs on the screen, but instead of viewing someone else execute an object-directed grasp, participants executed object-directed grasps of real-life versions of the handles and knobs on boxes situated on a table in front of them, though they kept their gaze on the images of the boxes on the screen (Fig. S1). All participants executed the grasp actions with their right hand (i.e., their dominant hand), and all reach/grasps in the Action Observation condition were performed by an actor’s right hand. In the Control condition, participants viewed static images of the same boxes shown in the Action Execution and Action Observation conditions, but with the handles/knobs removed to eliminate the possibility of imagined grasping. Importantly, given the visual stimulus was supplied by the computer screen above participants’ heads for all conditions, all three conditions were designed such that they elicited the same shifts in visual attention (see description below), so as to control for the possibility of confounding attentional differences across conditions. Each trial began with a jittered fixation cross (500–1500 ms) to direct initial gaze, followed by the appearance of two boxes (1000 ms) on the screen. A toy then appeared over one of the two screen boxes (500 ms) as an informative cue to indicate which box would be reached for/grasped (either by an actor’s hand in the Action Observation Condition, or by the participant who reached and grasped a corresponding real-life box in the Action Execution condition). The experimental event differed depending on trial type (either execute action, observe action, or observe scene).
Specifically, for Action Observation, the actor’s hand appeared in the center bottom of the screen for 500 ms and then the actor reached for the previously cued location (1500 ms). Participants were instructed to look at the fixation cross when it appeared, and then to watch the hand reach and grasp the target (previously cued) object. To mimic the visual and attentional shifts associated with the Action Observation condition, for Action Execution, a fixation cross appeared in the center bottom of the screen for 500 ms to orient the participants’ attention to the same location of where the actor’s hand appeared in the Action Observation Condition. The fixation cross then disappeared and the participant looked to the previously cued box on the screen while they reached for and grasped the corresponding real-life box in front of them (1500 ms). In this way, the visual and attentional experience in the Action Execution condition matched that in the Action Observation condition except in the Execution condition the participant did not observe the action but rather executed it themselves. The Control condition matched the Action Observation and Execution conditions except no action was executed or observed. For this condition, a fixation cross appeared at the center bottom of the screen (500 ms) (just as in the Action Execution Condition). The fixation cross then disappeared and the participants shifted their attention to look at the previously cued box (just as in other conditions). Which box was cued (left or right) as well as which box contained either a knob or a handle was fully counter balanced across trials. Note that handles and knobs each required slightly different grasping actions, which deliberately increased the complexity of the task to keep participants engaged, and critically forced participants to look at the screen to determine the required action, thus controlling their visual experience across conditions. The slightly different grasping actions were not conditions of interest but rather utilized to maintain participants’ engagement in the grasp task. As such, all execute trials are analyzed together regardless of the type of grasp.
Trials were presented in blocks of sets of five consecutive trials with the same condition. At the beginning of each block, participants were reminded of which experimental condition they had to perform by receiving instructions to either “Reach” or “Look” (2000-2500 ms2 ; Fig. 1). An experimenter stood beside the participants (next to the scanner bore) to ensure that participants placed their hand in the center of the two boxes upon receiving the “Reach” reminder, and that they kept their hands still in their lap upon receiving the “Look” reminder. The task consisted of a total of 8 blocks or 40 trials per condition divided into 4 functional runs of approximately 4 min. All stimuli were presented using Matlab with the Psychophysics Toolbox extensions, Version 3 (PTB-3) (Brainard, 1997; Kleiner et al., 2007; Pelli, 1997). Participants received an approximately 45-minute training session to ensure that they understood the task and could perform it accurately as well as practice how to complete the task and remain motionless in the scanner. In addition, as noted, most participants (81.4%) completed the same task while EEG data were collected before performing the task in the scanner (Mean time between tasks = 5.65; SD = 22.09 days). Results of the EEG study will be reported elsewhere.
2.4. Behavioral tasks
Only children completed the behavioral tasks.
2.4.1. Motor skills
In order to assess the children’s motor skills, we used the pegboard task from the Movement Assessment Battery for Children (MABC; Henderson and Sugden, 1992). The test requires the children to pick up a small peg and place it into a small hole (in a grid of holes) as quickly as possible. It is a standard measure of motor skills for this age group. One hand holds the box of loose pegs and the other hand places the pegs, one at a time, into the holes (in any order). Children practice with four pegs. Practice pegs are removed and test data is calculated on 12 pegs. Children first complete practice and test trials by moving pegs with their non-dominant hand, and then complete another practice and test round with their dominant hand. The task requires both speed and accuracy of hand movements to the target, and manipulation of small objects. As stipulated in the MABC manual, the task is scored as the total time (in seconds) it takes children to accurately complete this action (place all the pegs in the holes). As such, faster times represent better motor skills. We utilized the non-dominant hand as it was performed first and it provided more variability than the dominant hand. Children took on average 38.71 s to complete the pegboard task (SD = 8.74, min = 24.72, max = 54.00).
