Abstract
The levels of organic pollutants, such as optical brightener (OB) compounds, in the global environment have been increasing in recent years. The toxicological effects and signal transduction systems associated with OB toxicity have not been thoroughly studied. The ubiquitin–proteasome system (UPS) plays a crucial role in regulating multiple essential cellular processes, and proteasome-associated cysteine deubiquitinases (DUBs), UCHL5 and USP14, are two major regulators for (de)ubiquitiation and stability of many important target proteins. Therefore, potential inhibition of UCHL5 and USP14 activities by some environmental chemicals might cause in vivo toxicity. In the current study we hypothesize that electrophilic OB compounds, such as DAST, FB-28 and FB-71, can interact with the catalytic triads (CYS, HIS, and ASP) of UCHL5 and USP14 and inhibit their enzymatic activities, leading to cell growth suppression. This hypothesis is supported by our findings presented in this study. Results from in silico computational docking and ubiquitin vinyl sulfone assay confirmed the UCHL5/USP14-inhibitory activities of these OB compounds that have potencies in an order of: FB71 > FB-28 > DAST. Furthermore, inhibition of these two proteasomal DUBs by OBs resulted in cell growth inhibition and apoptosis induction in two human breast cancer cell models. In addition, we found that OB-mediated DUB inhibition triggers a feedback reaction in which expression of UCHL5 and USP14 proteins is increased to compromise the suppressed activities. Our study suggests that these commonly used OB compounds may target and inhibit proteasomal cysteine DUBs, which should contribute to their toxicological effects in vivo.
Keywords: Fluorescent brightening agent, environmental pollutant, molecular target, degradation, post-translational modification
Introduction
The ubiquitin-proteasome system (UPS) is responsible for degradation of mis-regulated and damaged proteins in order to maintain protein homeostasis, and plays an essential role in cell proliferation, aging, apoptosis, and other major cellular processes (Meiners et al., 2018). A major regulatory step in the UPS is ubiquitination, a post-translational modification that consists of the addition of the highly conserved 76 amino acid ubiquitin to a target protein via an adenosine triphosphate (ATP)-dependent process. First, the ubiquitin-activating enzyme (E1) forms a transient high-energy thiol with ubiquitin’s C-terminal glycine residue in an ATP-dependent manner, followed by the transfer of ubiquitin to the active-site cysteine residue on the ubiquitin conjugation enzyme (E2). Ubiquitination is then completed when E2 binds with an ubiquitin ligase (E3) conjugating ubiquitin to the target substrate. The E1, E2 and E3 enzymes add an extra layer in the regulation of cellular functions (Magnani et al., 2018).
The ubiquitination process can be reversed by a group of enzymes called deubiquitinases (DUBs) that remove ubiquitin chains from the target protein, increasing or decreasing its proteasomal degradation dependent on the context. USP14 and UCHL5 are two DUBs, which are associated with 19S proteasome.
Alterations in the UPS system have been involved in the pathogenesis of several disorders including neurodegenerative, cancer, or immune diseases (Cheon et al., 2018). Most recently, UCHL5 and USP14 have been attracted research interest because their roles in cell proliferation, tumor development and drug resistance.
Since the 90’s, optical brighteners (OBs), otherwise known as fluorescent whitening agents (FWAs), have been incorporated into the commercial world and their uses are constantly increasing; from the textile, plastic, laundry detergent, and paper industries to personal care and cosmetic products. The OBs are hydrophilic, water-soluble compounds mainly derived from coumarins, diphenyl pyrazoline, heterocyclic dicarboxylic acid, naphthalene dicarboxylic acid, and diaminostilbenedisulphonic acid, the latter being the most used (Stensby, 1968; Suwiński, 2008; Steber, 2007). The characteristic that makes these compounds attractive is that they transform ultraviolet (UV) light waves to enhance blue light and minimize the amount of yellow light reflected to make objects appear whiter. Therefore, they do not get clothes or other objects any cleaner, but instead make them appear whiter and brighter. Notably, they also remain on the clothes due to their ability to resist heat and even chemicals like bleach (Burckett et al., 2007; Dorlars et al., 1975). In addition, due to the increasing demands on the appearance of cleaning and whiteness required of the human being the main user, the use of OBs has increased, ultimately generating greater exposure by having direct contact with the materials that have been treated with these compounds. This process has led to a continuous growth of this industry, which goes hand in hand with population growth and the quality of life of different socio-economic sectors. The process also lead to an increase in the demand for detergents, especially in the products for personal and household care, taking approximately 56% of the world’s production of detergents for this purpose (Àlvarez, 2004).
There is limited information available about commonly used OB agents (Shadkami et al., 2011) and their toxicology (Pedrazzani, 2012). The main justification for carrying out this study is the huge consumption of OB agents as a daily-use product. Given the daily contact we have with these compounds and the interest in the UPD and DUBs in various aspects of cellular processes, health and disease including cancer, the current study has been promoted. We hypothesize that UCHL5 and USP14 are molecular targets of electrophilic OB compounds, such as DAST, Fluorescent Brightener 28 (FB-28) and FB-71 (Figure 1). We then performed computational docking and biochemical assays to test the hypothesis. Our results indicate that these OB compounds can target and inhibit these proteasomal DUBs’ activities, and this inhibitory mechanism might explain at least in part the toxicological effects of OBs in vivo.
Figure 1.
