Abstract
Irregular menstrual cycles due to anovulation are well-described in the first few years after menarche, but the normal developmental trajectory from anovulatory to mature ovulatory cycles during adolescence remains undefined. This paper presents our very limited understanding of this final stage of female reproductive axis development and why additional research in this area is critical to the health of women.
Long and irregular menstrual cycles are common in the first 1–2 years after menarche, but the majority of girls settle into a regular pattern of cycles (24–38-day interval) by late adolescence 1. The achievement of the mature ovulatory cycle of a fertile woman is arguably one of the most critical milestones of adolescence, yet the physiological mechanisms underlying this developmental transition are not well understood. This knowledge gap has hampered our ability to distinguish abnormal (e.g. polycystic ovarian syndrome [PCOS]) from normal developmental trajectories. The observation that most adult women with oligomenorrhea of unknown etiology report symptoms dating back to early adolescence 2,3 suggests further that the early post-menarchal period may represent a critical window when preventative measures must be instituted to safeguard reproductive health in adulthood. The purpose of this article is to review what little we do know about reproductive physiology in the early post-menarchal years and to highlight major knowledge gaps to inform future research into adolescent menstruation.
The early post-menarchal years: when do girls first achieve ovulatory cycles?
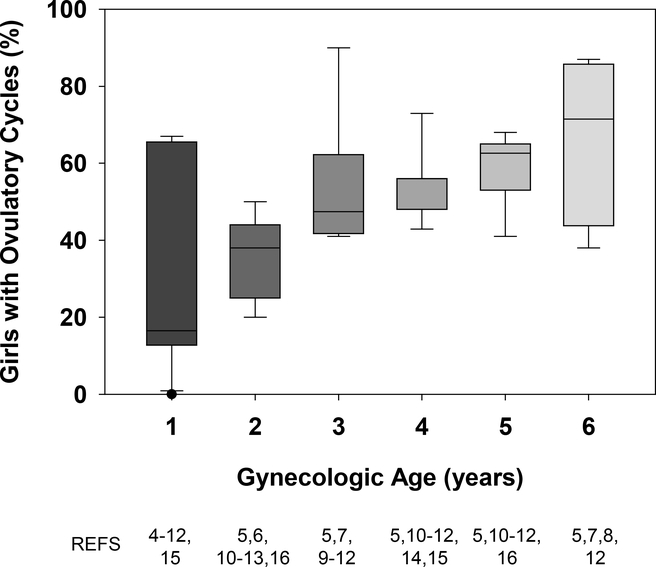
There is a general consensus that in the first 1–2 gynecologic years, the majority of menstrual cycles are anovulatory. The gynecologic age at which ovulatory cycles are first achieved, however, is less clear. There is a small, and relatively old, body of literature on this topic, and the estimates encompass a broad range of gynecologic ages (Fig 1) 4–16. This inconsistency stems from differences in the number of cycles monitored per individual and potentially to differences in subject ethnicity, socio-economic status, body composition, and average age at menarche 17. There are also significant differences in the sensitivity of the methods used to identify ovulatory cycles:
Figure 1.
Box and whisker plots demonstrating variability among 14 studies in the reported percentage of girls with ovulatory cycles according to gynecologic age (years since menarche). In these studies, ovulatory status had been determined using either urine 6,11, serum 5,10,16, or saliva hormone tests 12,14,15, cycle length 4,8,9,13, or a shift in basal body temperature 7. References used to calculate summary statistics for each gynecologic year are listed at the bottom of the figure.
(1). Cycle length
Cycle lengths of 25–35 days strongly predict ovulation in adult women 18, but cycle length is a poor marker of ovulation in adolescents 13,19,20.
(2). Basal body temperature (BBT) charting
BBT charting is an ovulation detection method that relies on the subtle increase in basal (resting) body temperature that occurs in the presence of progesterone (P4). While simple and non-invasive, it is an unreliable method to detect or confirm ovulation and is recommended only in an adjunctive capacity (reviewed in 21). Bauman 22 reported that BBT charting was only 22% accurate compared with serum hormone profiles, and this figure dropped to <5% for ovulatory cycles with short luteal phase lengths. At least one early study relied entirely on BBT to classify adolescent menstrual cycles 7.
