Abstract
Osteoclasts (OC) are large, multinucleated bone resorbing cells originating from the bone marrow myeloid lineage, and share a common progenitor with macrophages and dendritic cells. Bone marrow cells (BMC) are a common source for in vitro osteoclastogenesis assays but are a highly heterogeneous mixture of cells. Protocols for in vitro osteoclastogenesis vary considerably thus hindering interpretation and comparison of results between studies. Macrophage colony-stimulation factor (M-CSF) pretreatment is commonly used to expand OC progenitors (OCP) in BMC cultures prior to in vitro differentiation. However, failure of osteoclastogenesis of M-CSF primed bone marrow myeloid blasts has been reported. In this study, we used a simple method of differential adherence to plastic to enrich OCP from mouse BMCs. We found that M-CSF pretreatment of plastic adherent BMCs (adBMC) increased the number of CD11b−F4/80+ macrophages and decreased the number of CD11b+ monocytes resulting in decreased OC formation. M-CSF pretreatment of purified c-Kit+ progenitors weakly inhibited OC formation, whereas M-CSF pretreatment of purified c-Kit-CD11b+ progenitors promoted formation of large OC. M-CSF pretreatment increased the proliferation of both purified c-Kit+ and c-Kit-CD11b+ cells, and increased the percentage of CD11b−F4/80+ cells from c-Kit+ progenitors. In addition, M-CSF pretreatment increased the percentage of CD11b+F4/80− cells from purified c-Kit-CD11b+ cells. M-CSF pretreatment increased the percentage of CD14+CD16+ intermediate monocytes and subsequent OC formation from human 2adBMCs, and increased OC formation of purified CD14+ cells. Together, these results indicate that in vitro OCP expansion in the presence of M-CSF and bone marrow stromal cells is dependent upon the developmental stage of myeloid cells, in which M-CSF favors macrophage differentiation of multi-potent progenitors, promotes monocyte maturation and supports differentiation of late stage OC progenitor cells.
Keywords: M-CSF, osteoclastogenesis, osteoclast progenitor, c-Kit, CD11b, CD14, CD16
INTRODUCTION
Osteoclasts (OC) are large, multinucleated, bone-resorbing cells that originate from the bone marrow myeloid/monocytes lineage. OCs play important roles in bone modeling, remodeling and homeostasis (Teitelbaum & Ross, 2003). As part of the immune system, the activity of OC also regulates immune cell development in bone marrow (Mansour et al., 2011). Dysregulation of OC differentiation and activation is associated with abnormal bone metabolism and remodeling, and contributes to bone diseases (Feng & McDonald, 2011; Teti et al., 1999). In vitro OC differentiation assays have not only advanced our understanding of the biology of OC, but also been a useful tool for developing treatments for metabolic bone diseases such as osteoporosis (Susa, Luong-Nguyen, Cappellen, Zamurovic, & Gamse, 2004).
M-CSF and RANKL are two essential factors supplied by bone marrow stromal/osteoblastic cells for OC differentiation and maturation both in vitro and in vivo (David et al., 1998; Suda, Jimi, Nakamura, & Takahashi, 1997; Takahashi, Udagawa, & Suda, 1999; Yasuda et al., 1998). Studies in the mouse have demonstrated that OCs, macrophages, and dendritic cells (DCs) share a common progenitor (Arai et al., 1999; Jacome-Galarza, Lee, Lorenzo, & Aguila, 2013; Miyamoto et al., 2001; Xiao et al., 2017). Multiple cell types including cells of the myeloid/monocyte lineage from bone marrow, spleen and peripheral blood, have been shown to form OC when treated with M-CSF and RANKL. A variety of in vitro OC differentiation methods exist with varying results across different protocols, reagents, and sources of OC progenitor cells (OCP), thus hindering interpretation and comparison of results between protocols. M-CSF, a cytokine involved in myeloid/macrophage differentiation, proliferation and survival, is commonly used to generate and expand OCP prior to in vitro OC differentiation (Kobayashi et al., 2000; Takeshita, Kaji, & Kudo, 2000). However, failure of OC differentiation by M-CSF primed bone marrow myeloid blasts has also been observed (De Vries et al., 2015), suggesting more study is needed on the role of M-CSF on OC differentiation.
In the present study, we used a simple sequential plastic adherence method to enrich OCP from mouse bone marrow cells (BMC), and tested the effect of M-CSF pretreatment on OC differentiation potential. The results show that both overnight plastic adherent mouse bone marrow cells (1adBMC) and a subsequent population of adherent cells derived from the overnight non-adherent cells (2adBMC) are enriched with OCP. Pretreatment with M-CSF markedly inhibited both 1adBMC and 2adBMC OC differentiation, while increasing expression of CD68 and F4/80 macrophage markers and the percentage of CD11b−F4/80+ cells. This effect was partially attributed to the presence of bone marrow stromal cells (BMSC) since M-CSF had no inhibitory effect on OC differentiation of purified c-Kit-CD11b+ OCP, and only mildly inhibited purified c-Kit+ early progenitor OC differentiation. Furthermore, incubation of human 2adBMCs with M-CSF increased the percentage of CD34-CD14+ monocytes, particularly the CD14+CD16+ intermediate monocytes, but did not change the percentage of CD14-CD16+ non-classical monocytes. M-CSF pretreatment also promoted OC formation of purified CD14+ cells. Together, these results show that M-CSF promotes the differentiation of myeloid progenitors towards the macrophage lineage at the expense of CD11b+ monocyte and OC differentiation in the presence of bone marrow stromal cells. However, M-CSF enhances OC differentiation of purified c-Kit-CD11b+ progenitor cells. These results suggest that OC differentiation experiments using M-CSF pretreatment should be interpreted with caution depending upon the preparation of BMC used for OC differentiation.
