Abstract
Hydrogen sulfide (Hydrogen2S) has been known as a gasotransmitter, and it contributes to various physiological and pathological processes. Multiple enzymes such as cystathionine-β-synthase (CBS), cystathionine-γ-lyase (CSE) and 3-Mercaptopyruvate sulfurtransferase (MST) produce endogenous H2S, and these are differentially expressed in the various tissue systems including the skeletal system. However, abnormal H2S production is associated with deregulation of the signaling cascade and imbalanced tissue homeostasis. Several studies have previously provided evidence showing the essential regulatory action of H2S in skeletal homeostasis. In this review, we have emphasized the novel function of H2S in both bone and skeletal muscle anabolism, in particular. Additionally, we also reviewed the molecular and epigenetic basis of H2S signaling in bone development and skeletal muscle function.
Keywords: Bone formation, Osteoclastogenesis, DNA methylation, Histone acetylation, skeletal muscle angiogenesis
Introduction
Hydrogen sulfide (H2S) is a gas with an odor similar to that of a rotten egg and has been considered as a toxic environmental pollutant (1, 2). In the last decades, it has been shown that H2S exists in the biological system and performs many biological functions, the primary function being maintaining physiological homeostasis. Like nitric oxide and carbon monoxide, it is also considered a gasotransmitter in the tissue system (3). Physiologically, H2S is an endogenously released gasotransmitter which is known to be generated in the nervous system, heart, kidneys, vasculature, brain, gastrointestinal tract, skeletal muscle, and bones (1, 4, 5). H2S is mainly produced in mammalian tissue by two pyridoxal-5′-phosphate (PLP)-dependent enzymes, cystathionine-β-synthase (CBS) and cystathionine-γ-lyase (CSE), in the transsulfuration pathway of homocysteine metabolism (1, 6) and also from 3-Mercaptopyruvate sulfurtransferase (3-MST). However, the synthesis of H2S and bio-distribution is dependent upon on the tissue-specific action. More detailed information on H2S biosynthesis is shown in Figure 1.
Figure 1. Endogenous H2S production, metabolism during methionine cycle.
First, Methionine is sequentially converted to L-cysteine through homocysteine intermediate. Methionine can also be resynthesized from homocysteine by methionine synthase (MS) activity. Second, H2S biosynthesis from L-cysteine, via the trans-sulfuration pathway by CBS, CSE, and CAT/3MST enzymes. H2S; hydrogen sulfide, CBS; cystathionine-beta-synthase, CSE; cystathionine-gamma-lyase, CAT; cysteine aminotransferase 3MST; 3-mercaptopyruvate sulfurtransferase, DAO; D-amino acid oxidase.
CBS is predominantly expressed in bone marrow mesenchymal stem cells (BMMSCs), the central nervous system, ileum, and kidney, as well as in the pancreatic islets. CSE is abundantly expressed in the heart, vascular endothelium, kidney, and vascular smooth muscle (1, 7, 8). Abnormal H2S production is linked to different pathophysiological disorders such as atherosclerosis, diabetes, hypertension, asthma and Alzheimer’s disease (1, 2, 9). We had previously reported that H2S maintains the bone anabolism and homeostasis via the epigenetic differentiation of BMMSCs (1, 10). A similar result was obtained from a study also suggesting that H2S is an important mediator of bone anabolism and the homeostasis of T cells in the immune system (11, 12, 13, 14). It has also been demonstrated that H2S functions as an antioxidant, and anti-inflammatory molecule, as well as balancing redox homeostasis and inducing antioxidant transcription factor Nrf2 (1, 10, 15). Several studies have shown that the abnormal production of H2S is associated with an array of pathological disturbances (10, 11, 13). However, the mechanistic basis of the function of H2S in the tissue system is not fully understood. Therefore, in this review, we particularly emphasized the recent advancement of research on H2S as it pertains to skeletal development, with the more precise molecular basis of H2S signaling in bone formation and skeletal muscle myogenesis.
Hydrogen Sulfide on BMMSCs Function and Bone Formation
Hyperhomocysteinemia
H2S as an endogenous gasotransmitter provides anti-inflammatory, anti-oxidative and anti-apoptotic effects that are closely related to skeletal development. Recent studies on the physiological and pathological role of H2S have clearly explained its osteo-protective effects in bone disease (1, 10, 11). Osteoporosis is a bone disease characterized by an imbalance of bone resorption and bone formation that causes bone fragility and increases the risk of fracture due to high intake of methionine through the typical western diet (16). A disturbance in the methionine metabolic pathway causes the elevation of serum Hcy, a condition called hyperhomocysteinemia (HHcy) (16). Both epidemiological and clinical studies have suggested that a patient having severe HHcy due to decreased expression of CBS could have a detrimental risk factor for the onset of bone loss and fracture (11). Considering this, we have previously investigated the potential role of H2S in reversing HHcy-induced bone loss using a high methionine diet (HMD) enhanced HHcy model in mice, which evaluated the potential role of H2S in reversing HHcy-induced bone loss (1). Results from our lab have shown that BMMSCs express the CBS protein and enhance H2S levels that maintain osteogenesis and bone formation in BMMSCs. In this study, the high methionine diet (HMD) fed mice developed HHcy phenotypes, leading to oxidative stress and further epigenetic changes in the CpG islands of the RANKL/OPG promoter through c-Jun/JNK signaling (1). Administration of an H2S donor (sodium hydrosulfide; NaHS prevent the HHcy-induced oxidative damage and bone loss, thus displaying an osteo-protective property. The detailed H2S mediated preventive action on bone formation during HHcy is depicted in Figure 2. Indeed, the study of Xu et al., (2011) reported that H2S protects against oxidative stress via inhibition of mitogen-activated protein kinase (MAPK) signaling in cultured osteoblastic MC3T3-E1 cells (17). Another study demonstrated that HHcy is associated with the bone loss by decreasing osteoblast activity in a rat model (18). This study demonstrated that Hcy induces phosphorylation of the protein phosphatase 2 A (PP2A) to inhibit FOXO1/P38 signaling and OPG synthesis. However, administration of N-acetyl cysteine reverses the HHcy mediated bone loss and reduction of bone quality (18). Also, Yang et al., (2014) confirmed that H2S prevented dexamethasone (Dex)-induced apoptosis in MC3T3-E1 cells via AMP-activated protein kinase (AMPK) signaling and inhibited ROS production (19).