(3). Progesterone (P4) measurements
Salivary P4 can be measured easily, but salivary hormone assays are known to suffer from greater variability than serum or urine assays 11,23. Our research group recently demonstrated, for example, that in adolescent girls, salivary P4 does not correlate well with serum P4 (at least by immunoassay), particularly at low levels (0–2 ng/mL)24. Measurement of serum P4 or its primary urinary metabolite, pregnanediol (Pd), is one of the most sensitive ovulation detection methods but timing is critical. In some studies, measurements were made only during the first and third weeks of a menstrual cycle (as opposed to weekly), potentially leading to mis-classification of long ovulatory cycles as anovulatory 25. In addition, previous studies relied on biochemical criteria for ovulation from the adult literature 6,11,12,26 (e.g., serum P4 > 3.7 ng/mL26), whereas we recently demonstrated that in adolescents, the threshold P4 value indicative of ovulation is much lower, at 1.65 ng/mL 24. Thus, many adolescent cycles previously classified as anovulatory may have instead been ovulatory with luteal insufficiency. For an in-depth review of the first gynecologic year, please see the recent paper by Gunn et al.27 in the December 2018 issue of JPAG.
One could argue that even those studies which employed the best methodology are no longer relevant to today’s adolescent girl: the vast majority of studies were performed in Euro-Caucasian girls with low or normal body weight and at a time (≈ 30 years ago) when breast development, and to a lesser extent, menarche, occurred at a significantly older age 28. Our current understanding of the early post-menarchal period in contemporary adolescent girls rests entirely on two observational studies that, while limited by relatively small sample sizes, have the advantage of intensive monitoring. Zhang et al. 29 studied 7 healthy girls in the New York City area with daily urine tests for LH, FSH, and estrogen and progesterone metabolites (E1C and Pd-3-glucuronide [PdG], respectively) for up to 2 years after menarche. Approximately 35% of cycles met biochemical criteria for ovulation, which the group defined as a ≥3-SD rise in each hormone above a 5-day moving average as well as a PdG rise ≥1.0 μg/mg Cr based on similar algorithms validated in adult women. In these early cycles, the luteal phase was short and/or PdG output was significantly lower than in adult women with ovulatory cycles (average PdG rise 5.1 μg/mg Cr)29. We recently conducted a cross-sectional study in 23 healthy girls (aged 12.8–17.6 years; average of 1.8 years post-menarche) from the Boston metropolitan area 24. Subjects were racially diverse (15 White, 5 Black, 3 Asian; 25% Hispanic) and 56% were overweight or obese. Subjects underwent serial reproductive hormone measurements (nearly every other day via blood or urine) and pelvic ultrasounds during 2 consecutive menstrual cycles. Data were compared with 65 adult historic controls with ovulatory cycles. Nearly 80% of girls demonstrated at least one ovulatory cycle, although as observed by Zhang et al., many cycles were characterized by luteal insufficiency. Thus, it is possible that girls are achieving ovulatory competence more rapidly than in the past, but it is difficult to make such comparisons given major differences in study methodology.
What is the normal maturational sequence of events from early post-menarchal anovulation to regular ovulatory cycles?
Cross-sectional studies in early post-menarchal girls have consistently demonstrated a progressive increase in P4 6,12,15,30,31 and/or days with an increased BBT 7 with advancing gynecologic age. While the rise in P4 suggests there may be a natural progression from 1) anovulatory cycles to 2) immature ovulatory cycles with inadequate luteal function to 3) mature ovulatory cycles, this possibility has not been systematically investigated. Instead, in previous studies, girls with intermediate levels of P4 (0.5–2 ng/mL), which we now know to be compatible with ovulation 24, were considered unclassifiable and excluded from analyses. An increase in P4 and/or estimated luteal phase length approaching normal adult parameters was also observed in two longitudinal case series based on urinary hormone measurements and cervical mucous changes 29,32. As these studies did not include sonographic data, it is unclear whether the increase in P4 reflects immature ovulatory cycles with a short luteal phase or a luteinized unruptured follicle, as these two types of immaturity may have overlapping P4 levels 24 yet different underlying pathophysiologies.
