Abstract
Objective:
To assess outcomes of endovascular reperfusion followed by delayed open aortic repair for stable patients with acute type A aortic dissection (ATAAD) and mesenteric malperfusion syndrome (MesMPS).
Methods:
Among 602 patients with ATAAD who presented to our center from 1996 to 2017, all 82 (14%) with MesMPS underwent upfront endovascular fenestration/stenting. Primary outcomes were in-hospital mortality and long-term survival. ATAAD patients with no malperfusion syndrome of any organ (n=419) served as controls.
Results:
In-hospital mortality of all comers with MesMPS was 39%. After endovascular fenestration/stenting, 20 (24%) MesMPS patients died from organ failure and 11 (13%) from aortic rupture before open aortic repair, 47 (58%) underwent aortic repair, and 4 (5%) survived without open repair. No patients died from aortic rupture during the second decade (2008-2017). The significant risk factors for death from organ failure after endovascular reperfusion were acute stroke (odds ratio (OR)= 23 (95% CI: 4, 144), p=0.0008), gross bowel necrosis at laparotomy (OR= 7 (1.4, 34), p=0.016), and serum lactate ≥6 mmol/L (OR= 13.5 (2, 97), p=0.0097). There was no significant difference in operative mortality (2.1% vs. 7.5%; p=0.50) or long-term survival between MesMPS patients who underwent open aortic repair after recovering from MesMPS and patients with no malperfusion syndrome.
Conclusions:
In ATAAD patients with MesMPS, endovascular fenestration/stenting and delayed open aortic repair achieved favorable short- and long-term outcomes. Surgeons should consider correcting mesenteric malperfusion before undertaking open aortic repair in MesMPS patients, especially those with acute stroke, gross bowel necrosis at laparotomy, or serum lactate ≥6 mmol/L.
INTRODUCTION
Acute type A aortic dissection (ATAAD) is a catastrophic event with an average operative mortality of 20-25%.1 Prompt surgical intervention (open aortic repair) is generally necessary to prevent death from aortic rupture. ATAAD patients presenting with concomitant dissection-related severe end-organ malperfusion, tissue/organ necrosis, and consequential failure (malperfusion syndrome, or MPS) have a significantly increased mortality. Mesenteric MPS (MesMPS), in particular, has been reported to have a very poor prognosis with in-hospital mortality ranging from 60 to 75%.1–12 The optimal management of ATAAD patients with MPS remains an unsettled issue. Emergent open aortic repair aims to prevent aortic rupture and death from hemorrhagic shock or tamponade. However, for patients with MesMPS the impending risk of bowel necrosis and septic shock might be the most immediate, life-threatening concern. Aortic repair usually achieves visceral reperfusion through surgical stabilization of the true lumen2 and resolves dynamic occlusion of the superior mesenteric artery (SMA), but it might fail in instances of static occlusion of the SMA, such as with SMA true or false lumen thrombosis. In addition, relief of the SMA obstruction does not cause immediate resolution of the already existing ischemic injury. In both cases, putting the patient through the major physiologic stress of a long operation on cardiopulmonary bypass (CPB) with circulatory arrest before correction of or recovery from MesMPS could increase the probability of a poor outcome.
At the University of Michigan, since 1996 we have adopted a different approach for ATAAD patients with MPS who are otherwise stable (no aortic rupture, no tamponade), which consists of upfront endovascular reperfusion (through fenestration/stenting) of the critically malperfused organ system(s), including abdominal viscera, by interventional radiology (IR), followed by open aortic repair at resolution of organ failure.13–16 Herein, we present the 20-year-long experience of our approach, focusing on patients with ATAAD and MesMPS. The primary objective was to assess short- and long-term outcomes of ATAAD patients with mesenteric MPS. A secondary objective was to identify risk factors for death from organ failure even after successful visceral reperfusion in order to differentiate patients for whom open aortic repair could achieve survival at discharge from those for whom it would likely be futile.
METHODS
This study was approved by the University of Michigan Institutional Review Board (Michigan Medicine, Ann Arbor, MI) and was in compliance with Health Insurance Portability and Accountability Act (HIPAA) regulations. A waiver of consent was obtained.
Patient population and data collection
Between July 1996 and January 2017, 602 patients presented to the University of Michigan with an ATAAD, defined as onset within 14 days of admission. Eighty-two (14%) patients had mesenteric malperfusion syndrome (MesMPS) and represent the focus of this study. Patients (n=419, 70%), who did not suffer from any (cerebrospinal, coronary, mesenteric, renal, or lower extremity) malperfusion syndrome (MPS) and underwent upfront open aortic repair, served as control. (See Supplemental Figure S1) Investigators obtained Society of Thoracic Surgery data elements from the University of Michigan Department of Cardiac Surgery Data Warehouse to determine pre-, intra-, and post-operative characteristics. Demographics, medical records, and operative reports were reviewed to supplement data collection. Survival was obtained through the National Death Index database through December 201517 and medical record review.
Diagnosis and management of ATAAD with mesenteric malperfusion syndrome
The diagnosis of MesMPS requires both clinical and laboratory features (abdominal pain, bloody diarrhea, tenderness to palpation, elevated lactate, metabolic acidosis) as well as radiographic demonstration of low or absent blood flow through the SMA with or without SMA thrombosis. Unless there was evidence of aortic rupture or cardiac tamponade (which were indications for immediate aortic repair), all patients with MesMPS were treated with upfront endovascular fenestration/stenting prior to open repair. If the SMA was dissected, SMA pressure was measured beyond the distal extent of the dissection. Angiographic confirmation of treatable MesMPS was documented by a significant systolic blood pressure gradient (>15 mmHg) between the ascending aorta and the distal SMA. In patients with aortic coarctation, a blood pressure differential of >20 mmHg has traditionally been considered significant, so we conservatively choose a gradient of systolic blood pressure of 15 mmHg as the criterion for malperfusion. Aortic fenestration and stenting were performed percutaneously by creating a tear in the dissection flap to balance the blood pressure and permit flow between the true and false lumens, as previously described.13–16 If the gradient between the ascending aorta and SMA persisted (>15 mmHg) after the aortic fenestration and stenting, further SMA stenting, thrombolysis, or suction thromboembolectomy was performed. (Supplemental Table S1) In dissected vessels with thrombosed false lumens, gradients after stenting might exceed 15 mmHg, but as long as absolute perfusion pressure was viable, i.e., >60 mmHg, post-dilation of stents was not performed.
