Summary
The neural crest is a fascinating embryonic population unique to vertebrates that is endowed with remarkable differentiation capacity. Thought to originate from ectodermal tissue, neural crest cells generate neurons and glia of the peripheral nervous system, and melanocytes throughout the body. However, the neural crest also generates many ectomesenchymal derivatives in the cranial region, including cell types considered to be of mesodermal origin such as cartilage, bone, and adipose tissue. These ectomesenchymal derivatives play a critical role in the formation of the vertebrate head, and are thought to be a key attribute at the center of vertebrate evolution and diversity. Further, aberrant neural crest cell development and differentiation is the root cause of many human pathologies, including cancers, rare syndromes, and birth malformations. In this review, we discuss the current findings of neural crest cell ontogeny, and consider tissue, cell, and molecular contributions toward neural crest formation. We further provide current perspectives into the molecular network involved during the segregation of the neural crest lineage.
Keywords: BMP, craniofacial development, embryonic stem cells, epigenetic, FGF, gene regulatory network, induction, multipotent, neural crest cells, neural plate border, specification, Wnt
1 |. INTRODUCTION
The neural crest is an embryonic, multipotent cell population that migrates extensively and gives rise to a multitude of derivatives throughout the body, including melanocytes, peripheral neurons and glia, and craniofacial bone, cartilage, and connective tissue. Neural crest cells are unique to vertebrates, and have defined the taxa by contributing to the evolution of key features of the predatory lifestyle, including a jaw, a larger brain enclosure, and paired sense organs (Gans & Northcutt, 1983; Northcutt, 2005). Owing to the broad contribution of neural crest cells to derivatives throughout the body, a large number of human health conditions are associated with improper neural crest development and differentiation. Collectively known as “neurocristopathies” (Bolande, 1974, 1997; Vega-Lopez, Cerrizuela, Tribulo, & Aybar, 2018), these include craniofacial malformations, rare diseases such as Waardenburg syndrome, and aggressive cancers such as neuroblastoma and melanoma (Etchevers, Amiel, & Lyonnet, 2006; Farlie, McKeown, & Newgreen, 2004; Watt & Trainor, 2014). Of particular clinical relevance, craniofacial malformations account for over one-third of all congenital birth defects (Twigg & Wilkie, 2015). Many distinct craniofacial syndromes exist, including cleft lip and cleft palate (which occurs in 1:700 live births in the United States [Leslie et al., 2015]), missing or improperly fused bones of the face and skull (including craniosynostosis), and malformed teeth and facial features (Trainor, 2010; Twigg & Wilkie, 2015). Since their discovery 150 years ago (His, 1868), neural crest biology has captivated the interests of scientists, and the origins, formation, and differentiation capacity of neural crest cells, as well as their associated pathologies, continue to be the subject of intense research.
Neural crest cells were first identified in chick embryos by Wilhelm His in 1868, who described them as a middle furrow or groove (“zwischenrinne”) surrounding the neural plate in early stages, and once the neural tube was formed, as a middle cord or thread (“zwischengstrang”) of tissue in-between the neural tube and the epidermis (Dupont, 2018; Garcia-Castro, 2011; His, 1868). Neural crest cells develop along most of the embryonic anteroposterior axis and exhibit specific differentiation capacities according to their axial identity. Therefore, neural crest cells are generally grouped as cranial (cephalic), vagal, trunk, and sacral according to their position within the anteroposterior axis of the embryo. While neural crest from these locations have been shown to contribute to a specific set of derivatives, elegant heterotopic grafting experiments performed in birds revealed the plasticity of pre-migratory neural crest cells, and the critical role of the unique environment surrounding migratory neural crest in differentiation (Le Douarin, 1980; Le Douarin, Creuzet, Couly, & Dupin, 2004; Noden, 1975, 1988; Rothstein, Bhattacharya, & SimõesCosta, 2018).
Neural crest cell development and migration occurs in a rostrocaudal wave with the neural crest from the cranial region being the first to undergo an epithelial-to-mesenchymal transition (EMT). Cranial neural crest cells migrate along a well-defined dorsolateral pathway, where they populate defined regions of the embryo and differentiate to give rise to much of the craniofacial skeleton, parasympathetic and sensory ganglia, and endocrine and pigment cells. At more caudal axial levels, trunk neural crest cells arise from the dorsal aspect of the neural tube and contribute to derivatives that include the peripheral neurons and glia, adrenomedullary cells, and pigment cells (Schlosser, 2008). Among the subpopulations of neural crest, it is conventionally thought that only cranial neural crest could form ectomesenchyme in amniotes, while anamniote trunk neural crest was capable of producing mesenchyme, in particular in the larval fin. How-ever, recent studies have challenged these notions. Using geneticbased lineage tracing in zebrafish, it was observed that the larval fin mesenchyme, originally thought to originate at least in part from the neural crest, is derived from the paraxial mesoderm (Lee, Knapik, Thiery, & Carney, 2013). Around the same time, lineage tracing in turtles revealed a second wave of migratory trunk neural crest cells that contribute to the plastron bones (Cebra-Thomas et al., 2013). These intriguing results suggest that anamniote trunk neural crest does not contribute to mesenchymal derivatives, while amniote trunk neural crest cells contributed to an ectomesenchymal derivate to some extent. These findings raise intriguing questions of neural crest plasticity and lineage restrictions that remain to be addressed.
Owing to the major contributions of the neural crest to the vertebrate body plan and related neurocristopathies, it is essential to understand the underlying molecular mechanisms required for the formation of this cell type. Numerous studies have explored the processes underlying neural crest induction in a variety of species, elaborating on the signaling pathways and tissues interactions leading to the formation of the neural crest. More recent research has focused on the earliest neural crest specification, providing a better perspective of the origins of this multipotent cell type. In this review, we focus on our current understanding of the induction of the neural crest from ectodermal tissue, and highlight the molecular events leading to the formation of neural crest through different stages of embryonic development. We pay special attention to recent evidence suggesting early neural crest specification events, and provide our perspective of a novel pre-border state during neural crest specification prior to the formation of neural plate border state.
2 |. EARLY STEPS IN NEURAL CREST FORMATION
Neural crest cell formation is a progressive process involving the combinatorial interactions between signaling pathways and transcription factors (Figure 1). The neural crest is considered to be of ectodermal origin, arising between the developing neural plate and the nonneural ectoderm, in a region termed as the neural plate border. There is considerable evidence supporting the role of inductive processes in neural crest formation, and signaling pathways including Wnt, BMP, FGF and Notch from neural and nonneural ectoderm, and mesoderm tissues have all been implicated (reviewed in Stuhlmiller & García-Castro, 2012a). However, this model has perplexed biologists when viewed in light of concepts of cell fate restrictions. As neural crest cells hold the capacity to differentiate into cells types commonly assumed to be derived from ectoderm and mesoderm germ layers, it would seem that their potential is even greater than the cells from which they are proposed to originate. While the fields of somatic cell reprogramming and stem cell biology have revealed the tremendous capacity of cells to dedifferentiate and acquire new fates (Gurdon, 1962; Gurdon, Byrne, & Simonsson, 2003; Jaenisch & Young, 2008), the principle of sequential segregation of potential during normal embryonic development is still well-regarded. Under this principle, progenitor cells generate derivatives with more restricted potential, and no other cell type apart from the neural crest has been suggested to bypass this principle. In this section, we discuss the evidence of the role of an induction mechanism in the formation of the neural crest, with details on the particular pathway components presented in the next section. We then discuss recent work that brings to the forefront new questions of the origins and earliest specification events of neural crest.
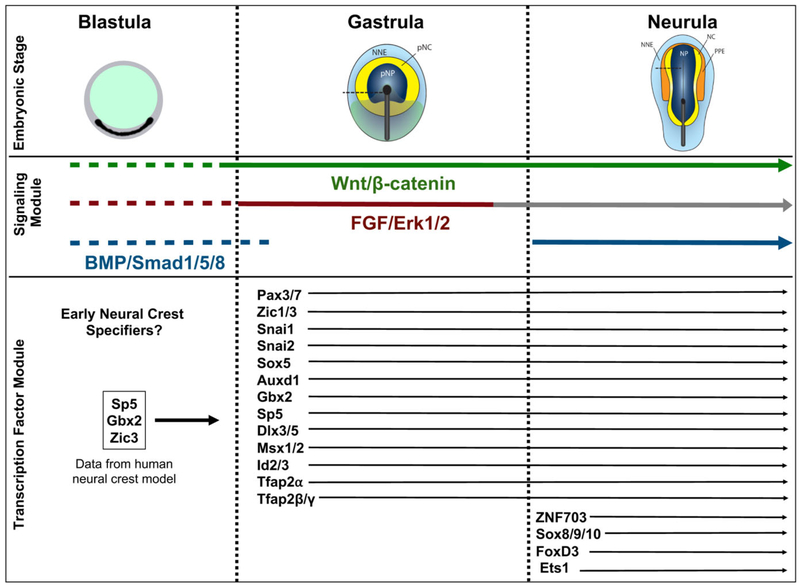
FIGURE 1.
Dynamics of signaling molecules and transcription factors regulating neural crest cell induction from blastula to neurula stage. Neural crest formation is viewed as a progressive inductive process initiating at blastula stage and continuing through gastrulation into neurula stage. This process is mediated by Wnt, FGF, and BMP signaling cues and transduced by transcription factors. Experimental data from works cited in this review were used to provide a temporal perspective of signaling and transcription factor modules involved during different phases of neural crest induction. Under the signaling module, dotted lines represent the potential role of these signaling molecules during blastula stages where their requirement is unknown. Based on expression patterns, BMP/Smad1/5/8 signaling is active at blastula stage (Faure, de Santa Barbara, Roberts, & Whitman, 2002) as well as Wnt and FGF/Erk1/2 signaling. During NC development, Wnt signaling is known to play a role during gastrula and neurula stages, FGF/Erk1/2 signaling during gastrula stage, and BMP/Smad1/5/8 signaling during neurula stage (denoted by solid lines), however, their role during pre-gastrula stage remains to be analyzed. FGF/Erk1/2 signaling is active during neurula stage; however, it is not suggested to be involved in NC development at this stage (grey line). BMP/Smad1/5/8 signaling is active during gastrula stage, but needs to be attenuated in cells specified towards NC fate, represented by the gap in the blue BMP signaling line. The transcription factor module provides a hierarchical view of the requirement of specific transcription factors during the different phases of neural crest induction. The transcription factors mentioned under blastula stage were identified from studies in a human neural crest model based on embryonic stem cells. The network of transcription factors involved in neural crest specification at blastula stage in animal models remains to be identified. NC = neural crest; NNE = nonneural ectoderm; NP = neural plate; pNC = prospective neural crest; pNP = prospective neural plate, PPE = preplacodal ectoderm). Schematics of gastrula and neurula chick embryos are adapted from Stuhlmiller & GarcííaCastro, 2012a
2.1 |. Tissue interactions and neural crest induction
The initial description by His, later confirmed by a wealth of studies in multiple organisms, located the neural crest and its precursors at the neural plate border, in-between the thicker medial neural plate and the thinner lateral nonneural ectoderm. Underneath these ectodermal cells, and in direct contact with them, a mesodermal layer is positioned. It seems logical to assume that the tissues surrounding the location where neural crest markers arise could be involved in their formation. This gave rise to the proposition of an inductive event underscoring neural crest formation. Classic induction refers to the interaction of two different cell types, whereby one elicits a signal that affects the development of the receiving cell, and triggers the appearance of a new, third cell type. Efforts to test these ideas provided considerable experimental evidence suggesting that an induction event was at play in neural crest cell formation.
While neural crest development has been studied in multiple model systems, our current understanding of the role of early inductive signaling relies primarily on work performed in chick and amphibian embryos. Molecular evidence from these systems supports a twostep model of neural crest induction. First, inductive signals from Wnt and FGF ligands are involved in the formation of the neural plate border (Garcia-Castro, Marcelle, & Bronner-Fraser, 2002; Kengaku & Okamoto, 1993; Mayor, Guerrero, & Martínez, 1997; Monsoro-Burq, Fletcher, & Harland, 2003; Patthey, Edlund, & Gunhaga, 2009; Stuhlmiller & García-Castro, 2012b). Second, during neurulation, Wnt, BMP, and Notch pathways are involved in activating and maintaining the expression of transcription factors necessary for the neural crest identity, termed “neural crest specifiers,” which then go on to activate specifiers of EMT and migration (de Croze, Maczkowiak, & MonsoroBurq, 2011; Liem, Tremml, Roelink, & Jessell, 1995; Marchant, Linker, Ruiz, Guerrero, & Mayor, 1998; Monsoro-Burq et al., 2003; Selleck, García-Castro, Artinger, & Bronner-Fraser, 1998; Tribulo, Aybar, Nguyen, Mullins, & Mayor, 2003) (reviewed in Prasad, Sauka-Spengler, & Labonne, 2012; Stuhlmiller & García-Castro, 2012a).
Numerous studies indicate that neural crest induction occurs as a result of interactions between the neural and nonneural ectoderm and/or ectoderm and mesoderm. Embryonic grafts or ex-vivo explants in axolotl, frog, and chick that replicate the boundaries between neural and nonneural ectoderm demonstrated that these interactions trigger the formation of neural crest cells, and that neural crest cells can be induced from both neural and nonneural ectoderm (Basch, Selleck, & Bronner-Fraser, 2000; Dickinson, Selleck, McMahon, & BronnerFraser, 1995; Labonne & Bronner-Fraser, 1998; Moury & Jacobson, 1990; Selleck & Bronner-Fraser, 1995; Yardley & García-Castro, 2012). In amphibians, the mesoderm was originally identified as the source of the neural crest inducing signals. Grafting and coculture experiments carried out in salamanders and later Xenopus revealed the capacity of the mesoderm to induce the expression of neural crest markers and neural crest derivatives (Mancilla & Mayor, 1996; Mayor, Morgan, & Sargent, 1995; Raven & Kloos, 1945). In the Xenopus gastrula, the prospective neural crest is situated above the dorsolateral marginal zone (prospective paraxial mesoderm), which expresses both Wnt and FGF ligands, the BMP antagonist Chordin, and numerous other signaling regulators (Hong, Park, & Saint-Jeannet, 2008; Mayor et al., 1995; Monsoro-Burq et al., 2003; Steventon, Araya, Linker, Kuriyama, & Mayor, 2009). Coculturing of the dorsolateral marginal zone together with naive animal caps result in the expression of neural crest markers (Bonstein, Elias, & Frank, 1998; Mancilla & Mayor, 1996). However, the requirement for mesoderm in neural crest induction has been debated. Reports in zebrafish indicate that the mesoderm is dispensable for neural crest induction, as Nodal mutant embryos lacking mesoderm retain the expression of neural crest markers (Ragland & Raible, 2004). Evidence in chick also indicates that mesoderm might not be required for neural crest induction. Chick nonneural ectoderm can respond to FGF signaling and activate neural crest marker expression in the absence of mesoderm induction (Yardley & García-Castro, 2012), and explants generated from gastrula stage chick embryos can generate neural crest cells in the absence of mesodermal markers (Basch, Bronner-Fraser, & García-Castro, 2006; Patthey et al., 2009; Patthey, Gunhaga, & Edlund, 2008). Together, these experiments collectively suggest that neural crest cells arise in multiple ways, perhaps through interactions of neural and nonneural ectoderm, mesoderm and nonneural ectoderm, or a combination of neural and nonneural ectoderm with signals from underlying mesoderm, depending upon species, developmental stage, and axial level. Importantly, however, the requirement of these tissue contributions in the induction of neural crest cells have not been fully confirmed in vivo, and thus, additional mechanisms might be at play. Further, the role of tissue induction in neural crest formation has not been demonstrated in mammals, and this is a crucial area of future exploration.
2.2 |. Evidence for the early specification of neural crest
The notion that neural crest cells are derived from the ectoderm is consistent with the position of neural crest precursors in the upper layer of the gastrulating embryo, and with their contribution to ectodermal derivatives such as melanocytes and peripheral neurons and glia. However, their ectomesenchymal contributions to mesoderm derivatives such as cartilage, bone, and adipocytes appear to defy current assumptions regarding lineage commitment and the sequential restriction of potential. Recognizing that the neural crest does not comply with the classic germ layer theory, Hall (Hall, 2000, 2018) proposed that the neural crest constitute a fourth germ layer. However, no molecular mechanism explaining their dual ectoderm and mesoderm potential had been described. Work focused on the origin and early specification of neural crest in chick embryos has offered a new perspective on this paradox. These studies revealed that neural crest cell specification is evident in stages of embryogenesis preceding the establishment of the neural plate, and can go on in the absence of either mesodermal or definitive neural ectoderm contributions (Basch et al., 2006; Patthey et al., 2008, 2009). Specification refers to the initiation of a differentiation program which will continue under permissive conditions. While the term specification can be used with regards to multiple processes and developmental time points—for example, the states of neural crest formation preceding maturation at the neural folds, or preceding the terminal differentiation of neural crest derivatives—in this context, early neural crest specification refers to early stages of development prior to the acquisition of the earliest known neural crest markers at the neural plate border. In particular, for chick embryos, this is prior to the expression of the paired domain transcription factor Pax7 (Basch et al., 2006). These studies examined early gastrula chick embryos at stages when the primitive streak is just formed and not fully grown (Hamburger and Hamilton stages 2 and 3), and interrogated the capacity of individual explants to express neural crest markers after isolated culture under noninductive conditions (Basch et al., 2006; Patthey et al., 2009). Importantly, at the stage in which the explant was generated (time zero), none of the explants expressed Pax7 or any other marker of neural crest. The test here was to assess if, after culturing the explants in isolation and under noninducing conditions, would the explants express neural crest markers? The results revealed that explants from a restricted region are able to express pre-migratory and migratory neural crest markers after culture. Importantly, this gastrula stage specification of neural crest occurs independently of neural and mesodermal tissue, as markers for these cell fates are not observed in explants of prospective neural crest, suggesting for the first time that neural crest cells could arise in the absence of the definitive neural and mesoderm tissue interactions previously reported (Basch et al., 2006). Interestingly, the prospective neural crest territory of the gastrula epiblast is found between the prospective neural plate and the prospective nonneural ectoderm or future epidermis (Patthey et al., 2009). Fate mapping further revealed that this territory contributes to neural crest cells in vivo (Basch et al., 2006; Ezin, Fraser, & Bronner-Fraser, 2009).
