Abstract
Herpes stromal keratitis (HSK) is a chronic immunoinflammatory condition which develops in response to recurrent herpes simplex virus-1 (HSV-1) infection of the cornea. Patients with HSK often demonstrate the concurrence of corneal desiccation and the loss of blink reflex. However, the relationship between severity of HSK, level of basal tears and inflammation of the lacrimal gland is mostly unexplored. In this study, we compared these variables in extraorbital lacrimal gland (EoLG) after corneal HSV-1 infection in the C57BL/6J mouse model. Our results showed a significant reduction in the volume of tears in infected eyes during the development of HSK. Extensive architectural damage to EoLG, presumably caused by a massive influx of interferon-gamma secreting T cells, was observed during clinical disease period of HSK. A positive correlation between the decrease in tear volume, severity of HSK and the damage to EoLG were evident in infected mice. The presence of infectious virus measured in EoLG during pre-clinical, but not clinical disease period of HSK, suggested that viral cytopathic effects are not the major contributors of extensive damage seen in EoLG. Furthermore, topical administration of lacritin peptide delayed but did not prevent the decrease in tears in HSV-1 infected mice, and had no significant effect in either reducing the severity of HSK or T cell infiltration in EoLG of infected mice. Together, our results showed an interplay between the severity of HSK, inflammation of EoLG, and the reduced level of tears after corneal HSV-1 infection.
Keywords: HSV-1, tears, lacrimal gland, lacritin and T cells
Herpes simplex virus-1 (HSV-1) is a double-stranded DNA virus, which establishes latency in the neuronal cell body of sensory ganglia. In the United States, more than 50% of humans are seropositive for HSV-1 (Xu et al., 2006). Upon primary mucosal infection, the virus uses retrograde axonal transport to infect neuronal cell bodies in the trigeminal ganglia (TG), where it establishes latent infection. Once reactivated from latency, HSV-1 can traffic via sensory nerves of the ophthalmic branch of TG to corneal tissue. The anterograde axonal transport of HSV-1 either results in asymptomatic viral shedding on the corneal surface or causes the development of herpes epithelial keratitis (HEK) (Darougar et al., 1985). Patients with HEK exhibit pain, photophobia, tearing, redness and blurred vision (Jones, 1958), but are effectively treated with antiviral drugs, such as trifluorothymidine (TFT) and acyclovir (ACV) (Pavan-Langston and Foster, 1977; Wilhelmus, 2007). On the other hand, recurrent corneal HSV-1 infection causes the development of herpes stromal keratitis (HSK) (Koujah et al., 2019). The salient clinical features of HSK involve the loss of corneal sensation and the development of corneal opacity and angiogenesis (Barron et al., 1994; Rowe et al., 2013). The loss of corneal sensation is likely the outcome of damage to sensory corneal nerve innervation as shown in patients with HSK (Hamrah et al., 2010). Recently, in a mouse model of HSK, loss of sensory nerve fibers was reported in the corneal epithelium of eyes with HSK (Chucair-Elliott et al., 2015; Yun et al., 2014). Sensory corneal nerves are involved in regulating aqueous tear production from the exocrine lacrimal gland (Meng and Kurose, 2013). Therefore, the damage to sensory corneal nerves is anticipated to have an effect on the production of aqueous tears from lacrimal gland. In this study, we measured the outcome of corneal HSV-1 infection on the level of basal tears, inflammation of the lacrimal gland, and their correlation with the severity of HSK in the C57BL/6J mouse model.