Post-IR management and open aortic repair (Figure 1)
Figure 1.
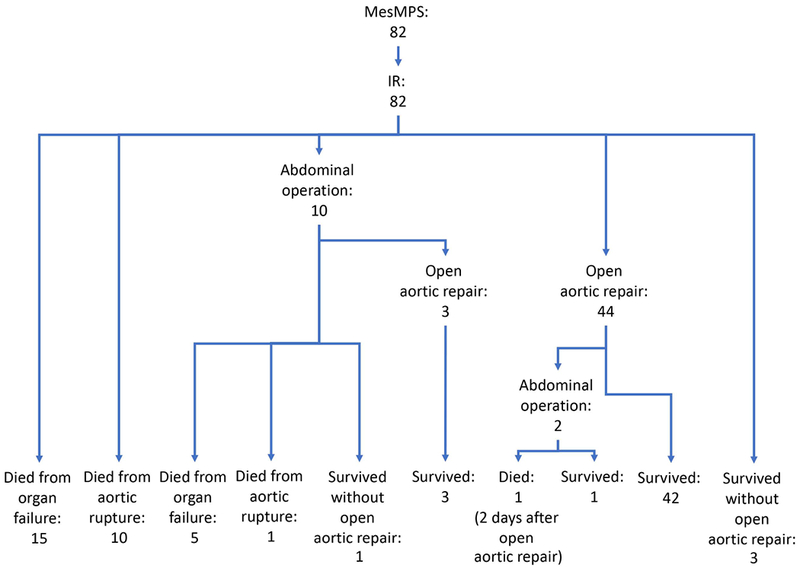
Management and short-term outcomes of patients with mesenteric malperfusion syndrome. IR = endovascular reperfusion by interventional radiology; MesMPS = mesenteric malperfusion syndrome.
After endovascular reperfusion of the SMA, the general surgery team evaluated the 82 patients to determine if an exploratory laparotomy was needed based on the clinical exam (abdominal tenderness), increasing lactate, and pneumatosis in the wall of the intestine on CT scan. If no laparotomy was indicated or laparotomy was performed but no necrotic bowel was identified, we waited for resolution of abdominal pain, normalization of lactate and metabolic acidosis, and then performed open aortic repair. If a patient had a resection of necrotic bowel, we waited for the patient to recover from metabolic acidosis, septic shock, and/or acute respiratory distress syndrome, and then performed open aortic repair. The cohort of patients with MesMPS who eventually underwent open aortic repair after recovering from MPS was defined as surgical MesMPS patients (n=47). The median time from fenestration/stenting to open aortic repair was 6 (interquartile range: 2-19) days (Table 1). The operative strategy for the surgical MesMPS patients was the same as for the ATAAD patients with no MPS (non-MPS patients).
Table 1.
Demographics and preoperative characteristics of all patients
| All patients (n= 501) | MesMPS (n = 82) | Non-MPS (n = 419) | p-value | |
|---|---|---|---|---|
| Admission variables | ||||
| Age on admission (years) | 59 (49, 68) | 59.5 (50, 68) | 59 (49, 68) | 0.97 |
| Gender (female) | 151 (30) | 22 (27) | 129 (31) | 0.48 |
| NYHA class | ||||
| III or IV | 99 (22) | 8 (10) | 91 (22) | 0.013 |
| CAD | 88 (18) | 17 (20) | 71 (17) | 0.41 |
| History of MI | 29 (5.8) | 9 (11) | 20 (4.8) | 0.03 |
| Previous cardiac surgery | 50 (10) | 13 (16) | 37 (8.8) | 0.04 |
| Hypertension | 351 (70) | 65 (81) | 286 (68) | 0.019 |
| COPD | 44 (8.8) | 7 (8.5) | 37 (8.8) | 0.93 |
| Smoking status | 0.96 | |||
| Never smoker | 232 (47) | 36 (46) | 196 (47) | |
| Former smoker | 126 (25) | 20 (25) | 106 (25) | |
| Current smoker | 138 (28) | 23 (29) | 115 (28) | |
| Diabetes | 27 (5.5) | 2 (2.7) | 25 (5.9) | 0.40 |
| Creatinine on admission (mg/dL) | 1.0 (0.8, 1.3) | 1.4 (1.0, 2.3) | 1.0 (0.8, 1.2) | <.0001 |
| Chronic kidney disease | 19 (3.8) | 8 (9.9) | 11 (2.6) | 0.006 |
| History of CVA | 14 (2.8) | 3 (3.7) | 11 (2.6) | 0.71 |
| PVOD | 57 (11) | 9 (11) | 48 (11) | 0.90 |
| Connective tissue disorder | 28 (5.6) | 2 (2.4) | 26 (6.2) | 0.29 |
| Aortic insufficiency | 0.97 | |||
| None | 130 (28) | 21 (28) | 109 (28) | |
| Trace/trivial | 54 (12) | 10 (13) | 44 (11) | |
| Mild | 94 (20) | 15 (20) | 79 (20) | |
| Moderate | 83 (18) | 14 (19) | 69 (18) | |
| Severe | 104 (22) | 15 (20) | 89 (22.8) | |
| Cardiogenic shock | 38 (7.8) | 3 (4.3) | 35 (8.4) | 0.23 |
| Acute stroke | 12 (2.4) | 10 (12) | 2 (0.5) | <.0001 |
| Acute paralysis | 6 (1.2) | 6 (7.3) | 0 (0) | <.0001 |
| Acute MI | 1 (0.2) | 1 (1.2) | 0 (0) | 0.16 |
| Tamponade | 42 (8.4) | 3 (3.7)a | 39 (9.3) | 0.09 |
| AKI | 80 (16) | 62 (76) | 18 (4.3) | <.0001 |
| Requiring new dialysis pre-operatively | 5 (1.0) | 5 (6.1) | 0 (0) | 0.0001 |
| Renal malperfusion | 55 (11) | 55 (67) | 0 (0) | |
| Lower extremity malperfusion | 53 (11) | 53 (65) | 0 (0) | |
| Bowel necrosis at laparotomy | 9 (1.8) | 9 (11) | 0 | |
| Max serum lactate before IR (mmol/L) | NA | 2.3 (1.2, 3.4) | NA | |
| Max serum lactate before IR ≥ 6 mmol/L | NA | 7 (8.5) | NA | |
| Management | ||||
| IR | 82 (16) | 82 (100) | 0 (0) | < 0.001 |
| Time from admission to IR (days) | NA | 0 (0, 1) | NA | NA |
| Open aortic repair | 466 (93) | 47 (57) | 419 (100) | < 0.001 |
| Time from admission to aortic repair (days) | 0 (0, 1) | 7 (3, 20) | 0 (0, 1) | <.0001 |
| Time from IR to aortic repair (days) | NA | 6 (2, 19) | NA | NA |
Data presented as median (interquartile range) for continuous variables and number (percentage) for categorical variables.