Figure 2: H2S epigenetically accelerates bone formation in HHcy mice model.
(A): A high methionine diet (HMD) induces the HHcy condition in mice by decreasing endogenous H2S production (1). HHcy condition activates C-Jun/JNK-p signaling and further transcriptionally regulates DNMT1 expression (2). Increased DNMT1 causes OPG promoter hypermethylation, leading to BMMSCs-derived osteoblast dysfunction (3). The upregulation of RANKL during HHcy increases osteoclastogenesis and bone loss (4). (B): Proposed mechanism of H2S signaling that reverses the HMD induced HHHcy phenotype (1). Exogenous H2S administration inhibits JNK-p-DNMT1 signaling (2). Decreased DNMT1 balances OPG-RANKL production in BMMSCs (3). The upregulation of OPG upon H2S administration increases osteogenesis and bone homeostasis (4).
CBS Deficiency Causes Bone Loss
H2S is important for BMMSCs function in that it maintains cell proliferation and differentiation (10, 11). H2S deficiency in BMMSCs attenuates osteogenesis and proliferation. Interestingly, CBS deficient (CBS+/−) mice have decreased serum and intracellular levels of H2S, causing a severe osteoporotic phenotype (10, 11). However, administration of H2S via an H2S donor (NaHS or GYY4137) can restore normal bone homeostasis. The biochemical data suggest that CBS deficient mice have increased levels of Hcy in the plasma, and this leads to oxidative damage and dysfunction of the BMMSCs (10). The mechanistic study revealed that H2S deficiency causes decreased intracellular Ca2+ influx due to reduced protein sulfhydration of cysteine residues on multiple Ca2+ transient receptor potential (TRP) channels (11). The reduced intracellular level of Ca2+ flux further downregulates PKC dependent Wnt/β-catenin signaling, leading to ablation of osteogenic differentiation of BMMSCs (11) (Figure 3B). Indeed, we have also provided evidence of the epigenetic role of H2S in CBS deficiency-induced bone loss (10). Our study strongly suggested that H2SS deficiency caused inhibition of HDAC3 activity and subsequent inflammation by enhancing oxidative damage (10). Mechanistically, inflammatory cytokines (IL-6, TNF-α) are transcriptionally activated by an acetylated lysine residue in histone (H3K27ac) of chromatin by binding to its promoter. Further, we demonstrate that IL-6 secreted by BMMSCs induces osteoclast differentiation and bone resorption (10). However, H2S administration in CBS+/− mice attenuated histone acetylation- dependent inflammatory signaling by restoring HDAC3 activity in BMMSCs and promoted bone formation via RUNX2 in a sulfhydration dependent manner (10). Collectively, restoration of H2S may provide a novel anti-osteoporotic property for CBS-deficiency induced metabolic osteoporosis (Figure 3A).
Figure 3: H2S deficiency accelerates bone loss in CBS-deficient mice.
A. Proposed mechanism for H2S mediated bone homeostasis in CBS+/− mice. CBS-deficiency in mice causes HHcy condition and decreased H2S production. This leads to inhibition of histone deacetylase activity through an oxidative stress mechanism. Inhibition of HDAC3 further epigenetically regulates histone acetylation (H3K27ac) and further decreases RunX2 sulfhydration and osteogenesis. In other words, H2S deficiency enhances osteoclastogenesis and bone loss. B. Proposed mechanism of H2S signaling in mesenchymal stem cell (MSC) function via regulating Ca+2 channel. The endogenous or exogenous H2S administration affects sulfhydration of calcium channels and calcium influx. This leads to β - catenin-mediated Runx2 dependent osteogenesis and bone homeostasis.
