Abstract
Introduction:
Epidemiologic studies have demonstrated an association between acetaminophen (APAP) use and the development of asthma symptoms. However, few studies have examined relationships between APAP-induced signaling pathways associated with the development of asthma symptoms. We tested the hypothesis that acute APAP exposure causes airway hyper-responsiveness (AHR) in human airways.
Methods:
Precision cut lung slice (PCLS) airways from humans and mice were used to determine the effects of APAP on airway bronchoconstriction and bronchodilation and to assess APAP metabolism in lungs.
Results:
APAP did not promote AHR in normal or asthmatic human airways ex vivo. Rather, high concentrations mildly bronchodilated airways pre-constricted with carbachol (CCh), histamine (His), or Immunoglobulin E (IgE) cross-linking. Further, the addition of APAP prior to bronchoconstrictors protected the airways from constriction. Similarly, in vivo treatment of mice with APAP (200 mg/kg IP) resulted in reduced bronchoconstrictor responses in PCLS airways ex vivo. Finally, in both mouse and human PCLS airways, exposure to APAP generated only low amounts of APAP-protein adducts, indicating minimal drug metabolic activity in the tissues.
Conclusion:
These findings indicate that acute exposure to APAP does not initiate AHR, that high-dose APAP is protective against bronchoconstriction, and that APAP is a mild bronchodilator.
Keywords: Acetaminophen, Airway hyper-responsiveness, Acetaminophen metabolism, Asthma, Precision Cut Lung Slice
Introduction
Acetaminophen (APAP) is the most commonly used drug for the treatment of pain and fever in the United States (Kaufman and others 2002). Over the last decade, a number of epidemiologic studies have reported an association between APAP use and the occurrence of pulmonary disease. Symptoms of asthma have been reported in adults and children treated with frequent doses of APAP and in infants exposed to APAP during pregnancy (Barr and others 2004; Etminan and others 2009b; Kennon-McGill and McGill 2018; Kreiner-Moller and others 2012; McBride 2011; Shaheen and others 2000). In addition, epidemiologic studies of asthma in children have shown a dramatic rise in asthma prevalence that started around the 1980s (Akinbami and others 2012; Prevention May 2011). Among other factors, the linkage of aspirin use and Reye syndrome in children (Arrowsmith and others 1987) led to increased APAP exposure in this population and has fueled the speculation that APAP might cause asthma or aggravate asthma symptoms. Some have suggested that asthmatics treated with APAP during febrile illnesses may be more likely to develop exacerbations of asthma, leading to increased mortality and morbidity (McBride 2011), and many primary care physicians are therefore hesitant to offer APAP as an antipyretic to asthma patients. The hesitancy stems from concerns that APAP may decrease airway glutathione setting up increased risk for oxidative damage, leading to acute bronchoconstriction. However, to date, the validity of a causal relationship between APAP treatment and exacerbations of asthma and the mechanisms whereby APAP exposure might affect the development of these exacerbations have not been investigated.
In addition to its beneficial effects, APAP is a dose-dependent hepatotoxin and is the leading cause of acute liver failure in the USA and Western Europe (Lee 2008). Following high-dose exposure to APAP, depletion of hepatic glutathione and the formation of the reactive intermediate N-acetyl-p-benzoquinone imine (NAPQI) are recognized as the initiating events in the pathogenesis of liver injury (Hinson and others 2010; McGill and Jaeschke 2013). Other contributory mechanisms of liver injury include oxidative and nitrosative stress, mitochondrial injury, and inflammatory responses (Hinson and others 2010; McGill and Jaeschke 2013). The enzyme primarily responsible for the oxidative metabolism of APAP in the liver is CYP2E1. APAP is also metabolized in extra-hepatic tissues containing CYP2E1 such as the nasal epithelium, the pancreas, and the lung. In addition, CYP3A4 and CYP1A2 participate in the metabolism of APAP. The relationship of the extra hepatic metabolism of APAP in human disease or injury in other tissues is unknown.
The following study was conducted to evaluate the relationship between APAP treatment and bronchoconstriction and bronchodilation in human airways. This was accomplished using human precision cut lung slice (PCLS) airways prepared from organ donor lungs collected by transplant teams that were processed and cultured ex vivo. PCLS airways retain physiological responses to relevant bronchoconstrictors and bronchodilators and maintain ciliary motility (An and others 2012; Cooper and others 2011; Cooper and others 2009; Kennedy JL 2018). Immunohistochemical studies using PCLS airways were performed to determine if APAP metabolism in the lung mirrored that in the liver. An in vivo murine model of APAP exposure in which hepatotoxicity is well documented was used to confirm the findings in human tissue.
Materials and Methods
Drugs and Reagents.
Unless otherwise specified, compounds were obtained from Sigma Chemical Co. (St. Louis, MO). Phosphate buffered saline, DMEM:F12, forskolin, Gills Hematoxylin II, antibiotics and Permount were acquired from Fisher Scientific, Inc. (Pittsburgh, PA). Anti-IgE and human IgE were from Calbiochem (San Diego, CA).
Preparation of human precision cut lung slices.
Human lungs donated for research were obtained from aborted organ transplants by the National Disease Research Interchange (http://www.ndriresource.org) and the Arkansas Regional Organ Recovery Agency (http://www.arora.org). Donated organs were de-identified and contained limited demographic (e.g., age, sex, race) and historical data (e.g., past medical history, cause of death). Inclusion criteria for acquisition of lung tissue included donors age <55 years with <10 pack years smoking history, a clear chest x-ray, and arterial pO2 over 100 on ≤ 100% FiO2 prior to organ collection. Those with positive tests for HIV, hepatitis B, and hepatitis C were excluded. The University of Arkansas for Medical Sciences Institutional Review Board determined that the use of human lungs for these studies does not constitute human subjects research as defined in the US code of federal regulations 45 CFR 46.