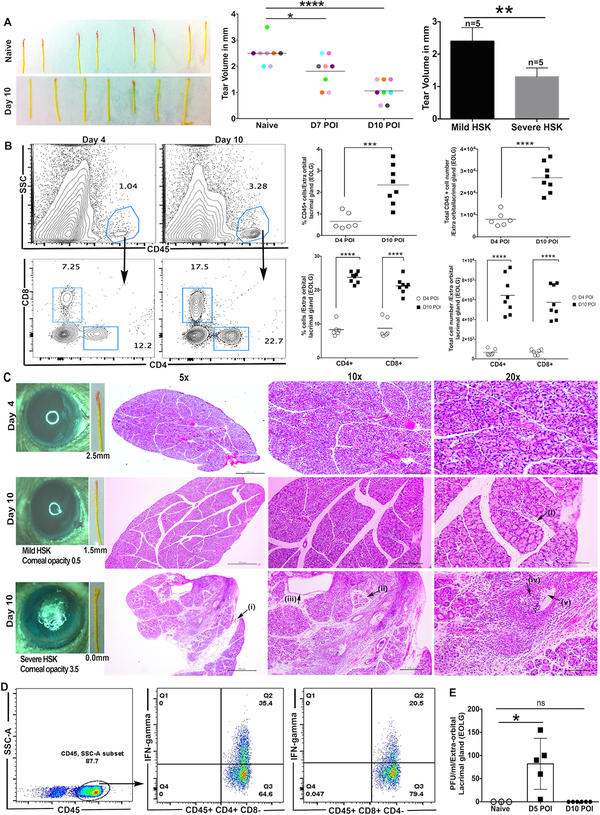
Female C57BL/6J mice were anesthetized, and mild scratching of the corneas was carried out with a 27G needle to disrupt the barrier of apical corneal epithelial (ACE) cells. Following this, 1×105 plaque forming units (PFU) of HSV-1 (McKrae) was topically applied to the corneal surface in 3μl of PBS. All animal experiments were carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals, and the experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Wayne State University. The volume of tears was measured before corneal HSV-1 infection and at different time-points after infection using the phenol red thread test. The thread test was carried out by holding the loose skin at the neck of the unanesthetized mouse. After the mouse acclimatized, a phenol red impregnated cotton thread with blunt tip was touched on to the ocular surface at the lateral canthus for 30 seconds, held by an eye dressing forcep (Miltex, 18–779). Appropriate care was taken to avoid touch stimulation of eyelashes and whiskers. When phenol red comes in contact with alkaline tears, its color changes from yellow to red. The red colored thread length was measured after the removal from the ocular surface. Measurement of tear level in the same eye before and after corneal HSV-1 infection demonstrated about 50% reduction in tear volume at 10-day post-infection, a time-point when development of corneal opacity was evident in infected eyes (Figure 1A). Furthermore, the level of tear reduction correlated with severity of HSK (corneal opacity ≥3.0) (Figure 1A), in keeping with human HSK study where alterations in tear composition have also been reported (Keijser et al., 2002; Ma and Lu, 2017).
Figure 1: Inflammatory cell infiltrate in EoLG and the decrease in tear fluid volume during clinical phase of HSK.