Three patients developed cardiac tamponade after IR procedure due to progression of disease.
AKI = acute kidney injury; CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; CVA = cerebrovascular accident; GFR = glomerular filtration rate as estimated using the Cockcroft-Gault formula; IR = endovascular procedure by interventional radiology; MI = myocardial infarction; MPS = malperfusion syndrome; NA = not applicable; Non-MPS = no malperfusion syndrome; NYHA = New York Heart Association; PVOD = peripheral vascular occlusive disease.
Statistical Analysis
Chi-square and Fisher exact tests were used for categorical variables and Wilcoxon rank-sum test was used for continuous variables, as appropriate. Multivariable logistic regression was used to determine the risk factors with odds ratio (OR) for in-hospital mortality in comparison of all patients with MesMPS vs. non-MPS after stepwise selection of variables, including group, age, gender, NYHA class III/IV, history of MI, previous cardiac surgery, CAD, COPD, cardiogenic shock, acute MI, acute stroke, acute renal failure, acute paralysis, and preoperative chronic renal failure based on clinical relevance. Multivariable logistic regression was also used to determine the risk factors and OR of mortality in comparison of patients who died from organ failure after endovascular reperfusion but before open repair vs. those who survived to open repair or discharge. Stepwise selection was performed for variables including age, acute stroke, renal malperfusion, extremity malperfusion, cardiogenic shock, bowel necrosis identified at laparotomy, and max serum lactate before IR ≥ 6 mmol/L. Long-term survival curves were estimated using the Kaplan-Meier method with the log-rank test in all patients with MesMPS vs. non-MPS patients, and surgical MesMPS patients vs. non-MPS patients. The Cox proportional hazard model was used to compare the long-term outcome in surgical MesMPS patients vs. non-MPS patients, but not in all patients with MesMPS vs. non-MPS due to violation of proportional hazard assumption. Stepwise selection of variables was used for the Cox model, including all the variables used for the logistic model for in-hospital mortality. P values (two-tailed) < 0.05 were considered statistically significant. All statistical analysis used SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Demographics of patients with mesenteric malperfusion syndrome vs no malperfusion syndrome.
As expected, patients with MesMPS were much sicker than non-MPS patients when they arrived at our hospital, including a significantly higher incidence of acute stroke, acute renal malperfusion, acute kidney injury with or without dialysis, acute extremity malperfusion, acute paralysis, bowel necrosis, chronic renal failure, hypertension, and previous myocardial infarction and cardiac surgery. (Table 1)
Overall outcomes of patients with ATAAD and mesenteric malperfusion syndrome
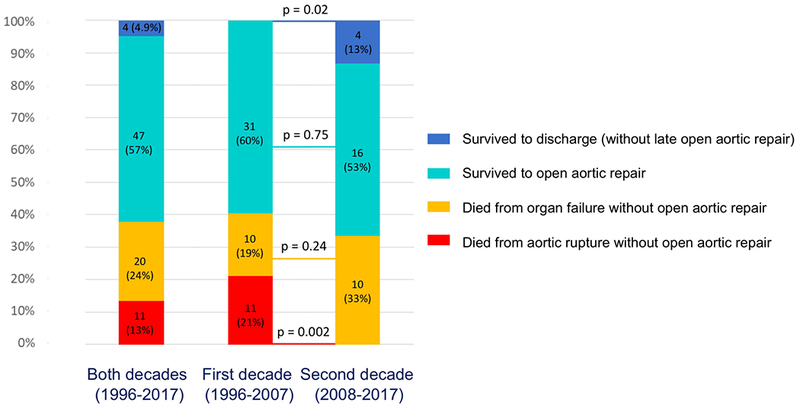
All 82 patients with ATAAD and MesMPS underwent upfront endovascular reperfusion by IR (Figure 1) (Supplemental Table S1). After endovascular fenestration/stenting, 38% of patients died before open aortic repair either from organ failure (n=20, 24.4%) or aortic rupture (n=11, 13.4%). The remaining patients survived and either underwent an open aortic repair (n=47, 57%; 44 patients had an operation before discharge, 3 patients had an operation at readmission within 1 year) or survived through discharge but never had an open aortic repair (n=4, 5%) (Figure 2). All deaths from aortic rupture (n=11) occurred during the first decade (1996-2007), with zero deaths from aortic rupture in the second decade (2008-2017), p=0.002 (Figure 2). One patient died after open aortic repair. The overall in-hospital mortality was 39% (Table 2).