In our recent cross-sectional studies in 23 early post-menarchal girls, we similarly observed different cycle patterns which reflect a continuum of P4 secretion: anovulatory cycles without follicle luteinization (8.7%), anovulatory cycles with luteinization (defined as P4 > 1 ng/mL or a 3-fold rise in urine Pd above the mean follicular phase level; 21.7%), ovulatory cycles with a short (5–9 day) luteal phase length (21.7%), and ovulatory cycles with a normal (10–14 day) luteal phase length (47.8%) (Fig 2). Girls with anovulatory cycles demonstrated heterogeneous hormone profiles and follicle development that ranged from minimal follicle growth to development of a dominant follicle, a pre-ovulatory estradiol (E2) surge, and a mid-cycle LH surge but no follicle rupture; instead, the dominant follicle(s) continued to secrete E2 and a small amount of P4 before menses occurred, consistent with the presence of luteinized unruptured follicles. While girls with ovulatory cycles (with normal or short luteal phase lengths) demonstrated the same overall patterning of LH, FSH, E2, and P4 secretion across the menstrual cycle as seen in mature ovulatory cycles in adults, they had lower gonadotropin levels than adults as well as signs of luteal insufficiency (low luteal P4 and E2). Girls with ovulatory cycles also had lower E2 and inhibin B levels (a granulosa cell marker) than adults, even after adjusting for both lower FSH levels and smaller follicle size, suggesting intrinsic ovarian immaturity in girls. Thus, there does not appear to be a single limiting factor in the development of normal, ovulatory cycles; rather, reproductive maturity requires the coordinated development of the hypothalamic, pituitary, and ovarian components of the reproductive axis.
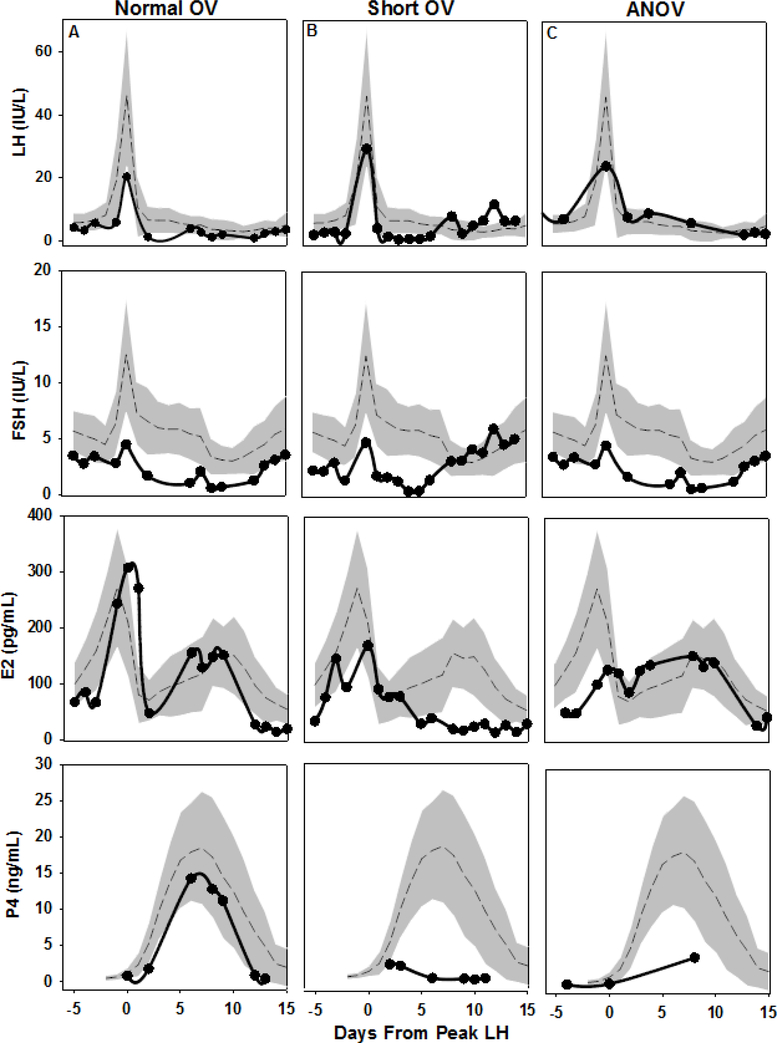
Figure 2.