Materials and Methods
Bone marrow cell collection and culture
C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and housed in the Maine Medical Center Research Institute (MMCRI) AAALAC accredited animal facility. Bone marrow cells (BMCs) were collected from 8- to 10-weeks old female or male mice, and pooled for testing. Briefly, mice were euthanized by isoflurane over-dose followed by cervical dislocation. Femurs and tibias were isolated and the ends were trimmed off. BMCs were collected by centrifugation of bones at 13,000rpm for 30 s in 1.5 ml microcentrifuge tubes (USA Scientific), followed by depletion of red blood cell (RBC) with RBC lysis buffer (Sigma-Aldrich, MO). Cells were cultured in α-MEM (Corning) containing 1× GlutaMax (Gibco), 10% of fetal bovine serum (FBS) (Atlantic Biologics) and 1× antimycotic-antibiotic mixture (Gibco). Fourteen to eighteen hours later, non-adherent cells were collected and replated onto 10cm tissue culture dishes. The first overnight adherent cells were replenished with fresh medium. Three days later, adherent cells (1adBMC from overnight culture, and 2adBMC from overnight non-adherent cells) were collected using trypsin or Accutase (Millipore), and counted for OC differentiation, FACS analysis, or magnetic beads cell purification procedures. The Institutional Animal Care and Use Committee of MMCRI approved all protocols involving animals.
Human bone marrow cells isolation
Human bone marrow cells were isolated from discard bony pieces from patients (age from 48 to 81-years old) undergoing hip replacement, and collection of this discarded surgical material was approved as non-human subjects research by the Maine Medical Center Institutional Review Board. Bone marrow cells were extracted by briefly vortexing bony pieces in Hank’s balanced salt solution (HBSS), and pelleted by centrifugation at 1200rpm for 5 min at RT. Red blood cells were lysed with RBC lysis buffer. Mononuclear cells were filtered through 40μM cell strainer and sequentially cultured in 10% FBS-α-MEM growth medium similar to mouse bone marrow cells.
Osteoclast cell differentiation
For mouse OC differentiation, sequential plastic adherent BMCs were digested with trypsin, counted, seeded in 96-well plates at the density of 5×105 cells/well, and cultured in OC differentiation medium (α-MEM containing, 10% FBS, 10ng/ml M-CSF (PeproTech) and 10ng/ml RANKL (AdventBio)) for 2 to 5 days unless otherwise specified.
Magnetic bead cell purification and Fluorescence-activated cell sorting (FACS) analysis
Adherent BMCs were digested with Accutase (Millipore), suspended in growth medium, pelleted by centrifugation at 1200 rpm for 5 min. Cells were resuspended in 0.1% BSA-PBS at a concentration 1×108 cells/ml for magnetic bead separation using EasySep kits (Stem Cell Technology) by labeling cells with APC or FITC -anti-mouse c-Kit, PE -anti-mouse CD11b, or APC -anti-human CD14 (Biolegend). For FACS analysis, 1×106 cells were labeled with APC-anti-mouse CD11b, APC/Cy7-anti-mouse F4/80, PE-anti-mouse-RANK (CD256), BrillantViolet421-anti-mouse CD115, PE-anti-human CD33, APC-anti-human CD34, FITC-anti-human CD14, BrillantViolet421-anti-human CD16, and analyzed by MACQuant Analyzer (Miltenyi Biotec).
Tartrate-resistant acid phosphatase (TRAP) staining
OC differentiation was assessed using a tartrate-resistant acid phosphatase (TRAP) staining kit (Sigma-Aldrich) according to manufacturer’s instructions. Cells were washed with PBS, and fixed with 2.5 % paraformaldehyde in PBS for 15 minutes, stained for 1 hour at 37°C, and then washed with PBS. OC were scored by positive staining for TRAP and by a multi-nuclear appearance (3 or more nuclei per cell).
RNA extraction and reverse transcriptase Quantitative PCR
Total RNA was extracted from BMC using RNeasy Plus (Qiagen). The purity and concentration of total RNA were measured with a NanoDrop Spectrophotometer (NanoDrop Technologies) at 260nm/280nm. The ratios of 260nm/280nm of all samples were between 1.8 and 2.0. ProtoScript M-MuLV first Strand cDNA Synthesis kit (New England Biolabs) was used to generate cDNA. Quantitative real-time PCR (qPCR) of target genes was performed using SYBR Green (BioRad) on an IQ5 Multicolor Real-Time PCR Detection System (BioRad) according to the manufacturer’s instructions. GAPDH was used as an internal reference in each reaction. Melting curve analyses using the program run in the step acquisition mode was used to verify the presence of a single amplification production. Primers for qPCR were obtained from Integrated DNA Technologies and had the following sequences: mPU.1: TCCAGCCTTACTGGACTACCA (forward), ATCCGATGGCTCTCGTTCCT (reverse); mCD11b: CTGAGACTGGAGGCAACCAT (forward), CAGGGTCTAAAGCCAGGTCA (reverse); mCD68: TCAGCTAAACTCCAATC (forward), TCCAGCCTGTTGTAACTGAG (reverse); mF4/80: CTTTGGCTATGGGCTTCCAGT (forward), GCAAGGAGGACAGAGTTTATCGTG (reverse); mGADPH: ACACATTGGGGGATGGAACA (forward), AACTTTGGCATTGTGGAAGG (reverse).
Statistics analysis
RT-qPCR, FACS analysis and TRAP+ quantification results are expressed as means from replicate experiments, and are verified by at least three independent experiments. Error bars represent the standard deviation. Comparisons between two groups were performed by Student’s t test. For multiple comparisons, Student’s t test in conjunction with ANOVA analysis was carried out (Graph Pad, Prism). P values ≤ 0.05 were considered statistically significant.
RESULTS
M-CSF pretreatment inhibits osteoclast differentiation of plastic adherent mouse bone marrow cells
Bone marrow is comprised of multiple cell types and is a major source of progenitor cells for OC differentiation assays. To investigate ways of optimizing OC differentiation assays, we used a simple strategy to separate mouse BMCs based on differential adherence to plastic. This included an overnight adherent population (adherent cells after 14-18 hours culture, hereafter reference as 1adBMC) and the non-adherent cells from this step were replated and cultured for three days (hereafter referred to as 2adBMC) (Figure 1A). We then tested the OC differentiation potential of these two populations. Both 1adBMC and 2adBMC were able to reproducibly differentiate into multinucleated TRAP positive OC within 3-5 days by induction with medium containing 10ng/ml of RANKL and 10ng/ml of M-CSF (Figure 1B, and data not shown), indicating that adherent BMC contain OC progenitor cells.
