Abstract
The stream of human consciousness persists during sleep, albeit in altered form. Disconnected from external input, the mind and brain remain active, at times creating the bizarre sequences of thought and imagery that comprise “dreaming”. Yet despite substantial effort towards understanding this unique state of consciousness, no reliable neurophysiological indicator of dreaming has been discovered. Here, we identified electroencephalographic (EEG) correlates of dreaming using a within-subjects design to characterize the EEG preceding awakenings from sleep onset, REM (rapid eye movement) sleep, and N2 (NREM Stage 2) sleep from which participants were asked to report their mental experience. During transition into sleep, compared to periods during which participants reported thinking, emergence of dream imagery was associated with increased absolute power below 7Hz. During later N2, dreaming conversely occurred during periods of decreased relative power below 1Hz, accompanied by an increase in relative power above 4Hz. No EEG predictors of dreaming were identified during REM. These observations suggest an inverted-u relationship between dreaming and the prevalence of low-frequency EEG rhythms, such that dreaming first emerges in concert with EEG slowing during the sleep-wake transition, but then disappears as high-amplitude slow oscillations come to dominate the recording during later N2 sleep.
1. Introduction
Dreaming1 is a unique state of consciousness intermittently present during sleep, during which perceptual imagery is generated in the absence of sensory input. Although modern neuroscience is increasingly endeavoring to describe the mechanisms of sleep consciousness, surprisingly, a reliable biomarker of dream experience has yet to be identified. Here, we examine EEG predictors of dream recall from both REM and NREM sleep throughout the night.
The discovery of rapid eye movement sleep (REM) in the 1950’s was originally hailed as a discovery of the brain basis of dreaming (Aserinsky & Kleitman, 1953). However, it was subsequently found that complex and vivid dreams are also common during NREM sleep, despite the presence of high-amplitude slow waves classically thought to signify a state of neural “inactivity” (Antrobus, 1983; Foulkes, 1962; Wamsley, Hirota, Tucker, Smith, & Antrobus, 2007). As REM-specific neurophysiology now appears insufficient to explain dreaming, more recent investigations have focused on candidate neural mechanisms common to both REM and NREM sleep.
Within-stage variability in NREM neurophysiology offers a particularly promising avenue for identifying EEG correlates of dreaming, with recent data contradicting the simplistic notion of NREM as a static state of “inactivity”. PET studies, for example, reveal that selected brain regions remain relatively active during NREM sleep (Nofzinger et al., 2002; Peigneux et al., 2004). NREM sleep regional cerebral blood flow (rCBF) is also highly variable across the night – in a number of cortical and subcortical regions, rCBF is inversely related to slow wave activity (power < 4hz), with increased rCBF seen during “lighter” epochs of NREM sleep containing less slow wave activity (Dang-Vu et al., 2005; Hofle et al., 1997). The NREM sleep EEG is itself highly variable across the night, with periods of N2 proximal to slow wave sleep containing large amounts of high-amplitude, slow wave activity, and epochs of “lighter” N2 proximal to waking or REM dominated by a higher-frequency theta EEG pattern.
Several recent studies have indeed reported within-stage EEG predictors of dream recall in both REM and NREM sleep. However, this literature has been highly inconsistent. The recall of dreaming during NREM, for example, has variously been associated with decreased alpha activity (Esposito, Nielsen, & Paquette, 2004; Marzano et al., 2011), increased alpha activity (Takeuchi, Ogilvie, Murphy, & Ferrelli, 2003), decreased spindle-frequency power (Chellappa, Münch, Knoblauch, & Cajochen, 2012), increased sleep spindling (Nielsen et al., 2016), and decreased delta power (Chellappa et al., 2012; Esposito et al., 2004; Scarpelli et al., 2017; Siclari et al., 2017). There is also wide variability in the claimed localization of these effects – for example, the association between dreaming and delta power has variously been reported to be expressed primarily over frontal regions (bilateral (Esposito et al., 2004)), left frontal and temporo-parietal cortex (Scarpelli et al., 2017), or posterior parietal / occipital cortex (Siclari et al., 2017)).
Methodological limitations and inconsistencies may explain this cross-study variability. First, several prior investigations have utilized between-subjects designs in which participants who recall dreams are compared to those who do not (Eichenlaub, Bertrand, Morlet, & Ruby, 2014; Marzano et al., 2011). This method is subject to substantial individual difference confounds, as participants with high dream recall (a stable trait) are known to differ from those with low dream recall on a number of dimensions, including personality traits (Hartmann, 1989; Hill, Diemer, & Heaton, 1997; Schredl, Nürnberg, & Weiler, 1996), and neurobiological traits (Eichenlaub, Nicolas, et al., 2014; Ruby et al., 2013). Studies have also varied substantially in their method of determining whether a participant dreamed, with some coding detailed open-ended subjective reports (Esposito et al., 2004), and others simply asking participants to report whether or “how much” they dreamed (Chellappa et al., 2012; Marzano et al., 2011; Scarpelli, Marzano, et al., 2015; Takeuchi et al., 2003).
Given our laboratory’s interest in the memory function of sleep (Brokaw et al., 2016; Wamsley, 2014), of particular interest to us have been recent reports that dream recall during NREM and REM sleep may be associated with EEG features similar to those predicting successful memory encoding in the awake state (Marzano et al., 2011; Scarpelli, Marzano, et al., 2015). In a 2011 paper using a between-subjects design, Marzano et al. reported that dream recall during REM sleep was associated with increased frontal theta power, and during NREM sleep was associated with reduced right temporal alpha power (Marzano et al., 2011). These observations arguably mirror EEG correlates of successful episodic encoding during wakefulness (Marzano et al., 2011).
In the current study, our goals were twofold. First, we aimed to investigate EEG correlates of dreaming during sleep onset, N2 and REM sleep in 5 a priori frequency bands using a “gold-standard” experimental approach -- a repeated-measures design in which each participant is awakened from PSG-defined sleep to provide open-ended verbal reports on their mental experience. Given the prior results of Marzano et al. (2011), we had a special interest in the alpha and theta bands. Second, we aimed to assess whether prior reports of EEG differences between participants who do and do not recall a dream may be attributed to individual differences by exploring whether we could replicate the interesting between-subjects effects reported by Marzano et al. (2011) in a within-subjects design, while also conducting the same analyses in a between-subjects fashion for comparative purposes.
2. Method
2.1. Participants
N = 40 participants age 18 to 23 successfully completed the study (mean age 20.5 +/− 1.3SD; 70% male; Table 1). Participants were recruited through advertisement on campus, and respondents were excluded from enrolling if they had a history of sleep or mental disorders, were currently using medications known to interfere with sleep, or had little or no prior experience playing 3D style video games (due to the goals of the larger study of which this research was a part). Participants were asked to maintain a regular sleep schedule for three consecutive nights prior to the experimental night (confirmed by sleep log), to refrain from recreational drugs or alcohol for 24hrs prior to their appointment, and not to drink caffeine after 10am on the day of the study. Participants received monetary compensation at the conclusion of the study. The study was approved by the IRB committee at Furman University in Greenville, SC.
Table 1.
Participant Demographics and Sleep Architecture (N = 40)
| Mean | ±SD | |
|---|---|---|
| Age (yrs) | 20.5 | 1.3 |
| Sex (% male) | 70% (28) | |
| Dream Recall Frequency | 2.4 | 0.8 |
| TST (min) | 460.1 | 36.1 |
| N1 (min) | 32.1 | 10.2 |
| N2 (min) | 230.7 | 47.7 |
| N3 (min) | 103.5 | 24.5 |
| REM | 89.3 | 21.8 |
Notes. Means ± SD; Habitual dream recall was measured on a 5-point self-report scale ranging from recalling dreams “less than once a year” to “every day”. TST = Total Sleep Time, N1 = Stage 1 sleep, N2 = Stage 2 sleep, N3 = Stage 3/slow wave sleep, REM = Rapid Eye Movement Sleep.