Serum LH, FSH, estradiol (E2), and progesterone (P4) levels during two consecutive menstrual cycles in 3 representative adolescent subjects (A, B, C). Hormone data for subjects A, B, and C are shown in the left, middle, and right columns, respectively. Each subject’s data are plotted against a backdrop of normative data from 65 historic adult controls with regular, ovulatory cycles. Days are centered to the midcycle LH peak in cycle 1 (day 0). Adult data are presented as mean (dashed line) ± 1 SD (shaded area). A) 13 ½ -year-old (gynecologic age of 9 mo) subject with a 33-day ovulatory cycle with a normal luteal phase length of 12 days. B) 15 ½-year-old (gynecologic age 2.8 years) subject with a 24-day ovulatory cycle with short luteal phase length of 5 days. C) 13-year-old (gynecologic age 7 mo) subject with a 42-day anovulatory cycle due to a luteinized unruptured follicle. Initial cycle dynamics were relatively normal with growth of a dominant follicle (to a 30-mm diameter), an increase in E2, and an LH surge of 24.2 IU/L, but ovulation did not occur. The unruptured follicle continued to produce E2 and luteinized (maximum P4 3.9 ng/mL) until menses occurred 12 days later. To convert serum E2 to SI units (pmol/L), multiply by 3.67; for serum P4 (nmol/L), multiply by 3.18.
While all 23 study participants were monitored during two consecutive menstrual cycles (cycle 1 and cycle 2), 7 participants (and one additional girl who did not enroll in the full protocol) completed a pilot study before cycle 1, and 2 participants underwent additional monitoring after cycle 2 to determine eligibility for a second research study (N. Shaw, unpublished data). Two key observations emerge from these semi-longitudinal data on reproductive axis maturation in the early post-menarchal period: 1) the path to regular, ovulatory cycles is not smooth and stepwise; rather, it is not uncommon for a normal ovulatory cycle to be followed by regression to an anovulatory cycle. This suggests that the identification of a single ovulatory cycle in an adolescent girl, as reported by many older studies, may not be as clinically meaningful as assumed and certainly does not signify reproductive maturity, and 2) there is significant variability, even among healthy, normoandrogenemic, urban adolescent girls, in the time needed to achieve normal ovulatory cycles. The cause(s) of this variability is unknown but may include differences in race, ethnicity, body weight, physical activity level, stress, and/or genetic background.
Can girls with true hormonal aberrations (e.g., early PCOS), be distinguished from girls with prolonged physiological anovulation of adolescence?
PCOS is typically diagnosed in adult women presenting with hirsutism, irregular periods, and/or infertility, but it is believed to first manifest peri-pubertally 33. Making the diagnosis during adolescence, however, when it might (one day) be possible to institute preventative measures, is very difficult because the cardinal features of PCOS in adult women, including hyperandrogenism, insulin resistance, anovulation/irregular menstrual cycles, and polycystic ovarian morphology (PCOM), are also normal, albeit transient, parts of female reproductive axis maturation 34. Current guidelines define androgen excess in adolescents as moderate to severe hirsutism, moderate to severe comedonal acne, or testosterone levels persistently greater than adult female normative values and define irregular menses as oligo-anovulation two or more years after menarche 35. They argue against the use of obesity, insulin resistance, PCOM, or AMH levels in making the diagnosis during adolescence 35. Longitudinal studies in adolescent girls have demonstrated that girls with irregular cycles 36–38, hyperandrogenemia 37,39, high LH levels or a fast LH pulse profile 40, enlarged ovaries 41, and/or obesity 36 are at increased risk for but are not destined to develop PCOS as young adults. Another biochemical hallmark of adult PCOS is 17-hydroxy-progesterone (17OHP) hyper-responsiveness to GnRH or hCG stimulation testing 42, consistent with the aberrant regulation of ovarian cytochrome P450c17 alpha that has been demonstrated in vitro in theca cells from polycystic human ovaries 43. Peri-menarchal girls with multiple PCOS risk factors (e.g., irregular cycles and hirsutism, acanthosis nigricans, obesity, severe acne, and/or enlarged ovaries) have higher basal 17OHP levels than age-matched, regularly cycling girls 44, and 75% have a 17OHP hyper-response to GnRH or hCG stimulation that persists into adulthood 45,46. It is unknown, however, whether increased 17OHP is also seen in girls with physiological anovulatory cycles due to reproductive axis immaturity or if 17OHP may represent an early and specific biomarker of impending PCOS.
