Abstract
Functional connectivity (FC) between the amygdala and the ventromedial prefrontal cortex underlies socioemotional functioning, a core domain of impairment in autism spectrum disorder (ASD). Although frontoamygdala circuitry undergoes dynamic changes throughout development, little is known about age-related changes in frontoamygdala networks in ASD. Here we characterize frontoamygdala resting-state FC in a cross-sectional sample (ages 7–25) of 58 typically developing (TD) individuals and 53 individuals with ASD. Contrary to hypotheses, individuals with ASD did not show different age-related patterns of frontoamygdala FC compared with TD individuals. However, overall group differences in frontoamygdala FC were observed. Specifically, relative to TD individuals, individuals with ASD showed weaker frontoamygdala FC between the right basolateral (BL) amygdala and the rostral anterior cingulate cortex (rACC). These findings extend prior work to a broader developmental range in ASD, and indicate ASD-related differences in frontoamygdala FC that may underlie core socioemotional impairments in children and adolescents with ASD.
Keywords: Autism spectrum disorder (ASD), Amygdala, Development, Resting-state functional connectivity, Adolescence, Prefrontal cortex
1. Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental condition characterized by deficits in communication and reciprocal social interaction as well as the presence of restricted and repetitive behaviors (American Psychiatric Association, 2000). Social interaction depends on the perception and evaluation of socioemotional information (Swartz et al., 2014a), processes subserved by the amygdala and its functional connectivity (FC) with other cortical and subcortical regions, in particular the ventromedial prefrontal cortex (vmPFC; Adolphs, 2008; Ochsner et al., 2012; Quirk and Beer, 2006; Swartz et al., 2014b). Atypical structure and function of the amygdala has been theorized to contribute to the social difficulties at the core of ASD (Baron-Cohen et al., 2000; Schultz, 2005). Although the amygdala has dense reciprocal connections with the prefrontal cortex (Amaral and Insausti, 1992; Ghashghaei et al., 2007; Johansen-Berg et al., 2008), few studies have used resting-state FC to examine differences in the functional interactions and organization of the amygdala in individuals with ASD (Kleinhans et al., 2016; Rausch et al., 2018; Shen et al., 2016; von dem Hagen et al., 2013). Moreover, the amygdala and its connections with the prefrontal cortex undergo dynamic changes during development (Decety and Michalska, 2010; Gabard-Durnam et al., 2014, 2018; Gee, 2016; Gee et al., 2013; Perlman and Pelphrey, 2011; Vink et al., 2014), yet little is known about age-related changes in intrinsic functional organization of frontoamygdala networks in individuals with ASD. The present study aimed to delineate age-related differences in frontoamygdala FC in children and adolescents with ASD compared with typically developing (TD) individuals and to assess associations between frontoamygdala FC and social impairment among individuals with ASD.
The amygdala is consistently implicated in socioemotional processing, including processing of emotions, emotion regulation, theory of mind, and eye gaze (Etkin et al., 2006; Hariri et al., 2003; Kim et al., 2011; Phelps et al., 2001; Zhang et al., 2013), all of which are impaired in individuals with ASD (Baron-Cohen et al., 2000). Across the lifespan, individuals with ASD show an atypical trajectory of amygdala structure, initially showing an excess of neurons during childhood, followed by a reduction in adulthood (Avino et al., 2018). Individuals with ASD also show atypical activation of the amygdala during a range of socioemotional tasks across the lifespan (Harms et al., 2010; Pelphrey et al., 2011). However, the nature of these findings has been largely mixed, with some evidence for both lower (Ashwin et al., 2007; Baron-Cohen et al., 2000; Bookheimer et al., 2008; Domes et al., 2013; Kleinhans et al., 2009, 2011; Perlman et al., 2011; Pierce et al., 2001; Pierce and Redcay, 2008) and higher (Dalton et al., 2005; Grelotti et al., 2005; Pelphrey et al., 2007; Tottenham et al., 2014; Zurcher et al., 2013) task-related amygdala activation in ASD, even in response to the same socially-evocative tasks (for a review, see Nomi and Uddin, 2015a). While the majority of the literature on amygdala-related socioemotional processing in ASD focuses on adults (e.g., Ashwin et al., 2007; Kleinhans et al., 2009; Pelphrey et al., 2007; Perlman et al., 2011), more recent studies have focused on youth with ASD and tend to show higher task-related amygdala activation in ASD (e.g., Dalton et al., 2005; Herrington et al., 2016; Tottenham et al., 2014). Yet, to our knowledge, age-related differences in amygdala function and socioemotional processes remain unexplored in ASD. Inconsistent findings in the extant work focusing on the amygdala as an isolated structure may stem from varying task demands and performance or failure to account for age-related differences (Piggot et al., 2004).
The amygdala is a heterogeneous region that has been cytoarchitectonically subdivided into basolateral (BL), centromedial (CM), and superficial (SF) subregions (Amunts et al., 2005; Price, 2003). These subregions support distinct functions, with the existing literature in humans focusing on the CM and BL subregions, which are two divisions of the main nuclei of the amygdala, based largely on both cellular architecture and structural connectivity in the primate brain (Jalbrzikowski et al., 2017; Mosher et al., 2010; Pape and Pare, 2010). Primarily a sensory input region, the BL amygdala is involved in evaluating the emotional content of incoming information (Davis and Whalen, 2001; Mosher et al., 2010; Pape and Pare, 2010; Sah et al., 2003). Conversely, the CM amygdala is primarily an output region and supports attention allocation and salience detection. Given the role of the BL in evaluating emotions, a process that is impaired in ASD, alterations of the BL amygdala may be especially pertinent to ASD symptom severity (Kleinhans et al., 2016).
The vmPFC is comprised of subregions with distinct functions including the ventral anterior cingulate cortex (vACC), rostral anterior cingulate cortex (rACC), subgenual cingulate cortex, anterior vmPFC, and medial orbitofrontal cortex (medial OFC; Mackey and Petrides, 2014). The rACC and anterior vmPFC play a particularly strong role in the cognitive control of emotion (Blair et al., 2007; Etkin et al., 2015, 2006; Hariri et al., 2000; Ochsner et al., 2004, 2012; Phelps and LeDoux, 2005; Phillips et al., 2003; Zald, 2003). FC between the amygdala and the vmPFC may be especially relevant to ASD, as this circuitry supports processes that are crucial for successful social interactions including emotional attention, learning, and regulation (Adolphs and Spezio, 2006; Ochsner et al., 2012; Phillips et al., 2003), all of which may be impaired in individuals with ASD (Adolphs et al., 2001; Ashwin et al., 2006; Baron-Cohen et al., 1999; Celani et al., 1999; Corbett et al., 2009; Critchley et al., 2000; Dalton et al., 2005; Davies et al., 1994; Macdonald et al., 1989; Schultz et al., 2000). The strong reciprocal connections between the amygdala and the vmPFC (Adolphs, 2008; Ochsner et al., 2012; Quirk and Beer, 2006; Swartz et al., 2014b) further suggest that assessing FC is critical to understanding amygdala-related alterations in ASD.
Resting-state fMRI has been used to index the brain’s functional architecture in typical development and to assess how it differs in clinical populations (Berking and Wupperman, 2012; Das et al., 2007; Uddin et al., 2011). Although the majority of studies exploring amygdala FC in ASD have examined task-based connectivity, few studies have observed alterations in amygdala FC in ASD using resting-state fMRI (Guo et al., 2016; Kleinhans et al., 2016; Rausch et al., 2018, 2016; Shen et al., 2016). Of these, recent studies of adolescents and adults with ASD have reported amygdala hypoconnectivity to visuospatial and superior parietal regions (Rausch et al., 2016), to the thalamus and putamen (Guo et al., 2016), and to the insula (von dem Hagen et al., 2013). Kleinhans et al. (2016) observed lower FC of the BL amygdala to the nucleus accumbens in adolescents and adults with ASD, but higher FC to the cerebellum, occipital pole, lateral occipital cortex, and superior frontal gyrus. Lastly, recent evidence suggests amygdala hypoconnectivity with the mPFC in preschool-aged children (Shen et al., 2016) and adolescents (Rausch et al., 2018) with ASD. While these studies converge on amygdala hypoconnectivity in ASD, most of the studies were conducted in adults. Several of these studies have combined adolescents and adults (Dalton et al., 2005; Zurcher et al., 2013) or focused on earlier development (Shen et al., 2016), while few studies have accounted for age-related differences (Pitskel et al., 2011; Tottenham et al., 2014). Given the neurodevelopmental nature of ASD and dynamic changes in amygdala FC with age, assessing amygdala FC across a broader age range of individuals with ASD will be important for further understanding the nature of amygdala abnormalities in ASD. This is the first study to date to investigate intrinsic frontoamygdala FC across a wide age range, including children, adolescents, and young adults with ASD.
Prior research using both task-based (Gee et al., 2013; Hare et al., 2008; McRae et al., 2012; Perlman et al., 2011; Wu et al., 2016) and resting-state fMRI (Gabard-Durnam et al., 2014, 2016; Qin et al., 2012) demonstrates that dramatic changes occur in the typical development of amygdala FC across childhood and adolescence. In cross-sectional studies, adults showed stronger frontoamygdala FC than children (Bunge et al., 2002; Casey et al., 1997; Decety and Michalska, 2010; Gabard-Durnam et al., 2014, 2016; Gee et al., 2013; Giedd et al., 1996; Nelson et al., 2005; Perlman and Pelphrey, 2011; Qin et al., 2012; Swartz et al., 2014a), a finding replicated in an independent study of TD individuals ages 4 to 23 (Gabard-Durnam et al., 2014). Gabard-Durnam et al. (2014) found an age-related increase in frontoamygdala FC, with the transition from childhood to adolescence being an important point of change in this circuit. In contrast, a recent longitudinal study of TD individuals ages 10 to 25 found age-related decreases in FC between the CM amygdala and the rACC, anterior vmPFC, and subgenual cingulate, and between the BL amygdala and rACC (Jalbrzikowski et al., 2017). Given dynamic changes in frontoamygdala FC across development, it is imperative to account for age when assessing frontoamygdala differences in individuals with ASD. Furthermore, a "two-hit" model of autism has been proposed (Picci and Scherf, 2015) whereby the transition into adolescence marks a time of particular vulnerability for youth with ASD due to the increased social demands and brain network changes that happen in parallel. This model underscores the importance of a developmental approach when assessing ASD-related differences in neural regions associated with socioemotional processes (Burrows et al., 2016).