Figure 1. M-CSF pretreatment decreases mouse bone marrow cells OC differentiation.
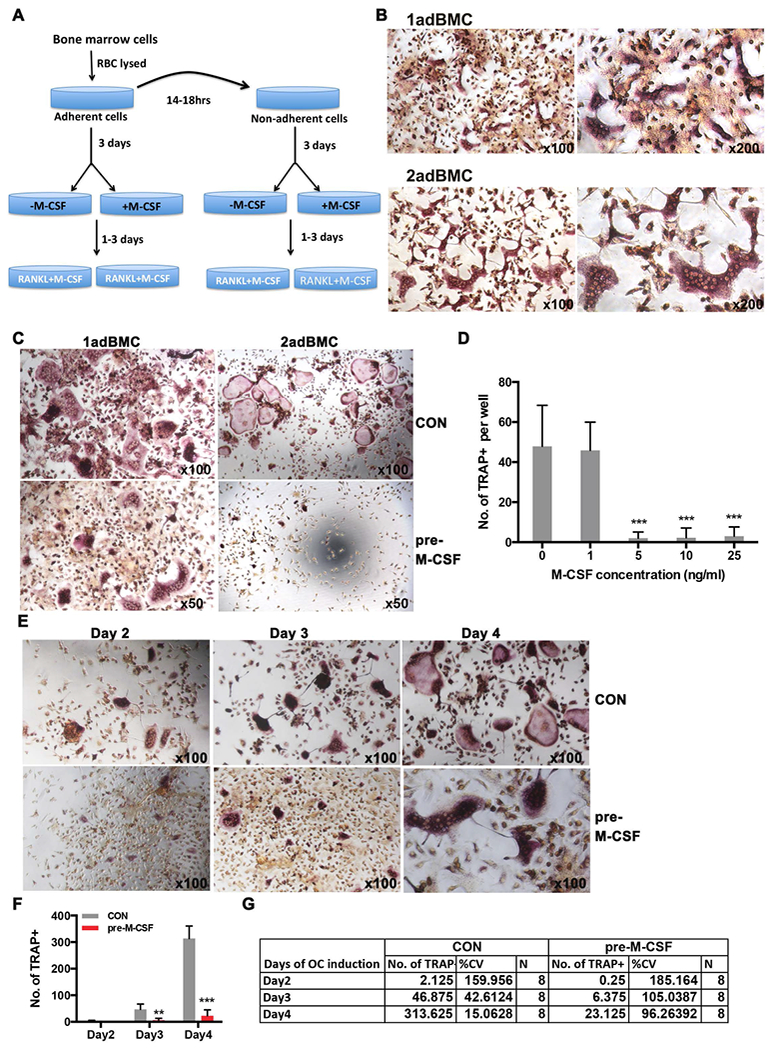
A) A schematic diagram of the OC differentiation protocol using mouse bone marrow cells (BMC). BMC were isolated from mouse femurs, red blood cells lysed and the total population adhered to plastic cell culture dishes for 14-18h (1adBMC). After 18 h the non-adherent cells were removed and replated onto plastic cell culture dishes (2adBMC). After three days, 1adBMC and 2adBMC were treated with or without M-CSF for 3 days, followed by addition of OC differentiation medium (M-CSF and RANKL).
B) TRAP staining shows that plastic adBMC can be effectively induced into multinuclear TRAP+ OC with M-CSF and RANKL, particularly the slow adherent cells (2adBMC) in the absence of M-CSF pretreatment. C). M-CSF pretreatment markedly decreased OC formation of 1adBMC and 2adBMC. D). Dose response of M-CSF pretreatment shows that pretreatment with as little as 5 ng/ml M-CSF was sufficient to inhibit OC formation of 2adBMC. E). Representative images of TRAP stained OC from a time course analysis of M-CSF pre-treatment shows M-CSF inhibited OC differentiation of 1adBMC. F). Quantification from time course analysis in E. G). Statistical analysis shows that M-CSF pre-treatment resulted in higher variation in the experiments from E. Note: **: p<0.01; ***: p<0.001.
To test the effect of M-CSF pretreatment on the differentiation potential of BMC into OC, 1adBMC and 2adBMC were treated with 10ng/ml of M-CSF for 3 days prior to induction OC differentiation. We observed that M-CSF pretreatment significantly increased the number of cells in both populations, however addition of OC differentiation medium induced significant cell death in M-CSF pretreated cells compared to controls (data not shown). Multinucleated TRAP+ OC was reduced in both M-CSF pretreated 1adBMC and 2adBMC compared to controls that did not receive M-CSF pretreatment (Figure 1C). Pretreatment of 1adBMC and 2adBMC with M-CSF for 3 days resulted in fewer OC compared to untreated controls, and this effect was dose dependent (Figure 1C and 1D, and data not shown). Pretreatment of 1adBMC with M-CSF reduced the formation of multinucleated TRAP+ OC in a time dependent manner as well (Figure 1E and F). In addition, the M-CSF pretreated groups had a higher coefficient of variation (%CV) compared to those of controls (Figure 1G).