Recently, these results in chick have been supported by studies in mammalian neural crest. In the first example of neural crest specification in mammals, intermediate explants generated from stage 3+ rabbit gastrulae (which do not express neural crest markers at this time point) and cultured in isolation and under noninductive conditions for 45 hr go on to express neural crest markers Pax7, Sox10, and Sox9 (Betters, Charney, & García-Castro, 2018). Further, recent work from our laboratory using a model of human cranial neural crest based on human embryonic stem cells suggests that human neural crest forms independently of neuroectoderm (PAX6+ cells), and without apparent contribution from mesodermal tissue (Leung et al., 2016). Broadly, these studies suggest that neural crest formation is underway during gastrulation and perhaps is initiated at an even earlier blastula stage, proposed here as a pre-border stage, as has been shown for the neural fate (Wilson, Graziano, Harland, Jessell, & Edlund, 2000; Wilson et al.,2001) and placodal fate (Trevers et al., 2017). If an induction event is at play, then it must involve the interaction of prospective tissues within the epiblast. Alternatively, inheritance of epigenetic factors (e.g., chromatin accessibility, RNA and/or protein modulators) could provide a unique environment responsive to signaling events in the epiblast leading to neural crest formation. Further characterization of the events leading to the earliest neural crest specification remains to be addressed.
In Xenopus, fate mapping has revealed a prospective neural crest territory in the Nieuwkoop and Faber stage 10 early gastrula that exist immediately above the dorsolateral marginal zone and between the prospective neural and nonneural ectoderm (Steventon et al., 2009). A recent study in Xenopus has also suggested a pre-gastrula origin of neural crest (Buitrago-Delgado, Nordin, Rao, Geary, & Labonne, 2015). This work proposed that prospective neural crest cells retain stemness markers from pluripotent epiblast cells. The authors advocate that neural crest cells retain the capacity to generate endodermal derivatives and equate neural crest cells to pluripotent epiblast cells. However, neural crest cells in vivo have never been shown to contribute to endodermal derivatives and their differentiation potential, as broad as it is, remains limited (e.g., neural crest cannot contribute to all the mesodermal derivatives) in comparison to the epiblast cells from which the three germ layers arise.
3 |. SIGNALING PATHWAYS INVOLVED IN NEURAL CREST INDUCTION
Signaling pathways play important roles in cell fate decisions. In the context of neural crest development, Wnt, BMP, FGF, and Notch signaling have been extensively shown to play critical roles during the formation, migration, and differentiation of neural crest. As such, we focus on the known roles of these signaling pathways during neural crest formation, and recent reports delineating their roles during early neural crest specification.
3.1 |. Wnt signaling pathway
The Wnt signaling pathway involves a family of secreted ligands, 19 Wnts in mammals, that elicit specific cellular responses after binding to transmembrane receptors. Wnt signaling plays crucial roles throughout embryonic development, as well as in degenerative diseases and cancer progression (Logan & Nusse, 2004; Moon, Kohn, De Ferrari, & Kaykas, 2004). The Wnt pathway can be subdivided into canonical and noncanonical pathways, with the former being the most significantly linked to neural crest formation. Canonical Wnt signaling involves the stabilization and nuclear localization of β-catenin, a protein with dual functions in cytoskeletal arrangement and transcriptional regulation. In the absence of the ligand, a destruction complex formed between APC and Axin sequesters β-catenin, which is subsequently phosphorylated and marked for degradation by GSK3β. In the presence of a Wnt ligand bound to a Frizzled/LRP receptor, the protein Dishevelled (DSH) is activated and inhibits the formation of the destruction complex, thereby promoting the stabilization of β-catenin, which translocates to nucleus and activates the transcription of target genes along with DNA bound TCF/LEF proteins.
Wnt signaling has been shown to be required for neural crest induction in fish, frog, chick, and human embryonic stem cell-derived neural crest cells. Various Wnt ligands including Wnt1, Wnt3a, Wnt6, Wnt7b, and Wnt8a are expressed in tissues involved in neural crest induction (Knecht & Bronner-Fraser, 2002). Gain-of-function experiments in Xenopus and chick have demonstrated that the ectopic expression of Wnt ligands, along with modulation of BMP signaling, induces neural plate border and neural crest marker expression (Chang & Hemmati-Brivanlou, 1998; Hong & Saint-Jeannet, 2007; Hong et al., 2008; Labonne & Bronner-Fraser, 1998; Monsoro-Burq, Wang, & Harland, 2005; Nichane et al., 2008; Saint-Jeannet, He, Varmus, & Dawid, 1997; Sasai, Mizuseki, & Sasai, 2001). Studies in chick and frog have also demonstrated that the inhibition of Wnt signaling results in the ablation of overall neural crest development (AbuElmagd, Garcia-Morales, & Wheeler, 2006; Basch et al., 2006; Chang & Hemmati-Brivanlou, 1998; Deardorff, Tan, Saint-Jeannet, & Klein, 2001; Elkouby et al., 2010; Garcia-Castro et al., 2002; Gutkovich et al., 2010; Hassler et al., 2007; Heeg-Truesdell & Labonne, 2006; Hong et al., 2008; Hong & Saint-Jeannet, 2007; Labonne & BronnerFraser, 1998; Li, Kuriyama, Moreno, & Mayor, 2009; Litsiou, Hanson, & Streit, 2005; Monsoro-Burq et al., 2005; Sakai et al., 2005; Sato, Sasai, & Sasai, 2005; Steventon et al., 2009; Tamai et al., 2000; Villanueva, Glavic, Ruiz, & Mayor, 2002; Wu, Yang, & Klein, 2005).
The specific Wnt ligands involved in neural crest development vary between species. In Xenopus, Wnt3a and Wnt8a are expressed beginning in the gastrula, with later expression restricted to the caudal neural plate and paraxial mesoderm (Elkouby et al., 2010; Hong et al., 2008; Steventon et al., 2009). In chick, Wnt3a and Wnt8a expression begins at blastula stages in the lateral epiblast. As development progresses, Wnt3a expression becomes restricted to the primitive streak in the gastrula, and further restricted to the dorsal aspect of the neural tube during later stages of development. Meanwhile, Wnt6 is expressed in the nonneural ectoderm, and is proposed to play a role in continued neural crest induction during later stages of development (Garcia-Castro et al., 2002; Schmidt, McGonnell, Allen, Otto, & Patel, 2007; Skromne & Stern, 2001; Wilson et al., 2001). In zebrafish, Wnt8, in particular Wnt8.1, is required for neural crest induction (Lewis et al., 2004). Perturbation studies in Xenopus, zebrafish, and chick have demonstrated the requirement of these Wnt ligands in the induction of neural crest specific genes (Elkouby et al., 2010; Lewis et al., 2004; Monsoro-Burq et al., 2005; Patthey et al., 2009; Schmidt et al., 2007; Steventon et al., 2009; Wilson et al., 2001). The role of Wnt signaling in mouse embryos has been explored by several groups, and it is clear that it contributes to steps following neural crest induction, including the migration and differentiation of neural crest derivatives (Ikeya, Lee, Johnson, McMahon, & Takada, 1997; van Amerongen & Berns, 2006). However, surprisingly, no direct evidence of the contribution of Wnt signaling to neural crest induction has been reported in mouse. The expression of Wnt1 and Wnt3a in mouse neural folds, along with double knockout studies, revealed defects in craniofacial development but not in early neural crest formation (Barriga, Trainor, Bronner, & Mayor, 2015; Ikeya et al., 1997; van Amerongen & Berns, 2006). More robust perturbations through a conditional knockout of β-catenin have relied on Wnt1-cre drivers, which are not expressed early enough to inform if the canonical Wnt/β-catenin pathway contributes to neural crest induction in mice (Barriga et al., 2015; Debbache, Parfejevs, & Sommer, 2018).
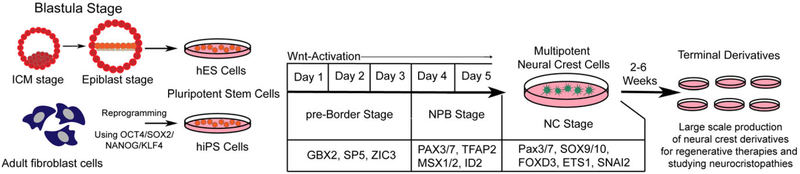
Few embryonic human neural crest studies have been reported (Betters, Liu, Kjaeldgaard, Sundström, & García-Castro, 2010; O’Rahilly & Müller, 2007), and while a modest molecular comparison toward other model organisms has been presented, these studies have not provided insight into the mechanisms underlying human neural crest formation. Access to early embryos to address human neural crest induction and the associated signaling events remain out of reach due to technical and ethical limitations. However, over the past decade, human pluripotent stem cells have emerged as an excellent surrogate to study human neural crest development. These studies have considered key roles for various signaling pathways (BMP, Neuregulin, and WNT, among others) in human neural crest formation (Betters, Murdoch, Leung, & García-Castro, 2014). A robust system was recently reported that enables highly efficient neural crest formation in a short time, with high expression of neural crest markers and differentiation potential toward neural crest terminal derivatives. Importantly, this model is launched by WNT activation (using the GSK3 small molecule inhibitor CHIR 99021, or Wnt3a), and depends on β-catenin function (Leung et al., 2016), and thus validates the requirement of WNT/β-catenin signaling during neural crest induction in humans.
In addition to the Wnt ligands themselves, multiple molecules have been suggested to play a role in modulating Wnt signaling during neural crest induction. The matrix metalloprotease ADAM13 promotes Wnt signaling by inhibiting the Wnt-repressive ephrinB signaling (Wei et al., 2010). A recent study further elaborated on the role of ADAM13 during neural crest induction. This study proposed a nonproteolytic role for ADAM19 in stabilizing ADAM13 to promote Wnt signaling during neural crest induction in Xenopus embryos (Li et al., 2018). ADAM19 loss-of-function and gain-of-function analyses in Xenopus embryos validated its requirement during neural crest induction in a Wnt-dependent manner (Li et al., 2018).
While much is known about the role of Wnt signaling in neural crest induction, precisely how the Wnt signal is effected to elicit the activation of neural crest specifiers remains unknown. A step in this direction, it was recently demonstrated in chick that the transcription factor Axud1 functions downstream of Wnt signaling to directly regulate FoxD3 expression by interacting with neural plate border specifiers Msx1 and Pax7 (Simões-Costa, Stone, & Bronner, 2015). While this study identifies a Wnt effector during neural crest development, it will be necessary to address direct targets of Wnt signaling associated with the earliest stages of neural crest induction and neural plate border formation. Another intriguing study recently revealed a novel role for the Dickkopf family glycoprotein Dkk2 in positively regulating Wnt/β-catenin signaling in a GSK3β independent manner (Devotta, Hong, & Saint-Jeannet, 2018). While the Dkk family is generally known as Wnt antagonists, this work in Xenopus demonstrated that Dkk2 acts through the LRP5/6 receptor to activate β-catenin and promote neural crest cell specification. Finally, a recent study in Xenopus suggests that the transcription factor Hes3 negatively regulates the effect of Wnt signaling arising from paraxial mesoderm at the neural plate-neural crest border, thereby promoting boundaries of gene expression (Hong & Saint-Jeannet, 2018). These studies are beginning to shed much-needed light on how the Wnt signaling pathway modulates neural crest cell induction. Further studies will be required to identify the direct transcriptional responses of Wnt/β-catenin signaling during neural crest induction.
3.2 |. Bone morphogenetic signaling
The TGF-β superfamily of signaling molecules—in particular bone morphogenetic proteins (BMPs) and nodal/activin signaling—play crucial roles in development by controlling growth and differentiation processes. BMPs are secreted proteins that bind to Type I and II BMP receptors, resulting in the phosphorylation and activation of the Smad1/5/8 transcriptional mediators. BMP signaling is known to play extensive roles in development and disease (Hill, 2001; Massagué, 1998; Massagué & Wotton, 2000; Wakefield & Roberts, 2002). During neural crest induction, it has been well-established that some level of BMP signaling is required. There are two models explaining the appropriate level of BMP signaling required for neural crest induction. In the gradient model, it has been suggested that an intermediate level of BMP signaling, along with other signaling pathways, induces neural crest at the border between neural and nonneural ectoderm (Labonne & Bronner-Fraser, 1998). This is consistent with other major findings in frogs whereby a high level of BMP signaling induces epidermis, and inhibition of BMP results in a neural fate (Baker, Beddington, & Harland, 1999; Wilson & Hemmati-Brivanlou, 1995). Similar experiments in chick have identified the role of BMP in neural crest induction in the absence of ectoderm from intermediate neural plate explants (Liem et al., 1995). The intermediate gradient of BMP signaling at the neural plate border is generated by antagonistic interactions between the high levels of BMP signals from nonneural ectoderm and the BMP signaling inhibitors Cerberus, Noggin, Chordin, and Follistatin secreted from the neural plate (Sauka-Spengler & Bronner-Fraser, 2008; Tribulo et al., 2003). However, additional signaling is required for the induction of neural crest, as intermediate levels of BMP signaling cannot alone induce neural crest in any vertebrate species (GarciaCastro et al., 2002; Labonne & Bronner-Fraser, 1998). In an alternative model, it is suggested that during gastrulation, the attenuation of BMP signaling forms a “zone of competence” to promote neural crest induction under the influence of Wnt and FGF signaling (Ragland & Raible, 2004; Steventon et al., 2009). This is then followed by the reactivation of BMP signaling at the neural plate border to promote the expression of neural plate border and neural crest genes. Precisely how the permissive levels of BMP signaling are established and maintained is a major question. Toward this end, the nuclear coactivator SNW1 was found to act upstream of BMP signaling and to regulate its effect in the restricted domain of the neural plate border in postgastrula Xenopus embryos (Wu, Ramel, Howell, & Hill, 2011). Further, a recent study in chick suggests the role of CKIP-1/Smurf1 in modulating the precise level of phospho-Smad1/5/8 at the neural plate border required to maintain neural crest induction (Piacentino & Bronner, 2018).
The role of BMP signaling in neural crest induction has also been explored in mammalian systems. In the mouse, BMP-receptor Alk2/3/5 or Tgfbr2-inducible knockout mouse lines driven by Wnt1-Cre or Pax3-Cre result in severe craniofacial, pharyngeal, and cardiac defects (Choudhary et al., 2006; Dudas, Sridurongrit, Nagy, Okazaki, & Kaartinen, 2004; Jia et al., 2007; Nie, Deng, Wang, & Jiao, 2008; Stottmann & Klingensmith, 2011). Interestingly, while neural plate border gene expression, including Msx1/2, AP2α, Pax3, and Sox9, was not affected at early stages (E8.5), their expression was downregulated at E9.5. This supports a role for BMP signaling in maintaining the expression of neural crest markers rather than stimulating their initial expression. A human model of neural crest based on embryonic stem cells induced through WNT signaling has also demonstrated that BMP is required for neural crest induction, but that high levels will inhibit their formation (Leung et al., 2016). Taken together, data from chick, Xenopus, zebrafish, mouse, and human models suggest a two phase model for BMP activity whereby, after initial neural crest induction by Wnt and FGF signaling, Wnt and BMP signaling is required for maintenance of neural crest induction.
3.3 |. Fibroblast growth factor signaling
FGF signaling plays crucial roles in mesoderm development, gastrulation movements, and embryonic patterning (Böttcher & Niehrs, 2005; Dorey & Amaya, 2010). There are 22 FGF genes in vertebrates that transduce signaling through receptor tyrosine kinase FGF receptors, and are modulated through extracellular matrix components. Intracellular signaling is transduced via phosphorylated receptors through a cascade of proteins including MAPK (ERK1/2), PKC, and PLCγ (Turner & Grose, 2010).
In Xenopus, FGF signaling through the mesoderm has been implicated in neural crest induction (Monsoro-Burq et al., 2003). Additional compelling evidence for the role of FGF signaling during neural crest induction comes from studies in the chick. Inhibition of FGF signaling (dnFgfr1/Mkp3) in the restricted perspective neural plate border region of the gastrula epiblast results in a loss of neural crest markers Pax7 and Snai2; however, inhibition of FGF signaling post-gastrulation does not affect these markers (Lunn, Fishwick, Halley, & Storey, 2007; Stuhlmiller & García-Castro, 2012b). This work demonstrated a direct role of FGF/MAPK signaling in the establishment of neural plate border cells, and demonstrates a cell autonomous requirement for ERK signaling during neural crest formation. Expression analysis also suggested a mesoderm independent role of FGF signaling, as FGFR1/4 are expressed in perspective neural crest epiblast during these stages, but not in the mesoderm (Lunn et al., 2007; Stuhlmiller & García-Castro, 2012b). Supporting these findings, recent work in rabbit supports a mesoderm-independent specification of neural crest, which is dependent upon FGF signaling (Betters et al., 2018). In this report, explants from the prospective neural crest territory in the gastrula rabbit embryo were unable to express the neural crest markers Pax7 and Sox10 in the presence of an FGF inhibitor.