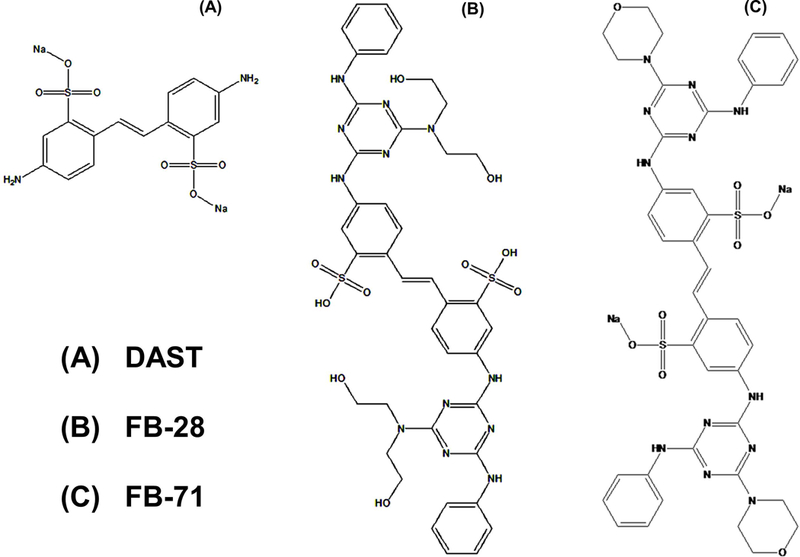
Chemical structures of OBs, DAST (A), FB-28 (B), and FB-71 (C).
Materials and methods
Materials
The optical brightener (OB) compounds DAST (462268 Aldrich), Fluorescence brightener #28 (F3543 Sigma), and Fluorescence brightener #71 (Toronto Research Chemicals) were purchased in the indicated companies, and dissolved in PBS (Fisher Scientific, USA, IL) at 1 mM concentration as a stock solution and diluted with cell culture medium when used. 3-(4,5-dimethylthiazole-2-yl)-2,5-dihenltetrazolium bromide (MTT) was purchased from Thermo Fisher (Waltham, MA). Ubiquitin-Vinyl Sulfone (Ub-VS; 250 μM) was obtained from Boston Biochem. UCHL5 polyclonal antibody and USP14 (D8Q6S) rabbit mAb were purchased from Proteintech™ (Rosemont, IL) and Cell Signaling Technology (Danvers, MA), respectively. The protein assay kit was from Bio-Rad Laboratories, Inc. (Hercules, CA).
Cell culture
The breast cancer MDA-MB-468 and MCF-7 cell lines were purchased from ATCC and grown in DMEM and RPMI medium (Gibco™, Gaithersburg, MD), respectively. All cell media were supplemented with 10% FBS (J R Scientific, Woodland, CA), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco™, Gaithersburg, MD). Cell culture experiments were performed at 37°C and 5% CO2.
Molecular docking
An in silico computational analysis was performed using molecular docking. The studies were conducted by employing USP14 and UCHL5 as receptors and DAST, Fluorescent Brightener 28 (FB-28), and Fluorescent Brightener 71 (FB-71) as ligands. The chemical structures of these OBs are shown in Figure 1. The USP14 (2AYO) and UCHL5 (3IHR) X-ray structures (proteasome 19S proteins) were retrieved from the RCSB Protein Data Bank. As for the ligands, DAST, FB-28, and FB-71, they were created using the ChemSketch software. Docking calculations to predict the interactions between DUBs and the ligands were carried out using the software AutoDock Vina. Targeting the reported active site residues of USP14 (CYS114, HIS435, and ASP451; Hu et al., 2005) and UCHL5 (GLN82, CYS88, HIS164, and ASP179; VanderLinden et al., 2015) by the OB compounds allowed for specific predictions. Through the AutoDock-specific algorithm, a predicted interaction resulted to a file in addition to a free energy score corresponding to the specified binding state. The grid coordinates were centered on the reported macromolecule’s catalytic active site and adjusted to cover the whole surface of the protein at that site. Each ligand/protein pair was docked by triplicate, and the averages of the best affinity scores (kcal/mol) of each protein–ligand pair were used to rank the complexes. The in silico predictions of the contact residues participating in the protein–ligand interactions of the complexes with the best affinity scores were identified using LigandScout 3.1.4 with default settings. The output files were visualized in PyMol 2.0 and analyzed for further interaction. The interactions that were generated between each of the 3 Optical Brightener ligand compounds and the active site residues are shown in Figure 2.
Figure 2.
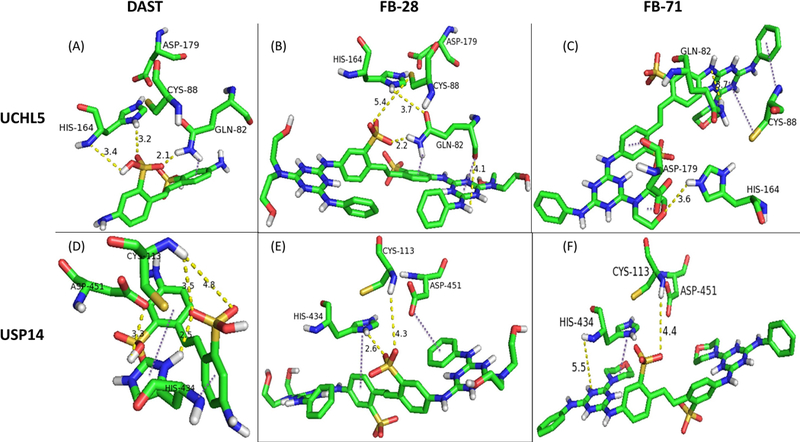
Results of interactions of ligands DAST, FB-28, and FB-71 with 19S-associated DUBs UCHL5 and USP14 through computational molecular docking. Docking studies were conducted between receptors UCHL5 (A-C) and USP14 (D-F) with ligands DAST, FB-28, and FB-71 (left, middle, and right columns, respectively, labeled above). The outputted predictions of the conformations (docking states) between known amino acid residues of known active sites of USP14 and UCHL5 and the ligands (Optical Brighteners) are displayed. DAST, 4,4’-Diamino-2,2’-stilbenedisulfonic acid; DUB, deubiquitinating enzyme; FB-28, Fluorescent Brightener 28; FB-71, Fluorescent Brightener 71.