Ovariectomy (OVX)
Postmenopausal osteoporosis is a common skeletal disease associated with the declining level of estrogen, leading to bone loss and increased risk of fracture (12, 20, 21,22). Due to the lack of estrogen, the bone resorption process is primarily increased by osteoclast maturation (23). This led to both trabecular and cortical bone changes after estrogen deficiency (23, 24). Other have shown that genetic factors may potentially modulate bone loss subsequent to estrogen deficiency using different inbred strains of mice (24). However, future research is still needed to delineate the genetic factors that govern the skeletal changes to estrogen deficiency. For laboratory practice in a small animal such as rats or mice, the acute effect of menopause is modeled by ovariectomy (OVX), which intensifies the bone resorption by increasing osteoclast formation (23, 25). However, the mechanism of preventing osteoclast- mediated bone loss by restoring bone formation needs to be addressed. The work of Grassi et al., (2015), investigated whether estrogen deficiency impairs the H2S level and the role of H2S in OVX induced bone loss (12). Grassi et.al. showed that administration of the GYY4137 (H2Sdonor) increases bone formation and completely prevents both trabecular and cortical bone loss caused by ovariectomy via restoring the level of H2S and increasing BMMSC osteogenic differentiation (12). Mechanistic studies showed that GYY4137 increases osteoblastogenesis through the activation of the Wnt signaling cascade by increased production of the Wnt ligands Wnt16, Wnt2b, Wnt6, and Wnt10b in the bone marrow (12). Further, in vitro treatment with 17b-estradiol in human BM stromal cells (hSCs), upregulates the expression of CBS and CSE and produces normal H2S synthesis. Therefore, restoration of H2S levels could be a potential osteoprotective approach for postmenopausal osteoporosis (12).
Tissue regeneration and bone fracture healing
In the past decades, the advancement of research in mesenchymal stem cell (MSCs) transplantation has brought milestones in regenerative medicine, as it has been found that MSCs have a high potential for tissue regeneration. Additionally, H2S has recently been proposed as a modulator or inhibitor of cell viability/apoptosis in various organ systems. Recent studies demonstrate that administration of H2S could potentiate MSCs proliferation and survival by preventing multiple forms of stress (low oxygen, oxidative damage, or serum deprivation) induced apoptosis (26–30). The work of Fox et al., (2012) reported that H2S might represent a novel mechanism of cytoprotection in inflammatory joint pain and rheumatoid arthritis (26). H2S is also known to regulate MSC function through upregulating the expression of the antiapoptosis gene Bcl-2 to attenuate the hypoxia-mediated effect. (30). Recent studies reported that H2S (GYY4137) promotes bone fracture healing in the rabbit model of distraction osteogenesis (31). However, the mechanistic basis of H2S mediated bone healing is still unclear. The work of Zheng et al., (2017) reported that Cystathionine gamma-lyase enzyme (CSE) is the major expressed enzyme generating H2S in osteoblasts. Mechanistically, the CSE-H2S system promotes increased osteogenesis activity via RUNX2 sulfhydration as a novel transactivation regulator, thereby promoting bone healing. These findings suggest that modulation of H2S metabolism or H2S donors might serve as a therapeutic approach for treating osteoporosis or other bone diseases. However, the molecular mechanism needs to be investigated.
CSE-H2S induces bone fracture healing using a fixed bone fracture model in the rat (32). Zheng et al., also showed that using microcomputed tomography scanning and 3D reconstruction, those bone fracture lesions were well repaired with increasing trabecular numbers and reducing the trabecular spacing (32). Furthermore, H2S is able to prevent inflammatory cell filtration and helps in the deposition of more collagen and osteocytes. Mechanistically, the CSE-H2S system promotes increased osteogenesis activity via RUNX2 sulfhydration as a novel transactivation regulator, thereby promoting bone healing. These findings suggest that modulation of H2S metabolism or H2S donors might serve as a therapeutic approach for treating osteoporosis or other bone diseases, including HHcy induced bone injury or bone fracture in individuals. However, more research is needed to understand further the role of H2S on transplanted MSCs and direct administration in clinical practice.
Periodontal disease and orthodontics
Mesenchymal stem cells (MSCs) have been identified from the specialized craniofacial tissue such as exfoliated deciduous teeth, apical papillae, and gingiva (2, 33–35). Dental pulp stem cells (DPSCs), also enriched in tooth pulp, exhibit self-renewal, and multilineage differentiation potential, as observed in BMMSCs (2). However, DPSCs more specifically undergo odontogenic lineage, and play an important role in tooth development (2, 36). Several studies have reported the novel function of H2S in these dental stem cells (36). The work of Cen et al., (2016) demonstrates that an optimal concentration of endogenous H2S is required for periodontal ligament stem cell (PDLSC) osteogenesis via Wnt/β-catenin signaling (37). Other studies also showed that H2S is indispensable for PDLSCs and involved in osteogenic and adipogenic differentiation. Interestingly, CBS enzyme is the main source of endogenous H2S in PDLSC (38). This study indicates that H2S is required for periodontal tissue homeostasis. Periodontal inflammation and alveolar bone remodeling are involved in tooth movement (39). In this study, CSE-H2S system contributes to osteoclastogenesis during bone remodeling induced by mechanical loading (39). The data demonstrate that CSE-H2S was produced endogenously during osteoclast formation and orthodontic tooth movement (OTM) and played a pro-inflammatory role. Furthermore, using CSE+/− mice, they confirmed that CSE-H2S is essential for bone remodeling induced by mechanical loading (39). In addition to H2S mediated periodontal tissue remodeling, the work of Pu et al., (2017) investigated the effect of H2S on the alveolar bone remodeling that is associated with tooth movement (40). The data provided evidence that H2S was caused to increase in the rate of tooth movement in vivo by promoting osteogenesis and osteoclastogenesis in alveolar bone (40). This finding provides a novel understanding of how to increase tooth movement and shorten the treatment time, demonstrating the potential therapeutic value of H2S as orthodontic treatment.