PCLS airways were produced as previously described (An and others 2012; Cooper and others 2009; Kennedy JL 2018). Briefly, lungs were received in organ preservation solution on wet ice within 12–36 h of surgical resection. After warming, the lungs were inflated with 1.8% molten low melting point agarose (Amresco, Solon OH) in PBS at 37°C. After inflation, the agarose was allowed to harden at 4–7°C, and cores containing cross-sectioned airways were cut into 0.75mm thick slices using a Compresstome (Precisionary Instruments, San Jose CA). Slices containing human airways <3 mm in diameter were chosen and cultured in multi-well plates for 24 h with continuous agitation at 37°C, 5% CO2 in 200 μL DMEM:F12 culture medium supplemented with 1 mM glutathione and antibiotics (10,000 units/mL penicillin, 10,000 μg/mL streptomycin, and 25 μg/mL Amphotericin B, 0.2% Primocin (Invivogen, San Diego, CA)) for 24 hours. Suitable airways within slices were selected on the basis of the following criteria: presence of a full smooth muscle wall, presence of beating cilia, and unshared muscle walls at airway branch points to eliminate possible counteracting contractile forces. Each slice contained ~98% parenchyma tissue; hence, all airways situated on a slice had sufficient parenchymal tissue to impart basal tone. It is our experience that airway diameter does not correlate with whether or not an airway will contract (supplemental figure 1). After 24 hours, slices were maintained in medium with antibiotics but without glutathione with media changes at 3-day intervals. Airway viability was confirmed microscopically by evidence of ciliary motility and bronchoconstriction following treatment with 1 μM carbachol (CCh) prior to experiments and after treatments. Airways were excluded from experiments if they did not respond with at least 20% contraction to 1 μM CCh. Experiments were initiated within 48–72 hours after receiving the lungs.
Assays for bronchoconstriction, bronchodilation, and bronchoprotection in human PCLS airways.
Airway cross-sectional areas were measured in living PCLS airways using a Zeiss Axiovert microscope, CCD camera, and customized image analysis tools in ImageJ (Abramoff 2004). Bronchoconstriction was calculated based on areas measured before and after treatment with 1μM CCh, 1μM histamine (His), or anti-IgE for 15 minutes. For some experiments, dose responses via cumulative addition of CCh and/or His were used (1–100000nM). For the Anti-IgE experiments, PCLS airways were incubated for 18 hours with 5μg/mL of human myeloma IgE (Calbiochem). Bound IgE receptors were then crosslinked with 20μg/mL of goat anti-human IgE (Sigma) to activate the receptors.
Importantly, we studied both the ability of APAP to bronchodilate (CCh added prior to the addition of APAP) and bronchoprotect (pretreatment of airways with APAP and then secondarily adding CCh). For bronchodilation experiments in human airways, slices were treated with a dose response to APAP (50μM-3000μM) in the presence of either 1μM CCh, 1μM His, or anti-IgE. Bronchodilation for the asthma donor lung and the APAP overdose donor lung was performed using 2μM isoproterenol and 2μM forskolin. In other experiments, Human PCLS airways were treated with either 5mM APAP or 1μM albuterol in the presence of CCh to evaluate the bronchodilatory effects of these interventions. The percent reversal of bronchoconstriction was used to calculate bronchodilation. Treatment with 2μM forskolin to activate adenylyl cyclase provided a positive control for bronchodilation. Time-lapse photography for the bronchodilatory studies of APAP was performed using a Zeiss Axiovert microscope equipped with a CCD camera. In order to study the bronchoprotection of airways afforded by APAP, 5mM APAP was added to slices prior to adding 1μM CCh. Airways treated with 1μM CCh alone or pretreated with 1μM albuterol served as controls for these experiments.
Experimental Animals.
For in vivo studies, eight-week old C57Bl/6J mice were obtained from Jackson Laboratory (Bar Harbor, ME). For ex vivo PCLS studies, six-week old male B6C3F1 mice were obtained from Harlan Sprague Dawley, Inc. (Indianapolis, IN). All animal experiments were conducted according to the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences. The institution’s Animal Care and Use Committee approved protocols for animal experimentation. Mice were acclimatized one week prior to experiments and fed ad libitum. Animals were housed 3 per cage and maintained on a 12 h light/dark cycle. On the day prior to experiments, mice were fasted overnight and dosing studies began at 0800 the following morning. Food was returned to the mice 4 h after APAP. Mice were treated with APAP (10, 25, 100, or 300 mg/kg IP in saline) and sacrificed at 1 h after APAP (n=3 per group). Other mice received saline (n=3). Animals were anesthetized with CO2 for blood sampling. Blood was removed from the retro-orbital plexus, allowed to coagulate at room temperature, centrifuged, and the serum was used for measurement of alanine aminotransferase (ALT). Mice were then euthanized in a CO2 atmosphere followed by cervical dislocation and removal of the livers and lungs. The livers and lungs were weighed, and a portion was preserved in formalin for paraffin embedding and histological sectioning. The remaining tissues were snap frozen in liquid nitrogen and stored at −80° C for additional analyses.
Bronchoconstriction assay using mouse airways.