Ubiquitin Vinyl Sulfone (Ub-VS) Assay
This assay was used to detect the inhibitory effect of OBs on 19S-associated USP14 and UCHL5, as described (D’Arcy et al., 2011; Ahmed et al., 2018). The breast cancer cells were seeded in 60-mm dishes and incubated at 37°C and 5% CO2. When the cell confluence reached about 80%, the cells were either untreated or treated with OBs at indicated concentrations for 3 h. Cell lysates (60 μg per sample) were mixed with Ub-VS (1 μM) for 30 min at 37°C in a DUB buffer [50 mM Tris-HCl (PH 7.5), 250 mM sucrose, 5 mM of MgCl2, 1 mM of Phenyl methane sulfonyl fluoride (Sigma Aldrich), 2 mM ATP (ThermoFisher Scientific, Waltham, MA, USA) and 0.2 mM DTT (1,4-Dithiothreitol, Sigma Aldrich) in dd H2O], followed by SDS gel electrophoresis and immunoblotting with USP14 and UCHL5 antibodies.
Western Blot Assay
The Western blot was performed as described (Ahmed et al., 2018). MDA-MB-468 and MCF-7 breast cancer cells were seeded in 60-mm dishes and either untreated or treated with PBS or 15 µM of DAST, FB-28, and FB-71. After a treatment time of 12 h, the cells were harvested in lysis buffer and the protein concentration of the supernatant was determined by the bicinchoninic acid (BCA) assay. An equivalent amount of protein extracts (60 µg) were separated using 10% of SDS gel electrophoresis (SDS-PAGE; Bio-Rad, Hercules, CA) under reducing conditions and 105 V for 120 minutes. The proteins were transferred to a PVDF membrane (Milpore corp., Billerica, MA) using freshly made transfer buffer at 11 mV for 1 h and blocked with 5% non-fat milk in TBST buffer for 1 h, followed by incubation in the primary, polyclonal antibody anti-UCHL5 (1:500 dilution), anti-USP14 (D8Q6S) rabbit mAb (1:500 dilution), or purified mouse anti-uman PARP (1:500 dilution). The membranes were incubated with the appropriate HRP conjugated secondary antibody (1:2000 dilution) for 2 h, followed by using the ECL 8 chemiluminescence detection system (Amersham Bioscience, Piscataway, NJ). The membranes were also re-probed with a monoclonal antibody raised against β-actin (HRP-conjugated beta Actin Monoclonal antibody, 1: 10,000 dilution) as an internal control for protein loading and normalization between samples.
Cell viability assay
The method of reduction of the tetrazolium salt, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), to a blue formazan crystal was used to evaluate the cytotoxic effect of OBs in breast cancer cell lines grown in a 96-well plate. Following treatment with OBs at different concentrations for different times, MTT (to final concentration of 0.5 mg/mL) was added to each well, and incubated for 4 h at 37 °C, followed by dissolving the blue formazan crystals in DMSO. Absorbance was measured at 750 nm. Cell viability was calculated as a percentage of vehicle control using GraphPad Prism v.5.0.
Results
Autodock modeling suggests OBs’ ability to interact with and inhibit 19S proteasome-associated DUBs, UCHL5 and USP14
We hypothesized that several electrophilic optical brighteners can interact with the catalytic triads (CYS, HIS, and ASP) of the proteasomal cysteine deubiquitinases (DUBs) UCHL5 and USP14 (D’Arcy et al., 2011; Hu et al., 2005), ultimately leading to inhibition of their activities. To test this hypothesis, docking analysis was carried out for three OBs, DAST, FB-28 and FB-71 (Fig. 1) with UCHL5 and USP14, using AutoDock Vina (Smith et al., 2004; Trott and Olson, 2010; Wang et al., 2016). Figure 2A depicts the potential interactions between DAST and UCHL5 with a free energy score of −5.6 kcal/mol; DAST shows multiple interactions with the UCHL5 catalytic triad. For example, the HIS164 nitrogen forms a hydrogen bond with the DAST’s hydroxyl group. The two oxygen atoms in the sulfonic acid group of DAST form hydrogen bonds with the imidazole ring of HIS164 and the α-amino group of GLN82. Although docking did not display any interaction between DAST and CYS88, potential interactions were noted between the α-amino group of GLN82 and the aromatic ring of the DAST (Figure 2A, a dark purple dashed line).
Figure 2B displays the docking results of predicted interactions between FB-28 and the active site of UCHL5. FB-28 is an analog of DAST with 2 additional triazine rings added symmetrically to the parent molecule DAST (Fig. 1B). The free energy score of the FB-28-UCHL5 complex was −6.5 kcal/mol (Figure 2B), suggesting a potentially higher potency of FB-28 than DAST (−5.6 kcal/mol, Figure 2A). Unlike the DAST-UCHL5 complex, the sulfonate group in FB-28 showed possible interaction with CYS88 through electrostatic interactions. Additionally, the carbonyl oxygen of GLN82, the oxyanion hole residue (Nishio et al., 2009); displayed possible interaction with the triazine ring of the FB-28 ligand different from the parent DAST molecule. Additional hydrogen bonding interactions were noted between FB-28 and UCHL5 while similar interactions found in the DAST-UCHL5 complex were observed in the FB-28-UCHL5 complex.