A. Phenol-red thread images demonstrate the measurement of the volume of tear fluid in uninfected (naive) eyes and HSV-1 infected eyes at 10-day post-infection. The color change in thread from yellow to red depicts the tear volume. Data shown is the representation of three independent experiments. Scatter plot with colored dots represents the level of basal tears in an individual eye prior to and after the corneal HSV-1 infection. Each colored dot represents the tear volume in an individual eye prior to infection (Naive), and on 7-day and 10-day post-ocular infection (POI). Data shown is the sum of two independent experiments. (n= 4 eyes per group). The p values were calculated using one-way ANOVA followed by the Bonferroni’s multiple comparison test. (****p<0.0001 and *p=0.0347). Bar diagram depicts the level of tears in eyes with severe and mild HSK (n= 5 eyes per group). The p value was calculated using unpaired nonparametric Mann-Whitney test (**p=0.0079). B. Representative FACS plots denote the frequency of CD45+ cells (top panel) and CD4/CD8 T cells (bottom panel) in EoLG of HSV-1 infected mice on 4 and 10-day post-infection. Scatter plots demonstrate the frequency and absolute number of total leukocytes (CD45) and CD4/CD8 T cells in individual EoLG of infected mice on 4-day and 10-day POI. Data were analyzed using unpaired two-tailed student’s t-test with Welch’s correction (*** p=0.0008, ****p<0.0001). C. Representative hematoxylin and eosin (H&E) staining in the paraffin sections of EoLG excised at 4-day and 10-day post-infection. At 10-day post-infection, the inflammatory cell influx was compared between EoLG excreting tears to eyes with mild or severe HSK. Eye images depict the severity of corneal opacity. Labeled areas in EoLG excreting tears to eyes with severe HSK demonstrate (i) fatty infiltration, (ii) acinar atrophy and fibrosis, (iii) ductal dilation, (iv) inflammatory infiltrate in duct lumen, (v) stasis of tear fluid. (D) Representative FACS plots denoting the frequency of IFN-gamma secreting CD4 and CD8 T cells in EoLG providing tears to HSK developing eyes at 10-day post-infection. (E) Scatter plot with bar diagram shows the number of plaque forming units (PFU) of HSV-1 in EoLG at 5- and 10-day post-infection. *p=0.0357 was calculated by carrying out unpaired non-parametric Mann-Whitney test.
The reduction in the level of tears and the alterations in the composition of the tear film in HSV-1 infected eyes could be the outcome of the development of inflammation in the lacrimal gland. The lacrimal gland is the major contributor to the aqueous layer of the tear film and lacrimal gland inflammation has been reported to promote aqueous-deficient dry eye, clinically known as keratoconjunctivitis sicca (KCS) (Williamson et al., 1973). Unlike humans, the main lacrimal gland in rats and mice is extra-orbital and is located just below the ear (Dartt, 2009; Ding et al., 2001). The cellular elements (acinar, ductal and myoepithelial cells [MECs]) of lacrimal gland in mouse, rat, rabbit, and humans are similar with most abundant tear protein secretory acinar cells (Rios et al., 2005). Acinar lumens converge to form excretory ducts lined by cuboidal cells that release electrolytes and water (Walcott et al., 2005). MECs play critical roles in the lacrimal gland development and homeostasis, and the contractile capacity of MECs are involved in the propulsion of lacrimal gland secretion (Makarenkova and Dartt, 2015). The lacrimal gland also contains a sizeable number of lymphocytes, plasma cells, dendritic cells, mast cells and macrophages (Zierhut et al., 2002). Increased infiltration of the inflammatory cells in the lacrimal gland is considered to contribute to tissue damage and lacrimal gland dysfunction (Zoukhri, 2006).
Therefore, we next measured the lacrimal gland leukocytic influx in HSV-1 infected mice using flow cytometry. Single cell suspensions of lacrimal gland from individual mice were prepared by incubating pre-minced gland tissue with 50μg of liberase TL in 200μl of RPMI 1640 media with antibiotics at 37°C for 45 minutes on a tissue disruptor (1500rpm). Cells were stained using the fluorochrome conjugated antibodies Percp-Cy5.5 conjugated- anti-CD8a (53–6.7), PE- Cy7 conjugated anti-CD45 (30-F11), and APC conjugated anti-CD4 (RM4–5), all were purchased from BD biosciences, San Diego, CA. At the end of cell surface staining, samples were acquired using a LSRFortessa flow cytometer (BD Biosciences, San Jose, CA), and the data were analyzed using the FlowJo software (Ashland, OR, USA. V8.8.7). Our results showed that in comparison to EoLG excised from HSV-1 infected mice during pre-clinical disease period (at 4-day post-infection), the EoLG excised during clinical disease period (at 10-day post-infection) had a significantly increased frequency and the absolute number of CD45+ leukocytes (Figure 1B). The staining of leukocytic subpopulation showed an increased frequency and the absolute number of CD4 and CD8 T cells in EoLG of infected mice during clinical than pre-clinical disease period (Figure 1B). An increased focal or scattered infiltration of T cells reported in Sjogren’s syndrome and sarcoidosis, respectively are associated with lacrimal gland inflammation, and insufficient tearing (Fox and Kang, 1992).