2.4.2. Action representation ability
To evaluate children’s ability to represent others’ actions, we used a task designed to capture children’s ability to mentally represent different hand positions and object-directed hand actions (Bowman et al., 2017). This task has been used in prior childhood assessments of action representation. This task consists of 8 items. For each item, children were asked to pick the hand (out of four possible options) that is in the best shape to grab the handle. Correct responses on each item required understanding of how both body orientation (of the hand in relation to the object) and grasping position (relative positions of fingers, palm, and wrist) should be optimized to grasp differentially shaped and oriented handles. This task was also timed and children were instructed to select their response as fast as they could. Reaction Time (RT) and accuracy are measured. Practice items were presented to ensure task understanding. Given that most children in this age range display high levels of accuracy, we operationalized action representation ability as the sum of the RT to select their responses. As such, faster RTs indicate better action representation ability. Children took on average 39.57 s to complete the action representation task (SD = 9.52, min = 24.06, max = 59.04).
2.5. Data analyses
Surface-based fMRI analyses were performed using the Analysis of Functional NeuroImages (AFNI) (Cox, 1996) and surface-mapping (SUMA) programs (Saad and Reynolds, 2012). Cortical surface models were created from the structural MRI data using the automated pipeline from Freesurfer (version 5.1.0 with RedHat 6.3 Linux terminal). This pipeline developed by Dale, Fischl, and colleagues has been widely used and documented elsewhere (Dale et al., 1999; Desikan et al., 2006; Fischl and Dale, 2000; Fischl et al., 2002, 1999; Fischl et al., 1999b). The output of this pipeline was used by SUMA to generate a 2D mesh surface representation of the brain for each subject. These surface models were aligned to the structural data producing an aligned surface volume.
Before projecting to the surface, the functional time series were preprocessed in the volume domain. The preprocessing steps were slice-timing correction and co-registration of each functional volume and the anatomical volumes to the first volume of the functional timeseries using an affine registration. Six motion parameters (x,y,z and roll, pitch, yaw) were calculated at this step and volumes that displayed excessive motion (>1 mm framewise displacement) were censored and subsequently excluded from regression analyses. Moreover, runs that contained excessive motion were not utilized in further analyses. Excessive motion consisted of greater than 4 mm total frame displacement across a run or greater than 10% of outlier time points (>1 mm frame displacement). For three children one run was excluded and for two children two runs were excluded. Although on average children displayed more motion than adults, t(37) = 3.39, p = .002, the results presented below remain significant when mean FD is included as a covariate in all analyses to control for average motion.
The preprocessed time series aligned to the individual’s surface volume were then projected to a 2D standardized surface mesh (MNI N27, 36,0002 surface nodes per hemisphere) using the participant’s own anatomy. Intensities were normalized to a mean of 100. Data were then smoothed on the surface using a 5 mm full-width half maximum Gaussian smoothing kernel. Smoothing was performed on the surface as it has been shown to result in greater spatial accuracy compared to smoothing on the volume where signal from non-adjacent voxels can be smoothed due to the way the cortex is folded (e.g., smoothing across gyri).
For each participant, a general linear model (GLM) was used to estimate a parameter for each of the three conditions (Action Observation, Action Execution, and Control). We used REML estimation methods to account for the temporal autocorrelation in the time series. Each condition of interest was modeled using AFNI’s duration modulated block function with each block beginning after the instruction cue and ending at the end of the last trial of the block. Blocks were approximately 23 s and contained 5 trials of the same condition. In addition to the regressors for each condition, we included nuisance regressors in the model including polynomial trends to detrend the time series as well as twelve motion regressors (i.e., roll, pitch, yaw, x, y, z and their derivatives) to model the residual effects of motion. Contrasts were estimated for each effect of interest (i.e., Action Observation vs. Control and Action Execution vs. Control).
The coefficients and t-values for each contrast were brought to second-level analyses using mixed effect models (3dMEMA; (Chen et al., 2012). We estimated the effect of each condition for each node for children and adults separately. To correct for multiple comparisons, we used Monte Carlo simulations on the cortical surface to estimate the minimum cluster size. Specifically, noise was generated within the functional volume and then mapped to the surface and smoothed to reach the target smoothness. Simulations were then run on these smoothed surface data. Using a voxel threshold of p < 0.005, this approach suggested that a minimum cluster size of 88 mm2 on the surface maintained an overall alpha < .05. All results discussed used this cluster correction. However, we also present two additional analyses. First, we indicate in Table 1 regions that also survive when using a more stringent cluster correction (voxel threshold of p < .001 and cluster size > 55 mm2). Second, estimating spatial autocorrelations when volumetric data are projected to and smoothed on the surface is a complicated problem and to our knowledge no cluster-correction method currently exists to account for this. Thus, we also performed the same analyses in the volume, in which we account for the spatial autocorrelation when estimating the minimum cluster size. Results from these analyses were similar to the one presented on the surface (see Supplementary Data for details).