Figure 2C shows potential interactions between FB-71 another DAST analog (Fig. 1C), and UCHL5, with a predicted free energy score of −7.8 kcal/mol. This decrease in free energy (−5.6 kcal/mol vs. −6.5 kcal/mol for DAST and FB-28, respectively) signifies a potentially stronger interaction of the FB-71 ligand than the parent DAST and the analog FB-28. This could be attributed to the large size and specific side chains of the FB-71 ligand allowing it to interact with all 4 residues of the known active site of UCHL5 (Nishio et al., 2009). Docking studies on FB-71-UCHL5 complex showed multiple interactions that differ significantly from the other ligands. One such interaction was between the sulfur of the CYS88 and one of the heterocyclic triazine rings of FB-71. Additional interaction was also noted between the α-amino group of CYS88 and a pheny ring of FB-71 adjacent to the triazine ring. Two additional interactions were noted between the two carbonyl oxygen atoms of ASP179 and the 2 rings, one phenyl ring and one morpholine ring on the opposing side of the symmetric FB-71 ligand. In addition, hydrogen bonding interactions with GLN82 and HIS164 further stabilize interaction between FB-71 and the UCHL5 active site.
Docking analysis showed that these three OBs not only interact strongly with UCHL5, but also with USP14 enzyme through similar interactions. Free energy score predictions were also similar, but a significant decrease was seen with the DAST-USP14 complex in comparison to the FB-71-USP14 complex (−5.7 vs. −8.3 kcal/mol) (Table 1). Figure 2D suggests a combination of hydrophobic and other interactions between DAST and all three residues (CYS114, HIS435, and ASP451; Hu et al., 2005) in the active site of USP14 with a predicted free energy score of −5.7 kcal/mol. The interactions form between the imidazole ring of the HIS434 and one phenyl ring of the DAST ligand as well as the α-amino nitrogen of the HIS434 and the other phenyl ring of the DAST ligand. Additionally, several hydrogen bonding interactions were noted between DAST and the CYS113 and ASP451 residues that may have stabilized interaction between DAST and the USP14 active site.
Table 1.
Summary of results from DAST, FB-28 and FB-71 in computational docking, ubiquitin vinyl sulfone assay and MTT in human breast cancer cells.
| OB compound | Free energy of docking to UCHL5 (kcal/mol) | Inhibition of UCHL5 in breast cancer cells | Free energy of docking to USP14 (kcal/mol) | Inhibition of USP14 in breast cancer cells | MTT IC50 values (μM in MBA-MD-468 cells) | MTT IC50 values (μM in MCF7 cells) |
|---|---|---|---|---|---|---|
| DAST | −5.6 | + | −5.7 | + | 28 | 10 |
| FB-28 | −6.5 | ++ | −6.3 | + | 12 | 9 |
| FB-71 | −7.8 | +++ | −8.3 | + | 10 | 9 |
The free energy values of the reactions between UCHL5 and DAST, FB-28 and FB-71 were −5.6, −6.5, and −7.8 kcal/mol, respectively, and reactions between USP14 and DAST, FB-28, and FB-71 were similar at −5.7, −6.3, and −8.3 kcal/mol, respectively. The levels of inhibition of UCHL5 or USP14 activities by these OBs in breast cancer cells were based on the data of Figure 3, in the order of +++ > ++ > +. In MDA-MB468 cells, IC50 values of DAST, FB-28 and FB-71 were 28, 12, and 10 μM, respectively, at 72 h. In MCF7 cells, IC50 values of DAST, FB-28 and FB-71 were 10, 9, and 9 μM, respectively at 72 h. DAST, 4,4’-Diamino-2,2’-stilbenedisulfonic acid; DUB, deubiquitinating enzyme; FB-28, Fluorescent Brightener 28; FB-71, Fluorescent Brightener 71.
Figure 2E examines interactions between FB-28 and the three residues of the USP14 active site. The free energy score difference between the FB-28-USP14 complex and the FB-28-UCHL5 complex in Figure 2B was not significant (−6.3 kcal/mol and −6.5 kcal/mol, respectively). The possible interactions include the two different aromatic rings of the symmetric FB-28 ligand separately interacting with the HIS434 and ASP451 residues. Further stabilization between FB-28 and the USP14 active site through hydrogen bonding with CYS113 and HIS434 may have occurred.
Figure 2F describes possible hydrophobic and hydrogen bonding interactions in the FB-71-USP14 complex. The free energy prediction for the FB-71-USP14 complex was slightly more significant than the FB-71-UCHL5 complex shown in Figure 2C (−8.3 kcal/mol and −7.8 kcal/mol, respectively) explaining possibly stronger interactions between FB-71 and USP14. The imidazole ring of the HIS434 residue may interact with the morpholine ring of FB-71 providing a possible hydrophobic interaction. Possible stabilizing interactions are shown through hydrogen bonds between the α-amino group on the CYS113 and sulfonate oxygen of FB-71 as well as the α-amino group of HIS434 and the nitrogen atom of triazine ring on FB-71. In addition, the table 1 show IC50 values in order to compare the inhibition potencies orders of the OB obtained with the in silico and the biological techniques.
In summary, docking results suggest that these three OB compounds may be inhibitors of UCHL5 and USP14 with potencies in the order of: FB-71 > FB-28 > DAST (Table 1).