To determine whether infiltration of leukocytes in EoLG of HSV-1 infected mice is scattered or focal, paraffin sections of EoLG from HSV-1 infected mice were stained with hematoxylin and eosin. As shown in Figure 1C, a widespread influx of mononuclear cells was seen in the EoLG that is supplying tear fluid to eye with severe HSK at 10-day post-infection. The inflammatory infiltrates seen in the acinar cell region, and the lumen of the lacrimal gland duct were associated with extensive architectural damage to the lobules as evident by acinar cell atrophy, periacinar fibrosis, fatty infiltration, and interlobular duct dilation (Figure 1C). We also noted the presence of eosinophilic amorphous material in some ductal lumen suggesting the stasis of tear film (Figure 1C). On the other hand, the EoLG, which is supplying tears to the eyes with mild HSK showed infiltration of fewer inflammatory cells in acinar cell region during clinical disease period at 10-day post-infection. No extensive architectural damage to acini and the ductal cells was seen in these EoLG. Furthermore, EoLG of infected mice during pre-clinical disease period (at 4-day post-infection) also did not show any extensive damage to acinar cells in the lobular region (Figure 1C).
The histopathological changes detected in EoLG of infected mice at 10-day post-infection are likely the outcome of the increased level of pro-inflammatory cytokines such as interferon-gamma (IFN-g) secreted by infiltrating T cells (Pitcher et al., 2011). Therefore, we next determined if infiltrating T cells in the inflamed lacrimal gland are producing pro-inflammatory IFN-g cytokine? The single cell suspension of the EoLGs excised at 10-day post-infection was stimulated with PMA and Ionomycin for 6hrs., followed by intracellular cytokine staining for IFN-γ. Our flow cytometry data as shown in Figure 1D demonstrates the presence of IFN-γ producing CD4 and CD8 T cells in inflamed lacrimal gland. We next asked why these inflammatory cells are pulled into the lacrimal gland? One possible explanation could be impairment of afferent sensory neural regulation of lacrimal gland secretion during the development of HSK. Sensory corneal nerves regulate the secretion of neurotransmitters such as acetylcholine and norepinephrine from parasympathetic and sympathetic lacrimal gland nerves (Dartt, 2009). Degeneration of sensory nerves in corneas during the clinical phase of HSK is likely to cause a decrease in the release of neurotransmitters from the nerves in EoLG, and this could have a negative effect on the secretion of tear fluid from acinar and ductal cells resulting in the development of inflammation. Earlier, pharmacological blockade of neurotransmitter (acetylcholine) receptor has been shown to cause inflammation and lymphocytic infiltration in EoLG of C57BL/6 mice (Pitcher et al., 2011). It is also possible that HSV-1 infection of EoLG causes the massive influx of inflammatory cells. To address this, EoLG was excised from HSV-1 infected mice at 5-day and 10-day post-infection. EoLG was cut into smaller pieces, and the latter were co-incubated with Vero cells for 48 hr as a readout of HSV-1 viral infectivity. Experiments were performed in 75cm2 flasks in DMEM + 2% FBS growth medium. Co-culturing of Vero cells with lacrimal gland excised from infected mice at-5-day post-infection showed the alterations in Vero cell morphologies and after 48 hours of co-culture, the Vero cells became smooth and rounded and started losing cell-cell contact (data not shown). These features are the outcome of HSV-1 cytopathy, which ultimately kills the infected cells (Roizman, 1962). In contrast, Vero cells cultured with EoLG excised at 10-day post-infection were unaffected and grew as confluent cell monolayer. Indeed, via the Vero cell plaque assay, virus was detected at 5-day but not 10-day post-infection (Figure 1E).