Figure 2.
Short-term outcomes of patients with mesenteric malperfusion syndrome (MesMPS) after endovascular reperfusion by interventional radiology (IR). IR = endovascular reperfusion by interventional radiology; MesMPS = mesenteric malperfusion syndrome.
Table 2.
Outcomes after procedure by interventional radiology (IR) or open repair (OR) of patients with mesenteric malperfusion syndrome (MesMPS) or no malperfusion syndrome (Non-MPS)
| All patients (n=501) | MesMPS (n=82) | Non-MPS (n=419) | p value | |
|---|---|---|---|---|
| Reoperation for bleeding | 43 (8.6) | 5 (6.1) | 38 (9.1) | 0.37 |
| Tamponade | 10 (2.0) | 0 (0) | 10 (2.4) | 0.38 |
| Postoperative MI | 8 (1.6) | 2 (2.4) | 6 (1.4) | 0.62 |
| Atrial fibrillation | 165 (33) | 24 (29) | 141 (34) | 0.43 |
| New-onset CVA | 33 (6.6) | 3 (3.7) | 30 (7.2) | 0.24 |
| New-onset paraplegia | 3 (0.6) | 1 (1.2) | 2 (0.5) | 0.42 |
| Pneumonia | 82 (16) | 17 (21) | 65 (16) | 0.25 |
| Reintubation | 28 (5.6) | 4 (4.9) | 24 (5.7) | 1.0 |
| Tracheostomy | 17 (3.4) | 14 (3.4) | 3 (3.7) | 0.75 |
| Post-op AKI | 46 (9.2) | 3 (3.7) | 43 (10) | 0.06 |
| Requiring new dialysis | 19 (3.8) | 1 (1.2) | 18 (4.3) | 0.34 |
| Total LOS (days) | 11 (7, 18) | 18 (4, 28) | 10 (7, 16) | 0.12 |
| In-hospital mortality | 62 (12) | 32 (39) | 30 (7.2) | <.0001 |
In the MesMPS group, any complications after interventional radiology (IR) procedures or open repair (OR) were recorded as outcomes. In Non-MPS group, any complications after open repair were recorded as outcomes. Data presented as median (interquartile range) for continuous variables and number/total number (percentage) for categorical variables. AKI = acute kidney injury; CVA = cerebrovascular accident; LOS = length of stay; MesMPS = mesenteric malperfusion syndrome; MI = myocardial infarction; MPS = malperfusion syndrome Non-MPS = no malperfusion syndrome.
Risk factors for “death from organ failure after resolution of malperfusion”
The MesMPS patients who died from organ failure after endovascular revascularization had significantly more cardiogenic shock, acute stroke, and higher lactate levels compared to those who survived to open aortic repair or discharge. (Table 3) Multivariable logistic regression showed the significant independent risk factors for death from organ failure were acute stroke (OR = 23 (95% CI: 4, 144), p=0.0008), gross bowel necrosis at laparotomy (OR = 7 (1.4, 34), p=0.016), and serum lactate ≥ 6 mmol/L (OR = 13.5 (2, 97), p=0.0097).
Table 3.
Clinical condition of patients with mesenteric malperfusion syndrome divided into three groups based on the outcome of endovascular reperfusion
| Death from organ failure (n=20) | Survival* (n=51) |
Death from aortic rupture (n=11) | p-value | |
|---|---|---|---|---|
| Age on admission (years) | 58 (47-73) | 57 (51-65) | 63 (58-69) | 0.65 |
| Gender (female) | 8 (40) | 12 (24) | 2 (18) | 0.27 |
| CAD | 5 (28) | 11 (22) | 1 (10) | 0.11 |
| History of MI | 4 (21) | 4 (7.8) | 1 (10) | 0.20 |
| Previous cardiac surgery | 3 (15) | 9 (18) | 1 (9.1) | 1 |
| Hypertension | 16 (84) | 44 (86) | 5 (50) | 1 |
| COPD | 1 (5.0) | 5 (9.8) | 1 (9.1) | 0.67 |
| Diabetes | 0 (0) | 2 (3.9) | 0 (0) | 1 |
| Creatinine on admission (mg/dL) | 1.4 (1.1, 2.5) | 1.4 (1.0, 2.6) | 1.3 (1.1, 1.4) | 0.88 |
| GFR on admission (mL/min/1.73 m2) | 67 (42-93) | 69 (47-96) | 62 (48-92) | 0.98 |
| Chronic kidney disease | 3 (16) | 5 (9.8) | 0 (0) | 0.67 |
| History of CVA | 0 (0) | 3 (5.9) | 0 (0) | 0.55 |
| PVOD | 2 (10) | 7 (14) | 0 (0) | 1 |
| Cardiogenic shock | 3 (20) | 0 (0) | 0 (0) | 0.01 |
| Acute stroke | 8 (40) | 2 (3.9) | 0 (0) | < 0.001 |
| Acute paralysis | 2 (10) | 4 (7.8) | 0 (0) | 1 |
| Acute MI | 0 (0) | 0 (0) | 1 (9.1) | 1 |
| Pre-operative CPR | 0 (0) | 1 (2.0) | 0 (0) | 1 |
| AKI | 16 (80) | 38 (75) | 8 (73) | 0.76 |
| Requiring new dialysis pre-operatively | 1 (5.0) | 4 (7.8) | 0 (0) | 1.0 |
| Renal malperfusion | 15 (75) | 33 (65) | 7 (64) | 0.65 |
| Lower extremity malperfusion | 11 (55) | 36 (71) | 6 (55) | 0.33 |
| Bowel necrosis at laparotomy | 5 (25) | 4 (7.8) | 0 (0) | 0.12 |
| Max serum lactate before IR (mmol/L) | 3.0 (2.2, 6.8) | 1.6 (1.2, 3.2) | 2.2 (1.6, 2.7) | 0.02 |
| Max serum lactate before IR ≥ 6 mmol/L | 5 (31) | 2 (5) | 0 (0) | 0.02 |
Survival: means patients survived to open aortic repair or discharge without open repair. P value indicates the difference between the groups of death from organ failure and survival to open aortic repair or hospital discharge.