Inhibition of activities of UCHL5 and USP14 by OBs in human breast cancer cells
To confirm the proteasomal DUB-inhibitory activities of the three OBs, DAST, FB-28 and FB-71, as predicted by our computational modeling (Fig. 2), we performed a ubiquitin-Vinyl Sulfone (Ub-VS) assay. Ub-VS, a potent and irreversible inhibitor of UCHL5 and USP14 (and other DUBs), is able to bind to the active site, shifting UCHL5 protein from 37 to 45 kD, or USP14 protein from 60 to 70 kD; in the presence of an inhibitor to such a DUB, the super-shifts can be inhibited (D’Arcy et al., 2011).
We first chose human breast cancer MDA-MB-468 cell line as a working model. MDA-MB-468 cells were treated with either the solvent PBS, or 2.5 μM of DAST, FB-28 or FB-71 for 3 h. The prepared cell lysates were incubated with Ub-VS, followed by SDS gel electrophoresis and Western blot assay using specific UCHL5 and USP14 antibodies. In non-treated (NT) or PBS-treated cells, addition of Ub-VS in vitro completely super-shifted the original bands of UCHL5 and USP14 (37 and 60 kD, respectively) to higher positions (47 and 70 kD, respectively) (Figure 3A, top and bottom panels, lanes 2, 3 vs. 1, 4; *, a possible non-specific band). However, in the presence of an OB compound, especially, FB-28 or FB-71, we detected decreased levels of UCHL5-Ub-VS conjugates and simultaneously increased levels of unbound UCHL5 protein (Figure 3A, top panel, lanes 5–8), indicating inhibition of UCHL5 ubiquitin-binding activity by these OBs. The rank of these three OBs to inhibit the UCHL5 activity is: FB-71 > FB-28 > DAST (Fig. 3A, top panel, lanes 5–8), identical to that of the potencies predicted by the in silica computational docking (Fig. 2). However, we found that all the three OBs only partially inhibited the ubiquitin-binding activity of USP14 (Fig. 3A, bottom panel, lanes 5–8).
Figure 3.
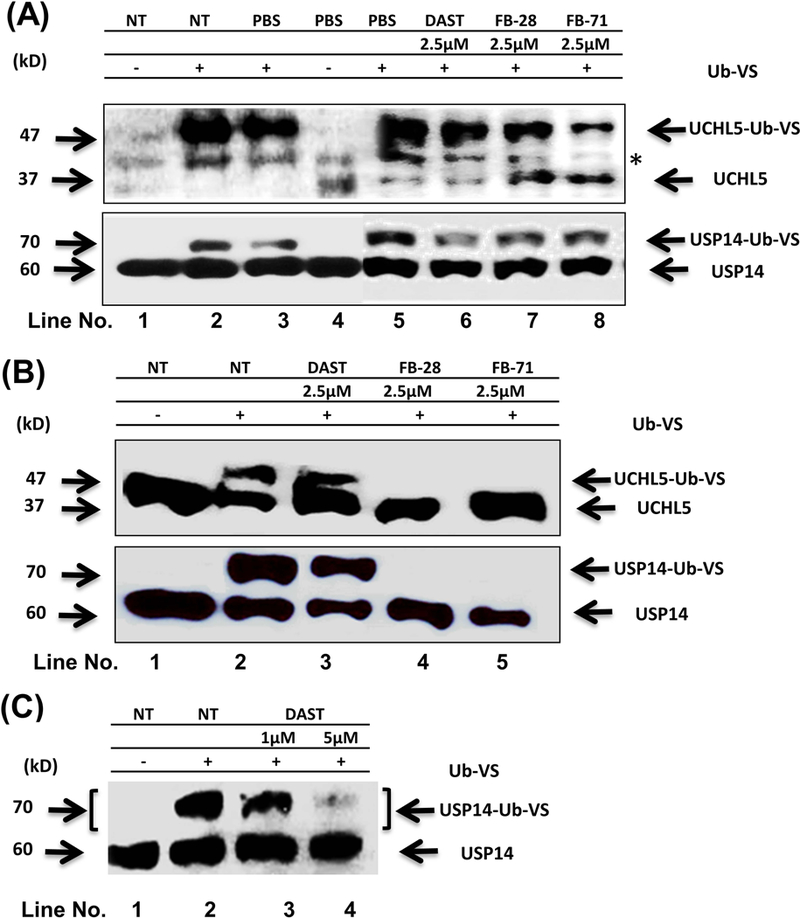
Inhibition of active site-directed labeling of proteasomal DUBs by DAST, FB-28 and FB-71. Breast cancer cells MDA-MB-468 (A) and MCF-7 (B, C); were either not treated (NT, -) or treated for 3 h with either PBS or an OB at indicated concentration. After that, 60 μg cell lysates were subsequently labelled with Ub-VS (1 μM) in vitro for 30 min at 37°C followed by SDS gel electrophoresis and immunoblotting with USP14 and UCHL5 antibodies to evaluate the inhibitory activity of these OBs on the 19S-associated deubiquitinases USP14 and UCHL5. The upper bands represent the active USP14 or UCHL5 bound by Ub-VS (UCHL5-Ub-VS and USP14-Ub-VS conjugates), while the lower bands are unbound UCHL5 and USP14. In panel A, * indicates a possible non-specific band.
We then used another human breast cancer line MCF-7 to confirm the biochemical inhibition of UCHL5 and USP14 by these three OB compounds. MCF-7 cells were either untreated or treated with 2.5 μM of DAST, FB-28 or FB-71 for 3 h, followed by the Ub-VS assay as described above. We found that FB-28 and FB-71 at 2.5 μM were able to completely inhibit the ubiquitin-binding activity of both UCHL5 and USP14, while DAST at the same concentration had only a partial inhibitory effect (Figure 3B, top and bottom panels, lanes 3–5 vs. 1–2). However, in another experiment, DAST at 5 μM was able to inhibit the activity of USP14 almost completely (Fig. 3C). These Ub-VS assay results have confirmed the inhibitory activities of the three OB compounds with potencies in the rank of: FB-71, FB-28 > DAST (Table 1).