Table 1.
Differences in activation between children and adults for Action Execution and Action Observation.
| Region | H | # nodes | Area (mm2) | Peak node | x | y | z | t-value | |
|---|---|---|---|---|---|---|---|---|---|
| Action Execution > Control | |||||||||
| Children > Adults | |||||||||
| 1 | Parahippocampal gyrus /Medial temporal* | LH | 199 | 287.59 | 6958 | −33 | −31 | −8 | −5.78 |
| Adults > Children | |||||||||
| 2 | Postcentral sulcus and gyrus | RH | 102 | 133.08 | 18532 | 22 | −40 | 72 | 3.73 |
| Action Observation > Control | |||||||||
| Adults > Children | |||||||||
| 1 | Middle occipital gyrus/Lunate sulcus/Occipital Pole* | LH | 87 | 437.55 | 29887 | −13 | −96 | 2 | 4.88 |
| 2 | Intraparietal sulcus/Superior occipital gyrus and sulcus* | LH | 114 | 277.89 | 21231 | −22 | −76 | 33 | 3.58 |
| 3 | Lateral occipito-temporal sulcus* | LH | 39 | 192.70 | 31540 | −36 | −74 | −1 | 4.97 |
| 4 | Temporo-occipital incisure | LH | 38 | 143.81 | 31828 | −44 | −67 | −3 | 3.21 |
| 5 | Postcentral sulcus | LH | 38 | 92.24 | 22859 | −38 | −34 | 40 | 4.98 |
| 6 | Middle occipital gyrus/Superior occipital gyrus* | RH | 28 | 135.40 | 29756 | 24 | −99 | 6 | 5.93 |
| 7 | Middle occipital gyrus | RH | 27 | 122.57 | 30225 | 44 | −75 | 15 | 3.81 |
| 8 | Middle occipital sulcus and lunate sulcus | RH | 25 | 104.22 | 29751 | 24 | −91 | 2 | 4.00 |
Note: Region names are based on the Freesurfer parcellation (Destrieux et al., 2010). x, y, z = MNI coordinates for the peak node within each cluster; t-values = t-value for the peak node. LH = Left hemisphere; RH = Right hemisphere. Clusters are thresholded at alpha <0.05 (corrected; voxel threshold of p < .005 and cluster size >88 mm2). *Cluster also survives a more stringent threshold (voxel threshold of p < .001 and cluster size >55 mm2).
In order to identify the MNS at the group-level, we performed a conjunction analysis to determine the neural regions that were significantly active (p < .05 corrected) during both Action Execution as well as Action Observation when each was independently contrasted to the Control condition (Nichols et al., 2005). To further evaluate the development and function of the MNS, we examined individual differences in the extent of the concurrence of activation during Action Execution and Action Observation by performing a conjunction analysis across the whole brain at the individual level (p < .005 uncorrected). To control for overall levels of activation, we used a ratio or the percentage of nodes displaying significant activation for both conditions (i.e., overlap) while adjusting for all the significant nodes across either condition. This was done by dividing the number of nodes displaying the conjunction by the total number of significantly active nodes (i.e., nodes active just for one or both conditions). We tested if the percentage of nodes displaying the conjunction was related to age, motor skills, and action representation ability by performing zero-order correlations. Moreover, in an exploratory analysis, we examined if the extent of the execution-observation overlap mediated the relation between age and either motor skills or action representation in a regression framework. Separate models were tested for each behavioral outcome (i.e., motor skills and action representation). We estimated the indirect effects of the MNS activation using the PROCESS macro for SPSS (Model 4) to determine the 95% bootstrap bias corrected confidence intervals (Hayes, 2017).
3. Results
3.1. Neural correlates of action execution
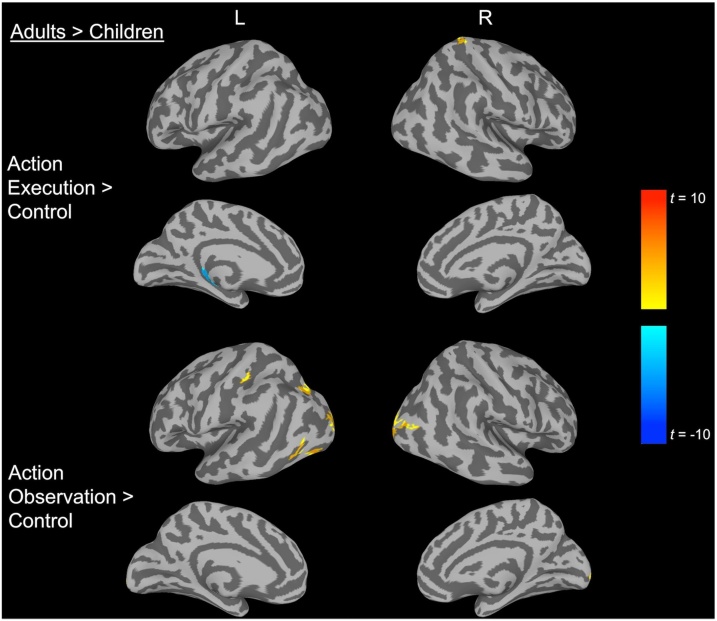
To determine which areas were active during action execution, we contrasted Action Execution with the Control condition for adults and children separately. As displayed in Table S1 and Fig. S2, for adults, we observed several large clusters that were more active during Action Execution compared to Control, encompassing the primary motor cortex, superior parietal, inferior parietal lobe (intraparietal sulcus and postcentral sulcus), superior precentral gyrus (dorsal premotor), inferior precentral gyrus (ventral premotor), and STS/inferior temporal. As illustrated in Table S1 and Fig. S2, children displayed a similar network of activation. Indeed only two clusters reached significance when evaluating differences in activation to Action Execution between children and adults. As shown in Fig. 2 and Table 1, adults displayed significantly higher activity in the right superior parietal region (postcentral gyrus and sulcus). In contrast, children exhibited higher activity in the left medial temporal region (parahippocampal gyrus). These results held for the volume analysis (see Supplementary Data; Fig. S4).