AKI = acute kidney injury; CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; CPR = cardiopulmonary resuscitation; CVA = cerebrovascular accident; GFR = glomerular filtration rate as estimated using the Cockcroft-Gault formula; IR = endovascular procedure by interventional radiology; MI = myocardial infarction; MPS = malperfusion syndrome; NYHA = New York Heart Association; PVOD = peripheral vascular occlusive disease.
Short-term outcomes of patients with mesenteric malperfusion syndrome vs no malperfusion syndrome.
The in-hospital mortality was 5-times higher in the MesMPS group (n=82) compared to the non-MPS group (n=419) (39% vs. 7.2%, p<0.001). All other post-procedural (IR or open repair) outcomes in the MesMPS group were not significantly different from the post-operative outcomes in the non-MPS group despite the fact that patients with MesMPS were much sicker at admission. (Table 2) Multivariable logistic regression showed the significant risk factors for in-hospital mortality were MesMPS (OR = 5 (2.4, 10.6), p<0.0001), age (OR = 1.03 (1, 1.05), p=0.046), cardiogenic shock (OR = 8 (3.5, 14), p<0.001), and acute stroke (OR = 12 (2.6, 59), p=0.002).
Comparison of surgical outcomes between MesMPS and non-MPS patients
Although surgical MesMPS patients were still significantly sicker at admission (Supplemental Table S2), there was no significant difference in the complexity of the operation (Supplemental Table 3) or the operative (30-day post-operative or in-hospital) mortality (2.1 vs. 7.4%; p = 0.23) between the surgical MesMPS vs. non-MPS groups (Table 4). However, surgical MesMPS patients required more blood transfusions intra-operatively (Supplemental Table S3), and had a longer post-operative (after open aortic repair) hospital stays (Table 4).
Table 4.
Post-operative outcomes of patients with or without mesenteric malperfusion syndrome (only patients who underwent open aortic repair)
| All patients (n=466) | MesMPS (n=47) | Non-MPS (n=419) | p value | |
|---|---|---|---|---|
| Reoperation for bleeding | 43 (9.2) | 5 (11) | 38 (9.1) | 0.79 |
| Tamponade | 10 (2.1) | 0 (0) | 10 (2.3) | 0.61 |
| Peri-operative MI | 6 (1.2) | 0 (0) | 6 (1.4) | 1.0 |
| Atrial fibrillation | 162 (35) | 21 (45) | 141 (34) | 0.14 |
| DSWI | 12 (2.5) | 0 (0) | 12 (2.8) | 0.62 |
| New-onset CVA | 33 (7.1) | 3 (6.4) | 30 (7.2) | 1.0 |
| New-onset paraplegia | 2 (0.4) | 0 (0) | 2 (0.5) | 1.0 |
| Pneumonia | 75 (16) | 10 (21) | 65 (16) | 0.31 |
| Reintubation | 27 (5.8) | 3 (6.4) | 24 (5.7) | 0.75 |
| Tracheostomy | 14 (3.0) | 0 (0) | 14 (3.3) | 0.38 |
| Post-op AKI | 45 (9.7) | 2 (4) | 43 (10) | 0.29 |
| Requiring new dialysis | 19 (4) | 1 (2) | 18 (4) | 0.71 |
| Post-op LOS (days) | 10 (7, 16) | 16 (10, 23) | 10 (7, 15) | <.0001 |
| Intraoperative mortality | 4 (0.8) | 0 (0) | 4 (0.9) | 1.0 |
| In-hospital mortality | 31 (6.7) | 1 (2.1) | 30 (7.2) | 0.35 |
| 30-day mortality | 25 (5.4) | 1 (2.1) | 24 (5.7) | 0.49 |
| Operative mortality a | 32 (6.9) | 1 (2.1) | 31 (7.4) | 0.23 |
Data presented as median (interquartile range) for continuous variables and number/total number (percentage) for categorical variables.
: Operative mortality: Defined as in-hospital mortality or mortality within 30 days after open repair.
AKI = acute kidney injury; CVA = cerebrovascular accident; DSWI = deep sternal wound infection; LOS = length of stay; MesMPS = mesenteric malperfusion syndrome; MI = myocardial infarction; MPS = malperfusion syndrome; Non-MPS = no malperfusion syndrome.
Long-term survival
MesMPS patients (n=82) had worse overall long-term survival than non-MPS patients due to a much higher in-hospital mortality (10-year survival: 41% (95% CI: 29%, 53%) vs. 64% (57%, 69%), p<0.001). (Figure 3A) However, there was no significant difference in long-term survival between surgical MesMPS patients (n=47) and non-MPS patients (10-year survival: 69% (50%, 82%) vs. 64% (57%, 69%), p=0.60). (Figure 3B) Cox proportional hazard model showed that the risk factors for all-time mortality after surgery in surgical MesMPS patients and non-MPS patients were age (hazard ratio (HR) = 1.04 (95% CI: 1.02, 1.05), p<0.001), CAD (HR = 1.8 (1.3, 2.7), p=0.001), and acute paralysis (HR = 7.3 (1.6, 33), p=0.01). MesMPS at admission was no longer a risk factor after patients were successfully treated with endovascular fenestration/stenting and recovered from MPS (HR=0.8 (0.4, 1.4), p=0.37).
Figure 3.