Hydrogen Sulfide on skeletal muscle development
The emerging evidence suggests that H2S has a multifaceted biological role and acts as an antiinflammatory, anti-oxidative and anti-apoptotic molecule (17, 41, 42). However, the exact biological role of these effects on skeletal muscle function in the pathological setting remained to be studied. Recent evidence demonstrates that H2S may protect from the mitochondrial damage associated with skeletal muscle dysfunction, as mitochondria provide the energy sources for the skeletal muscle function (43). Therefore, future study to be warranted to understand the detailed mechanistic role of H2S in reversing skeletal muscle myopathy and dysfunction.
Species-specific expression of H2S producing enzymes (CBS, CSE) is well documented in skeletal muscles (44). For example, human skeletal muscle expresses ample amounts of these enzymes, whereas mouse skeletal muscle expresses these enzymes in much smaller amounts (44, 45), but these enzymes were present at a detectable level in rat skeletal muscles (46). However, the species-specific contribution of H2S production in the skeletal muscle system is not clear. To ameliorate the paucity of knowledge about the role of H2S in muscle function, several pieces of evidence have been put forth to understand the physiological function of H2S in skeletal muscle wasting and homeostasis. It was suggested from one study that diabetic patients have a lower level of H2S in the plasma, potentially leading to skeletal muscle myopathy (47). Studies by Parsanathan et al., (2017) using high glucose- induced C2C12 myoblasts, demonstrated that H2S donors significantly upregulate the CSE expression and restoration of normal H2S levels (47). Further, using the CSE knockdown approach, the data demonstrate that expression of the glucose transporter type 4 (GLUT4) transporter and the key transcription factors (VDR, PGC1a, PPARa, and PPARy) were decreased in C2C12 myotubes (47). Another study also explored that H2S has a protective role in the diaphragmatic muscle function of type 1 diabetic rats (48). The data demonstrate found that H2S could improve the diaphragm contractility and ultrastructural damage of diaphragmatic muscle (48). Furthermore, the mechanistic study showed that H2S administration increases the activity of superoxide dismutase (SOD) and the ratio of Bcl2/Bax mRNA levels, indicating that H2S administration in diabetic rats promotes an anti-apoptotic mechanism (48).
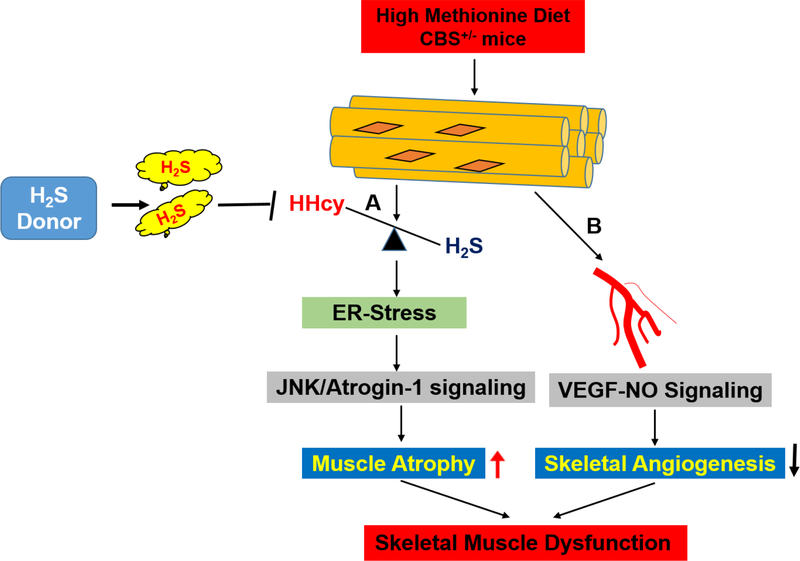
In another recent study from our lab, we demonstrated that CBS-deficient mice having hyperhomocysteinemia (HHcy) that can cause skeletal muscle dysfunction (49). We found CBS-deficiency inhibits H2S production and induces HHcy, causing redox imbalance and endoplasmic reticulum (ER) stress in the skeletal muscle in vivo. Furthermore, the data revealed that HHcy was detrimental to skeletal muscle, particularly the gastrocnemius and quadriceps muscle weights and muscle atrophy, via JNK/Atrogen 1 signaling. Administration of an H2S donor, such as NaHS, is beneficial in mitigating HHcy-mediated skeletal injury incited by oxidative/ER-stress responses in CBS+/− mouse models (49) (Figure 4A). Others have reported that children born with CBS homozygous mutation (CBS+/−) die after the age of 15–16 years, however children can survive with the heterozygous mutation (CBS+/−), and the single functional allele is able to produce sufficient CBS enzyme to produce at least some of the H2S required for proper physiological function (49).
Figure 4: H2S promotes skeletal muscle development in CBS-deficient mice.
A. The proposed mechanism of H2S mediated recovery of skeletal muscle dysfunction via ER stress-dependent muscle atrophy. HHcy causes an ER stress response via the oxidative stress-dependent mechanism. This leads to activation of JNK-Atrogen 1 signaling and subsequently causes muscle atrophy. B. The proposed model of HHcy mediated ablation of skeletal muscle angiogenesis. HHcy causes decreased endothelial NO production via VEGF-eNOS signaling. This leads to disruption of endothelium in skeletal muscle. H2S administration reverses the above effect in HHcy mice model.