Fig. 2.
Developmental differences (Children vs. Adults) in activation for Action Execution and Action Observation.
3.2. Neural correlates of action observation
We investigated the active areas during Action Observation by contrasting this condition to the Control condition independently for children and adults. As depicted in Table S2 and Fig. S3, adults displayed widespread bilateral activation including superior parietal, inferior parietal lobe (intraparietal sulcus and postcentral sulcus), superior precentral gyrus (dorsal premotor), inferior precentral gyrus (ventral premotor), STS/inferior temporal, middle and inferior occipital gyrus and sulcus. Children displayed an analogous network of activation, except they did not display significant activation in inferior precentral gyrus (ventral premotor) and the left inferior parietal lobe cluster was divided into two clusters. When evaluating age-related differences by contrasting children and adults in Action Observation, adults displayed significantly higher activation in the left inferior parietal lobe (intraparietal sulcus and postcentral sulcus) and other visual areas in the occipital lobe (Fig. 2 and Table 1). No clusters survived in which children displayed greater activation compared to adults. While these age-related differences were significant at a more stringent threshold on the surface (Table 1), they were not present when using volumetric analyses (see Supplementary Data; Fig. S4).
3.3. Shared activation of action execution and action observation
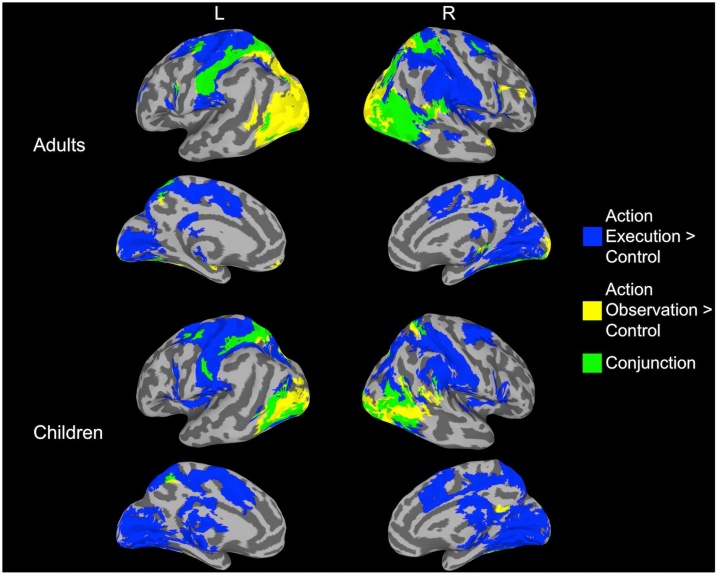
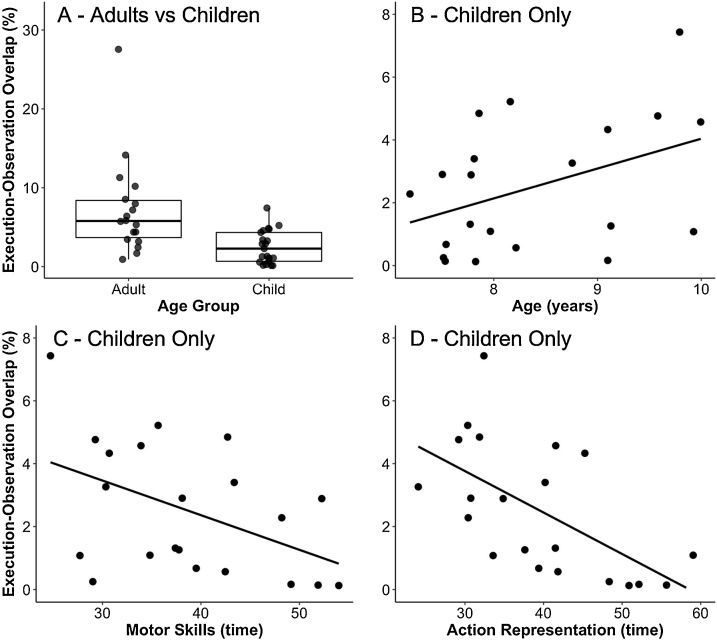
In order to examine the MNS, we determined the neural regions that were significantly active during both Action Execution as well as Action Observation when each was contrasted to the Control condition. As displayed in Fig. 3, the conjunction analyses in adults revealed that several areas displayed overlapping activation (i.e., activation during both conditions), including several areas previously identified as part of the MNS such as superior parietal, inferior parietal lobe (intraparietal sulcus and postcentral sulcus), superior precentral gyrus (dorsal premotor), inferior precentral gyrus (ventral premotor), and STS/inferior temporal. For children, the conjunction analyses revealed similar results. In order to quantify age differences in the overlap/conjunction, we examined if individual differences in the extent of the execution-observation overlap was related to age. Because the adult group contained an outlier (> 3 SDs from the mean), we performed a nonparametric test, which is robust to outliers or non-normal distributions. As expected, a Mann-Whitney test revealed that adults displayed significantly more overlap in the individual-level conjunction analyses compared to children, Medianadults = 5.78; Medianchildren = 2.28; U = 313, p < .001 (Fig. 4A). In the same vein, when examining age as a continuous measure within the child group, results revealed a similar relation with age such that older participants displayed a larger conjunction, although it was a non-significant trend (Fig. 4B), r(19) = .41, p = .064.
Fig. 3.
Brain regions significantly active for Action Execution (blue), Action Observation (yellow), and the overlap, or conjunction, of the two (green).
Fig. 4.
The four graphs show the percent of nodes displaying overlapping activation for execution and observation by (A) age group, (B) children’s age in years, (C) children’s motor skills, and (D) children’s action representation ability.
Functional Significance of Shared Activation for Action Execution and Action Observation
Finally, to evaluate the functional role of the MNS, we examined if individual differences in the amount of overlap across conditions was related to children’s motor skill and action representation ability. As hypothesized, results revealed that individuals who displayed more overlap across conditions (suggestive of increased mirror-like activity) had better motor skills (Fig. 4C), r(19) = −.46, p = .036, and action representation abilities (Fig. 4D), r(19) = −.60, p = .004.