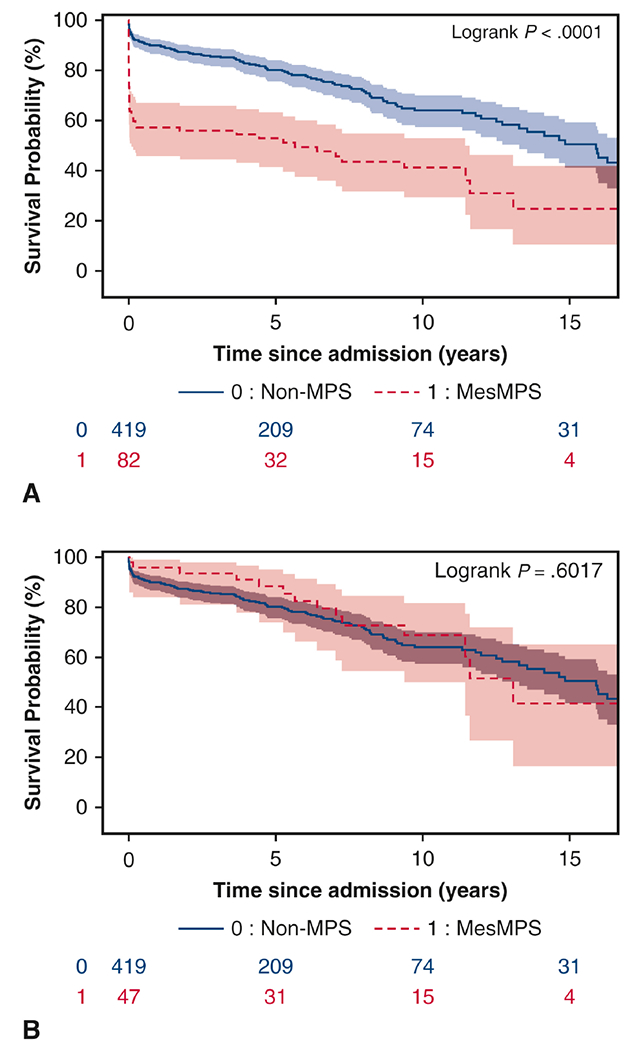
Overall long-term survival of patients with mesenteric malperfusion syndrome vs. those with no (mesenteric or non-mesenteric) malperfusion syndrome (non-MPS): A. Survival since hospital admission, all MesMPS patients (n=82) vs. non-MPS patients; B. Survival since open aortic repair, surgical MPS patients (n=47) who underwent open aortic repair vs. non-MPS patients.
MPS = malperfusion syndrome; Non-MPS = no malperfusion syndrome.
DISCUSSION
In this study, we reported our 20-year-long term experience of managing ATAAD with MesMPS treated with endovascular reperfusion and delayed open aortic repair. The key findings include: (1) in-hospital mortality for all MesMPS patients (after endovascular reperfusion, with or without open repair) was 39%, which was 5 times higher than that in non-MPS patients. (2) Twenty-four percent of MesMPS patients died of organ failure despite adequate endovascular revascularization. The significant risk factors of death from organ failure were acute stroke, gross bowel necrosis at laparotomy, and maximum lactate ≥ 6 mmol/L. (3) After endovascular revascularization and recovery from MesMPS, the postoperative short- and long-term survival in surgical MesMPS patients was not significantly different from that in non-MPS patients. (Video)
There are two critical issues in patients with ATAAD and MesMPS: (1) rupture of the proximal aorta and (2) mesenteric ischemia/necrosis, which can both result in death. Traditional teaching predicates immediate open aortic repair for all ATAAD patients, even with MesMPS, with the goal to protect the patient from aortic rupture and to resolve the mesenteric malperfusion. This strategy may work for dynamic obstruction of the SMA if no end-organ damage (malperfusion syndrome) has occurred; however, once end-organ damage is present or static occlusion of the SMA due to thrombosis of the false lumen in the SMA is present, the mesenteric issue cannot reliably be resolved by open aortic repair alone. More than 50% of MesMPS patients in our study needed SMA stenting, focal thrombolysis, or suction thromboembolectomy to resolve the obstruction of the SMA (Supplemental Table S1). Some institutions perform SMA stenting after open repair if patients were suspected to have ongoing mesenteric ischemia and malperfusion.21 However, this approach could be too late if patients already have compromised bowel upon arrival. With the traditional approach, the operative mortality in ATAAD with MesMPS is above 50%.1–12
Due to this limitation of the traditional approach (upfront open aortic repair), we developed our strategy to treat MesMPS with initial endovascular fenestration/stenting followed by open aortic repair upon recovery from mesenteric MPS. There are several advantages to such an approach. First, we are able to resolve the obstruction of the SMA and other visceral branches immediately and adequately with a minimally-invasive percutaneous procedure, avoiding CPB and hypothermic circulatory arrest. The procedure itself has minimal negative impact due to trauma on these very sick patients. In addition, we can treat SMA occlusion with stenting, thrombolysis with local fibrinolysis (tissue plasminogen activator), or suction thromboembolectomy (Supplemental Table S1). At the end of the procedure, we measure the blood pressure in each visceral branch (such as the SMA, celiac artery, and renal arteries) and in the iliac arteries to confirm that arterial obstruction has been adequately relieved. We accept a blood pressure gradient between the ascending aorta and arterial branch below 15 mmHg. Second, our approach avoids futile open aortic repair, which may consume more resources than a percutaneous procedure alone. Twenty patients in this cohort died from organ failure even after arterial obstruction of all the viscera and extremities was resolved (Figures 1 and 2, Supplemental Table S4). Assuming an open aortic repair could have resolved the malperfusion, it is very likely that these patients would have died from organ failure due to the prolonged period of MesMPS. Third, we were able to treat all the MesMPS patients with this approach even if they had mesenteric malperfusion for >24 hours, as long as they did not have aortic rupture or cardiac tamponade. We do not turn down any patients with MesMPS no matter how sick they are. We give every patient a chance of recovery by reperfusion of his/her mesentery. Finally, our approach provides very favorable long-term survival in MesMPS patients after endovascular reperfusion and subsequent open aortic repair, which was very similar to ATAAD patients without MPS (Figure 3b).