Bone marrow stromal cells contribute to the inhibitory effect of M-CSF on OC differentiation
Bone marrow stromal cells (BMSC) have supportive roles in hematopoiesis and exert immunoregulatory activities (Alkhouli et al., 2013; Uccelli, Moretta, & Pistoia, 2008). FACS analysis showed that both 1adBMC and 2adBMC contain a small fraction of c-Kit+ hematopoietic progenitors and a moderate number of CD11b+ myeloid/monocytes (Figure 2A, and data not shown). M-CSF pretreatment of 1adBMC for 24 hours resulted in a decrease in the percentage of CD11b+ myeloid/monocytes (Figure 2A and B). To determine whether BMSC contribute to the inhibitory effect of M-CSF pretreatment on OC formation of adBMC, we sequentially purified c-Kit+ hematopoietic progenitors and c-Kit-CD11b+ myeloid progenitors using APC-anti-c-Kit and PE-anti-CD11b respectively. FACS analysis demonstrated that the purity of both c-Kit+ and c-Kit-CD11b+ cell fractions was greater than 90% (Figure 2C). MTT analyses showed that M-CSF treatment stimulates the proliferation of both purified c-Kit+ and c-Kit-CD11b+ cells (Figure 2D). Pretreatment of purified c-Kit+ cells with M-CSF for 24 hours prior to the addition of 10 ng/ml RANKL, reduced the number of TRAP+ OC, whereas pretreatment of purified c-Kit-/CD11b+ cells with M-CSF for 24 hours had little effect on differentiation of TRAP+ OC, although c-Kit-CD11b+ cells pretreated with M-CSF seemed to produced larger OC (Figure 2E and G). These data suggest that c-Kit-CD11b− stromal cells have an inhibitory effect on OC differentiation of c-Kit+ and/or c-Kit-CD11b+ cells when pretreated with M-CSF.
Figure 2. Bone marrow stromal cells contribute to inhibitory effect of M-CSF on bone marrow cell OC differentiation.
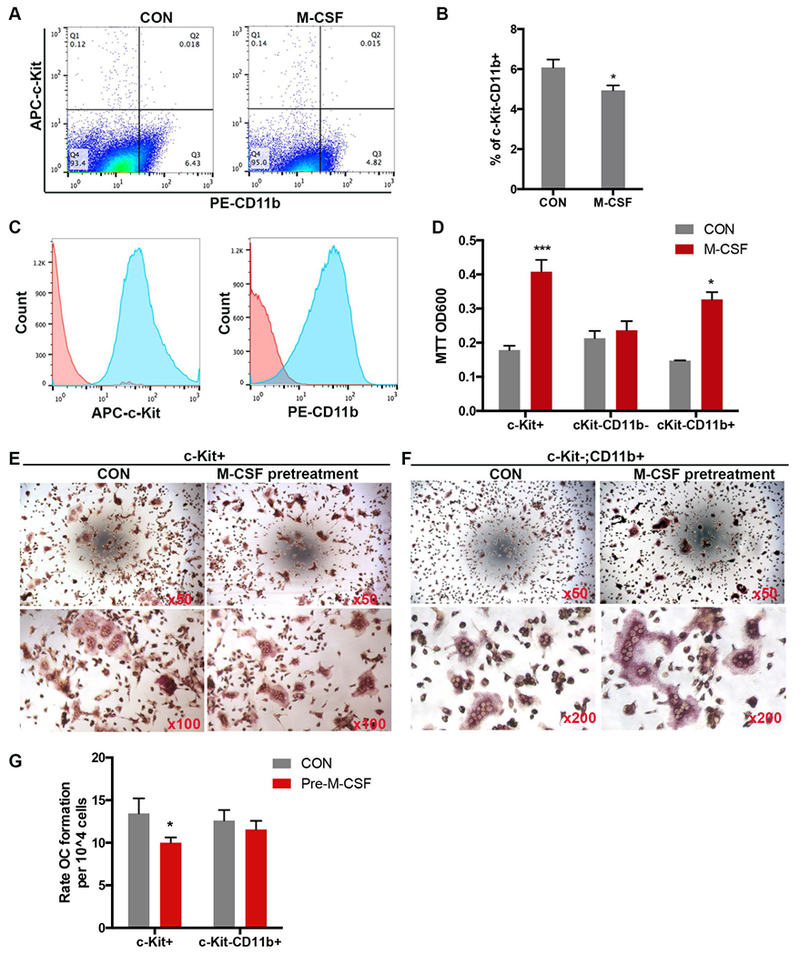
A). Representative FACS analysis shows that M-CSF pretreatment decreased the percentage of c-Kit-CD11b+ cell in 1adBMC. B) Quantification of the percentage of c-Kit−CD11b+ from 1adBMC treated with or without 10ng/ml M-CSF for 24 h. C). FACS analysis showing the purity of APC-c-Kit+ and PE-CD11b+ magnetic bead-purified cells. D). MTT assay shows M-CSF stimulated c-Kit+ and c-Kit-CD11b+ cell proliferation. E and F). Representative TRAP staining images from c-Kit+ or c-Kit-CD11b+ cells subjected to M-CSF pretreatment and then OC differentiation. G). Quantification of multinuclear TRAP+ cells from E, F. Note: *: p<0.05; ***: p<0.001.
M-CSF promotes macrophage differentiation of c-Kit+ early progenitors
Myeloid progenitors can be differentiated into OC, macrophages or dendritic cells depending on which cytokines they are exposed to, and M-CSF plays an important role in macrophage development. We next examined the affect of M-CSF on the macrophage differentiation of adBMC. Because 1adBMC and 2adBMC both have similar responses to M-CSF, we cultured BMC for 3 days in growth medium, and then replaced with the growth medium with or without 10 ng/ml M-CSF for 24 hours. FACS analyses show that M-CSF treatment for 24 h significantly increased the percentage of CD11b−F4/80+ macrophage-like cells (Figure 3A, B). Sequential magnetic bead purification with APC-anti-c-Kit and PE-anti-CD11b showed that M-CSF treatment of c-Kit+ early progenitors for 24 hours results in a significant increase in the expression of PU.1, CD11b, CD68 and F4/80 mRNA indicating that M-CSF drives c-Kit+ cells toward the macrophage lineage (Figure 3C). FACS analysis showed that M-CSF treatment of c-Kit+ cells increased the percentage of CD11b−F4/80+ cells and decreased the percentage of CD11b+F4/80− cells (Figure 3D). In contrast, M-CSF treatment had no effect on CD68 and F4/80 mRNA expression but increased CD11b mRNA level in c-Kit-CD11b+ cells (Figure 3B). FACS analyses also showed a higher proportion of F4/80−CD11b+ myeloid/monocytes and a lower proportion of F4/80+CD11b− cells in M-CSF treated c-Kit-CD11b+ cells compared to controls (Figure 3F).
Figure 3. M-CSF promotes c-Kit+ early progenitors towards macrophage differentiation.
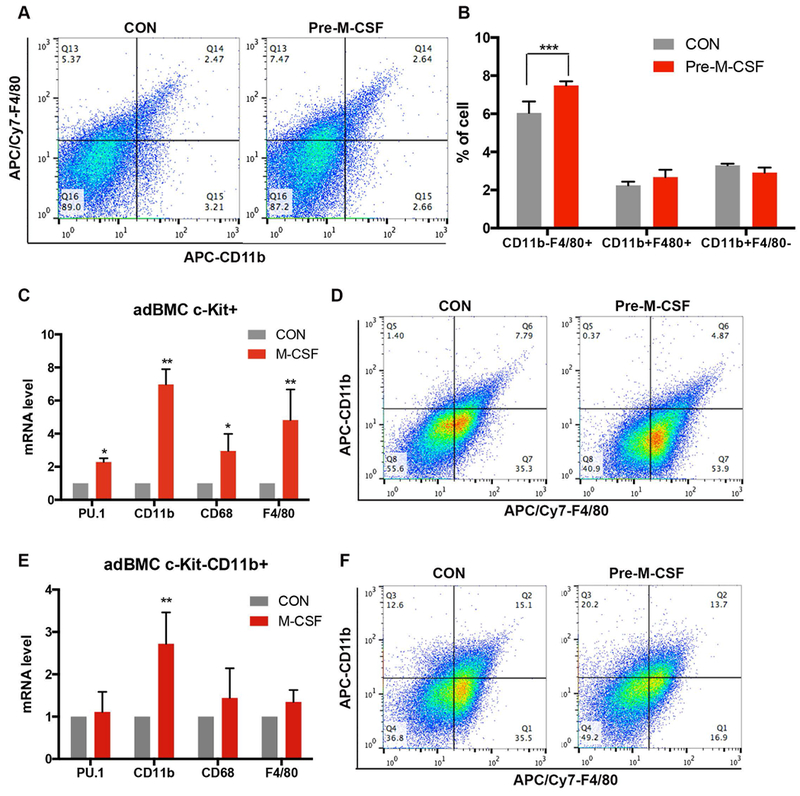
A). Representative FACS analysis shows that M-CSF pretreatment of combined mouse 1adBMC and 2adBMC altered the distribution of F4/80+ CD11− and F4/80−CD11b+ monocyte populations. B). Quantification from A shows M-CSF pretreatment significantly increased CD11 b-F4/80+ macrophages in combined mouse 1adBMC and 2adBMC cultures. C). RT-qPCR analyses show that M-CSF pretreatment of c-Kit+ cells significantly increased the expression of PU.1, CD11b, CD68, F4/80. D). Representative FACS analysis diagrams to show M-CSF pre-treatment of beads separated c-Kit+ cells increased CD11b−F4/80+, decreased CD11b+F4/80− and CD11b+F4/80+ cells population. E). RT-qPCR analyses show that M-CSF pretreatment of c-Kit-CD11b+ cells increased CD11b expression, but has no effect on PU.1, CD11b, CD68, F4/80 expression. F). Representative FACS analysis to show M-CSF pre-treatment of beads separated c-Kit-CD11b+ cells increased CD11b+F4/80−, but decreased CD11b−F4/80+ cells population. Note: *: p<0.05; **: p<0.01; ***: p<0.001.
M-CSF pretreatment promotes the development of human CD14+CD16+ intermediate monocytes and OC differentiation
Next we sought to examine whether M-CSF pretreatment had an effect on human BMCs similar to mouse BMCs. The majority human 1adBMC (overnight adherent cells) from discard hip replacement specimens were fibroblast-like stromal cells with little OC differentiation potential (data not shown), whereas the 2adBMC were enriched with OCPs. Therefore, we analyzed the response of 2adBMC to M-CSF. Human BMC were cultured in growth medium for 16-18 hours and the non-adherent cells were collected and cultured with or without 15 ng/ml of M-CSF. After 3 days, the 2adBMC treated with or without M-CSF were collected for FACS analysis to determine cellular composition using CD34 (hematopoietic progenitor), CD33 (myeloid/monocyte), CD14 and CD16 (monocyte) surface markers. The results showed that the 2adBMC contained few CD34+ progenitors (data not shown) as well as CD33+ early myeloid/monocytes (Figure 4A). M-CSF pretreatment for 3 days markedly increased the percentage of CD14+CD33− (Figure 4A). CD14 and CD16 are markers of monocytes and have been used to identify OCP in human bone marrow cells, although this remains controversial (Chiu et al., 2010; Komano, Nanki, Hayashida, Taniguchi, & Miyasaka, 2006). FACS analysis showed that M-CSF treatment markedly increased the percentage of this intermediate CD14+CD16+ monocyte subset, and decreased the percentage of the non-classical CD14-CD16+ monocyte subset, but had little effect on the classical CD14+CD16− subset (Figure 4B).
Figure 4. M-CSF promotes human bone marrow cells CD14+CD16+ development and OC differentiation.
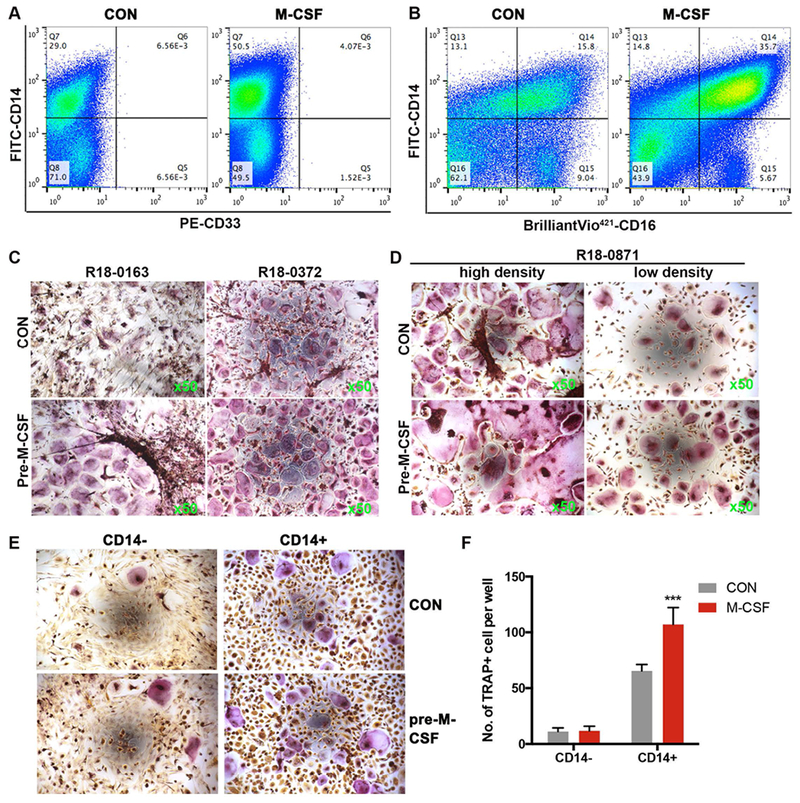
A) Representative FACS diagrams show that the number of CD33+ cells is low in human 2adBMC. M-CSF pretreatment increased the percentage of CD14+ cells in 2adBMC. B) FACS analyses of monocyte subsets based on CD14 and CD16 expression show M-CSF pretreatment of human 2adBMC markedly increased the percentage of CD14+CD16+ intermediate monocyte, but decreased the percentage of CD14−CD16+ non-classical monocytes. C) Representative TRAP stained images show M-CSF pretreatment increased OC differentiation of human 2adBMC. D) Representative TRAP stained images show that the effect of M-CSF pretreatment on human bone marrow cells OC differentiation is not cell density-independent. E) Representative images show that CD14+ but not CD14− 2adBMC efficiently form TRAP+ OCs, and M-CSF pretreatment increased CD14+ cell OC differentiation. F) Quantification of multinuclear TRAP+ cells from F. Note: ***: p<0.001.
Next, we examine the effect of M-CSF on human BMC OC differentiation. Overnight non-adherent human BMCs were cultured with or without 15 ng/ml of M-CSF. Three days later, the 2adBMC were subjected for OC differentiation. M-CSF pretreatment promoted human 2adBMC OC differentiation in all tested samples (12 samples) (Figure 4C, and data not shown), and this effect was cell density-independent (Figure 4D). We then tested the OC differentiation potential of magnetic bead purified CD14+ or CD16+ 2adBMC. We found that both purified CD14+ and CD16+ cells can be efficiently differentiated into OC within 10 days by 10 ng/ml RANKL and 10 ng/ml M-CSF (data not shown). Because more than half of the CD14+ cells are also CD16+, and because M-CSF treatment increased the CD14+CD16+ cell population (Figure 4B), and because CD14+ and CD16+ cells have OC forming potential, we tested the effect of M-CSF pretreatment on OC differentiation of purified CD14+ cells. Our results show that M-CSF pretreatment of CD14+ cells resulted in significant OC differentiation, whereas pretreatment CD14− cells with M-CSF produced very few TRAP+ OC (Figure 4E-F).
Discussion
In vitro osteoclastogenesis is a convenient way of studying OC biology and for developing and screening therapeutic drugs for OC related bone diseases (Susa et al., 2004). Inconsistency in OC differentiation exists across different protocols, reagents, and even among replicates within a single experiment, thus hindering the interpretation of results (Marino, Logan, Mellis, & Capulli, 2014). Bone marrow is a common source of progenitors for OC assays, but is heterogeneous in its cellular composition. M-CSF is commonly used to expand OCPs from this mixed population of cells. However, M-CSF priming has been shown to cause complete loss of OC differentiation of osteoclast precursors (De Vries et al., 2015). M-CSF is a cytokine that has an important role in macrophage development. Therefore, it is important to evaluate the effect of M-CSF pretreatment on the differentiation potential of OCPs from bone marrow in order to improve and standardize OC differentiation assays using bone marrow as a source of OC progenitors.
Numerous protocols for culturing osteoclasts have been described in the literature with many variations. In some protocols, overnight adherent bone marrow cells were used for OCs differentiation, in other protocols overnight non-adherent bone marrow cells were used for OC differentiation (Cody et al., 2011; Dai et al., 2018; Nakamura et al., 2007; Takeshita et al., 2000). Our observations show that both overnight adherent and non-adherent mouse BMCs contain OCPs, however M-CSF pretreatment did not expand OCPs in the presence of BMSC, but instead promoted macrophage development. In contrast, the majority of overnight plastic adherent human BMCs are fibroblast-like stromal cells with no OC differentiation potential, the overnight non-adherent cells slowly adhered to plastic plates and M-CSF promoted their adherence and subsequent OC differentiation. The difference in M-CSF pretreatment on mouse adBMC (including 1adBMC and 2adBMC) and on human 2adBMC is likely due to less contamination by bone marrow stromal cells of human 2adBMC than mouse adBMC. Whether this phenomenon is due to the source of human bone marrow cells (older adults with osteoarthritis) remains to be determined.
OC, macrophages and dendritic cells share common myeloid progenitors (Jacome-Galarza et al., 2013; Miyamoto et al., 2001; Wiktor-Jedrzejczak et al., 1990). OC and macrophages depend on M-CSF for their proliferation and differentiation. Mice deficient in M-CSF (op/op mice) show a complex phenotype that involves severe osteopetrosis due to defects in OC development and are deficient in macrophages. However, the lineage relationship between macrophages and OC has not yet been firmly established. OCs develop through a sequential process beginning with c-Kit+ hematopoietic progenitors, c-kit+CD11b+ cells then to c-Kit-CD11b+ cells and then to OC (Takeshita et al., 2000). Takeshita and colleagues reported that high doses of M-CSF induced proliferation of bone marrow monocytes and these cells differentiated into OC in the presence of M-CSF and soluble RANKL (sRANKL). FACS analysis of this bone marrow monocyte population showed that Mac-1-F4/80dull cells had the greatest OC differentiation potential, whereas Mac-1-F4/80+ cells had the lowest OC differentiation potential (Takeshita et al., 2000). Similarly, De Vries et al., reported that M-CSF priming of bone marrow myeloid precursors caused a loss of OC differentiation of myeloid blasts when cultured on plastic, and OC potential was restored when myeloid cells were cultured on bone in the presence of M-CSF and sRANKL (De Vries et al., 2015). In the present study, we show that M-CSF pretreatment of plastic adherent BMC promoted macrophage proliferation and differentiation and inhibited the OC differentiation. We also show that M-CSF pretreatment weakly inhibited OC formation of purified c-Kit+ progenitors, but increased expression of mRNA of macrophage markers CD68 and F4/80, and increased the number of CD11b−F4/80+ cells and decreased CD11b+F4/80− cells, which give rise to OC. Conversely, M-CSF pretreatment accelerated OC formation from more mature c-Kit-CD11b+ OCPs, and increased the expression of CD11b mRNA with little effect on the expression of other macrophage markers. These results suggest that M-CSF pretreatment regulates OC differentiation of OCP depending upon their stage of development (Figure 5).
Figure 5. Schematic of proposed effect of M-CSF on bone marrow OCP development and OC differentiation.
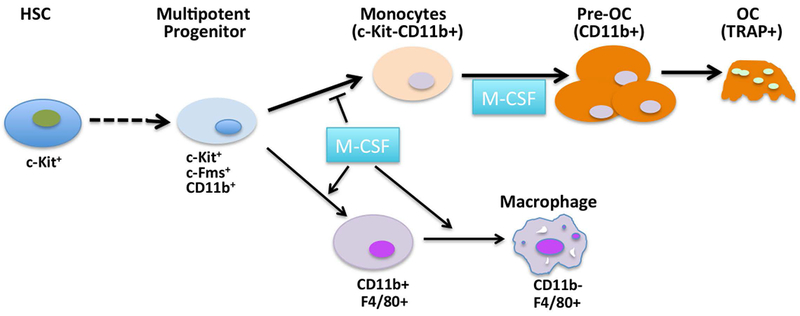
OC, share a common progenitor with macrophages, and develop sequentially from c-Kit+ cells via c-Kit+CD11b+ cells to c-Kit-CD11b+ cells and then to pre-OC. M-CSF promotes macrophage development of early myeloid progenitors at the expense of OCP expansion, while promoting late OC progenitor development and OC differentiation.
The monocyte markers CD14 and CD16 have been used to identify human OCP, though this remains controversial. It has been reported that in health individuals, OC are derived from peripheral blood CD14+ monocytes (Komano et al., 2006; Lari, Kitchener, & Hamilton, 2009), but in psoriatic arthritis patients from CD16+ monocytes (Chiu et al., 2010). Up-regulation of CD16 occurs on CD14+ monocytes during in vitro osteoclastogenesis, suggesting both CD14 and CD16 are markers of OC progenitors in humans. We observed that both magnetic bead-purified CD14+ and CD16+ cells from 2adBMC from arthritis hip replacement bone efficiently formed TRAP+ OCs. Because half of the CD14+ cells also express CD16+ upon culturing on plastic dishes, and the CD14+CD16+ monocytes subset is generally thought to be more mature than the CD14+CD16− subset (Fingerle et al., 1993; Ziegler-Heitbrock, 2007), our results agree with those of Chiu et al., that human osteoclastogenesis is a consecutive process from CD14+ pro-osteoclast that transition into CD14+CD16+ monocytes (pre-osteoclast) and then OCs. M-CSF pretreatment expands the CD14+CD16+ pool of monocytes and therefore increases OC formation. These results also agree with the observation in mouse BMC that M-CSF promotes c-Kit-CD11b+ monocytes maturation and osteoclastogenesis.
In summary, our findings show that adhering bone marrow cells to plastic dishes enriches OCPs, and M-CSF pretreatment promotes macrophage development at the expense of OCPs expansion and differentiation in the presence of bone marrow stromal cells. M-CSF pretreatment of early myeloid progenitors drives development toward the macrophage lineage while inhibiting OC formation. However, M-CSF promotes osteoclastogenesis of more mature OCP, thus the effects of M-CSF on osteoclastogenesis are myeloid stage specific. Our studies therefore suggest that use of M-CSF pretreatment does not promote expansion of bone marrow OC progenitors in the presence of bone marrow stromal cells and the use of M-CSF pretreatment of BMC in OC differentiation assays should be used and interpreted with caution
Acknowledgments
We thank Dr. Anne Breggia, Ivette F. Emery and the staff of the MMCRI BioBank for procurement of discarded human bone material for this study. This research also utilized the services of the Flow cytometry and Molecular phenotyping Cores supported by NIH/NIGMS P30GM106391 (R. Friesel, PI), with additional support from NIH/NIGMS U54GM115516 (C. Rosen and G Stein, PIs). This work was supported in part by funding from Maine Medical Center.
Footnotes
Conflicts of interest: The authors declare that they have no conflicts of interest.