Moreover, to further explore the role of the MNS, we evaluated whether the execution-observation overlap mediated the relations between age and either of the two behavioral measures (i.e., motor skills and action representation). This was done in two separate models by estimating the indirect effects of the execution-observation overlap in the relations between i) age and motor skills as well as ii) age and action representation. Indirect effects indicated the extent to which the overlap in action execution and action observation activity mediated the relations between age and the behavioral outcomes. For the relation between age and motor skills, r(19) = −.58, p = .006, the execution-observation overlap did not mediate this relation as the indirect effect of the overlap was not significant, −1.1 (SE = 1.12), 95% CIs [−4.45, 0.33]). Indeed, when age was included in the same model, the relation between the extent of the overlap and motor skills was no longer significant, b = −1.12, t(18) = −1.33, p = .200, while the relation between age and motor skills remained significant, b = −4.49, t(18) = −2.30, p = .033. Although the relation between age and action representation was not significant, r(19) = −.19, p = .412, the execution-observation overlap marginally mediated the relation between age and action representation as suggested by an indirect effect estimate of −2.72 (SE = 1.96), 95% CIs [−7.77, 0.03]. The relation between the extent of the execution-observation overlap and action representation remained significant when age was in the same model, b = −2.86, t(18) = −3.04, p = .007; while the relation with age was not significant, b = 0.73, t(18) = 0.34, p = .740.
4. Discussion
Despite considerable research on the MNS in adults, little is known about its development, location, and functional significance in childhood. In the current study, we utilized a novel task to investigate the neural regions involved in both action execution and action observation as well as their overlap in children, as a way to examine MNS activity. Moreover, we evaluated age-related differences in brain activation by comparing children and adults. Finally, we examined the functional significance of overlapping observation-execution activation in childhood by relating the extent of the execution-observation overlap to motor and action representation abilities.
4.1. Neural correlates of action observation and action execution
In line with previous studies examining the brain regions involved in action observation and/or action execution in children (Biagi et al., 2016; Ohnishi et al., 2004; Reynolds et al., 2019, 2015; Wadsworth et al., 2018, 2017), children displayed similar activation as the “mature” activation maps during Action Observation and Execution. Although activation maps were highly similar for children and adults, we did observe age-related differences during action execution and action observation. For Action Execution, adults, in contrast to children, displayed greater activation in the right superior parietal lobe. For Action Observation, adults displayed more activation compared to children within the occipital cortex and on the left inferior parietal lobe (intraparietal sulcus and postcentral sulcus), a region commonly involved in action execution. However, this parietal region, did not survive cluster correction on the supplementary volume analyses (see Supplementary Data). As such, we caution against strong interpretations of age-related differences in this region. In sum, these findings suggest that the activation maps for action execution and action observation are largely similar for children and adults. However, despite gross similarities in activation between children and adults, some brain regions did exhibit differences in activation (Table 1), suggesting potential developmental changes between middle childhood and adulthood.