2.2. Procedure
Participants signed informed consent prior to filling out a demographics form, Epworth sleepiness scale (Johns, 1991), and a 3-day retrospective sleep log. High-density electrode caps (BrainProducts) were used to record EEG throughout the night (58 EEG electrodes placed following the international 10–10 system). During recording, EEG signals were referenced to the contralateral mastoid to aid on-line sleep staging. Additionally, muscle tone was monitored using electromyography (EMG; bipolar chin leads) and eye movement was monitored using electrooculography (EOG; left and right outer canthus). All data were recorded at 400Hz using a Grass-Telefactor AURA amplifier with a high-pass filter at 0.1Hz. After applying the electrodes, participants were randomly assigned to either train on a virtual maze navigation task (Wamsley, Tucker, Payne, & Stickgold, 2010; Wamsley, Tucker, Payne, Benavides, & Stickgold, 2010) or complete a control task (the psychomotor vigilance task (PVT) (Dinges & Powell, 1985)). In both groups, participants also underwent a 7min eyes-closed baseline EEG recording both before and after task performance. These training procedures were a part of a larger study investigating memory reactivation following a spatial learning task and its effect on memory consolidation. The experimental group was trained on the maze navigation task prior to sleep and was tested on the same task the next morning, while the control group was trained on the PVT prior to sleep and performed maze navigation task the next morning. Results of this behavioral testing are not discussed here but will be reported in a subsequent paper. The two tasks did not substantially influence dream content as only one mentation report was judged by raters to be task-related. Dream recall rates were comparable between groups - participants completing the maze task recalled dreams from 78.8% of awakenings, as compared to 79.2% of awakenings among participants completing the control task.
Prior to sleep, participants were given instructions on how to provide open-ended mentation reports throughout the night. Specifically, they were asked to verbally describe “everything that was going through your mind just before I called” whenever they heard the prompt “please report now”. Participants were instructed to provide as much detail as possible on their experience, regardless of whether they considered it to be a “dream” or not, and were instructed that if they cannot remember their experience or were not having an experience, they should state this instead.
Figure 1 illustrates the timeline for dream report collection during the night. Participants lay down to begin a 9hr sleep opportunity at approximately 11pm. After participants entered sleep, indicated by online PSG (polysomnographic) monitoring, they were awoken periodically to provide verbal reports on their mental experience. Following our prior work, we maximized the number of report samples per participant by conducting a large number of awakenings during the sleep onset period (during which dream recall is high and participants are able to fall back to sleep quickly) combined with a smaller number of awakenings later in the night (Wamsley, Perry, Djonlagic, Reaven, & Stickgold, 2010). This method has the advantage of yielding a high number of datapoints per participant without exposing participant to substantial sleep deprivation. Up to 10 “sleep onset” dream reports were obtained during the first hour of the night, collected after 30, 60, or 90 seconds of elapsed sleep (order of latency counterbalanced across participants). As a result, sleep onset reports occurred during a mix of NREM stages -- 61% of sleep onset reports were obtained from N1, 38% from N2, and 2% from N3. Then, at least one hour after the last sleep onset awakening, one N2 sleep report (following at least 10min of PSG-defined N2) and one REM sleep dream report (following at least 5min of PSG-defined REM) were collected, also in counterbalanced order. N2 and REM report awakenings were separated by at least 30min. Participants were then allowed to sleep uninterrupted until being awoken at approximately 8am the next morning to provide a final dream report, regardless of sleep stage. These morning reports were also classified as either “REM” or “N2” reports according to the last sleep stage present prior to awakening, and analyzed along with other REM and N2 reports. As described below, 3 of these morning reports were excluded, either because the participant was in slow wave sleep rather than N2 prior to awakening, or because EEG data prior to awakening were unusable. Although systematic awakenings from slow wave sleep would be of theoretical interest, concerns regarding the possible introduction of sleep deprivation and the low rate of dream recall reported during this stage in previous studies precluded conducting a larger number of nocturnal awakenings (Foulkes, 1962). At each report time point, participants were first awoken by calling their name, and then heard a standardized prompt “Please report now”. Verbal reports were digitally recorded and transcribed for subsequent analysis.
Figure 1. Dream Report Collection.
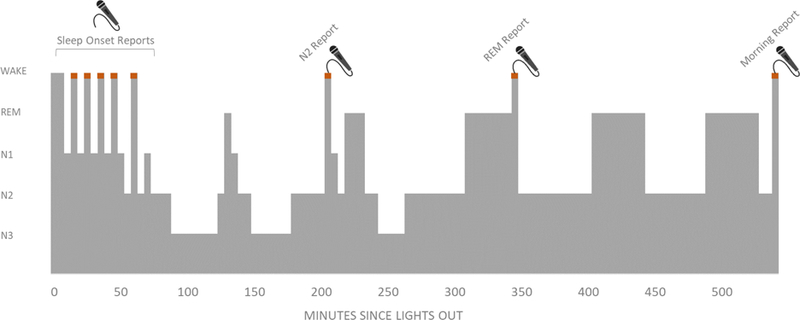
While spending the night in the sleep laboratory, participants were awakened up to 13 times to report on their current subjective experience. Up to 10 awakenings were made from the sleep onset phase, within the first hour of the night. Additional awakenings from REM and N2 sleep were conducted later in the night. To avoid time-of-night confounds, order of REM/N2 awakenings was counterbalanced across subjects. A final subjective report was collected upon morning awakening, regardless of sleep stage.
2.3. Dream Report Coding
In this study, we define a “dream” as any mental experience recalled from sleep. Within this broad category, we further discriminated between dream reports that contained perceptual imagery, and those that contained thought in the absence of any perceptual imagery. A total of 428 reports were coded for the presence of thought and imagery by 2 independent judges, who were blind to sleep stage and experimental condition. For each report, judges assessed first whether the report contained a description of any mental content, and if so, whether or not that mental content contained perceptual imagery. Reports that contained no mental content (e.g. “I can’t remember” or “There was nothing”) are referred to as “No Content” reports. Reports containing perceptual imagery are referred to as “Imagery” reports. Reports with content but without any perceptual imagery are referred to as “Thought” reports. Inter-rater agreement for whether the report contained content was 97.4% and for whether or not the report contained imagery was 90.9%. Disagreements were resolved by a 3rd judge, who was also blind to sleep stage and experimental condition.
2.4. EEG Analysis
PSG data were scored for sleep stage following the standardized criteria established by the American Academy for Sleep Medicine (AASM) (Iber, Ancoli-Israel, Chesson, & Quan, 2007). Awakenings which did not meet the target sleep stage criteria described above were excluded from further analysis. Quantitative EEG analyses focused on characterizing the spatial-frequency content of the EEG prior to awakenings that yielded No Content, Imagery, and Thought reports, and were carried out using BrainVision Anlayzer 2 (Brain Products, GmbH), and the EEGLab toolbox for MatLab (Delorme et al., 2011). Artifacts were rejected via a combination of visual inspection/removal of artifact-laden trials, and rejection of artifactual independent components. Noisy EEG channels were removed and, where possible, interpolated using spherical splines. For each participant, all useable electrodes were included for analysis (mean useable number of electrodes = 56.3 +/− 2.0SD).
We then examined the power spectrum preceding the moment of each report awakening, calculating mean power spectral density (µV2/Hz) in five a priori frequency bands: slow oscillation (all frequencies < 1Hz remaining after the aforementioned 0.1Hz high pass filtering), delta (1–4Hz), theta (4–7Hz), alpha (8–12Hz), and beta (13–35Hz). For each participant, peak alpha frequency was defined as the frequency at which power was maximal within the 8–12Hz range, for the average spectra across all electrodes.