For ex vivo studies using mouse airways, lungs were harvested and PCLS airways were prepared as previously described (Muldrew and others 2002). After preparing slices, the airways were placed in 200 μL DMEM:F12 culture medium supplemented with antibiotics. Viability of airways was confirmed using similar measures to the human airways, including ciliary motility and visual inspection of tissue. These airways were evaluated for bronchoconstriction using 20μM CCh after 72 hours.
Biochemistry.
Serum ALT levels were measured using an ACE Alera chemistry analyzer (Alfa Wassermann, West Caldwell, NJ). Measurement of hepatic glutathione was performed using a modified Tietz method (McGill and Jaeschke 2015).
Quantification of APAP protein adducts.
Homogenates of liver and lung tissue from APAP and control mice were used for quantification of APAP protein adducts. Tissues were homogenized in 10 volumes of cold PBS and clarified by centrifugation at 10,000xG. The resulting sample was assayed for APAP protein adducts using a validated analytical HPLC assay with coulometric electrochemical detection (HPLC-EC) (Muldrew, et al). Results were reported as nanomoles of APAP-Cys adduct per mg protein.
Immunohistochemical studies with antibody to APAP protein adducts.
The intracellular and tissue localization of APAP-protein adducts as functions of time and treatment were determined by immunohistochemical detection of adducts using specific antisera as described previously (Roberts and others 1991).
Statistical Analysis.
For the human PCLS airways bronchoconstriction and bronchodilation assays, results are expressed as means ± SE. A p value of 0.05 was considered significant for all analyses. Comparisons of numerous slices between individual lungs were performed by one-way analysis of variance followed by the Tukey HSD post-hoc test. After collation of all lung slice points for each individual lung, non-parametric analysis (Kruskal Wallis and Mann Whitney) was used for analysis of data that was not normally distributed, including the bronchoprotection and bronchodilation assays. SPSS Version 10.0 (SPSS Inc., Chicago, IL) was used for all statistical analyses.
Results
Acetaminophen does not induce the airway hypersensitivity response.
First, we sought to determine if APAP enhances bronchoconstriction. Human PCLS airways respond to increasing doses of CCh with luminal narrowing that is readily monitored microscopically (Fig 1A). Airways from a donor without a history of asthma (normal) were treated with or without 5 mM APAP for 72 hours ex vivo and response to CCh was evaluated in a cumulative dose response assay (Fig. 1B). There was no significant difference in CCh-induced bronchoconstriction between APAP-treated and untreated PCLS airways.
Figure 1: APAP does not promote airway hyper-responsiveness (AHR) in human airways ex vivo.
Airways in PCLS were cultured in the presence or absence of 5mM APAP for 72 h and sensitivity to a cumulative dose response of carbachol (CCh) was evaluated (1–100000nM). After APAP washout, dose response curves were constructed by measuring the cross-sectional areas of airways in micrographs (A) taken 10–15 minutes after the addition of increasing doses of CCh and plotted (B). Data are expressed as mean±SE for n = 8 independent airways from one donor.
Acetaminophen is a mild bronchodilator and is bronchoprotective against CCh bronchoconstriction.
An alternative mechanism of AHR for reductions in airflow that characterize asthma is impaired bronchodilation. Accordingly, we evaluated the effects of APAP on bronchodilation. Human PCLS airways treated with CCh, His, or IgE crosslinking to promote bronchoconstriction can be bronchodilated with β-agonists, including isoproterenol and albuterol (Cooper and others 2011). Different bronchoconstrictors were utilized to determine if there was a specific pathway by which APAP was working. CCh is a cholinomimetic drug that binds and activates acetylcholine receptors on smooth muscle, while His binds directly to its receptor also located on the smooth muscle(Chand and others 1980). Anti-IgE has an indirect effect on airway contractility by cross-linking IgE receptors leading to the generation of His (Wohlsen and others 2001). Bronchoconstriction after exposure to 1μM CCh, 1μM His, or Anti-IgE was reversed with increasing doses of APAP (Fig. 2A). APAP treatment resulted in 25±10% bronchodilation at a concentration (1 mM) considered subtoxic and 60±5% bronchodilation at a concentration (5 mM) considered toxic, based on previous in vitro studies of hepatocytes (James and others 2009). Interestingly, in a time-lapse video microscopy study, PCLS airway bronchodilation by APAP persisted for at least 24 hours (Fig. 2B). This finding is important because, in contrast to β-agonist-mediated bronchodilation where tachyphylaxis/desensitization can be an issue, bronchodilation by APAP was sustained. After exposure to 1μM CCh, 5mM APAP caused significant bronchodilation compared to CCh alone (APAP Mean 31.41%, SD 25.64, and CCh Mean 56.29%, SD 11.08; p<0.05) (Figure 3A).
Figure 2: APAP is an acute bronchodilator with persistent effects.
Human PCLS airways were bronchoconstricted ex vivo by crosslinking IgE with anti-IgE, by treatment with 1mM histamine (His), or by treatment with 1 μM carbachol (CCh). Increasing doses of APAP were added and airway cross-sectional areas were measured to evaluate bronchodilation (A), which was observed in all cases at doses of 2 and 5 mM APAP. Data are expressed as mean±SE for n = 8 independent PCLS airways from one human donor. The duration of APAP-induced bronchodilation from one slice was measured by time-lapse video microscopy (B). Addition of 1 μM CCh resulted in airway occlusion and this was rapidly and persistently reversed after addition of 5 mM APAP.