4.2. Shared activation of action execution and action observation
Identifying age-related differences (and similarities) in Action Execution and Action Observation conditions, however, does not speak directly to age-related change in the extent of shared neural processing of action execution and observation. Importantly, because the current study was able to identify “Action Observation” regions that were also involved during Action Execution, we could probe development of overlapping activation between conditions. To identify shared activation between execution and observation, we performed conjunction analyses between conditions of Action Observation and Execution – both at the group level and within individuals. Similar to the activation results, children and adults displayed similar regions of overlap. Across children and adults, the areas displaying overlap across execution and observation have been identified as part of the MNS in previous fMRI studies, including superior parietal, inferior parietal lobe (intraparietal sulcus and postcentral sulcus), superior precentral gyrus (dorsal premotor), inferior precentral gyrus (ventral premotor), and STS/inferior temporal (Molenberghs et al., 2012). Somewhat surprisingly, we also found overlap across conditions for temporal-occipital regions. Although not traditionally considered as part of the MNS, several studies have also found overlap in activation during observation and execution of actions in areas involved in processing visual information (Caspers et al., 2010; Molenberghs et al., 2012), suggesting further integration between the action observation network and the MNS. While the group conjunction results only allow for a qualitative comparison between groups, the individual-level conjunction analyses showed that the extent of the execution-observation overlap was significantly smaller for children compared to adults. This finding is in line with the developmental literature examining the MNS using EEG, where age-related increases in mu-rhythm desynchronization are also observed (Cannon et al., 2016; Thorpe et al., 2016).
4.3. Functional significance of shared activation for action execution and action observation
In the current study, we also found evidence for the functional significance of the MNS–insofar as this system is indexed by overlapping activity in action-execution and action-observation conditions in our task. Specifically, the extent of the execution-observation overlap was related to behavioral assessments of children’s motor skills and action representation abilities. These findings are in line with the mu-rhythm desynchronization literature, in which patterns of brain activity thought to index an underlying MNS are related to motor experience and/or motor skills as well as young children’s ability to understand actions (Cannon et al., 2016; Filippi et al., 2016; Gerson et al., 2015; van Elk et al., 2008; Woodward and Gerson, 2014). Together, these findings suggest that the MNS may play a role in the ability to execute fine-motor actions as well as the capacity to represent and understand others’ actions.
Intriguingly, when exploring the role of the MNS on the relations between age and motor skills and action representation, our findings suggest a possible dissociation between motor skills and action representation as related to MNS development. Although both motor skills and action representation ability were directly related to the execution-observation overlap, improvements in motor skills were better captured by age than the execution-observation overlap. These findings suggest that age (or other unmeasured factors correlated with age in this sample) may be a larger driver of fine motor skill development in middle childhood than potential neural mirroring processes. On the other hand, the relation between the execution-observation overlap and action representation was independent of age, likely reflecting individual differences due to other factors (e.g., social and cognitive abilities). Moreover, when exploring whether execution-observation overlap (our index of an underlying MNS) mediated the link between age and action representation, the marginally significant indirect effect suggests that the development of the MNS may be one process underlying age-related changes in action representation.
4.4. Limitations and future directions
The findings of the current study should be considered in light of several limitations. The main limitation is the nature of the sample. The sample size is relatively small, especially for the correlation analyses within the children group. In addition, we employed a cross-sectional sample comparing children to adults to carry out a first fMRI examination of developmental differences in the MNS. Given the nature of the sample, the current findings should be considered as preliminary, but promising, evidence regarding the development of the MNS and its functional significance. Future studies should replicate these findings using a larger sample with an age-stratified longitudinal design, which would allow studying within-person change in a wide age range.
We used a novel experimental design to incorporate action execution within the scanner bore. However, a limitation of our approach is that there was a visual-tactile discrepancy for the Action Execution condition as participants had to maintain their visual attention on the screen while reaching for the objects. This added to the complexity of the task, requiring participants to learn to adapt to this discrepancy. This may have been especially challenging for younger participants. Moreover, in our experimental setup the accuracy and reaction time could not be recorded during the execution condition from the MRI-safe box and table setup. These issues are mitigated by the fact that the actions used in this study consisted of relatively simple actions that all participants could perform accurately. On the other hand, the simplicity of the actions may be a reason why we observed relatively few differences in activation between children and adults. It is possible that including more complex actions would enhance age differences in performance and activation.
Finally, although we tried to match our experimental conditions as closely as possible and attempted to control for visual shifts in attention across conditions, significant activation in the same areas across action execution and action observation could arise from other non-action-specific cognitive processes such as inhibition of irrelevant information, choice discrimination, and predictive coding. Future studies could include assessments of these domain-general covariates to further isolate brain activity and behavioral skills specific to the motor/action domain.