Why does this stage of development deserve greater attention?
Irregular menstruation affects > 2.5 million reproductive-aged women in the US each year 47, and like many adult diseases, it often has its roots in childhood. Until we understand normal hormone dynamics during the transition to mature ovulatory cycles, we will remain unable to precisely differentiate, and potentially treat, girls with anovulatory cycles who are at high-risk for long term irregular cycles and infertility from those who will eventually establish regular cycles. The interval from menarche to regular ovulatory cycles also represents a time of unopposed estrogen exposure (i.e. E2 without P4), which is an established risk factor for endometrial cancer. The incidence of endometrial cancer is increasing worldwide, potentially due to the obesity epidemic as well as reproductive factors such as a trend for fewer pregnancies48. While endometrial cancer is primarily a disease of postmenopausal women, the incidence rate has increased by approximately 2% per year over the past 10 years in the US in both pre-menopausal and postmenopausal women48, and cases have been reported in women with PCOS as young as 8.5 gynecologic years49. Increased exposure to unopposed estrogens through obesity, earlier menarche, and anovulatory cycles would be expected to increase the risk of endometrial cancer among today’s youth, but this has not been systematically investigated.
Mapping a research agenda for adolescent menstrual cycles.
There is a critical need to investigate and understand the normal developmental trajectory from menarche to regular ovulatory cycles in a contemporary adolescent cohort. We should not expect that one pattern will fit all; as such, studies must include subjects of diverse ethnicities and with a spectrum of body weights. It is also important to consider environmental factors that may be unique to the adolescent microcosm such as sleep restriction, drug and alcohol use, and energy balance. As we gain a fundamental understanding of this important developmental period, we must also begin to think carefully and creatively about potential interventions, beyond lifestyle modification or birth control pills, to divert the subset of adolescents who may otherwise be destined for irregular cycles and infertility as adults. Finally, we should consider how best to manage girls at high risk for endometrial hyperplasia and cancer and, particularly, whether the current practice of prescribing hormonal therapy for anovulatory cycles is sufficient.
Fortunately, there appears to be growing support for research on adolescent reproductive health. In 2016, six years after launching the Global Strategy for Women’s and Children’s Health, the United Nations recognized the special health needs of adolescents to be a critical priority and changed the title to the Global Strategy for Women’s, Children’s, and Adolescents’ Health50. In 2017, NICHD/NIH released an RFA devoted to characterization of the adolescent reproductive transition, and the current Trans-NIH Strategic Plan for Women’s Health Research includes research into reproductive function in girls and women, including normal and dysfunctional menstruation, among its major goals.
In summary, the early post-menarchal years represent an important yet understudied stage of reproductive development. Future studies are necessary to delineate the precise maturational changes that occur at each level of the reproductive axis as a girl begins to establish ovulatory cycles as well as the genetic and/or environmental factors that modulate this process. Only after establishing what “normal” is can we begin to identify and treat girls who are poised to develop PCOS and other menstrual conditions as adults.
Acknowledgements
This work was supported, in part, by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01-ES103315) and by Grant Number 1UL1TR001102. NDS is also supported as a Lasker Clinical Research Scholar (1SI2ES025429-01). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources, the National Center for Advancing Translational Science or the National Institutes of Health.