In another study, the role of FGF4 in neural crest induction was examined by implanting beads soaked in FGF4 in regions of chick nonneural ectoderm (Yardley & García-Castro, 2012). Following treatment, the authors observed an upregulation of early neural crest markers, without apparent mesoderm contribution or acquiring definitive neural character. Interestingly, treatment with FGF first launched the upregulation of BMP4 and Wnt8c, prior to the detection of neural crest associated transcripts. The contribution of nonneural ectoderm under inductive signals to form neural crest has been suggested in Xenopus and chick using nonneural ectoderm-neural plate explant juxtapositions (Mancilla & Mayor, 1996; Ruffins & Bronner-Fraser, 2000; Selleck & Bronner-Fraser, 1995; Streit & Stern, 1999). Finally, a recent study in human ES cell-derived neural crest model system has also demonstrated the requirement of FGF signaling during neural crest induction, as the inhibition of FGF signaling (using PD173074) resulted in inhibition of neural crest induction even in presence of Wnt signaling (Leung et al., 2016).
Despite the studies in Xenopus and chick embryos, and a model of human neural crest, a clear role for FGF signaling has yet to be established in mouse and zebrafish. Several FGF ligands are known to have a role in pre-gastrula mouse embryos (Arman, Haffner-Krausz, Chen, Heath, & Lonai, 1998; Feldman, Poueymirou, Papaioannou, Dechiara, & Goldfarb, 1995; Meyers, Lewandoski, & Martin, 1998), but a regulatory role for FGF during mouse neural crest induction still remains to be seen (Frank et al., 2002). Further, mice lacking FGF receptors and zebrafish embryos lacking mesoderm undergo normal neural crest development (Jones & Trainor, 2005). The lack of a defined role for FGF signaling in mouse mutants can perhaps be explained based on the functional redundancy between the FGF factors, and will require an analysis of all the FGF signaling receptors and ligands to decipher the role of FGF signaling in neural crest development. In zebrafish, the requirement of FGF signaling can be explained based on heterochrony, where FGF signaling might play a transient role much earlier in development than analyzed
3.4 |. Notch signaling
Notch signaling involves Notch receptor binding to a membranebound ligand to activate a cascade of proteolysis that results in the release of the Notch intracellular domain (NICD). The NICD then translocates to the nucleus and functions as a transcription factor in conjunction with a CSL protein (Kopan & Ilagan, 2009). During neural crest induction, Notch signaling has been shown to function upstream of BMP4 in frog and chick embryos (Endo, Osumi, & Wakamatsu, 2002). In zebrafish, Notch signaling has been implicated in neural crest development through the restriction of the neural fate. Studies at earlier stages in zebrafish have identified Prdm1a as a Notch/Delta target that is necessary for neural plate border specification by antagonizing the pro-neural factor Olig4 (Filippi et al., 2005; Hernandez-Lagunas, Powell, Law, Grant, & Artinger, 2011; Hernandez-Lagunas et al., 2005). Studies in zebrafish addressing the role of Notch signaling during neural crest development using loss-of-function analysis demonstrate an effect on the formation of trunk neural crest cells but not cranial neural crest cells (Cornell & Eisen, 2005). Thus, it appears that Notch might not be responsible for induction of neural crest at all axial levels in zebrafish.
The role of Notch signaling during neural crest induction in mouse has not yet been identified. However, Notch signaling does play a crucial role during later neural crest development, as Delta-1 null mice exhibit defects in neural crest cell migration and differentiation (De Bellard, Ching, Gossler, & Bronner-Fraser, 2002). These variations in the role of Notch signaling between vertebrates could be explained based on the redundancy of Notch signaling components and functional redundancy between signaling pathways involved in neural crest development that might vary between species.
3.5 |. Crosstalk between signaling pathways
Neural crest induction involves crosstalk between Wnt, FGF, and BMP pathways. As discussed, each of these pathways play a crucial role in neural crest induction and continued formation of the neural crest. Wnt has been suggested to modulate BMP signaling. Studies in the chick have shown that Wnt3a can induce BMP expression in gastrula stage neural explants, while inhibition of Wnt signaling in neural crest explants results in the downregulation of BMP4 (Patthey et al., 2009). These experiments provide a possible mechanistic role of Wnt and BMP signaling in neural crest induction, where initial inhibition of BMP and the activation of Wnt is required for neural crest induction in almost all species. However, activation of BMP through Wnt provides signal to neural crest specifiers at the neural plate border needed for continued neural crest induction. The modulation of BMP signaling through FGF/MAPK signaling has been demonstrated in Xenopus (Branney, Faas, Steane, Pownall, & Isaacs, 2009; Fletcher & Harland, 2008; Kudoh, Concha, Houart, Dawid, & Wilson, 2004), chick, and zebrafish, where FGF signaling positively regulates the BMP inhibitors Chordin and Noggin, negatively regulates BMP ligand, positively regulates the BMP modulator SNW1, and importantly, MAPK and GSK3 (independent of Wnt and downstream of MAPK) has been shown to negatively regulate Smad1 (Furthauer, 2004; Hardy, Yatskievych, Konieczka, Bobbs, & Antin, 2011; Kretzschmar, Liu, Hata, Doody, & Massagué, 1997; Wilson et al., 2000; Wilson et al., 2001). Together, these studies suggest significant crosstalk between FGF and Wnt pathways that induces the neural crest gene regulatory network (GRN) at the neural plate border during gastrulation. Further, these pathways function to modulate the BMP signaling required for activation and continued expression of neural crest specifier genes at the neural plate border.
4 |. TRANSCRIPTIONAL NETWORK UNDERLYING NEURAL CREST CELL FORMATION
As discussed above, neural crest induction is thought to be mediated by the actions of WNT, BMP, FGF, and Notch signaling molecules, which function in the activation and maintenance of the gene regulatory network controlling the establishment of the neural plate border and the formation of the neural crest. The neural plate border is characterized by the expression of transcription factors including Zic1, Pax3/7, and Gbx2, among others, which are involved in activating a cascade of factors that lead to the maintenance of the neural crest state and later migratory and differentiation networks. Integrative efforts from numerous models including Xenopus, chick, zebrafish, and mouse, and more recently in basal vertebrates such as lamprey and hagfish (Nikitina, Sauka-Spengler, & Bronner-Fraser, 2008; Ota, Kuraku, & Kuratani, 2007; Sauka-Spengler, Meulemans, Jones, & Bronner-Fraser, 2007), and other mammalian models such as rabbit (Betters et al., 2018), and human embryonic stem cell-based systems (Leung et al., 2016), have informed a neural crest GRN representing the hierarchical and combinatorial interactions between signaling molecules and transcription factors governing neural crest formation (Prasad et al., 2012; Rogers & Nie, 2018; Sauka-Spengler & BronnerFraser, 2008; Simoes-Costa & Bronner, 2015).
4.1 |. Neural plate border specifiers
During neural crest development, the neural plate border cells receive inducing signals and begin to express a set transcription factors known as neural plate border specifiers. As development proceeds, these factors activate a battery of neural crest specifier transcription factors. A number of neural plate border specifier genes have been the focus of investigation, including Zic1, Msx1/2, Pax3/7, Dlx5, and Gbx2, as well as AP2α and SP5 (Park et al., 2013; Prasad et al., 2012; SaukaSpengler & Bronner-Fraser, 2008; Simoes-Costa & Bronner, 2015). As such, these transcription factors are generally considered to be some of the earliest players in neural crest cell formation. The Xenopus model has been particularly powerful in addressing the gene network underlying neural plate border formation, as signaling and transcription factor expression can be modulated through microinjection. In Xenopus, the expression of msxl, pax3 and zicl were found to be induced by BMP, Wnt, and FGF signals (Monsoro-Burq et al., 2005; Sato et al., 2005). Additional work further revealed that both BMP and FGF can separately activate zicl and pax3 expression (Hong & Saint-Jeannet, 2007). However, both signals might be required in combination for the endogenous specification of neural crest. FGF8 and Wnt have been shown to converge and activate pax3 expression (Monsoro-Burq et al., 2005), while lower levels of BMP signaling regulates dlx5 expression (Luo, Matsuo-Takasaki, & Sargent, 2001). Wnt signaling directly activates gbx2 to initiate the expression of other neural plate border specifier genes, including msx1 and pax3, thereby translating the Wnt signaling input during neural crest induction (Li et al., 2009). Finally, a conserved cis-regulatory module has been identified in mouse and Drosophila which mediates intermediate levels of BMP signaling to activate Msx2 promoter (Brugger et al., 2004).
The paired domain transcription factors Pax3 and Pax7 are part of a nine factor family in mammals which play crucial and diverse roles during development (Mayran, Pelletier, & Drouin, 2015). Expression of Pax3 and Pax7 is found at the neural plate border in most vertebrates (Huang & Saint-Jeannet, 2004; Monsoro-Burq, 2015), and therefore, these factors have been the subject of much focus. In chick, Pax7 has been identified as the earliest marker of the neural crest, and was shown to be necessary for its formation (Basch et al., 2006). A recent report in rabbit also identified Pax7 as the earliest factor specifically expressed in the neural plate border (Betters et al., 2018), and future perturbation studies will be required to address its function. Perturbation experiments in Xenopus have identified FGF, Wnt, and retinoids as regulators of Pax7 expression (Maczkowiak et al., 2010). Consistent with these findings, in chick, beads soaked in Wnt or BMP inhibitors and placed in the prospective Pax7 expression domain revealed a decrease in Pax7 expression (Basch et al., 2006), and FGF signaling via Erk1/2 has been shown to be required for Pax7 expression (Stuhlmiller & García-Castro, 2012b). The direct regulation of Pax7 expression is a major question. In chick, this has been shown to occur through cMyb binding to a Pax7 enhancer (Vadasz, Marquez, Tulloch, Shylo, & Garcia-Castro, 2013), and studies in Xenopus indicate that Pax3 directly activates its own expression and that of pax7 (Maczkowiak et al., 2010; Plouhinec et al., 2014).
Interestingly, the functional roles of Pax3 and Pax7 appear divergent between Xenopus and amniotes. In Xenopus, is has been suggested that Pax3 elicits its functions on neural crest formation from its expression domain in the ectoderm, while the role of Pax7 stems from inductive processes in the paraxial mesoderm (Maczkowiak et al., 2010; Sato et al., 2005). In mouse embryos, Pax3 and Pax7 appear to play redundant roles (Relaix, Rocancourt, Mansouri, & Buckingham, 2004). However, this study also suggests that neural crest cells arise in the absence of either Pax3 or Pax7. Recently, through Pax3/7 double knockouts and a Pax3 dominant negative, it appears that the function of Pax3/7 are dispensable for mouse neural crest formation (Zalc, Rattenbach, Auradé, Cadot, & Relaix, 2015).
Elucidating the regulatory relationships between neural plate border transcription factors has been an area of intense study. Work in Xenopus has made great strides in delineating the regulatory network activated by Pax3 and Zic1 at the neural plate border (Plouhinec et al., 2014). This study indicates a widespread role for these two transcription factors in regulating a host of neural plate border and neural crest genes. Interestingly, Plouhinec et al. (2014) further uncovered a role for Pax3 and Zic1 in modulating the transcriptional output of Wnt and retinoic acid signaling pathways by regulating the expression of axin2 and cycp26c1. Another downstream target of Pax3 and Zic1 identified in Xenopus is Tfap2e. Perturbation analysis in whole Xenopus embryos as well as naive animal caps revealed that Tfap2e is required for neural crest progenitor formation (Hong, Devotta, Lee, Park, & Saint-Jeannet, 2014). Finally, a recent study identified the zinc finger transcriptional repressor Znf703 as a downstream target of Pax3 and Zic1 in Xenopus (Hong & Saint-Jeannet, 2017). While the expression of Znf703 spans the ectoderm and neural plate, this study identifies its role at the neural plate border in regulating the neural crest specifier genes Snail2 and Sox10 downstream of Pax3 and Zic1
4.2 |. Neural crest specifiers
Transcription factors expressed in the neural plate border, along with signaling events at this stage, activate and/or maintain the expression of neural crest specifier genes. Among the best characterized neural crest specifiers are Sox8, Sox9, Sox10, FoxD3, Snai1/2, cMyc, and the Id genes. In addition to the expression of these genes, continued expression of neural plate border specifiers, such as Pax3/7 and AP2α, is also observed, and together these factors control the cell fate decision toward the pre-migratory and migratory neural crest state. Interestingly, in the past few years, studies have reported on the expression of some of these specifiers, including Snai1 and Sox5, during the late blastula to early gastrula stages of neural crest development, suggesting an even earlier role for some of these factors in neural crest formation (Buitrago-Delgado et al., 2015). These transcription factors have been well-studied in regards to their repetitive use throughout neural crest development, from early specification to differentiation into terminal derivatives. Here, we discuss key neural crest specifiers and their regulatory role during continued neural crest specification.
4.2.1 |. SoxE/D transcription factors
SoxE (Sox8, Sox9, and Sox10) and SoxD (Sox5) families are known to play a crucial role during neural crest formation and later migration. SoxE genes are expressed at the neural plate border following neural crest induction in a specific temporal manner that differs between species. In chick, Sox9, Sox10, and Sox5 expression precedes the expression of Sox8 (Cheung & Briscoe, 2003; Perez-Alcala, Nieto, & Barbas, 2004; Southard-Smith, Kos, & Pavan, 1998). In Xenopus, all three SoxE genes are coexpressed, and a recent report suggests blastula stage expression of sox5 (Aoki et al., 2003; Buitrago-Delgado et al., 2015; Spokony, Aoki, Saint-Germain, Magner-Fink, & SaintJeannet, 2002). The expression of Sox9 and Sox10 genes has been well-characterized during different stages of neural crest development. In Xenopus and chick, Sox10 is expressed transiently in cranial neural crest migrating toward the pharyngeal arches, but is not observed in the ectomesenchymal derivatives of cranial crest (Cheng, Cheung, Abu-Elmagd, Orme, & Scotting, 2000; Spokony et al., 2002). By contrast, in chick and mouse embryos, Sox9 is restricted to neural crest cells migrating into pharyngeal arches that differentiate into cranial skeletal elements and in cardiac derivatives (Cheung & Briscoe, 2003; Montero et al., 2002). In human embryos, SOX10 expression along with PAX7, AP2α, and SOX9 has been documented in premigratory and migratory neural crest cells (Betters et al., 2010). In early rabbit embryos, Sox9 marks both pre-migratory and migratory cranial and trunk neural crest cells, while Sox10 appears specific to migratory neural crest (Betters et al., 2018). Sox8 expression overlaps with both Sox9 and Sox10 in several neural crest domains, while the expression of Sox9 and Sox10 is nonoverlapping. Thus, based on the expression of Sox8 in different species, it has been suggested to play a redundant role in neural crest development that can be substituted by other SoxE genes (Cheung & Briscoe, 2003).
The role and regulation of Sox factors during neural crest development has been analyzed in much detail using perturbation and cis-regulatory analysis. In avian embryos, Sox9 has been implicated in the maintenance of an undifferentiated state of neural crest, and is required for trunk neural crest development (Cheung et al., 2005; Cheung & Briscoe, 2003). In chick, Sox5 has been implicated as an modulator of Sox10 expression, and induces RhoB expression leading to cytoskeletal changes required for EMT (Perez-Alcala et al., 2004). A recent study in Xenopus has described an even earlier role for Sox5 in regulating the expression of neural plate border and neural crest specifiers by modulating BMP signaling through direct interaction with Smad1/4 and regulating the expression of targets such as msx1, pax3, and zic1 (Nordin & Labonne, 2014). It has been further demonstrated in Xenopus that the expression of sox9 can be induced by AP2α and a combination of Gbx2 and Zic1 (Li et al., 2009; Luo et al., 2001). In zebrafish, a cis-regulatory element in the first intron of sox10 containing functional Tcf/Lef sites along with SoxE and FoxD3 binding sites implicates the combinatorial regulation of sox10 (Dutton et al., 2008). In Xenopus embryos, both sox9 and sox10 expression can be repressed by Id transcription factors (Light, Vernon, Lasorella, Iavarone, & Labonne, 2005), and functional studies involving loss and gain-of function have also identified Sox9 and Slug as activators of sox10 expression in the neural crest (Aoki et al., 2003). Although these perturbation experiments identify the role of different transcription factors in sox9 and sox10 regulation, it does not exclude the possible involvement other factors. Finally, direct regulation of Sox10 by Ets1, cMyb and Sox9 in chick cranial neural crest has been documented (Betancur, Bronner-Fraser, & Sauka-Spengler, 2010).
During neural crest induction and migration, SoxE factors (Sox9 and Sox10) are involved in the maintenance of the neural crest multipotent cell state by inhibiting differentiation. During later development, as neural crest cells migrate and are exposed to local environmental signals, Sox10 plays a vital role in terminal differentiation of neural crest into melanocytes and glia (Aoki et al., 2003; Stolt, Lommes, Hillgärtner, & Wegner, 2008), while Sox9 initiates ectomesenchymal differentiation (Lefebvre, Huang, Harley, Goodfellow, & de Crombrugghe, 1997). The partial redundancies between the Sox genes suggests a complex functional conservation, which can be deciphered by analyzing the interactions between them, their protein partners, and target genes during different stages of neural crest development.