5. Conclusion
The current study investigates the development, location, and function of the MNS using a novel fMRI paradigm that examines both action execution and observation within the same child while controlling for visual attention. The findings suggest that both children and adults display a spatial overlap when performing actions and when observing the same action. This overlap implies “mirroring” processes, in which the same brain regions used to execute one’s own action are engaged when perceiving others’ actions. We found that the extent of this overlap increased with age, suggesting developmental changes in MNS across childhood to adulthood. Moreover, this overlap was related to motor and action representation abilities in childhood. The current data highlight the importance of examining the MNS from a developmental perspective, which can offer valuable insight into its function across development.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgement
This work was supported by the National Institutes of Health (P01HD064653 to NAF).
Footnotes
Three adult participants had 113 volumes per run because they were presented a slightly different (i.e., shorter) instruction cue (see below). However, the duration of the experimental stimuli was identical across participants.
Three adult participants were consistently presented with 2000 ms instructions. The rest of the participants were presented with instructions that varied from 2000–2500 ms.
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2019.100655.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Biagi L., Cioni G., Fogassi L., Guzzetta A., Sgandurra G., Tosetti M. Action observation network in childhood: a comparative fMRI study with adults. Dev. Sci. 2016;19(6):1075–1086. doi: 10.1111/desc.12353. [DOI] [PubMed] [Google Scholar]
- Bowman L.C., Thorpe S.G., Cannon E.N., Fox N.A. Action mechanisms for social cognition: behavioral and neural correlates of developing Theory of Mind. Dev. Sci. 2017;20(5) doi: 10.1111/desc.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard D.H. The psychophysics toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Calero C.I., Salles A., Semelman M., Sigman M. Age and gender dependent development of Theory of Mind in 6- to 8-years old children. Front. Hum. Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon E.N., Simpson E.A., Fox N.A., Vanderwert R.E., Woodward A.L., Ferrari P.F. Relations between infants’ emerging reach-grasp competence and event-related desynchronization in EEG. Dev. Sci. 2016;19(1):50–62. doi: 10.1111/desc.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S., Zilles K., Laird A.R., Eickhoff S.B. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 2010;50(3):1148–1167. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Saad Z.S., Nath A.R., Beauchamp M.S., Cox R.W. FMRI group analysis combining effect estimates and their variances. Neuroimage. 2012;60(1):747–765. doi: 10.1016/j.neuroimage.2011.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Destrieux C., Fischl B., Dale A., Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53(1):1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pellegrino G., Fadiga L., Fogassi L., Gallese V., Rizzolatti G. Understanding motor events: a neurophysiological study. Exp. Brain Res. 1992;91(1):176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Ferrari P.F., Tramacere A., Simpson E.A., Iriki A. Mirror neurons through the lens of epigenetics. Trends Cognit. Sci. 2013;17(9):450–457. doi: 10.1016/j.tics.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi C.A., Cannon E.N., Fox N.A., Thorpe S.G., Ferrari P.F., Woodward A.L. Motor system activation predicts goal imitation in 7-month-Old infants. Psychol. Sci. 2016;27(5):675–684. doi: 10.1177/0956797616632231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis: II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Tootell R.B., Dale A.M. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp. 1999;8(4):272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fox N.A., Bakermans-Kranenburg M.J., Yoo K.H., Bowman L.C., Cannon E.N., Vanderwert R.E. Assessing human mirror activity with EEG mu rhythm: a meta-analysis. Psychol. Bull. 2016;142(3):291. doi: 10.1037/bul0000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V., Fadiga L., Fogassi L., Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119(2):593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Gerson S.A., Bekkering H., Hunnius S. Short-term motor training, but not observational training, alters neurocognitive mechanisms of action processing in infancy. J. Cognit. Neurosci. 2015 doi: 10.1162/jocn_a_00774. [DOI] [PubMed] [Google Scholar]
- Hayes A.F. Guilford Publications; 2017. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. [Google Scholar]
- Henderson S.E., Sugden D.A. The Psychological Corporation; London: 1992. The Movement Assessment Battery for Children. [Google Scholar]
- Hickok G. Eight problems for the mirror neuron theory of action understanding in monkeys and humans. J. Cognit. Neurosci. 2009;21(7):1229–1243. doi: 10.1162/jocn.2009.21189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G. WW Norton & Company; 2014. The Myth of Mirror Neurons: The Real Neuroscience of Communication and Cognition. [Google Scholar]