Footnotes
Disclosures
The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Treloar AE, Boynton RE, Behn BG, Brown BW. Variation of the human menstrual cycle through reproductive life. Int J Fertil 1967;12:77–126. [PubMed] [Google Scholar]
- 2.Southam AL, Richart RM. The prognosis for adolescents with menstrual abnormalities. AmJ ObstetGynecol 1966;94:637–45. [DOI] [PubMed] [Google Scholar]
- 3.Gardner J Adolescent menstrual characteristics as predictors of gynaecological health. Ann Hum Biol 1983;10:31–40. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization multicenter study on menstrual and ovulatory patterns in adolescent girls. II. Longitudinal study of menstrual patterns in the early postmenarcheal period, duration of bleeding episodes and menstrual cycles. World Health Organization Task Force on Adolescent Reproductive Health. J Adolesc Health Care 1986;7:236–44. [PubMed] [Google Scholar]
- 5.Apter D Serum steroids and pituitary hormones in female puberty: a partly longitudinal study. Clin Endocrinol (Oxf) 1980;12:107–20. [DOI] [PubMed] [Google Scholar]
- 6.Borsos A, Lampe L, Balogh A, Csoknyay J, Ditroi F, Szekely P. Ovarian function after the menarche and hormonal contraception. Int J Gynaecol Obstet 1988;27:249–53. [DOI] [PubMed] [Google Scholar]
- 7.Doring GK. The incidence of anovular cycles in women. J Reprod Fertil 1969:77–81. [Google Scholar]
- 8.Flug D, Largo RH, Prader A. Menstrual patterns in adolescent Swiss girls: a longitudinal study. Ann Hum Biol 1984;11:495–508. [DOI] [PubMed] [Google Scholar]
- 9.Legro RS, Lin HM, Demers LM, Lloyd T. Rapid maturation of the reproductive axis during perimenarche independent of body composition. J Clin Endocrinol Metab 2000;85:1021–5. [DOI] [PubMed] [Google Scholar]
- 10.Lemarchand-Beraud T, Zufferey MM, Reymond M, Rey I. Maturation of the hypothalamo-pituitary-ovarian axis in adolescent girls. J Clin Endocrinol Metab 1982;54:241–6. [DOI] [PubMed] [Google Scholar]
- 11.Metcalf MG, Skidmore DS, Lowry GF, Mackenzie JA. Incidence of ovulation in the years after the menarche. J Endocrinol 1983;97:213–9. [DOI] [PubMed] [Google Scholar]
- 12.Read GF, Wilson DW, Hughes IA, Griffiths K. The use of salivary progesterone assays in the assessment of ovarian function in postmenarcheal girls. J Endocrinol 1984;102:265–8. [DOI] [PubMed] [Google Scholar]
- 13.Vollman RF. The menstrual cycle. MajorProblObstetGynecol 1977;7:1–193. [PubMed] [Google Scholar]
- 14.Vuorento T, Huhtaniemi I. Daily levels of salivary progesterone during menstrual cycle in adolescent girls. Fertil Steril 1992;58:685–90. [PubMed] [Google Scholar]
- 15.Wilson DW, Read GF, Hughes IA, Walker RF, Griffiths K. Hormone rhythms and breast cancer chronoepidemiology: salivary progesterone concentrations in pre- and post-menarchal girls and in normal premenopausal women. Chronobiol Int 1984;1:159–65. [DOI] [PubMed] [Google Scholar]
- 16.Winter JS, Faiman C. The development of cyclic pituitary-gonadal function in adolescent females. J Clin Endocrinol Metab 1973;37:714–8. [DOI] [PubMed] [Google Scholar]
- 17.Apter D, Vihko R. Early menarche, a risk factor for breast cancer, indicates early onset of ovulatory cycles. J Clin Endocrinol Metab 1983;57:82–6. [DOI] [PubMed] [Google Scholar]
- 18.Magyar DM, Boyers SP, Marshall JR, Abraham GE. Regular menstrual cycles and premenstrual molimina as indicators of ovulation. Obstet Gynecol 1979;53:411–4. [PubMed] [Google Scholar]
