Abstract
Depression is characterized by altered sensitivity to rewards, with recent evidence suggesting that the ability to sustain responses to rewards over the course of long experimental tasks is diminished. Most work on sustained reward responsiveness has taken a categorical approach and focused on major depressive disorder. However, impairments in reward sensitivity are also found at lower levels of symptom severity and may be relevant for understanding basic mechanisms linking reward processing abnormalities to depression. The current study took a dimensional approach to examine the relation between depression symptoms and sustained reward responsiveness by examining how early neural responses to rewards and losses change over a short time course, i.e., during the experiment. In a sample of 45 unselected undergraduates, changes in the amplitude of the reward positivity (RewP) and feedback negativity (FN) were examined over the course of a simple gambling task using multilevel modeling (MLM). Amplitude of the RewP was sustained and amplitude of the FN increased over the course of the task. Unlike prior work focused on clinical populations, depression symptoms in this unselected sample were associated with enhanced RewP and FN responding over the course of the task. Results echo prior work that underscores the importance of examining changes in response to reward over the course of trials and further suggests that sustained responses to both rewards and losses vary in relation to symptom level.
1. Introduction
A wealth of research has linked depression to deficits in reward sensitivity. Recent findings have related depression symptoms to impaired reward learning (Henriques & Davidson, 2000; Pizzagalli, Iosifescu, Hallett, Ratner & Fava, 2008; Liu et al., 2011), diminished willingness to complete physically difficult tasks in order to obtain rewards (Treadway, Buckholtz, Schwartzman & Zald, 2009; Treadway, Bossaller, Shelton & Zald, 2012) and blunted neural responses to rewards relative to non rewards (Epstein et al., 2006; Foti & Hajcak, 2009; Foti, Weinberg, Dien & Hajcak, 2011). Depression symptoms have also been linked to abnormal patterns of sustained reward responding, with converging evidence from functional neuroimaging (Heller et al., 2009; Heller et al., 2014), contingency-based reward learning (Liu et al., 2011) and event-related potential (ERP) studies (Brush, Ehmann, Hajcak, Selby & Alderman, 2018), suggesting that individuals with higher levels of depression show more rapidly diminishing responses to rewards over the course of long experimental tasks.
However, most research on relations between sustained reward responding and depression has been limited to major depressive disorder (MDD), despite evidence that abnormalities to reward sensitivity are not unique to clinical samples and have been reported in relation to elevated depression symptoms in the general population. For example, Pizzagalli, Jahn and O’Shea (2005) have demonstrated that participants with elevated depression symptoms but no MDD diagnosis develop weak or non existent response biases to rewards relative to participants with low levels of depression. Additionally, Liu and colleagues (2011) have reported diminished tendencies to develop response biases towards rewards in both major depression and sub-syndromal depression relative to control individuals. Variations in behavioral measures of reward sensitivity have also been linked to structural differences in brain regions implicated in reward processing (Harvey, Pruessner, Czechowska & Lepage, 2007) and to differences in electrophysiological indices of reward sensitivity (Bress & Hajcak, 2013; Liu et al., 2014) both in the general population and in MDD, supporting the view that reward processing abnormalities are related to depression symptoms dimensionally, not only within formal diagnostic categories.
Based on these and similar findings, including changes to reward sensitivity observed in other disorders (e.g., schizophrenia; Gard, Kring, Gard, Horan & Green, 2007), recent work has proposed that abnormalities to reward sensitivity are not limited to major depression but relate dimensionally to varying levels of dysfunction within dopaminergic brain circuits that mediate reward appraisal (Whitton, Treadway & Pizzagalli, 2015; Dillon, Rosso, Pechtel, Killgore, Rauch, & Pizzagalli, 2014). Indeed, there has recently been increased interest in conceptualizing reward sensitivity as a dimensional construct independent from the diagnostic entities defined by the Diagnostic and Statistical Manual for Mental Disorders (DSM; Dillon, et al., 2014; Woody & Gibb, 2015). More broadly, the National Institute of Mental Health’s (NIMH) Research Domain Criteria (RDoC) initiative has emphasized the value of dimensional approaches to studying neurobiological and psychological constructs in relation to features of psychopathology, beyond the limitations of variance in putative mechanisms imposed by narrowly defined disorder categories (Cuthbert, 2014; Sanislow, 2016). The ultimate goal of this dimensional approach is to more fully capture the variance of disrupted mechanisms in psychopathology research, and ultimately to aid in improving approaches for clinical diagnosis and treatment of clinical problems such as depression (Kozak & Cuthbert, 2016). While a growing number of studies have applied this framework to the study of clinical features of depressive disorders by linking variation in symptom levels to specific neural measures (e.g., Nusslock, Walden & Harmon-Jones, 2015), relatively little research to date has examined varying levels of depression symptoms in relation to indices of reward sensitivity, despite the benefit that such information would provide for clarifying depression mechanisms and suggesting new approaches to treatment (Woody & Gibb, 2015).
Such dimensional research is particularly lacking for examining relationships between depression and sustained reward responsiveness, despite the fact that sustained responses to rewards are a key component of the RDoC framework in the Positive Valence Systems domain (National Institute of Mental Health, n.d.) and appear to be altered in patients with MDD (Heller et al., 2009; Liu et al., 2011). Aberrant sustained responses to rewards have been proposed as a core contributor to depression symptoms, particularly anhedonia (Tomarken & Keener, 1998), and are relevant for understanding how individuals sustain positive affect across long periods in their daily lives (Heller et al., 2009). Understanding altered neural correlates of reward sensitivity in general populations is important for drawing conclusions about patterns seen in clinical populations. For instance, altered neural sensitivity to rewards has been identified as a risk factor for clinical depression (Bress & Hajcak, 2013).
However, because no studies to date have investigated whether sustained reward responsiveness is linked to depression symptoms outside of patient groups, current understanding of this aspect of reward sensitivity remains restricted to individuals with high levels of depression severity. To address this gap, the present study examined the relation between sustained reward responding and depression symptoms in an unselected sample, using two commonly studied electrophysiological measures of reward sensitivity, the reward positivity (RewP) and the feedback negativity (FN). We were particularly interested in changes in RewP and FN over the time course of experimental trials.