Treatment with OB compounds increases expression of cellular UCHL5 and USP14 proteins
We previously reported that inhibition of USP-14 and UCHL5 activities by isothiocyanates caused increased levels of UCHL5 and USP14 proteins (Othman, et al., 2018). We tested whether the OB compounds can affect expression levels of these two proteasomal DUBs. MDA-MB-468 and MCF-7 cell lines were treated 15 μM of DAST, FB-28 or FB-71 for 12 hours, followed by measuring levels of UCHL5 and USP14 proteins in Western blotting. We found that exposure to any one of the three OB compounds could increase protein levels of UCHL5 and USP14 in both cell lines (Fig. 4A–B, a-b, lanes 2–4 vs. 1). These results suggest that inhibition of UCHL5/USP14 by OBs triggers a feedback reaction in which protein expression of these DUBs is subsequently increased to compromise the suppressed DUB activities. Our data further supports the conclusion that OBs are inhibitors of the proteasomal cysteine DUBs, UCHL5 and USP14.
Figure 4.
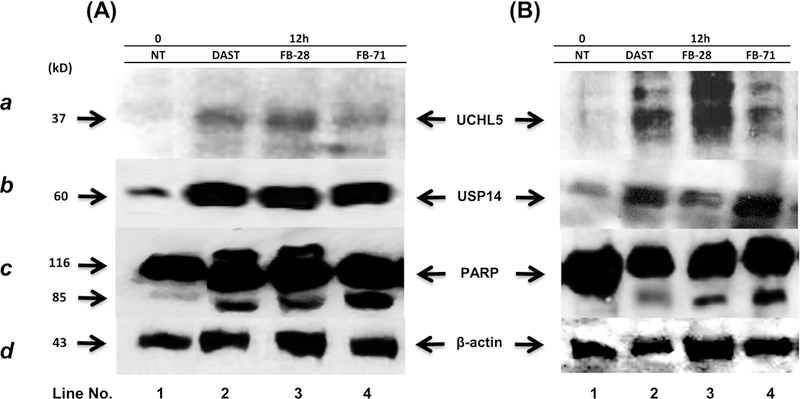
Effects of OBs on levels of 19S-DUBs and PARP in breast cancer MDA-MB-468 (A) and MCF-7 (B) cells. MDA-MB-468 and MCF-7 cell lines were treated with PBS or 15 μM of DAST, FB-28 and FB-71 for 12 hours. The cells were harvested, and protein lysates were extracted for electrophoresis, immunoblotting for UCHL5 (a), USP14 (b) and PARP (c) as well as β-actin (d; as an internal control for protein loading and normalization between samples).
OBs inhibit cell growth and induce cell death
So far our computational modeling and biochemical confirmation results have demonstrated that OB compounds are inhibitors of proteasomal DUBs UCHL5 and USP14 (Figs. 2–3). It has been reported that both UCHL5 and USP14 play important roles in regulating cell growth and proliferation (Liub et al., 2015; Arpalahti, et al., 2017; Liao, et al., 2018). We then hypothesize that inhibition of these two proteasomal DUBs by OBs would lead to inhibition of proliferation and/or induction of cell death.
To test this hypothesis, MDA-MB-468 and MCF-7 cell lines were treated with either the control solvent PBS or DAST, FB-28, or FB-71 at various concentration for 24, 48, and 72 hours, followed by MTT assay. The data shows that OBs inhibit cell proliferation in dose- and time-dependent manners (Fig. 5). By using Probit analysis, we determined IC50 values of these OBs, and the results showed that at 72 h, IC50 values of DAST, FB-28 and FB-71 in MDA-MB-468 cells are 28, 12 and 10 μM, respectively, and in MCF-7 cells, are 10, 9 and 9 μM, respectively (Table 1).
Figure 5.
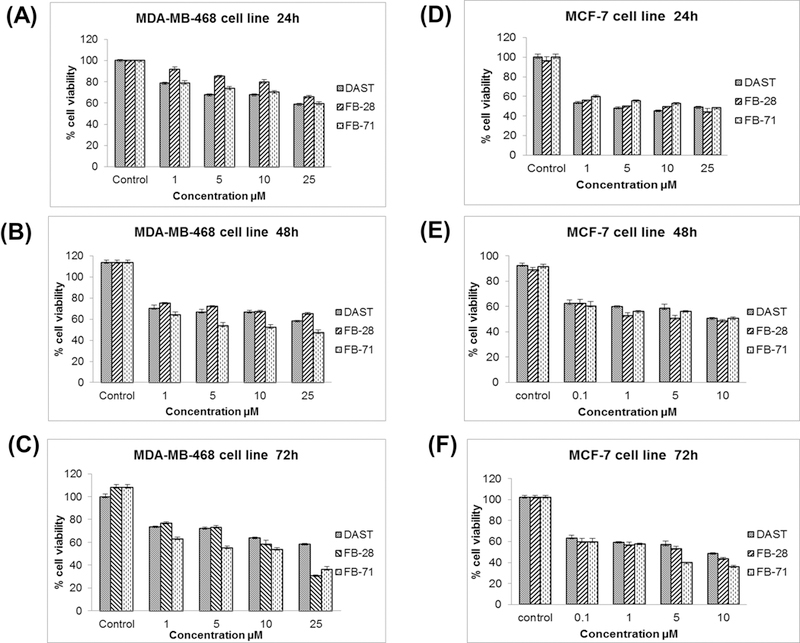
Cytotoxicity effect of OBs DAST, FB-28, and FB-71 in breast cancer cells. Breast cancer cell lines MBA-MD-468 (A-C) and MCF-7 (D-F) were seeded in a 96-well plate treated with different concentrations (0, 1, 5, 10 and 25 µM) of OBs and then cell viability was evaluated by MTT assay after an incubation time of 24h, 48h and 72h. The total percentage of MTT in control cells is taken as 100%.