Sleep onset awakenings were analyzed separately from later night N2 awakenings due to well-described quantitative and qualitative differences between sleep onset and later night NREM dreaming (Foulkes & Vogel, 1965; Vogel, 1991). For 30/60/90s sleep onset awakenings, the timeframe analyzed consisted of all artifact-free sleep preceding the awakening. For later-night awakenings, the timeframe analyzed consisted of all artifact-free time during the 2min preceding awakening. Power spectra were calculated using fast Fourier transform (FFT), utilizing all artifact-free 4sec segments, with 50% segment overlap (Hanning window).
For each participant, all observations in one dream recall category (No Content/Thought/Imagery) were averaged, with participant averages used as the unit of analysis for subsequent statistical comparisons. For spectral power, we calculated both absolute and relative power (absolute power/total power in the 0–100Hz range). To reduce the influence of extreme values, for each electrode and frequency band, power data were converted to z-scores and data points with values > 2SD from the mean were excluded from analysis. Of 82,895 spectral analysis data points, 3,815 (4.6%) were rejected as extreme (> 2SD from mean). As rejected points were widely distributed across participants, electrodes, times, and frequency bands, this data cleaning did not result in the exclusion of any participant or electrode in its entirety. The resulting power values were then log-transformed (log10(x+1-min(x))) to normalize the distributions.
In this paper, we report both absolute and relative power measures for all comparisons. The reasons for this are twofold. First, the prior literature connecting EEG spectral power to cognitive processes in sleep (and in wake) has been inconsistent in the power metrics that have been reported, which creates an impediment to drawing conclusions across studies. By reporting results for both absolute and relative power, we ensure that our findings can more easily be compared to those of others or included in future meta-analyses. Second, it remains unknown whether absolute or relative power is the most cognitively relevant metric in studies of dream recall, and thus in our view it is not possible to a priori select one of these metrics as the definitively appropriate measure.
2.5. Statistical Analysis.
Hierarchical linear models were used to compare spectral power across No Content, Thought, and Imagery reports. Recall category was a repeated factor, grouped by subject. To test for the overall effect of recall category (No Content/Thought/Imagery) on the EEG power spectrum, hierarchical linear models were first conducted on the mean slow oscillation, delta, theta, alpha, and beta power averaged across all electrode sites.
Excluding Thought reports from analyses of N2 and REM sleep awakenings (which were exceedingly infrequent outside of sleep onset - see Results), there were a total of 7 analyzed report types per participant: Sleep Onset - No Content, Sleep Onset - Thought, Sleep Onset - Imagery, N2 - No Content, N2 - Imagery, REM - No Content, and REM - Imagery. Participants contributed an average of 3.6 +/− 0.7SD of these 7 possible report types. The numbers of reports contributed in each recall category are further summarized in Table 2. In these analyses, the restricted maximum likelihood method is used to estimate model parameters in the presence of missing data, enabling participants with missing observations for one or more of these 7 report types to still contribute to the model, in contrast to the listwise deletion approach typically employed in repeated measures analysis of variance.
Table 2.
Dream Reports by Sleep State
| Reports Elicited | No Content | Thought | Imagery | |
|---|---|---|---|---|
| Sleep Onset | 312 | 43(13.8%) | 20(6.4%) | 249(79.8%) |
| NREM | 67 | 32(47.8%) | 2*(3.0%) | 33(49.3%) |
| REM | 49 | 14(28.6%) | 2*(4.1%) | 33(67.3%) |
| Total | 428 | 89(20.8%) | 24(5.6%) | 315(73.6%) |
Notes. Total number and (%) of reports collected in each category.
The low number of Thought reports elicited from REM and NREM prohibited meaningful analysis of EEG correlates of this category of experience.
We report t-tests comparing model estimated marginal means across recall categories. Type I error was controlled using the Benjamini-Hochberg method of controlling false discovery rate (Benjamini & Hochberg, 1995; Thissen, Steinberg, & Kuang, 2002), an adaptive method that results in an adjusted critical p-value that differs for each set of comparisons conducted. These comparisons were carried out separately for sleep onset, N2, and REM awakenings, with the above-described hierarchical linear model approach followed by false discovery rate correction applied in each state.
To assess the topography of these effects, we additionally ran pairwise comparisons of the estimated marginal means separately for each recording site. Type I error was again controlled using the Benjamini-Hochberg method of controlling false discovery rate. Here, adjusted p-values were set separately for sleep onset, N2, and REM analyses, and separately for absolute vs. relative power analyses. Topographic plots using interpolation by spherical splines were generated to display mean differences between recall categories, flagging electrodes at which the comparisons remained significant after correction for multiple comparisons.
2.6. Between-Subjects Comparison of Participants who Recall vs. Do Not Recall a Dream Upon Morning Awakening
We hypothesize that prior reports of EEG differences between participants who recall vs. do not recall a dream in the morning may be attributed in part to individual differences. Thus, we also conducted a separate between-subjects analysis of morning dream recall, following the approach of Marzano et al. (2011), for comparison to the outcome of our primary analyses. Replicating the analyses described in Marzano et al. (2011), we classified each participant as either a “recaller” or “non-recaller” based solely on their morning-collected dream report. Following Marzano et al., the power spectrum of the 5min of EEG just prior to morning awakening was then examined as a predictor of whether a dream would be subsequently recalled, and only “Imagery” reports were classified as a successful recall of a dream. Each participant was classified as having awoken from REM or N2 sleep in the morning, based on the last sleep stage scored prior to awakening. The number of participants awakening from REM sleep (n = 10) was insufficient for further analysis. In participants awakening from N2, the last 5min of artifact-free sleep prior to awakening were extracted and passed to EEGLab (Delorme et al., 2011) for further analysis. The total number of participants included in this analysis was n = 27 (one participant who awoke from SWS was excluded, another participant was excluded because a morning report was not successfully collected, and a third was excluded because they were the sole person with a Thought report from morning N2). The power spectrum preceding Imagery reports was then compared to the spectrum preceding No Content reports using permutation tests. Again, correction for multiple comparisons was applied by controlling the false discovery rate (Delorme et al., 2011).
3. Results
3.1. Dream Report Characteristics
Overall, N = 428 experimental awakenings were conducted, 339 (79.2%) of which yielded a report of thought or imagery. On average, each participant contributed 10.8 +/− 2.9SD reports. Table 2 describes the incidence of Thought, Imagery and No Content reports across state. Because Thought reports were exceedingly rare outside of sleep onset, N2 and REM analyses were restricted to comparing Imagery and No Content reports.
At sleep onset, the duration of sleep prior to awakening did not impact dream recall (86.4% recall at 30s, 85.6% recall at 60s, and 86.7% recall at 90s; χ2 = 0.06, p = 0.97) or the presence of imagery (89.9% of content-filled reports contained imagery at 30s, 94.4% at 60s, and 92.3% at 90s; χ2 = 1.25, p = 0.56).
3.2. Dream Imagery at Sleep Onset is accompanied by an Increase in Absolute Power Below 7Hz
Absolute power was significantly greater preceding Imagery, as compared to Thought reports in the slow oscillation (t20 = 3.42, p = 0.003), delta (t22 = 2.91, p = 0.008), and theta (t19 = 2.95, p = 0.008) frequencies (comparison of estimated marginal mean power across all electrode sites, adjusted significance threshold of p<0.0083; see Figure 2A). This comparison did not survive false discovery rate correction in the alpha band (t21 = 2.47, p = 0.02). After correction for multiple comparisons, increased power preceding Imagery vs. Thought reports remained statistically significant at the majority of individual electrodes for the slow oscillation, delta, and theta bands (Figure 2C).
Figure 2. Dream Imagery at Sleep Onset is Accompanied by an Increase in Absolute Power.