The RewP and FN are ERP components elicited by feedback indicating rewards versus non rewards, respectively. The RewP is characterized as a relative positivity in the ERP waveform following rewards that is absent following losses (Proudfit, 2015; Levinson, Speed, Infantolino & Hajcak, 2017), and the FN is characterized as a relative negativity following losses and not rewards (Holroyd & Coles, 2002; Foti & Hajcak, 2009; Proudfit, 2015). The difference between RewP and FN is known as ∆RewP (rewards minus losses; Levinson et al., 2017). Variability in ∆RewP between individuals is more strongly explained by differences in RewP than differences in FN (Bress & Hajcak, 2013; Foti et al., 2011), suggesting that this ERP measure indexes neural sensitivity to rewards (Proudfit, 2015). Importantly, the RewP appears blunted in MDD relative to healthy individuals (Foti & Hajcak, 2009; Foti et al., 2011; Foti, Carlson, Sauder & Proudfit, 2014). The RewP and FN are maximal at frontocentral sites and peak 250–350 ms following presentation of feedback regarding whether an outcome was favorable or unfavorable (Foti & Hajcak, 2009). Source localization and functional neuroimaging studies have linked ∆RewP to increased striatal and medial prefrontal activation during favorable versus unfavorable outcomes (Foti, Weinberg, Dien & Hajcak, 2011; Carlson, Foti, Mujica-Parodi, Harmon-Jones & Hajcak, 2011), suggesting that RewP reflects activity in reward-encoding brain circuitry (Proudfit, 2015). Further, within-task RewP amplitudes show less increase over time at higher levels of depression severity when including patients with MDD (Brush et al., 2018), making RewP and FN useful for examining alterations to sustained reward responses in relation to depression.
Using a widely utilized gambling task, the current study investigated how early responses to both rewards and non rewards (losses) change across trials as a function of depression symptoms, using RewP and FN amplitude to track how responding changed over the course of the task. In order to investigate the temporal dynamics of the RewP/FN, we used multilevel modeling (MLM), similar to prior analyses of within-task ERP amplitude changes (Brush et. al, 2018; Volpert-Esmond, Merkle, Levsen, Ito & Bartholow, 2017). Depression symptoms were assessed dimensionally across an unselected undergraduate sample, consistent with other studies examining reward sensitivity outside of patient groups (Pizzagalli, Jahn & O’Shea, 2005; Bress & Hajcak, 2013). In light of findings in MDD (Brush et al., 2018), it was hypothesized that RewP would show more negative within-task amplitude changes relative to FN for participants with relatively high levels of depression symptoms.
2. Method
2.1. Participants
Fifty-two university students (38 females) were recruited using electronic and print advertisements. Seven participants were deemed not to have usable EEG data for any trial of the task and were thus excluded from MLM analysis, for reasons as follows: difficulties performing the task (n = 2), problems during EEG recording that compromised data quality (n = 4), and experimenter error (n = 1). The final sample consisted of 45 participants (34 females) with a mean age of 20.20 (SD = 1.25). The racial breakdown of the participant sample was as follows: 51.1% White, 35.6% Asian, 2.2% American Indian, 2.2% Black and 6.7% more than one race. 2.2% of participants did not volunteer their racial identity. Hispanic participants comprised 8.9% of the final sample. The university institutional review board approved the study, and informed consent was obtained from each participant prior to beginning the procedure. Participants were compensated $20.00 in total for their time. No participants chose to discontinue participation.
2.2. Personality Assessment Inventory – Depression Scale
Depression was assessed using the depression scale of the Personality Assessment Inventory (PAI-DEP; Morey, 1991), a measure of personality and psychopathology. The PAI-DEP is highly correlated with other depression measures such as the Beck Depression Inventory (Morey, 2003; Hill, Musso, Jones, Pella & Gouvier, 2013). It includes three subscales, each corresponding to a distinct category of depression symptoms: affective (A), cognitive (C) and physiological (P). Individual item scores on the PAI-DEP range from 1 to 4, and scores of 1 are recoded into 0, 2 into 1, 3 into 2 and 4 into 3. Eight items are reverse-scored because they reflect the absence of depression symptoms (#6, 9, 17, 18, 19, 20, 22, and 23). Total and subscale raw scores are converted into T scores, which reflect norms from a sample of 1,000 adults living in the community in the United States (Morey, 2003), and range from 20 to 120. Scores below 59T indicate minimal depression, corresponding to <13 on the BDI-II; 59–69T indicates mild to moderate difficulty (BDI-II: 14–19); over 70T indicates moderate depression and merits clinical attention (BDI-II > 20); over 81T is indicative of severe depression (BDI-II > 29; Beck, Steer & Brown, 1996; Morey, 1991, p. 28). Three participants did not answer one or two questions on the PAI-DEP. For those participants, mean substitutions were used in order to obtain total scores. The internal consistency of the PAI-DEP for the current sample was high (α = 0.84).
2.3. Doors Task
A simple gambling task, the “doors task” (Foti & Hajcak, 2009), was used to elicit the RewP and FN. The task was administered using Presentation software on a Pentium 4 class computer with a 19-in. monitor. Each trial consisted of the presentation of two identical doors, and participants were instructed to choose the door they believed there was a prize behind. Fifty percent of trials resulted in rewards and presented positive feedback in the form of a green arrow pointing upward, which signaled that the participant had chosen correctly and would receive an additional $0.50. The remaining 50% of trials resulted in losses and presented negative feedback in the form of a red arrow pointing down, which signaled that the participant had chosen incorrectly and would lose $0.25. In total, the task consisted of 60 trials, with reward and loss trials ordered randomly. On each trial, the doors were presented and participants were instructed to choose one door within four seconds. They were told to click the left mouse button to select the left door and the right mouse button to select the right door. After responding, a fixation cross was presented for 1000 ms. Feedback was then presented for 2000 ms. A fixation cross was presented again for 1500 ms and was followed by a screen that read “Click for next round” that remained visible until participants clicked (see Figure 1). All participants were compensated an additional $7.50 for their earnings in the doors task, which were the same across all participants.
Figure 1.
Overview of the simple guessing paradigm (doors task) used to elicit the FN. Two doors are presented and participants are instructed to select either the left or right door within four seconds, using the left and right buttons on a mouse. A fixation cross is then presented for 1000ms, following which participants receive feedback on whether they guessed incorrectly (red downward arrow) and lost 25 cents, or guessed correctly (green upward arrow) and won 50 cents. Feedback is presented for 2000ms, following which a fixation cross is presented for 1500ms. Participants are then instructed to begin the next trial.
2.4. Electrophysiological Recording and Data Processing
During the doors task, electroencephalograph (EEG) activity was recorded using the BioSemi Active Two system (BioSemi, Amsterdam, Netherlands) with 64 electrodes arranged based on the 10–20 system. Electrodes were placed on the left and right mastoids, and data were referenced to the average of these channels during offline processing. Two electrodes were placed one centimeter to the posterior of each eye on the face to record horizontal eye movements, and two electrodes were placed above and below the left eye to record vertical eye movements.