- Kleiner M., Brainard D., Pelli D., Ingling A., Murray R., Broussard C. What’s new in psychtoolbox-3. Perception. 2007;36(14):1. [Google Scholar]
- Marshall P.J., Meltzoff A.N. Neural mirroring systems: exploring the EEG mu rhythm in human infancy. Dev. Cognit. Neurosci. 2011;1(2):110–123. doi: 10.1016/j.dcn.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs P., Cunnington R., Mattingley J.B. Brain regions with mirror properties: a meta-analysis of 125 human fMRI studies. Neurosci. Biobehav. Rev. 2012;36(1):341–349. doi: 10.1016/j.neubiorev.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Mukamel R., Ekstrom A.D., Kaplan J., Iacoboni M., Fried I. Single-neuron responses in humans during execution and observation of actions. Curr. Biol. 2010;20(8):750–756. doi: 10.1016/j.cub.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T., Brett M., Andersson J., Wager T., Poline J.-B. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25(3):653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Ohnishi T., Moriguchi Y., Matsuda H., Mori T., Hirakata M., Imabayashi E. The neural network for the mirror system and mentalizing in normally developed children: an fMRI study. Neuroreport. 2004;15(9):1483–1487. doi: 10.1097/01.wnr.0000127464.17770.1f. [DOI] [PubMed] [Google Scholar]
- Oosterhof N.N., Wiestler T., Downing P.E., Diedrichsen J. A comparison of volume-based and surface-based multi-voxel pattern analysis. NeuroImage. 2011;56(2):593–600. doi: 10.1016/j.neuroimage.2010.04.270. [DOI] [PubMed] [Google Scholar]
- Paulus M., Hunnius S., van Elk M., Bekkering H. How learning to shake a rattle affects 8-month-old infants’ perception of the rattle’s sound: electrophysiological evidence for action-effect binding in infancy. Dev. Cognit. Neurosci. 2012;2(1):90–96. doi: 10.1016/j.dcn.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli D.G. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spatial Vision. 1997;10(4):437–442. [PubMed] [Google Scholar]
- Reynolds J.E., Licari M.K., Billington J., Chen Y., Aziz-Zadeh L., Werner J. Mirror neuron activation in children with developmental coordination disorder: a functional MRI study. Int. J. Dev. Neurosci. 2015;47:309–319. doi: 10.1016/j.ijdevneu.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Reynolds J.E., Billington J., Kerrigan S., Williams J., Elliott C., Winsor A.M. Mirror neuron system activation in children with developmental coordination disorder: a replication functional MRI study. Res. Dev. Disabil. 2019;84:16–27. doi: 10.1016/j.ridd.2017.11.012. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Fadiga L., Gallese V., Fogassi L. Premotor cortex and the recognition of motor actions. Cognit. Brain Res. 1996;3(2):131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Saad Z.S., Reynolds R.C. Suma. Neuroimage. 2012;62(2):768–773. doi: 10.1016/j.neuroimage.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D.J., Grosbras M.-H., Leonard G., Pike G.B., Paus T. Development of functional connectivity during adolescence: a longitudinal study using an action–observation paradigm. J. Cognit. Neurosci. 2011;23(12):3713–3724. doi: 10.1162/jocn_a_00112. [DOI] [PubMed] [Google Scholar]
- Shaw D.J., Grosbras M.-H., Leonard G., Pike G.B., Paus T. Development of the action-observation network during early adolescence: a longitudinal study. Social Cognit. Affect. Neurosci. 2012;7(1):64–80. doi: 10.1093/scan/nsq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate V., Johnson M.H., Karoui I.E., Csibra G. Motor system activation reveals infants’ on-line prediction of others’ goals. Psychol. Sci. 2010;21(3):355–359. doi: 10.1177/0956797610362058. [DOI] [PubMed] [Google Scholar]
- Thorpe S.G., Cannon E.N., Fox N.A. Spectral and source structural development of mu and alpha rhythms from infancy through adulthood. Clin. Neurophysiol. 2016;127(1):254–269. doi: 10.1016/j.clinph.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elk M., van Schie H.T., Hunnius S., Vesper C., Bekkering H. You’ll never crawl alone: neurophysiological evidence for experience-dependent motor resonance in infancy. Neuroimage. 2008;43(4):808–814. doi: 10.1016/j.neuroimage.2008.07.057. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F., Baetens K. Understanding others’ actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage. 2009;48(3):564–584. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Wadsworth H.M., Maximo J.O., Lemelman A.R., Clayton K., Sivaraman S., Deshpande H.D. The Action Imitation network and motor Imitation in children and adolescents with autism. Neuroscience. 2017;343:147–156. doi: 10.1016/j.neuroscience.2016.12.001. [DOI] [PubMed] [Google Scholar]
- Wadsworth H.M., Maximo J.O., Donnelly R.J., Kana R.K. Action simulation and mirroring in children with autism spectrum disorders. Behav. Brain Res. 2018;341:1–8. doi: 10.1016/j.bbr.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.-C., Magasi S.R., Bohannon R.W., Reuben D.B., McCreath H.E., Bubela D.J. Assessing dexterity function: a comparison of two alternatives for the NIH toolbox. J. Hand Ther. 2011;24(4):313–321. doi: 10.1016/j.jht.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward A.L., Gerson S.A. Mirroring and the development of action understanding. Philos. Trans. R. Soc. B. 2014;369(1644) doi: 10.1098/rstb.2013.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.