4.2.2 |. Snai zinc-finger transcription factors
The Snail family of transcription factors has been well-characterized in development and disease. Snai1 and Snai2 are paralogous transcription factors that emerged in vertebrates as a result of a gene duplication event from a single Snail gene in protochordates (Manzanares, Locascio, & Nieto, 2001). Snai1 and Snai2 expression switches between different species, suggesting an orthologous role of these two factors. Expression of Snai1 and Snai2 in pre-migratory neural crest, and in the tail bud of mouse, chick, zebrafish, lizard, and turtle shows some conserved but diverse patterns. Snai1 is expressed in pre-migratory neural crest in mouse and zebrafish, while in chick and lizard, Snai2 is expressed (Locascio, Manzanares, Blanco, & Nieto, 2002). A report in Xenopus also revealed the expression of snail in blastula stage embryos (Buitrago-Delgado et al., 2015), suggesting an earlier role of Snai1 in neural crest specification. Interestingly, a report profiling the transcriptomes of tissue derived from early gastrula Xenopus embryos identifies almost fivefold higher levels of snail in vegetal tissue compared to animal cap tissue (Blitz et al., 2016), implicating Snai1 in mesendoderm formation. This earlier expression of snail suggests its role in the early development of multiple cell fates, while snai2 expression in Xenopus is not detected until the neural plate border stage. These findings allude to potentially segregated roles of these two genes during neural crest development.
Snai2 expression patterns have been well-characterized during embryonic development in mouse, chicken, Xenopus, and zebrafish. Snai2 is involved in both specification and migration of neural crest in chick and Xenopus embryos (Labonne & Bronner-Fraser, 1998). Snai2 in chick, and Snai1 in other vertebrate embryos, regulates the delamination of neural crest cells. Studies in avian embryos indicate that the overexpression of Snai2 can induce neural crest at the cranial level, but not in the trunk (del Barrio & Nieto, 2002). Snai2 is regulated by a complex set of signaling inputs and transcription factors. Direct regulation of Snai2 has been demonstrated to be under the control of combinatorial signaling inputs including Wnt and intermediate levels of BMP signaling (Conacci-Sorrell et al., 2003). Supporting this regulation, snai2 expression is induced in Xenopus animal caps following Wnt8 overexpression and the attenuation of high levels of BMP (Kee & Bronner-Fraser, 2005). A regulatory element in the mouse Snai2 promoter consists of Smad1 and Tcf/Lef1 sites, confirming the role of BMP and Wnt signaling in modulating its expression, while the Xenopus snai2 promoter consists of a functional LEF-1 binding site (Sakai et al., 2005; Vallin et al., 2001). In addition, Notch signaling through the Hairy2 transcription factor regulates snai2 in Xenopus (Glavic, Silva, Aybar, Bastidas, & Mayor, 2004), while neural plate border specifiers Zic1, Msx1, and Pax3/7 also induce snai2 expression in Xenopus and chick in the presence of Wnt signaling (Meulemans & Bronner-Fraser, 2004; Sato et al., 2005; Tribulo et al., 2003). Although these functional studies suggest different regulators of Snai2 expression in different species, it does not rule out the role of other factors that might cooperatively play a role in modulating Snai2 expression.
4.2.3 |. Additional neural crest specifier transcription factors
The functions of Sox and Snail family transcription factors are almost indispensable to neural crest development. However, as suggested earlier, a host of other transcription factors is required for proper development of neural crest cells. Unlike other cell fates such as endoderm (Charney, Paraiso, Blitz, & Cho, 2017) and hematopoietic stem cells (Swiers, Patient, & Loose, 2006), the neural crest field is still relatively new in terms of the identity of regulatory factors involved in neural crest formation, and the GRN through which they function. However, in the past two decades, a large number of transcription factors have been identified to play a role in regulating different stages of neural crest formation. We discuss the role of these additional neural crest specifiers in this section.
cMyc and Id factors play a crucial role in transducing signals from the neural plate border specifiers. Id (inhibitor of differentiation) genes are downstream targets of the BMP signaling pathway, and in Xenopus and lamprey, Id factors have been found to be downstream of cMyc (Light et al., 2005; Nikitina, Tong, & Bronner, 2011). Both Id and cMyc are involved in maintenance of the neural crest multipotent state by regulating the expression of proliferation and differentiation genes. In Xenopus, knockdown of Id3 through injection of morpholino oligonucleotides results in cell cycle inhibition and the loss of the neural crest cell progenitor pool (Kee & Bronner-Fraser, 2005). The initial expression of cMyc and Id genes could be triggered by other upstream activators such as AP2α and/or Zic1, and expression of Id genes is maintained in pre-migratory neural crest by cMyc (Nikitina etal., 2011).
The winged helix transcription factor FoxD3 plays a central role in the maintenance of neural crest cell multipotency by functioning to prevent early differentiation (Lister et al., 2006; Teng, Mundell, Frist, Wang, & Labosky, 2008). The regulation of FoxD3 has been wellcharacterized in chick embryos, with the identification of two separate enhancers that control the expression of FoxD3 in cranial or trunk neural crest (Simões-Costa, McKeown, Tan-Cabugao, Sauka-Spengler, & Bronner, 2012). This study further identifies Pax7, Msx1/2, and Ets1 as upstream regulators of FoxD3 expression in both cranial and trunk neural crest, while Zic1 appears to specifically regulate FoxD3 expression in the trunk (Simões-Costa et al., 2012). In Xenopus embryos, Notch signaling through Hairy2, Msx1, and a combination of Zic1, Pax3/7, and Wnt signaling induces foxd3 expression (Sato et al., 2005; Tribulo et al., 2003; Wettstein, Turner, & Kintner, 1997). In most model systems, the direct targets of FoxD3 are not known; however, functional studies in chick and mouse have revealed that ectopic expression of FoxD3 leads to the induction of cadherin-7 and β1-integrin (Cheung et al., 2005). A study in Xenopus has identified an autoregulatory feedback loop, whereby ectopic expression of FoxD3 represses its own expression (Pohl & Knochel, 2001). Importantly, a recent study in zebrafish has characterized the direct targets of FoxD3 in great detail using RNA-Seq, ATAC-Seq, and Foxd3 ChIP-Seq. This work proposes a temporally regulated bimodal role of FoxD3 in neural crest specification, by first priming the neural crest genes for activation during specification and migration, and later acting as a transcriptional repressor to inhibit certain cells fates (Lukoseviciute et al., 2018).
The role of adapter proteins in transcriptional regulatory complexes during neural crest formation is just starting to unfold. Adapter proteins are involved in transcriptional regulation by interacting with other transcription factors, but do not bind to DNA directly. In Xenopus embryos, Ajuba Lim proteins (Ajuba, Limd1, and Wtip) act as corepressors along with Snai1 and Snai2 during neural crest formation (Langer et al., 2008; Ochoa, Salvador, & Labonne, 2012). In another example, ectopic expression of the LIM domain transcription factor LMO4 activates snai1 and snai2 expression in the neural plate border and nonneural ectoderm (Ochoa et al., 2012). During later neural crest formation, LMO4 cooperates with Snai1 and Snai2 to act as a corepressor (Ochoa et al., 2012). Similar studies in chick embryos have validated the role of Lmo4 as a Snai co-factor in later neural crest migration, as well as in neural crest pathology (Ferronha et al., 2013).
cMyb is known to regulate Pax7 expression in chick at neural plate border stages (Vadasz et al., 2013), and was also found to regulate the expression of the neural crest specifier snai2 (Karafiat et al., 2005). Gene expression analysis following cMyb knockdown in chick revealed downregulation of Pax7 and Twist1, among others, and an upregulation of Zic1 (Betancur, Simões-Costa, Sauka-Spengler, & Bronner, 2014), placing cMyb as a regulator of neural plate border and neural crest specifier genes. Ets1, on the other hand, is known to be a downstream effector of FGF/Erk signaling, and can transduce FGF/Erk signaling effects (Nelson et al., 2010). As a cell cycle regulator, Ets1 regulates G1/S transition during neural crest delamination (Fafeur et al., 1997; Sauka-Spengler & Bronner-Fraser, 2008; Theveneau, Duband, & Altabef, 2007). The cranial and vagal neural crest specific bHLH transcription factor Twist1 is another neural crest specifier that modulates later neural crest development. However, little is known regarding the direct regulation of Twist1 and its downstream targets. In Xenopus embryos, mis-expression of Snai2, FoxD3, and Zic1 transcription factors induces twist1 expression (Meulemans & Bronner-Fraser, 2004; Sasai, Mizuseki, & Sasai, 2001), and Notch signaling has also been implicated in regulating twist1 expression in Xenopus (Cornell & Eisen, 2005). Another transcription factor, Gli2, has been recently implicated in neural crest induction in Xenopus. Through gain-of-function and loss-of-function analyses, Gli2 was found to affect the expression of neural plate border and neural crest genes including pax3, zic1, msx1, snai2, foxd3, and sox10 (Cerrizuela, VegaLopez, Palacio, Tribulo, & Aybar, 2018). It remains to be seen how Gli2 mediated responses integrates with other signaling cascades (such as Wnt/β-catenin and FGF signaling) that function in the regulation of these genes.
Hox genes have also been implicated in neural crest development, but due to the functional redundancy of the Hox gene clusters, a definitive role of particular Hox genes during neural crest development has been difficult to ascertain. However, loss-of-function studies have revealed that Hox genes are broadly involved in neural crest patterning and migration (Trainor & Krumlauf, 2000). Hox genes display unique expression patterns in the rhombencephalic neural crest, and are involved in the positional identity of the pharyngeal arches (Minoux & Rijli, 2010; Santagati, Minoux, Ren, & Rijli, 2005). Interestingly, at least two studies have suggested a role of anterior Hox genes in the specification of neural crest from neural progenitors. In embryonic stem cell derived neural stem cells, Hoxb1 can activate Msx1/2 and Snai1 expression (Gouti & Gavalas, 2008), and ectopic expression of the anterior Hox gene Hoxb1 in chick embryos induces a neural crest fate from neural cells which is accompanied by a loss of proliferation and changes in cell adhesion and induction of EMT (Gouti, Briscoe, & Gavalas, 2011). This induction appears to be BMP-dependent, and requires intermediate levels of Notch signaling and the repression of Hes5. In the same report, other anterior Hox genes were also able to induce neural crest, but were unable to potentiate neural crest EMT, while posterior Hox genes were unable to induce the neural crest fate (Gouti et al., 2011). Thus, anterior Hox genes can be implicated in neural crest formation and EMT in combination with other signaling pathways.
Finally, a recent study in chick lends support to the axial identity of transcription factors involved in the continued induction of neural crest (Simões-Costa & Bronner, 2016). This study identified Brn3c, Dmbx1, and Lhx5 as factors expressed earlier than the neural crest specifiers Sox8, Tfap2b, and Ets1, and which regulate their expression in cranial neural crest cells (Simoes-Costa & Bronner, 2015; SimõesCosta & Bronner, 2016).
Based on the studies described above using perturbations, cis-regulatory analysis, and known temporal expression patterns of neural crest specifiers, a GRN of neural crest induction has been proposed (Prasad et al., 2012; Sauka-Spengler & Bronner-Fraser, 2008; SimoesCosta & Bronner, 2015). Here, we have provided an updated version of the neural crest GRN, incorporating a novel pre-border state during pre-gastrula stages of development (Figure 2). While we have to acknowledge species-specific differences between expression and function of these transcription factors, a combinatorial role of these specifiers during neural crest induction has been well agreed upon. Functionally, it appears that there is more divergence across species in the role of specific transcription factors functioning during early neural crest specification and induction, compared to later terminal differentiation. Teasing out the specific roles and redundancies of core neural crest specifiers, and the identification of novel players, is required for a complete understanding of the neural crest-GRN.
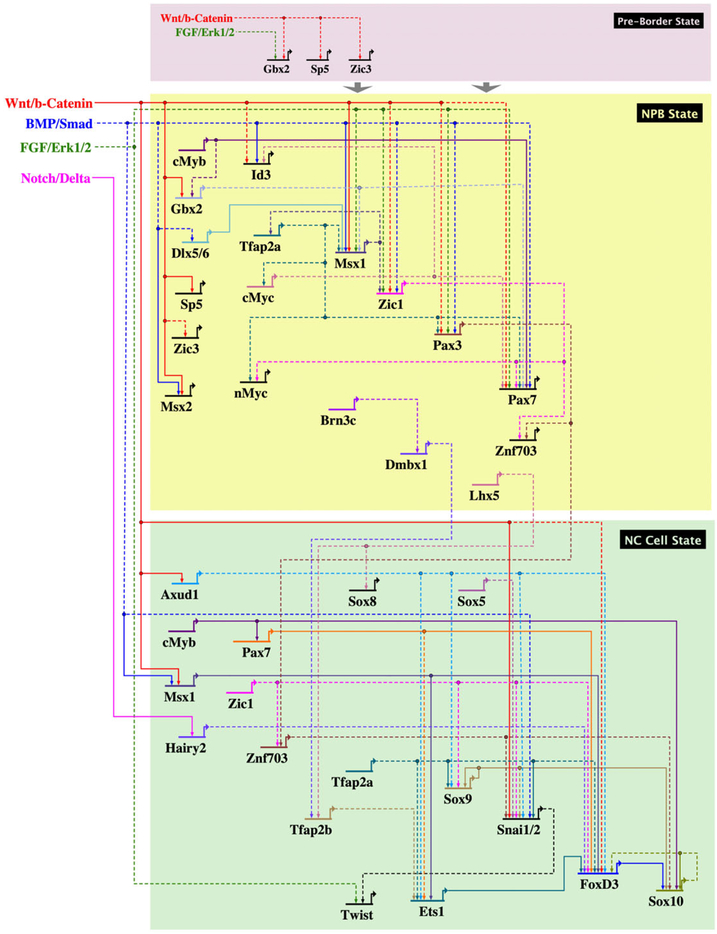
FIGURE 2.
Gene regulatory network governing the specification and formation of neural crest cells. Proposed gene regulatory network involved in neural crest induction based on available literature. The recently proposed pre-border state based on the human neural crest model system depicts potential signaling pathways that may be involved at this stage according to their known expression patterns in different species. The transcription factors at this stage were identified from studies in human neural crest specification. The neural plate border (NPB) and subsequent neural crest (pre-migratory) states follow the pre-border state, and depict an updated version of the neural crest gene regulatory network compiled from studies in chick, mouse, human, Xenopus, and zebrafish model systems. The GRN model was built using BioTapestry software (Longabaugh et al., 2009)
5 |. EPIGENETIC CONTROL OF NEURAL CREST FORMATION
In addition to signal transduction and transcription factor binding, gene regulation involves the regulation of higher order chromatin structure via histone modifications and the recruitment of general transcriptional activation or repression machinery. The presence of histone variants, modifications of histones, and ATP-dependent chromatin remodeling regulates the chromatin structure. In eukaryotes, the nucleosome is composed of a histone core consisting of H2A, H2B, H3, and H4 that are wrapped by genomic DNA of around 146 bp. Post-translational modifications of histone tails involves methylation, acetylation, phosphorylation, ubiquitination, sumoylation, ADP ribosylation, deimination, and the non-covalent proline isomerization (Berger, 2007; Gibney & Nolan, 2010). The regulation of gene expression is mediated by the recruitment of transcriptional activators and repressors to specific genomic locations. These genomic loci are marked by chromatin modifications that are signatures of open/closed (active/repressed) chromatin. H3K4me3, H3K27ac, and H3K36me3 are known to be transcriptional activation marks, while H3K9me3 and H3K27me3 are known to be repressive marks (Berger, 2007).
A comprehensive study in human embryonic stem cell-derived neural crest cells identified transcriptionally active and inactive enhancers using H3K4me1, H3K27ac, and H3K27me3 ChIP-Seq (Rada-Iglesias et al., 2011). This work elaborated on poised enhancers associated with the H3K27me3 mark that are known to be involved in gastrulation, neurulation, and mesoderm formation, and validated that their spatial and temporal activity in zebrafish recapitulates endogenous gene expression (Rada-Iglesias et al., 2011). This work demonstrates the importance of epigenetic analysis to identify developmentally functional enhancers associated with gene regulation. Another approach for identifying cis-regulatory modules is through the identification of conserved regulatory regions. This approach was used in a study of human and chimp neural crest cells derived from induced pluripotent stem cells. By combining ChIP-Seq for histone marks, ATAC-Seq to delineate open chromatin regions, and RNA-Seq to identify gene expression changes, the authors identified cis-regulatory elements involved in the formation of cranial neural crest cells and the craniofacial skeleton (Prescott et al., 2015). Further, a recent study in zebrafish made use of a transgenic FoxD3 reporter line coupled with high-throughput sequencing techniques to identify a wide array of enhancer modules for numerous known and novel neural plate border and neural crest specifier genes (Lukoseviciute et al., 2018). Importantly, this work made use of an elegant biotagging approach (Trinh et al., 2017) to specifically isolate cells expressing FoxD3. As neural crest cells develop within a complex and heterogenous cellular environment, the use of genomic approaches on neural crest cells from animal models depends on the use of such sorting techniques, including protein tagging or enhancer/promoter driven fluorescence.