There is a difference between mesenteric malperfusion and mesenteric malperfusion syndrome (MesMPS). Malperfusion is inadequate blood flow to an end organ, i.e., ongoing arterial obstruction. The organ could be ischemic but not necrotic, especially at the early stage. MPS is the late stage of malperfusion and the malperfused end organ already has cell/tissue/organ death and malfunction, such as necrotic gut for mesenteric malperfusion. MPS is the indication for IR evaluation and possible treatment, but radiographic (such as CT angiogram) malperfusion alone is not. The patients who died from organ failure after endovascular revascularization in our study all had very late stage malperfusion and unsalvageable end-organ death, even after the mesenteric malperfusion was resolved by IR. Many of them had multi-organ arterial obstruction. (Table S4) Any intervention most likely would have been futile, including IR and open repair. MesMPS includes all those patients with existing bowel damage and reperfusion injury, which are not eliminated by restoring arterial perfusion. However, when patients with MPS (late stage of malperfusion) come to us, we never know who will survive and who will not survive after revascularization. IR is less traumatic than open aortic repair, and at least gives those patients a chance to recover; maybe a better chance in those borderline patients due to much less traumatic impact on the patients. This is why we recommend IR first for patients with MPS, especially because the risk of aortic rupture decreased dramatically with our current management.
A similar approach to ATAAD patients with MesMPS has also been adapted by other groups either through an endovascular approach10,22 or open SMA bypass23,24 with good outcomes. Driven by the concern for impending aortic rupture, those groups performed open aortic repair immediately after mesenteric reperfusion. The reasons to delay open aortic repair in our approach are as follows: patients may have a necrotic bowel requiring bowel resection, patients may need time to recover from multi-organ failure, such as ARDS or septic shock from necrotic bowel, and some patients may die from organ failure even after visceral and/or extremity malperfusion are resolved (as we saw in our study). For these patients, immediate open aortic repair would be futile.
There is always the risk of proximal aortic rupture and death from hemorrhage or tamponade while waiting for delayed open aortic repair during recovery from mesenteric ischemia. Indeed, we had 11 (13%) patients die from aortic rupture. All the cases of aortic rupture occurred in the first decade (1996-2007). As we gained more experience managing ATAAD patients after endovascular fenestration/stenting, we had no deaths from aortic rupture during the second decade (2008-2017) (Figure 2), likely due to aggressive blood pressure control (goal: systolic < 110 mmHg), better care in the intensive care unit, and earlier secondary open aortic repair (median waiting time: 7 days in the 1st decade vs. 4 days in the 2nd decade, p=0.44).
In order to better predict the outcomes of ATAAD patients with MesMPS, we aimed to identify variables associated with mortality from organ failure even after successful endovascular visceral reperfusion. We found that acute stroke, gross bowel necrosis at laparotomy, and a serum lactate ≥ 6 mmol/L were significant independent risk factors of death from organ failure with a high odds ratio (7-23), which are consistent with other reports.25–27 Therefore, it might be valuable for the treating clinician to gauge the appropriateness vs. futility of open aortic repair when patients present with an acute stroke, bowel necrosis, or high serum lactate in the setting of ATAAD with MesMPS.
The MesMPS patients that recovered from endovascular reperfusion and subsequently underwent open aortic repair are a highly selected group. This group of patients had no more visceral or extremity malperfusion and had recovered from most complications (except renal failure) of MesMPS by the time they underwent open aortic repair. It is not surprising that the short- and long-term outcomes were comparable to non-MPS ATAAD patients (Table 4, Figure 3B). Actually, this is exactly our goal of treating ATAAD patients with MesMPS: to reperfuse abdominal viscera as soon as possible, essentially “converting” a patient with MesMPS to a non-MPS patient, and improve survival in this difficult-to-treat and dreadful disease.
This study is limited by a single-center retrospective experience, although, we report one of the largest cohorts of ATAAD patients with MesMPS. Since the severity of MesMPS would be expected to correlate with duration of SMA obstruction, it might have been helpful to include duration of symptoms as one of the variables associated with mortality from organ failure. Although serum lactate might be a surrogate for duration of SMA obstruction, lactate might be falsely low in the setting where superior mesenteric venous outflow is reduced due to celiac artery and SMA obstruction. Our institution is one of only a few centers using this approach for ATAAD patients with MesMPS, wherein the mesenteric arterial occlusion is corrected and the patient is allowed to recover from malperfusion before open aortic repair. Our results may not be reproducible at other institutions. The sample size of patients who died from organ failure before open repair is still small, which prevented us, for instance, from building a robust multivariable quantitative prognostic score for risk of death from organ failure.
CONCLUSION
In ATAAD patients with MesMPS, endovascular fenestration/stenting followed by delayed open repair achieved favorable short- and long-term outcomes. For MesMPS patients with acute stroke, bowel necrosis at laparotomy, or serum lactate ≥ 6 mmol/L, the risk of dying from organ failure increased dramatically, and therefore, caution should be exercised when offering open aortic repair to those patients. Surgeons should keep in mind that not every untreated ATAAD will rupture, but every untreated MesMPS will cause patient’s death.
Supplementary Material
Discussion of the management of ATAAD patients with mesenteric malperfusion syndrome (MesMPS), including indications for upfront endovascular revascularization, detailed technique of fenestration and stenting of the dissected aorta and its branches, timing for delayed open aortic repair, and short- and long-term outcomes.
Supplemental Figure S1: CONSORT flow diagram of patients’ selection.
CENTRAL MESSAGE.