- 19.Apter D, Vihko R. Serum pregnenolone, progesterone, 17-hydroxyprogesterone, testosterone and 5 alpha-dihydrotestosterone during female puberty. JClinEndocrinolMetab 1977;45:1039–48. [DOI] [PubMed] [Google Scholar]
- 20.Jay N, Mansfield MJ, Blizzard RM, et al. Ovulation and menstrual function of adolescent girls with central precocious puberty after therapy with gonadotropin-releasing hormone agonists. J Clin Endocrinol Metab 1992;75:890–4. [DOI] [PubMed] [Google Scholar]
- 21.Barron ML, Fehring RJ. Basal body temperature assessment: is it useful to couples seeking pregnancy? MCN Am J Matern Child Nurs 2005;30:290–6; [DOI] [PubMed] [Google Scholar]
- 22.Bauman JE. Basal body temperature: unreliable method of ovulation detection. Fertil Steril 1981;36:729–33. [DOI] [PubMed] [Google Scholar]
- 23.Vining RF, McGinley RA. Hormones in saliva. Crit Rev Clin Lab Sci 1986;23:95–146. [DOI] [PubMed] [Google Scholar]
- 24.Sun BZ, Kangarloo T, Adams JM, et al. Healthy Post-Menarchal Adolescent Girls Demonstrate Multi-Level Reproductive Axis Immaturity. J Clin Endocrinol Metab 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vihko R, Apter D. Endocrine characteristics of adolescent menstrual cycles: impact of early menarche. J Steroid Biochem 1984;20:231–6. [DOI] [PubMed] [Google Scholar]
- 26.Lee PA, Xenakis T, Winer J, Matsenbaugh S. Puberty in girls: correlation of serum levels of gonadotropins, prolactin, androgens, estrogens, and progestins with physical changes. J Clin Endocrinol Metab 1976;43:775–84. [DOI] [PubMed] [Google Scholar]
- 27.Gunn HM, Tsai MC, McRae A, Steinbeck KS. Menstrual Patterns in the First Gynecological Year: A Systematic Review. J Pediatr Adolesc Gynecol 2018;31:557–65 e6. [DOI] [PubMed] [Google Scholar]
- 28.Sorensen K, Mouritsen A, Aksglaede L, Hagen CP, Mogensen SS, Juul A. Recent secular trends in pubertal timing: implications for evaluation and diagnosis of precocious puberty. Horm Res Paediatr 2012;77:137–45. [DOI] [PubMed] [Google Scholar]
- 29.Zhang K, Pollack S, Ghods A, et al. Onset of ovulation after menarche in girls: a longitudinal study. J Clin Endocrinol Metab 2008;93:1186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemarchand-Beraud T, Zufferey M, Reymond M, Rey-Stocker I.Pituitary responsiveness to LHRH and TRH in adolescent girls. Bull Schweiz Akad Med Wiss 1978;34:241–54. [PubMed] [Google Scholar]
- 31.Venturoli S, Porcu E, Fabbri R, et al. Postmenarchal evolution of endocrine pattern and ovarian aspects in adolescents with menstrual irregularities. Fertil Steril 1987;48:78–85. [DOI] [PubMed] [Google Scholar]
- 32.Brown JB. Pituitary control of ovarian function--concepts derived from gonadotrophin therapy. Aust N Z J Obstet Gynaecol 1978;18:46–54. [DOI] [PubMed] [Google Scholar]
- 33.Yen SSC. REVIEW ARTICLE: THE POLYCYSTIC OVARY SYNDROME. Clin Endocrinol (Oxf) 1980;12:177–208. [DOI] [PubMed] [Google Scholar]
- 34.Carmina E, Oberfield SE, Lobo RA. The diagnosis of polycystic ovary syndrome in adolescents. AmJ ObstetGynecol 2010;203:201–5. [DOI] [PubMed] [Google Scholar]
- 35.Witchel SF, Oberfield S, Rosenfield RL, et al. The Diagnosis of Polycystic Ovary Syndrome during Adolescence. Horm Res Paediatr 2015. [DOI] [PubMed] [Google Scholar]