Specific histone modifiers have also been implicated in neural crest formation. JmjD2A, part of the Jumonji family of histone demethylases, is expressed in the neural plate border and demethylates H3K9me3 to activate the expression of neural crest specifiers Sox9, Sox10, FoxD3, and Snai2 in the chick embryo (Strobl-Mazzulla, Sauka-Spengler, & Bronner-Fraser, 2010). CHD7, a chromodomain helicase, was identified to activate the expression of neural crest specifiers, SOX9, TWIST1, and SNAI2 in human embryonic stem cellderived neural crest cells by associating with their enhancers (Bajpai et al., 2010). Histone acetyl transferases (HATs) and histone deacetylases (HDACs) regulate the addition and removal of acetyl groups from histones (Carrozza, Utley, Workman, & Côté, 2003; Hsieh, Nakashima, Kuwabara, Mejia, & Gage, 2004), resulting in changes to DNA accessibility. While not much is known about the role of HATs during different stages of neural crest development, a few studies have begun to explore the roles of HDACs. The inducible knockout of Hdac8 in neural crest cells of mouse embryos results in craniofacial defects, and this was suggested to be mediated by the specific repression of Otx2 and Lhx1 transcription factors (Haberland, Mokalled, Montgomery, & Olson, 2009). Finally, a recent study in Xenopus reported that increased levels of HDAC1 are required for neural crest specification during pre-gastrula stage (Rao & Labonne, 2018).
In the past few years, significant effort has been directed toward elucidating the epigenetic landscape underlying neural crest formation in multiple model systems. As discussed above, these studies have provided promising results that further our understanding of the cis-regulatory modules and chromatin landscape that function to regulate the expression of neural plate border and neural crest specifiers. These studies provide direct insight into the regulatory network that orchestrates the neural crest developmental program. Further effort in this direction is required to better define the regulatory network involved in neural crest cell fate specification.
6 |. NEURAL CREST LINEAGE SEGREGATION
The classical view of neural crest induction defines a neural and nonneural ectodermal contribution to form neural crest. The ectodermal derivation of neural crest is consistent with their contribution to ectodermal derivatives such as pigment cells, and peripheral neurons and glia. But at the same time, neural crest contributes to ectomesenchymal derivatives in the cranial region, including bone, cartilage, and fat cells known to be derived from the mesoderm in other parts of the body. This multipotent nature of the neural crest, in particular its ability to contribute to derivatives of two different germ layers, has been a topic of scientific discussion for quite some time. A step toward explaining this multipotential came from Basch et al. (2006), which described a model of early neural crest specification during gastrulation and prior to the formation of definitive germ layers. Further support for this study has come from additional studies in chick, and in rabbit embryos describing the gastrula stage specification of neural crest (Betters et al., 2018; Patthey et al., 2008). Recent studies using human neural crest derived from human embryonic stem cells also support an early neural crest specification, independent of definitive neural and mesodermal tissue (Leung et al., 2016). Taken together, these studies point to a model of pre-gastrula neural crest specification, where neural crest cells are derived from a pluripotent state. Such a model whereby neural crest cells emerge from a state of wide differentiation potential and retain a unique multipotent state which enables them to make ectomesenchymal contributions would explain the vast potential of the neural crest.
While the recent studies discussed above have suggested an earlier model for neural crest specification, it does not discount the prior model of definitive ectodermal contribution toward neural crest induction. It is plausible that neural crest cells arise in multiple ways, and perhaps the earliest anterior neural crest emerge independently from neural and nonneural ectoderm and mesoderm interactions, while later neural crest arises through those interactions. Interestingly, recent work by several groups has suggested that in the trunk territory of the embryo, neural crest may arise from an axial progenitor or neuromesodermal precursors (NMP) (Wymeersch et al., 2016). However, the tissue interactions and signaling mechanisms responsible for such neural crest origin have not been described and are an interesting avenue of future exploration.
Another model of neural crest specification was recently proposed in Xenopus (Buitrago-Delgado et al., 2015), and reports on the blastula stage expression of neural plate border and neural crest genes pax3, zicl, sox5, and snail along with expression of pluripotency genes, oct25/60, sox2 and vent2. The expression patterns of these specifier transcription factors do suggest blastula stage specification of neural crest. However, the model proposed in this study suggests the retention of pluripotency in these cells, rather than blastula stage specification of a neural crest-specific program. While the maintenance of pluripotency in prospective neural crest cells is an intriguing theory, given the changes in cell fate and signaling in the surrounding tissue that will soon segregate into definitive ectoderm and mesoderm, it seems unlikely that neural crest cells retain a differentiation potential equivalent to that of pluripotent epiblast cells of the blastula. The prospective neural crest cells at the blastula stage are likely not yet committed toward a neural crest cell fate, but based on unpublished data from the Garcia-Castro lab in chick embryos at blastula stage, it has been proposed that these cells are specified toward the neural crest cell fate.
Given that prospective neural crest cells at the blastula stage express pluripotency genes along with neural plate border and neural crest specifier genes, it can be suggested that these cells are open to multiple potential. A recent study in chick embryos also identified co-expression of some of the pluripotency factors along with neural crest specifier genes at neurula stages of neural crest induction (Lignell, Kerosuo, Streichan, Cai, & Bronner, 2017). This study further suggests a cooperative role of pluripotency factors along with neural crest specifiers during neural crest induction. Further, a recent study in Xenopus interrogating single cell gene expression (RNA-Seq) from blastula to neurula stages of development has demonstrated the expression of the neural crest genes Foxd3, cMyc, Id3, Tfap2, Ets1, Snail along with pluripotency factors ventx2 and oct25 in most of the ectodermal cells, as well as during non-pluripotent states in the endoderm and mesoderm (Briggs et al., 2018). Given the expression of these factors in non-pluripotent cells, this study suggests that these factors may not be imparting pluripotency to prospective neural crest cells at blastula stage, but instead function to coordinate lineage commitment (Wang, Oron, Nelson, Razis, & Ivanova, 2012). In support of these findings, a recent study in zebrafish has suggested the presence of a network functioning during neural crest formation that is paralogous to the epiblast pluripotency regulatory network (Lukoseviciute et al., 2018). A study in a human model of neural crest development has also identified this early stage of neural crest specification as a pre-border state, with co-expression of pluripotency and neural crest factors (Leung et al., 2016). Support for a pre-border stage was also recently identified with a transcriptomic profile in pregastrula stage chick embryos and identified coexpression of neural plate border genes along with pluripotency genes (Trevers et al., 2017). Based on these recent findings on neural crest specification, it can be suggested that there is an operational GRN for neural crest specification prior to gastrulation that shares factors from the pluripotency GRN. However, the GRN for prospective neural crest is distinct from the pluripotency GRN, suggesting a segregation of neural crest lineage prior to gastrulation.
7 |. PERSPECTIVES
Formation of the neural crest has been studied extensively in multiple model systems, in particular Xenopus, chick, zebrafish, and mouse. While these integrated efforts have revealed broad conservation in the formation of neural crest cells, some major discrepancies remain. For example, while current models indicate that the mesoderm is dispensable for neural crest formation in chick, zebrafish, and human embryonic stem cellbased models of neural crest, ectodermmesoderm interactions appear to be required in Xenopus. Further, major questions regarding mammalian neural crest cell specification and formation remain unanswered. Due to its small size and unique gastrula morphology, neural crest cell specification assays have not been performed in the mouse—the main model of mammalian neural crest development. Further, the role of signaling pathways (e.g., Wnt, FGF) and major transcription factors (e.g., Pax3/7, Snai1/2) that have been shown to be required for neural crest formation in other model systems have either not been tested or deemed dispensable in murine neural crest formation (Barriga et al., 2015). The appreciation for variation among mammalian species has been boosted by recent findings in mammalian early cell lineage decisions (Berg et al., 2011; Rossant, 2011). Given this, we believe it will be critical to explore major questions of neural crest formation in alternative mammalian systems, including ontogeny, tissue contributions, and the role of signaling pathways and transcription factors. One such model system is the rabbit, which develops as a large, flat blastodisc with a standard germ layer arrangement. Importantly, this early rabbit development is morphologically similar to human embryos. Used in many classic embryology studies (Waddington & Waterman, 1933), today the rabbit is widely used in biomedical research as a model for human disease (Shiomi, 2009). Further, while the evolutionary distances separating primates and rabbit and rodents are similar, rabbit gene sequences are closer to human than to rodent, as rodent sequences evolved more rapidly (Graur, Duret, & Gouy, 1996; Margulies et al., 2007). Some initial progress has been made in characterizing rabbit neural crest cell specification and formation (Betters et al., 2018), further studies using the rabbit, or other complementary models of mammalian neural crest formation, are needed.
Recently, the enormous progress in pluripotent stem cell biology has permeated to neural crest studies (Chambers, Mica, Lee, Studer, & Tomishima, 2016; Fukuta et al., 2014; Hackland et al., 2017; Leung et al., 2016; Mica, Lee, Chambers, Tomishima, & Studer, 2013; RadaIglesias et al., 2012; Umeda et al., 2015). These models, to a great extent, have confirmed the findings of the few human embryological studies (Betters et al., 2010; O’Rahilly & Müller, 2007), which remain restricted due to technical and ethical limitations. Furthermore, a recent human neural crest model recapitulates the progression of neural crest development seen in model organisms, from the inductive signaling events mediated by WNTs, to the temporal expression (and function) of neural plate border, neural fold, and migratory markers (Leung et al., 2016) (Figure 3). The human neural crest model has also been successfully used to explore neural crest based pathologies with great success (Fattahi et al., 2016; Lee et al., 2012; Mica et al., 2013). Current progress has made the human neural crest model more efficient and offering today an unprecedented access to unlimited, synchronized biological material, enabling “omic” scale studies representing an outstanding platform to advance both basic and translational research.
FIGURE 3.
Stem cell-based model of human neural crest development. Schematic depicting the neural crest differentiation protocol using human embryonic stem cells (hES) or human induced pluripotent stem cells (hiPS) as described in Leung et al. (2016). The neural crest induction from hES/hiPS cells begins with the activation of Wnt signaling using the GSK3 inhibitor CHIR99021, and neural crest cell formation is complete on Day 5. Different phases of neural crest development, including pre-border, neural plate border (NPB) and neural crest state, have been designated based on the in vivo expression of known neural crest markers during 5 days of induction. The large amounts of synchronous neural crest cells obtained at Day 5 can be used to obtain all known neural crest derivatives (Leung et al., 2016) to address neural crest pathologies
The study of the formation of the neural crest has a rich history, but it is clear that major questions remain regarding the origins, signaling pathways, and transcription factors involved in defining the earliest stages of neural crest specification. Taken together, the current literature points to an early specification of neural crest during blastula stages, with the coexpression of pre-border and pluripotency factors. This pre-border state has been elaborated on in the human model of neural crest induction from hES cells (Leung et al., 2016) as well as in chick embryo (Trevers et al., 2017). Neural crest induction continues through gastrula and neurula stages of embryonic development through inductive signals of Wnt, BMP, FGF and a host of neural plate border and neural crest specifier transcription factors. The current state of neural crest induction has been focused on the molecular changes during gastrula and neurula stages. Much remains to be addressed regarding the signaling pathways and transcription factors required during the earliest neural crest specification during blastula stage.
ACKNOWLEDGMENTS
We apologize to researchers whose work we were unable to include due to space limitations. This work is supported by the National Institute of Dental & Craniofacial Research of the National Institutes of Health Award Number R01DE017914 to M.I.G.C., and the National Institute of Dental & Craniofacial Research of the National Institutes of Health Award Number F32DE027862 to R.M.C.
Funding information
National Institute of Dental and Craniofacial Research, Grant/Award Numbers: R01DE017914, F32DE027862
REFERENCES
- Abu-Elmagd M, Garcia-Morales C, & Wheeler GN (2006). Frizzled7 mediates canonical Wnt signaling in neural crest induction. Developmental Biology, 298, 285–298. 10.1016/j.ydbio.2006.06.037 [DOI] [PubMed] [Google Scholar]
- Aoki Y, Saint-Germain N, Gyda M, Magner-Fink E, Lee Y-H, Credidio C, & Saint-Jeannet J-P (2003). Sox10 regulates the development of neural crest-derived melanocytes in Xenopus. Developmental Biology, 259, 19–33. 10.1016/S0012-1606(03)00161-1 [DOI] [PubMed] [Google Scholar]
- Arman E, Haffner-Krausz R, Chen Y, Heath JK, & Lonai P (1998). Targeted disruption of fibroblast growth factor (FGF) receptor 2 suggests a role for FGF signaling in pregastrulation mammalian development. Proceedings of the National Academy of Sciences, 95, 5082–5087. 10.1073/pnas.95.9.5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajpai R, Chen DA, Rada-Iglesias A, Zhang J, Xiong Y, Helms J..Wysocka J (2010). CHD7 cooperates with PBAF to control multipotent neural crest formation. Nature, 463, 958–962. 10.1038/nature08733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JC, Beddington RS, & Harland RM (1999). Wnt signaling in Xenopus embryos inhibits bmp4 expression and activates neural development. Genes & Development, 13, 3149–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barriga EH, Trainor PA, Bronner M, & Mayor R (2015). Animal models for studying neural crest development: Is the mouse different? Development, 142, 1555–1560. 10.1242/dev.121590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basch ML, Bronner-Fraser M, & García-Castro MI (2006). Specification of the neural crest occurs during gastrulation and requires Pax7. Nature, 441, 218–222. 10.1038/nature04684 [DOI] [PubMed] [Google Scholar]
- Basch ML, Selleck MA, & Bronner-Fraser M (2000). Timing and competence of neural crest formation. Developmental Neuroscience, 22, 217–227. 10.1159/000017444 [DOI] [PubMed] [Google Scholar]
- Berg DK, Smith CS, Pearton DJ, Wells DN, Broadhurst R, Donnison M, & Pfeffer PL (2011). Trophectoderm lineage determination in cattle. Developmental Cell, 20, 244–255. 10.1016/j.devcel.2011.01.003 [DOI] [PubMed] [Google Scholar]
- Berger SL (2007). The complex language of chromatin regulation during transcription. Nature, 447,407–412. 10.1038/nature05915 [DOI] [PubMed] [Google Scholar]
- Betancur P, Bronner-Fraser M, & Sauka-Spengler T (2010). Genomic code for Sox10 activation reveals a key regulatory enhancer for cranial neural crest. Proceedings of the National Academy of Sciences of the United States of America, 107, 3570–3575. 10.1073/pnas.0906596107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancur P, Simões-Costa M, Sauka-Spengler T, & Bronner ME (2014). Expression and function of transcription factor cMyb during cranial neural crest development. Mechanisms of Development, 132, 38–43. 10.1016/j.mod.2014.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betters E, Charney RM, & García-Castro MI (2018). Early specification and development of rabbit neural crest cells. Developmental Biology, 21, 316–320. 10.1016/j.ydbio.2018.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betters E, Liu Y, Kjaeldgaard A, Sundström E, & García-Castro MI(2010). Analysis of early human neural crest development. Developmental Biology, 344, 578–592. 10.1016/j.ydbio.2010.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betters E, Murdoch B, Leung AW, & García-Castro MI (2014). Human neural crest cells and stem cell-based models In Neural crest cells (pp. 395–412). Academic Press; 10.1016/B978-0-12-401730-6.00019-3 [DOI] [Google Scholar]
