Abstract
Primates must balance the need to monitor other conspecifics to gain social information while not losing other resource opportunities. We consolidate evidence across the fields of primatology, psychology, and neuroscience to examine individual, population, and species differences in how primates, particularly macaques, monitor conspecifics. We particularly consider the role of serotonin in mediating social competency via social attention, aggression and dominance behaviors. Finally, we consider how the evolution of variation in social tolerance, aggression, and social monitoring might be explained by differences in serotonergic function in macaques.
Keywords: Non-human primates, macaques, serotonin, social monitoring, social information
Graphical/Visual Abstract and Caption
Cover Figure: Serotonin may play a role in balancing macaques’ need to monitor others and monitor the environment, ultimately mediating differences in social monitoring across individuals, populations, and species of macaques.
Introduction
Many species of primates, including humans, live in complex social environments, and must monitor other individuals in order to avoid conflict, share resources, and maintain status within their social groups. Primates thus allocate extensive cognitive resources to monitor conspecifics. However, primates must flexibly balance the need to gain information about social partners with the need to exploit food sources and avoid predation and environmental risks. This review consolidates evidence across the fields of primatology, psychology, and neuroscience to discuss the variability in monitoring others and a potential neuromodulator pathway that underlies this variability.
Individuals within a species vary in their social monitoring strategies. Social monitoring also varies across different populations of the same species, and also across different species. These differences in strategies seem to be dependent on the amount of social monitoring required for an individual to avoid conflict and maintain its dominance rank. Given that serotonin plays a critical role in regulating aggression and dominance rank, we suggest that serotonin, at least in part, also plays a role in social monitoring. We discuss evidence for this hypothesis, synthesizing pharmacological, clinical, and neuroscientific research on serotonin and social attention. Finally, we consider how evolutionary differences in dominance hierarchy despotism and social monitoring may be linked to differences in serotonergic function and genetics across species, particularly in macaques.
Monitoring Others Helps Individuals Navigate Social Environments
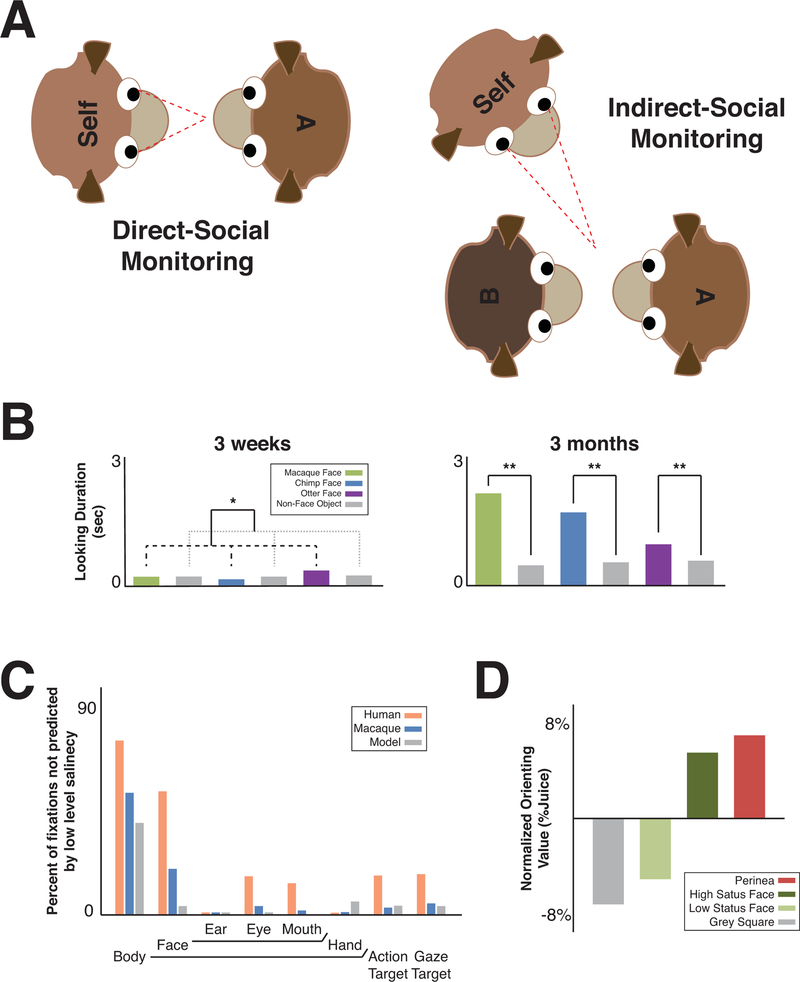
For the purposes of this review, we define monitoring others as the act of attending to, looking at, or watching conspecifics. The social information that can be gleaned via monitoring others is not only information directed from conspecific ‘A’ to oneself (direct-social monitoring), but also information that is independent of oneself, i.e. directed from conspecific ‘A’ to a third conspecific, conspecific ‘B’ (indirect-social monitoring, or third-party monitoring) (Figure 1A).
Figure 1: Monitoring others for gaining social information in primates.
A) Many primate species gather social information by either monitoring others when information is directed from a conspecific ‘A’ to themselves (direct-social monitoring) or when information is communicated between conspecific ‘A’ to conspecific ‘B’ (indirect-social or third-party monitoring). B) Rhesus macaques detect faces as early as 3 weeks of age, and this detection becomes biased towards conspecific faces by 3 months. When 3-week-old macaques were presented with an image of a macaque, chimpanzee, or otter face in an array with 9 non-face objects they looked at faces (colored bars connected with black dashed lines) longer than non-face objects (grey bars connected with grey dashed lines). By 3 months, they also looked at macaque faces significantly longer than chimpanzee faces and otter faces. *p < .05. **p < .016. Reproduced and adapted with permission from(Simpson et al., 2017). C) When humans and rhesus macaques view natural scene videos featuring rhesus macaques, humans, or cartoon characters, gaze fixation locations are not simply predicted by a model that only considers low-level visual characteristics (e.g., contrast, stimulus orientation). Instead, the viewed locations often feature social agents and the targets of agents’ actions and attention. Reproduced and adapted with permission from(Shepherd et al., 2010). D) Rhesus macaques are willing to forgo juice rewards in order to view female perinea and high-ranking male faces, but require extra juice rewards in order to choose to view low-ranking male faces and non-social stimuli. Reproduced and adapted with permission from(Deaner et al., 2005).
Primatologists historically have studied what primates know about others. Through this work, we know that monkeys can, and do, use social information to represent social relationships including kinship, dominance, and even facial expressions(Cheney & Seyfarth, 1990, 1999; Parr & Heintz, 2009; Parr, Winslow, Hopkins, & de Waal, 2000; Pokorny & de Waal, 2009; Shepherd, Deaner, & Platt, 2006). Monitoring both direct- and indirect- social information allows monkeys to more quickly represent social relationships and this can help them determine which individuals will reciprocate prosocial behavior, preventing them from wasting resources on unreciprocated altruistic behavior(Trivers, 1971). Even more importantly, gaining social information and representing social relationships allows individuals to navigate complex social environments while reducing the need for potentially threatening direct interactions. This is true for many species, but especially for the species of primates that live in complex social groups organized into linear dominance hierarchies(Rowell, 1974). In these societies, like those of macaques, it is crucial for each individual to know their relative dominance rank; aggressing towards a higher-ranking animal can result in injury or death(J. D. Higley, P. T. Mehlman, S. B. Higley, et al., 1996; Silk, 2007) while inappropriately submitting to lower-ranking animals can result in a loss of resources or decrease in dominance status(Pusey, Williams, & Goodall, 1997; Rowell, 1974; Sapolsky, 2005; Wittig & Boesch, 2003). Physical cues such as size and age are not the most reliable predictors of dominance rank in some primate species, including macaques(Bernstein & Gordon, 1980; Maestripieri & Wallen, 1997). In these species, where age and size are not perfect predictors of rank, individuals must monitor species-typical social signals(F. de Waal & Luttrell, 1985) to communicate and decipher dominance hierarchies. For example, macaque dominance hierarchies seem to be reflected and signaled by differences in facial expressions(Maestripieri, 1997; Partan, 2002) and social gaze dynamics(Dal Monte, Piva, Morris, & Chang, 2016).
Indeed, social primates are adapted to seek out, value, and use social information related to faces(Anderson, 1998). Infant rhesus macaques as young as 3 weeks of age are more likely to detect faces and look longer at faces relative to non-social stimuli. By the age of 3 months, this face-prioritizing visual system has been tuned specifically to conspecific faces relative to other primate and animal faces(Simpson et al., 2017) (Figure 1B). In addition, when viewing dynamic video clips of naturalistic scenes, humans and macaques direct their attention to social agents even when these locations are not predicted by a model that only considers low-level visual characteristics (e.g., contrast, stimulus orientation)(Shepherd, Steckenfinger, Hasson, & Ghazanfar, 2010) (Figure 1C).