The present study aims to characterize age-related differences in frontoamygdala FC among children, adolescents, and young adults with ASD and to examine the relationship between frontoamygdala FC and social impairment in ASD. Based on existing literature on amygdala FC in ASD (Etkin et al., 2015; Kleinhans et al., 2016; Rausch et al., 2018), we hypothesized that individuals with ASD would display lower frontoamygdala FC compared with TD individuals. We anticipated that these group differences would be specific to the right amygdala and to BL amygdala FC with rACC and anterior vmPFC, consistent with prior literature on right-lateralized socioemotional processing (Cahill, 2003) and vmPFC subregion functions (e.g., Etkin et al., 2015; Ochsner et al., 2004; Jalbrzikowski et al., 2017). We further hypothesized that TD individuals and individuals with ASD would show different age-related patterns of amygdala FC. Based on earlier cross-sectional work in TD individuals (Gabard-Durnam et al., 2014) and adolescents with ASD (Rausch et al., 2018), we anticipated that TD individuals would show a linear age-related increase in frontoamygdala FC and that individuals with ASD would show a positive quadratic age-related pattern, such that there would be group differences across the lifespan but that adolescents with ASD would show the largest difference in FC relative to TD adolescents. Lastly, we hypothesized that lower frontoamygdala FC would be associated with greater social impairment in ASD. Consistent with the two-hit model of autism (Picci and Scherf, 2015), we predicted that group differences in amygdala FC and relationships with social impairment would be strongest during adolescence.
2. Methods and materials
2.1. Participants
Resting-state fMRI data from 111 participants from the Autism Brain Imaging Data Exchange first and second releases (ABIDE-I, ABIDE-II; http://fcon_1000.projects.nitrc.org/indi/abide/; Di Martino et al., 2017, 2013) were analyzed. Only data collected at the New York University (NYU) Langone Medical Center were utilized due to the wide age range of individuals with ASD and large sample of TD individuals available in this dataset. To facilitate comparison with previous comprehensive studies of amygdala FC development (Gabard-Durnam et al., 2014; Jalbrzikowski et al., 2017), participants ages 7–25 were included in the current sample (Table 1). Participants from the ABIDE-I release included in the present study are a subsample of participants used in prior studies by our group (Dajani and Uddin, 2016; Farrant and Uddin, 2016; Nomi and Uddin, 2015a, 2015b). Since the release of ABIDE-II, subjects from the same site (Sample 1) were added in order to increase the current sample size. Data collection and sharing procedures were approved by the institutional review board at NYU. Written informed consent and/or informed assent were obtained from each participant and their parent, where applicable.
Table 1.
Participant characteristics and demographic information for ASD and TD groups.
| Measure | ASD (n = 53) |
TD (n = 58) |
||||
|---|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | t-value | p-value | |
| Sex | 46 M : 7 F | – | 52 M : 6 F | – | χ2 = 0.22 | .639 |
| Age (years) | 12.57 (4.33) | 7.13–24.41 | 12.34 (4.46) | 7.11–23.35 | 0.28 | .782 |
| Full-scale IQ | 108.70 (17.28) | 78–148 | 112.07 (14.69) | 80–139 | −1.11 | .269 |
| Verbal IQ | 106.32 (15.84) | 77–139 | 113.22 (13.93) | 80–142 | −2.44 | .016 |
| Perceptual IQ | 110.00 (18.19) | 79–149 | 108.14 (15.90) | 67–137 | 0.58 | .566 |
| Mean FD (mm) | 0.14 (0.05) | 0.06–0.25 | 0.13 (0.05) | 0.07–0.26 | 1.24 | .219 |
| Data removed scrubbing (%) | 0.05 (0.06) | 0.00–0.23 | 0.04 (0.05) | 0.00–0.24 | 0.64 | .525 |
| ADOS SA | 8.81 (4.03) | 3–20 | – | – | – | – |
Demographic and mean IQ scores are shown for the ASD and TD groups. M, Male; F, Female; FD, Framewise displacement; ADOS SA, Autism Diagnostic Observation Schedule social affect score (Gotham algorithm).
Participants were included in the ASD group if they had a prior clinician’s DSM-IV-TR diagnosis of Autistic Disorder, Asperger’s Disorder, or Pervasive Developmental Disorder Not-Otherwise-Specified or DSM-5 diagnosis of Autism Spectrum Disorder, for those enrolled after the DSM-5 release in 2013. ASD diagnoses were confirmed using Overall Total scores on the Autism Diagnostic Observation Schedule- Generic (ADOS-G; Lord et al., 2000) for ABIDE-I subjects and the Autism Diagnostic Observation Schedule- Second Edition (ADOS-2; Lord et al., 2012) for ABIDE-II subjects, in which higher scores signify a higher level of impairment. The ADOS consists of four modules (1–4), one of which is selected to be administered depending on the child’s or adult’s developmental stage and verbal fluency. Of the 53 participants with ASD included in the present study, a total of 41 were administered module 3 and 12 were administered module 4. Though the modules contain some overlapping items, only scores from module 3 were examined in the present study because the tasks and scoring of modules 3 and 4 are considered incompatible (i.e., the modules consist of different tasks, and distinct items contribute to the scoring algorithms) (Hus and Lord, 2014; Lord et al., 2000). Social affect, restricted and repetitive behaviors, total, and severity scores were calculated using the Gotham algorithm for ADOS-G for ABIDE-I subjects and the Gotham algorithm for ADOS-2 for ABIDE-II subjects (Gotham et al., 2009). These two algorithms use identical items regardless of ADOS version. The Gotham algorithm was selected due to its improved psychometric properties compared to the “classic” ADOS-G algorithm (Gotham et al., 2009; Lord et al., 2012). The social affect score from the Gotham algorithm was included as a dependent variable in further analyses. This score sums specific items in the Reciprocal Social Interaction and Language and Communication sections. Finally, when possible, the ASD diagnosis was further confirmed with the Autism Diagnostic Interview-Revised (ADI-R; Lord et al., 1994). TD individuals had no current Axis-I disorders, assessed using the Kiddie Schedule for Affective Disorders and Schizophrenia for Children- Present and Lifetime Version (K-SADS-PL; Kaufman et al., 1997) for individuals under 18 years of age and the Structured Clinical Interview for DSM-IV-TR Axis-I Disorders, Non-patient Edition (SCID-I/NP; First et al., 1995) for adults over 18 years of age. All participants were right-handed as assessed with the Edinburgh Handedness Inventory (Oldfield, 1971).
Subjects were excluded if their in-scanner mean rotational or translational motion in any of the 6 rigid directions was greater than 2 mm or 2°, respectively, or if the percentage of data that would need to be scrubbed at a 0.5 mm threshold exceeded 25%, resulting in the removal of 10 subjects (Power et al., 2012). Motion was assessed for each individual using a summary measure of the 6 rigid parameters called mean framewise displacement (FD), calculated as in Power et al. (2012). In order to match the ASD and TD groups on in-scanner motion and FSIQ, assessed using Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999) two additional ASD subjects were excluded based on excessive motion (>3 standard deviations from the mean motion within each ABIDE subsample). In addition, manual one-to-one matching based on age and sex was computed to remove the fewest subjects possible without biasing the sample. Eight additional subjects were excluded from further analyses due to excessive signal loss or distortion in the ventral anterior frontal cortex (defined as outliers in temporal signal-to-noise ratio (tSNR); Welvaert and Rosseel, 2013). See Supplementary materials for detailed information regarding this assessment of data quality. Following these procedures, there were no group differences in mean FD (p = 0.219, see Table 1 and Supplementary Table S1). The final sample consisted of a total of 111 participants, with 81 participants from ABIDE-I and 30 from ABIDE-II. Of the combined sample, 53 participants were in the ASD group and 58 were in the TD group. 50 out of the 53 participants in the ASD sample met the clinical cutoff (total score >7) on the ADOS, and the remaining three participants (who scored 6, 6, and 5 respectively on the ADOS) met criteria on the ADI-R for ASD. The final ASD and TD groups did not differ significantly on age, sex, mean FD, full-scale IQ (FSIQ), or performance IQ (PIQ). See Table 1 for participant demographics and group comparisons.
2.2. fMRI data acquisition
Functional magnetic resonance imaging (fMRI) data for ABIDE-I were acquired at NYU on a 3 T Allegra scanner using the following parameters: TR = 2000 ms; TE = 15 ms; flip angle = 90°; FOV = 240 mm; voxel size = 3 × 3 × 4 mm; number of slices = 33; 4 mm slice thickness. For ABIDE-II data, fMRI data were also acquired at on a 3 T Allegra scanner with all the same parameters as ABIDE-I except the following: TE = 30 ms; flip angle = 82°; number of slices = 34; 3 mm slice thickness. Resting-state fMRI scans for both ABIDE-I & ABIDE-II were acquired over 6 min (180 volumes). For each subject in ABIDE-I, a high-resolution T1-weighted structural scan was acquired with the following parameters: TR = 2530 ms; TE = 3.25 ms; scan time = 8:07 min; flip angle = 7; 128 slices; 1 vol FOV = 256 mm. For the structural scan, all parameters remained unchanged in ABIDE-II except the TR = 3250 ms. See (Di Martino et al., 2017, 2013) and the ABIDE website (http://fcon_1000.projects.nitrc.org/indi/abide/abide_II.html) for more details on the data acquisition.