- Blitz IL, Paraiso KD, Patrushev I, Chiu WTY, Cho KWY, & Gilchrist MJ (2016). A catalog of Xenopus tropicalis transcription factors and their regional expression in the early gastrula stage embryo. Developmental Biology, 426, 409–417. 10.1016/j.ydbio.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolande R (1974). The neurocristopathies: A unifying concept of disease arising in neural crest maldevelopment. Human Pathology, 5, 409–429. 10.1016/S0046-8177(74)80021-3 [DOI] [PubMed] [Google Scholar]
- Bolande RP (1997). Neurocristopathy: Its growth and development in 20 years. Pediatric Pathology & Laboratory Medicine, 17, 1–25. [PubMed] [Google Scholar]
- Bonstein L, Elias S, & Frank D (1998). Paraxial-fated mesoderm is required for neural crest induction in Xenopus embryos. Developmental Biology, 193, 156–168. 10.1006/dbio.1997.8795 [DOI] [PubMed] [Google Scholar]
- Böttcher RT, & Niehrs C (2005). Fibroblast growth factor signaling during early vertebrate development. Endocrine Reviews, 26, 63–77. 10.1210/er.2003-0040 [DOI] [PubMed] [Google Scholar]
- Branney PA, Faas L, Steane SE, Pownall ME, & Isaacs HV (2009). Characterisation of the fibroblast growth factor dependent Transcriptome in early development. PLoS One, 4, e4951 10.1371/journal.pone.0004951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs JA, Weinreb C, Wagner DE, Megason S, Peshkin L, Kirschner MW, & Klein AM (2018). The dynamics of gene expression in vertebrate embryogenesis at single-cell resolution. Science, 360, eaar5780 10.1126/science.aar5780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugger SM, Merrill AE, Torres-Vazquez J, Wu N, Ting M-C,Cho JY-M,… Maxson R (2004). A phylogenetically conserved cis-regulatory module in the Msx2 promoter is sufficient for BMP dependent transcription in murine and Drosophila embryos. Development, 131, 5153–5165. 10.1242/dev.01390 [DOI] [PubMed] [Google Scholar]
- Buitrago-Delgado E, Nordin K, Rao A, Geary L, & Labonne C (2015). NEURODEVELOPMENT. Shared regulatory programs suggest retention of blastula-stage potential in neural crest. Cell, 348, 1332–1335. 10.1126/science.aaa3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrozza MJ, Utley RT, Workman JL, & Côté J (2003). The diverse functions of histone acetyltransferase complexes. Trends in Genetics, 19, 321–329. 10.1016/S0168-9525(03)00115-X [DOI] [PubMed] [Google Scholar]
- Cebra-Thomas JA, Terrell A, Branyan K, Shah S, Rice R, Gyi L.,…Gilbert SF (2013). Late-emigrating trunk neural crest cells in turtle embryos generate an osteogenic ectomesenchyme in the plastron. Developmental Dynamics, 242, 1223–1235. 10.1002/dvdy.24018 [DOI] [PubMed] [Google Scholar]
- Cerrizuela S, Vega-Lopez GA, Palacio MB, Tribulo C, & Aybar MJ (2018). Gli2 is required for the induction and migration of Xenopus laevis neural crest. Mechanisms of Development, 154, 219–239. 10.1016/j.mod.2018.07.010 [DOI] [PubMed] [Google Scholar]
- Chambers SM, Mica Y, Lee G, Studer L, & Tomishima MJ (2016). Dual-SMAD inhibition/WNT activation-based methods to induce neural crest and derivatives from human pluripotent stem cells. Methods in Molecular Biology, 1307, 329–343. 10.1007/7651_2013_59 [DOI] [PubMed] [Google Scholar]
- Chang C, & Hemmati-Brivanlou A (1998). Neural crest induction by Xwnt7B in Xenopus. Developmental Biology, 194, 129–134. 10.1006/dbio.1997.8820 [DOI] [PubMed] [Google Scholar]
- Charney RM, Paraiso KD, Blitz IL, & Cho KWY (2017). A gene regulatory program controlling early Xenopus mesendoderm formation: Network conservation and motifs. Seminars in Cell and Developmental Biology, 66, 12–24. 10.1016/j.semcdb.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y-C, Cheung M, Abu-Elmagd MM, Orme A, & Scotting PJ (2000). Chick Sox10, a transcription factor expressed in both early neural crest cells and central nervous system. Developmental Brain Research, 121, 233–241. 10.1016/S0165-3806(00)00049-3 [DOI] [PubMed] [Google Scholar]
- Cheung M, & Briscoe J (2003). Neural crest development is regulated by the transcription factor Sox9. Development, 130, 5681–5693. 10.1242/dev.00808 [DOI] [PubMed] [Google Scholar]
- Cheung M, Chaboissier M-C, Mynett A, Hirst E, Schedl A, & Briscoe J (2005). The transcriptional control of trunk neural crest induction, survival, and delamination. Developmental Cell, 8, 179–192. 10.1016/j.devcel.2004.12.010 [DOI] [PubMed] [Google Scholar]
- Choudhary B, Ito Y, Makita T, Sasaki T, Chai Y, & Sucov HM (2006). Cardiovascular malformations with normal smooth muscle differentiation in neural crest-specific type II TGFβ receptor (Tgfbr2) mutant mice. Developmental Biology, 289, 420–429. 10.1016/j.ydbio.2005.11.008 [DOI] [PubMed] [Google Scholar]
- Conacci-Sorrell M, Simcha I, Ben-Yedidia T, Blechman J, Savagner P, & Ben-Ze’ev A (2003). Autoregulation of E-cadherin expression by cadherin-cadherin interactions: The roles of beta-catenin signaling, slug, and MAPK. The Journal of Cell Biology, 163, 847–857. 10.1083/jcb.200308162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell RA, & Eisen JS (2005). Notch in the pathway: The roles of notch signaling in neural crest development. Seminars in Cell and Developmental Biology, 16, 663–672. 10.1016/j.semcdb.2005.06.009 [DOI] [PubMed] [Google Scholar]
- De Bellard ME, Ching W, Gossler A, & Bronner-Fraser M (2002). Disruption of segmental neural crest migration and ephrin expression in Delta-1 null mice. Developmental Biology, 249, 121–130. 10.1006/dbio.2002.0756 [DOI] [PubMed] [Google Scholar]
- de Croze N, Maczkowiak F, & Monsoro-Burq AH (2011). Reiterative AP2a activity controls sequential steps in the neural crest gene regulatory network. Proceedings of the National Academy of Sciences of the United States of America, 108, 155–160. 10.1073/pnas.1010740107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deardorff MA, Tan C, Saint-Jeannet JP, & Klein PS (2001). A role for frizzled 3 in neural crest development. Development, 128, 3655–3663. [DOI] [PubMed] [Google Scholar]
- Debbache J, Parfejevs V, & Sommer L (2018). Cre-driver lines used for genetic fate mapping of neural crest cells in the mouse: An overview. Genesis, 56, e23105–e23108. 10.1002/dvg.23105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Barrio MG, & Nieto MA (2002). Overexpression of snail family members highlights their ability to promote chick neural crest formation. Development, 129, 1583–1593. [DOI] [PubMed] [Google Scholar]
- Devotta A, Hong CS, & Saint-Jeannet J-P (2018). Dkk2 promotes neu-ral crest specification by activating Wnt/β-catenin signaling in a GSK3β independent manner. eLife, 7, e34404 10.7554/eLife.34404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson ME, Selleck MA, McMahon AP, & Bronner-Fraser M (1995). Dorsalization of the neural tube by the non-neural ectoderm. Development, 121, 2099–2106. [DOI] [PubMed] [Google Scholar]
- Dorey K, & Amaya E (2010). FGF signalling: Diverse roles during early vertebrate embryogenesis. Development, 137, 3731–3742. 10.1242/dev.037689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudas M, Sridurongrit S, Nagy A, Okazaki K, & Kaartinen V (2004). Craniofacial defects in mice lacking BMP type I receptor Alk2 in neural crest cells. Mechanisms of Development, 121, 173–182. 10.1016/j.mod.2003.12.003 [DOI] [PubMed] [Google Scholar]
- Dupont J-C (2018). Historical perspective on neuroembryology: Wilhelm His and his contemporaries. Genesis, 56, e23218 10.1002/dvg.23218 [DOI] [PubMed] [Google Scholar]
- Dutton JR, Antonellis A, Carney TJ, Rodrigues FS, Pavan WJ, Ward A, & Kelsh RN (2008). An evolutionarily conserved intronic region controls the spatiotemporal expression of the transcription factor Sox10. BMC Developmental Biology, 8, 105 10.1186/1471-213X-8-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkouby YM, Elias S, Casey ES, Blythe SA, Tsabar N, Klein PS,…Frank D (2010). Mesodermal Wnt signaling organizes the neural plate via Meis3. Development, 137, 1531–1541. 10.1242/dev.044750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y, Osumi N, & Wakamatsu Y (2002). Bimodal functions of notchmediated signaling are involved in neural crest formation during avian ectoderm development. Development, 129, 863–873. [DOI] [PubMed] [Google Scholar]
- Etchevers HC, Amiel J, & Lyonnet S (2006). Molecular bases of human neurocristopathies. Advances in Experimental Medicine and Biology, 589, 213–234. 10.1007/978-0-387-46954-6_14 [DOI] [PubMed] [Google Scholar]
- Ezin AM, Fraser SE, & Bronner-Fraser M (2009). Fate map and morphogenesis of presumptive neural crest and dorsal neural tube. Developmental Biology, 330, 221–236. 10.1016/j.ydbio.2009.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fafeur V, Tulasne D, Queva C, Vercamer C, Dimster V, Mattot V,…Vandenbunder B (1997). The ETS1 transcription factor is expressed during epithelial-mesenchymal transitions in the chick embryo and is activated in scatter factor-stimulated MDCK epithelial cells. Cell Growth & Differentiation, 8, 655–665. [PubMed] [Google Scholar]
- Farlie PG, McKeown SJ, & Newgreen DF (2004). The neural crest: Basic biology and clinical relationships in the craniofacial and enteric nervous systems. Birth Defects Research. Part C, Embryo Today, 72, 173–189. 10.1002/bdrc.20013 [DOI] [PubMed] [Google Scholar]
- Fattahi F, Steinbeck JA, Kriks S, Tchieu J, Zimmer B, Kishinevsky S, … Studer L (2016). Deriving human ENS lineages for cell therapy and drug discovery in Hirschsprung disease. Nature, 531, 105–109. 10.1038/nature16951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure S, de Santa Barbara P, Roberts DJ, & Whitman M (2002). Endogenous patterns of BMP signaling during early Chick development. Developmental Biology, 244, 44–65. 10.1006/dbio.2002.0579 [DOI] [PubMed] [Google Scholar]
- Feldman B, Poueymirou W, Papaioannou VE, Dechiara TM, & Goldfarb M (1995). Requirement of FGF-4 for postimplantation mouse development. Science, 267, 246–249. 10.1126/science.7809630 [DOI] [PubMed] [Google Scholar]
- Ferronha T, Rabadán MA, Gil-Guiñon E, Le Dréau G, de Torres C, & Martí E (2013). LMO4 is an essential cofactor in the Snail2-mediated epithelial-to-mesenchymal transition of neuroblastoma and neural crest cells. The Journal of Neuroscience, 33, 2773–2783. 10.1523/JNEURUSCI.4511-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi A, Tiso N, Deflorian G, Zecchin E, Bortolussi M, & Argenton F(2005). The basic helix-loop-helix olig3 establishes the neural plate boundary of the trunk and is necessary for development of the dorsal spinal cord. Proceedings of the National Academy of Sciences, 102, 4377–4382. 10.1073/pnas.0407284102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher RB, & Harland RM (2008). The role of FGF signaling in the establishment and maintenance of mesodermal gene expression in Xenopus. Developmental Dynamics, 237, 1243–1254. 10.1002/dvdy.21517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DU, Fotheringham LK, Brewer JA, Muglia LJ, TristaniFirouzi M, Capecchi MR, & Moon AM (2002). An Fgf8 mouse mutant phenocopies human 22q11 deletion syndrome. Development, 129,4591–4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuta M, Nakai Y, Kirino K, Nakagawa M, Sekiguchi K, Nagata S,…Toguchida J (2014). Derivation of mesenchymal stromal cells from pluripotent stem cells through a neural crest lineage using small molecule compounds with defined media. PLoS One, 9, e112291 10.1371/journal.pone.0112291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furthauer M (2004). Fgf signalling controls the dorsoventral patterning of the zebrafish embryo. Development, 131, 2853–2864. 10.1242/dev.01156 [DOI] [PubMed] [Google Scholar]
- Gans C, & Northcutt RG (1983). Neural crest and the origin of vertebrates: A new head. Science, 220, 268–273. 10.1126/science.220.4594.268 [DOI] [PubMed] [Google Scholar]
- Garcia-Castro M (2011). Early origin and differentiation capacity of the neural crest In Topics in animal and plant development (pp. 55–74). Transworld Research Network: Kerala, India. [Google Scholar]
- Garcia-Castro MI, Marcelle C, & Bronner-Fraser M (2002). Ectodermal Wnt function as a neural crest inducer. Science, 297, 848–851. 10.1126/science.1070824 [DOI] [PubMed] [Google Scholar]
- Gibney ER, & Nolan CM (2010). Epigenetics and gene expression. Heredity (Edinb), 105, 4–13. 10.1038/hdy.2010.54 [DOI] [PubMed] [Google Scholar]
- Glavic A, Silva F, Aybar MJ, Bastidas F, & Mayor R (2004). Interplay between notch signaling and the homeoprotein Xiro1 is required for neural crest induction in Xenopus embryos. Development, 131, 347–359. 10.1242/dev.00945 [DOI] [PubMed] [Google Scholar]
- Gouti M, Briscoe J, & Gavalas A (2011). Anterior HoxGenes interact with components of the neural crest specification network to induce neural crest fates. Stem Cells, 29, 858–870. 10.1002/stem.630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouti M, & Gavalas A (2008). Hoxb1 controls cell fate specification and proliferative capacity of neural stem and progenitor cells. Stem Cells, 26, 1985–1997. 10.1634/stemcells.2008-0182 [DOI] [PubMed] [Google Scholar]
- Graur D, Duret L, & Gouy M (1996). Phylogenetic position of the order Lagomorpha (rabbits, hares and allies). Nature, 379, 333–335. 10.1038/379333a0 [DOI] [PubMed] [Google Scholar]
- Gurdon JB (1962). The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. Journal of Embryology and Experimental Morphology, 10, 622–640. [PubMed] [Google Scholar]
- Gurdon JB, Byrne JA, & Simonsson S (2003). Nuclear reprogramming and stem cell creation. Proceedings of the National Academy of Sciences, 100 (suppl 1), 11819–11822. 10.1073/pnas.1834207100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkovich YE, Ofir R, Elkouby YM, Dibner C, Gefen A, Elias S, & Frank D (2010). Xenopus Meis3 protein lies at a nexus downstream to Zic1 and Pax3 proteins, regulating multiple cell-fates during early nervous system development. Developmental Biology, 338, 50–62. 10.1016/j.ydbio.2009.11.024 [DOI] [PubMed] [Google Scholar]
- Haberland M, Mokalled MH, Montgomery RL, & Olson EN (2009). Epigenetic control of skull morphogenesis by histone deacetylase 8. Genes & Development, 23, 1625–1630. 10.1101/gad.1809209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackland JOS, Frith TJR, Thompson O, Marin Navarro A, GarcíaCastro MI, Unger C, & Andrews PW (2017). Top-down inhibition of BMP signaling enables robust induction of hPSCs into neural crest in fully defined, Xeno-free conditions. Stem Cell Reports, 9, 1043–1052. 10.1016/j.stemcr.2017.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BK (2000). The neural crest as a fourth germ layer and vertebrates as quadroblastic not triploblastic. Evolution & Development, 2, 3–5. 10.1046/j.1525-142X.2000.00032.x [DOI] [PubMed] [Google Scholar]
- Hall BK (2018). Germ layers, the neural crest and emergent organization in development and evolution. Genesis, 56, e23103 10.1002/dvg.23103 [DOI] [PubMed] [Google Scholar]
- Hardy KM, Yatskievych TA, Konieczka JH, Bobbs AS, & Antin PB (2011). FGF signalling through RAS/MAPK and PI3K pathways regulates cell movement and gene expression in the chicken primitive streak without affecting E-cadherin expression. BMC Developmental Biology, 11, 20 10.1186/1471-213X-11-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassler C, Cruciat CM, Huang YL, Kuriyama S, Mayor R, & Niehrs C (2007). Kremen is required for neural crest induction in Xenopus and promotes LRP6-mediated Wnt signaling. Development, 134, 4255–4263. 10.1242/dev.005942 [DOI] [PubMed] [Google Scholar]
- Heeg-Truesdell E, & Labonne C (2006). Neural induction in Xenopus requires inhibition of Wnt-beta-catenin signaling. Developmental Biology, 298, 71–86. 10.1016/j.ydbio.2006.06.015 [DOI] [PubMed] [Google Scholar]
- Hernandez-Lagunas L, Choi IF, Kaji T, Simpson P, Hershey C,Zhou Y,…Artinger KB (2005). Zebrafish narrowminded disrupts the transcription factor prdm1 and is required for neural crest and sensory neuron specification. Developmental Biology, 278, 347–357. 10.1016/j.ydbio.2004.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Lagunas L, Powell DR, Law J, Grant KA, & Artinger KB (2011). prdm1a and olig4 act downstream of notch signaling to regulate cell fate at the neural plate border. Developmental Biology, 356, 496–505. 10.1016/j.ydbio.2011.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CS (2001). TGF-β signalling pathways in early Xenopus development. Current Opinion in Genetics & Development, 11, 533–540. 10.1016/s0959-437x(00)00229-x [DOI] [PubMed] [Google Scholar]
- His W, 1868. Untersuchungen über die erste Anlage des Wirbelthierleibes: die erste Entwickelung des Hühnchens im Ei. Leipzig: Vogel. [Google Scholar]