EEG data were recorded with a sampling rate of 1024 Hz and filtered online with a low-pass 100 Hz filter and a high-pass 0.16 Hz filter. Each electrode was measured online relative to a common mode sense electrode that formed a monopolar channel. The signal was amplified by a gain of one at each electrode. All data were processed offline using BrainVision Analyzer 2 (Brain Products GmbH, Munich, Germany). After being re-referenced to the average of the left and right mastoid electrodes, data were filtered with Butterworth zero phase half-amplitude filters with a low cutoff of 0.1 Hz, a high cutoff of 30 Hz, and a maximal slope of 24 dB/oct, applied simultaneously. Data were segmented into response-locked epochs that include 200 ms before feedback and 800 ms after. Ocular corrections were performed using the Gratton and Coles algorithm (Gratton, Coles, & Donchin, 1983). Artifacts were detected and rejected through semi-automatic inspection. Segments falling outside of the following parameters were automatically marked for rejection: a maximal voltage step of 50 µV/ms, a maximal difference of 300 µV between the highest and lowest points in an interval of 200 ms, and activity below 0.5 µV for 100 ms. Additional artifacts were removed through manual inspection. Trials were then segmented based on whether they were reward or loss trials. Activity in the 200 ms window prior to stimulus onset served as the baseline. On both reward and loss trials, the FN was quantified as the mean activity from 250 to 350 ms following feedback for each of nine electrode sites (Cz, Fz, FCz, F1, F2, FC1, FC2, C1 and C2). We selected these electrodes because they correspond to the frontal and central sites on the 64-channel cap and encompass the region where the FN has previously been shown to be maximal (Foti, Weinberg, Dien & Hajcak, 2011). We selected multiple frontocentral sites (i.e., not only FCz and Cz) to account for individual-level heterogeneity in RewP/FN responding and improve power to model variance between electrode sites, consistent with prior studies (Volpert-Esmond et al., 2017). In contrast to previous studies that have analyzed the RewP/FN as an average difference score between reward and loss trials (e.g. Foti & Hajcak, 2009), the current study calculated the RewP and FN trial-by-trial for each participant individually, consistent with other time-based ERP analyses (Von Gunten, Volpert-Esmond, & Bartholow, 2018; Volpert-Esmond et al., 2017).
2.5. Statistical Analysis
All statistical analyses were performed using R (R Foundation for Statistical Computing, Vienna, Austria). Of the total available trials (30 trials per condition), all participants had at least 28 usable trials per condition (in total, a minimum of 57 available trials per participant). Grand-averaged response-locked waveforms were created using all available trials per condition and per participant. Mean amplitude values for the RewP, FN and ∆RewP were quantified using the same time windows as trial-level analyses. A paired t test with a two-tailed alpha level of .05 was used to examine amplitude differences between grand-averaged RewP and FN amplitude. Bivariate Pearson correlations were computed to assess relationships between grand-averaged RewP, FN, ∆RewP and total and subscale scores on the PAI-DEP.
In order to examine interactions between changes to RewP and FN amplitude over the course of the task and depression symptoms, we used multilevel modeling (MLM), which can account for baseline response differences at the beginning of the task and model variance specific to both subjects and electrode sites (Baayen, Davidson & Bates, 2008). This approach increases power to detect fixed effects of individual-level predictor variables on trial-level responding and does not require any averaging of data across experimental trials (Vossen, Van Breukelen, Hermens, Van Os & Lousberg, 2011). MLM is also robust to missing trial-level data, making it suitable for use with ERPs (Goldstein, 2011). Moreover, the MLM approach has been previously used to investigate trial-level changes to FN/RewP in the context of depression (Brush et al., 2018).
A total of six models were implemented to examine predictive effects of depression level on within-task RewP/FN changes both within and across experimental conditions and at varying levels of depression severity. For all models, trial number (1–30) and experimental condition (effect-coded as 1 for reward and −1 for loss) were modeled as level 1 (trial-level) predictors of within-subject RewP/FN amplitude, and depression score (PAI-DEP) was modeled as a level 2 (individual-level) predictor. Model 1 examined whether depression score moderated the effects of experimental condition on within-task RewP/FN amplitude changes, and included cross-level interactions between depression, experimental condition and trial number. In order to determine whether the results of Model 1 were driven primarily by RewP, FN or both RewP and FN, Models 2 and 3 examined the relation between total depression score and within-task RewP/FN amplitude changes for RewP and FN separately (Model 2 = RewP, Model 3 = FN), including cross-level interactions between depression and trial. For these models, the primary interaction term of interest was Trial × Depression. Finally, a follow-up analysis of simple slopes was conducted to evaluate the trajectory of within-task change (increasing, decreasing or sustaining) for both RewP and FN at varying levels of depression.
In all models, the trial variable was shifted to correspond with the intercept (t=0), meaning that values for trial ranged from 0 to 29. The depression score was standardized to have a variance of one and a mean of zero for all models. In addition, all models included subject, electrode and trial as random effects. In Model 1, slopes and intercepts for experimental condition were allowed to vary by subject, and only intercepts were allowed to vary between electrode sites, consistent with prior analyses (Volpert-Esmond et al., 2017). In Models 2 and 3, only intercepts, and not slopes, were allowed to vary between electrode sites and subjects, as experimental condition was not included as a fixed effect in these models. Intercepts were also allowed to vary by trial for all models. Intraclass correlations and estimated variances associated with each of these random effects, for both RewP and FN, are reported in Table 3. All models used an unstructured covariance matrix and restricted maximum likelihood estimation (REML) and allowed for covariances between random slopes and intercepts. Finally, Satterwhaite approximations were used to estimate degrees of freedom and obtain two-tailed p-values for each predictor and interaction term in all models. Model specifications are included in the supplementary material.
Table 3.
Intraclass correlations and variances associated with subject, electrode and trial for RewP and FN
| Subject | Electrode | Trial | Residual | |
|---|---|---|---|---|
| RewP | ||||
| ICC | .260 | .029 | .014 | |
| Variance | 84.3 | 9.34 | 4.40 | 225.7 |
| FN | ||||
| ICC | .227 | .027 | .025 | |
| Variance | 65.0 | 7.85 | 7.04 | 207.0 |
Note: ICCs and variances were calculated separately for RewP and FN, using intercept-only multilevel models (i.e., models without predictors) for which RewP/FN amplitude were included as dependent variables (see Supplementary Material for model specifications). ICC values represent the proportion of total variance in mean RewP/FN amplitude accounted for by each grouping variable at level 1.
All MLM analyses were carried out using R packages lme4 (Bates, Mächler, Bolker & Walker, 2015), lmerTest (Kuznetsova, Brockhoff, & Christensen, 2017) and jtools (Long, 2018). We used R model specification procedures similar to Volpert-Esmond, Merkle, Levsen, Ito and Bartholow (2017) and referenced the code that these authors made available online (at https://github.com/hiv8r3/MLM-ERP/) to assist with our analyses.