Even though our results showed the presence of infectious virus in EoLG during pre-clinical but not clinical disease period of HSK, how did HSV-1 applied topically to the cornea gained access to the lacrimal gland? One likely explanation is through innervation of the lacrimal gland, inclusive of parasympathetic and sympathetic fibers and the lacrimal nerve, which is a sensory branch of the ophthalmic trigeminal nerve (V1). After corneal HSV-1 infection, virus via retrograde axonal transport travels to the cell body of sensory neurons in the trigeminal ganglia (TG). During active viral replication in TG, virus may traffic via V1 branch of the TG to the EoLG and infect different cell types of the lacrimal gland. This anterograde trafficking pattern of HSV-1 to the lacrimal gland might also occur during viral reactivation in the latent TG, and HSV-1 infected lacrimal gland could be the source of asymptomatic viral shedding detected in human tears on the corneal surface (Kaufman et al., 2005).
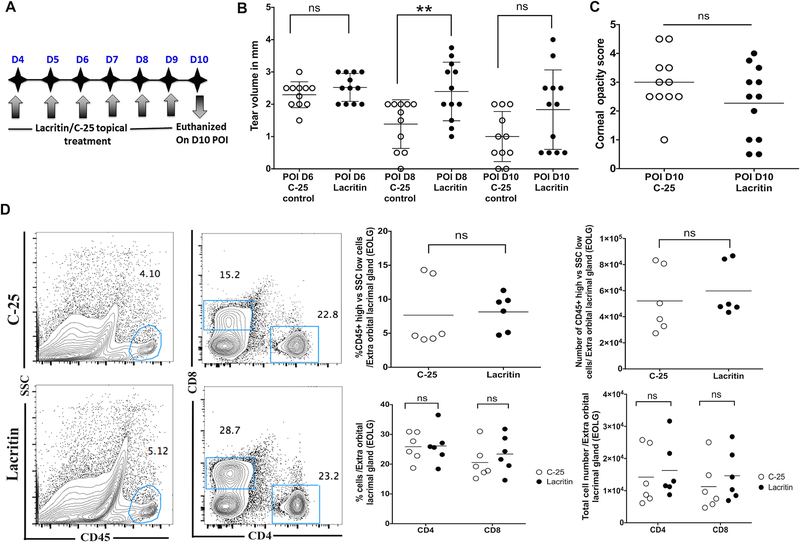
Tears play an important role in maintaining health of the ocular surface and hydration of the cornea. Reduced tear levels as shown in HSK developing eyes (Figure 1A) are likely to cause the corneal desiccation, whereas the protection of the infected corneas from desiccation is shown to reduce the severity of HSK (Yun et al., 2014). Therefore, the approach to increase tears in HSV-1 infected eyes could have an effect in ameliorating the severity of HSK. Recently, an endogenous tear glycoprotein Lacritin, which is secreted by acinar cells has been identified as a novel secretion-enhancing factor (Sanghi et al., 2001). Topical administration of lacritin is reported to promote tear secretion, restore ocular surface integrity and reduce focal CD4 T cell infiltration of the lacrimal gland in a mouse model of aqueous-deficient dry eye disease (Vijmasi et al., 2014; Wang et al., 2013). Therefore, we explored the therapeutic potential of lacritin in HSK. Drops of bacterial recombinant lacritin or C-25 (an inactive lacritin truncation mutant) were applied four times a day at a dose of 4μM, which is effective in normal rabbits and dry eye mouse (Samudre et al., 2011; Vijmasi et al., 2014). The treatment was given from 4-day through 10-day post-corneal infection with tear levels measured at 6, 8 and 10-day post-corneal infection. On 10-day post-infection, HSK severity was assessed by measuring corneal opacity using a hand-held slit lamp microscope as quantitated via a 0 to 5 scale (Gaddipati et al., 2016). EoLGs were excised from both groups of infected mice, and flow cytometry was performed as described above. A schematic of the treatment regimen is shown in Figure 2A. As shown in Figure 2B, HSV-1 infected eyes receiving the topical lacritin treatment, showed a significantly higher level of tears than C-25 treated control group of infected eyes at 8-day post-infection. However, the effect of lacritin attenuated with the progress of time, and on 10-day post-infection no significant difference in the level of tears was measured between treated and control groups of infected eyes. No significant difference in the frequency and the number of leukocytes infiltrating EoLG of infected mice was detected on 10-day post-infection, when compared between both groups of mice (Figure 2D), suggesting that lacritin treatment alone is not efficient in controlling HSV-1 induced inflammation of the EoLG. Recent neurophysiology studies reveal that lacritin contributes to basal tearing by acting directly on low threshold, cold sensory afferent typical of TRPM8 neurons (Dallacasagrande et al., 2016). Damaging of sensory nerves in HSK developing corneas would negate the beneficial effect of lacritin. Similarly, lacritin treatment given alone was not effective in significantly reducing the severity of HSK (corneal opacity) (Figure 2C). The inability of lacritin treatment to reduce the severity of HSK suggests that multiple immunopathogenic processes are involved in the development of HSK (Streilein et al., 1997) and increasing tear secretion alone is not sufficient to ameliorate the severity of HSK. In HSK, the corneal stroma is heavily infiltrated with immune cells (Thomas et al., 1997), and the latter causes stromal inflammation. Therefore, lacritin treatment in association with an approach to control stromal inflammation could be more effective in reducing the severity of HSK.
Figure 2. Topical application of lacritin protein transiently increases the tears in HSV-1 infected eyes, but did not reduce HSK severity and the inflammatory influx in EoLG.
A. Schematic representing the treatment plan with topical application of lacritin and C-25 control peptide. B. Scatter plot represents the volume of tears measured between C-25 treated (open circles, n=11 eyes) and lacritin treated groups of infected mice (closed circles, n=12 eyes) at 6-, 8-, and 10-day post-ocular infection (POI). A significant increase in the tear volume is shown in lacritin treated mice at 8-day POI (**p=0.0084 calculated using unpaired t-test with Welch’s correction). Data shown are derived from two similar experiments. C. Scatter plot shows the corneal opacity score of individual eye from lacritin and C-25 treated groups of mice at 10-day POI. Data shown are pooled from two similar experiments. (open circles, n= 11 eyes and closed circles, n= 12 eyes) D. Representative FACS plots show the frequencies of CD45 and CD4/CD8 T cells in EoLG derived from C-25 and lacritin treated groups of infected mice at 10-day POI. Scatter plots demonstrate frequency and absolute number of CD45 and CD4/CD8 T cells in the lacrimal gland from both groups of infected mice at 10-day POI. Data shown in C and D were analyzed using unpaired two-tailed student’s t-test with Welch’s correction (ns represent p>0.05).
Taken together, our results showed that eyes with severe HSK had reduced level of tears, and the EoLGs connected to severe HSK eyes demonstrated an extensive architectural damage and a massive influx of inflammatory T cells. The presence of infectious virus in EoLG suggests that virus may initiate immunoinflammatory reaction, which persists even after the clearance of the infectious virus from the lacrimal gland. Future studies should look into the trafficking pathway of HSV-1 to EoLG and if the other ocular glands such as intra-orbital lacrimal gland and harderian gland also get affected after corneal HSV-1 infection. Based on our findings, the investigation of the possible contribution of HSV-1 induced lacrimal gland inflammation in human HSK is likely warranted.
Acknowledgements
The authors would like to thank Subhash Gaddipati for technical assistance during the measurement of the tear fluid.
Funding sources
Study was supported by funds received from National Eye Institute Grants EY022417, EY029690 (SS), and EY024327, EY026171 (GWL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
GWL is cofounder of TearSolutions, Inc, that is currently testing the efficacy of a lacritin synthetic peptide for Sjogren’s syndrome dry eye in a phase 2 clinical trial. RLM is contractor of TearSolutions.
References:
- Barron BA, Gee L, Hauck WW, Kurinij N, Dawson CR, Jones DB, Wilhelmus KR, Kaufman HE, Sugar J, Hyndiuk RA, et al. , 1994. Herpetic Eye Disease Study. A controlled trial of oral acyclovir for herpes simplex stromal keratitis. Ophthalmology 101, 1871–1882. [DOI] [PubMed] [Google Scholar]
- Chucair-Elliott AJ, Zheng M, Carr DJ, 2015. Degeneration and regeneration of corneal nerves in response to HSV-1 infection. Invest Ophthalmol Vis Sci 56, 1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallacasagrande V, Hirata H, Mizerska KK, van Kuppevelt TH, McKown RL, Laurie GW, 2016. Lacritin acutely enhances corneal nerve sensitivity to ocular surface dryness as a key stimulus for basal tear production: Implications for dry eye disease. Invest Ophth Vis Sci 57. [Google Scholar]
- Darougar S, Wishart MS, Viswalingam ND, 1985. Epidemiological and clinical features of primary herpes simplex virus ocular infection. Br J Ophthalmol 69, 2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartt DA, 2009. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res 28, 155–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C, Walcott B, Keyser KT, 2001. Neuronal nitric oxide synthase and the autonomic innervation of the mouse lacrimal gland. Invest Ophthalmol Vis Sci 42, 2789–2794. [PubMed] [Google Scholar]
- Fox RI, Kang HI, 1992. Pathogenesis of Sjogren’s syndrome. Rheum Dis Clin North Am 18, 517–538. [PubMed] [Google Scholar]
- Gaddipati S, Rao P, Jerome AD, Burugula BB, Gerard NP, Suvas S, 2016. Loss of Neurokinin-1 Receptor Alters Ocular Surface Homeostasis and Promotes an Early Development of Herpes Stromal Keratitis. J Immunol 197, 4021–4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrah P, Cruzat A, Dastjerdi MH, Zheng L, Shahatit BM, Bayhan HA, Dana R, Pavan-Langston D, 2010. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology 117, 1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BR, 1958. The clinical features of viral keratitis and a concept of their pathogenesis. Proc R Soc Med 51, 917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman HE, Azcuy AM, Varnell ED, Sloop GD, Thompson HW, Hill JM, 2005. HSV-1 DNA in tears and saliva of normal adults. Invest Ophthalmol Vis Sci 46, 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keijser S, van Best JA, Van der Lelij A, Jager MJ, 2002. Reflex and steady state tears in patients with latent stromal herpetic keratitis. Invest Ophthalmol Vis Sci 43, 87–91. [PubMed] [Google Scholar]
- Koujah L, Suryawanshi RK, Shukla D, 2019. Pathological processes activated by herpes simplex virus-1 (HSV-1) infection in the cornea. Cell Mol Life Sci 76, 405–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Lu Y, 2017. Bilateral tear film alterations in patients with unilateral quiescent herpes simplex keratitis. Acta Ophthalmol 95, 629–633. [DOI] [PubMed] [Google Scholar]
- Makarenkova HP, Dartt DA, 2015. Myoepithelial Cells: Their Origin and Function in Lacrimal Gland Morphogenesis, Homeostasis, and Repair. Curr Mol Biol Rep 1, 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng ID, Kurose M, 2013. The role of corneal afferent neurons in regulating tears under normal and dry eye conditions. Exp Eye Res 117, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavan-Langston D, Foster CS, 1977. Trifluorothymidine and idoxuridine therapy of ocular herpes. Am J Ophthalmol 84, 818–825. [DOI] [PubMed] [Google Scholar]
- Pitcher JD 3rd, De Paiva CS, Pelegrino FS, McClellan AJ, Raince JK, Pangelinan SB, Rahimy E, Farley WJ, Stern ME, Li DQ, Pflugfelder SC, 2011. Pharmacological cholinergic blockade stimulates inflammatory cytokine production and lymphocytic infiltration in the mouse lacrimal gland. Invest Ophthalmol Vis Sci 52, 3221–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios JD, Horikawa Y, Chen LL, Kublin CL, Hodges RR, Dartt DA, Zoukhri D, 2005. Age-dependent alterations in mouse exorbital lacrimal gland structure, innervation and secretory response. Exp Eye Res 80, 477–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B, 1962. Polykaryocytosis induced by viruses. Proc Natl Acad Sci U S A 48, 228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe AM, St Leger AJ, Jeon S, Dhaliwal DK, Knickelbein JE, Hendricks RL, 2013. Herpes keratitis. Prog Retin Eye Res 32, 88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samudre S, Lattanzio FA Jr., Lossen V, Hosseini A, Sheppard JD Jr., McKown RL, Laurie GW, Williams PB, 2011. Lacritin, a novel human tear glycoprotein, promotes sustained basal tearing and is well tolerated. Invest Ophthalmol Vis Sci 52, 6265–6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanghi S, Kumar R, Lumsden A, Dickinson D, Klepeis V, Trinkaus-Randall V, Frierson HF Jr., Laurie GW, 2001. cDNA and genomic cloning of lacritin, a novel secretion enhancing factor from the human lacrimal gland. J Mol Biol 310, 127–139. [DOI] [PubMed] [Google Scholar]
- Streilein JW, Dana MR, Ksander BR, 1997. Immunity causing blindness: five different paths to herpes stromal keratitis. Immunol Today 18, 443–449. [DOI] [PubMed] [Google Scholar]
- Thomas J, Gangappa S, Kanangat S, Rouse BT, 1997. On the essential involvement of neutrophils in the immunopathologic disease: herpetic stromal keratitis. J Immunol 158, 1383–1391. [PubMed] [Google Scholar]
- Vijmasi T, Chen FY, Balasubbu S, Gallup M, McKown RL, Laurie GW, McNamara NA, 2014. Topical administration of lacritin is a novel therapy for aqueous-deficient dry eye disease. Invest Ophthalmol Vis Sci 55, 5401–5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcott B, Birzgalis A, Moore LC, Brink PR, 2005. Fluid secretion and the Na+-K+−2Cl-cotransporter in mouse exorbital lacrimal gland. Am J Physiol Cell Physiol 289, C860–867. [DOI] [PubMed] [Google Scholar]
- Wang N, Zimmerman K, Raab RW, McKown RL, Hutnik CM, Talla V, Tyler M.F.t., Lee JK, Laurie GW, 2013. Lacritin rescues stressed epithelia via rapid forkhead box O3 (FOXO3)-associated autophagy that restores metabolism. J Biol Chem 288, 18146–18161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmus KR, 2007. Therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database Syst Rev, CD002898. [DOI] [PubMed] [Google Scholar]
- Williamson J, Gibson AA, Wilson T, Forrester JV, Whaley K, Dick WC, 1973. Histology of the lacrimal gland in keratoconjunctivitis sicca. Br J Ophthalmol 57, 852–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, Berman SM, Markowitz LE, 2006. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 296, 964–973. [DOI] [PubMed] [Google Scholar]
- Yun H, Rowe AM, Lathrop KL, Harvey SA, Hendricks RL, 2014. Reversible nerve damage and corneal pathology in murine herpes simplex stromal keratitis. J Virol 88, 7870–7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierhut M, Dana MR, Stern ME, Sullivan DA, 2002. Immunology of the lacrimal gland and ocular tear film. Trends Immunol 23, 333–335. [DOI] [PubMed] [Google Scholar]
- Zoukhri D, 2006. Effect of inflammation on lacrimal gland function. Exp Eye Res 82, 885–898. [DOI] [PMC free article] [PubMed] [Google Scholar]