- Hong C-S, Devotta A, Lee Y-H, Park BY, & Saint-Jeannet J-P (2014). Transcription factor AP2 epsilon (Tfap2e) regulates neural crest specification in Xenopus. Developmental Neurobiology, 74, 894–906. 10.1002/dneu.22173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C-S, Park BY, & Saint-Jeannet J-P (2008). Fgf8a induces neural crest indirectly through the activation of Wnt8 in the paraxial mesoderm. Development, 135, 3903–3910. 10.1242/dev.026229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C-S, & Saint-Jeannet J-P (2007). The activity of Pax3 and Zic1 regulates three distinct cell fates at the neural plate border. Molecular Biology of the Cell, 18, 2192–2202. 10.1091/mbc.e06-11-1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C-S, & Saint-Jeannet J-P (2017). Znf703, a novel target of Pax3 and Zic1, regulates hindbrain and neural crest development in Xenopus. Genesis, 55, e23082 10.1002/dvg.23082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C-S, & Saint-Jeannet J-P (2018). The b-HLH transcription factor Hes3 participates in neural plate border formation by interfering with Wnt/β-catenin signaling. Developmental Biology, 442, 162–172. 10.1016/j.ydbio.2018.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J, Nakashima K, Kuwabara T, Mejia E, & Gage FH (2004). Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proceedings of the National Academy of Sciences, 101, 16659–16664. 10.1073/pnas.0407643101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, & Saint-Jeannet J-P (2004). Induction of the neural crest and the opportunities of life on the edge. Developmental Biology, 275, 1–11. 10.1016/j.ydbio.2004.07.033 [DOI] [PubMed] [Google Scholar]
- Ikeya M, Lee SM, Johnson JE, McMahon AP, & Takada S (1997). Wnt signalling required for expansion of neural crest and CNS progenitors. Nature, 389, 966–970. 10.1038/40146 [DOI] [PubMed] [Google Scholar]
- Jaenisch R, & Young R (2008). Stem cells, the molecular circuitry of Pluri potency and nuclear reprogramming. Cell, 132, 567–582. 10.1016/j.cell.2008.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Q, McDill BW, Li S-Z, Deng C, Chang C-P, & Chen F (2007). Smad signaling in the neural crest regulates cardiac outflow tract remodeling through cell autonomous and non-cell autonomous effects. Developmental Biology, 311, 172–184. 10.1016/j.ydbio.2007.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NC, & Trainor PA (2005). Role of morphogens in neural crest cell determination. Journal of Neurobiology, 64, 388–404. 10.1002/neu.20162 [DOI] [PubMed] [Google Scholar]
- Karafiat V, Dvorakova M, Krejci E, Kralova J, Pajer P, Snajdr P,…Dvorak M (2005). Transcription factor c-Myb is involved in the regulation of the epithelial-mesenchymal transition in the avian neural crest. Cellular and Molecular Life Sciences, 62, 2516–2525. 10.1007/s00018-005-5297-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee Y, & Bronner-Fraser M (2005). To proliferate or to die: Role of Id3 in cell cycle progression and survival of neural crest progenitors. Genes & Development, 19, 744–755. 10.1101/gad.1257405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kengaku M, & Okamoto H (1993). Basic fibroblast growth factor induces differentiation of neural tube and neural crest lineages of cultured ectoderm cells from Xenopus gastrula. Development, 119, 1067–1078. [DOI] [PubMed] [Google Scholar]
- Knecht AK, & Bronner-Fraser M (2002). Induction of the neural crest: A multigene process. Nature Reviews. Genetics, 3, 453–461. 10.1038/nrg819 [DOI] [PubMed] [Google Scholar]
- Kopan R, & Ilagan MXG (2009). The canonical notch signaling path-way: Unfolding the activation mechanism. Cell, 137, 216–233. 10.1016/j.cell.2009.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar M, Liu F, Hata A, Doody J, & Massagué J (1997). The TGF-beta family mediator Smad1 is phosphorylated directly and activated functionally by the BMP receptor kinase. Genes & Development, 11, 984–995. 10.1101/gad.11.8.984 [DOI] [PubMed] [Google Scholar]
- Kudoh T, Concha ML, Houart C, Dawid IB, & Wilson SW (2004). Combinatorial Fgf and bmp signalling patterns the gastrula ectoderm into prospective neural and epidermal domains. Development, 131, 3581–3592. 10.1242/dev.01227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonne C, & Bronner-Fraser M (1998). Neural crest induction in Xenopus: Evidence for a two-signal model. Development, 125, 2403–2414. [DOI] [PubMed] [Google Scholar]
- Langer EM, Feng Y, Zhaoyuan H, Rauscher FJ III, Kroll KL, & Longmore GD (2008). Ajuba LIM proteins are snail/slug corepressors required for neural crest development in Xenopus. Developmental Cell, 14, 424–436. 10.1016/j.devcel.2008.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin NM (1980). The ontogeny of the neural crest in avian embryo chimaeras. Nature, 286, 663–669. 10.1038/286663a0 [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Creuzet S, Couly G, & Dupin E (2004). Neural crest cell plasticity and its limits. Development, 131, 4637–4650. 10.1242/dev.01350 [DOI] [PubMed] [Google Scholar]
- Lee G, Ramirez CN, Kim H, Zeltner N, Liu B, Radu C,… Studer L (2012). Large-scale screening using familial dysautonomia induced pluripotent stem cells identifies compounds that rescue IKBKAP expression. Nature Biotechnology, 30, 1244–1248. 10.1038/nbt.2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RTH, Knapik EW, Thiery JP, & Carney TJ (2013). An exclusively mesodermal origin of fin mesenchyme demonstrates that zebrafish trunk neural crest does not generate ectomesenchyme. Development, 140, 2923–2932. 10.1242/dev.093534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V, Huang W, Harley VR, Goodfellow PN, & de Crombrugghe B (1997). SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Molecular and Cellular Biology, 17, 2336–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie EJ, Taub MA, Liu H, Steinberg KM, Koboldt DC, Zhang Q, … Murray JC (2015). Identification of functional variants for cleft lip with or without cleft palate in or near PAX7, FGFR2, and NOG by targeted sequencing of GWAS loci. American Journal of Human Genetics, 96, 397–411. 10.1016/j.ajhg.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AW, Murdoch B, Salem AF, Prasad MS, Gomez GA, & García-Castro MI (2016). WNT/β-catenin signaling mediates human neural crest induction via a pre-neural border intermediate. Development, 143, 398–410. 10.1242/dev.130849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JL, Bonner J, Modrell M, Ragland JW, Moon RT, Dorsky RI, & Raible DW (2004). Reiterated Wnt signaling during zebrafish neural crest development. Development, 131, 1299–1308. 10.1242/dev.01007 [DOI] [PubMed] [Google Scholar]
- Li B, Kuriyama S, Moreno M, & Mayor R (2009). The posteriorizing gene Gbx2 is a direct target of Wnt signalling and the earliest factor in neural crest induction. Development, 136, 3267–3278. 10.1242/dev.036954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Perfetto M, Neuner R, Bahudhanapati H, Christian L, Mathavan K,…Wei S (2018). Xenopus ADAM19 regulates Wnt signaling and neural crest specification by stabilizing ADAM13. Development, 145, dev158154. 10.1242/dev.158154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem KF, Tremml G, Roelink H, & Jessell TM (1995). Dorsal differentiation of neural plate cells induced by bmp-mediated signals from epidermal ectoderm. Cell, 82, 969–979. 10.1016/00928674(95)90276-7 [DOI] [PubMed] [Google Scholar]
- Light W, Vernon AE, Lasorella A, Iavarone A, & Labonne C (2005). Xenopus Id3 is required downstream of Myc for the formation of multipotent neural crest progenitor cells. Development, 132, 1831–1841. 10.1242/dev.01734 [DOI] [PubMed] [Google Scholar]
- Lignell A, Kerosuo L, Streichan SJ, Cai L, & Bronner ME (2017). Identification of a neural crest stem cell niche by spatial genomic analysis. Nature Communications, 8, 1830 10.1038/s41467017-01561-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister JA, Cooper C, Nguyen K, Modrell M, Grant K, & Raible DW(2006). Zebrafish Foxd3 is required for development of a subset of neural crest derivatives. Developmental Biology, 290, 92–104. 10.1016/j.ydbio.2005.11.014 [DOI] [PubMed] [Google Scholar]
- Litsiou A, Hanson S, & Streit A (2005). A balance of FGF, BMP and WNT signalling positions the future placode territory in the head. Development, 132, 4051–4062. 10.1242/dev.01964 [DOI] [PubMed] [Google Scholar]
- Locascio A, Manzanares M, Blanco MJ, & Nieto MA (2002). Modularity and reshuffling of snail and slug expression during vertebrate evolution. Proceedings of the National Academy of Sciences, 99, 16841–16846. 10.1073/pnas.262525399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, & Nusse R (2004). The Wnt signaling pathway in development and disease. Annual Review of Cell and Developmental Biology, 20, 781–810. 10.1146/annurev.cellbio.20.010403.113126 [DOI] [PubMed] [Google Scholar]
- Longabaugh WJ, Davidson EH, & Bolouri H (2009). Visualization, documentation, analysis, and communication of large-scale gene regulatory networks. Biochimica et Biophysica Acta, 1789, 363–374. 10.1016/j.bbagrm.2008.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukoseviciute M, Gavriouchkina D, Williams RM, Hochgreb-Hägele T,Senanayake U, Chong-Morrison V,… SaukaSpengler T (2018).From pioneer to repressor: Bimodal foxd3 activity dynamically remodels neural crest regulatory landscape in vivo. Developmental Cell, 47, 608–628.e6. 10.1016/j.devcel.2018.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn JS, Fishwick KJ, Halley PA, & Storey KG (2007). A spatial and temporal map of FGF/Erk1/2 activity and response repertoires in the early chick embryo. Developmental Biology, 302, 536–552. 10.1016/j.ydbio.2006.10.014 [DOI] [PubMed] [Google Scholar]
- Luo T, Matsuo-Takasaki M, & Sargent TD (2001). Distinct roles for distal-less genes Dlx3 and Dlx5 in regulating ectodermal development in Xenopus. Molecular Reproduction and Development, 60, 331–337. 10.1002/mrd.1095 [DOI] [PubMed] [Google Scholar]
- Maczkowiak F, Matéos S, Wang E, Roche D, Harland R, & Monsoro Burq AH (2010). The Pax3 and Pax7 paralogs cooperate in neural and neural crest patterning using distinct molecular mechanisms, in Xenopus laevis embryos. Developmental Biology, 340, 381–396. 10.1016/j.ydbio.2010.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancilla A, & Mayor R (1996). Neural crest formation in Xenopus laevis: Mechanisms of Xslug induction. Developmental Biology, 177, 580–589. 10.1006/dbio.1996.0187 [DOI] [PubMed] [Google Scholar]
- Manzanares M, Locascio A, & Nieto MA (2001). The increasing complexity of the snail gene superfamily in metazoan evolution. Trends in Genetics, 17,178–181. 10.1016/S0168-9525(01)02232-6 [DOI] [PubMed] [Google Scholar]
- Marchant L, Linker C, Ruiz P, Guerrero N, & Mayor R (1998). The inductive properties of mesoderm suggest that the neural crest cells are specified by a BMP gradient. Developmental Biology, 198, 319–329. 10.1006/dbio.1998.8902 [DOI] [PubMed] [Google Scholar]
- Margulies EH, Cooper GM, Asimenos G, Thomas DJ, Dewey CN,Siepel A,…Sidow A (2007). Analyses of deep mammalian sequence alignments and constraint predictions for 1% of the human genome. Genome Research, 17, 760–774. 10.1101/gr.6034307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J (1998). TGF-beta signal transduction. Annual Review of Biochemistry, 67, 753–791. 10.1146/annurev.biochem.67.1.753 [DOI] [PubMed] [Google Scholar]
- Massagué J, & Wotton D (2000). Transcriptional control by the TGF beta/Smad signaling system. The EMBO Journal, 19, 1745–1754. 10.1093/emboj/19.8.1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor R, Guerrero N, & Martínez C (1997). Role of FGF and noggin in neural crest induction. Developmental Biology, 189, 1–12. 10.1006/dbio.1997.8634 [DOI] [PubMed] [Google Scholar]
- Mayor R, Morgan R, & Sargent MG (1995). Induction of the prospective neural crest of Xenopus. Development, 121, 767–777. [DOI] [PubMed] [Google Scholar]
- Mayran A, Pelletier A, & Drouin J (2015). Pax factors in transcription and epigenetic remodelling. Seminars in Cell and Developmental Biology, 44, 135–144. 10.1016/j.semcdb.2015.07.007 [DOI] [PubMed] [Google Scholar]
- Meulemans D, & Bronner-Fraser M (2004). Gene-regulatory interactions in neural crest evolution and development. Developmental Cell, 7, 291–299. 10.1016/j.devcel.2004.08.007 [DOI] [PubMed] [Google Scholar]
- Meyers EN, Lewandoski M, & Martin GR (1998). An Fgf8 mutant allelic series generated by Cre and Flp-mediated recombination. Nature Genetics, 18, 136–141. 10.1038/ng0298-136 [DOI] [PubMed] [Google Scholar]
- Mica Y, Lee G, Chambers SM, Tomishima MJ, & Studer L (2013). Modeling neural crest induction, melanocyte specification, and disease-related pigmentation defects in hESCs and patient-specific iPSCs. Cell Reports, 3, 1140–1152. 10.1016/j.celrep.2013.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoux M, & Rijli FM (2010). Molecular mechanisms of cranial neural crest cell migration and patterning in craniofacial development. Development, 137, 2605–2621. 10.1242/dev.040048 [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq AH (2015). PAX transcription factors in neural crest development. Seminars in Cell and Developmental Biology, 44, 87–96. 10.1016/j.semcdb.2015.09.015 [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq AH, Fletcher RB, & Harland RM (2003). Neural crest induction by paraxial mesoderm in Xenopus embryos requires FGF signals. Development, 130, 3111–3124. 10.1242/dev.00531 [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq A-H, Wang E, & Harland R (2005). Msx1 and Pax3 cooperate to mediate FGF8 and WNT signals during Xenopus neural crest induction. Developmental Cell, 8, 167–178. 10.1016/j.devcel.2004.12.017 [DOI] [PubMed] [Google Scholar]
- Montero JA, Giron B, Arrechedera H, Cheng YC, Scotting P,Chimal-Monroy J,… Hurle JM (2002). Expression of Sox8, Sox9 and Sox10 in the developing valves and autonomic nerves of the embryonic heart. Mechanisms of Development, 118, 199–202. [DOI] [PubMed] [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, & Kaykas A (2004). WNT and beta-catenin signalling: Diseases and therapies. Nature Reviews. Genetics, 5, 691–701. 10.1038/nrg1427 [DOI] [PubMed] [Google Scholar]
- Moury JD, & Jacobson AG (1990). The origins of neural crest cells in the axolotl. Developmental Biology, 141, 243–253. 10.1016/0012-1606(90)90380-2 [DOI] [PubMed] [Google Scholar]
- Nelson ML, Kang H-S, Lee GM, Blaszczak AG, Lau DKW, McIntosh LP, & Graves BJ (2010). Ras signaling requires dynamic properties of Ets1 for phosphorylation-enhanced binding to coactivator CBP. Proceedings of the National Academy of Sciences of the United States of America, 107, 10026–10031. 10.1073/pnas.0915137107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichane M, de Crozé N, Ren X, Souopgui J, Monsoro-Burq AH, & Bellefroid EJ (2008). Hairy2-Id3 interactions play an essential role in Xenopus neural crest progenitor specification. Developmental Biology, 322, 355–367. 10.1016/j.ydbio.2008.08.003 [DOI] [PubMed] [Google Scholar]
- Nie X, Deng C-X, Wang Q, & Jiao K (2008). Disruption of Smad4 in neural crest cells leads to mid-gestation death with pharyngeal arch, craniofacial and cardiac defects. Developmental Biology, 316, 417–430. 10.1016/j.ydbio.2008.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitina N, Sauka-Spengler T, & Bronner-Fraser M (2008). Dissecting early regulatory relationships in the lamprey neural crest gene network. Proceedings of the National Academy of Sciences of the United States of America, 105, 20083–20088. 10.1073/pnas.0806009105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitina N, Tong L, & Bronner ME (2011). Ancestral network module regulating prdm1 expression in the lamprey neural plate border. Developmental Dynamics, 240, 2265–2271. 10.1002/dvdy.22720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noden DM (1975). An analysis of migratory behavior of avian cephalic neural crest cells. Developmental Biology, 42, 106–130. 10.1016/0012-1606(75)90318-8 [DOI] [PubMed] [Google Scholar]
- Noden DM (1988). Interactions and fates of avian craniofacial mesenchyme. Development, 103, 121–140. [DOI] [PubMed] [Google Scholar]
- Nordin K, & Labonne C (2014). Sox5 Is a DNA-binding cofactor for BMP R-Smads that directs target specificity during patterning of the early ectoderm. Developmental Cell, 31, 374–382. 10.1016/j.devcel.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcutt RG (2005). The new head hypothesis revisited. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution, 304, 274–297. doi: 10.1002/jez.b.21063 [DOI] [PubMed] [Google Scholar]
- Ochoa SD, Salvador S, & Labonne C (2012). The LIM adaptor protein LMO4 is an essential regulator of neural crest development. Developmental Biology, 361, 313–325. 10.1016/j.ydbio.2011.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rahilly R, & Müller F (2007). The development of the neural crest in the human. Journal of Anatomy, 211, 335–351. 10.1111/j.1469-7580.2007.00773.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota KG, Kuraku S, & Kuratani S (2007). Hagfish embryology with reference to the evolution of the neural crest. Nature, 446, 672–675. 10.1038/nature05633 [DOI] [PubMed] [Google Scholar]
- Park D-S, Seo J-H, Hong M, Bang W, Han J-K, & Choi S-C (2013). Role of Sp5as an essential early regulator of neural crest specification in xenopus. Developmental Dynamics, 242, 1382–1394. 10.1002/dvdy.24034 [DOI] [PubMed] [Google Scholar]
- Patthey C, Edlund T, & Gunhaga L (2009). Wnt-regulated temporal control of BMP exposure directs the choice between neural plate border and epidermal fate. Development, 136, 73–83. 10.1242/dev.025890 [DOI] [PubMed] [Google Scholar]