3. Results
3.1. PAI-DEP
T scores on the PAI-DEP ranged from 38–92. The mean score was 51.81 (SD = 10.34), similar to the general population (i.e., 50T; Morey, 2003), and scores on the PAI-DEP were normally distributed, with skewness of 0.40 (SE = 0.016) and kurtosis of −0.57 (SE = 0.032). Thus, the distribution of depression levels in this sample was representative of the general population, consistent with the goal of the study not to limit putative depression mechanisms and advance knowledge about the range of disruptions observed in clinically relevant neural systems. Two participants (4.4%) had scores in the range for considerable dysphoria meriting clinical attention (≥70T; Morey, 1991, p. 28). The mean PAI-DEP affective subscale score was 52.05 (SD = 9.89), the mean cognitive subscale score was 52.66 (SD = 12.31), and the mean physiological subscale score was 50.21 (SD = 9.29).
3.2. RewP/FN
Grand-averaged RewP, FN and ΔRewP waveforms are presented in Figure 2. Amplitudes of both the RewP and FN were maximal at FCz relative to other electrode sites. Descriptive statistics for the RewP, FN and ΔRewP, including for the first and last ten trials of the experiment, are provided in Table 1. As expected, grand-averaged amplitudes were more positive for rewards (RewP) than for losses (FN; (t(44) = 2.63, p = 0.01, d = .315). Bivariate Pearson correlations between depression scores (PAI-DEP, total and subscales) and grand-averaged RewP/FN amplitudes are reported in Table 2. ΔRewP was significantly positively correlated with total and affective depression scores on the PAI-DEP, in contrast to some prior studies which have reported negative correlations (Foti & Hajcak, 2009; Brush et al., 2018).
Figure 2.
(A) Grand-averaged RewP and FN across all participants in response to rewards and losses at electrodes Cz, Fz, FCz, F1, F2, FC1, FC2, C1 and C2 (left). The onset of the stimulus is denoted by a solid line. Dotted lines indicate the interval in which the mean RewP/FN amplitude was quantified (250-350 ms). Positive amplitudes are plotted downward per ERP convention. (B) The scalp topography (right) representing the difference waveform (∆RewP; gain-loss difference) for the interval 250-350ms following onset of the stimulus.
Table 1.
Descriptive statistics for the RewP and FN at first and last 10 trials of the experiment
| Trial 1-10 Mean (SD) | Trial 21-30 Mean (SD) | Overall Mean (SD) | |
|---|---|---|---|
| RewP (Rewards) | 17.28 (16.98) | 15.97 (19.09) | 16.25 (18.01) |
| FN (Losses) | 12.44 (14.92) | 8.85 (19.35) | 10.74 (16.99) |
| ΔRewP (Reward-Loss) | 4.84 (19.71) | 7.12 (23.41) | 5.51 (19.75) |
Note: All values are averaged across electrode sites Cz, Fz, FCz, F1, F2, FC1, FC2, C1 and C2.
Table 2.
Bivariate correlations between RewP/FN and scores on the PAI_DEP
| ERP | PAI-DEP – Total | PAI-DEP - Affective | PAI-DEP - Cognitive | PAI-DEP - Physiological |
|---|---|---|---|---|
| RewP | .20 | .22 | .17 | .09 |
| FN | .02 | .07 | .04 | −.05 |
| ΔRewP | .36* | .32* | .28 | .28 |
Note:
p < 0.05
To assess the within-task reliability of the RewP/FN in the current sample, we computed Cronbach’s alpha and split-half reliability statistics across all 30 trials of the task. For split-half reliability, correlations between odd- and even-numbered trials were examined for RewP and FN separately, and the Spearman-Brown corrected formula was used. Both FN and RewP exhibited high reliability in the current sample (RewP: α = 0.91, split-half = 0.94; FN: α = 0.90, split-half = 0.92), consistent with previous findings and supporting the use of the RewP/FN as a valid individual difference measure of reward and loss sensitivity (Levinson, Speed, Infantolino & Hajcak, 2017).
3.3. Multilevel Modeling
The results of multilevel modeling analyses, including model fit parameters, are reported in Table 4. For Model 1, the main effect of Trial was significant, indicating that on average, amplitude decreased over the course of the task when collapsed across RewP and FN (b = −.075, SE = .032, t(25.9) = −2.26, p = 0.032). The main effect of depression at the beginning of the experiment (i.e., intercept) was also significant (b = 2.58, SE = 1.11, t(76.6) = 2.31, p = 0.023), as was the effect of condition (reward vs. loss; b = 1.64, SE = .350, t(67.7) = 4.69, p < 0.001), indicating that both greater depression symptoms and reward trials (i.e., RewP) were associated with more positive amplitudes. Additionally, a significant Trial × Condition interaction was observed, indicating that within-task changes (slopes) were more positive for reward trials (RewP), compared to loss trials (FN; b = .075, SE = .011, t(24180) = 6.56, p < 0.001). The Trial × Depression and Condition × Depression interactions were not significant. Further, a significant Trial × Condition × Depression interaction was observed indicating that depression score moderated the effect of experimental condition on RewP/FN amplitude changes over the course of the task (b = .064, SE = .011, t(24180) = 5.80, p < 0.001).1,2
Table 4.
Results of multilevel models predicting within-task changes to RewP and FN amplitude over time.
| Variable | b | SE | df | t | p |
|---|---|---|---|---|---|
| Model 1: PAI-DEP - Total | |||||
| Intercept | 14.3 | 1.69 | 44.8 | 8.51 | <0.001* |
| Trial | −.075 | .032 | 25.9 | −2.26 | 0.032* |
| Condition | 1.64 | .350 | 67.7 | 4.69 | <0.001* |
| Depression | 2.58 | 1.11 | 76.6 | 2.31 | 0.023* |
| Trial × Condition | .075 | .011 | 24180 | 6.56 | <0.001* |
| Trial × Depression | −.002 | .011 | 24180 | −.209 | 0.834 |
| Condition × Depression | .034 | .344 | 75.4 | .098 | 0.923 |
| Trial × Condition × Depression | .064 | .011 | 24180 | 5.80 | <0.001* |
| Model 2: PAI-DEP – Total, RewP Only | |||||
| Intercept | 16.0 | 1.89 | 50.2 | 8.43 | <0.001* |
| Trial | <.001 | .048 | 25.8 | .006 | 0.995 |
| Depression | 2.74 | 1.22 | 79.9 | 2.25 | 0.027* |
| Trial × Depression | .062 | .016 | 12070 | 3.92 | <0.001* |
| Model 3: PAI-DEP – Total, FN Only | |||||
| Intercept | 12.8 | 1.75 | 54.7 | 7.27 | <0.001* |
| Trial | −.153 | .051 | 26.2 | −2.98 | 0.006* |
| Depression | 1.28 | 1.24 | 45.8 | 1.03 | 0.310 |
| Trial × Depression | −.066 | .015 | 12070 | −4.37 | <0.001* |
Note:
p < 0.05,
p < 0.01,
p < 0.001.