2.3. Data preprocessing
Data were preprocessed using the Data Processing Assistant for Resting-State fMRI- Advanced edition (DPARSF-A; http://rfmri.org/DPARSF; Chao-Gan and Yu-Feng, 2010). This toolbox combines tools from SPM8 (http://www.fil.ion.ucl.ac.uk/spm), FSL (http://fsl.fmrib.ox.ac.uk/), and the toolbox for Data Processing & Analysis of Brain Imaging (DPABI, http://rfmri.org/DPABI).
Functional images underwent removal of the first four timepoints, slice time correction, spatial realignment, nuisance covariate regression [linear trend, 24 motion parameters (Friston et al., 1996), white matter (WM), and cerebrospinal fluid (CSF) signal], band-pass filtering (0.01–0.08 Hz), normalization to standard stereotaxic space (based on the Montreal Neurological Institute (MNI) 152 2 mm template), motion scrubbing for TR-to-TR motion greater than a 0.5 mm threshold (Power et al., 2012), and spatial smoothing using a 6 mm full-width half-maximum Gaussian kernel to increase the signal-to-noise ratio prior to statistical analysis. 24 motion parameter regression was used because it has been shown to be superior to 6 parameter motion regression for mitigating the effects of motion artifacts (Fair et al., 2012; Friston et al., 1996; Power et al., 2014; Satterthwaite et al., 2013; Yan et al., 2013). The 24 motion parameters included the 6 standard motion variables (i.e., 3 rigid motion directions (x, y, z) and 3 rotation directions (pitch, roll, yaw) for each timepoint, 6 head motion parameters one time point before, and the 12 corresponding squared items (Friston et al., 1996). Global signal regression (GSR) was not applied in the primary analyses for a number of reasons. First, electrophysiological recordings in macaques have demonstrated that the global signal also includes neural components (Schölvinck et al., 2010); therefore, regressing this signal out amounts to removing some true neural activity. Second, after GSR, correlation values are centered on zero, which can lead to spurious negative correlation values (Murphy et al., 2009). Third, in simulated data, GSR has been shown to artificially introduce correlations between brain regions and substantially distort group differences in inter-regional correlations (Saad et al., 2012). Fourth, differences in caffeine intake can affect global signal such that caffeine leads to widespread decreases in connectivity and global signal amplitude (Wong et al., 2012). Fifth, and most importantly, it has been demonstrated in a study of ASD that GSR leads to a reversal in the direction of group correlation differences relative to other preprocessing approaches, with a higher incidence of both long-range and local connectivity differences that favor the ASD group (Gotts et al., 2013). In the current work, the primary goal was to interpret group differences in connectivity; thus we chose not to use GSR in the primary analysis. In order to assess the robustness of the results, data were also analyzed with two additional modified pipelines (i.e., with global signal regression, without smoothing) (See Supplementary Tables S4–S7). Structural images were skull-stripped, manually coregistered to the functional images using AC-PC alignment, and segmented into gray matter, WM, and CSF components. Based on visual inspection, all images passed quality checks after brain extraction, coregistration, and normalization steps.
2.4. Region of interest selection
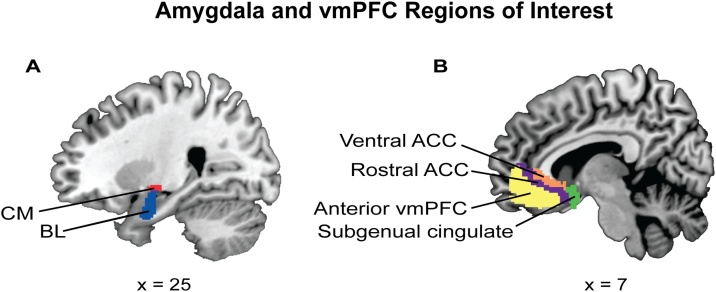
Amygdala region of interest (ROI) selection included subregions identified based on previous studies (Gabard-Durnam et al., 2014; Jalbrzikowski et al., 2017; Qin et al., 2012), which were derived from FSL’s Juelich histological atlas: stereotaxic probabilistic maps of cytoarchitectonic boundaries generated by Amunts et al. (2005). Consistent with a recent study examining amygdala FC across development, maps of the CM and BL amygdala subregions were used, excluding the superficial subregion given a lack of literature on this subregion (Jalbrzikowski et al., 2017). Each amygdala subregion was thresholded at 50% probability, and every voxel was assigned to only one subregion (Fig. 1; Gabard-Durnam et al., 2014; Jalbrzikowski et al., 2017). The right and left hemisphere ROIs were considered separately.
Fig. 1.
Amygdala and vmPFC regions of interest (ROIs).
(A) Amygdala subregions examined in the present study were selected from FSL’s Juelich histological atlas (Eikhoff et al., 2007) and thresholded at fifty percent; each voxel was assigned to only one subregion. The subregions included the centromedial amygdala (CM; red) and the basolateral amygdala (BL; blue). The superficial amygdala was not included, consistent with previous studies (Jalbrzikowski et al., 2017). (B) The ventromedial prefrontal cortex (vmPFC) subregions used in the present study were derived from Mackey and Petrides (2014), implemented in AFNI and converted to MNI space. The subregions included the ventral anterior cingulate cortex (ACC; orange), the rostral ACC (purple), the anterior vmPFC (yellow), and the subgenual cingulate (green) (for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
Consistent with a previous study of CM and BL amygdala FC across development, we determined vmPFC ROIs for each subject using AFNI’s Mackey vmPFC atlas of asymmetric and probabilistic cytoarchitectonic maps generated by Mackey and Petrides (2014). This atlas provides eight vmPFC subregions, extending from the frontal pole to the septal region (Mackey and Petrides, 2014): the subgenual cingulate (BA 25), the rACC (caudal portion of BA 32), the vACC (rostral BA 24), the anterior vmPFC (dorsal portion of BA 11 and rostral-most portion of BA 32), two subregions of the anterior medial orbitofrontal cortex (OFC; ventral anterior portion of BA 11), and two of the posterior medial orbitofrontal cortex (ventral posterior portion of BA 11). All four OFC subregions were excluded from further analyses due to dropout and/or low signal quality in the majority of subjects as evidenced via quantitative assessment of tSNR (see Supplementary Fig. S2) and visual inspection of raw fMRI images. Therefore, a total of two amygdala subregions and four vmPFC subregions were used in analyses (Fig. 1).
2.5. Individual-level analysis
Individual-level seed-to-seed FC matrices were generated by correlating the right amygdala BL and CM subregions’ average timeseries with each of the four vmPFC subregions using the REST toolbox V1.8 (http://restfmri.net). The Fisher-transformed correlation coefficients were carried forward to the multiple regression analyses.
2.6. Group-level analyses
Group-level analyses were conducted to address two primary questions: 1) whether age-related changes in frontoamygdala FC differed in individuals with ASD compared with TD individuals, and 2) whether frontoamygdala FC related to social impairment in children with ASD and how this association may vary as a function of age. For each aim, we conducted a set of multiple regression models to test each of eight possible amygdala subregion-vmPFC subregion FC pairs for each hemisphere. To address the first aim, both TD and ASD individuals were included, and the following independent variables were modeled: percent of volumes scrubbed (centered), diagnostic group (0 = TD, 1 = ASD), age (centered), age-squared, and an age x diagnosis interaction term. To reduce multicollinearity, the age-squared term was calculated as the square of the centered age variable. For aim 1, the age-squared x diagnosis interaction term was omitted due to high multicollinearity (Variance Inflation Factor >5 in all models).
To address the second aim, we conducted a regression analysis examining the effect of age on the relationship between frontoamygdala FC and ADOS Gotham algorithm social affect (ADOS SA) scores. Only individuals with ASD with scores on module 3 of the ADOS were included (ages 7.1–14.6; n = 43). The following independent variables were modeled: percent of volumes scrubbed (centered), ADOS SA score (centered), age (centered), age-squared, and age x ADOS SA and age-squared x ADOS SA interaction terms. The age-squared term was computed in the same manner as described above. To address the second aim, the only predictors of interest were ADOS SA and the age x ADOS SA and age-squared x ADOS SA interaction terms. All analyses controlled for the percentage of volumes scrubbed for each individual.
To control for multiple comparisons for the eight seed to seed FC models, all tests of significance for beta values were thresholded at a false discovery rate (FDR) of p < .05. FDR correction was implemented in R (www.r-project.org/) and applied separately for each regression model. For all regression model results, standardized betas and associated t statistics are reported.
3. Results
3.1. Age x diagnostic group on frontoamygdala FC
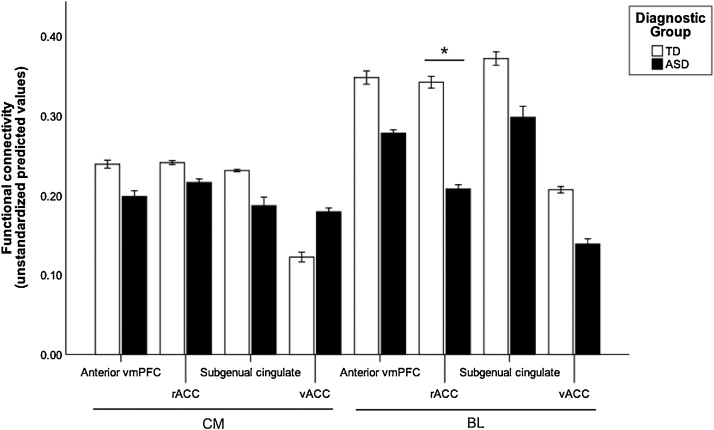
To investigate the relationship between frontoamygdala FC, diagnostic status, and age, a multiple regression analysis was conducted. Contrary to hypotheses, there were no significant interactions of age x diagnosis or age-squared x diagnosis (p > .05, FDR-corrected, Table 2). Consistent with hypotheses, we observed diagnostic group differences in right BL amygdala-rACC FC when controlling for age and motion (R-squared = 0.13, B = −0.30, t(108) = −3.28, p = .011, FDR-corrected), such that individuals with ASD had weaker FC than TD individuals (TD: M = 0.34, SD = 0.21; ASD: M = 0.21, SD = 0.23; Fig. 2). The group difference in BL amygdala-rACC FC remained significant when we implemented different preprocessing steps including no spatial smoothing (p = .001, FDR-corrected) and adding global signal regression (p = .033, uncorrected; Supplementary Tables S4–S7).
Table 2.
Seed to seed results: age x diagnosis on frontoamygdala connectivity.
| Centromedial amygdala |
Basolateral amygdala |
|||||||
|---|---|---|---|---|---|---|---|---|
| vACC | rACC | anterior vmPFC | subgenual cingulate | vACC | rACC | anterior vmPFC | subgenual cingulate | |
|
Right hemisphere | ||||||||
| Diagnostic group | 0.120 | −0.073 | −0.117 | −0.105 | −0.166 | −0.300* | −0.157 | −0.144 |
| Age | 0.085 | −0.030 | 0.035 | −0.082 | 0.134 | 0.067 | 0.173 | −0.102 |
| Age-squared | −0.207 | −0.002 | 0.002 | 0.201 | −0.045 | 0.172 | 0.091 | 0.371* |
| Age x Diagnostic group | 0.120 | 0.134 | 0.085 | 0.132 | 0.089 | −0.015 | −0.091 | 0.056 |
|
Left hemisphere | ||||||||
| Diagnostic group | 0.051 | −0.063 | −0.039 | −0.138 | 0.098 | 0.001 | 0.004 | 0.032 |
| Age | 0.009 | −0.158 | −0.164 | −0.091 | 0.020 | −0.131 | 0.059 | 0.000 |
| Age-squared | −0.024 | 0.038 | 0.141 | 0.095 | 0.140 | 0.148 | 0.120 | 0.146 |
| Age x Diagnostic group | 0.160 | 0.170 | 0.086 | 0.028 | 0.041 | 0.136 | −0.027 | −0.026 |
Standardized beta values for each regression model between right and left amygdala subregions and vmPFC subregions. Each model included a nuisance covariate for percentage of data scrubbed.
= p < .05, FDR-corrected.
Fig. 2.
Group differences in right frontoamygdala FC. FC strength for centromedial (CM) and basolateral (BL) amygdala subregions with each vmPFC subregion in the TD (white) and ASD (black) diagnostic groups. BL-rACC FC strength was significantly weaker in ASD compared to TD individuals, p = .011, FDR-corrected. There were no other group differences in frontoamygdala FC prior to or following FDR correction. Error bars represent ±1 standard error. The y-axis shows unstandardized predicted values that were extracted from the regression model. FC values are Fisher’s r to z transformed values.
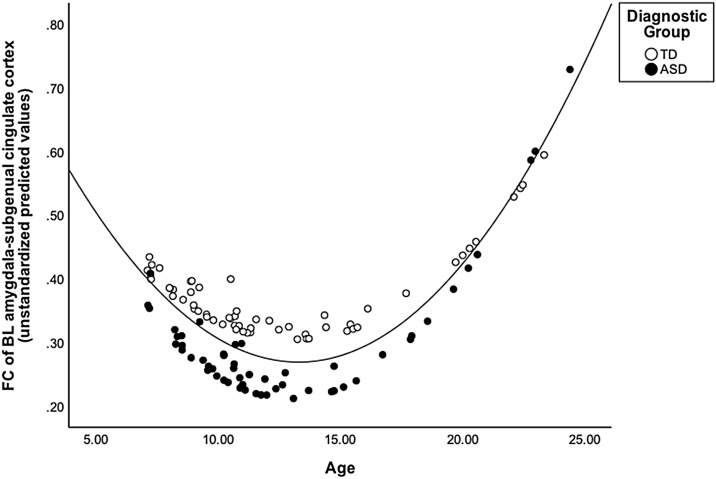
We also observed a significant relationship between age-squared and right BL amygdala-subgenual cingulate FC when controlling for diagnostic group and motion (R-squared = 0.13, B = 0.37, t(108) = 2.86, p = .041, FDR-corrected), such that adolescents showed the lowest FC compared to both children and adults (i.e., a U-shaped pattern, Fig. 3). This result was also evident when preprocessing was conducted without smoothing (p = .033, uncorrected) and with global signal regression (p < .001, FDR-corrected). There were no other group differences in seed to seed FC. There were no significant predictors in any model using left hemisphere seeds.
Fig. 3.
Quadratic association between age and frontoamygdala FC. FC of the BL amygdala-subgenual cingulate showed a main effect of age-squared, controlling for diagnostic group and age, p = .041, FDR-corrected, such that adolescents showed weaker FC than children and adults. The y-axis shows unstandardized predicted values that were extracted from the regression model. FC values are Fisher’s r to z transformed values. Diagnostic group distinction (i.e., dot color) is shown for illustrative purposes only; there was no significant interaction between diagnostic group and age.
3.2. Frontoamygdala FC and ASD social impairment
To investigate the relationship between frontoamygdala FC and social impairment in children and adolescents with ASD, a multiple regression analysis was used. No results survived FDR correction. Here we report preliminary results at an exploratory threshold of p <.05, uncorrected. In the right BL-rACC FC model, there was a preliminary main effect of ADOS SA (R-squared = 0.18, B = −0.52, t(34) = −2.32, p = 0.027, uncorrected) and an interaction between age-squared and ADOS SA (R squared = 0.18, B = 0.59, t(34) = 2.18, p = .036, uncorrected). For the right CM-subgenual cingulate FC model, there was a main effect of age-squared, controlling for ADOS SA and age (R squared = 0.16, B = 0.39, t(34) = 2.15, p = .039, uncorrected; Table 3). Given that these effects (i.e., the main effect of age-squared for CM-subgenual cingulate FC and the main effect of ADOS SA and age-squared x ADOS SA interaction for BL-rACC FC) did not survive FDR correction for multiple comparisons, they were not interrogated further. There were no significant predictors in any model investigating social impairment using left hemisphere seeds.
Table 3.
Seed to seed results: age x ADOS SA on frontoamygdala connectivity.
| Centromedial amygdala |
Basolateral amygdala |
|||||||
|---|---|---|---|---|---|---|---|---|
| vACC | rACC | anterior vmPFC | subgenual cingulate | vACC | rACC | anterior vmPFC | subgenual cingulate | |
|
Right hemisphere | ||||||||
| ADOS SA | −0.084 | −0.083 | −0.028 | 0.045 | −0.284 | −0.519† | −0.281 | −0.159 |
| Age | 0.070 | −0.051 | 0.047 | −0.266 | 0.005 | −0.089 | −0.014 | −0.318 |
| Age-squared | −0.176 | 0.070 | 0.087 | 0.389† | −0.027 | 0.255 | 0.206 | 0.217 |
| ADOS SA x age | −0.308 | −0.377 | −0.308 | −0.214 | −0.287 | −0.326 | −0.133 | −0.135 |
| ADOS SA x age-squared | 0.104 | 0.146 | 0.044 | 0.116 | 0.369 | 0.592† | 0.236 | 0.249 |
|
Left hemisphere | ||||||||
| ADOS SA | 0.115 | −0.037 | 0.218 | −0.159 | −0.346 | −0.326 | 0.031 | −0.040 |
| Age | 0.115 | −0.086 | −0.236 | −0.126 | −0.165 | −0.096 | −0.057 | −0.117 |
| Age-squared | −0.148 | 0.019 | 0.144 | 0.076 | 0.259 | 0.129 | 0.135 | −0.042 |
| ADOS SA x age | −0.185 | −0.260 | −0.247 | −0.348 | −0.274 | −0.375 | −0.140 | −0.042 |
| ADOS SA x age-squared | −0.210 | −0.113 | −0.187 | 0.033 | 0.188 | 0.288 | −0.138 | −0.142 |
Standardized beta values for each regression model between right and left amygdala subregions and vmPFC subregions. ADOS social affect score (ADOS SA) (Gotham algorithm) was used for subjects with ASD who were administered module 3 (n = 43; Gotham et al., 2009). Each model included a nuisance covariate for percentage of data scrubbed.
= p < .05, uncorrected.
4. Discussion
Situated at the core of the brain’s socioemotional networks, the amygdala and its interactions with the vmPFC are critical for domains impaired in ASD such as socioemotional processing and regulation. Given dynamic changes in frontoamygdala FC across typical development, assessing FC differences in individuals with ASD across a broad age range was a central focus of the present study. To our knowledge, this was the first study to investigate age-related changes in frontoamygdala networks in ASD and the relation of changes to social impairment across childhood and adolescence. Relative to TD individuals, individuals with ASD exhibited weaker frontoamygdala FC, and this group difference was specific to the right hemisphere BL amygdala and its FC with the rACC. These findings provide evidence for differences in intrinsic amygdala FC among individuals with ASD across a broad age range.
Resting-state fMRI studies are optimally suited to examine changes in large-scale brain networks across development and between clinical populations, as they may be less affected by confounds of differences in task demands and strategies (Fox and Raichle, 2007). However, existing studies of FC in ASD have examined a single age group or have not accounted for age-related changes in FC (Guo et al., 2016; Hahamy et al., 2015; Kleinhans et al., 2016; Rausch et al., 2018; Shen et al., 2016), precluding the opportunity to inform developmental change in ASD. Consistent with two previous studies of frontoamygdala FC in preschool-age children with ASD (Shen et al., 2016) and adolescents with ASD (Rausch et al., 2018), we observed weaker frontoamygdala FC in ASD. Though both of these studies examined youth with ASD, they did not assess for age-related changes or control for age. The present study also incorporated rigorous motion correction (i.e., Friston 24 motion parameters regression, motion scrubbing, and controlling for differences in percent scrubbed in regression analyses) and found highly similar correlations of age and motion parameters between ASD and TD individuals (Supplementary Table S1). Lastly, the present findings were consistent when data were analyzed using two alternative preprocessing pipelines (i.e., without smoothing the fMRI data, and using global signal regression). Here, in a large cross-sectional sample that is uniquely equipped to examine age-related differences across a wide age range (i.e., 7–25 years old), we found converging evidence for weaker frontoamygdala FC in individuals with ASD, relative to TD counterparts.
FC between the amygdala and subregions of the vmPFC has been implicated in many higher-order socioemotional processes, including appraising emotions (Adolphs, 2002), eye gaze (Spezio et al., 2007), understanding others’ perspectives (Völlm et al., 2006), and emotion regulation (Etkin et al., 2006; Hariri et al., 2000; Kim et al., 2011; Phelps and LeDoux, 2005; Zhang et al., 2013). Individuals with autism exhibit difficulty with social interactions including abnormal face processing (Adolphs et al., 2001; Ashwin et al., 2006; Baron-Cohen et al., 1999; Critchley et al., 2000; Dalton et al., 2005; Davies et al., 1994; Schultz et al., 2000) and automatic processing and judgement of emotions (Celani et al., 1999; Macdonald et al., 1989; Wang et al., 2004). In the present study, lower frontoamygdala FC in individuals with ASD was observed, specifically between the right BL amygdala and the rACC, two regions that have previously been implicated in socioemotional processes including the evaluation of emotional content of incoming information (Dolan and Vuilleumier, 2003; Sah et al., 2003) and the cognitive control of emotion (Etkin et al., 2015, 2011), both of which are crucial for successful social interactions. No other regions showed a group difference in FC after controlling for multiple comparisons, suggesting this diagnostic group difference may be specific to the right hemisphere BL amygdala-rACC FC. This lateralization is consistent with our hypotheses based on extant literature implicating the right amygdala in emotion regulation and social functioning (Adolphs and Spezio, 2006; Cahill, 2003; Ochsner et al., 2012), as well as lateralization in prior findings of frontoamygdala FC (e.g., Jalbrzikowski et al., 2017). The finding of lower BL amygdala-rACC FC in ASD builds on existing literature reporting frontoamygdala differences in individuals with ASD (Rausch et al., 2018; Shen et al., 2016) by extending the age range of this finding to middle childhood and identifying interactions between the BL amygdala and rACC as a specific aspect of frontoamygdala circuitry that may be implicated in ASD.
Contrary to our hypotheses, there was no evidence for group differences in the age-related trajectory of frontoamygdala FC. Here, we had expected to find age-related changes in right BL-rACC FC in ASD given the involvement of these regions in socioemotional processes that are implicated in ASD (Dolan and Vuilleumier, 2003; Etkin et al., 2015; Kleinhans et al., 2016; Sah et al., 2003). However, we did observe non-linear age-related changes in frontoamygdala FC between the BL amygdala and subgenual cingulate cortex in the entire sample, when controlling for diagnostic status. Specifically, BL amygdala-subgenual cingulate FC displayed a quadratic U-shaped pattern with age, such that adolescents had weaker FC than both children and adults. We did not have a priori hypotheses about amygdala FC with the subgenual cingulate, though prior evidence suggests that this circuitry may undergo change during typical development (Jalbrzikowski et al., 2017; Kelly et al., 2009), and it has also been implicated in depression (Johansen-Berg et al., 2008; Murphy et al., 2016) and anxiety (Coombs et al., 2014). Although the present ABIDE sample does not provide measures of anxiety or depression symptoms, comorbidities between ASD with anxiety and depression (Brereton et al., 2006; Burrows et al., 2017a; Simonoff et al., 2008; White et al., 2009) suggest this is a relevant circuit to examine with regard to co-occurring ASD and internalizing disorders.
Previous studies examining the typical development of frontoamygdala FC at rest have demonstrated mixed results, reporting both age-related increases (Gabard-Durnam et al., 2014; Qin et al., 2012; Alarcón et al., 2015) and decreases (Jalbrzikowski et al., 2017). These inconsistent findings may stem from diverging methods, with one previous study focusing on cross-sectional development of the bilateral amygdala to whole-brain FC (Gabard-Durnam et al., 2014) and the other using longitudinal data to examine amygdala FC with vmPFC subregions (Jalbrzikowski et al., 2017). Here, to examine FC between specific amygdala and vmPFC subregions we followed the recent methods of Jalbrzikowski et al. (2017) as closely as possible. Jalbrzikowski et al. (2017) found age-related decreases in right hemisphere BL-rACC, CM-rACC, CM-vmPFC, and CM-subgenual cingulate FC in TD individuals. A key difference is that the data available in the present study were cross-sectional and not longitudinal, which may have contributed to discrepancies in our findings. Taken together, the current findings contribute to a mixed literature on typical and atypical frontoamygdala development and suggest that future research would benefit from longitudinal investigations to further clarify the nature of frontoamygdala development in ASD.
A strength of the present study is that social impairment in youth with ASD was assessed using an in vivo clinician-administered observational measure of current social and communication symptoms, which could provide enhanced ecological validity relative to self-report measures of social symptoms. However, the ADOS assessment is not without limitations. The ADOS has been shown to display consistency insofar as establishing whether an individual meets criteria for an ASD diagnosis, yet test-retest reliability and inter-rater reliability for individual items are less robust (Lord et al., 2000). Relevant to the present study, reliability is adequate and relatively higher for social items than for restricted and repetitive behavior items across modules (Lord et al., 2000). In the present study, data from only one site were used, increasing the likelihood that the reported scores were consistently obtained. Because the primary goal of the present study was to investigate ASD-related differences in amygdala FC given its role in socioemotional processing, we selected a severity score specific to social impairment, as opposed to a more general measure of symptom severity. Due to differences between ADOS modules 3 and 4, only subjects with the ADOS module 3 (i.e., ages 14.6 or younger in this sample) were included in the analyses of frontoamygdala FC and social impairment.
Given that adolescence is a particularly salient time for social functioning, we hypothesized that frontoamygdala FC would be related to social impairment in youth with ASD in an age-dependent manner. Although BL amygdala-rACC FC, the same network that showed lower FC in individuals with ASD compared to TD individuals, had a non-linear age-related association with social impairment among ASD youth at an uncorrected statistical threshold, this finding did not survive correction for multiple comparisons. Thus we have avoided interpreting the effect further and note that the inability to combine across ADOS modules may have constrained statistical power for our analysis of social impairment. Thus, while we did not observe associations between frontoamygdala FC and social impairment, examining potential age-related changes in the relationship between frontoamygdala FC and social impairment in ASD may be an important avenue for future research with a larger sample size and perhaps a more robust measure of social impairment.
The current study has several limitations that should be addressed in future research on frontoamygdala circuitry in ASD. Although resting-state fMRI studies may help to minimize developmental differences in task performance, the process of “resting” itself could differ across development, and may be particularly challenging for younger children. Future work may capitalize on recent developments in the use of movie viewing paradigms to improve compliance in young children (Vanderwal et al., 2015). Given the primary goal to examine age-related change across childhood through early adulthood in ASD, the current study specifically benefited from the broad age range of participants at the NYU site in the ABIDE consortium. A replication sample with a similarly broad age range was not available within ABIDE; thus, replication of the results reported here will be important. Our findings regarding frontoamygdala connectivity in ASD extend prior work from adulthood and adolescence to middle childhood. Future research will also benefit from examining early childhood, when ASD is typically diagnosed.
The present findings contribute to the growing literature implicating the amygdala as an important locus of dysfunction in individuals with ASD and highlight alterations in frontoamygdala circuitry, specifically interactions between the BL amygdala and rACC, in ASD. Disruptions in intrinsic frontoamygdala connectivity may contribute to ASD-related social deficits, though ongoing research will be necessary to further assess this relationship. As part of its role in socioemotional development, frontoamygdala circuitry has also been associated with anxiety symptoms during childhood and adolescence (Blackford and Pine, 2012; Gee et al., 2013; Jalbrzikowski et al., 2017; Kujawa et al., 2016; Gee et al., 2016). Youth with ASD are at heightened risk for co-occurring anxiety, which often emerges among individuals with ASD during childhood and adolescence (Sukhodolsky et al., 2008; White et al., 2009). Children and adolescents with ASD who have more severe social deficits, as well as insight into these difficulties, are particularly vulnerable to developing symptoms of anxiety (Burrows et al., 2017b; Sukhodolsky et al., 2008). Increasingly, interventions for youth with ASD have focused on targeting comorbid symptoms of anxiety (Sze and Wood, 2008; Wood et al., 2009; White et al., 2009; Storch et al., 2013). Future research may elucidate associations between frontoamygdala FC, ASD-related social impairment, and comorbid anxiety in youth with ASD (Burrows et al., 2017a), as well as a potential pathway from social difficulties to anxiety (e.g., Bellini, 2006), in ways that inform clinical translation for interventions aimed at targeting both ASD and comorbid anxiety.
Conflict of Interest
None.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2018.12.001.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Adolphs R. Neural systems for recognizing emotion. Curr. Opin. Neurobiol. 2002;12(2):169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Fear, faces, and the human amygdala. Curr. Opin. Neurobiol. 2008;18(2):166–172. doi: 10.1016/j.conb.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R., Spezio M. Role of the amygdala in processing visual social stimuli. Prog. Brain Res. 2006;156:363–378. doi: 10.1016/S0079-6123(06)56020-0. [DOI] [PubMed] [Google Scholar]
- Adolphs R., Sears L., Piven J. Abnormal processing of social information from faces in autism. J. Cogn. Neurosci. 2001;13(2):232–240. doi: 10.1162/089892901564289. [DOI] [PubMed] [Google Scholar]
- Alarcón G., Cservenka A., Rudolph M.D., Fair D.A., Nagel B.J. Developmental sex differences in resting state functional connectivity of amygdala sub-regions. Neuroimage. 2015;115:235–244. doi: 10.1016/j.neuroimage.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral D.G., Insausti R. Retrograde transport of D-[3H]-aspartate injected into the monkey amygdaloid complex. Exp. Brain Res. 1992;88(2):375–388. doi: 10.1007/BF02259113. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . American Psychiatric Association; Washington, D.C: 2000. Diagnostic Criteria from DSM-IV-TR. [Google Scholar]
- Amunts K., Kedo O., Kindler M., Pieperhoff P., Mohlberg H., Shah N.J. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat. Embryol. (Berl.) 2005;210(5-6):343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Ashwin C., Chapman E., Colle L., Baron-Cohen S. Impaired recognition of negative basic emotions in autism: a test of the amygdala theory. Soc. Neurosci. 2006;1(3-4):349–363. doi: 10.1080/17470910601040772. [DOI] [PubMed] [Google Scholar]
- Ashwin C., Baron-Cohen S., Wheelwright S., O’Riordan M., Bullmore E.T. Differential activation of the amygdala and the’ social brain’ during fearful face-processing in Asperger Syndrome. Neuropsychologia. 2007;45(1):2–14. doi: 10.1016/j.neuropsychologia.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Avino T.A., Barger N., Vargas M.V., Carlson E.L., Amaral D.G., Bauman M.D., Schumann C.M. Neuron numbers increase in the human amygdala from birth to adulthood, but not in autism. Proc. Natl. Acad. Sci. U. S. A. 2018;115(14):3710–3715. doi: 10.1073/pnas.1801912115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S., Ring H.A., Wheelwright S., Bullmore E.T., Brammer M.J., Simmons A., Williams S.C. Social intelligence in the normal and autistic brain: an fMRI study. Eur. J. Neurosci. 1999;11(6):1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Ring H.A., Bullmore E.T., Wheelwright S., Ashwin C., Williams S.C. The amygdala theory of autism. Neurosci. Biobehav. Rev. 2000;24(3):355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Bellini S. The development of social anxiety in adolescents with autism spectrum disorders. Focus Autism Other Dev. 2006;21(3):138–145. [Google Scholar]
- Berking M., Wupperman P. Emotion regulation and mental health: recent findings, current challenges, and future directions. Curr. Opin. Psychiatry. 2012;25(2):128–134. doi: 10.1097/YCO.0b013e3283503669. [DOI] [PubMed] [Google Scholar]
- Blackford J.U., Pine D.S. Neural substrates of childhood anxiety disorders: a review of neuroimaging findings. Child Adolesc. Psychiatr. Clin. N. Am. 2012;21(3):501–525. doi: 10.1016/j.chc.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair K.S., Smith B.W., Mitchell D.G., Morton J., Vythilingam M., Pessoa L. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35(1):430–440. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer S.Y., Wang A.T., Scott A., Sigman M., Dapretto M. Frontal contributions to face processing differences in autism: evidence from fMRI of inverted face processing. J. Int. Neuropsychol. Soc. 2008;14(6):922–932. doi: 10.1017/S135561770808140X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brereton A.V., Tonge B.J., Einfeld S.L. Psychopathology in children and adolescents with autism compared to young people with intellectual disability. J. Autism Dev. Disord. 2006;36(7):863–870. doi: 10.1007/s10803-006-0125-y. [DOI] [PubMed] [Google Scholar]
- Bunge S.A., Dudukovic N.M., Thomason M.E., Vaidya C.J., Gabrieli J.D. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33(2):301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows C.A., Laird A.R., Uddin L.Q. Functional connectivity of brain regions for self- and other-evaluation in children, adolescents and adults with autism. Dev. Sci. 2016;19(4):564–580. doi: 10.1111/desc.12400. [DOI] [PubMed] [Google Scholar]
- Burrows C.A., Timpano K.R., Uddin L.Q. Putative brain networks underlying repetitive negative thinking and comorbid internalizing problems in autism. Clin. Psychol. Sci. 2017;5(3):522–536. doi: 10.1177/2167702616683506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows C.A., Usher L.V., Mundy P.C., Henderson H.A. The salience of the self: self-referential processing and internalizing problems in children and adolescents with autism spectrum disorder. Autism Res. 2017;10(5):949–960. doi: 10.1002/aur.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L. Sex- and hemisphere-related influences on the neurobiology of emotionally influenced memory. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27(8):1235–1241. doi: 10.1016/j.pnpbp.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Trainor R.J., Orendi J.L., Schubert A.B., Nystrom L.E., Giedd J.N. A developmental functional MRI study of prefrontal activation during performance of a Go-No-Go task. J. Cogn. Neurosci. 1997;9(6):835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Celani G., Battacchi M.W., Arcidiacono L. The understanding of the emotional meaning of facial expressions in people with autism. J. Autism Dev. Disord. 1999;29(1):57–66. doi: 10.1023/a:1025970600181. [DOI] [PubMed] [Google Scholar]
- Chao-Gan Y., Yu-Feng Z. DPARSF: a MATLAB toolbox for "Pipeline" data analysis of resting-state fMRI. Front. Syst. Neurosci. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs G., Loggia M.L., Greve D.N., Holt D.J. Amygdala perfusion is predicted by its functional connectivity with the ventromedial prefrontal cortex and negative affect. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0097466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett B.A., Carmean V., Ravizza S., Wendelken C., Henry M.L., Carter C., Rivera S.M. A functional and structural study of emotion and face processing in children with autism. Psychiatry Res. 2009;173(3):196–205. doi: 10.1016/j.pscychresns.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley H.D., Daly E.M., Bullmore E.T., Williams S.C., Van Amelsvoort T., Robertson D.M. The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123(Pt. 11):2203–2212. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- Dajani D.R., Uddin L.Q. Local brain connectivity across development in autism spectrum disorder: a cross-sectional investigation. Autism Res. 2016;9(1):43–54. doi: 10.1002/aur.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton K.M., Nacewicz B.M., Johnstone T., Schaefer H.S., Gernsbacher M.A., Goldsmith H.H. Gaze fixation and the neural circuitry of face processing in autism. Nat. Neurosci. 2005;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P., Kemp A.H., Flynn G., Harris A.W., Liddell B.J., Whitford T.J. Functional disconnections in the direct and indirect amygdala pathways for fear processing in schizophrenia. Schizophr. Res. 2007;90(1–3):284–294. doi: 10.1016/j.schres.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Davies S., Bishop D., Manstead A.S., Tantam D. Face perception in children with autism and Asperger’s syndrome. J. Child Psychol. Psychiatry. 1994;35(6):1033–1057. doi: 10.1111/j.1469-7610.1994.tb01808.x. [DOI] [PubMed] [Google Scholar]
- Davis M., Whalen P.J. The amygdala: vigilance and emotion. Mol. Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Decety J., Michalska K.J. Neurodevelopmental changes in the circuits underlying empathy and sympathy from childhood to adulthood. Dev. Sci. 2010;13(6):886–899. doi: 10.1111/j.1467-7687.2009.00940.x. [DOI] [PubMed] [Google Scholar]
- Di Martino A., Yan C.G., Li Q., Denio E., Castellanos F.X., Alaerts K. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol. Psychiatry. 2013 doi: 10.1038/mp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A., O’Connor D., Chen B., Alaerts K., Anderson J.S., Assaf M. Enhancing studies of the connectome in autism using the autism brain imaging data exchange II. Sci. Data. 2017;4:170010. doi: 10.1038/sdata.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan R.J., Vuilleumier P. Amygdala automaticity in emotional processing. Ann. N. Y. Acad. Sci. 2003;985:348–355. doi: 10.1111/j.1749-6632.2003.tb07093.x. [DOI] [PubMed] [Google Scholar]
- Domes G., Heinrichs M., Kumbier E., Grossmann A., Hauenstein K., Herpertz S.C. Effects of intranasal oxytocin on the neural basis of face processing in autism spectrum disorder. Biol. Psychiatry. 2013;74(3):164–171. doi: 10.1016/j.biopsych.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Etkin A., Egner T., Peraza D.M., Kandel E.R., Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51(6):871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Büchel C., Gross J.J. The neural bases of emotion regulation. Nat. Rev. Neurosci. 2015;16(11):693–700. doi: 10.1038/nrn4044. [DOI] [PubMed] [Google Scholar]