Studies by Veeranki et al., (2015) demonstrated the mechanistic basis of HHcy induced skeletal muscle weakness and fatigability through mitochondrial dysfunction and epigenetic alternation using CBS+/− mice (50). CBS+/− can cause a reduction in the number of large muscle fibers, and it reduced mitochondrial ATP production with a decrease in mitochondrial transcription factor A (mtTFA) expression, and, consequently, the reduction of muscular dystrophin level in skeletal muscle (50). The molecular alteration observed in CBS+/− in mice was reversed after physical treadmill exercise. These results suggest that exercise plays a causal role in reversing the HHcy mediated effect in skeletal muscles in CBS+/− mice (50). Another study has reported that treadmill exercise regulates endogenous H2S generation and expression of CSE enzyme, thereby attenuating inflammation in the skeletal muscles of obese rats (51), indicating that treadmill exercise could enhance the H2S synthesis in skeletal muscle to combat H2S deficiency associated skeletal muscle dysfunction and weakness. Studies by Du et.al also revealed that H2S could be endogenously generated by rats’ skeletal muscles (H2S:(2.06 ± 0.43) nmol/mg) and its level was, indeed, down-regulated, mediated through increased oxidative damage in the skeletal muscle of rats with ischemic reperfusion (I-R) skeletal muscle injury (52). However, H2S treatment significantly protected rat skeletal muscle against I-R injury (52).
Hydrogen Sulfide on skeletal muscle angiogenesis
Angiogenesis is the process of new capillary growth from the pre-existing vasculature, improving blood flow under ischemic conditions and accelerating wound healing. Therefore, therapeutic angiogenesis might be suggested as an alternative approach in the treatment of ischemia. The proangiogenic function of H2S and its capacity for the improvement of regional blood flow in ischemic organs is still unknown, though the work of Wang et al., (2010) reports that H2S is a new gasotransmitter promoting angiogenesis in a rat model of hindlimb ischemia (53). Wang et al. found that H2S donor (NaHS) administration significantly increased collateral vessel growth, capillary density, and blood flow in ischemic hindlimb skeletal muscles compared with the controls, and there was a subsequent increase in vascular endothelial growth factor (VEGF) expression and vascular endothelial growth factor receptor 2 (VEGFR2) phosphorylation. Mechanistically, the proangiogenic effect of NaHS resulted in VEGF dependent VEGFR2-Protein kinase B (Akt) signaling in skeletal muscle cells, and improved the regional blood flow (53).
The earlier report suggests that CBS is an important Hcy metabolizing enzyme, actively participating in the transsulfuration pathway of methionine-Hcy metabolism (54). However, mice with heterozygous CBS deficiency (CBS+/−) develop the mild to severe HHcy phenotype (55). Taking this into account, Majumdar et al., (2018) used an HHcy mouse model (CBS+/−) to investigate the effect of H2S on neoangiogenesis in ischemic skeletal muscle (56). The data suggested that H2S donor (GYY4137) administration significantly improved collateral vessel density and blood flow in hindlimb femoral artery ligation (FAL) or ischemic hindlimb skeletal muscles of CBS+/− mice compared with WT mice. The mechanistic study revealed that the GYY4137 treatment augmented VEGF-eNOS-NO signaling in skeletal muscle cells via an HHcy antagonizing effect, and GYY4137 could serve as a potential neo-angiogenic modulator to treat the angiogenic defect in hindlimb ischemia of the skeletal muscle in CBS+/− mice (56) (Figure 4B). In another study, it was reported that restoration or administration of H2S improves bone marrow (BM) cell function and subsequent preservation of skeletal muscle architecture in a diabetic type-2 FAL mice model (db/db+FAL) (57). In vitro data showed that treatment of H2S donor diallyl trisulfide (DATS) or overexpression of CSE restored H2S synthesis and BMC angiogenic activity in high glucose (HG)-treated BMCs. In vivo administration of DATS or CSE-overexpressing BMCs greatly improved blood perfusion, capillary/arteriole density, and skeletal muscle architecture in ischemic hind limbs of db/db mice. Mechanistically, DATS administration in BMC upregulates NO signaling mediated angiogenesis and restores skeletal muscle function (57).
Future challenges and Conclusive Remarks
H2S, a colorless irritant gas and considered as a toxic gas and environment hazard (58). It exhibits different effects in a dose-dependent manner. At low doses, it is beneficial and is highly toxic in high doses. Till date, there is no antidote available to combat or treat the H2S toxicity in pathophysiological settings (59). In particular, a knowledge gap exists about the physiological and pathological role of H2S in the past decades. However, H2S based therapy remained to be a great challenge for the development of suitable H2S donors with good tissue-specific action (59). To understand the mechanistic role of H2S as well as safe use of H2S, a new method must be developed with a low limit of detection. This might help in measuring the H2S concentration in diseased tissue and organs at the clinics. Further, the use of the cost-effective animal model that mimics the human condition following acute H2S inhalation might be needed to understand the mechanistic response to candidate H2S donors. Accumulated evidence suggests that H2S gas plays a wide variety of roles in both the physiological and pathological processes of the skeletal system. The data found that H2S is known to regulate BMMSCs and skeletal muscle function, ensuring bone and skeletal muscle homeostasis. H2S also plays a crucial role in cell proliferation and differentiation of BMMSCs in the HHcy mouse model. Another study also demonstrates that H2S regulates BMMSCs function in both OVX and bone fracture mouse models. During skeletal muscle homeostasis, H2S is known to regulate the skeletal muscle function by regulating muscle angiogenesis. Mechanistic insight suggests that H2S governs key cellular signaling pathways, protein sulfhydration, and epigenetic remodeling of chromatin landscapes in the skeletal tissue. Therefore, future research is warranted to make a thorough evaluation of the physiological and pathophysiological roles of H2S in the skeletal tissue and further novel H2S releasing drugs to be discovered for use as a therapeutic module in clinical settings.
Highlights.
H2S promotes bone formation in hyperhomocysteinemia mice model via epigenetic DNA methylation.
H2S supplementation prevents cystathionine β-synthase deficiency induced Bone loss through histone acetylation-dependent action in mice.
H2S administration promotes bone formation in ovariectomy-induced mice model through Wingless/integrated signaling.
H2S mitigates hyperhomocysteinemia caused skeletal muscle dysfunction mediated through endoplasmic reticulum stress and c-Jun N-terminal kinase/Atrogen 1 signaling.
H2S supplementation improves skeletal muscle angiogenesis and regional blood flow in cystathionine β-synthase deficiency mice via vascular endothelial growth factor–nitric oxide signaling.
Acknowledgments
This study was supported, in part, by NIH grants HL-107640 and AR-067667 to NT. The authors are grateful to Jessica Ison for English editing of the manuscript.
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Behera J, George AK, Voor MJ, Tyagi SC, Tyagi N, Hydrogen sulfide epigenetically mitigates bone loss through OPG/RANKL regulation during hyperhomocysteinemia in mice, Bone 114 (2018) 90–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang R, Liu Y, Shi S, Hydrogen Sulfide Regulates Homeostasis of Mesenchymal Stem Cells and Regulatory T Cells, J Dent Res 95 (2016) 1445–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang R, Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev 92 (2012) 791–896. [DOI] [PubMed] [Google Scholar]
- 4.Wang R, The gasotransmitter role of hydrogen sulfide, Antioxid. Redox Signal 5 (2003) 493–501. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Rose P, Moore PK, Hydrogen sulfide and cell signaling, Annu Rev Pharmacol Toxicol 51 (2011) 169–187. [DOI] [PubMed] [Google Scholar]
- 6.Gadalla MM, Snyder SH, Hydrogen sulfide as a gasotransmitter, J Neurochem 113 (2010) 14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimura H, The physiological role of hydrogen sulfide and beyond, Nitric Oxide 41 (2014) 4–10. [DOI] [PubMed] [Google Scholar]
- 8.Polhemus DJ, Lefer DJ, Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease, Circ Res 114 (2014) 730–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, Rose P, Moore PK, Hydrogen sulfide and cell signaling, Annu Rev Pharmacol Toxicol. 51 (2011) 169–187. [DOI] [PubMed] [Google Scholar]
- 10.Behera J, Kimberly KE, Voor MJ, Naira M, Tyagi SC, Tyagi N, Hydrogen Sulfide Promotes Bone Homeostasis by Balancing Inflammatory Cytokine Signaling in CBS-Deficient Mice through an Epigenetic Mechanism, Scientific Reports (2018) 15226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Yang R, Liu X, Zhou Y, Qu C, Kikuiri T, Wang S, Zandi E, Du J, Ambudkar IS, et al. 2014. Hydrogen sulfide maintains mesenchymal stem cell function and bone homeostasis via regulation Of Ca(2+) channel sulfhydration, Cell Stem Cell 15(1):66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grassi F, Tyagi AM, Calvert JW, Gambari L, Walker LD, Yu M, Robinson J, Li JY, Lisignoli G,Vaccaro C, Adams J, Pacifici R, Hydrogen Sulfide Is a Novel Regulator of Bone Formation Implicated in the Bone Loss Induced by Estrogen Deficiency, J Bone Miner Res 31 (2016) 949–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh SA, Li MO, TETs link hydrogen sulfide to immune tolerance, Immunity 43 (2005) 211–213. [DOI] [PubMed] [Google Scholar]
- 14.Yang R, Qu C, Zhou Y, Konkel JE, Shi S, Liu Y, Chen C, Liu S, Liu D, Chen Y, Hydrogen sulfide promotes Tet1-and Tet2-mediated Foxp3 demethylation to drive regulatory T cell differentiation and maintain immune homeostasis, Immunity 43 (2015) 251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gambari L, Lisignoli G, Cattini L, Manferdini C, Facchini A, Grassi F, Sodium hydrosulfide inhibits the differentiation of osteoclast progenitor cells via NRF2-dependent mechanism, Pharmacol Res 87 (2014) 99–112. [DOI] [PubMed] [Google Scholar]
- 16.van Meurs JB, Dhonukshe-Rutten RA, Pluijm SM, van der KL, de Jonge R, Lindemans J, de Groot LC, Hofman A, Witteman JC, van Leeuwen JP, Breteler MM, Lips P, Pols HA, Uitterlinden AG Homocysteine levels and the risk of osteoporotic fracture. N Engl J Med 350 (2004) 2033–41. [DOI] [PubMed] [Google Scholar]
- 17.Xu ZS, Wang XY, Xiao DM, Hu LF, Lu M, Wu ZY, Bian JS Hydrogen sulfide protects MC3T3-E1 osteoblastic cells against H2O2-induced oxidative damage—implications for the treatment of osteoporosis, Free Radic Biol Med 50 (2011) 1314–23. [DOI] [PubMed] [Google Scholar]
- 18.Vijayan V, Khandelwal M, Manglani K, Singh RR, Gupta S, Surolia A, Homocysteine alters the osteoprotegerin/RANKL system in the osteoblast to promote bone loss: pivotal role of the redox regulator forkhead O1, Free Radic Biol Med 61 (2013) 72–84. [DOI] [PubMed] [Google Scholar]
- 19.Yang M, Huang Y, Chen J, Chen YL, Ma JJ, Shi PH, Activation of AMPK participates hydrogen sulfide-induced cyto-protective effect against dexamethasone in osteoblastic MC3T3-E1 cells, Biochem Biophys Res Commun 454 (2014) 42–7. [DOI] [PubMed] [Google Scholar]
- 20.Meng-Xia J, Qi Y Primary osteoporosis in postmenopausal women. Chronic Dis Transl Med 1 (2015) 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riggs BL, Melton LJ 3rd, Evidence for two distinct syndromes of involutional osteoporosis, Am J Med 75 (1983) 899–901. [DOI] [PubMed] [Google Scholar]
- 22.Riggs BL, Melton LJ 3rd, Involutional osteoporosis, N Engl J Med 314 (1986) 1676–86. [DOI] [PubMed] [Google Scholar]
- 23.Weitzmann MN, Pacifici R, Estrogen deficiency and bone loss: an inflammatory tale, J Clin Invest 116 (2006) 1186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouxsein ML, Myers KS, Shultz KL, Donahue LR, Rosen CJ, Beamer WG Ovariectomy-Induced Bone Loss Varies Among Inbred Strains of Mice. J Bone Miner Res 20 (2005) 1085–92. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S, Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts, Cell 130 (2007) 811–23. [DOI] [PubMed] [Google Scholar]
- 26.Fox B, Schantz JT, Haigh R, Wood ME, Moore PK, Viner N, Spencer JP, Winyard PG, Whiteman M, Inducible hydrogen sulfide synthesis in chondrocytes and mesenchymal progenitor cells: is H2S a novel cytoprotective mediator in the inflamed joint? J Cell Mol Med. 16 (2012) 896–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo Z, Li CS CS, Wang CM, Xie YJ, Wang AL, CSE/H2S system protects mesenchymal stem cells from hypoxia and serum deprivationinduced apoptosis via mitochondrial injury, endoplasmic reticulum stress and PI3K/Akt activation pathways, Mol Med Rep 12 (2015) 21282134. [DOI] [PubMed] [Google Scholar]
- 28.Xie X, Sun A, Zhu W, Huang Z, Hu X, Jia J, Zou Y, Ge J, Transplantation of mesenchymal stem cells preconditioned with hydrogen sulfide enhances repair of myocardial infarction in rats, Tohoku J Exp Med 226 (2012) 29–36. [DOI] [PubMed] [Google Scholar]
- 29.Zhao Y, Wei H, Kong G, Shim W, Zhang G, Hydrogen sulfide augments the proliferation and survival of human induced pluripotent stem cell-derived mesenchymal stromal cells through inhibition of BKCa, Cytotherapy 15 (2013) 1395–1405. [DOI] [PubMed] [Google Scholar]
- 30.Li C, Guo Z, Guo B, Xie Y, Yang J, Wang A, Inhibition of the endogenous CSE/H(2)S system contributes to hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells, Mol Med Rep 9 (2019) 2467–2472. [DOI] [PubMed] [Google Scholar]
- 31.Jiang X, Chen Y, Lu K, Zhang H, Fan X, GYY4137 promotes bone formation in a rabbit distraction osteogenesis model: a preliminary report, J Oral Maxillofac Surg 73 (2015) 732.e1–e6. [DOI] [PubMed] [Google Scholar]
- 32.Zheng Y, Liao F, Lin X, Zheng F, Fan J, Cui Q, Yang J, Geng B, Cai JC, Cystathionine c-Lyase-Hydrogen Sulfide Induces Runt-Related Transcription Factor 2 Sulfhydration, Thereby Increasing Osteoblast Activity to Promote Bone Fracture Healing, Antioxid Redox Signal 27 (2017) 742–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S, Investigation of multipotent postnatal stem cells from human periodontal ligament, Lancet 364 (2004) 149–155. [DOI] [PubMed] [Google Scholar]
- 34.Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, Huang GT, Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study, J Endod. 34 (2008) 166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Shi S, Le AD, Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis, J Immunol 183 (2009) 7787–7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang GT, Gronthos S, Shi S, Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine, J Dent Res 88 (2009) 792–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cen SD, Yu WB, Ren MM, Chen LJ, Sun CF, Ye ZL, Deng H, Hu RD, Endogenous hydrogen sulfide is involved in osteogenic differentiation in human periodontal ligament cells, Arch Oral Biol 68 (2016) 1–8. [DOI] [PubMed] [Google Scholar]
- 38.Su Y, Liu D, Liu Y, Zhang C, Wang J, Wang SP, Physiologic levels of endogenous hydrogen sulfide maintain the proliferation and differentiation capacity of periodontal ligament stem cells, J Periodontol 86 (2015) 1276–86. [DOI] [PubMed] [Google Scholar]
- 39.Mo S, Hua Y, Cystathionine gamma lyase-H2S contributes to osteoclastogenesis during bone remodeling induced by mechanical loading, Biochem Biophys Res Commun 501 (2018) 471–477. [DOI] [PubMed] [Google Scholar]
- 40.Pu H, Hua Y, Hydrogen sulfide regulates bone remodeling and promotes orthodontic tooth movement, Mol Med Rep 16 (2017) 9415–9422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang JY Ding YP, Wang Z, Kong Y, Gao R, Chen G Hydrogen sulfide therapy in brain diseases: from bench to bedside. Med Gas Res 7 (2017) 113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei HJ, Xu JH, Li MH, Tang JP, Zou W, Zhang P, Wang L, Wang CY, Tang XQ, Hydrogen sulfide inhibits homocysteine-induced endoplasmic reticulum stress and neuronal apoptosis in rat hippocampus via upregulation of the BDNF-TrkB pathway, Acta Pharmacol Sin 35 (2014) 707–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bitar MS, Nader J, Al-Ali W, Madhoun AA, Arefanian H, Al-Mulla F Hydrogen Sulfide Donor NaHS Improves Metabolism and Reduces Muscle Atrophy in Type 2 Diabetes: Implication for Understanding Sarcopenic Pathophysiology. Oxid Med Cell Longev (2018) 6825452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veeranki S, Tyagi SC, Role of hydrogen sulfide in skeletal muscle biology and metabolism, Nitric Oxide 46 (2015) 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen NC, Yang F, Capecci LM, Gu Z, Schafer AI, Durante W, Yang XF, Wang H, Regulation of homocysteine metabolism and methylation in human and mouse tissues, FASEB J 24 (2010) 2804–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DU JT, Li W, Yang JY, Tang CS, Li Q, Jin HF, Hydrogen sulfide is endogenously generated in rat skeletal muscle and exerts a protective effect against oxidative stress, Chin Med J (Engl) 126 (2013) 930–6. [PubMed] [Google Scholar]
- 47.Parsanathan R, Jain SK, Hydrogen sulfide increases glutathione biosynthesis, and glucose uptake and utilization in C2C12 mouse myotubes, Free Radic Res 52 (2018) 288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang R, Jia Q, Guo X, Liu X, Ma S, Gao Q, Guan S, Protective effects of hydrogen sulfide on diaphragmatic muscle of Type 1 diabetic rats and its anti-apoptotic mechanisms, Zhong Nan Da Xue Xue Bao Yi Xue Ban 40 (2015) 1173–8. [DOI] [PubMed] [Google Scholar]
- 49.Majumder A, Singh M, Behera J, Theilen NT, George AK, Tyagi N, Metreveli N, Tyagi SC, Hydrogen sulfide alleviates hyperhomocysteinemia-mediated skeletal muscle atrophy via mitigation of oxidative and endoplasmic reticulum stress injury, Am J Physiol Cell Physiol 2018. August 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Veeranki S, Winchester LJ, Tyagi SC, Hyperhomocysteinemia associated skeletal muscle weakness involves mitochondrial dysfunction and epigenetic modifications, Biochim Biophys Acta 1852 (2015) 732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu D, Lu Z, Li M, Feng Z, Liu A, Jin D, Ji A, Li Y, Treadmill exercise increases cystathionine Y-lyase expression and decreases inflammation in skeletal muscles of high-fat diet-induced obese rats, Int J Clin Exp Med 9 (2016) 23119–23126. [Google Scholar]
- 52.DU JT, Li W, Yang JY, Tang CS, Li Q, Jin HF, Hydrogen sulfide is endogenously generated in rat skeletal muscle and exerts a protective effect against oxidative stress, Chin Med J (Engl) 126 (2013) 930–6. [PubMed] [Google Scholar]
- 53.Wang MJ, Cai WJ, Li N, Ding YJ, Chen Y, Zhu YC, The hydrogen sulfide donor NaHS promotes angiogenesis in a rat model of hind limb ischemia, Antioxid Redox Signal 12 (2010) 1065–77. [DOI] [PubMed] [Google Scholar]
- 54.Kumar A, Palfrey HA, Pathak R, Kadowitz PJ, Gettys TW, Murthy SN, The metabolism and significance of homocysteine in nutrition and health, Nutr Metab (Lond) (2017) 14:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tyagi N, Qipshidze N, Sen U, Rodriguez W, Ovechkin A, Tyagi SC, Cystathionine beta synthase gene dose dependent vascular remodeling in murine model of hyperhomocysteinemia, Int J Physiol Pathophysiol Pharmacol 3 (2011) 210–22. [PMC free article] [PubMed] [Google Scholar]
- 56.Majumder A, Mahavir S, George AK, Behera J, Tyagi N, Tyagi SC, Hydrogen sulfide improves postischemic neoangiogenesis in the hind limb of cystathionine-b-synthase mutant mice via PPAR-c/VEGF axis, Physiol Rep 6 (2018) e13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheng Z, Garikipati VN, Nickoloff E, Wang C, Polhemus DJ, Zhou J, Benedict C, Khan M, Verma SK, Rabinowitz JE, Lefer D, Kishore R, Restoration of Hydrogen Sulfide Production in Diabetic Mice Improves Reparative Function of Bone Marrow Cells, Circulation 134 (2016) 14671483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rumbeiha W, Whitley E, Anantharam P, Kim DS, Kanthasamy A A, Acute hydrogen sulfide- induced neuropathology and neurological sequelae: challenges for translational neuroprotective research, Ann N Y Acad Sci 1378 (2016) 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu D, Hu Q, Zhu Y Therapeutic application of hydrogen sulfide donors: the potential and challenges. Front Med 10 (2016) 18–27. [DOI] [PubMed] [Google Scholar]
- 60.Zheng Y, Xingyue J, Kaili J, Wang B, Hydrogen sulfide prodrugs—a review, Acta Pharmaceutica Sinica B, (2015) 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]