Laboratory studies also indicate that adult rhesus macaques readily recognize individuals(Pokorny & de Waal, 2009), their facial expressions(Parr & Heintz, 2009) and statuses(Shepherd et al., 2006). Consistent with the value attached to social information, rhesus macaques will work in order to receive social information(Andrews, Bhat, & Rosenblum, 1995; Turrin, Fagan, Dal Monte, & Chang, 2017) and even forgo juice and food rewards in order to gain valuable social information(Deaner, Khera, & Platt, 2005) (Figure 1D). Macaques also reflexively follow the gaze of conspecifics and humans(Drayton & Santos, 2017; Mosher, Zimmerman, & Gothard, 2014; Putnam, Roman, Zimmerman, & Gothard, 2016).
Overall, primates strategically devote cognitive resources to maximize the information they gain by monitoring others. In a naturalistic scene viewing task, macaques were found to view conspecifics earlier and for a longer period of time when they exhibited directed eye gaze and redder sex skin, which both convey particularly important social information(Solyst & Buffalo, 2014). Furthermore, macaques frequently direct attention to the eye region of faces(Dal Monte et al., 2016), scan faces conveying agonistic and affiliative expressions differently(Nahm, Perret, Amaral, & Albright, 1997) and attend to photos of novel conspecifics more than familiar conspecifics(Gothard, Erickson, & Amaral, 2004), likely to maximize gained social information. When foraging for social information, rhesus macaques decide to explore or exploit “patches” that offer varying amounts of novel social information(Turrin et al., 2017), indicating that they employ a strategy in seeking social information. This strategy seems to be guided by social value. For instance, macaques are more willing to forgo juice rewards to observe the perinea of fecund females and the faces of high ranking macaques(Deaner et al., 2005) and more readily follow the gaze of high dominance status individuals(Shepherd et al., 2006).
While social information is important, monitoring others for too long of a period can be risky. For example, direct eye gaze towards another macaque is considered to be a threat(Maestripieri, 1997). Thus, animals also gain dominance related information via attending to third party or to other-other interactions(Qu, Ligneul, Van der Henst, & Dreher, 2017). This allows individuals avoid having to learn about dominance hierarchies via direct competition and thus limit fighting and antagonistic interactions. Rhesus macaques are able to use behavioral cues to identify, responding via joystick, which of two unfamiliar conspecifics in artificially created videos was the dominant individual.(Bovet & Washburn, 2003) Another study demonstrated that macaques were not only able to learn which of two novel conspecifics was the dominant individual, but were also able to remember and transfer, responding via touchscreen, this dominance relationship to new videos where the recorded animals provided no dominance information(Paxton et al., 2010).
Monitoring Others – Variability Between Individuals, Populations, and Species
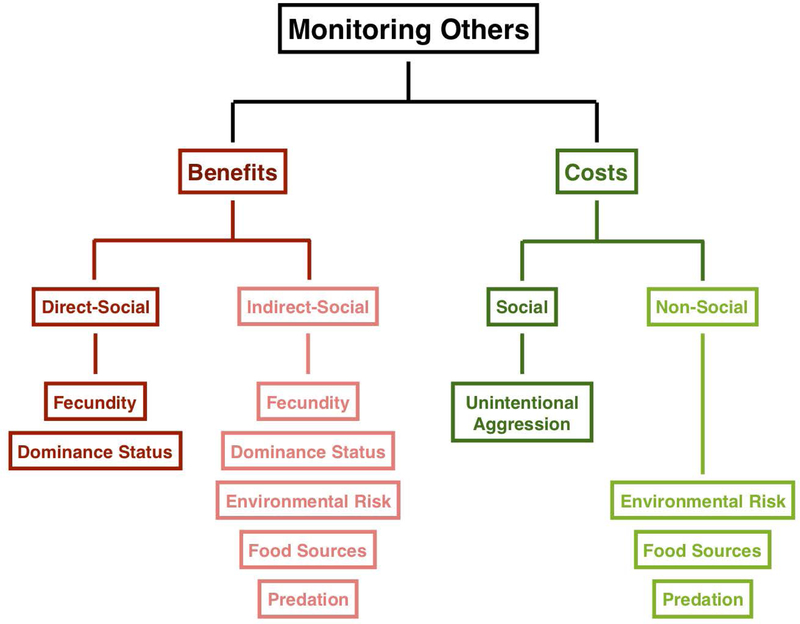
All individuals must judiciously balance the benefits and costs of monitoring conspecifics. Monitoring others can yield valuable information about fecundity, dominance ranks, and third-party environment information. However, excessive monitoring of conspecifics can lead to accidentally aggressing to conspecifics, and prevents individuals from scanning their environment for food or potential threats (Figure 2). This balancing act is reflected as variation in monitoring behaviors, and can be observed in the laboratory and in the wild, across individuals, populations, and species.
Figure 2: The costs and benefits of social monitoring.
Primates must balance the costs and benefits associated with social monitoring. From direct-social monitoring, primates can gain information about conspecifics fecundity and dominance status. From indirect-social monitoring they can learn not only about fecundity and dominance status, but also environmental risk, food sources, and predation. However, monitoring others for too long can result in the social cost of unintentional aggression. It can also cause a reduction in environmental monitoring which can cause animals to learn less about environmental risk, food sources, and predation.
Third party monitoring can manifest differently across individuals within a given population according to age, gender, and dominance rank. As rhesus macaques age they tend to modulate gaze following, increasing gaze following into adolescence and then decreasing gaze following into adulthood(Rosati, Arre, Platt, & Santos, 2016). This is likely because juvenile rhesus depend on monitoring other-other interactions to learn relative dominance rankings, but this need decreases with age as individuals gain more knowledge of and become more established in their dominance ranks(Rosati et al., 2016). Adult rhesus males are more vigilant and monitor conspecifics more than females(K. Watson et al., 2015), likely because the dominance ranking of males are more volatile than female rhesus, who exhibit inherited dominance ranks(Holekamp & Smale, 1991). However, adult females follow gaze more readily than adult males(Rosati et al., 2016). Moreover, as macaques age they also modulate rates of direct gaze. Bonnet macaques exhibit increased eye contact from infancy to adolescence, and then decrease direct gaze into adulthood, likely because adult macaques punish direct gaze more as macaques age(Coss, Marks, & Ramakrishnan, 2002).
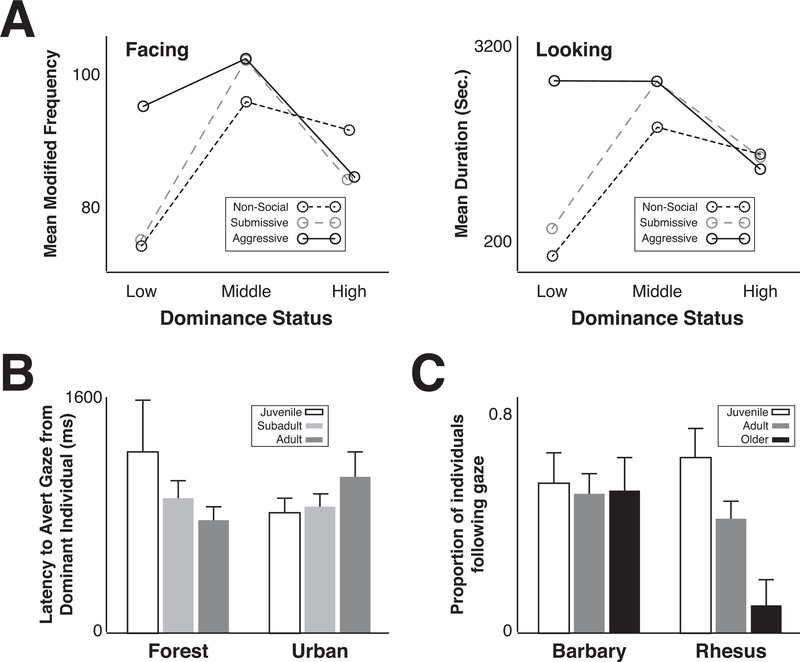
An individual’s dominance rank also strongly influences patterns of social monitoring. Haude found that middle ranking rhesus macaques looked at conspecifics more frequently, but that each of these looking events was for a shorter period of time than low or high-ranking individuals(Haude, Graber, & Farres, 1976). This is likely because the dominance rankings of middle ranking individuals are more volatile, requiring them to be more vigilant towards conspecifics, hence the increased number of looks. However, middle ranking individuals also need to avoid accidentally aggressing to others during their heightened vigilance, hence the relative short duration of each looking event in order to prevent accidental direct eye contact. Capitanio and colleagues observed a similar effect in pigtailed macaques; middle ranking females spent a relatively longer period of time monitoring conspecifics compared to low- and high- ranking females(J. P. Capitanio, Boccia, & Colaiannia, 1985) (Figure 3A). In addition, middle-ranking pigtailed macaques attended most to videos that featured the next highest-ranking females, particularly when they exhibited submissive gestures, which is incongruent to the relative dominance status between the stimulus female and the tested female. Furthermore, the middle-ranking macaques increased aggression in the home cage after viewing these videos, but not other types of videos(J. Capitanio, 1987). Thus, macaques modulate rates of social monitoring depending on their relative dominance relationships and use social information to update their own dominance ranks.
Figure 3: Social monitoring in primates differs across individuals, populations, and species.