- 36.van Hooff MH, Voorhorst FJ, Kaptein MB, Hirasing RA, Koppenaal C, Schoemaker J. Predictive value of menstrual cycle pattern, body mass index, hormone levels and polycystic ovaries at age 15 years for oligo-amenorrhoea at age 18 years. Hum Reprod 2004;19:383–92. [DOI] [PubMed] [Google Scholar]
- 37.West S, Lashen H, Bloigu A, et al. Irregular menstruation and hyperandrogenaemia in adolescence are associated with polycystic ovary syndrome and infertility in later life: Northern Finland Birth Cohort 1986 study. Hum Reprod 2014;29:2339–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung PW, Chan SS, Yiu KW, Lao TT, Chung TK. Menstrual disorders in a Paediatric and Adolescent Gynaecology Clinic: patient presentations and longitudinal outcomes. Hong Kong Med J 2011;17:391–7. [PubMed] [Google Scholar]
- 39.Apter D, Vihko R. Endocrine determinants of fertility: serum androgen concentrations during follow-up of adolescents into the third decade of life. J Clin Endocrinol Metab 1990;71:970–4. [DOI] [PubMed] [Google Scholar]
- 40.Venturoli S, Porcu E, Fabbri R, et al. Longitudinal evaluation of the different gonadotropin pulsatile patterns in anovulatory cycles of young girls. J Clin Endocrinol Metab 1992;74:836–41. [DOI] [PubMed] [Google Scholar]
- 41.Venturoli S, Porcu E, Fabbri R, et al. Longitudinal change of sonographic ovarian aspects and endocrine parameters in irregular cycles of adolescence. Pediatr Res 1995;38:974–80. [DOI] [PubMed] [Google Scholar]
- 42.Barnes RB, Rosenfield RL, Burstein S, Ehrmann DA. Pituitary-Ovarian Responses to Nafarelin Testing in the Polycystic Ovary Syndrome. N Engl J Med 1989;320:559–65. [DOI] [PubMed] [Google Scholar]
- 43.Gilling-Smith C, Willis DS, Beard RW, Franks S. Hypersecretion of androstenedione by isolated thecal cells from polycystic ovaries. J Clin Endocrinol Metab 1994;79:1158–65. [DOI] [PubMed] [Google Scholar]
- 44.Apter D, Butzow T, Laughlin GA, Yen SS. Accelerated 24-hour luteinizing hormone pulsatile activity in adolescent girls with ovarian hyperandrogenism: relevance to the developmental phase of polycystic ovarian syndrome. J Clin Endocrinol Metab 1994;79:119–25. [DOI] [PubMed] [Google Scholar]
- 45.Rosenfield RL. The Diagnosis of Polycystic Ovary Syndrome in Adolescents. Pediatrics 2015;136:1154–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenfield RL, Ghai K, Ehrmann DA, Barnes RB. Diagnosis of the polycystic ovary syndrome in adolescence: comparison of adolescent and adult hyperandrogenism. J Pediatr Endocrinol Metab 2000;13 Suppl 5:1285–9. [PubMed] [Google Scholar]
- 47.Kjerulff KH, Erickson BA, Langenberg PW. Chronic gynecological conditions reported by US women: findings from the National Health Interview Survey, 1984 to 1992. Am J Public Health 1996;86:195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lortet-Tieulent J, Ferlay J, Bray F, Jemal A. International Patterns and Trends in Endometrial Cancer Incidence, 1978–2013. J Natl Cancer Inst 2018;110:354–61. [DOI] [PubMed] [Google Scholar]
- 49.Rosenfield RL, Ehrmann DA, Littlejohn EE. Adolescent polycystic ovary syndrome due to functional ovarian hyperandrogenism persists into adulthood. J Clin Endocrinol Metab 2015;100:1537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuruvilla S, Bustreo F, Kuo T, et al. The Global strategy for women’s, children’s and adolescents’ health (2016–2030): a roadmap based on evidence and country experience. Bull World Health Organ 2016;94:398–400. [DOI] [PMC free article] [PubMed] [Google Scholar]