Reference
- Alkhouli N, Mansfield J, Green E, Bell J, Knight B, Liversedge N, … Winlove CP (2013). The mechanical properties of human adipose tissues and their relationships to the structure and composition of the extracellular matrix. Am J Physiol Endocrinol Metab, 305(12), E1427–1435. doi: 10.1152/ajpendo.00111.2013 [DOI] [PubMed] [Google Scholar]
- Arai F, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K, … Suda T (1999). Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J Exp Med, 190(12), 1741–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YG, Shao T, Feng C, Mensah KA, Thullen M, Schwarz EM, & Ritchlin CT (2010). CD16 (FcRgammaIII) as a potential marker of osteoclast precursors in psoriatic arthritis. Arthritis Res Ther, 12(1), R14. doi: 10.1186/ar2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody JJ, Rivera AA, Liu J, Liu JM, Douglas JT, & Feng X (2011). A simplified method for the generation of human osteoclasts in vitro. Int J Biochem Mol Biol, 2(2), 183–189. [PMC free article] [PubMed] [Google Scholar]
- Dai Q, Han Y, Xie F, Ma X, Xu Z, Liu X, … Wang J (2018). A RANKL-based Osteoclast Culture Assay of Mouse Bone Marrow to Investigate the Role of mTORC1 in Osteoclast Formation. J Vis Exp(133). doi: 10.3791/56468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David JP, Neff L, Chen Y, Rincon M, Horne WC, & Baron R (1998). A new method to isolate large numbers of rabbit osteoclasts and osteoclast-like cells: application to the characterization of serum response element binding proteins during osteoclast differentiation. J Bone Miner Res, 13(11), 1730–1738. doi: 10.1359/jbmr.1998.13.11.1730 [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoenmaker T, Aerts D, Grevers LC, Souza PP, Nazmi K, … Everts V (2015). M-CSF priming of osteoclast precursors can cause osteoclastogenesis-insensitivity, which can be prevented and overcome on bone. J Cell Physiol, 230(1), 210–225. doi: 10.1002/jcp.24702 [DOI] [PubMed] [Google Scholar]
- Feng X, & McDonald JM (2011). Disorders of bone remodeling. Annu Rev Pathol, 6, 121–145. doi: 10.1146/annurev-pathol-011110-130203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingerle G, Pforte A, Passlick B, Blumenstein M, Strobel M, & Ziegler-Heitbrock HW (1993). The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood, 82(10), 3170–3176. [PubMed] [Google Scholar]
- Jacome-Galarza CE, Lee SK, Lorenzo JA, & Aguila HL (2013). Identification, characterization, and isolation of a common progenitor for osteoclasts, macrophages, and dendritic cells from murine bone marrow and periphery. J Bone Miner Res, 28(5), 1203–1213. doi: 10.1002/jbmr.1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, … Suda T (2000). Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med, 191(2), 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komano Y, Nanki T, Hayashida K, Taniguchi K, & Miyasaka N (2006). Identification of a human peripheral blood monocyte subset that differentiates into osteoclasts. Arthritis Res Ther, 8(5), R152. doi: 10.1186/ar2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lari R, Kitchener PD, & Hamilton JA (2009). The proliferative human monocyte subpopulation contains osteoclast precursors. Arthritis Res Ther, 11(1), R23. doi: 10.1186/ar2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Anginot A, Mancini SJ, Schiff C, Carle GF, Wakkach A, & Blin-Wakkach C (2011). Osteoclast activity modulates B-cell development in the bone marrow. Cell Res, 21(7), 1102–1115. doi: 10.1038/cr.2011.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino S, Logan JG, Mellis D, & Capulli M (2014). Generation and culture of osteoclasts. Bonekey Rep, 3, 570. doi: 10.1038/bonekey.2014.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Ohneda O, Arai F, Iwamoto K, Okada S, Takagi K, … Suda T (2001). Bifurcation of osteoclasts and dendritic cells from common progenitors. Blood, 98(8), 2544–2554. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, … Kato S (2007). Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell, 130(5), 811–823. doi: 10.1016/j.cell.2007.07.025 [DOI] [PubMed] [Google Scholar]
- Suda T, Jimi E, Nakamura I, & Takahashi N (1997). Role of 1 alpha,25-dihydroxyvitamin D3 in osteoclast differentiation and function. Methods Enzymol, 282, 223–235. [DOI] [PubMed] [Google Scholar]
- Susa M, Luong-Nguyen NH, Cappellen D, Zamurovic N, & Gamse R (2004). Human primary osteoclasts: in vitro generation and applications as pharmacological and clinical assay. J Transl Med, 2(1), 6. doi: 10.1186/1479-5876-2-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Udagawa N, & Suda T (1999). A new member of tumor necrosis factor ligand family, ODF/OPGL/TRANCE/RANKL, regulates osteoclast differentiation and function. Biochem Biophys Res Commun, 256(3), 449–455. doi: 10.1006/bbrc.1999.0252 [DOI] [PubMed] [Google Scholar]
- Takeshita S, Kaji K, & Kudo A (2000). Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J Bone Miner Res, 15(8), 1477–1488. doi: 10.1359/jbmr.2000.15.8.1477 [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL, & Ross FP (2003). Genetic regulation of osteoclast development and function. Nat Rev Genet, 4(8), 638–649. doi: 10.1038/nrg1122 [DOI] [PubMed] [Google Scholar]
- Teti A, Migliaccio S, Taranta A, Bernardini S, De Rossi G, Luciani M, … Bianco P (1999). Mechanisms of osteoclast dysfunction in human osteopetrosis: abnormal osteoclastogenesis and lack of osteoclast-specific adhesion structures. J Bone Miner Res, 14(12), 2107–2117. doi: 10.1359/jbmr.1999.14.12.2107 [DOI] [PubMed] [Google Scholar]
- Uccelli A, Moretta L, & Pistoia V (2008). Mesenchymal stem cells in health and disease. Nat Rev Immunol, 8(9), 726–736. doi: 10.1038/nri2395 [DOI] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW Jr., Ahmed-Ansari A, Sell KW, Pollard JW, & Stanley ER (1990). Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A, 87(12), 4828–4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Palomero J, Grabowska J, Wang L, de Rink I, van Helvert L, & Borst J (2017). Macrophages and osteoclasts stem from a bipotent progenitor downstream of a macrophage/osteoclast/dendritic cell progenitor. Blood Adv, 1(23), 1993–2006. doi: 10.1182/bloodadvances.2017008540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, … Suda T (1998). Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A, 95(7), 3597–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock L (2007). The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol, 81(3), 584–592. doi: 10.1189/jlb.0806510 [DOI] [PubMed] [Google Scholar]