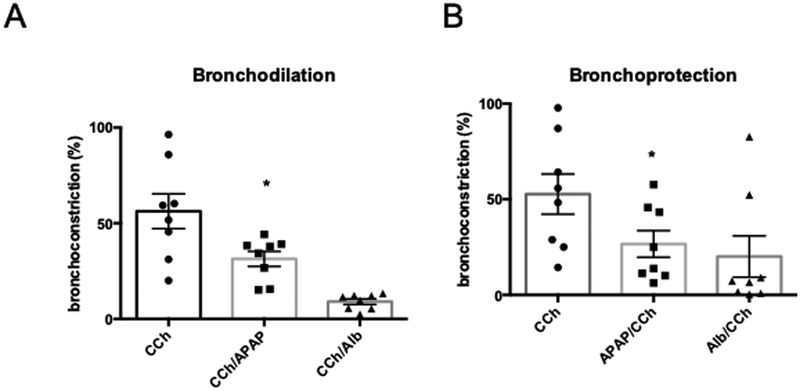
Figure 3: Comparison of APAP-induced bronchoprotection and bronchodilation to β-agonists.
Human PCLS airways were treated with 1μM carbachol (CCh) before treatment with 1μM albuterol (Alb) or 5 mM APAP for 1hour as indicated. The percent bronchoprotection or bronchodilation was calculated from measurements of airway cross-sectional area and compared to parallel PCLS airways treated with CCh alone. Data are expressed as mean±SE for n = 8 independent PCLS airways from one human donor.
Pre-incubation with the beta-agonists albuterol prior to treatment with CCh abrogates bronchoconstriction, a phenomenon referred to as bronchoprotection (figure 3B). The bronchoprotective effects of APAP were evaluated by preincubation of PCLS airways with 5 mM APAP for 60 minutes. Like the beta-agonists, CCh-induced bronchoconstriction was reduced by APAP (APAP Mean 26.65%, SD 19.62, CCh Mean 52.68%, SD 29.69; p<0.05) (figure 3B). Similarly, both beta-agonists and APAP promoted bronchodilation. However, the bronchodilatory and bronchoprotective effects of APAP were modest compared to the beta-agonists.
The bronchodilatory effects of acetaminophen on normal and asthmatic airways are comparable.
The preceding results indicate that in airways from normal individuals, a likely effect of APAP is to enhance airflow. Given that the concerns raised by clinicians are related to asthmatic children, we also evaluated the effects of APAP on airway bronchodilation from a single donor with fatal asthma (Fig. 4). 1μM His was used to bronchoconstrict airways from this donor, and treatment with APAP led to dose-dependent bronchodilation (Fig. 4A). Similar to airways prepared from normal donors, maximal APAP dilation was less than that induced by 2μM isoproterenol or 2μM forskolin; however, 2mM and 5mM doses of APAP did lead to significant bronchodilation effects in these airways (2mM APAP mean 59.40%, SD 29.26, 5mM APAP mean 49.82%, SD 26.11, His mean 65.42%, SD 23.59; His compared to either 2 or 5mM APAP, p<0.05) (Fig. 4A). While human in vivo studies of APAP overdose using PCLS airways are not feasible, we did procure one donor with a history of APAP overdose (unknown quantity of ingestion) approximately 4 weeks prior to organ collection. The APAP overdose was not the cause of death for this patient, and the donor did not have a history of asthma. PCLS airways from this donor were evaluated for bronchodilation by APAP, and results were similar to those noted in normal (Figs. 1–3) and asthmatic donor lungs (Fig. 4A) (2mM APAP mean 47.13%, SD 14.91, 5mM APAP mean 32.38%, SD 11.69, His mean 57.94%, SD 15.25; His compared to either 2 or 5mM APAP, p<0.05) (Fig. 4B).
Figure 4: Bronchoconstriction and bronchodilation in PCLS airways from donors with fatal asthma and APAP overdose donor.
A) PCLS airways (n=8) from a single human lung donor with history of fatal asthma were treated with 1 μM of histamine (His) for 15 minutes and then increasing doses of APAP were added at 15-minute intervals. Airway luminal area was measured after each incubation. APAP at 2 and 5mM decreased bronchoconstriction and increased bronchodilation (p<0.05), though not on the order of either isoproterenol or forskolin. B). PCLS (n=47) from a single human donor with history of APAP overdose were treated as above. Similarly, APAP at doses of 2 and 5mM decreased bronchoconstriction (caused bronchodilation) (p<0.05), though not on the order of isoproterenol or forskolin. Data are expressed as mean±SE. *p<0.05 vs. His alone.
Hepatotoxic doses of APAP in mice appear bronchoprotective when evaluated ex vivo.
To evaluate the effect of administration of toxic doses of APAP on bronchoconstriction, we used a well-characterized mouse model of APAP toxicity. Mice were treated with saline, 100, 200, or 300 mg/kg APAP and sacrificed 1 or 24 hours after APAP administration (3 mice/group) (McGill and others 2013). After sacrifice, mouse PCLS airways were prepared and cultured for 72 hours and treated with 20μM CCh to evaluate bronchoconstrictor responses (Fig. 5). Airways from mice treated with APAP for 1 hour were less responsive to CCh than airways from saline-treated mice. Airways from mice treated with APAP for 24 hours were also less responsive to CCh. Thus, similar to human airways, APAP treatment in vivo did not induce AHR and may even be bronchoprotective in mouse airways ex vivo.
Figure 5. APAP treatment in in vivo murine models is bronchoprotective ex vivo.
C57Bl/6J mice were treated with saline (0 m/kg) or the indicated doses of APAP for 1 hour (black circles) or 24 hours (red squares). After euthanasia, precision cut lung slices were prepared and bronchoconstrictor responses to 1μM CCh were measured ex vivo. Data are expressed as mean±SD for n = 11–21 mouse PCLS airways from three donors.
Multiple doses of acetaminophen can cause oxidative stress in mouse lungs.
It has been hypothesized that the pulmonary effects of APAP are due to depletion of glutathione in the lungs and resulting oxidative stress (Kennon-McGill and McGill 2018). To test this hypothesis, mice were treated with saline vehicle or either once with a toxic (300 mg/kg) dose of APAP or multiple times (every 3h for a total of 12 h) with a sub-toxic dose (100 mg/kg), and tissue samples were collected for measurement of glutathione (Fig. 6). Blood samples were collected at 1 hour and prepared into serum for determination of serum ALT measures. No differences were found in ALT measurements between the APAP treatment and saline controls (data not shown), as would be expected at 1 hour, an early time point Total glutathione (GSH+GSSG) was decreased in both lungs and liver at 1 h post-APAP, though the decrease in lungs was much less (28% vs. 86% of control for lung and liver tissue, respectively) (Fig. 6A). The time course of GSH+GSSG levels in the lungs revealed that GSH remained decreased until 12 h post-APAP before returning to control values (Fig. 6B). Importantly, although a single treatment with 100 mg/kg APAP had no effect on lung GSH+GSSG or GSSG, multiple treatments did reduce GSH+GSSG levels and increase GSSG (Fig. 6C, D).
Figure 6. Markers of oxidative stress in the lungs are increased in mice following APAP administration.
C57Bl/6J mice were treated either once with PBS vehicle, 100, or 300 mg/kg APAP, or multiple times with 100 mg/kg APAP. (A) Lung and liver total glutathione 1 h after treatment with 300 mg/kg APAP. (B) Lung total glutathione at multiple time points after treatment with 300 mg/kg APAP. (C) Lung total glutathione after single or multiple doses of PBS or 100 mg/kg APAP. (D) Lung GSSG after single or multiple doses of PBS or 100 mg/kg APAP. Data expressed as mean±SE for n = 3–6 mice per group. *p<0.05 vs. Veh.
Acetaminophen-protein adducts are detectable in mouse airways and human PCLS airways.
Although our data demonstrate that APAP decreases GSH+GSSG levels in the lung after large doses, the decrease is modest compared to those noted in the liver. Thus, if APAP-protein adducts form in the lungs after APAP exposure, it likely only occurs in a single specific cell type. To determine if APAP-protein adducts are detectable in lungs after sub-toxic or toxic doses of APAP and to define the cellular localization of APAP metabolism, we performed immunohistochemistry for acetaminophen-protein adducts in lungs from the APAP-treated mice and in APAP-treated human PCLS airways. Dark brown staining indicative of adducts was detected in airway epithelial cells from mice treated with 100 or 300 mg/kg APAP compared to saline controls (Fig. 7). Similar results were obtained from the human PCLS airways (Fig. 8). The majority of the epithelial staining was observed in the small to mid-size airways and in the alveoli. To determine the extent of APAP-protein binding, we also measured APAP-protein adducts in whole tissue homogenates from several mice treated with varied doses of APAP using HPLC with electrochemical detection. Although adducts were detectable in lungs after APAP treatment, the concentrations were far lower than in liver (5.8±3% of liver values). Together, these data demonstrate APAP-protein adducts can occur in both mouse and human airways after exposure to high doses of APAP, but adducts are detectable only at low levels.
Figure 7: Immunohistochemical analysis of APAP protein adducts in mouse lung.
C57Bl/6J mice were treated with saline, 100, 200 or 300 mg/kg APAP and euthanized 4 h later. Lung tissue from control (saline) and APAP-treated mice were stained for APAP protein adducts and examined microscopically with a 40x objective. Increased brown staining indicative of APAP-adducts is observed in the airway epithelium from mice treated with 200 and 300 mg/kg APAP.
Figure 8: Immunohistochemical detection of APAP protein adducts in human precision cut lung slices.
Airways in PCLS were treated with 5 mM APAP for 24 h, fixed and stained for APAP-protein adducts. Serial sections were processed in the absence (control) and presence (anti-APAP adducts) of primary antibody.
Discussion
The present studies used human and mouse PCLS airways ex vivo to evaluate the effects of APAP on airway function mediated by smooth muscle contractility. Based on epidemiological observations (Amberbir and others 2014; Barr and others 2004; Etminan and others 2009a; Kreiner-Moller and others 2012; Perzanowski and others 2010), we hypothesized that APAP would promote airway hyper-responsiveness. This is an important clinical issue as some physicians are reluctant to use APAP during febrile illnesses in pediatric patients with asthma or a family history of asthma due to concern for eliciting an asthma exacerbation (Lowe and others 2010; McBride 2011; Newson and others 2000; Shaheen and others 2000). However, our experiments suggest that acute treatment with APAP does not promote acute AHR ex vivo. This finding confirms the work of Soferman, et al, in which they performed a double-blind placebo-controlled study of 42 asthmatic children and 21 healthy controls. In that study, physical examination, spirometry results, and fractional exhaled nitric oxide levels remained similar before and after both APAP and saline placebo (Soferman and others 2013). In fact, in our studies, rather than promoting AHR, we observed that APAP had mild bronchodilator effects at high concentrations. These findings are also similar to a study by Sheehan, et al. in which the authors determined that there was no increased risk for asthma exacerbations in young children exposed to APAP (Sheehan and others 2016; Sheehan and Phipatanakul 2016). Finally, our results are also comparable to those from a previous study in which APAP attenuated inflammatory responses in mice sensitized to house dust mite exposure (Smith and others 2016). However, there were no measures of lung function or AHR in that study.
It is important to note that the concentrations at which bronchodilation was achieved were relatively high. The therapeutic range for serum APAP concentration is 10 – 20 μg/mL (65 – 130 μM), though concentrations up to 50 μg/mL (325 μM) are generally considered safe in humans. We observed bronchodilatory effects beginning at 300–400 μM. Thus, it is unlikely that typical therapeutic doses of APAP promote bronchodilation, though high therapeutic doses might have that effect. Nevertheless, our data indicate that APAP does not acutely induce bronchoconstriction.
Human PCLS airways provide an excellent platform for functional studies of airway contractility because the slices maintain the 3-dimensional architecture and integrity of lung. In human PCLS airways, treatment with APAP for 72 hours did not promote AHR. Contrary to our original hypothesis, acute treatment with APAP bronchodilated pre-constricted airways, an effect that persisted for hours. Further, pretreatment with APAP was bronchoprotective in both human and mouse airways. Bronchodilation by APAP was observed in airways from both normal and asthmatic donors as well as in airways from a donor with a recent history of APAP overdose. We postulate that a potential mechanism for bronchoprotection and bronchodilation occurs through bitter receptors (TAS2R) because (1) APAP has been shown to activate TAS2R39 in HEK293 cells (Meyerhof and others 2010) and (2) TAS2R has been shown to have bronchodilatory properties in both mouse and human airways (Deshpande et al., 2010; Kohl et al., 2013), including PCLS airways (An and others 2012). While significant compared to vehicle, the magnitude of bronchodilation by APAP was not as robust as that induced with a short-acting β-agonists albuterol). Taken together, the functional results indicate that APAP does not enhance human airway bronchoconstriction or inhibit bronchodilation, but instead leads to mild bronchodilation.
Although glutathione depletion and oxidative stress have been proposed as mechanisms to explain the effects of APAP in the lungs, we observed only modest depletion of glutathione in mouse lung after administration of high doses of APAP. While repeated treatments with a high dose of APAP caused increased reactive oxygen species formation in the lung tissue based on GSSG content, that dose was still larger than a typical human daily dose which would equate to 48–57 mg/kg/day for a 70 kg individual treated with 3–4 grams per day. Finally, although we have demonstrated for the first time that APAP-protein binding occurs in non-hepatic tissues in humans (human airway epithelial cells), the adduct levels were very low. Based on those data, we cannot rule out the possibility that repeated therapeutic use of APAP depletes glutathione and causes minor chronic tissue injury, but it appears to be an unlikely scenario, especially when considering Morris, et al. In this study, the authors determined that systemic levels of glutathione were not different between young children exposed to APAP with and without asthma exacerbations (Morris and others 2018).
Our results seemingly contradict current epidemiological reports that link APAP to the etiology of asthma (Amberbir and others 2014; Barr and others 2004; Beasley and others 2008; Beasley and others 2011; Etminan and others 2009a; Kennon-McGill and McGill 2018; Kreiner-Moller and others 2012; Lowe and others 2010; McBride 2011; Rebordosa and others 2008; Roberts and others 1991). Much of the data for those reports has been derived from observational studies, which are limited due to confounding by indication, a problem in which symptoms of an underlying disorder are mistakenly considered a side effect of treatment (Kennon-McGill and McGill 2018; Signorello and others 2002). In this case, viral and bacterial infections lead to fever, which is treated with APAP. It is possible that it is the viral infections, rather than exposure to APAP, that is associated with wheeze and asthma, as well as exacerbations of asthma. As an example, a recent study of 1,490 mother/child pairs revealed that APAP use in infants increased the risk for asthma in early childhood (unadjusted OR 1.21). However, when the authors adjusted for respiratory tract infections in early childhood, the effect was almost abolished (Sordillo and others 2015), and those findings were consistent with others refuting the APAP asthma association (Schnabel and others 2010).
Importantly, the present study was not designed to evaluate the impact of APAP on the development of an asthma phenotype. Rather, we focused on the potential for acute effects of APAP on promoting AHR. Acute exposure to APAP may not be sufficient to promote AHR, whereas chronic exposure may have different effects, which we did not study. Furthermore, our study was not designed to evaluate an IgE-mediated allergic response to APAP. Drug allergy to APAP is considered a rare event, but it has been documented. One might expect that a person that is allergic to APAP would have AHR in our platform after exposure to the offending drug. An experiment such as this would require exposure to the drug and generation of IgE and a second exposure that would cause release of His and ultimately AHR. Based on clinical experience, this rare event is usually not the reason that many general pediatricians avoid APAP in the asthma population, and, therefore, we chose to evaluate the acute effects of single exposure with APAP on airways.
Overall, our results suggest that acute exposure to APAP does not induce AHR, but is instead protective at high doses, reducing or even reversing airway contractile responses. Thus, we suggest that it is unnecessary to exclude the use of APAP in children with asthma or a family history of asthma.
Supplementary Material
Acknowledgments
This research was supported, in part, by the Arkansas Children’s Research Institute, by the Arkansas Biosciences Institute, and by the University of Arkansas for Medical Sciences College of Medicine Children’s University Medical Group Fund Grant Program (JK). Additional support was provided by the AASLD Foundation Pinnacle Research Award (MRM).
Funding Details: Dr. Kennedy is supported by grants from the NIH NIAID (K08AI121345–01A1), NIH NCATS (UL1TR000039, KL2TR000063), the University of Arkansas for Medical Sciences Clinician Scientist Program, Arkansas Biosciences Institute, and the Arkansas Children’s Research Institute Marion B. Lyon New Scientist Development Award. Drs. Panettieri, Koziol-White, and Kurten disclose funding by the National Institutes of Health (P01 HL114471).
Footnotes
Disclosure Statement: The authors have no relevant conflicts of interest.
References:
- Abramoff MD, Magalhaes, Paulo J; Ram, Sunanda J (2004). Image processing with Image J. Biophotonics International, 11, 36–42. [Google Scholar]
- Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, Liu X. (2012). Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS data brief, 1–8. [PubMed] [Google Scholar]
- Amberbir A, Medhin G, Hanlon C, Britton J, Davey G, Venn A. (2014). Effects of early life paracetamol use on the incidence of allergic disease and sensitization: 5 year follow-up of an Ethiopian birth cohort. PloS one, 9, e93869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An SS, Wang WC, Koziol-White CJ, Ahn K, Lee DY, Kurten RC, Panettieri RA Jr., Liggett SB. (2012). TAS2R activation promotes airway smooth muscle relaxation despite beta(2)-adrenergic receptor tachyphylaxis. Am J Physiol Lung Cell Mol Physiol, 303, L304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrowsmith JB, Kennedy DL, Kuritsky JN, Faich GA. (1987). National patterns of aspirin use and Reye syndrome reporting, United States, 1980 to 1985. Pediatrics, 79, 858–63. [PubMed] [Google Scholar]
- Barr RG, Wentowski CC, Curhan GC, Somers SC, Stampfer MJ, Schwartz J, Speizer FE, Camargo CA Jr. (2004). Prospective study of acetaminophen use and newly diagnosed asthma among women. Am J Respir Crit Care Med, 169, 836–41. [DOI] [PubMed] [Google Scholar]
- Beasley R, Clayton T, Crane J, von Mutius E, Lai CK, Montefort S, Stewart A, Group IPTS. (2008). Association between paracetamol use in infancy and childhood, and risk of asthma, rhinoconjunctivitis, and eczema in children aged 6–7 years: analysis from Phase Three of the ISAAC programme. Lancet, 372, 1039–48. [DOI] [PubMed] [Google Scholar]
- Beasley RW, Clayton TO, Crane J, Lai CK, Montefort SR, Mutius E, Stewart AW, Group IPTS. (2011). Acetaminophen use and risk of asthma, rhinoconjunctivitis, and eczema in adolescents: International Study of Asthma and Allergies in Childhood Phase Three. Am J Respir Crit Care Med, 183, 171–8. [DOI] [PubMed] [Google Scholar]
- Chand N, Dhawan BN, Srimal RC, Rahmani NH, Shukla RK, Altura BM. (1980). Reactivity of trachea, bronchi, and lung strips to histamine and carbachol in rhesus monkeys. J Appl Physiol Respir Environ Exerc Physiol, 49, 729–34. [DOI] [PubMed] [Google Scholar]
- Cooper PR, Kurten RC, Zhang J, Nicholls DJ, Dainty IA, Panettieri RA. (2011). Formoterol and salmeterol induce a similar degree of beta2-adrenoceptor tolerance in human small airways but via different mechanisms. British journal of pharmacology, 163, 521–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper PR, Lamb R, Day ND, Branigan PJ, Kajekar R, San Mateo L, Hornby PJ, Panettieri RA Jr. (2009). TLR3 activation stimulates cytokine secretion without altering agonist-induced human small airway contraction or relaxation. Am J Physiol Lung Cell Mol Physiol, 297, L530–7. [DOI] [PubMed] [Google Scholar]
- Etminan M, Sadatsafavi M, Jafari S, Doyle-Waters M, Aminzadeh K, Fitzgerald JM. (2009a). Acetaminophen use and the risk of asthma in children and adults: a systematic review and metaanalysis. Chest, 136, 1316–23. [DOI] [PubMed] [Google Scholar]
- Etminan M, Sadatsafavi M, Jafari S, Doyle-Waters M, Aminzadeh K, FitzGerald JM. (2009b). Acetaminophen use and the risk of asthma in children and adults: a systematic review and metaanalysis. Chest, 136, 1316–23. [DOI] [PubMed] [Google Scholar]
- Hinson JA, Roberts DW, James LP. (2010). Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol, 369–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James LP, Letzig L, Simpson PM, Capparelli E, Roberts DW, Hinson JA, Davern TJ, Lee WM. (2009). Pharmacokinetics of acetaminophen-protein adducts in adults with acetaminophen overdose and acute liver failure. Drug metabolism and disposition: the biological fate of chemicals, 37, 1779–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. (2002). Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. Jama, 287, 337–44. [DOI] [PubMed] [Google Scholar]
- Kennedy JLK-WC, Jeffus S, Rettiganti MR, Fisher P, Kurten M, Eze A, House S, Sikes JD, Askew E, Putt C, Panettieri RA, Jones SM, Kurten RC. (2018). Effects of rhinovirus 39 infection on airway hyperresponsiveness to carbachol in human airways precision cut lung slices. J Allergy Clin Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennon-McGill S, McGill MR. (2018). Extrahepatic Toxicity of Acetaminophen: Critical Evaluation of the Evidence and Proposed Mechanisms. J Clin Transl Res, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiner-Moller E, Sevelsted A, Vissing NH, Schoos AM, Bisgaard H. (2012). Infant acetaminophen use associates with early asthmatic symptoms independently of respiratory tract infections: the Copenhagen Prospective Study on Asthma in Childhood 2000 (COPSAC(2000)) cohort. J Allergy Clin Immunol, 130, 1434–6. [DOI] [PubMed] [Google Scholar]
- Lee WM. (2008). Etiologies of acute liver failure. Semin Liver Dis, 28, 142–52. [DOI] [PubMed] [Google Scholar]
- Lowe AJ, Carlin JB, Bennett CM, Hosking CS, Allen KJ, Robertson CF, Axelrad C, Abramson MJ, Hill DJ, Dharmage SC. (2010). Paracetamol use in early life and asthma: prospective birth cohort study. Bmj, 341, c4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride JT. (2011). The association of acetaminophen and asthma prevalence and severity. Pediatrics, 128, 1181–5. [DOI] [PubMed] [Google Scholar]
- McGill MR, Jaeschke H. (2013). Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res, 30, 2174–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Jaeschke H. (2015). A direct comparison of methods used to measure oxidized glutathione in biological samples: 2-vinylpyridine and N-ethylmaleimide. Toxicol Mech Methods, 25, 589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Lebofsky M, Norris HR, Slawson MH, Bajt ML, Xie Y, Williams CD, Wilkins DG, Rollins DE, Jaeschke H. (2013). Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: dose-response, mechanisms, and clinical implications. Toxicology and applied pharmacology, 269, 240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, Appendino G, Behrens M. (2010). The molecular receptive ranges of human TAS2R bitter taste receptors. Chemical senses, 35, 157–70. [DOI] [PubMed] [Google Scholar]
- Morris CR, Mauger DT, Suh JH, Phipatanakul W, Sheehan WJ, Moy JN, Paul IM, Szefler SJ, Jackson DJ, Fitzpatrick AM and others. (2018). Glutathione and arginine levels: Predictors for acetaminophen-associated asthma exacerbation? J Allergy Clin Immunol, 142, 308–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldrew KL, James LP, Coop L, McCullough SS, Hendrickson HP, Hinson JA, Mayeux PR. (2002). Determination of acetaminophen-protein adducts in mouse liver and serum and human serum after hepatotoxic doses of acetaminophen using high-performance liquid chromatography with electrochemical detection. Drug metabolism and disposition: the biological fate of chemicals, 30, 446–51. [DOI] [PubMed] [Google Scholar]
- Newson RB, Shaheen SO, Chinn S, Burney PG. (2000). Paracetamol sales and atopic disease in children and adults: an ecological analysis. Eur Respir J, 16, 817–23. [DOI] [PubMed] [Google Scholar]
- Perzanowski MS, Miller RL, Tang D, Ali D, Garfinkel RS, Chew GL, Goldstein IF, Perera FP, Barr RG. (2010). Prenatal acetaminophen exposure and risk of wheeze at age 5 years in an urban low-income cohort. Thorax, 65, 118–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevention CDC. (May 2011). Vital Signs. May.
- Rebordosa C, Kogevinas M, Sorensen HT, Olsen J. (2008). Pre-natal exposure to paracetamol and risk of wheezing and asthma in children: a birth cohort study. Int J Epidemiol, 37, 583–90. [DOI] [PubMed] [Google Scholar]
- Roberts DW, Bucci TJ, Benson RW, Warbritton AR, McRae TA, Pumford NR, Hinson JA. (1991). Immunohistochemical localization and quantification of the 3-(cystein-S-yl)-acetaminophen protein adduct in acetaminophen hepatotoxicity. The American journal of pathology, 138, 359–71. [PMC free article] [PubMed] [Google Scholar]
- Schnabel E, Heinrich J, Group LS. (2010). Respiratory tract infections and not paracetamol medication during infancy are associated with asthma development in childhood. J Allergy Clin Immunol, 126, 1071–3. [DOI] [PubMed] [Google Scholar]
- Shaheen SO, Sterne JA, Songhurst CE, Burney PG. (2000). Frequent paracetamol use and asthma in adults. Thorax, 55, 266–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan WJ, Mauger DT, Paul IM, Moy JN, Boehmer SJ, Szefler SJ, Fitzpatrick AM, Jackson DJ, Bacharier LB, Cabana MD and others. (2016). Acetaminophen versus Ibuprofen in Young Children with Mild Persistent Asthma. N Engl J Med, 375, 619–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan WJ, Phipatanakul W. (2016). Acetaminophen versus Ibuprofen in Mild Persistent Asthma. N Engl J Med, 375, 2099–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorello LB, McLaughlin JK, Lipworth L, Friis S, Sorensen HT, Blot WJ. (2002). Confounding by indication in epidemiologic studies of commonly used analgesics. Am J Ther, 9, 199–205. [DOI] [PubMed] [Google Scholar]
- Smith GJ, Thrall RS, Cloutier MM, Manautou JE, Morris JB. (2016). Acetaminophen Attenuates House Dust Mite-Induced Allergic Airway Disease in Mice. J Pharmacol Exp Ther, 358, 569–79. [DOI] [PubMed] [Google Scholar]
- Soferman R, Tsivion A, Farber M, Sivan Y. (2013). The effect of a single dose of acetaminophen on airways response in children with asthma. Clin Pediatr (Phila), 52, 42–8. [DOI] [PubMed] [Google Scholar]
- Sordillo JE, Scirica CV, Rifas-Shiman SL, Gillman MW, Bunyavanich S, Camargo CA Jr., Weiss ST, Gold DR, Litonjua AA. (2015). Prenatal and infant exposure to acetaminophen and ibuprofen and the risk for wheeze and asthma in children. J Allergy Clin Immunol, 135, 441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlsen A, Uhlig S, Martin C. (2001). Immediate allergic response in small airways. Am J Respir Crit Care Med, 163, 1462–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.