In the final version of all models, subject, electrode and trial were included as random effects. Slopes and intercepts for experimental condition were allowed to vary between subjects, and intercepts were allowed to vary between electrodes and trials. Satterwhaite approximations were used to estimate degrees of freedom on which p-values were calculated. Condition (reward vs. loss) was effect-coded (Reward = 1, Loss = −1). Trial number was rescaled to range from 0 to 29. For reduced models containing only fixed effects, random effects of subject and electrode and no interactions, AIC values were as follows: Model 1 = 200581.3; Model 2 = 100780.0; Model 3 = 99787.3. Including interactions improved the fit of all models (for Model 1, AIC without interactions = 200522.5; for Model 2, AIC with interactions = 100773.4; for Model 3, AIC with interactions = 99777.1). Finally, the inclusion of trial as a random effect further improved the fit of all models, yielding final AIC values as follows: Models 1= 200372.6; Model 2 = 100621.0; Model 3 = 99571.1.
In order to clarify whether the Trial × Condition × Depression interaction observed in Model 1 was driven by RewP, FN or both RewP and FN, Models 2 and 3 were run to examine the prediction of depression scores on amplitude changes for the RewP and FN separately. RewP amplitudes showed more positive slopes in relation to higher depression scores, as evidenced by a significant Trial × Depression interaction in Model 2 (b = .062, SE = .016, t(12070) = 3.92, p < 0.001). The same interaction term in Model 3 revealed the opposite pattern, with FN amplitudes showing more negative slopes in relation to higher depression symptoms (b = −.066, SE = .015, t(12070) = −4.37, p < 0.001). However, although the main effect of trial was not significant in Model 2 (RewP; b < .001, SE = .048, t(25.8) = .006, p = 0.995), it was significant and negative in Model 3 (FN; b = −.153, SE = .051, t(26.2) = −2.98, p = 0.006), indicating that amplitudes only changed over the course of trials for the FN. Overall, these results suggest that greater levels of depression are associated with enhanced responses to rewards and diminished responses to losses over the course of the experiment. Variance in amplitude attributable to trial, subject and electrode differences was similar between the RewP and FN (see Table 3). .
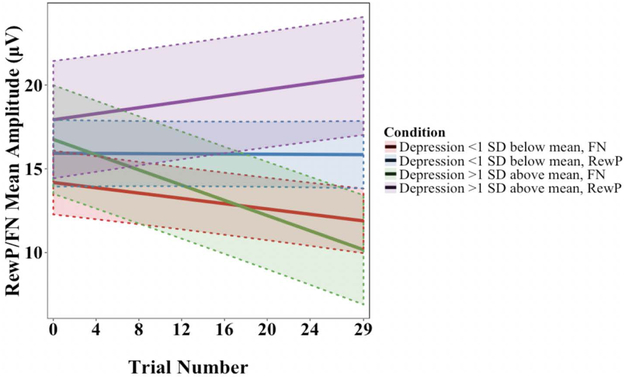
The results of follow-up comparisons of simple slopes between participants with PAI-DEP scores more than one standard deviation above the mean (≥62T, within the range for mild to moderate depression) and more than one standard deviation below the mean (≤42T) for both the RewP and FN are reported in Table 5. For these analyses, a significant positive slope for the RewP was considered as increasing, given that the RewP is a positive-going component, whereas a significant negative slope for the FN was considered as increasing, given that the FN is a negative-going component. Responses that were neither increasing nor decreasing were considered to be sustained. Slopes were significantly negative for loss trials and neither positive nor negative for reward trials, indicating that FN amplitude increased over the course of the task, whereas RewP amplitude was sustained (i.e., neither increased nor decreased). In addition, elevated depression symptoms (≥1 SD above the mean) were associated with a more positive RewP slope estimate and a more negative FN slope estimate than were lower levels (≤ 1 SD below the mean). However, RewP slopes were neither significantly positive nor negative in either elevated or reduced depression, whereas FN slopes were significantly negative regardless of depression level. In addition, FN, but not RewP slopes were significantly different between participants with elevated versus reduced depression scores, indicating greater within-task increases to FN at higher levels of depression. These findings elaborated upon the results of Models 2 and 3 by indicating that RewP amplitudes were sustained overall regardless of depression symptoms, but enhanced (more positive change over the course of the task) at higher levels of depression symptoms relative to lower levels. FN amplitudes increased over the course of the task and were also enhanced at higher levels of depression symptoms. The slopes and standard errors associated with each trial and condition, at varying levels of depression severity, are depicted in Figure 3.
Table 5.
Unstandardized coefficients and 95% confidence intervals for the simple slope of trial on RewP/FN mean amplitude as a function of experimental condition (reward vs. loss) and total depression score on the PAI-DEP.
| Depression ≥ 1 SD Above Mean | Depression ≤ 1 SD Below Mean | |
|---|---|---|
| RewP | .120 [−.007, .247] | .020 [−.043, .083] |
| FN | −.516* [−.643, −.389] | −.159* [−.222, −.096] |
Note: Slopes are presented separately for subjects who had depression scores greater than or equal to one standard deviation above the mean on the PAI-DEP (≥62.0) and less than or equal to one standard deviation below the mean (≤42.0). Asterisks denote slope estimates for which for which the confidence interval does not cross 0.
Figure 3.
The slopes associated with mean change in RewP/FN amplitude over the course of the task, plotted separately for reward (RewP) vs. loss (FN) conditions and for participants with relatively elevated depression (≥1 SD above the mean, ≥62T) and those with relatively low depression scores (≤1 SD below the mean, ≤42T), calculated from Model 1. As the FN is a negative deflection, negative slopes indicate that the FN became larger over the course of the task. As the RewP is a positive deflection, more positive slopes indicate that RewP became larger over the course of the task. Shaded areas represent ±1 standard error in model predictions.
4. Discussion
The present study examined individual differences in how neural responses to rewards and losses are sustained over the course of an experimental task and the relation to depression symptoms in an unselected sample. The RewP and FN were used to index neural responding to reward and loss stimuli, respectively. Consistent with our hypotheses, we found evidence of a relationship between depression symptoms and changes to both reward and loss sensitivity over the course of a simple gambling task. However, contrary to our expectations, the amplitude of the RewP was sustained and the amplitude of the FN increased. In addition, elevated symptoms of depression were associated with enhanced responses to both rewards and losses when examined across trials of the task. Taken together, these findings suggest that when examined in the general population, elevated levels of depression may be associated with potentiation of both reward and loss responses over the course of short experiments.