- Patthey C, Gunhaga L, & Edlund T (2008). Early development of the central and peripheral nervous systems is coordinated by Wnt and BMP signals. PLoS One, 3, e1625 10.1371/journal.pone.0001625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Alcala S, Nieto MA, & Barbas JA (2004). LSox5 regulates RhoB expression in the neural tube and promotes generation of the neural crest. Development, 131, 4455–4465. 10.1242/dev.01329 [DOI] [PubMed] [Google Scholar]
- Piacentino ML, & Bronner ME (2018). Intracellular attenuation of BMP signaling via CKIP-1/Smurf1 is essential during neural crest induction. PLoS Biology, 16, e2004425 10.1371/journal.pbio.2004425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouhinec J-L, Roche DD, Pegoraro C, Figueiredo AL,Maczkowiak F, Brunet LJ,…Monsoro-Burq AH (2014). Pax3 and Zic1 trigger the early neural crest gene regulatory network by the direct activation of multiple key neural crest specifiers. Developmental Biology, 386,461–472. 10.1016/j.ydbio.2013.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl BS, & Knochel W (2001). Overexpression of the transcriptional repressor FoxD3 prevents neural crest formation in Xenopus embryos. Mechanisms of Development, 103, 93–106. 10.1016/S0925-4773(01)00334-3 [DOI] [PubMed] [Google Scholar]
- Prasad MS, Sauka-Spengler T, & Labonne C (2012). Induction of the neural crest state: Control of stem cell attributes by gene regulatory, post-transcriptional and epigenetic interactions. Developmental Biology, 366, 10–21. 10.1016/j.ydbio.2012.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SL, Srinivasan R, Marchetto MC, Grishina I, Narvaiza I,Selleri L,… Wysocka J (2015). Enhancer divergence and cis-regulatory evolution in the human and chimp neural crest. Cell, 163, 68–83. 10.1016/j.cell.2015.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada-Iglesias A, Bajpai R, Prescott S, Brugmann SA, Swigut T, & Wysocka J (2012). Epigenomic annotation of enhancers predicts transcriptional regulators of human neural crest. Stem Cell, 11, 633–648. 10.1016/j.stem.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada-Iglesias A, Bajpai R, Swigut T, Brugmann SA, Flynn RA, & Wysocka J (2011). A unique chromatin signature uncovers early developmental enhancers in humans. Nature, 470, 279–283. 10.1038/nature09692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JW, & Raible DW (2004). Signals derived from the underlying mesoderm are dispensable for zebrafish neural crest induction. Developmental Biology, 276, 16–30. 10.1016/j.ydbio.2004.08.017 [DOI] [PubMed] [Google Scholar]
- Rao A, & Labonne C (2018). Histone deacetylase activity has an essential role in establishing and maintaining the vertebrate neural crest. Development, 145, dev163386. 10.1242/dev.163386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven CP, & Kloos J (1945). Induction by medial and lateral pieces of the archenteron roof, with special reference to the determination of the neural crest. Acta morphologica Neerlando-Scandinavica, 5, 348–362. [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, & Buckingham M (2004). Divergent functions of murine Pax3 and Pax7 in limb muscle development. Genes & Development, 18, 1088–1105. 10.1101/gad.301004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CD, & Nie S (2018). Specifying neural crest cells: From chromatin to morphogens and factors in between. Wiley Interdisciplinary Reviews: Developmental Biology, 7, e322–e323. 10.1002/wdev.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant J (2011). Developmental biology: A mouse is not a cow. Nature, 471, 457–458. 10.1038/471457a [DOI] [PubMed] [Google Scholar]
- Rothstein M, Bhattacharya D, & Simões-Costa M (2018). The molecular basis of neural crest axial identity. Developmental Biology, In Press 10.1016/j.ydbio.2018.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffins S, & Bronner-Fraser M (2000). A critical period for conversion of ectodermal cells to a neural crest fate. Developmental Biology, 218, 13–20. 10.1006/dbio.1999.9555 [DOI] [PubMed] [Google Scholar]
- Saint-Jeannet JP, He X, Varmus HE, & Dawid IB (1997). Regulation of dorsal fate in the neuraxis by Wnt-1 and Wnt-3a. Proceedings of the National Academy of Sciences, 94, 13713–13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai D, Tanaka Y, Endo Y, Osumi N, Okamoto H, & Wakamatsu Y (2005). Regulation of slug transcription in embryonic ectoderm by beta-catenin-Lef/Tcf and BMP-Smad signaling. Development, Growth & Differentiation, 47, 471–482. 10.1111/jM440-169X.2005.00821.x [DOI] [PubMed] [Google Scholar]
- Santagati F, Minoux M, Ren S-Y, & Rijli FM (2005). Temporal requirement of Hoxa2 in cranial neural crest skeletal morphogenesis. Development, 132, 4927–4936. 10.1242/dev.02078 [DOI] [PubMed] [Google Scholar]
- Sasai N, Mizuseki K, & Sasai Y (2001). Requirement of FoxD3-class signaling for neural crest determination in Xenopus. Development, 128, 2525–2536. [DOI] [PubMed] [Google Scholar]
- Sato T, Sasai N, & Sasai Y (2005). Neural crest determination by coactivation of Pax3 and Zic1 genes in Xenopus ectoderm. Development, 132, 2355–2363. 10.1242/dev.01823 [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, & Bronner-Fraser M (2008). A gene regulatory network orchestrates neural crest formation. Nature Reviews. Molecular Cell Biology, 9, 557–568. 10.1038/nrm2428 [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Meulemans D, Jones M, & Bronner-Fraser M(2007). Ancient evolutionary origin of the neural crest gene regulatory network. Developmental Cell, 13, 405–420. 10.1016/j.devcel.2007.08.005 [DOI] [PubMed] [Google Scholar]
- Schlosser G (2008). Do vertebrate neural crest and cranial placodes have a common evolutionary origin? BioEssays, 30, 659–672. 10.1002/bies.20775 [DOI] [PubMed] [Google Scholar]
- Schmidt C, McGonnell IM, Allen S, Otto A, & Patel K (2007). Wnt6 controls amniote neural crest induction through the non-canonical signaling pathway. Developmental Dynamics, 236, 2502–2511. 10.1002/dvdy.21260 [DOI] [PubMed] [Google Scholar]
- Selleck MA, & Bronner-Fraser M (1995). Origins of the avian neural crest: The role of neural plate-epidermal interactions. Development, 121, 525–538. [DOI] [PubMed] [Google Scholar]
- Selleck MA, García-Castro MI, Artinger KB, & Bronner-Fraser M (1998). Effects of Shh and Noggin on neural crest formation demonstrate that BMP is required in the neural tube but not ectoderm. Development, 125, 4919–4930. [DOI] [PubMed] [Google Scholar]
- Shiomi M (2009). Rabbit as a model for the study of human diseases In Rabbit biotechnology: rabbit genomics, transgenesis, cloning and models (pp. 49–63). Dordrecht: Springer; Netherlands. 10.1007/978-90-481-2227-1_7 [DOI] [Google Scholar]
- Simoes-Costa M, & Bronner ME (2015). Establishing neural crest identity: A gene regulatory recipe. Development, 142, 242–257. 10.1242/dev.105445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões-Costa M, & Bronner ME (2016). Reprogramming of avian neural crest axial identity and cell fate. Science, 352, 1570–1573. 10.1126/science.aaf2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões-Costa M, Stone M, & Bronner ME (2015). Axud1 integrates Wnt signaling and transcriptional inputs to drive neural crest formation. Developmental Cell, 34, 544–554. 10.1016/j.devcel.2015.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões-Costa MS, McKeown SJ, Tan-Cabugao J, SaukaSpengler T, & Bronner ME (2012). Dynamic and differential regulation of stem cell factor FoxD3 in the neural crest is encrypted in the genome. PLoS Genetics, 8, e1003142 10.1371/journal.pgen.1003142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skromne I, & Stern CD (2001). Interactions between Wnt and Vg1 signalling pathways initiate primitive streak formation in the chick embryo. Development, 128, 2915–2927. [DOI] [PubMed] [Google Scholar]
- Southard-Smith EM, Kos L, & Pavan WJ (1998). Sox10 mutation disrupts neural crest development in Dom Hirschsprung mouse model. Nature Genetics, 18, 60–64. 10.1038/ng0198-60 [DOI] [PubMed] [Google Scholar]
- Spokony RF, Aoki Y, Saint-Germain N, Magner-Fink E, & SaintJeannet J-P (2002). The transcription factor Sox9 is required for cranial neural crest development in Xenopus. Development, 129, 421–432. [DOI] [PubMed] [Google Scholar]
- Steventon B, Araya C, Linker C, Kuriyama S, & Mayor R (2009). Differential requirements of BMP and Wnt signalling during gastrulation and neurulation define two steps in neural crest induction. Development, 136, 771–779. 10.1242/dev.029017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolt CC, Lommes P, Hillgärtner S, & Wegner M (2008). The transcription factor Sox5 modulates Sox10 function during melanocyte development. Nucleic Acids Research, 36, 5427–5440. 10.1093/nar/gkn527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stottmann RW, & Klingensmith J (2011). Bone morphogenetic protein signaling is required in the dorsal neural folds before neurulation for the induction of spinal neural crest cells and dorsal neurons. Developmental Dynamics, 240, 755–765. 10.1002/dvdy.22579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit A, & Stern CD (1999). Establishment and maintenance of the border of the neural plate in the chick: Involvement of FGF and BMP activity. Mechanisms of Development, 82, 51–66. [DOI] [PubMed] [Google Scholar]
- Strobl-Mazzulla PH, Sauka-Spengler T, & Bronner-Fraser M (2010). Histone demethylase JmjD2A regulates neural crest specification. Developmental Cell, 19, 460–468. 10.1016/j.devcel.2010.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlmiller TJ, & García-Castro MI (2012a). Current perspectives of the signaling pathways directing neural crest induction. Cellular and Molecular Life Sciences, 69, 3715–3737. 10.1007/s00018-012-0991-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlmiller TJ, & García-Castro MI (2012b). FGF/MAPK signaling is required in the gastrula epiblast for avian neural crest induction. Development, 139, 289–300. 10.1242/dev.070276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiers G, Patient R, & Loose M (2006). Genetic regulatory networks programming hematopoietic stem cells and erythroid lineage specification. Developmental Biology, 294, 525–540. 10.1016/j. ydbio.2006.02.051 [DOI] [PubMed] [Google Scholar]
- Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y,…He X (2000). LDL-receptor-related proteins in Wnt signal transduction. Nature, 407, 530–535. 10.1038/35035117 [DOI] [PubMed] [Google Scholar]
- Teng L, Mundell NA, Frist AY, Wang Q, & Labosky PA (2008). Requirement for Foxd3 in the maintenance of neural crest progenitors. Development, 135, 1615–1624. 10.1242/dev.012179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theveneau E, Duband J-L, & Altabef M (2007). Ets-1 confers cranial features on neural crest delamination. PLoS One, 2, e1142 10.1371/journal.pone.0001142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor PA (2010). Craniofacial birth defects: The role of neural crest cells in the etiology and pathogenesis of Treacher Collins syndrome and the potential for prevention. American Journal of Medical Genetics. Part A, 152A, 2984–2994. 10.1002/ajmg.a.33454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor PA, & Krumlauf R (2000). Patterning the cranial neural crest: Hindbrain segmentation and Hox gene plasticity. Nature Reviews. Neuroscience, 1,116–124. 10.1038/35039056 [DOI] [PubMed] [Google Scholar]
- Trevers KE, Prajapati RS, Hintze M, Stower MJ, Strobl AC, Tambalo M,…Streit A (2017). Neural induction by the node and placode induction by head mesoderm share an initial state resembling neural plate border and ES cells. Proceedings of the National Academy of Sciences of the United States of America, 115, 355–360. 10.1073/pnas.1719674115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribulo C, Aybar MJ, Nguyen VH, Mullins MC, & Mayor R (2003). Regulation of Msx genes by a Bmp gradient is essential for neural crest specification. Development, 130, 6441–6452. 10.1242/dev.00878 [DOI] [PubMed] [Google Scholar]
- Trinh LA, Chong-Morrison V, Gavriouchkina D, Hochgreb-Hägele T, Senanayake U, Fraser SE, & Sauka-Spengler T (2017). Biotagging of specific cell populations in zebrafish reveals gene regulatory logic encoded in the nuclear transcriptome. Cell Reports, 19, 425–440. 10.1016/j.celrep.2017.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner N, & Grose R (2010). Fibroblast growth factor signalling: From development to cancer. Nature Reviews. Cancer, 10, 116–129. 10.1038/nrc2780 [DOI] [PubMed] [Google Scholar]
- Twigg SRF, & Wilkie AOM (2015). New insights into craniofacial malformations. Human Molecular Genetics, 24, R50–R59. 10.1093/hmg/ddv228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda K, Oda H, Yan Q, Matthias N, Zhao J, Davis BR, & Nakayama N (2015). Long-term expandable SOX9+ chondrogenic ectomesenchymal cells from human pluripotent stem cells. Stem Cell Reports, 4, 712–726. 10.1016/j.stemcr.2015.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadasz S, Marquez J, Tulloch M, Shylo NA, & Garcia-Castro MI(2013). Pax7 is regulated by cMyb during early neural crest development through a novel enhancer. Development, 140, 3691–3702. 10.1242/dev.088328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallin J, Thuret R, Giacomello E, Faraldo MM, Thiery JP, & Broders F (2001). Cloning and characterization of three Xenopus slug promoters reveal direct regulation by Lef/beta-catenin signaling. Journal of Biological Chemistry, 276, 30350–30358. 10.1074/jbc.M103167200 [DOI] [PubMed] [Google Scholar]
- van Amerongen R, & Berns A (2006). Knockout mouse models to study Wnt signal transduction. Trends in Genetics, 22, 678–689. 10.1016/j.tig.2006.10.001 [DOI] [PubMed] [Google Scholar]
- Vega-Lopez GA, Cerrizuela S, Tribulo C, & Aybar MJ (2018). Neurocristopathies: New insights 150 years after the neural crest discovery. Developmental Biology, In Press 10.1016/j.ydbio.2018.05.013 [DOI] [PubMed] [Google Scholar]
- Villanueva S, Glavic A, Ruiz P, & Mayor R (2002). Posteriorization by FGF, Wnt, and retinoic acid is required for neural crest induction. Developmental Biology, 241, 289–301. 10.1006/dbio.2001.0485 [DOI] [PubMed] [Google Scholar]
- Waddington CH, & Waterman AJ (1933). The development in vitro of young rabbit embryos. Journal of Anatomy, 67, 355–370. [PMC free article] [PubMed] [Google Scholar]
- Wakefield LM, & Roberts AB (2002). TGF-beta signaling: Positive and negative effects on tumorigenesis. Current Opinion in Genetics & Development, 12, 22–29. [DOI] [PubMed] [Google Scholar]
- Wang Z, Oron E, Nelson B, Razis S, & Ivanova N (2012). Distinct lineage specification roles for NANOG, OCT4, and SOX2 in human embryonic stem cells. Cell Stem Cell, 10, 440–454. 10.1016/j.stem.2012.02.016 [DOI] [PubMed] [Google Scholar]
- Watt KEN, & Trainor PA (2014). Neurocristopathies. In Neural Crest Cells (pp. 361–394). Academic Press; 10.1016/b978-0-12-401730-6.00018-1 [DOI] [Google Scholar]
- Wei S, Xu G, Bridges LC, Williams P, White JM, & Desimone DW (2010). ADAM13 induces cranial neural crest by cleaving class B Ephrins and regulating Wnt signaling. Developmental Cell, 19, 345–352. 10.1016/j.devcel.2010.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettstein DA, Turner DL, & Kintner C (1997). The Xenopus homolog of Drosophila suppressor of hairless mediates notch signaling during primary neurogenesis. Development, 124, 693–702. [DOI] [PubMed] [Google Scholar]
- Wilson PA, & Hemmati-Brivanlou A (1995). Induction of epidermis and inhibition of neural fate by bmp-4. Nature, 376, 331–333. 10.1038/376331a0 [DOI] [PubMed] [Google Scholar]
- Wilson SI, Rydström A, Trimborn T, Willert K, Nusse R, Jessell TM, & Edlund T (2001). The status of Wnt signaling regulates neural and epidermal fates in the chick embryo. Nature, 411, 325–330. 10.1038/35077115 [DOI] [PubMed] [Google Scholar]
- Wilson SI, Graziano E, Harland R, Jessell TM, & Edlund T (2000). An early requirement for FGF signalling in the acquisition of neural cell fate in the chick embryo. Current Biology, 10,421–429. [DOI] [PubMed] [Google Scholar]
- Wu J, Yang J, & Klein PS (2005). Neural crest induction by the canonical Wnt pathway can be dissociated from anterior-posterior neural patterning in Xenopus. Developmental Biology, 279, 220–232. 10.1016/j.ydbio.2004.12.016 [DOI] [PubMed] [Google Scholar]
- Wu MY, Ramel M-C, Howell M, & Hill CS (2011). SNW1 is a critical regulator of spatial BMP activity, neural plate border formation, and neural crest specification in vertebrate embryos. PLoS Biology, 9, e1000593–e1000522. 10.1371/journal.pbio.1000593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymeersch FJ, Huang Y, Blin G, Cambray N, Wilkie R, Wong FCK, & Wilson V (2016). Position-dependent plasticity of distinct progenitor types in the primitive streak. eLife, 5, e10042 10.7554/eLife.10042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley N, & García-Castro MI (2012). FGF signaling transforms nonneural ectoderm into neural crest. Developmental Biology, 372, 166–177. 10.1016/j.ydbio.2012.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalc A, Rattenbach R, Auradé F, Cadot B, & Relaix F (2015). Pax3 and Pax7 play essential safeguard functions against environmental stressinduced birth defects. Developmental Cell, 33, 56–66. 10.1016/j.devcel.2015.02.006 [DOI] [PubMed] [Google Scholar]