A) Left panel: The mean frequency of positioning the body to directly face videos of non-social stimuli, conspecifics exhibiting dominant expressions, and conspecifics exhibiting submissive expressions, for low-, middle-, and high-ranking female pigtailed macaques. Right panel: The mean look duration to these same categories of videos for the same female pigtailed macaques. Middle-ranking females faced the social videos more, and looked at the videos longer, compared to low- and high-ranking females. Reproduced and adapted with permission from(J. P. Capitanio et al., 1985). B) While bonnet macaques living in the forest avert eye contact with conspecifics more quickly as they age, bonnet macaques living in urban environments do not. Reproduced and adapted with permission from(Coss et al., 2002). C) As despotic rhesus macaques age, fewer of them follow the gaze of an experimenter while tolerant barbary macaques maintain juvenile rates of gaze following throughout their lives. Reproduced and adapted with permission from(Rosati & Santos, 2017).
Not surprisingly, social monitoring can also vary across populations and across species. Bonnet macaque populations living in the forest decrease direct gaze as they age, while populations living in urban environments maintain juvenile levels of direct gaze into adulthood(Coss et al., 2002) (Figure 3B). This difference is likely driven by an increased demand to monitor the environment for predators in the forest relative to urban settings. In addition, bonnet macaques in urban settings live in smaller territories and thus live in closer physical proximity to conspecifics, increasing the likelihood of fighting. For this reason, bonnet macaques in urban settings devote more time than their forest counterparts to monitoring conspecifics to avoid ingroup fighting(Coss et al., 2002). Just as bonnet macaques typically decrease directed gaze with age, rhesus macaques decrease gaze following with age. In contrast, the barbary macaque, a highly tolerant species with relatively flat dominance hierarchies, maintains juvenile levels of gaze following into adulthood(Rosati & Santos, 2017) (Figure 3C). This is perhaps because barbary macaques are punished less for direct gaze behavior and do not fear social repercussions relative to their more despotic relatives, i.e. the rhesus and bonnet macaques. Overall, in primate societies, we find variability between individuals, populations, and species in their social monitoring behaviors, with these differences being driven by socioecological and sociobiological distinction.
Serotonin and Monitoring Others
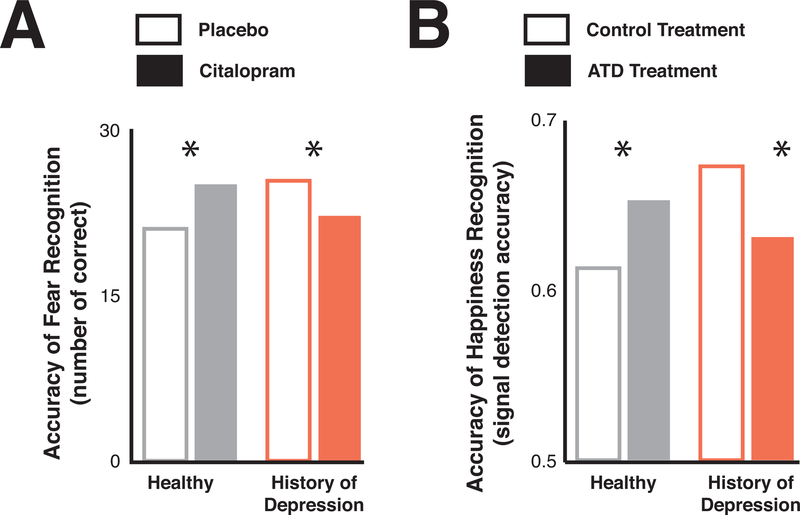
The serotonergic system appears to modulate processes underlying social behaviors and social monitoring and does so in a manner dependent on the prior history of individuals. Modulating central serotonin function with Selective Serotonin Reuptake Inhibitors (SSRIs), precursor augmentations (Acute Tryptophan Depletion (ATD), tryptophan loading, 5-Hydroxytrytophan (5-HTP) loading), or receptor agonism/antagonism has been shown to impact impulsivity, cognitive biases, attention, learning, and memory, with these effects being much stronger for social stimuli, affective processing, and emotional recognition(Mendelsohn, Riedel, & Sambeth, 2009; Merens, Van der Does, & Spinhoven, 2007; Riedel, Klaassen, & Schmitt, 2002; Silber & Schmitt, 2010; Young, 1996). A lot of research, particularly clinical research, has focused on how modulating central serotonin impacts attentional biases to different classes of emotional stimuli and suggest that decreasing central serotonin with ATD causes negative attentional biases(Fusar-Poli et al., 2007; Klaassen, Riedel, Deutz, & Van Praag, 2002; Munafò, Hayward, & Harmer, 2006; Robinson, Cools, Crockett, & Sahakian, 2010; Roiser et al., 2008), particularly in those at risk of depression, like recovered depressed individuals(Booij et al., 2005; Hayward, Goodwin, Cowen, & Harmer, 2005) or those with a family history of depression(Marsh et al., 2006; van der Veen, Evers, Deutz, & Schmitt, 2007). On the other hand, increasing central serotonin with acute SSRIs and serotonergic precursor loading seems to modulate eye gaze patterns(Jonassen, Chelnokova, Harmer, Leknes, & Landrø, 2015) and decrease negative attentional biases and negative emotional recognition(C. Harmer, Bhagwagar, et al., 2003; C. Harmer, Rogers, Tunbridge, Cowen, & Goodwin, 2003; C. J. Harmer, Mackay, Reid, Cowen, & Goodwin, 2006; C. J. Harmer, Shelley, Cowen, & Goodwin, 2004; Jonassen et al., 2015; Luciana, Burgund, Berman, & Hanson, 2001; Murphy, Longhitano, Ayres, Cowen, & Harmer, 2006), particularly for those at deficit prior to intervention(Bhagwagar, Cowen, Goodwin, & Harmer, 2004). Other studies have investigated the effects of serotonin manipulation on emotional recognition and shown that risk factors and baseline differences in behavior can impact the effect of serotonergic modulations(Bhagwagar et al., 2004; Hayward et al., 2005; Robinson et al., 2010). A single dose of the SSRI citalopram increases the recognition of fearful faces in healthy volunteers but decreases fear recognition in subjects with a history of depression(Bhagwagar et al., 2004) (Figure 4A). Furthermore, decreasing central concentrations with low dose ATD increases the recognition of happy faces in healthy volunteers, but instead decreases the recognition of happy emotions in recovered depressed patients(Hayward et al., 2005) (Figure 4B).
Figure 4: Serotonin differentially impacts emotional recognition dependent on individual differences.
A) The SSRI citalopram (filled bars), compared to placebo (open bars), causes a bidirectional effect on fear recognition depending on whether subjects had a history of depression (red bars; decreases fear recognition due to citalopram) or were healthy volunteers (grey bars; increases fear recognition due to citalopram). Reproduced and adapted with permission from(Bhagwagar et al., 2004). B) ATD (filled bars versus placebo open bars) causes a bidirectional effect on the recognition of happy emotions dependent on if subjects were healthy (left graph, grey bars, an increase due to ATD) or recovered depressed (right graph, red bars, a decrease due to ATD). Reproduced and adapted with permission from(Hayward et al., 2005).
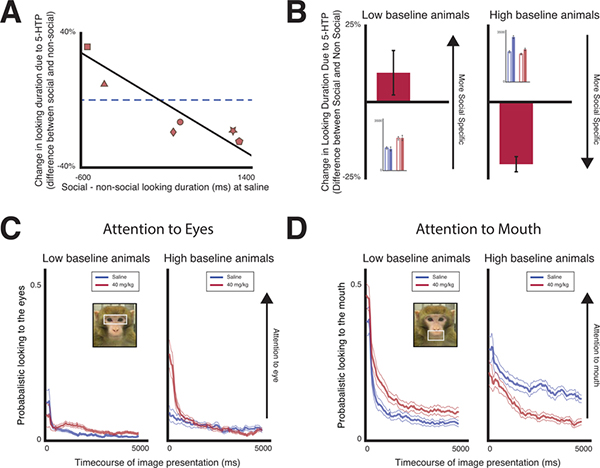
Mirroring the individual variability in serotonin manipulations in human subjects, a recent study from our lab reported that increasing central serotonin via acute administration of the serotonergic precursor 5-HTP modulated rhesus macaques’ attention to images in a bidirectional manner(Weinberg-Wolf et al., 2018). 5-HTP increased looking duration in subjects with low starting or baseline attention, yet decreased it in those with high baseline attention. Importantly, while 5-HTP modulated looking duration to both social and nonsocial images, 5-HTP had a greater effect on attention to social images (Figure 5A). In animals that, at baseline, directed less attention to social images, 5-HTP increased attention to social images more than to non-social images. On the other hand, in high baseline social attention animals, 5-HTP decreased attention specifically to social images. Moreover, 5-HTP had the largest impact on attention to facial features that are salient and convey important social information, the eyes and mouth (Figure 5B). 5-HTP increased looking to the eyes and mouth in animals with low baseline attention, but decreased looking to these same regions in animals with high baseline attention. In addition, these effects were greatest for expressive compared to neutral faces, and faces with direct gaze compared to averted gaze meaning that 5-HTP seems to modulate attention to social stimuli as a function of how salient facial expression are and also the relative value of the information they convey. Overall, these findings suggest that central serotonergic function may balance the benefits and costs of monitoring conspecifics dependent on differences between individuals.
Figure 5: Increasing central serotonin with 5-HTP modulates social attention.
A) Baseline differences in how long rhesus macaques look at social and non-social images are negatively correlated with the differences in how 40mg/kg 5-HTP changes looking duration to social and non-social images relative to saline (blue dashed line). Each shape represents an individual subject’s data. B) The average differences in the changes in looking duration to social and non-social images due to 5-HTP for low and high baseline animals. The inset shows the raw looking duration to social (filled bars) and non-social images (open bars) for low and high baseline animals during saline (blue) and 5-HTP (red) sessions. Adapted and reproduced with permission from(Weinberg-Wolf et al., 2018). C) Average time courses of 5-HTP’s bi-directional effect on attention to the mouth for low and high baseline animals. 5-HTP increases attention to the mouth region in low baseline animals but decreases it in high baseline animals. Saline data shown in blue, and 40mg/kg 5-HTP in red. Adapted and reproduced with permission from(Weinberg-Wolf et al., 2018). D) Average time courses of 5-HTP’s effect on attention to conspecific eyes for low and high baseline animals. While 5-HTP has a large effect on attention to the mouth, it only modestly increases attention to the eye region in low baseline animals while modestly decreases it in high baseline animals. Saline data shown in blue and 40mg/kg 5-HTP in red. Adapted and reproduced with permission from(Weinberg-Wolf et al., 2018).
Dominance and Aggression: The Role of Central Serotonin
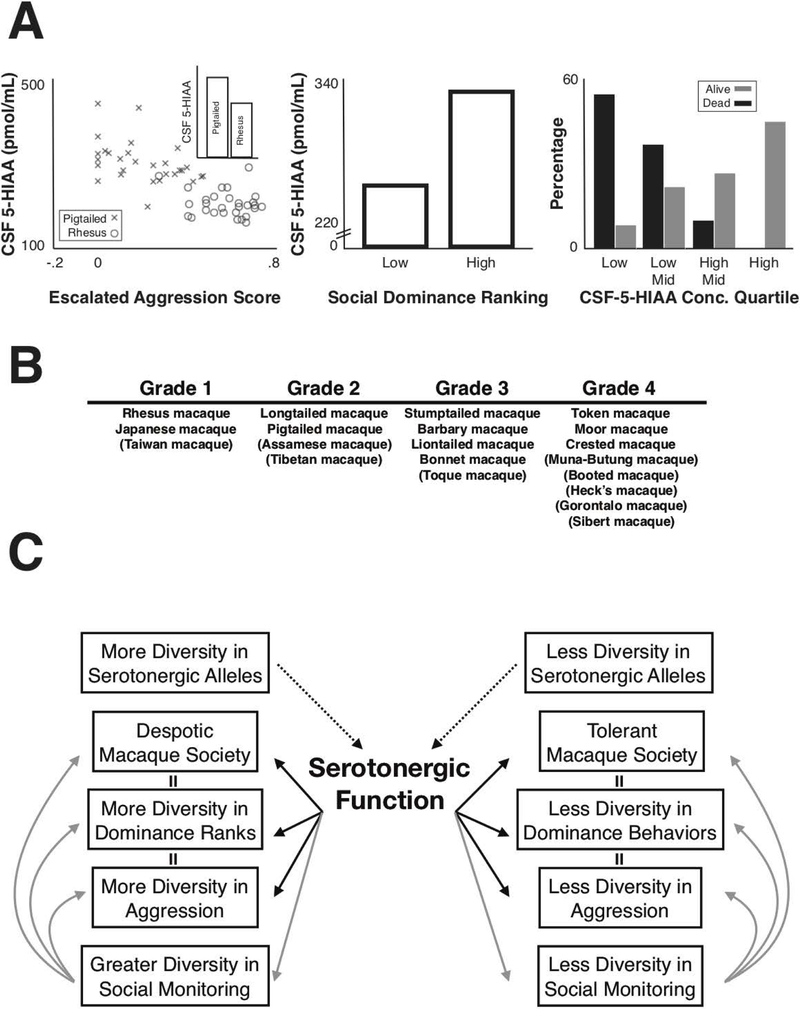
While monitoring others is a crucial component of dominance hierarchies, the establishment and maintenance of these hierarchies often relies on some level of aggressive behaviors. It ensures that individuals can maintain access to limited resources and defend themselves against others(Chiao, 2010; A. S. Clarke & Boinski, 1995; F. B. de Waal, 1986; Qu et al., 2017; Rowell, 1974). However, high levels of aggression are maladaptive and can lead to low social competency and impaired quality of life(F. B. de Waal, 1986; J. D. Higley, P. T. Mehlman, S. B. Higley, et al., 1996; Rowell, 1974). Central serotonin has been implicated repeatedly, and across many species, in regulating maladaptive aggression(Coccaro, 1992; Coccaro et al., 1997; Ferrari, Palanza, Parmigiani, de Almeida, & Miczek, 2005; Garattini, Giacalone, & Valzelli, 1967; Gibbons, Barr, Bridger, & Liebowitz, 1979; Linnoila et al., 1983; Manuck et al., 1998; Valzelli, 1971). While researchers have found that central concentrations of the serotonin metabolite 5-hydroxyindoleacetic Acid (5-HIAA) are overall inversely correlated with aggression in rhesus macaques(J. D. Higley et al., 1992), low CSF 5-HIAA concentrations have been linked specifically to only one subtype of aggression: impulsive aggression, or aggression that is unprovoked or unproductive and will not help an individual maintain access to resources or secure dominance status(J. Higley & Linnoila, 1997). On the other hand, productive aggression, used to maintain dominance rank, has been positively correlated with concentrations of testosterone in male macaques(J. D. Higley, P. T. Mehlman, R. E. Poland, et al., 1996). This relationship is further supported by extensive work, in multiple species, showing that low CSF 5-HIAA concentrations correlate with poor impulse control, impaired social functioning, severe wounding, and even mortality in primates(Fairbanks, Melega, Jorgensen, Kaplan, & McGuire, 2001; J. Higley, S. King, et al., 1996; J. Higley & Linnoila, 1997; J. Higley, Suomi, & Linnoila, 1996; J. D. Higley, Linnoila, & Suomi, 1994; J. D. Higley et al., 1992; J. D. Higley, P. T. Mehlman, S. B. Higley, et al., 1996; J. D. Higley, P. T. Mehlman, R. E. Poland, et al., 1996; P. Mehlman et al., 1994; P. T. Mehlman et al., 1997; Taub & Vickers, 1995; G. Westergaard, Mehlman, Westergaard, Suomi, & Higley, 1999; G. C. Westergaard et al., 2003; Zajicek et al., 2000) (Figure 6A). As a consequence of these impairments, primates with low CSF 5-HIAA concentrations are less likely to acquire and maintain social dominance than those with high CSF 5-HIAA concentrations(J. Higley, S. King, et al., 1996; J. D. Higley et al., 1992; J. D. Higley, P. T. Mehlman, R. E. Poland, et al., 1996; Howell et al., 2013; Kaplan, Manuck, Fontenot, & Mann, 2002; P. T. Mehlman et al., 1997; G. Westergaard et al., 1999; Zajicek et al., 2000) (Figure 6A).
Figure 6: Diversity in social tolerance in macaque species is related to diversity in serotonergic function, and potentially impacts diversity in dominance rank, aggression, and social monitoring.
A) Left Panel: Central concentrations of the serotonin metabolite (5-HIAA) are inversely correlated with escalated aggression scores, or the proportion of all aggressive acts that were characterized by high-intensity aggression, in rhesus macaques and pigtailed macaques. The inset shows that rhesus macaques have lower average CSF-5-HIAA than pigtailed macaques. Reproduced and adapted with permission from(G. Westergaard et al., 1999). Middle Panel: High-ranking female macaques exhibit relatively higher central concentrations of 5-HIAA than low-ranking females. Reproduced and adapted with permission from(J. Higley, S. King, et al., 1996). Right Panel: Animals with relatively lower concentrations of CSF 5-HIAA are more likely to have died 4 years after sample collection than animals with relatively higher concentrations of CSF 5-HIAA. Reproduced and adapted with permission from(J. D. Higley, P. T. Mehlman, S. B. Higley, et al., 1996). B) Macaque species can be categorized according to their social tolerance, varying from grade 4 (most tolerant) to grade 1 (most despotic). Reproduced and adapted with permission from(Bernard Thierry, 2007). C) A summary figure illustrating the known relationships between diversity in serotonergic genetics, serotonergic function, social tolerance, and diversity in dominance rank, aggression, and social monitoring across species of macaques. Dotted lines represent the impact of diversity in serotonergic alleles on serotonergic function. Solid black lines represent the impact of serotonergic function on aggression, dominance status, and social tolerance. Grey lines represent the relationship between serotonergic function and social monitoring and the relationship between social monitoring and aggression, dominance status, and social tolerance.
The Relationship Between Despotism and Variability in Serotonergic Alleles
There are 19 sub-species of macaques, each with slightly different styles of dominance hierarchy. Highly despotic species have extremely steep hierarchies whereby a dominant individual almost always wins contests with their subordinates. On the other hand, the dominance hierarchies of tolerant species are less steep and thus dominant and subordinate individuals win contests more evenly(Balasubramaniam et al., 2012; Petit, Abegg, & Thierry, 1997; B Thierry, 1985; Bernard Thierry, 2007; Bernard Thierry, Iwaniuk, & Pellis, 2000). These differences in despotism are categorized along a scale of 1–4 with one being the most despotic and four being the least despotic (Balasubramaniam et al., 2012; Bernard Thierry, 2007) (Figure 6B). Rhesus macaques are one of the sub-species with the most despotic, or steep, dominance hierarchies(Balasubramaniam et al., 2012; Bernard Thierry, 2007). They exhibit more extreme and higher rates of aggression, along with higher rates of species typical threat and submissive gestures compared to less despotic species, like the Tonkean macaques (Macaca tonkeana), who instead exhibit higher rates of affiliative gestures(Petit et al., 1997; Bernard Thierry, 2007). CSF 5-HIAA concentrations are inversely related to aggression levels across macaque species; species with relatively more despotic hierarchies, like rhesus, exhibit relatively lower concentrations of CSF 5-HIAA compared to species with slightly less despotic hierarchies, like pigtailed macaques(G. Westergaard et al., 1999). Not surprisingly, the relationship between individual variations in 5-HIAA and aggressive behaviors seen within a species(J. D. Higley et al., 1992), holds true across species as well (Figure 6A). It is likely that diversity in serotonergic function may contribute to regulating aggression and dominance relationships across individuals, population, and species.
Because of the pressure to know and defend dominance rank, we hypothesize that species with more despotic dominance hierarchies have developed greater intra-group diversity in social monitoring strategies because of greater intra-group differences in social threat(K. Watson et al., 2015). Concomitantly, despotic species of macaques exhibit more variation in genes related to serotonergic function, like the 5-HTT gene encoding for the serotonin transporter(Dobson & Brent, 2013; Wendland et al., 2006), indicating a relationship between variation in socially competent dominance-related behavior and the diversity in serotonergic function (Figure 6C). The relationship between individual differences in these genotypes and monitoring others, vigilance, and dominance has been investigated extensively. While the genetic architecture of these complex behavioral traits is polygenic in nature, evidence suggests that individual differences in these social behaviors are heritable and may be related to genetic variation in the serotonergic pathway(Beevers, Ellis, Wells, & McGeary, 2010; Beevers et al., 2011; Boll & Gamer, 2014; Brent et al., 2013; Canli & Lesch, 2007; Dobson & Brent, 2013; Duncan & Keller, 2011; Gibboni, Zimmerman, & Gothard, 2009; Hariri & Holmes, 2006; K. Watson et al., 2015; K. K. Watson, Ghodasra, & Platt, 2009). Overall, a plausible evolutionary hypothesis is that species that have more diversity in social monitoring due to higher levels of hierarchy despotism also exhibit greater diversity in the genetic encoding of the serotonergic pathway.
Interactions between the Central Serotonin and Oxytocin Systems in Social Monitoring
Oxytocin has been widely implicated in social cognition, influencing multiple stages of social processing, including social attention and valuation(Piva & Chang, 2018). Oxytocin has been shown to increase attention to social stimuli(Dal Monte et al., 2017; Parr et al., 2016), particularly to the eye region(Dal Monte, Noble, Costa, & Averbeck, 2014; Kotani et al., 2017), and to also increase gaze following(Putnam et al., 2016; Tollenaar, Chatzimanoli, van der Wee, & Putman, 2013). Increasing central OT also seems to amplify social preferences(Chang, Barter, Ebitz, Watson, & Platt, 2012), regulate social vigilance(Ebitz, Watson, & Platt, 2013; Landman, Sharma, Sur, & Desimone, 2014) and differentially impact attention and brain activation to different facial expressions(Domes, Steiner, Porges, & Heinrichs, 2013; Liu et al., 2015; Parr, Modi, Siebert, & Young, 2013).
Oxytocin and serotonin are increasingly being studied in tandem. Research has shown that OT receptors are expressed in serotonergic cells in the rodent DRN(Pagani et al., 2015; Yoshida et al., 2009), and that serotonin transporter-containing fibers overlap with oxytocin labeled cells in non-human primates(Emiliano, Cruz, Pannoni, & Fudge, 2007). It has also been shown that increasing central serotonin release via multiple methods, including serotonin receptor agonists, induces OT release in rodents, non-human primates, and humans(Bagdy & Kalogeras, 1993; Jørgensen, Riis, Knigge, Kjaer, & Warberg, 2003; Marazziti et al., 2012). Mottolese and colleagues also found that administering OT, compared to a placebo, increases serotonin binding potential in regions associated with social processing, attention, and valuation in humans: DRN, amygdala/hippocampal complex, insula, and orbitalfrontal cortex(Mottolese, Redouté, Costes, Le Bars, & Sirigu, 2014). This relationship is also present in the macaque brain(Lefevre, Richard, et al., 2017). However, the recruitment of 5-HT by OT seems to be heavily blunted in individuals with Autism Spectrum Disorders, suggesting that the interaction between OT and 5-HT is crucial for typical social behaviors, including monitoring others(Lefevre, Mottolese, et al., 2017). In addition, Dölen and colleagues found that in mice, coordinated activity between OT and 5-HT in the nucleus accumbens is required for social interactions to be reinforcing(Dölen, Darvishzadeh, Huang, & Malenka, 2013). Thus, it has been hypothesized that both oxytocin and serotonin impact social attention and social monitoring in tandem. It would be interesting to further explore the interaction between OT and 5-HT and their effect on social behaviors, specifically social monitoring and aggressive behaviors, in the future.
Conclusions and Future Directions
In this focused article, we examined the relative costs and benefits primates must consider when monitoring other conspecifics. In doing so, we discussed the methods by which primates gather social information and the importance of this information. Monitoring others is guided by its relative costs; accidental aggression to conspecifics and lost opportunities to monitor the environment and gain information about food sources, predation, and environmental risk. Therefore, the ability to balance social and environmental monitoring requires flexibility, a core aspect of behavior that serotonin has been implicated in regulating(H. Clarke, Dalley, Crofts, Robbins, & Roberts, 2004; H. Clarke et al., 2005; Matias, Lottem, Dugue, & Mainen, 2017). Indeed, primates are required to flexibly shift between obtaining useful social information from group members and acquiring valuable information about potential threats and resource opportunities from the environment. Evidence across primatology and psychology supports that social monitoring can greatly vary due to age, gender, and dominance status. Furthermore, it seems that variability in social tolerance explains how, and why, social monitoring differs across populations and across macaque species. The evolutionarily plausible account we present is that individual, population, and species variability in social monitoring and dominance behaviors seems to be, at least in part, linked to variability in serotonergic function and the genetic encoding of the serotonergic pathway. The central serotonin system may have thus played a significant role in shaping social monitoring in primate societies.
Acknowledgements:
This work was supported by the National Institute of Mental Health (R01 MH110750). We thank Amrita Nair for helpful comments.
References:
- Anderson JR (1998). Social stimuli and social rewards in primate learning and cognition. Behavioural processes, 42(2–3), 159–175. [DOI] [PubMed] [Google Scholar]
- Andrews MW, Bhat MC, & Rosenblum LA (1995). Acquisition and long-term patterning of joystick selection of food-pellet vs social-video reward by bonnet macaques. Learning and Motivation, 26(4), 370–379. [Google Scholar]
- Bagdy G, & Kalogeras KT (1993). Stimulation of 5-HT1A and 5-HT2/5-HT1C receptors induce oxytocin release in the male rat. Brain research, 611(2), 330–332. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam KN, Dittmar K, Berman CM, Butovskaya M, Cooper MA, Majolo B, . . . De Waal F (2012). Hierarchical Steepness, Counter‐Aggression, and Macaque Social Style Scale. American Journal of Primatology, 74(10), 915–925. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Ellis AJ, Wells TT, & McGeary JE (2010). Serotonin transporter gene promoter region polymorphism and selective processing of emotional images. Biological psychology, 83(3), 260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers CG, Marti CN, Lee H-J, Stote DL, Ferrell RE, Hariri AR, & Telch MJ (2011). Associations between serotonin transporter gene promoter region (5-HTTLPR) polymorphism and gaze bias for emotional information. Journal of abnormal psychology, 120(1), 187. [DOI] [PubMed] [Google Scholar]
- Bernstein IS, & Gordon TP (1980). The social component of dominance relationships in rhesus monkeys (Macaca mulatta). Animal behaviour, 28(4), 1033–1039. [Google Scholar]
- Bhagwagar Z, Cowen PJ, Goodwin GM, & Harmer CJ (2004). Normalization of enhanced fear recognition by acute SSRI treatment in subjects with a previous history of depression. American Journal of Psychiatry, 161(1), 166–168. [DOI] [PubMed] [Google Scholar]
- Boll S, & Gamer M (2014). 5-HTTLPR modulates the recognition accuracy and exploration of emotional facial expressions. Frontiers in behavioral neuroscience, 8, 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij L, Van der Does AW, Haffmans PJ, Riedel WJ, Fekkes D, & Blom MJ (2005). The effects of high-dose and low-dose tryptophan depletion on mood and cognitive functions of remitted depressed patients. Journal of Psychopharmacology, 19(3), 267–275. [DOI] [PubMed] [Google Scholar]
- Bovet D, & Washburn DA (2003). Rhesus macaques (Macaca mulatta) categorize unknown conspecifics according to their dominance relations. Journal of Comparative Psychology, 117(4), 400. [DOI] [PubMed] [Google Scholar]
- Brent LJ, Heilbronner SR, Horvath JE, Gonzalez-Martinez J, Ruiz-Lambides A, Robinson AG, . . . Platt ML (2013). Genetic origins of social networks in rhesus macaques. Scientific reports, 3, 1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, & Lesch K-P (2007). Long story short: the serotonin transporter in emotion regulation and social cognition. Nature neuroscience, 10(9), 1103–1109. [DOI] [PubMed] [Google Scholar]
- Capitanio J (1987). Influences of early history and current circumstances on social attention and knowledge in macaques. Primate Rep, 18, 11–16. [Google Scholar]
- Capitanio JP, Boccia ML, & Colaiannia DJ (1985). The influence of rank on affect perception by pigtailed macaques (Macaca nemestrina). American Journal of Primatology, 8(1), 53–59. [DOI] [PubMed] [Google Scholar]
- Chang SW, Barter JW, Ebitz RB, Watson KK, & Platt ML (2012). Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta). Proceedings of the National Academy of Sciences, 109(3), 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney DL, & Seyfarth RM (1990). The representation of social relations by monkeys. Cognition, 37(1), 167–196. [DOI] [PubMed] [Google Scholar]
- Cheney DL, & Seyfarth RM (1999). Recognition of other individuals’ social relationships by female baboons. Animal behaviour, 58(1), 67–75. [DOI] [PubMed] [Google Scholar]
- Chiao JY (2010). Neural basis of social status hierarchy across species. Current opinion in neurobiology, 20(6), 803–809. [DOI] [PubMed] [Google Scholar]
- Clarke AS, & Boinski S (1995). Temperament in nonhuman primates. American Journal of Primatology, 37(2), 103–125. [DOI] [PubMed] [Google Scholar]
- Clarke H, Dalley J, Crofts H, Robbins T, & Roberts A (2004). Cognitive inflexibility after prefrontal serotonin depletion. Science, 304(5672), 878–880. [DOI] [PubMed] [Google Scholar]
- Clarke H, Walker S, Crofts H, Dalley J, Robbins T, & Roberts AC (2005). Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. Journal of Neuroscience, 25(2), 532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF (1992). Impulsive aggression and central serotonergic system function in humans: an example of a dimensional brain-behavior relationship. International clinical psychopharmacology, 7(1), 3–12. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Kavoussi RJ, Trestman RL, Gabriel SM, Cooper TB, & Siever LJ (1997). Serotonin function in human subjects: intercorrelations among central 5-HT indices and aggressiveness. Psychiatry research, 73(1), 1–14. [DOI] [PubMed] [Google Scholar]
- Coss RG, Marks S, & Ramakrishnan U (2002). Early environment shapes the development of gaze aversion by wild bonnet macaques (Macaca radiata). Primates, 43(3), 217–222. [DOI] [PubMed] [Google Scholar]
- Dal Monte O, Noble PL, Costa VD, & Averbeck BB (2014). Oxytocin enhances attention to the eye region in rhesus monkeys. Frontiers in neuroscience, 8(41). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Monte O, Piva M, Anderson KM, Tringides M, Holmes AJ, & Chang SW (2017). Oxytocin under opioid antagonism leads to supralinear enhancement of social attention. Proceedings of the National Academy of Sciences, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Monte O, Piva M, Morris JA, & Chang SW (2016). Live interaction distinctively shapes social gaze dynamics in rhesus macaques. Journal of neurophysiology, 116(4), 1626–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal F, & Luttrell LM (1985). The formal hierarchy of rhesus macaques: an investigation of the bared‐teeth display. American Journal of Primatology, 9(2), 73–85. [DOI] [PubMed] [Google Scholar]
- de Waal FB (1986). The integration of dominance and social bonding in primates. The Quarterly review of biology, 61(4), 459–479. [DOI] [PubMed] [Google Scholar]
- Deaner RO, Khera AV, & Platt ML (2005). Monkeys pay per view: adaptive valuation of social images by rhesus macaques. Current Biology, 15(6), 543–548. [DOI] [PubMed] [Google Scholar]
- Dobson SD, & Brent LJ (2013). On the evolution of the serotonin transporter linked polymorphic region (5-HTTLPR) in primates. Frontiers in human neuroscience, 7, 588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G, Darvishzadeh A, Huang KW, & Malenka RC (2013). Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature, 501(7466), 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Steiner A, Porges SW, & Heinrichs M (2013). Oxytocin differentially modulates eye gaze to naturalistic social signals of happiness and anger. Psychoneuroendocrinology, 38(7), 1198–1202. [DOI] [PubMed] [Google Scholar]
- Drayton LA, & Santos LR (2017). Do rhesus macaques, Macaca mulatta, understand what others know when gaze following? Animal behaviour, 134, 193–199. [Google Scholar]
- Duncan LE, & Keller MC (2011). A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. American Journal of Psychiatry, 168(10), 1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebitz RB, Watson KK, & Platt ML (2013). Oxytocin blunts social vigilance in the rhesus macaque. Proceedings of the National Academy of Sciences, 110(28), 11630–11635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emiliano AB, Cruz T, Pannoni V, & Fudge JL (2007). The interface of oxytocin-labeled cells and serotonin transporter-containing fibers in the primate hypothalamus: a substrate for SSRIs therapeutic effects? Neuropsychopharmacology, 32(5), 977. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Melega WP, Jorgensen MJ, Kaplan JR, & McGuire MT (2001). Social impulsivity inversely associated with CSF 5-HIAA and fluoxetine exposure in vervet monkeys. Neuropsychopharmacology, 24(4), 370–378. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Palanza P, Parmigiani S, de Almeida RM, & Miczek KA (2005). Serotonin and aggressive behavior in rodents and nonhuman primates: predispositions and plasticity. European Journal of Pharmacology, 526(1), 259–273. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Allen P, Lee F, Surguladze S, Tunstall N, Fu CH, . . . McGuire PK (2007). Modulation of neural response to happy and sad faces by acute tryptophan depletion. Psychopharmacology, 193(1), 31. [DOI] [PubMed] [Google Scholar]
- Garattini S, Giacalone E, & Valzelli L (1967). Isolation, aggressiveness and brain 5‐hydroxytryptamine turnover. Journal of Pharmacy and Pharmacology, 19(5), 338–339. [DOI] [PubMed] [Google Scholar]
- Gibboni RR, Zimmerman PE, & Gothard KM (2009). Individual differences in scanpaths correspond with serotonin transporter genotype and behavioral phenotype in rhesus monkeys (Macaca mulatta). Frontiers in behavioral neuroscience, 3, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons JL, Barr GA, Bridger WH, & Liebowitz SF (1979). Manipulations of dietary tryptophan: effects on mouse killing and brain serotonin in the rat. Brain research, 169(1), 139–153. [DOI] [PubMed] [Google Scholar]
- Gothard KM, Erickson CA, & Amaral DG (2004). How do rhesus monkeys (Macaca mulatta) scan faces in a visual paired comparison task? Animal cognition, 7(1), 25–36. [DOI] [PubMed] [Google Scholar]
- Hariri AR, & Holmes A (2006). Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends in cognitive sciences, 10(4), 182–191. [DOI] [PubMed] [Google Scholar]
- Harmer C, Bhagwagar Z, Perrett D, Völlm B, Cowen P, & Goodwin G (2003). Acute SSRI administration affects the processing of social cues in healthy volunteers. Neuropsychopharmacology, 28(1), 148. [DOI] [PubMed] [Google Scholar]
- Harmer C, Rogers R, Tunbridge E, Cowen P, & Goodwin G (2003). Tryptophan depletion decreases the recognition of fear in female volunteers. Psychopharmacology, 167(4), 411–417. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Mackay CE, Reid CB, Cowen PJ, & Goodwin GM (2006). Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biological psychiatry, 59(9), 816–820. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Shelley NC, Cowen PJ, & Goodwin GM (2004). Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. American Journal of Psychiatry, 161(7), 1256–1263. [DOI] [PubMed] [Google Scholar]
- Haude RH, Graber JG, & Farres AG (1976). Visual observing by rhesus monkeys: some relationships with social dominance rank. Learning & Behavior, 4(2), 163–166. [DOI] [PubMed] [Google Scholar]
- Hayward G, Goodwin GM, Cowen PJ, & Harmer CJ (2005). Low-dose tryptophan depletion in recovered depressed patients induces changes in cognitive processing without depressive symptoms. Biological psychiatry, 57(5), 517–524. [DOI] [PubMed] [Google Scholar]
- Higley J, King S, Hasert M, Champoux M, Suomi S, & Linnoila M (1996). Stability of interindividual differences in serotonin function and its relationship to severe aggression and competent social behavior in rhesus macaque females. Neuropsychopharmacology, 14(1), 67–76. [DOI] [PubMed] [Google Scholar]
- Higley J, & Linnoila M (1997). Low central nervous system serotonergic activity is traitlike and correlates with impulsive behavior. Annals of the New York Academy of Sciences, 836(1), 39–56. [DOI] [PubMed] [Google Scholar]
- Higley J, Suomi S, & Linnoila M (1996). A Nonhuman Primate Model of Type II Alcoholism? Part 2. Diminished Social Competence and Excessive Aggression Correlates with Low Cerebrospinal Fluid 5‐Hydroxyindoleacetic Acid Concentrations. Alcoholism: Clinical and Experimental Research, 20(4), 643–650. [DOI] [PubMed] [Google Scholar]
- Higley JD, Linnoila M, & Suomi SJ (1994). Ethological contributions. Handbook of aggressive and destructive behavior in psychiatric patients, 17–32. [Google Scholar]
- Higley JD, Mehlman P, Taub D, Higley S, Suomi SJ, Linnoila M, & Vickers J (1992). Cerebrospinal fluid monoamine and adrenal correlates of aggression in free-ranging rhesus monkeys. Archives of General Psychiatry, 49(6), 436–441. [DOI] [PubMed] [Google Scholar]
- Higley JD, Mehlman PT, Higley SB, Fernald B, Vickers J, Lindell SG, . . . Linnoila M (1996). Excessive mortality in young free-ranging male nonhuman primates with low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations. Archives of General Psychiatry, 53(6), 537–543. [DOI] [PubMed] [Google Scholar]
- Higley JD, Mehlman PT, Poland RE, Taub DM, Vickers J, Suomi SJ, & Linnoila M (1996). CSF testosterone and 5-HIAA correlate with different types of aggressive behaviors. Biological psychiatry, 40(11), 1067–1082. [DOI] [PubMed] [Google Scholar]
- Holekamp KE, & Smale L (1991). Dominance acquisition during mammalian social development: the “inheritance” of maternal rank. American Zoologist, 31(2), 306–317. [Google Scholar]
- Howell BR, Godfrey J, Gutman DA, Michopoulos V, Zhang X, Nair G, . . . Sanchez MM (2013). Social subordination stress and serotonin transporter polymorphisms: associations with brain white matter tract integrity and behavior in juvenile female macaques. Cerebral Cortex, 24(12), 3334–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonassen R, Chelnokova O, Harmer C, Leknes S, & Landrø N (2015). A single dose of antidepressant alters eye-gaze patterns across face stimuli in healthy women. Psychopharmacology, 232(5), 953–958. [DOI] [PubMed] [Google Scholar]
- Jørgensen H, Riis M, Knigge U, Kjaer A, & Warberg J (2003). Serotonin receptors involved in vasopressin and oxytocin secretion. Journal of neuroendocrinology, 15(3), 242–249. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Fontenot MB, & Mann JJ (2002). Central nervous system monoamine correlates of social dominance in cynomolgus monkeys (Macaca fascicularis). Neuropsychopharmacology, 26(4), 431–443. [DOI] [PubMed] [Google Scholar]
- Klaassen T, Riedel W, Deutz N, & Van Praag H (2002). Mood congruent memory bias induced by tryptophan depletion. Psychological medicine, 32(1), 167–172. [DOI] [PubMed] [Google Scholar]
- Kotani M, Shimono K, Yoneyama T, Nakako T, Matsumoto K, Ogi Y, . . . Ikeda K (2017). An eye tracking system for monitoring face scanning patterns reveals the enhancing effect of oxytocin on eye contact in common marmosets. Psychoneuroendocrinology, 83, 42–48. [DOI] [PubMed] [Google Scholar]
- Landman R, Sharma J, Sur M, & Desimone R (2014). Effect of distracting faces on visual selective attention in the monkey. Proceedings of the National Academy of Sciences, 111(50), 18037–18042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre A, Mottolese R, Redouté J, Costes N, Le Bars D, Geoffray M-M, . . . Sirigu A (2017). Oxytocin fails to recruit serotonergic neurotransmission in the autistic brain. Cerebral Cortex, 1–10. [DOI] [PubMed] [Google Scholar]
- Lefevre A, Richard N, Jazayeri M, Beuriat P-A, Fieux S, Zimmer L, . . . Sirigu A (2017). Oxytocin and serotonin brain mechanisms in the nonhuman primate. Journal of Neuroscience, 37(28), 6741–6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnoila M, Virkkunen M, Scheinin M, Nuutila A, Rimon R, & Goodwin FK (1983). Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sciences, 33(26), 2609–2614. [DOI] [PubMed] [Google Scholar]
- Liu N, Hadj-Bouziane F, Jones KB, Turchi JN, Averbeck BB, & Ungerleider LG (2015). Oxytocin modulates fMRI responses to facial expression in macaques. Proceedings of the National Academy of Sciences, 112(24), E3123–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Burgund ED, Berman M, & Hanson KL (2001). Effects of tryptophan loading on verbal, spatial and affective working memory functions in healthy adults. Journal of Psychopharmacology, 15(4), 219–230. [DOI] [PubMed] [Google Scholar]
- Maestripieri D (1997). Gestural communication in macaques: usage and meaning of nonvocal signals. Evolution of communication, 1(2), 193–222. [Google Scholar]
- Maestripieri D, & Wallen K (1997). Affiliative and submissive communication in rhesus macaques. Primates, 38(2), 127–138. [Google Scholar]
- Manuck SB, Flory JD, McCaffery JM, Matthews KA, Mann JJ, & Muldoon MF (1998). Aggression, impulsivity, and central nervous system serotonergic responsivity in a nonpatient sample. Neuropsychopharmacology, 19(4), 287–299. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Baroni S, Giannaccini G, Betti L, Massimetti G, Carmassi C, & Catena-Dell’Osso M (2012). A link between oxytocin and serotonin in humans: supporting evidence from peripheral markers. European Neuropsychopharmacology, 22(8), 578–583. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Buzas B, Soliman N, Richell RA, Vythilingham M, . . . Blair R (2006). Impaired recognition of fear facial expressions in 5-HTTLPR S-polymorphism carriers following tryptophan depletion. Psychopharmacology, 189(3), 387–394. [DOI] [PubMed] [Google Scholar]
- Matias S, Lottem E, Dugue GP, & Mainen ZF (2017). Activity patterns of serotonin neurons underlying cognitive flexibility. Elife, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlman P, Higley J, Faucher I, Lilly A, Taub D, Vickers J, . . . Linnoila M (1994). Low CSF 5-HIAA concentrations and severe aggression and impaired impulse control in nonhuman primates. Am J Psychiatry, 151(1), 0. [DOI] [PubMed] [Google Scholar]
- Mehlman PT, Higley JD, Fernald BJ, Sallee FR, Suomi SJ, & Linnoila M (1997). CSF 5-HIAA, testosterone, and sociosexual behaviors in free-ranging male rhesus macaques in the mating season. Psychiatry research, 72(2), 89–102. [DOI] [PubMed] [Google Scholar]
- Mendelsohn D, Riedel WJ, & Sambeth A (2009). Effects of acute tryptophan depletion on memory, attention and executive functions: a systematic review. Neuroscience & Biobehavioral Reviews, 33(6), 926–952. [DOI] [PubMed] [Google Scholar]
- Merens W, Van der Does AW, & Spinhoven P (2007). The effects of serotonin manipulations on emotional information processing and mood. Journal of affective disorders, 103(1), 43–62. [DOI] [PubMed] [Google Scholar]
- Mosher CP, Zimmerman PE, & Gothard KM (2014). Neurons in the monkey amygdala detect eye contact during naturalistic social interactions. Current Biology, 24(20), 2459–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottolese R, Redouté J, Costes N, Le Bars D, & Sirigu A (2014). Switching brain serotonin with oxytocin. Proceedings of the National Academy of Sciences, 111(23), 8637–8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Hayward G, & Harmer C (2006). Selective processing of social threat cues following acute tryptophan depletion. Journal of Psychopharmacology, 20(1), 33–39. [DOI] [PubMed] [Google Scholar]
- Murphy SE, Longhitano C, Ayres RE, Cowen PJ, & Harmer CJ (2006). Tryptophan supplementation induces a positive bias in the processing of emotional material in healthy female volunteers. Psychopharmacology, 187(1), 121–130. [DOI] [PubMed] [Google Scholar]
- Nahm FK, Perret A, Amaral DG, & Albright TD (1997). How do monkeys look at faces? Journal of cognitive neuroscience, 9(5), 611–623. [DOI] [PubMed] [Google Scholar]
- Pagani JH, Williams Avram S, Cui Z, Song J, Mezey É, Senerth JM, . . . Young WS (2015). Raphe serotonin neuron‐specific oxytocin receptor knockout reduces aggression without affecting anxiety‐like behavior in male mice only. Genes, Brain and Behavior, 14(2), 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr LA, Brooks JM, Jonesteller T, Moss S, Jordano JO, & Heitz TR (2016). Effects of chronic oxytocin on attention to dynamic facial expressions in infant macaques. Psychoneuroendocrinology, 74, 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr LA, & Heintz M (2009). Facial expression recognition in rhesus monkeys, Macaca mulatta. Animal behaviour, 77(6), 1507–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr LA, Modi M, Siebert E, & Young LJ (2013). Intranasal oxytocin selectively attenuates rhesus monkeys’ attention to negative facial expressions. Psychoneuroendocrinology, 38(9), 1748–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr LA, Winslow JT, Hopkins WD, & de Waal F (2000). Recognizing facial cues: individual discrimination by chimpanzees (Pan troglodytes) and rhesus monkeys (Macaca mulatta). Journal of Comparative Psychology, 114(1), 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partan SR (2002). Single and multichannel signal composition: facial expressions and vocalizations of rhesus macaques (Macaca mulatta). Behaviour, 139(8), 993–1027. [Google Scholar]
- Paxton R, Basile BM, Adachi I, Suzuki WA, Wilson ME, & Hampton RR (2010). Rhesus monkeys (Macaca mulatta) rapidly learn to select dominant individuals in videos of artificial social interactions between unfamiliar conspecifics. Journal of Comparative Psychology, 124(4), 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit O, Abegg C, & Thierry B (1997). A comparative study of aggression and conciliation in three cercopithecine monkeys (Macaca fuscata, Macaca nigra, Papio papio). Behaviour, 134(5), 415–432. [Google Scholar]
- Piva M, & Chang SW (2018). An integrated framework for the role of oxytocin in multistage social decision‐making. American Journal of Primatology, e22735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokorny JJ, & de Waal FB (2009). Monkeys recognize the faces of group mates in photographs. Proceedings of the National Academy of Sciences, 106(51), 21539–21543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusey A, Williams J, & Goodall J (1997). The Influence of Dominance Rank on the Reproductive Success of Female Chimpanzees. Science, 277(5327), 828–831. doi: 10.1126/science.277.5327.828 [DOI] [PubMed] [Google Scholar]
- Putnam P, Roman J, Zimmerman P, & Gothard K (2016). Oxytocin enhances gaze-following responses to videos of natural social behavior in adult male rhesus monkeys. Psychoneuroendocrinology, 72, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu C, Ligneul R, Van der Henst J-B, & Dreher J-C (2017). An Integrative Interdisciplinary Perspective on Social Dominance Hierarchies. Trends in cognitive sciences, 21(11), 893–908. [DOI] [PubMed] [Google Scholar]
- Riedel WJ, Klaassen T, & Schmitt JA (2002). Tryptophan, mood, and cognitive function. Brain, behavior, and immunity, 16(5), 581–589. [DOI] [PubMed] [Google Scholar]
- Robinson O, Cools R, Crockett M, & Sahakian B (2010). Mood state moderates the role of serotonin in cognitive biases. Journal of Psychopharmacology, 24(4), 573–583. [DOI] [PubMed] [Google Scholar]
- Roiser JP, Levy J, Fromm SJ, Wang H, Hasler G, Sahakian BJ, & Drevets WC (2008). The effect of acute tryptophan depletion on the neural correlates of emotional processing in healthy volunteers. Neuropsychopharmacology, 33(8), 1992–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati AG, Arre AM, Platt ML, & Santos LR (2016). Rhesus monkeys show human-like changes in gaze following across the lifespan. Proc. R. Soc. B, 283(1830), 20160376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati AG, & Santos LR (2017). Tolerant Barbary macaques maintain juvenile levels of social attention in old age, but despotic rhesus macaques do not. Animal behaviour, 130, 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell TE (1974). The concept of social dominance. Behavioral biology, 11(2), 131–154. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM (2005). The influence of social hierarchy on primate health. Science, 308(5722), 648–652. [DOI] [PubMed] [Google Scholar]
- Shepherd SV, Deaner RO, & Platt ML (2006). Social status gates social attention in monkeys. Current Biology, 16(4), R119–R120. [DOI] [PubMed] [Google Scholar]
- Shepherd SV, Steckenfinger SA, Hasson U, & Ghazanfar AA (2010). Human-monkey gaze correlations reveal convergent and divergent patterns of movie viewing. Current Biology, 20(7), 649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber B, & Schmitt J (2010). Effects of tryptophan loading on human cognition, mood, and sleep. Neuroscience & Biobehavioral Reviews, 34(3), 387–407. [DOI] [PubMed] [Google Scholar]
- Silk JB (2007). Social components of fitness in primate groups. Science, 317(5843), 1347–1351. [DOI] [PubMed] [Google Scholar]
- Simpson EA, Jakobsen KV, Damon F, Suomi SJ, Ferrari PF, & Paukner A (2017). Face detection and the development of own‐species bias in infant macaques. Child development, 88(1), 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solyst JA, & Buffalo EA (2014). Social relevance drives viewing behavior independent of low-level salience in rhesus macaques. Frontiers in neuroscience, 8, 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub D, & Vickers J (1995). Correlation of CSF 5-HIAA concentration with sociality and the timing of emigration in free-ranging primates. Am J Psychiatry, 1(52), 907. [DOI] [PubMed] [Google Scholar]
- Thierry B (1985). Patterns of agonistic interactions in three species of macaque (Macaca mulatta, M fascicularis, M tonkeana). Aggressive Behavior, 11(3), 223–233. [Google Scholar]
- Thierry B (2007). Unity in diversity: lessons from macaque societies. Evolutionary Anthropology: Issues, News, and Reviews, 16(6), 224–238. [Google Scholar]
- Thierry B, Iwaniuk AN, & Pellis SM (2000). The influence of phylogeny on the social behaviour of macaques (Primates: Cercopithecidae, genus Macaca). Ethology, 106(8), 713–728. [Google Scholar]
- Tollenaar MS, Chatzimanoli M, van der Wee NJ, & Putman P (2013). Enhanced orienting of attention in response to emotional gaze cues after oxytocin administration in healthy young men. Psychoneuroendocrinology, 38(9), 1797–1802. [DOI] [PubMed] [Google Scholar]
- Trivers RL (1971). The evolution of reciprocal altruism. The Quarterly review of biology, 46(1), 35–57. [Google Scholar]
- Turrin C, Fagan NA, Dal Monte O, & Chang SW (2017). Social resource foraging is guided by the principles of the Marginal Value Theorem. Scientific reports, 7(1), 11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valzelli L (1971). Further aspects of the exploratory behaviour in aggressive mice. Psychopharmacology, 19(1), 91–94. [DOI] [PubMed] [Google Scholar]
- van der Veen FM, Evers EA, Deutz NE, & Schmitt JA (2007). Effects of acute tryptophan depletion on mood and facial emotion perception related brain activation and performance in healthy women with and without a family history of depression. Neuropsychopharmacology, 32(1), 216–224. [DOI] [PubMed] [Google Scholar]
- Watson K, Li D, Brent L, Horvath J, Gonzalez-Martinez J, Ruíz-Lambides A, . . . Platt M (2015). Genetic influences on social attention in free-ranging rhesus macaques. Animal behaviour, 103, 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson KK, Ghodasra JH, & Platt ML (2009). Serotonin transporter genotype modulates social reward and punishment in rhesus macaques. PloS one, 4(1), e4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg-Wolf H, Fagan NA, Anderson GM, Tringides M, Dal Monte O, & Chang SW (2018). The effects of 5-hydroxytryptophan on attention and central serotonin neurochemistry in the rhesus macaque. Neuropsychopharmacology, 43(7), 1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland JR, Lesch K-P, Newman TK, Timme A, Gachot-Neveu H, Thierry B, & Suomi SJ (2006). Differential functional variability of serotonin transporter and monoamine oxidase a genes in macaque species displaying contrasting levels of aggression-related behavior. Behavior genetics, 36(2), 163–172. [DOI] [PubMed] [Google Scholar]
- Westergaard G, Mehlman P, Westergaard G, Suomi S, & Higley J (1999). CSF 5-HIAA and aggression in female macaque monkeys: species and interindividual differences. Psychopharmacology, 146(4), 440–446. [DOI] [PubMed] [Google Scholar]
- Westergaard GC, Suomi SJ, Chavanne TJ, Houser L, Hurley A, Cleveland A, . . . Higley JD (2003). Physiological correlates of aggression and impulsivity in free-ranging female primates. Neuropsychopharmacology, 28(6), 1045. [DOI] [PubMed] [Google Scholar]
- Wittig RM, & Boesch C (2003). Food Competition and Linear Dominance Hierarchy Among Female Chimpanzees of the Taï National Park. International Journal of Primatology, 24(4), 847–867. doi: 10.1023/a:1024632923180 [DOI] [Google Scholar]
- Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, Onaka T, & Nishimori K (2009). Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. Journal of Neuroscience, 29(7), 2259–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SN (1996). Behavioral effects of dietary neurotransmitter precursors: basic and clinical aspects. Neuroscience & Biobehavioral Reviews, 20(2), 313–323. [DOI] [PubMed] [Google Scholar]
- Zajicek KB, Price CS, Shoaf SE, Mehlman PT, Suomi SJ, Linnoila M, & Higley JD (2000). Seasonal variation in CSF 5-HIAA concentrations in male rhesus macaques. Neuropsychopharmacology, 22(3), 240–250. [DOI] [PubMed] [Google Scholar]