These findings stand in contrast to previous work indicating that sustained responsiveness to rewards is diminished in the context of clinical levels of depression in patient populations (Heller et al., 2009; Heller et al., 2014; Liu et al., 2011; Brush et al., 2018). In particular, these results differ from those reported by Brush and colleagues (2018), who found positive linear growth in RewP across the doors task for healthy individuals, but blunted, non significant slopes for patients with MDD. In addition, these authors reported reduced sensitivity to rewards over time (i.e., less positive RewP slopes) at higher levels of depression. By contrast, in the current sample, RewP amplitudes were sustained throughout the task (non significant slope) regardless of depression level, and slopes were more positive for individuals with higher levels of depression compared to those with lower levels of depression. In addition, negative FN slopes were observed in the current sample at both high and low levels of depression, with higher depression level predicting enhancement of the FN (i.e., more negative slopes) across time.
The finding that elevated depression symptoms were associated with enhanced RewP amplitudes over the course of the experiment suggests that higher levels of depression may be related to greater ability to sustain sensitivity to rewards over short time intervals. Although RewP slopes were not significantly different from zero at higher levels of depression, a significant and positive Trial × Depression interaction was observed when examining within-task RewP amplitude changes (Model 2), and slope estimates were more positive at higher versus lower depression, indicating that higher depression level predicted relative enhancement of the RewP (more positive/less negative slope) across time compared to lower depression. This result provides tentative evidence for potentiation of responses to rewards over short time periods in relation to elevated depression symptoms examined outside of patient groups.
The results of the present study also suggest that the relationship between sustained neural responsiveness to rewards and depression level varies when differing distributions of symptoms are examined. Because prior studies have only examined relations between sustained reward responses and depression symptoms when compared between MDD and healthy samples (Heller et al., 2009) or when aggregated across MDD and healthy samples (Brush et al., 2008), it is possible that the current finding indicates a distinct pattern that only emerges when examining depression symptoms that are normally distributed around the general population mean. Such dimensional approaches to studying depression increase statistical power and may yield insight into basic biological disorder mechanisms not observed in clinical samples which use narrow criteria and show restricted symptom variability (Kozak & Cuthbert, 2016; Woody & Gibb, 2015). Thus, the dimensional approach of the current study may have contributed to differences observed between its results and those reported in studies of patients only.
In the current study, the degree of change in the RewP over time was moderated by depression, with greater depression symptoms associated with more positive slopes. This result may indicate that greater reactivity of dopaminergic brain circuitry involved in the early stages of reward processing is a neurobiological correlate of depression, at least when examined in the general population. The RewP has been proposed to reflect reward-specific activation of brain areas such as ventral tegmental area and ventral striatum (Foti et al., 2014; Proudfit, 2015), suggesting that activity in these regions may sustain over the course of consecutive reward presentations for individuals with higher depression symptoms. Furthermore, this result suggests potential future avenues for research on sustained reward responsiveness in depression—for example, investigating whether sustained elevation in activity of reward-encoding brain regions relates to depression risk or to common biological vulnerability between depression and other reward-related disorders.
The fact that FN amplitudes (to losses) were observed to increase during the experiment more greatly for individuals with higher levels of depression is consistent with prior research linking depression to altered neural responses to loss-related feedback (Mies et al., 2011; Santesso et al., 2008; Steele, Kumar & Ebmeier, 2007). In particular, these findings are consistent with those of Santesso and colleagues (2008) and Mies and colleagues (2011), who reported larger FN amplitudes to negative feedback for individuals with major depression and remitted depression, respectively, relative to healthy individuals. The findings of the current study expand upon this evidence by suggesting that neural sensitivity to loss feedback becomes more pronounced over the course of consecutive feedback presentations. Moreover, this result may implicate reactivity of the anterior cingulate cortex, a brain region which participates in generating the FN (Foti, Weinberg, Bernat & Proudfit, 2015) and exhibits aberrant processing of negative feedback in depression (Santesso et al., 2008), as a neurobiological correlate of depression.
In the current study, ∆RewP (reward – loss) was positively, rather than negatively correlated with both total and affective depression scores, indicating that even when grand-averaged, rather than trial-level, amplitudes were examined, greater neural sensitivity to reward feedback was related to greater depression, particularly affective symptoms. This result is in contrast to prior work demonstrating negative associations between ∆RewP and depression symptom level (e.g., Foti & Hajcak, 2009; Brush et al., 2018). Further, it suggests that in unselected samples showing a normal distribution of depression symptoms around the general population mean, higher depression is related to stronger differentiation of midbrain and basal ganglia responses to rewards relative to non rewards, as the ∆RewP is proposed to reflect the extent of reward-specific relative to non reward activation in these areas (Proudfit, 2015; Levinson et al., 2017). Thus, in unselected samples, higher symptoms of depression may predict both generally potentiated responses to rewards in these brain areas as well as increasing responses to both reward and loss responses over short time intervals. The fact that this result contrasts with those reported in prior studies again suggests that neural reward sensitivity and depression are not always inversely associated and may differ dependent on the range and level of symptoms examined.
Finally, the results of this study contribute to a growing literature using multilevel models to examine within-task changes to ERPs. While it is common to average amplitudes across subjects, trials, and electrodes when examining components such as the RewP and FN, the multilevel modeling approach implemented in this sample and in other ERP datasets (Brush et al., 2018; Volpert-Esmond et al., 2017; Vossen et al., 2011) allows for the variance attributable to these factors to be estimated and controlled for when examining effects of interest. Future work on the temporal dynamics of reward responding should continue to explore this statistical approach in the context of RewP/FN sustainment across experimental trials.
One limitation of the current study is the fact that all participants were undergraduate students with a relatively narrow age range. Future studies should examine whether the patterns observed within this sample also exist across the lifespan. The study was also limited by the fact that it did not assess competing influences on reward sensitivity outside of depression. For example, it did not account for symptoms of substance use disorders, which are known to alter sensitivity to rewards, including how reward responses change over the course of repeated presentations (Koob & Le Moal, 1997; Robinson & Berridge, 2001). Therefore, future studies should investigate important individual difference factors, including symptoms of other mental disorders and indices of reward sensitivity, which may alter patterns of reward and loss response sustainment. As well, the doors task is relatively brief—approximately ten minutes—which is considerably shorter than some studies that have examined sustained reward responses (e.g. Heller et al., 2009, who tracked changes over 37 minutes) but comparable to others (Brush et al., 2018). Additional research is needed to clarify the temporal dynamics of reward sensitivity over varying timescales, ranging from short to long, and their relation to depression severity. Finally, the size of the final sample in the current study was 45, which may have been underpowered to detect small effects; however, this sample size is similar to other studies using MLM (e.g., the MDD group examined by Brush and colleagues, 2018), and the range of symptoms examined was sufficient to examine differences in responding at various levels of depression, consistent with the aim of the study.