Acute type A aortic dissection with mesenteric malperfusion syndrome should be treated with endovascular revascularization (fenestration/stenting) and delayed open aortic repair to improve outcomes.
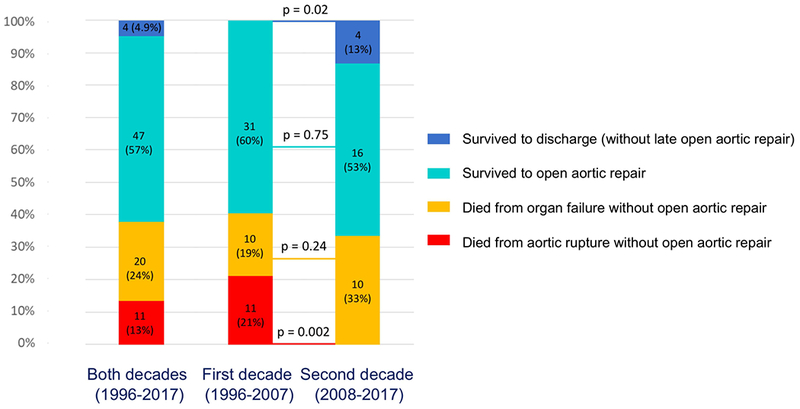
Short-term outcomes of patients with MesMPS after percutaneous endovascular reperfusion.
PERSPECTIVE STATEMENT.
Upfront endovascular fenestration/stenting in acute type A aortic dissection with mesenteric malperfusion syndrome can resolve visceral malperfusion quickly and adequately, and improve the chance of recovery from critical organ failure and survival with subsequent open aortic repair. This approach should be considered before open aortic repair in patients with mesenteric malperfusion syndrome.
AKNOWLEDGEMENT
The authors would like to thank the support from the Data Warehouse in the Department of Cardiac Surgery led by Dr. Donald Likosky, including Jeremy Wolverton, Amy Geltz, Mary Barry, Mary Ryzak, Brett Cross, and other team members as well as the support from the Department of Cardiac Surgery and the Frankel Cardiovascular Center at the University of Michigan.
Funding source: Dr. Yang is supported by the NHLBI of NIH K08HL130614, R01HL141891, Phil Jenkins and Darlene & Stephen J. Szatmari Funds. Dr. Patel is supported by the Joe D. Morris Collegiate Professorship, the David Hamilton Fund, and the Phil Jenkins Breakthrough Fund in Cardiac Surgery. Dr. Deeb is supported by the Herbert Sloan Collegiate Professorship, Jamie Buhr Fund, and Richard Nerod Fund.
GLOSSARY OF ABBREVIATIONS
- ACP
antegrade cerebral perfusion
- AKI
acute kidney injury
- ATAAD
acute type A aortic dissection
- CABG
coronary artery bypass graft
- CAD
coronary artery disease
- CPB
cardiopulmonary bypass
- CVA
cerebrovascular accident
- GFR
glomerular filtration rate
- HCA
hypothermic circulatory arrest
- IR
interventional radiology
- MesMPS
mesenteric malperfusion syndrome
- MI
myocardial infarction
- MPS
malperfusion syndrome
- non-MPS
no malperfusion syndrome
- OR
odds ratio
- PVOD
peripheral vascular occlusive disease
- RCP
retrograde cerebral perfusion
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: Dr. David Williams is on the Medical Advisory Board of Boston Scientific and Drs. David Williams and Himanshu Patel are consultants with Gore on an unrelated device. No conflict of interests related to this study.
Date and number of IRB approval: 9/26/2016 and HUM00119716
REFERENCES
- 1.Di Eusanio M, Trimarchi S, Patel HJ, Hutchison S, Suzuki T, Peterson MD, et al. Clinical presentation, management, and short-term outcome of patients with type A acute dissection complicated by mesenteric malperfusion: observations from the International Registry of Acute Aortic Dissection. J Thorac Cardiovasc Surg 2013;145:385–390.e1. [DOI] [PubMed] [Google Scholar]
- 2.Girardi LN, Krieger KH, Lee LY, Mack CA, Tortolani AJ, Isom OW. Management strategies for type A dissection complicated by peripheral vascular malperfusion. Ann Thorac Surg 2004;77:1309–14; discussion 1314. [DOI] [PubMed] [Google Scholar]
- 3.Yagdi T, Atay Y, Engin C, Mahmudov R, Tetik O, Iyem H, et al. Impact of organ malperfusion on mortality and morbidity in acute type A aortic dissections. J Card Surg 2006;21:363–9. [DOI] [PubMed] [Google Scholar]
- 4.Geirsson A, Szeto WY, Pochettino A, McGarvey ML, Keane MG, Woo YJ, et al. Significance of malperfusion syndromes prior to contemporary surgical repair for acute type A dissection: outcomes and need for additional revascularizations. Eur J Cardiothorac Surg 2007;32:255–62. [DOI] [PubMed] [Google Scholar]
- 5.Santini F, Montalbano G, Casali G, Messina A, Iafrancesco M, Luciani GB, et al. Clinical presentation is the main predictor of in-hospital death for patients with acute type A aortic dissection admitted for surgical treatment: a 25 years experience. Int J Cardiol 2007;115:305–11. [DOI] [PubMed] [Google Scholar]
- 6.Augoustides JG, Geirsson A, Szeto WY, Walsh EK, Cornelius B, Pochettino A, et al. Observational study of mortality risk stratification by ischemic presentation in patients with acute type A aortic dissection: the Penn classification. Nat Clin Pract Cardiovasc Med 2009;6:140–6. [DOI] [PubMed] [Google Scholar]
- 7.Girdauskas E, Kuntze T, Borger MA, Falk V, Mohr FW. Surgical risk of preoperative malperfusion in acute type A aortic dissection. J Thorac Cardiovasc Surg 2009;138:1363–9. [DOI] [PubMed] [Google Scholar]
- 8.Pacini D, