Abstract
Trametinib is a MEK inhibitor approved both as a single agent and in combination with dabrafenib for the treatment of BRAF V600E or V600K mutated melanoma. It is a once-daily oral medication that was approved based on progression-free survival and overall survival advantage compared to chemotherapy. Most common side effects include rash, diarrhea, peripheral edema, and fatigue. When used in combination with dabrafenib, pyrexia and nausea are also common. Most side effects can be managed effectively with dose interruptions, supportive care, and/or dose reductions. Ongoing trials are investigating the use of targeted therapy in combination with immunotherapy for cutaneous melanoma and other malignancies. The treatment landscape for metastatic melanoma continues to evolve. However, targeted therapy with trametinib remains a fast-acting and efficacious option, particularly when used in combination with dabrafenib.
Melanoma accounts for just 1% of all skin cancers, but the majority of skin cancer deaths (American Cancer Society, 2018). The incidence of melanoma has increased significantly over the past 30 years, with a 3% increase per year between 2005 and 2014 among those age 50 and older (American Cancer Society, 2018). Fortunately, during this same time period, the 5-year overall survival rate increased as well (American Cancer Society, 2018). Several targeted therapies and immunotherapies have been approved by the US Food and Drug Administration (FDA) since 2011, including therapies targeting the MAP kinase pathway, anti–PD-1 antibodies, anti–CTLA-4 antibodies, and an oncolytic virus therapy. Although immunotherapy is now the mainstay of metastatic melanoma treatment, targeted therapies continue to have an important role. Approximately 50% of melanomas have activating mutations in serine/threonine-protein kinase B-Raf (BRAF), which is a constituent of the MAP kinase signal-transduction pathway and provides an actionable therapeutic target (Flaherty et al., 2012). The MEK inhibitor trametinib (Mekinist) was initially approved as a single agent and then as a combination therapy with dabrafenib (Tafinlar). Here we focus primarily on the mechanism of action, clinical trial data, adverse events, and patient management for single-agent trametinib.
INDICATION
In 2013, the FDA approved trametinib as a single agent for the treatment of BRAF V600E or BRAF V600K mutation–positive unresectable or metastatic melanoma. One year later, the FDA granted accelerated approval to trametinib plus dabrafenib (a BRAF inhibitor) for use in combination for the same indication. Presently, trametinib has both single-agent and combination-therapy approval in the metastatic setting; however, it is more commonly prescribed in combination due to improved efficacy. Of note, dabrafenib and trametinib are also approved in metastatic non–small cell lung cancer, anaplastic thyroid cancer, and most recently in 2018 for the adjuvant treatment of melanoma.
MECHANISM OF ACTION
The MAP kinase pathway regulates the proliferation and survival of tumor cells in many different cancers (Flaherty et al., 2012). The pathway progresses through the Ras/Raf/MEK/ERK kinases, providing multiple targetable mutations for cancer therapy. Activated BRAF phosphorylates and activates MEK, which then activates downstream targets. Trametinib is an orally available, reversible, selective inhibitor of MEK1/MEK2 activation and kinase activity (Kim et al., 2013). In vitro studies showed that trametinib decreases cell proliferation, causes G1 cell-cycle arrest, and induces apoptosis. However, MEK inhibitors suppress ERK signaling in both tumor and normal cells, and therefore on-target toxicities limit the doses that can be safely administered (Chapman, Solit, & Rosen, 2014).
ADMINISTRATION
Trametinib is administered orally as a 2-mg tablet once daily at the same time each day. Tablets are available in 2-mg or 0.5-mg strengths in the event that a patient requires a dose reduction. Medication can be ordered through specialty pharmacies and shipped directly to the patient’s home. It should be kept refrigerated and taken on an empty stomach at least 1 hour before or 2 hours after a meal (Novartis Pharmaceuticals Corporation, 2018). Keeping medication refrigerated can be a challenge for patients who travel, but small, insulated coolers can serve as a portable solution. Oral medication adherence can be improved through patient education, counseling, and support. The Oncology Nursing Society offers an Oral Adherence Toolkit for nurses and providers on their website that includes strategies and resources to facilitate adherence (Oncology Nursing Society, 2009).
CLINICAL STUDIES
The phase II clinical trial of trametinib consisted of two cohorts including patients with metastatic BRAF-mutant melanoma previously treated with a BRAF inhibitor (cohort A) vs. those treated with chemotherapy and/or immunotherapy (BRAF inhibitor–naive; cohort B; Kim et al., 2013). There was significant clinical activity in the BRAF inhibitor–naive cohort, with 25% of patients achieving at least a partial response and 51% of patients with stable disease. There were no objective responses in cohort A, indicating that sequential monotherapy was not effective in patients who had developed resistance to BRAF inhibitors (Kim et al., 2013).
In the phase III open-label trial, patients with metastatic melanoma with a BRAF V600E or BRAF V600K mutation were randomized to receive either trametinib at 2 mg orally once daily or chemotherapy (dacarbazine or paclitaxel) every 3 weeks. Median progression-free survival was 4.8 months in the trametinib group and 1.5 months in the chemotherapy group (Flaherty et al., 2012). At 6 months, the overall survival was 81% in the trametinib group and 67% in the chemotherapy group (with crossover). Trametinib was approved based on the documented progression-free and overall survival.
ONGOING RESEARCH AND FUTURE STUDIES
Ongoing clinical trials seek to investigate the use of targeted therapies such as trametinib in combination with immunotherapies, including anti–PD-1 antibodies and anti–CTLA-4 antibodies. The challenges seen with these combination trials have included a significant increase in the rates and severity of known toxicities. Trametinib has also shown unique activity in uveal melanoma and is under investigation in a study of trametinib alone vs. in combination with GSK2141795 (NCI #9445). Finally, trametinib is under investigation for use in other BRAF-mutant cancers, including colon cancer and multiple myeloma.
ADVERSE SIDE EFFECTS
In the phase III trial, at least 15% of patients reported adverse events. The most common side effects with single-agent trametinib include rash, diarrhea, peripheral edema, fatigue, and acneiform dermatitis. Decreased ejection fraction or ventricular dysfunction has been observed in approximately 7% of patients, with reports of grade 3 cardiac toxicities due to the drug. Ocular events have been reported in 9% of patients receiving trametinib, with the most prevalent symptom being blurred vision. Other observed ocular toxicities include chorioretinopathy, central serous retinopathy, and retinal vein occlusion (Infante et al., 2012). Dose interruptions due to adverse events occurred in 35% of patients, and dose reductions due to adverse events occurred in 27% of patients in the trametinib arm (Flaherty et al., 2012). It has been our experience that many toxicities are well managed with short dose interruptions, such as a 2- or 3-day hold. Dose reductions are also efficacious, but may require a new prescription for 0.5-mg tablets; this can cause a delay in therapy while waiting for prescription approval and delivery. Targeted symptom management, including doxycycline for acneiform dermatitis or compression stockings for peripheral edema, can improve tolerability (Table 1–Table 3).
Table 1.
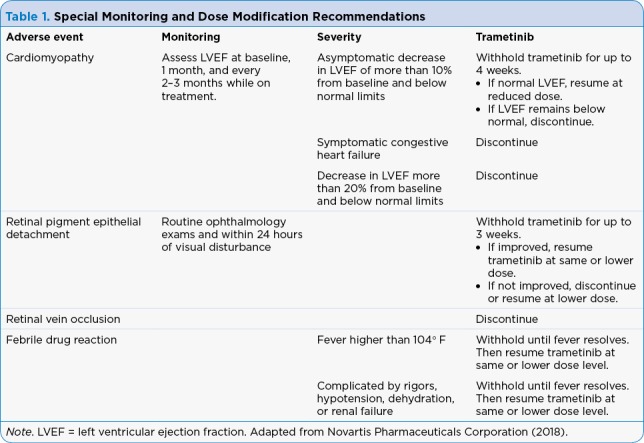
Special Monitoring and Dose Modification Recommendations
Table 2.
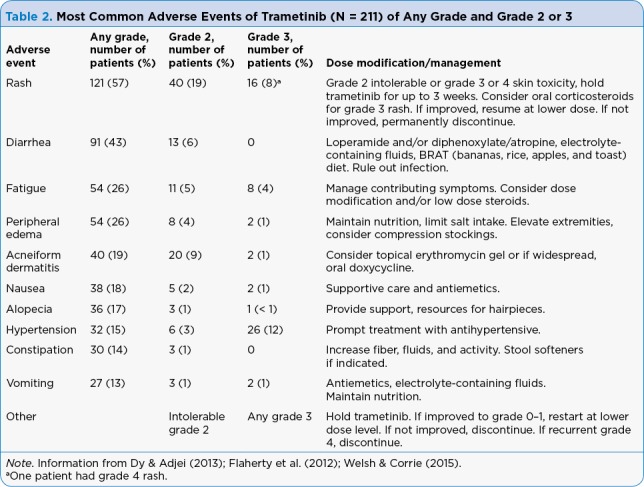
Most Common Adverse Events of Trametinib (N = 211) of Any Grade and Grade 2 or 3
Table 3.

Recommended Trametinib Dose Reductions for Toxicity (Single-Agent Trametinib)
Combination Dabrafenib and Trametinib Adverse Events
The combination of dabrafenib and trametinib compared to a single-agent BRAF inhibitor in randomized controlled trials for patients with BRAF-mutated metastatic melanoma demonstrated the improved efficacy of combination therapy. The most common side effects reported in the phase III open-label COMBI-v trial investigating dabrafenib plus trametinib vs. vemurafenib (Zelboraf) were pyrexia, nausea, diarrhea, chills, fatigue, headache, and vomiting. Permanent treatment discontinuation due to adverse events was similar in both groups: 13% in the combination group vs. 12% in the vemurafenib arm. Pyrexia and decreased ejection fraction were the most common reasons for permanent treatment discontinuation in the combination arm (Lugowska, Kosela-Paterczyk, Kozak, & Rutkowski, 2015). However, pyrexia can be managed with dose interruptions, reductions, nonsteroidal anti-inflammatory drugs (NSAIDs), oral corticosteroids, and supportive care (Dy & Adjei, 2013). Of note, there was a lower incidence of cutaneous squamous cell carcinoma and keratoacanthomas in the combination dabrafenib and trametinib group (1%) vs. the single-agent vemurafenib group (18%; Robert et al., 2015).
IMPLICATIONS FOR THE ADVANCED PRACTICE PROVIDER
Providers prescribing trametinib should be aware of the common and serious potential side effects of treatment and counsel patients on side-effect reporting and management. Pyrexia seen with trametinib can be particularly concerning for patients accustomed to neutropenic fever protocols during chemotherapy. Additional education regarding fever management and differentiation between drug fever and neutropenic fever can prevent unnecessary emergency room visits. For many patients, brief treatment interruptions for 24 to 48 hours result in the resolution of fevers. Consider low-dose prednisone (10 mg daily) for pyrexia refractory to treatment interruption and NSAIDs. Ocular complaints should be evaluated immediately as retinal vein occlusion, while rare, can occur and can result in permanent vision loss.
Trametinib can cause both lower extremity edema as well as cardiomyopathy, and these two adverse events are not necessarily related. If a patient presents with lower extremity edema, that does not definitively portend cardiac causes. Follow prescribing information recommendations for monitoring ejection fraction. Providers should keep in mind the significant proportion of dose interruptions or reductions in the phase III trial and not hesitate to hold the drug and potentially dose reduce for intolerable adverse events.
The average monthly cost of trametinib is approximately $10,000, and the manufacturer does offer a co-pay assistance program (us.tafinlarmekinist.com/advanced-melanoma/patient-support/cost-support). Specialty pharmacies are generally adept at assisting in the insurance approval process.
SUMMARY
Metastatic melanoma remains an aggressive and difficult malignancy to treat. Within the rapidly evolving treatment landscape, targeted therapy with trametinib is a fast-acting and efficacious treatment option, particularly when combined with dabrafenib. Side effects are tolerable with proper monitoring and management. Although immunotherapy has offered exciting advances in the durability of response and overall response, these therapies do not work immediately. Time to response can be crucial, particularly in patients with significant disease burden, and trametinib may induce rapid therapeutic responses.
Footnotes
Ms. Hoffner has served as a consultant for Bristol-Myers Squibb and Merck. Ms. Benchich has no conflicts of interest to disclose.
References
- 1.American Cancer Society. Atlanta, GA: American Cancer Society.; 2018. Cancer Facts and Figures 2018. Retrieved from https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2018.html. [Google Scholar]
- 2.Chapman Paul B, Solit David B, Rosen Neal. Combination of RAF and MEK inhibition for the treatment of BRAF-mutated melanoma: feedback is not encouraged. Cancer cell. 2014;26:603–604. doi: 10.1016/j.ccell.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Dy G K, Adjei A A. Understanding, recognizing, and managing toxicities of targeted anticancer therapies. CA: A Cancer Journal for Clinicians. 2013;63(4):249–279. doi: 10.3322/caa.21184. [DOI] [PubMed] [Google Scholar]
- 4.Flaherty Keith T, Robert Caroline, Hersey Peter, Nathan Paul, Garbe Claus, Milhem Mohammed, Demidov Lev V, Hassel Jessica C, Rutkowski Piotr, Mohr Peter, Dummer Reinhard, Trefzer Uwe, Larkin James M G, Utikal Jochen, Dreno Brigitte, Nyakas Marta, Middleton Mark R, Becker Jürgen C, Casey Michelle, Sherman Laurie J, Wu Frank S, Ouellet Daniele, Martin Anne-Marie, Patel Kiran, Schadendorf Dirk. Improved survival with MEK inhibition in BRAF-mutated melanoma. The New England journal of medicine. 2012;367:107–114. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 5.Infante Jeffrey R, Fecher Leslie A, Falchook Gerald S, Nallapareddy Sujatha, Gordon Michael S, Becerra Carlos, DeMarini Douglas J, Cox Donna S, Xu Yanmei, Morris Shannon R, Peddareddigari Vijay G R, Le Ngocdiep T, Hart Lowell, Bendell Johanna C, Eckhardt Gail, Kurzrock Razelle, Flaherty Keith, Burris Howard A, Messersmith Wells A. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. The Lancet. Oncology. 2012;13:773–781. doi: 10.1016/S1470-2045(12)70270-X. [DOI] [PubMed] [Google Scholar]
- 6.Kim Kevin B, Kefford Richard, Pavlick Anna C, Infante Jeffrey R, Ribas Antoni, Sosman Jeffrey A, Fecher Leslie A, Millward Michael, McArthur Grant A, Hwu Patrick, Gonzalez Rene, Ott Patrick A, Long Georgina V, Gardner Olivia S, Ouellet Daniele, Xu Yanmei, DeMarini Douglas J, Le Ngocdiep T, Patel Kiran, Lewis Karl D. Phase II study of the MEK1/MEK2 inhibitor Trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:482–489. doi: 10.1200/JCO.2012.43.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lugowska Iwona, Koseła-Paterczyk Hanna, Kozak Katarzyna, Rutkowski Piotr. Trametinib: a MEK inhibitor for management of metastatic melanoma. OncoTargets and therapy. 2015;8:2251–2259. doi: 10.2147/OTT.S72951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novartis Pharmaceuticals Corporation. Mekinist (trametinib) package insert. 2018 Retrieved from https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/mekinist.pdf.
- 9.Oncology Nursing Society. Tools for oral adherence toolkit. 2009 Retrieved from https://www.ons.org/sites/default/files/oral%20adherence%20toolkit.pdf.
- 10.Robert Caroline, Karaszewska Boguslawa, Schachter Jacob, Rutkowski Piotr, Mackiewicz Andrzej, Stroiakovski Daniil, Lichinitser Michael, Dummer Reinhard, Grange Florent, Mortier Laurent, Chiarion-Sileni Vanna, Drucis Kamil, Krajsova Ivana, Hauschild Axel, Lorigan Paul, Wolter Pascal, Long Georgina V, Flaherty Keith, Nathan Paul, Ribas Antoni, Martin Anne-Marie, Sun Peng, Crist Wendy, Legos Jeff, Rubin Stephen D, Little Shonda M, Schadendorf Dirk. Improved overall survival in melanoma with combined dabrafenib and trametinib. The New England journal of medicine. 2015;372:30–39. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 11.Welsh Sarah J, Corrie Pippa G. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Therapeutic advances in medical oncology. 2015;7:122–136. doi: 10.1177/1758834014566428. [DOI] [PMC free article] [PubMed] [Google Scholar]