In summary, the results of the present study suggest that when assessed in an unselected sample, higher levels of depression symptoms are associated with enhancement of the RewP and FN over the course of an experiment compared with lower levels of depression, in contrast to previous findings in clinical depression. These results may indicate that brain regions involved in processing reward and loss feedback exhibit greater activity over time among individuals with higher levels of depression symptoms. Further, they contribute to our understanding of the pathophysiology of depression as a dimensional construct independent from formal diagnostic categories, suggesting that patterns of sustained neural responding differ depending on whether symptoms are examined in clinical versus relatively healthy unselected samples exhibiting a normal distribution of depression severity. Moreover, the results of the present study may indicate that the range and level of depression examined are factors that influence relations between depression and sustained neural reward and loss sensitivity, considering that the present sample exhibited lower overall levels of depression than studies that have included patients (i.e., Brush et al., 2018). Taken together, these findings contribute to scientific understanding of neurobiological mechanisms of depression symptoms and suggest opportunities for future research on sustained reward and loss responsiveness in depression.
Supplementary Material
Acknowledgments
The authors would like to thank the reviewers of this manuscript for their helpful feedback on the statistical methods used and interpretation of the results found in the current study.
Funding
Support for this research was provided by NIH MH073708 and Wesleyan University.
Footnotes
Conflict of interest:
The authors declare no conflicts of interest.
To clarify relations between experimental condition and within-task RewP/FN amplitude change at varying levels of depression, the results of Model 1 were further explored by shifting the depression variable to examine changes at high (+1 SD above mean) versus low (−1 SD) levels of depression in two separate models. Significant Trial × Condition interactions in both models indicated that condition moderated the relationship between trial and amplitude such that RewP amplitudes became more positive over the task (i.e., enhanced, as the RewP is positive-going) at higher compared to lower depression, and FN amplitudes became more negative over the task (also enhanced, as the FN is negative-going) at higher compared to lower depression. These results are consistent with the simple slopes analysis (Table 5). See Supplementary Table 1 more information on these additional models.
Results of Model 1 were also further investigated by examining interactions between specific domains of depression (subscales on the PAI-DEP) and within-task changes to RewP/FN amplitude. Three separate models were fit using an identical structure to Model 1, but substituting total depression score for each of the individual subscales on the PAI-DEP (affective, cognitive, physiological). These models all revealed significant Trial × Condition × Depression interactions when substituting total score for each of the three subscales of the PAI-DEP, indicating that the prediction of depression level on within-task RewP and FN amplitude change was driven by elevations to a wide range of depression symptoms and was thus not attributable to any one domain of symptoms alone. See Supplementary Table 2 for more information on these subscale-focused models.
References
- American Psychiatric Association. (1994). Diagnostic and Statistical Manual of Mental Disorders (4th ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Baayen RH, Davidson DJ, & Bates DM (2008). Mixed-effects modeling with crossed random effects for subjects and items. Journal of Memory and Language, 59(4), 390–412. 10.1016/j.jml.2007.12.005 [DOI] [Google Scholar]
- Bates D, Mächler M, Bolker B, & Walker S (2014). Fitting linear mixed-effects modelsusing lme4. Journal of Statistical Software, 67(1), 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Beck AT, Steer RA, Brown GK (1996): Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Bress JN, & Hajcak G (2013). Self‐report and behavioral measures of reward sensitivity predict the feedback negativity. Psychophysiology, 50(7), 610–616. 10.1111/psyp.12053 [DOI] [PubMed] [Google Scholar]
- Brush CJ, Ehmann PJ, Hajcak G, Selby EA, & Alderman BL (2018). Using multilevel modeling to examine blunted neural responses to reward in major depression. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 10.1016/j.bpsc.2018.04.003 [DOI] [PubMed] [Google Scholar]
- Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, & Hajcak G (2011). Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: A combined ERP and fMRI study. Neuroimage, 57(4), 1608–1616. 10.1016/j.neuroimage.2011.05.037 [DOI] [PubMed] [Google Scholar]
- Cuthbert BN (2014). The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry, 13(1), 28–35. 10.1002/wps.20087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon DG, Rosso IM, Pechtel P, Killgore WD, Rauch SL, & Pizzagalli DA (2014). Peril and pleasure: An RDOC‐inspired examination of threat responses and reward processing in anxiety and depression. Depression and Anxiety, 31(3), 233–249. 10.1002/da.22202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, ... & Silbersweig DA (2006). Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. American Journal of Psychiatry, 163(10), 1784–1790. 10.1176/ajp.2006.163.10.1784 [DOI] [PubMed] [Google Scholar]
- Foti D, & Hajcak G (2009). Depression and reduced sensitivity to non-rewards versus rewards: Evidence from event-related potentials. Biological Psychology, 81(1), 1–8. 10.1016/j.biopsycho.2008.12.004 [DOI] [PubMed] [Google Scholar]
- Foti D, Carlson JM, Sauder CL, & Proudfit GH (2014). Reward dysfunction in major depression: Multimodal neuroimaging evidence for refining the melancholic phenotype. NeuroImage, 101, 50–58. 10.1016/j.neuroimage.2014.06.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Weinberg A, Dien J, & Hajcak G (2011). Event‐related potential activity in the basal ganglia differentiates rewards from nonrewards: Temporospatial principal components analysis and source localization of the feedback negativity. Human Brain Mapping, 32(12), 2207–2216. 10.1002/hbm.21182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Weinberg A, Bernat EM, & Proudfit GH (2015). Anterior cingulate activity to monetary loss and basal ganglia activity to monetary gain uniquely contribute to the feedback negativity. Clinical Neurophysiology, 126(7), 1338–1347. 10.1016/j.clinph.2014.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, & Green MF (2007). Anhedonia in schizophrenia: Distinctions between anticipatory and consummatory pleasure. Schizophrenia Research, 93(1–3), 253–260. 10.1016/j.schres.2007.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein H (2011). Multilevel statistical models (Vol. 922). Chichester, UK: John Wiley & Sons. [Google Scholar]
- Gratton G, Coles MG, & Donchin E (1983). A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology, 55(4), 468–484. 10.1016/0013-4694(83)90135-9 [DOI] [PubMed] [Google Scholar]