- Fair D.A., Nigg J.T., Iyer S., Bathula D., Mills K.L., Dosenbach N.U. Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Front. Syst. Neurosci. 2012;6:80. doi: 10.3389/fnsys.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant K., Uddin L.Q. Atypical developmental of dorsal and ventral attention networks in autism. Dev. Sci. 2016;19(4):550–563. doi: 10.1111/desc.12359. [DOI] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W., Davies M., Borus J. The structured clinical interview for Dsm-Iii-R personality-disorders (Scid-Ii) .2. Multisite test-retest reliability study. J. Pers. Disord. 1995;9(2):92–104. [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Williams S., Howard R., Frackowiak R.S., Turner R. Movement-related effects in fMRI time-series. Magn. Reson. Med. 1996;35(3):346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Gabard-Durnam L.J., Flannery J., Goff B., Gee D.G., Humphreys K.L., Telzer E. The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. Neuroimage. 2014;95:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam L.J., Gee D.G., Goff B., Flannery J., Telzer E., Humphreys K.L. Stimulus-elicited connectivity influences resting-state connectivity years later in human development: a prospective study. J. Neurosci. 2016;36(17):4771–4784. doi: 10.1523/JNEUROSCI.0598-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam L.J., O’Muircheartaigh J., Dirks H., Dean D.C., III, Tottenham N., Deoni S. Human amygdala functional network development: a cross-sectional study from 3 months to 5 years of age. Dev. Cogn. Neurosci. 2018;34:63–74. doi: 10.1016/j.dcn.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G. Sensitive periods of emotion regulation: influences of parental care on frontoamygdala circuitry and plasticity. New Dir. Child Adolesc. Dev. 2016;2016(153):87–110. doi: 10.1002/cad.20166. [DOI] [PubMed] [Google Scholar]
- Gee D.G., Humphreys K.L., Flannery J., Goff B., Telzer E.H., Shapiro M. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J. Neurosci. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Fetcho R.N., Jing D., Li A., Glatt C.E., Drysdale A.T. Individual differences in frontolimbic circuitry and anxiety emerge with adolescent changes in endocannabinoid signaling across species. Proc. Natl. Acad. Sci. U. S. A. 2016;113(16):4500–4505. doi: 10.1073/pnas.1600013113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei H.T., Hilgetag C.C., Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34(3):905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Snell J.W., Lange N., Rajapakse J.C., Casey B.J., Kozuch P.L. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cereb. Cortex. 1996;6(4):551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Gotham K., Pickles A., Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J. Autism Dev. Disord. 2009;39(5):693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotts S.J., Saad Z.S., Jo H.J., Wallace G.L., Cox R.W., Martin A. The perils of global signal regression for group comparisons: a case study of Autism Spectrum disorders. Front. Hum. Neurosci. 2013;7:356. doi: 10.3389/fnhum.2013.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grelotti D.J., Klin A.J., Gauthier I., Skudlarski P., Cohen D.J., Gore J.C. fMRI activation of the fusiform gyrus and amygdala to cartoon characters but not to faces in a boy with autism. Neuropsychologia. 2005;43(3):373–385. doi: 10.1016/j.neuropsychologia.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Guo X., Duan X., Long Z., Chen H., Wang Y., Zheng J. Decreased amygdala functional connectivity in adolescents with autism: a resting-state fMRI study. Psychiatry Res. Neuroimaging. 2016;257:47–56. doi: 10.1016/j.pscychresns.2016.10.005. [DOI] [PubMed] [Google Scholar]
- Hahamy A., Behrmann M., Malach R. The idiosyncratic brain: distortion of spontaneous connectivity patterns in autism spectrum disorder. Nat. Neurosci. 2015;18(2):302–309. doi: 10.1038/nn.3919. [DOI] [PubMed] [Google Scholar]
- Hare T.A., Tottenham N., Galvan A., Voss H.U., Glover G.H., Casey B.J. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol. Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri A.R., Bookheimer S.Y., Mazziotta J.C. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11(1):43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hariri A.R., Mattay V.S., Tessitore A., Fera F., Weinberger D.R. Neocortical modulation of the amygdala response to fearful stimuli. Biol. Psychiatry. 2003;53(6):494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Harms M.B., Martin A., Wallace G.L. Facial emotion recognition in autism spectrum disorders: a review of behavioral and neuroimaging studies. Neuropsychol. Rev. 2010;20(3):290–322. doi: 10.1007/s11065-010-9138-6. [DOI] [PubMed] [Google Scholar]
- Herrington J.D., Miller J.S., Pandey J., Schultz R.T. Anxiety and social deficits have distinct relationships with amygdala function in autism spectrum disorder. Soc. Cogn. Affect. Neurosci. 2016;11(6):907–914. doi: 10.1093/scan/nsw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hus V., Lord C. The autism diagnostic observation schedule, module 4: revised algorithm and standardized severity scores. J. Autism Dev. Disord. 2014;44(8):1996–2012. doi: 10.1007/s10803-014-2080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalbrzikowski M., Larsen B., Hallquist M.N., Foran W., Calabro F., Luna B. Development of white matter microstructure and intrinsic functional connectivity between the Amygdala and ventromedial prefrontal cortex: associations with anxiety and depression. Biol. Psychiatry. 2017;82(7):511–521. doi: 10.1016/j.biopsych.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H., Gutman D.A., Behrens T.E., Matthews P.M., Rushworth M.F., Katz E. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb. Cortex. 2008;18(6):1374–1383. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kelly A.M., Di Martino A., Uddin L.Q., Shehzad Z., Gee D.G., Reiss P.T. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb. Cortex. 2009;19(3):640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kim M.J., Loucks R.A., Palmer A.L., Brown A.C., Solomon K.M., Marchante A.N., Whalen P.J. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav. Brain Res. 2011;223(2):403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans N.M., Johnson L.C., Richards T., Mahurin R., Greenson J., Dawson G., Aylward E. Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. Am. J. Psychiatry. 2009;166(4):467–475. doi: 10.1176/appi.ajp.2008.07101681. [DOI] [PubMed] [Google Scholar]
- Kleinhans N.M., Richards T., Johnson L.C., Weaver K.E., Greenson J., Dawson G., Aylward E. fMRI evidence of neural abnormalities in the subcortical face processing system in ASD. Neuroimage. 2011;54(1):697–704. doi: 10.1016/j.neuroimage.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans N.M., Reiter M.A., Neuhaus E., Pauley G., Martin N., Dager S., Estes A. Subregional differences in intrinsic amygdala hyperconnectivity and hypoconnectivity in autism spectrum disorder. Autism Res. 2016;9(7):760–772. doi: 10.1002/aur.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A., Wu M., Klumpp H., Pine D.S., Swain J.E., Fitzgerald K.D. Altered development of amygdala-anterior cingulate cortex connectivity in anxious youth and young adults. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2016;1(4):345–352. doi: 10.1016/j.bpsc.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Rutter M., Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C., Risi S., Lambrecht L., Cook E.H., Leventhal B.L., DiLavore P.C. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C., Rutter M., DiLavore P., Risi S., Gotham K., Bishop S. Western Psychological Corporation; Los Angeles, CA: 2012. Autism Diagnostic Observation Schedule- 2nd Edition (ADOS-2) [Google Scholar]
- Macdonald H., Rutter M., Howlin P., Rios P., Le Conteur A., Evered C., Folstein S. Recognition and expression of emotional cues by autistic and normal adults. J. Child Psychol. Psychiatry. 1989;30(6):865–877. doi: 10.1111/j.1469-7610.1989.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Mackey S., Petrides M. Architecture and morphology of the human ventromedial prefrontal cortex. Eur. J. Neurosci. 2014;40(5):2777–2796. doi: 10.1111/ejn.12654. [DOI] [PubMed] [Google Scholar]
- McRae K., Gross J.J., Weber J., Robertson E.R., Sokol-Hessner P., Ray R.D. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Soc. Cogn. Affect. Neurosci. 2012;7(1):11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher C.P., Zimmerman P.E., Gothard K.M. Response characteristics of basolateral and centromedial neurons in the primate amygdala. J. Neurosci. 2010;30(48):16197–16207. doi: 10.1523/JNEUROSCI.3225-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K., Birn R.M., Handwerker D.A., Jones T.B., Bandettini P.A. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44(3):893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E.R., Barch D.M., Pagliaccio D., Luby J.L., Belden A.C. Functional connectivity of the amygdala and subgenual cingulate during cognitive reappraisal of emotions in children with MDD history is associated with rumination. Dev. Cogn. Neurosci. 2016;18:89–100. doi: 10.1016/j.dcn.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E.E., Leibenluft E., McClure E.B., Pine D.S. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol. Med. 2005;35(2):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Nomi J.S., Uddin L.Q. Face processing in autism spectrum disorders: from brain regions to brain networks. Neuropsychologia. 2015;71:201–216. doi: 10.1016/j.neuropsychologia.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomi J.S., Uddin L.Q. Developmental changes in large-scale network connectivity in autism. Neuroimage Clin. 2015;7:732–741. doi: 10.1016/j.nicl.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Ray R.D., Cooper J.C., Robertson E.R., Chopra S., Gabrieli J.D., Gross J.J. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23(2):483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Silvers J.A., Buhle J.T. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann. N. Y. Acad. Sci. 2012;1251:E1–24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pape H.C., Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol. Rev. 2010;90(2):419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey K.A., Morris J.P., McCarthy G., Labar K.S. Perception of dynamic changes in facial affect and identity in autism. Soc. Cogn. Affect. Neurosci. 2007;2(2):140–149. doi: 10.1093/scan/nsm010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey K.A., Shultz S., Hudac C.M., Vander Wyk B.C. Research review: constraining heterogeneity: the social brain and its development in autism spectrum disorder. J. Child Psychol. Psychiatry. 2011;52(6):631–644. doi: 10.1111/j.1469-7610.2010.02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S.B., Pelphrey K.A. Developing connections for affective regulation: age-related changes in emotional brain connectivity. J. Exp. Child Psychol. 2011;108(3):607–620. doi: 10.1016/j.jecp.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S.B., Hudac C.M., Pegors T., Minshew N.J., Pelphrey K.A. Experimental manipulation of face-evoked activity in the fusiform gyrus of individuals with autism. Soc. Neurosci. 2011;6(1):22–30. doi: 10.1080/17470911003683185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E.A., LeDoux J.E. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., O’Connor K.J., Gatenby J.C., Gore J.C., Grillon C., Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat. Neurosci. 2001;4(4):437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol. Psychiatry. 2003;54(5):504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Picci G., Scherf K.S. A two-hit model of autism: adolescence as the second hit. Clin. Psychol. Sci. 2015;3(3):349–371. doi: 10.1177/2167702614540646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K., Redcay E. Fusiform function in children with an autism spectrum disorder is a matter of "Who". Biol. Psychiatry. 2008;64(7):552–560. doi: 10.1016/j.biopsych.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K., Muller R.A., Ambrose J., Allen G., Courchesne E. Face processing occurs outside the fusiform’ face area’ in autism: evidence from functional MRI. Brain. 2001;124(Pt 10):2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Piggot J., Kwon H., Mobbs D., Blasey C., Lotspeich L., Menon V. Emotional attribution in high-functioning individuals with autistic spectrum disorder: a functional imaging study. J. Am. Acad. Child Adolesc. Psychiatry. 2004;43(4):473–480. doi: 10.1097/00004583-200404000-00014. [DOI] [PubMed] [Google Scholar]
- Pitskel N.B., Bolling D.Z., Hudac C.M., Lantz S.D., Minshew N.J., Vander Wyk B.C., Pelphrey K.A. Brain mechanisms for processing direct and averted gaze in individuals with autism. J. Autism Dev. Disord. 2011;41(12):1686–1693. doi: 10.1007/s10803-011-1197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Mitra A., Laumann T.O., Snyder A.Z., Schlaggar B.L., Petersen S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J.L. Comparative aspects of amygdala connectivity. Ann. N. Y. Acad. Sci. 2003;985:50–58. doi: 10.1111/j.1749-6632.2003.tb07070.x. [DOI] [PubMed] [Google Scholar]
- Qin S., Young C.B., Supekar K., Uddin L.Q., Menon V. Immature integration and segregation of emotion-related brain circuitry in young children. Proc. Natl. Acad. Sci. U. S. A. 2012;109(20):7941–7946. doi: 10.1073/pnas.1120408109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk G.J., Beer J.S. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr. Opin. Neurobiol. 2006;16(6):723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Rausch A., Zhang W., Haak K.V., Mennes M., Hermans E.J., van Oort E. Altered functional connectivity of the amygdaloid input nuclei in adolescents and young adults with autism spectrum disorder: a resting state fMRI study. Mol. Autism. 2016;7:13. doi: 10.1186/s13229-015-0060-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch A., Zhang W., Beckmann C.F., Buitelaar J.K., Groen W.B., Haak K.V. Connectivity-based parcellation of the amygdala predicts social skills in adolescents with autism spectrum disorder. J. Autism Dev. Disord. 2018;48(2):572–582. doi: 10.1007/s10803-017-3370-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad Z.S., Gotts S.J., Murphy K., Chen G., Jo H.J., Martin A., Cox R.W. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2(1):25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P., Faber E.S., Lopez De Armentia M., Power J. The amygdaloid complex: anatomy and physiology. Physiol. Rev. 2003;83(3):803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Satterthwaite T.D., Elliott M.A., Gerraty R.T., Ruparel K., Loughead J., Calkins M.E. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schölvinck M.L., Maier A., Ye F.Q., Duyn J.H., Leopold D.A. Neural basis of global resting-state fMRI activity. Proc. Natl. Acad. Sci. U. S. A. 2010;107(22):10238–10243. doi: 10.1073/pnas.0913110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R.T. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int. J. Dev. Neurosci. 2005;23(2–3):125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Schultz R.T., Gauthier I., Klin A., Fulbright R.K., Anderson A.W., Volkmar F. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch. Gen. Psychiatry. 2000;57(4):331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Shen M.D., Li D.D., Keown C.L., Lee A., Johnson R.T., Angkustsiri K. Functional connectivity of the amygdala is disrupted in preschool-aged children with autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2016;55(9):817–824. doi: 10.1016/j.jaac.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoff E., Pickles A., Charman T., Chandler S., Loucas T., Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47(8):921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Spezio M.L., Huang P.Y., Castelli F., Adolphs R. Amygdala damage impairs eye contact during conversations with real people. J. Neurosci. 2007;27(15):3994–3997. doi: 10.1523/JNEUROSCI.3789-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch E.A., Arnold E.B., Lewin A.B., Nadeau J.M., Jones A.M., De Nadai A.S. The effect of cognitive-behavioral therapy versus treatment as usual for anxiety in children with autism spectrum disorders: a randomized, controlled trial. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52(2):132–142 e132. doi: 10.1016/j.jaac.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Sukhodolsky D.G., Scahill L., Gadow K.D., Arnold L.E., Aman M.G., McDougle C.J. Parent-rated anxiety symptoms in children with pervasive developmental disorders: frequency and association with core autism symptoms and cognitive functioning. J. Abnorm. Child Psychol. 2008;36(1):117–128. doi: 10.1007/s10802-007-9165-9. [DOI] [PubMed] [Google Scholar]
- Swartz J.R., Carrasco M., Wiggins J.L., Thomason M.E., Monk C.S. Age-related changes in the structure and function of prefrontal cortex-amygdala circuitry in children and adolescents: a multi-modal imaging approach. Neuroimage. 2014;86:212–220. doi: 10.1016/j.neuroimage.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz J.R., Phan K.L., Angstadt M., Fitzgerald K.D., Monk C.S. Dynamic changes in amygdala activation and functional connectivity in children and adolescents with anxiety disorders. Dev. Psychopathol. 2014;26(4 Pt. 2):1305–1319. doi: 10.1017/S0954579414001047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze K.M., Wood J.J. Enhancing CBT for the treatment of autism Spectrum disorders and concurrent anxiety. Behav. Cogn. Psychol. 2008;36(Special Issue 4 (Anxiety of Childhood and Adolescence):403–409. [Google Scholar]
- Tottenham N., Hertzig M.E., Gillespie-Lynch K., Gilhooly T., Millner A.J., Casey B.J. Elevated amygdala response to faces and gaze aversion in autism spectrum disorder. Soc. Cogn. Affect. Neurosci. 2014;9(1):106–117. doi: 10.1093/scan/nst050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K.S., Ryali S., Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J. Neurosci. 2011;31(50):18578–18589. doi: 10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwal T., Kelly C., Eilbott J., Mayes L.C., Castellanos F.X. Inscapes: a movie paradigm to improve compliance in functional magnetic resonance imaging. Neuroimage. 2015;122:222–232. doi: 10.1016/j.neuroimage.2015.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M., Derks J.M., Hoogendam J.M., Hillegers M., Kahn R.S. Functional differences in emotion processing during adolescence and early adulthood. Neuroimage. 2014;91:70–76. doi: 10.1016/j.neuroimage.2014.01.035. [DOI] [PubMed] [Google Scholar]
- Völlm B.A., Taylor A.N., Richardson P., Corcoran R., Stirling J., McKie S. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. Neuroimage. 2006;29(1):90–98. doi: 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]
- von dem Hagen E.A., Stoyanova R.S., Baron-Cohen S., Calder A.J. Reduced functional connectivity within and between ‘social’ resting state networks in autism spectrum conditions. Soc. Cogn. Affect. Neurosci. 2013;8(6):694–701. doi: 10.1093/scan/nss053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A.T., Dapretto M., Hariri A.R., Sigman M., Bookheimer S.Y. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2004;43(4):481–490. doi: 10.1097/00004583-200404000-00015. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio, TX: 1999. Wechsler Abbreviated Scale of Intelligence. [Google Scholar]
- Welvaert M., Rosseel Y. On the definition of signal-to-noise ratio and contrast-to-noise ratio for FMRI data. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0077089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S.W., Oswald D., Ollendick T., Scahill L. Anxiety in children and adolescents with autism spectrum disorders. Clin. Psychol. Rev. 2009;29(3):216–229. doi: 10.1016/j.cpr.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C.W., Olafsson V., Tal O., Liu T.T. Anti-correlated networks, global signal regression, and the effects of caffeine in resting-state functional MRI. Neuroimage. 2012;63(1):356–364. doi: 10.1016/j.neuroimage.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J.J., Drahota A., Sze K., Har K., Chiu A., Langer D.A. Cognitive behavioral therapy for anxiety in children with autism spectrum disorders: a randomized, controlled trial. J. Child Psychol. Psychiatry. 2009;50(3):224–234. doi: 10.1111/j.1469-7610.2008.01948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Kujawa A., Lu L.H., Fitzgerald D.A., Klumpp H., Fitzgerald K.D. Age-related changes in amygdala-frontal connectivity during emotional face processing from childhood into young adulthood. Hum. Brain Mapp. 2016;37(5):1684–1695. doi: 10.1002/hbm.23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.G., Cheung B., Kelly C., Colcombe S., Craddock R.C., Di Martino A. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 2013;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald D.H. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res. Brain Res. Rev. 2003;41(1):88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- Zhang W., Schneider D.M., Belova M.A., Morrison S.E., Paton J.J., Salzman C.D. Functional circuits and anatomical distribution of response properties in the primate amygdala. J. Neurosci. 2013;33(2):722–733. doi: 10.1523/JNEUROSCI.2970-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurcher N.R., Donnelly N., Rogier O., Russo B., Hippolyte L., Hadwin J. It’s all in the eyes: subcortical and cortical activation during grotesqueness perception in autism. PLoS One. 2013;8(1) doi: 10.1371/journal.pone.0054313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.