We also investigated if DUB inhibition by OBs is associated with apoptotic cell death. During apoptosis, PARP is cleaved from the 116 kDa into 85 kDa and 25 kDa fragments (Lazebnik et al., 1994). In Figure 4A–B, three OB compounds increased the fragment of cleaved PARP (85 kDa) in both breast cancer cell lines. Taken together, our data indicate that OB-mediated DUB inhibition is functional, triggering signal transduction pathway and leading to growth inhibition and apoptosis in both tested cell models.
Discussion
Several studies have suggested that the proteasome is an important cellular target of different environmental chemical families (Shi et al., 2009; Liua et al., 2015; Chen et al., 2017), such as, we have also shown that 20S proteasome is a molecular target of environmental toxic organotins (Shi et al., 2009). The results of the current study have shown that proteasomal DUBs, UCHL5 and USP14 are two novel targets for commonly used OBs. The in silico analysis indicated that OBs can interact with these two 19S proteasome-associated DUBs. In UCHL5, HIS-164 and GLN-82 in the active site form interactions with DAST, FB-28, and FB-71. The latter also showed interaction with CYS-88 that is important in the activation or inhibition of UCH enzymes (Burgie et al., 2012). The rank of affinity based on the free energy was FB-71 > FB-28 > DAST.
Similar interactions were observed with USP14, which involved the formation of hydrogen bonds with the residues of the catalytic site, as predicted by the docking model (Fig. 2). Indeed, Ub-VS assays have confirmed that OBs can induce inhibition in the 19S proteasome-associated DUBs UCHL5 and USP14 in breast cancer MCF-7 and MDA-MB-468 cell lines in a sequence of inhibitory potency of FB-71 > FB-28 > DAST (Fig. 3). Congruent with these results, there was an increase in the levels of UCHL5 and USP14 as a consequence of inhibitory activity induced by OBs in the similar sequence. This indicates that OBs-mediated DUB triggers a feedback reaction in which cells tried to increases protein levels of these DUBs to compromise the inhibition. In addition, the OBs used in this research have shown their antiproliferative activity associated with their DUB-suppressing activities in a dose-dependent manner, suggesting that inhibition of UCHL5 and USP14 by OBs are functional, that would generate toxicity in normal tissues. It is possible that the more OB agents one exposes to, greater inhibition of cellular DUBs would happen in normal tissues, and more toxicity would be expected. However, this hypothesis should be tested by further studies.
Conclusion
There is no prior knowledge of OBs for a regulator of UPS. This study has shown that OBs have inhibitory activity to the two proteasomal cysteine DUBs, UCHL5 and USP14, in addition to its well-known roles in bio-pesticides, dyes, clothes, plastics, paper, and personal healthy products. Therefore, OB compounds as inhibitors of DUBs in normal tissues should induce cellular changes, including accumulation of polyubiquitinated proteins, decreased DUB activities, inhibition of cell growth and induction of programmed cell death (apoptosis). Under in vivo situation, these OB-caused reactions might result in toxicological effects. Our in vitro results in this study suggest that the commonly used OB compounds might be able to target and inhibit proteasomal cysteine DUBs in normal tissues, which could contribute to the toxicity associated with these chemicals.
Acknowledgments
This study was partially supported by a National Program for Doctoral Formation in Colombia (COLCIENCIAS, 647–2014; to I Castro), the National Cancer Institute grant R21CA184788 (to QP Dou), National Institutes of Health grant P30 CA022453 (to the Karmanos Cancer Institute at Wayne State University), and Karmanos internal funds (to QP Dou).