- Harvey PO, Pruessner J, Czechowska Y, & Lepage M (2007). Individual differences in trait anhedonia: A structural and functional magnetic resonance imaging study in non-clinical subjects. Molecular Psychiatry, 12(8), 767 10.1038/sj.mp.4002021 [DOI] [PubMed] [Google Scholar]
- Heller AS, Johnstone T, Shackman AJ, Light SN, Peterson MJ, Kolden GG, ... & Davidson RJ (2009). Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proceedings of the National Academy of Sciences, 106(52), 22445–22450. 10.1073/pnas.0910651106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, Johnstone T, Light SN, Peterson MJ, Kolden GG, Kalin NH, & Davidson RJ (2014). Relationships between changes in sustained fronto-striatal connectivity and positive affect in major depression resulting from antidepressant treatment. American Journal of Psychiatry. 10.1176/appi.ajp.2012.12010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques JB, & Davidson RJ (2000). Decreased responsiveness to reward in depression. Cognition & Emotion, 14(5), 711–724. 10.1080/02699930050117684 [DOI] [Google Scholar]
- Hill BD, Musso M, Jones GN, Pella RD, & Gouvier WD (2013). A psychometric evaluation of the STAI-Y, BDI-II, and PAI using single and multifactorial models in young adults seeking psychoeducational evaluation. Journal of Psychoeducational Assessment, 31(3), 300–312. 10.1177/0734282912462670 [DOI] [Google Scholar]
- Holroyd CB, & Coles MGH (2002). The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review, 109, 679–709. 10.1037//0033-295X.109.4.679 [DOI] [PubMed] [Google Scholar]
- Koob GF, & Le Moal M (1997). Drug abuse: hedonic homeostatic dysregulation. Science, 278(5335), 52–58. 10.1126/science.278.5335.52 [DOI] [PubMed] [Google Scholar]
- Kozak MJ, & Cuthbert BN (2016). The NIMH research domain criteria initiative: background, issues, and pragmatics. Psychophysiology, 53(3), 286–297. 10.1111/psyp.12518 [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, & Christensen RHB (2017). lmerTest package: Tests in linear mixed effects models. Journal of Statistical Software, 82(13). 10.18637/jss.v082.i13 [DOI] [Google Scholar]
- Levinson AR, Speed BC, Infantolino ZP, & Hajcak G (2017). Reliability of the electrocortical response to gains and losses in the doors task. Psychophysiology, 54(4), 601–607. 10.1111/psyp.12813 [DOI] [PubMed] [Google Scholar]
- Liu WH, Chan RC, Wang LZ, Huang J, Cheung EF, Gong QY, & Gollan JK (2011). Deficits in sustaining reward responses in subsyndromal and syndromal major depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 35(4), 1045–1052. 10.1016/j.pnpbp.2011.02.018 [DOI] [PubMed] [Google Scholar]
- Liu WH, Wang LZ, Shang HR, Shen Y, Li Z, Cheung EF, & Chan RC (2014). The influence of anhedonia on feedback negativity in major depressive disorder. Neuropsychologia, 53, 213–220 10.1016/j.neuropsychologia.2013.11.023 [DOI] [PubMed] [Google Scholar]
- Long JA (2018). jtools: Analysis and Presentation of Social Scientific Data. R Package Version 0.7.0, https://cran.r-project.org/package=jtools [Google Scholar]
- Mies GW, van der Veen FM, Tulen JH, Birkenhäger TK, Hengeveld MW, & van der Molen MW (2011). Drug-free patients with major depression show an increased electrophysiological response to valid and invalid feedback. Psychological medicine, 41(12), 2515–2525. 10.1017/S0033291711000778 [DOI] [PubMed] [Google Scholar]
- Morey LC (1991). The Personality assessment inventory: professional manual. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Morey LC (2003). Essentials of PAI assessment: John Wiley & Sons Inc., Hoboken, NJ. [Google Scholar]
- National Institute of Mental Health. (n.d.). Domain: Positive Valence Systems. Retrieved from https://www.nimh.nih.gov/research-priorities/rdoc/constructs/positive-valence-systems.shtml. [Google Scholar]
- Nusslock R, Walden K, & Harmon-Jones E (2015). Asymmetrical frontal cortical activity associated with differential risk for mood and anxiety disorder symptoms: An RDoC perspective. International Journal of Psychophysiology, 98(2), 249–261. 10.1016/j.ijpsycho.2015.06.004 [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, & Fava M (2008). Reduced hedonic capacity in major depressive disorder: Evidence from a probabilistic reward task. Journal of Psychiatric Research, 43(1), 76–87. 10.1016/j.jpsychires.2008.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, & O’Shea JP (2005). Toward an objective characterization of an anhedonic phenotype: A signal-detection approach. Biological Psychiatry, 57(4), 319–327. 10.1016/j.biopsych.2004.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfit GH (2015). The reward positivity: From basic research on reward to a biomarker for depression. Psychophysiology, 52(4), 449–459. 10.1111/psyp.12370 [DOI] [PubMed] [Google Scholar]
- Robinson TE, & Berridge KC (2001). Incentive‐sensitization and addiction. Addiction, 96(1), 103–114. 10.1046/j.1360-0443.2001.9611038.x [DOI] [PubMed] [Google Scholar]
- Sanislow CA (2016). Updating the research domain criteria. World Psychiatry, 15(3), 222–223. 10.1002/wps.20374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesso DL, Steele KT, Bogdan R, Holmes AJ, Deveney CM, Meites TM, & Pizzagalli DA (2008). Enhanced negative feedback responses in remitted depression. Neuroreport, 19(10), 1045 10.1097/WNR.0b013e3283036e73] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele JD, Kumar P, & Ebmeier KP (2007). Blunted response to feedback information in depressive illness. Brain, 130(9), 2367–2374. 10.1093/brain/awm150 [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, & Keener AD (1998). Frontal brain asymmetry and depression: A self-regulatory perspective. Cognition & Emotion, 12(3), 387–420. 10.1080/026999398379655 [DOI] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, & Zald DH (2009). Worth the ‘EEfRT’? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PloS One, 4(8), e6598 10.1371/journal.pone.0006598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Bossaller NA, Shelton RC, & Zald DH (2012). Effort-based decision-making in major depressive disorder: A translational model of motivational anhedonia. Journal of Abnormal Psychology, 121(3), 553 10.1037/a0028813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpert‐Esmond HI, Merkle EC, Levsen MP, Ito TA, & Bartholow BD (2017). Using trial‐level data and multilevel modeling to investigate within‐task change in event‐related potentials. Psychophysiology, 55(5), e13044 https://doi/org/10.1111/psyp.13044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Gunten CD, Volpert‐Esmond HI, & Bartholow BD (2018). Temporal dynamics of reactive cognitive control as revealed by event‐related brain potentials. Psychophysiology, 55(3), e13007 10.1111/psyp.13007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossen H, Van Breukelen G, Hermens H, Van Os J, & Lousberg R (2011). More potential in statistical analyses of event‐related potentials: A mixed regression approach. International Journal of Methods in Psychiatric Research, 20(3). 10.1002/mpr.348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton AE, Treadway MT, & Pizzagalli DA (2015). Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Current Opinion in Psychiatry, 28(1), 7 10.1097/YCO.0000000000000122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody ML, & Gibb BE (2015). Integrating NIMH research domain criteria (RDoC) into depression research. Current Opinion in Psychology, 4, 6–12. 10.1016/j.copsyc.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.