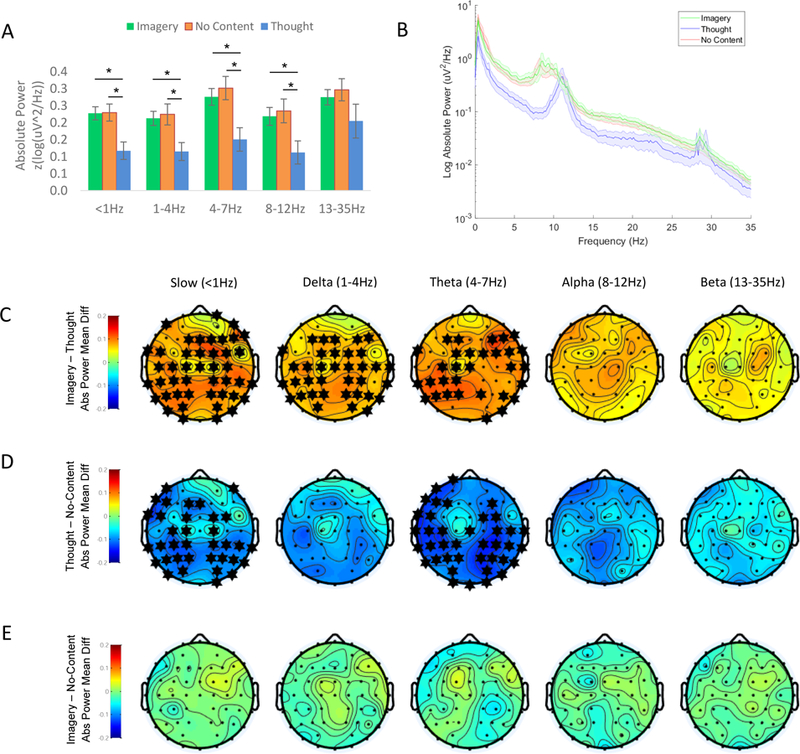
(A) Relative to Thought reports, both Imagery and No Content reports were preceded by an increase in absolute power prior to awakening. Mean ±SEM absolute power (log(z-uV2/Hz)). * = statistically significant for the mean across all electrodes (p < 0.05, uncorrected). (B) Power spectrum for No Content, Thought, and Imagery reports, averaged across electrodes. Shaded error bars +/−SEM. (C-E) Topographic plots of the estimated marginal mean difference in spectral power between conditions, derived from linear mixed models testing the effect of recall condition on power. * = statistically significant at this electrode following Benjamini-Hochberg correction for multiple comparisons. Values between electrodes were interpolated using spherical splines. (C) Imagery - Thought: The increase in absolute power preceding Imagery relative to Thought reports reached statistical significance at the majority of electrodes in the slow oscillation, delta, and theta bands. (D) Thought – No-Content: Absolute power was also greater preceding No-Content reports, relative to Thought reports, reaching statistical significance at the majority of electrodes in the slow oscillation and theta bands. (E) Imagery – No Content: The power spectrum preceding Imagery vs. No Content reports did not differ significantly at any electrode. Slow oscillation = < 1Hz, delta = 1–4Hz, theta = 4–7Hz, alpha = 8–12Hz, beta = 13–35Hz.
Absolute power was also significantly lower preceding Thought, as compared to No Content reports in the slow oscillation (t22 = 3.11, p = 0.005) and theta bands (t22 = 3.08, p = 0.005; comparisons of estimated marginal mean power across all electrodes with adjusted significance threshold of p < 0.0083; see Figure 2A). Power reductions preceding Thought vs. No-Content reports did not survive false discovery rate correction in the alpha (t23 = 2.49, p = 0.02), delta (t24 = 2.66, p = 0.013), or beta bands (t23 = 2.18, p = 0.04). Following correction for multiple comparisons, Thought reports were associated with lower absolute power than No Content reports at the majority of individual electrodes in the slow oscillation and theta bands. No electrode-level comparisons survived correction for multiple comparisons in other frequency bands (Figure 2D).
In contrast, No Content and Imagery reports did not differ in mean absolute power across electrodes, or in absolute power at any individual electrode (Figure 2E).
For relative power, no effects remained statistically significant following correction for multiple comparisons, with the adjusted significance threshold set to p < 0.0083.
In visually examining the power spectra of sleep onset reports illustrated in Figure 2B, we noted that the peak alpha frequency was numerically slower preceding Imagery and No Content reports, relative to Thought reports. Further exploring this effect, we found that it did not reach statistical significance. Preceding Thought reports, the mean alpha peak was at 10.5Hz ± 0.4SEM, in comparison to 9.5Hz ± 0.3SEM preceding Imagery (t18 = 1.91, p = 0.07 vs. Thought) and 9.4Hz ± 0.4SEM preceding No Content reports (t23 = 1.93, p = 0.07 vs. Thought).
3.3. Dreaming during N2 Sleep is Associated with Decreased Low-Frequency Power
During N2 sleep, Imagery was associated with a decrease in < 1Hz relative power (t50 = 3.46, p = 0.001), and concomitantly increased faster oscillations in the theta (t37 = 3.06, p = 0.004), alpha (t42 = 2.63, p = 0.012), and beta (t44 = 3.09, p = 0.004) bands (Figure 3A). After correction for multiple comparisons, these effects remained significant at the majority of individual electrodes for slow oscillation, theta, alpha and beta frequencies (Figure 3C). No effects were observed for absolute power.
Figure 3. N2 Sleep Dream Recall is Accompanied by a Shift Toward Higher-Frequency Power.
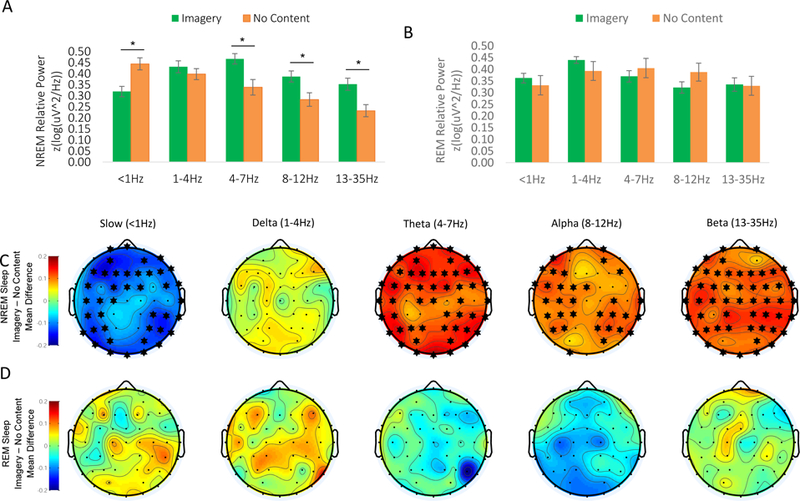
(A) Recall of Imagery from N2 sleep was associated with relatively decreased slow oscillation (< 1Hz) power, and increased faster oscillations in the theta, alpha, and beta bands. Means ±SEM relative power (log(z-uV2/Hz)/total power 0–100Hz). * = statistically significant for the mean across all electrodes (p < 0.05, uncorrected). (B) During REM sleep, Imagery and No Content reports did not differ significantly in any frequency band. Means ±SEM relative power (log(z-uV2/Hz)/total power 0–100Hz). (C-D) Topographic plots of the estimated marginal mean difference in spectral power between conditions, derived from linear mixed models testing the effect of recall condition on power. * = statistically significant at this electrode following Benjamini-Hochberg correction for multiple comparisons. Values between electrodes were interpolated using spherical splines. (C) N2 Imagery – No Content: Both the decrease in slow oscillation power and increase in > 4Hz power associated with recall of Imagery remained statistically significant at the majority of electrodes following correction for multiple comparisons. (D) REM Imagery – No Content: Following correction for multiple comparisons, the power spectrum preceding Imagery vs. No Content reports from REM sleep did not differ significantly at any electrode. Slow oscillation = < 1Hz, delta = 1–4Hz, theta = 4–7Hz, alpha = 8–12Hz, beta = 13–35Hz.
During REM sleep, Imagery and No Content reports did not differ significantly for any frequency band, for either absolute or relative power, across all electrodes or at any individual electrode (Figure 3B&D).
Because the power spectrum during NREM sleep is highly variable across the night (Kryger & Roth, 2016), we asked whether time of night or proximity to REM sleep might explain the inverse association between N2 dream recall and < 1Hz power, with the “lighter” N2 sleep that occurs closer to REM or later in the night accounting for dreaming in this stage. However, recall of dream imagery was not related to time of night (time since sleep onset = 194min ± 24 SEM for Imagery reports vs. 190min ± 14SEM for No Content reports; p = 0.87) or to proximity to REM sleep (mean distance from REM = 27min ± 7SEM for Imagery reports vs. 30min ± 7SEM for No Content reports; p = 0.79).
3.4. Between-Subjects Analysis of “Recallers” vs. “NonRecallers”
Finally, following Marzano et al. (2011), we conducted a between-subjects comparison of those who did vs. did not recall a dream upon awakening from N2 sleep in the morning (see Methods). N = 27 participants were included in this analysis (not including n = 10 additional participants who awoke from REM sleep, and n = 3 excluded for other reasons; see Method). Contrary to Marzano et al.’s report of decreased right temporal alpha power in participants recalling a dream from NREM, the only effect to survive correction for multiple comparisons was a spatially diffuse decrease in relative beta power in “recallers” (n = 17), relative to “non-recallers” (n = 10; Figure 4). However, because of a strong a priori hypothesis regarding alpha power based on Marzano et al.’s study (2011), we also ran uncorrected comparisons in this frequency band. Contrary to Marzano et al.’s observations, uncorrected for multiple comparisons, “recallers” showed increased relative power in the alpha band, particularly in right frontal regions, relative to “non-recallers” (Figure 4). There were no effects for absolute power.
Figure 4. Relative power of Pre-Awakening EEG in “Recallers” (n = 17) and “Non-Recallers” (n = 10).
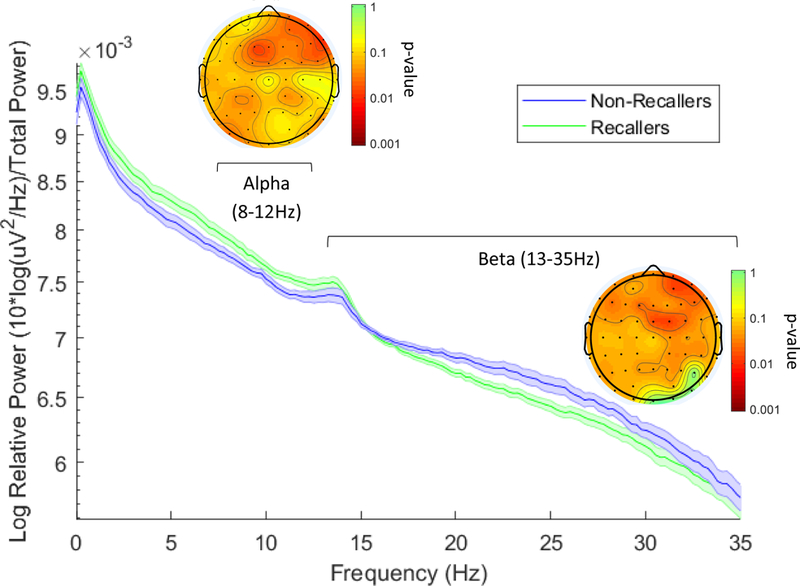
Relative power spectrum during the 5min prior to awakening in “recallers” and “non-recallers”, averaged across all electrodes. Shaded error bars +/− SEM. Topographic insets represent permutation test p-values comparing alpha and beta power prior to awakening. After correction for multiple comparisons, frontal beta power (13–35Hz) remained significantly lower in “recallers”, as compared to participants who did not recall dream imagery upon awakening. Uncorrected for multiple comparisons (see Method), frontal alpha power was significantly higher prior to morning awakening in participants who recalled a dream. Values between electrodes were interpolated using spherical splines. Slow oscillation = <1Hz, delta = 1–4Hz, theta = 4–7Hz, alpha = 8–12Hz, beta = 13–35Hz.
4. Discussion
We report that dreaming during NREM sleep is associated with global changes in the frequency content of the EEG. During N2, dreaming was associated with reduced < 1Hz activity and concomitantly increased power above 4Hz. Although differing in the specific frequency bands identified, this observation is broadly consistent with prior reports that dreaming is predicted by reduced low frequency power in the delta range (Esposito et al., 2004; Scarpelli et al., 2017; Siclari et al., 2017). As low-frequency power is inversely related to regional cerebral blood flow during sleep (Dang-Vu et al., 2005; Hofle et al., 1997), our observations support the longstanding hypothesis that dreaming outside of REM sleep occurs during periods of relatively heightened cortical “activation” (Antrobus, 1991; Scarpelli et al., 2017; Wamsley et al., 2007).
We additionally report the novel observation that at sleep onset, the association of dreaming with low-frequency power is in the opposite direction – dream imagery was associated with increased power in the slow-oscillation, delta, and theta bands, relative to wake-like thoughts. However, at sleep onset, imagery and no-content reports were associated with a similar spectral profile. Below, we speculate that no-content reports at sleep onset could signify a failure to recall dream imagery, rather than a lack of conscious experience. Contrary to our expectations, we failed to identify any EEG predictors of dreaming within REM sleep.
Together, these observations suggest that the dreaming state is supported by intermediate levels of low-frequency EEG power. We illustrate this possibility in a hypothetical model of the relationship between dreaming and low-frequency EEG power in Figure 5. At the onset of sleep, the transition from wake-like thought to dream imagery is associated with the global EEG slowing that characterizes sleep onset. Yet later in the night, dreaming outside of REM sleep occurs during periods of relatively reduced low-frequency power, and conscious experience disappears as low-frequency oscillations in the slow (< 1Hz) and delta (1–4Hz) bands come dominate the EEG. Thus, as illustrated in Figure 5, we proposed that dreaming may have an inverted u-shaped relationship with the prevalence of low-frequency EEG oscillations.
Figure 5. Hypothesized relationship between low-frequency EEG and dreaming.
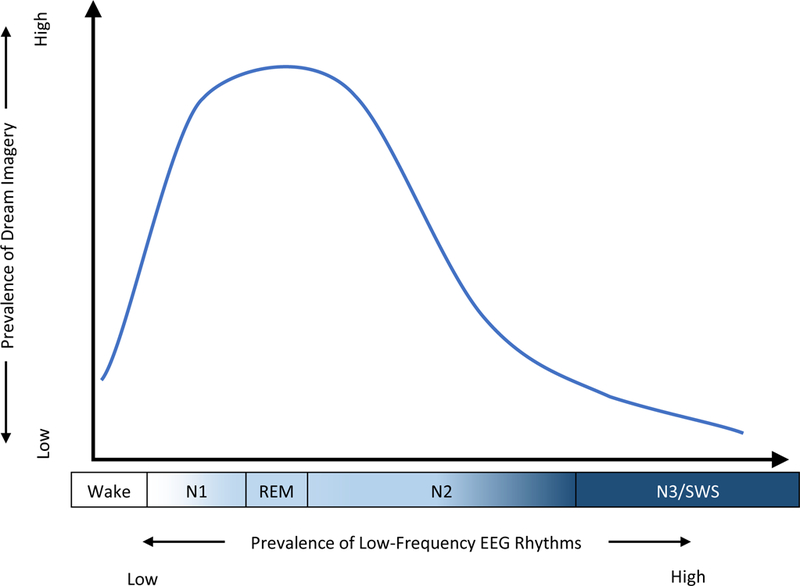
Our observations are consistent with the hypothesis that dreaming is associated with the intermediate levels of low-frequency EEG power which characterize N1, REM, and light N2 sleep. In this model, “low-frequency” is defined in relative terms – During wakefulness, when alpha (8–12Hz) and beta (13–35Hz) rhythms predominate, theta (4–7Hz) activity is relatively slow, yet during N2 sleep, theta is a relatively “fast” frequency in comparison to the delta (1–4Hz) and slow oscillations (1Hz) that dominate slow wave sleep.
There is sufficient reason to believe that excessively high-amplitude, low-frequency EEG in the slow (< 1Hz) and delta (1–4Hz) ranges is incompatible with conscious experience. Scalp-recorded EEG rhythms in these frequency bands result from hyper-synchronous underlying neuronal activity, in which the postsynaptic membrane potentials of millions of cortical neurons oscillate in synchrony. The < 1Hz cortical slow oscillation also powerfully modulates neuronal spiking, shifting the cortex between alternating hyperpolarized “down states”, characterized by neuronal silence, and “up states” during which firing rates approach waking levels (Steriade, 2006; Steriade, Timofeev, & Grenier, 2001). It has long been proposed that these highly “synchronized” EEG patterns signify loss of consciousness during the deepest epochs of slow wave sleep (Hobson, Pace-Schott, & Stickgold, 2000), and indeed, similar patterns of neuronal activity are implicated in the loss of consciousness under anesthesia (Purdon et al., 2013; Supp, Siegel, Hipp, & Engel, 2011).
Yet precisely why low-frequency, synchronized EEG rhythms coincide with loss of consciousness remains obscure. One recent theory attributes loss of consciousness during such states to reduced information-integration capacity resulting from the “bistable” neuronal firing patterns occurring during low-frequency dominated EEG (Nieminen et al., 2016; Tononi, 2004). Another longstanding hypothesis holds that dreaming requires a minimal level of cortical “activation” (Antrobus, 1991; Wamsley et al., 2007) which is only intermittently achieved during NREM, signified by the presence of faster-frequency EEG rhythms. This latter conjecture is supported by the fact that rCBF in a number of brain regions is indeed increased during periods of NREM with relatively decreased slow wave activity (Dang-Vu et al., 2005; Hofle et al., 1997).
Although excessive low-frequency activity may be incompatible with conscious experience, at the same time, sleep states known to have the highest dream recall (including REM, N1, and light N2) certainly contain more low-frequency power than wakefulness. This is consistent with the current observation that the emergence of imagery at sleep onset was associated with increased, rather than decreased low-frequency power. It may be that a moderate increase in slow oscillation and/or delta activity is necessary for dreaming to emerge. For example, in wake, delta activity has been proposed to support functional cortical deafferentation, inhibiting the processing of external sensory information in favor of attention to internally generated activity (Harmony, 2013; Harmony et al., 1996). In support of this notion, increased delta- and theta-frequency oscillations are also associated with mind wandering during waking tasks (Braboszcz & Delorme, 2011). In a similar fashion, low-frequency oscillations in the delta and theta ranges could function to inhibit external sensory processing at sleep onset, as mentation becomes increasingly hallucinatory and disconnected from the external environment (Foulkes & Vogel, 1965).
An alternative interpretation of our sleep onset observations could be that the association of Thought reports with relatively higher-frequency “wake-like” EEG indicates that these experiences occurred during a state of wakefulness. However, several factors argue against this interpretation. First, all sleep onset awakenings occurred during epochs of PSG-defined sleep; while the analyzed data might contain brief (seconds-long) periods of “wake-like” EEG, participants were not awake as currently defined by the AASM (Iber et al., 2007). Second, research dating back decades has established that brain state cannot be inferred on the basis of subjective report data -- Not only are thought-like reports common in sleep, but bizarre imagery is conversely common during wake (Foulkes, 1962; Wollman & Antrobus, 1987). For these reasons, we interpret our sleep onset observations as reflecting gradations of mind-brain activity occurring within sleep, rather than a contrast between sleep and wake.
One complexity of our observations is that the increase in low-frequency power associated with imagery at sleep onset is statistically significant only for absolute power, whereas the decrease in low-frequency power associated with imagery during N2 is significant only for relative power. This suggests that during sleep onset the absolute amplitude of the EEG (which increases dramatically as we move from the waking to sleep state) is the most critical factor predicting the nature of subjective experience. In contrast, during later-night N2 sleep, the most cognitively-relevant feature may instead be the relative proportion of the EEG dominated by low frequencies, independent of the absolute amplitude of those oscillations. Due to the relatively few studies which have to date described quantitative EEG predictors of dreaming, the consistency with which one vs. another metric predicts dream experience remains unknown, and would be a profitable focus of future research.
The effects we report are spatially global, rather than localized to any particular cortical region. In contrast, past EEG studies of dreaming have claimed a variety of topographically-specific effects (Fell et al., 2006; Marzano et al., 2011; Scarpelli et al., 2017; Siclari et al., 2017; Takeuchi et al., 2003), for example focused on central sites (Takeuchi et al., 2003), parietal-occipital lobe (Siclari et al., 2017), or frontal and temporo-parietal areas (Scarpelli et al., 2017). Yet despite variability in localization, the general observation that reduced power in the delta and/or slow oscillation bands predicts dream experience has remained a relatively consistent observation across studies, here again confirmed by our data. Thus, we speculate that dream experience is associated with a global change in cortical EEG rhythms, with inter-study variability in localization resulting primarily from sampling error, measurement error, and/or low statistical power.
Contrary to our hypotheses, we identified no significant EEG correlates of dreaming during REM sleep. In line with prior research, dream recall was substantially higher in REM than during N2 sleep (Nielsen, 2000), and as a result, the relatively small number of No-Content reports obtained could have caused us to be underpowered to detect an effect. Indeed, Figure 3B illustrates that alpha power was non-significantly lower (p = 0.17) during Imagery reports compared to No Content reports, consistent with two prior reports that decreased alpha predicts dreaming during REM sleep (Esposito et al., 2004; Takeuchi et al., 2003). Alternatively, the failure to detect EEG correlates of dreaming in this stage could be due to the fact that the n = 12 No Content reports collected from REM actually signify a failure of recall rather than a lack of conscious experience, and thus represent a similar pre-awakening brain state as Imagery reports. In support of this hypothesis, Siclari et al. (2017) found that participants who claimed to have dreamed but could not recall the content showed decreased low-frequency EEG power, just like those who recalled the content of the dream (Siclari et al., 2017). Similarly, we found no EEG difference between No-Content and Imagery reports at sleep onset, which could also indicate that participants who could not recall dreams still experienced dreaming.
The current study avoids some limitations of past research. For the sake of convenience, a number of prior studies assessed the presence of dreaming by simply asking the participants a question upon awakening about whether or “how much” they dreamed (Chellappa et al., 2012; Marzano et al., 2011; Scarpelli, D’Atri, Gorgoni, Ferrara, & Gennaro, 2015; Takeuchi et al., 2003). This approach prevents experimenters from operationally defining “dreaming”, instead relying on the participants’ own highly variable judgments about whether their experience meets the criteria for a “yes” answer. Importantly, it has long been established that asking people to report their “dreams” leads to drastically reduced estimates of NREM dreaming, in comparison to when participants are prompted to report “everything that was going through your mind” (Foulkes, 1962). Secondly, between-subjects comparisons of those who recall vs. fail to recall a dream (Eichenlaub, Bertrand, et al., 2014; Fell et al., 2006; Marzano et al., 2011) cannot determine whether dreaming depends on the patterns of brain activity prior to awakening, or alternatively, on individual differences between participants (Scarpelli, D’Atri, et al., 2015). Indeed, a strictly between-subjects analysis of our morning dream recall data yielded a very different pattern of results, suggesting that comparing the pre-awakening EEG of participants who do vs. do not recall a dream may strongly reflect individual differences. To overcome these issues, we employed a within-subjects paradigm, treating the participant as the unit of analysis and collecting dream reports using polysomnographically monitored laboratory awakenings from which participants provided an open-ended report on the content of all mental experience just prior to awakening. This is the first such study to be conducted across sleep onset, N2, and REM stages.
The current data do have some limitations of their own. First, a limitation common to all dream research is that, because no reliable physiological indicator of dreaming has yet been discovered, dream experience is only measurable via self-report. Thus, it is not possible to distinguish between the experience of a dream and the ability to recall and report that dream. Although some studies have asked participants to indicate if they had a dream that they cannot recall (Siclari et al., 2017), there is no independent evidence that participants’ self-assessment on this point is valid. As a result, the EEG effects we report here may be a result of the presence of dream experience during sleep, the successful retrieval of that experience from memory after sleep, or a combination of the two. Second, for practical reasons, we restricted NREM sleep data collection to the sleep onset period and to N2 sleep later in the night, and did not collect data from slow wave sleep. Finally, although this design takes a repeated-awakenings approach, the presence of missing data observations causes some participants included in the hierarchical linear models to contribute data in only one dream recall condition. If participants with missing reports differ systematically between conditions or from those with complete data, this could have introduced individual-differences confounds into our observations.
In summary, we report that the recall of dream experience from NREM sleep is associated with intermediate levels of low-frequency power – dream imagery emerges as low-frequency power increases at sleep onset, but then fades when high-amplitude, low-frequency EEG begins to dominate the recording during later NREM. These observations dovetail with others suggesting that while hypersynchronized low-frequency EEG may be incompatible with consciousness (Purdon et al., 2013; Supp et al., 2011), at the same time, moderate increases in delta-frequency oscillations could actually support attention to internally generated thought and imagery (Braboszcz & Delorme, 2011; Harmony, 2013; Harmony et al., 1996). This study thus helps to untangle inconsistent findings reported in the prior literature, suggesting levels of low-frequency EEG power are a consistently observed and parsimonious explanation for the waxing and waning of conscious experience during sleep. Future research should continue to investigate the mechanisms underlying the connection between low-frequency EEG power and conscious experience. Ultimately, understanding the brain correlates of dreaming during sleep will aid in isolating the conditions needed for the brain to support conscious experience during wakefulness.
Supplementary Material
Acknowledgement
This work was supported by NIMH grant R21MH098171
We thank Yvette Graveline, Graelyn Humiston, Alli Peipert, Sarah Wood, and Theodore Summer for assistance with data collection and data preprocessing, as well as Robert Stickgold and Michael Murphy for their contributions to the larger research project in the context of which these dream reports were collected.
Footnotes
Endnotes
Because complex mental experiences can be recalled from any stage of sleep, in this paper we use the term “dreaming” to refer to any mental experience recalled from polysomnographically defined sleep, regardless of sleep stage.
The authors declare no conflict of interest.
References
- Antrobus J (1983). REM and NREM sleep reports: comparison of word frequencies by cognitive classes. Psychophysiology, 20(5), 562–568. 10.1111/j.1469-8986.1983.tb03015.x [DOI] [PubMed] [Google Scholar]
- Antrobus J (1991). Dreaming: Cognitive processes during cortical activation and high afferent thresholds. Psychological Review, 98(1), 96–121. 10.1037/0033-295X.98.1.96 [DOI] [PubMed] [Google Scholar]
- Aserinsky E, & Kleitman N (1953). Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science (New York, N.Y.), 118(3062), 273–274. 10.1126/science.118.3062.273 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological), 57(1), 289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Braboszcz C, & Delorme A (2011). Lost in thoughts: neural markers of low alertness during mind wandering. NeuroImage, 54(4), 3040–3047. 10.1016/j.neuroimage.2010.10.008 [DOI] [PubMed] [Google Scholar]
- Brokaw K, Tishler W, Manceor S, Hamilton K, Gaulden A, Parr E, & Wamsley EJ (2016). Resting state EEG correlates of memory consolidation. Neurobiology of Learning and Memory, 130, 17–25. 10.1016/j.nlm.2016.01.008 [DOI] [PubMed] [Google Scholar]
- Chellappa SL, Münch M, Knoblauch V, & Cajochen C (2012). Age effects on spectral electroencephalogram activity prior to dream recall. Journal of Sleep Research, 21(3), 247–256. 10.1111/j.1365-2869.2011.00947.x [DOI] [PubMed] [Google Scholar]
- Dang-Vu TT, Desseilles M, Laureys S, Degueldre C, Perrin F, Phillips C, … Peigneux P (2005). Cerebral correlates of delta waves during non-REM sleep revisited. NeuroImage, 28(1), 14–21. 10.1016/j.neuroimage.2005.05.028 [DOI] [PubMed] [Google Scholar]
- Delorme A, Mullen T, Kothe C, Akalin Acar Z, Bigdely-Shamlo N, Vankov A, & Makeig S (2011). EEGLAB, SIFT, NFT, BCILAB, and ERICA: New Tools for Advanced EEG Processing [Research article] 10.1155/2011/130714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinges DF, & Powell JW (1985). Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behavior Research Methods, Instruments, & Computers, 17(6), 652–655. 10.3758/BF03200977 [DOI] [Google Scholar]
- Eichenlaub J-B, Bertrand O, Morlet D, & Ruby P (2014). Brain reactivity differentiates subjects with high and low dream recall frequencies during both sleep and wakefulness. Cerebral Cortex (New York, N.Y.: 1991), 24(5), 1206–1215. 10.1093/cercor/bhs388 [DOI] [PubMed] [Google Scholar]
- Eichenlaub J-B, Nicolas A, Daltrozzo J, Redouté J, Costes N, & Ruby P (2014). Resting brain activity varies with dream recall frequency between subjects. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 39(7), 1594–1602. 10.1038/npp.2014.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito MJ, Nielsen TA, & Paquette T (2004). Reduced Alpha power associated with the recall of mentation from Stage 2 and Stage REM sleep. Psychophysiology, 41(2), 288–297. 10.1111/j.1469-8986.00143.x [DOI] [PubMed] [Google Scholar]
- Fell J, Fernández G, Lutz MT, Kockelmann E, Burr W, Schaller C, … Helmstaedter C (2006). Rhinal-hippocampal connectivity determines memory formation during sleep. Brain: A Journal of Neurology, 129(Pt 1), 108–114. 10.1093/brain/awh647 [DOI] [PubMed] [Google Scholar]
- Foulkes D, & Vogel G (1965). Mental activity at sleep onset. Journal of Abnormal Psychology, 70(4), 231–243. 10.1037/h0022217 [DOI] [PubMed] [Google Scholar]
- Foulkes WD (1962). Dream reports from different stages of sleep. Journal of Abnormal and Social Psychology, 65, 14–25. 10.1037/h0040431 [DOI] [PubMed] [Google Scholar]
- Harmony T (2013). The functional significance of delta oscillations in cognitive processing. Frontiers in Integrative Neuroscience, 7, 83 10.3389/fnint.2013.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmony T, Fernández T, Silva J, Bernal J, Díaz-Comas L, Reyes A, … Rodríguez M (1996). EEG delta activity: an indicator of attention to internal processing during performance of mental tasks. International Journal of Psychophysiology, 24(1), 161–171. 10.1016/S0167-8760(96)00053-0 [DOI] [PubMed] [Google Scholar]
- Hartmann E (1989). Boundaries of dreams, boundaries of dreamers: thin and thick boundaries as a new personality measure. Psychiatric Journal of the University of Ottawa: Revue De Psychiatrie De l’Universite d’Ottawa, 14(4), 557–560. [PubMed] [Google Scholar]
- Hill CE, Diemer RA, & Heaton KJ (1997). Dream interpretation sessions: Who volunteers, who benefits, and what volunteer clients view as most and least helpful. Journal of Counseling Psychology, 44(1), 53–62. 10.1037/0022-0167.44.1.53 [DOI] [Google Scholar]
- Hobson JA, Pace-Schott EF, & Stickgold R (2000). Dreaming and the brain: Toward a cognitive neuroscience of conscious states. Behavioral and Brain Sciences, 23(6), 793–842. 10.1017/S0140525X00003976 [DOI] [PubMed] [Google Scholar]
- Hofle N, Paus T, Reutens D, Fiset P, Gotman J, Evans AC, & Jones BE (1997). Regional Cerebral Blood Flow Changes as a Function of Delta and Spindle Activity during Slow Wave Sleep in Humans. Journal of Neuroscience, 17(12), 4800–4808. 10.1523/JNEUROSCI.17-12-04800.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson A, & Quan S (2007). The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications Westchester, IL: American Academy of Sleep Medicine. [Google Scholar]
- Johns MW (1991). A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep, 14(6), 540–545. 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- Marzano C, Ferrara M, Mauro F, Moroni F, Gorgoni M, Tempesta D, … De Gennaro L (2011). Recalling and forgetting dreams: theta and alpha oscillations during sleep predict subsequent dream recall. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31(18), 6674–6683. 10.1523/JNEUROSCI.0412-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen TA (2000). A review of mentation in REM and NREM sleep: “covert” REM sleep as a possible reconciliation of two opposing models. The Behavioral and Brain Sciences, 23(6), 851–866; discussion 904–1121. 10.1017/S0140525X0000399X [DOI] [PubMed] [Google Scholar]
- Nielsen T, Carr M, Blanchette-Carrière C, Marquis L-P, Dumel G, Solomonova E, … Paquette T (2016). NREM sleep spindles are associated with dream recall. Sleep Spindles & Cortical Up States, 1(1). 10.1556/2053.1.2016.003 [DOI] [Google Scholar]
- Nieminen JO, Gosseries O, Massimini M, Saad E, Sheldon AD, Boly M, … Tononi G (2016). Consciousness and cortical responsiveness: a within-state study during non-rapid eye movement sleep. Scientific Reports, 6, 30932 10.1038/srep30932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nofzinger EA, Buysse DJ, Miewald JM, Meltzer CC, Price JC, Sembrat RC, … Moore RY (2002). Human regional cerebral glucose metabolism during non-rapid eye movement sleep in relation to waking. Brain: A Journal of Neurology, 125(Pt 5), 1105–1115. 10.1093/brain/awf103 [DOI] [PubMed] [Google Scholar]
- Peigneux P, Laureys S, Fuchs S, Collette F, Perrin F, Reggers J, … Maquet P (2004). Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron, 44(3), 535–545. 10.1016/j.neuron.2004.10.007 [DOI] [PubMed] [Google Scholar]
- Purdon PL, Pierce ET, Mukamel EA, Prerau MJ, Walsh JL, Wong KFK, … Brown EN (2013). Electroencephalogram signatures of loss and recovery of consciousness from propofol. Proceedings of the National Academy of Sciences of the United States of America, 110(12), E1142–E1151. 10.1073/pnas.1221180110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby P, Blochet C, Eichenlaub J-B, Bertrand O, Morlet D, & Bidet-Caulet A (2013). Alpha reactivity to first names differs in subjects with high and low dream recall frequency. Frontiers in Psychology, 4 10.3389/fpsyg.2013.00419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpelli S, D’Atri A, Gorgoni M, Ferrara M, & Gennaro LD (2015). EEG oscillations during sleep and dream recall: state- or trait-like individual differences? Frontiers in Psychology, 6, 605 10.3389/fpsyg.2015.00605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpelli S, D’Atri A, Mangiaruga A, Marzano C, Gorgoni M, Schiappa C, … Gennaro LD (2017). Predicting Dream Recall: EEG Activation During NREM Sleep or Shared Mechanisms with Wakefulness? Brain Topography, 30(5), 629–638. 10.1007/s10548-017-0563-1 [DOI] [PubMed] [Google Scholar]
- Scarpelli S, Marzano C, D’Atri A, Gorgoni M, Ferrara M, & De Gennaro L (2015). State- or trait-like individual differences in dream recall: preliminary findings from a within-subjects study of multiple nap REM sleep awakenings. Frontiers in Psychology, 6, 928 10.3389/fpsyg.2015.00928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schredl M, Nürnberg C, & Weiler S (1996). Dream recall, attitude toward dreams, and personality. Personality and Individual Differences, 20(5), 613–618. 10.1016/0191-8869(95)00216-2 [DOI] [Google Scholar]
- Siclari F, Baird B, Perogamvros L, Bernardi G, LaRocque JJ, Riedner B, … Tononi G (2017). The neural correlates of dreaming. Nature Neuroscience, 20(6), 872–878. 10.1038/nn.4545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M (2006). Grouping of brain rhythms in corticothalamic systems. Neuroscience, 137(4), 1087–1106. 10.1016/j.neuroscience.2005.10.029 [DOI] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, & Grenier F (2001). Natural waking and sleep states: a view from inside neocortical neurons. Journal of Neurophysiology, 85(5), 1969–1985. 10.1152/jn.2001.85.5.1969 [DOI] [PubMed] [Google Scholar]
- Supp GG, Siegel M, Hipp JF, & Engel AK (2011). Cortical hypersynchrony predicts breakdown of sensory processing during loss of consciousness. Current Biology: CB, 21(23), 1988–1993. 10.1016/j.cub.2011.10.017 [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Ogilvie RD, Murphy TI, & Ferrelli AV (2003). EEG activities during elicited sleep onset REM and NREM periods reflect different mechanisms of dream generation. Clinical Neurophysiology, 114(2), 210–220. 10.1016/S1388-2457(02)00385-1 [DOI] [PubMed] [Google Scholar]
- Thissen D, Steinberg L, & Kuang D (2002). Quick and Easy Implementation of the Benjamini-Hochberg Procedure for Controlling the False Positive Rate in Multiple Comparisons. Journal of Educational and Behavioral Statistics - J EDUC BEHAV STAT, 27, 77–83. 10.3102/10769986027001077 [DOI] [Google Scholar]
- Tononi G (2004). An information integration theory of consciousness. BMC Neuroscience, 5(1), 42 10.1186/1471-2202-5-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel GW (1991). Sleep-onset mentation. In The mind in sleep: Psychology and psychophysiology, 2nd ed (pp. 125–142). Oxford, England: John Wiley & Sons. [Google Scholar]
- Wamsley EJ (2014). Dreaming and Offline Memory Consolidation. Current Neurology and Neuroscience Reports, 14(3). 10.1007/s11910-013-0433-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley EJ, Hirota Y, Tucker MA, Smith MR, & Antrobus JS (2007). Circadian and ultradian influences on dreaming: A dual rhythm model. Brain Research Bulletin, 71(4), 347–354. 10.1016/j.brainresbull.2006.09.021 [DOI] [PubMed] [Google Scholar]
- Wamsley EJ, Perry K, Djonlagic I, Reaven LB, & Stickgold R (2010). Cognitive replay of visuomotor learning at sleep onset: temporal dynamics and relationship to task performance. Sleep, 33(1), 59–68. 10.1093/sleep/33.1.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley EJ, Tucker MA, Payne JD, & Stickgold R (2010). A brief nap is beneficial for human route-learning: The role of navigation experience and EEG spectral power. Learning & Memory, 17(7), 332 10.1101/lm.1828310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley EJ, Tucker M, Payne JD, Benavides JA, & Stickgold R (2010). Dreaming of a learning task is associated with enhanced sleep-dependent memory consolidation. Current Biology: CB, 20(9), 850–855. 10.1016/j.cub.2010.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman MC, & Antrobus JS (1987). Cortical arousal and mentation in sleeping and waking subjects. Brain and Cognition, 6(3), 334–346. 10.1016/0278-2626(87)90130-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