Footnotes
Conflict of Interest None
References
- Ahmed Z, Li X, Li F, Cheaito H, Patel K, Mosallam E, Elbargeesy G, Dou QP. (2018). Computational and biochemical studies of isothiocyanates as inhibitors of proteasomal cysteine deubiquitinases in human cancer cells. Journal of Cellular Biochemistry, July 17 10.1002/jcb.27157. [DOI] [PMC free article] [PubMed]
- Àlvarez M (2004). The Surfactant Industry: Worldwide Trends and Prospects in Colombia. Journal Palmas, 25, 340–353. [Google Scholar]
- Arpalahti L, Hagström J, Mustonen H, Lundin M, Haglund C, Holmberg C (2017). UCHL5 expression associates with improved survival in lymph-node-positive rectal cancer. Tumor Biology, 39 (7), 1–8. [DOI] [PubMed] [Google Scholar]
- Burckett J, Buzzaccarini F, Clerck K, Demeyere H, Labeque R, Lodewick R, Langenhove L (2007). Laundry Cleaning of Textiles. Handbook for Cleaning/Decontamination of Surfaces, 1, 57–102. [Google Scholar]
- Burgie E, Bingman C, Soni A, Phillips G (2012). Structural characterization of human Uch37. Proteins, 80 (2), 649–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yang Q, Xiao L, Tang D, Dou QP. Liu J (2017). Metal-based proteasomal deubiquitinase inhibitors as potential anticancer agents. Cancer Metastasis Reviews, 36 (4), 655–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon S, Dean M, Chahrour M (2018). The ubiquitin proteasome pathway in neuropsychiatric disorders. Neurobiology of Learning and Memory, In Press, Corrected Proof. 10.1016/j.nlm.2018.01.012. [DOI] [PubMed]
- D’Arcy P, Brnjic S, Olofsson M, Fryknäs M, Lindsten K, De Cesare M, Perego P, Sadeghi B, Hassan M, Larsson R, Linder S (2011). Inhibition of proteasome deubiquitinating activity as a new cancer therapy. Nature Medicine, 17, 1636–1640. [DOI] [PubMed] [Google Scholar]
- Dorlars A, Schellhammer C, Schroeder J (1975). Heterocycles as Structural Units in New Optical Brighteners. Angewandte Chemie International Edition, 14 (10), 665–679. [Google Scholar]
- Hu M, Li P, Song L, Jeffrey P, Chernova T, Wilkinson K, Cohen R, Shi Y (2005). Structure and mechanisms of the proteasome-associated deubiquitinating enzyme USP14. The EMBO Journal, 24, 3747–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazebnik Y, Kaufmann S, Desnoyers S, Poirier G, Earnshaw W (1994). Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature, 371 (6495), 346–347. [DOI] [PubMed] [Google Scholar]
- Liao Y, Xia X, Liu N, Cai J, Guo Z, Li Y, Jiang L, Dou QP, Tang D, Huang H, Liu J (2018). Growth arrest and apoptosis induction in androgen receptor-positive human breast cancer cells by inhibition of USP14-mediated androgen receptor deubiquitination. Oncogene, 37, 1896–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- aLiu N, Huang H, Ping Dou Q, Liu J (2015). Inhibition of 19S proteasome-associated deubiquitinases by metal-containing compounds. Oncoscience, 2 (5), 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- bLiu J, Shaik S, Dai X, Wu Q, Zhou X, Wang Z, Wei W (2015). Targeting the ubiquitin pathway for cancer treatment. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer, 1855 (1), 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani ND, Dada LA, Sznajder JI (2018). Ubiquitin-proteasome signaling in lung injury. Translational Research, In Press, Corrected Proof. 10.1016/j.trsl.2018.04.003. [DOI] [PMC free article] [PubMed]
- Meiners S, Evankovich J, Mallampalli RK (2018). The ubiquitin proteasome system as a potential therapeutic target for systemic sclerosis. Translational Research, In Press, Corrected Proof. 10.1016/j.trsl.2018.03.003. [DOI] [PMC free article] [PubMed]
- Nishio K, Kim S, Kawai K, Mizushima T, Yamane T, Hamazaki J, Murata S, Tanaka K, Morimoto Y (2009). Crystal structure of the de-ubiquitinating enzyme UCH37 (human UCH-L5) catalytic domain. Biochemical and Biophysical Research Communications, 390(3), 855–860. [DOI] [PubMed] [Google Scholar]
- Othman Z, Li X, Li F, Cheaito H, Patel K, Mohammed E, Hassan G, Dou Q (2018). Computational and Biochemical Studies of Isothiocyanates as Inhibitors of Proteasomal Cysteine Deubiquitinases in Human Cancer Cells. Journal of Cellular Biochemistry, 10.1002/jcb.27157. [DOI] [PMC free article] [PubMed]
- Pedrazzani R, Ceretti E, Zerbini I, Casale R, Gozio E, Bertanza G, Gelatti U, Donato F, Feretti D (2012). Biodegradability, toxicity and mutagenicity of detergents: Integrated experimental evaluations. Ecotoxicology and Environmental Safety 84 (1), 274–281. [DOI] [PubMed] [Google Scholar]
- Shadkami F, Helleur RJ, Sithole B (2011). The Analysis of Optical Brightening Agents in Paper Samples Using Liquid Chromatography with High-Resolution Mass Spectrometry. Journal of Wood Chemistry and Technology, 31(1), 42–57. [Google Scholar]
- Shi G, Chen D, Zhai G, Chen M, Cui Q, Zhou Q, He B, Dou QP, Jiang G (2009). The Proteasome is a Molecular Target of Environmental Toxic Organotins. Environmental Health Perspectives, 117 (3), 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D, Daniel K, Wang Z, Guida W, Chan T, Dou QP. (2004). Docking studies and model development of tea polyphenol proteasome inhibitors: applications to rational drug design. Proteins, 54 (1), 58–70. [DOI] [PubMed] [Google Scholar]
- Steber J (2007). The Ecotoxicity of Cleaning Product Ingredients. Handbook for Cleaning/Decontamination of Surfaces, 2, 721–746. [Google Scholar]
- Stensby PS (1968). Optical brighteners as detergent additives. Journal of the American Oil Chemists’ Society 45 (7), 497. [Google Scholar]
- Suwiński J, & Szczepankiewicz W (2008). 1,3,4-Oxadiazoles, In: Comprehensive Heterocyclic Chemistry III, 5, 397–466. [Google Scholar]
- Trott O, & Olson A (2010). Software news and update AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of Computational Chemistry, 31 (2), 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderLinden R, Hemmis C, Schmitt B, Ndoja A, Whitby F, Robinson H, Cohen R, Yao T, Hill C (2015). Structural Basis for the Activation and Inhibition of the UCH37 Deubiquitylase. Molecular Cell, 57(5), 901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Sun H, Yao X, Li D, Xu L, Li Y, Tiand S, Hou T (2016). Comprehensive evaluation of ten docking programs on a diverse set of protein–ligand complexes: the prediction accuracy of sampling power and scoring power. Physical Chemistry Chemical Physics, 18, 12964–12975. [DOI] [PubMed] [Google Scholar]


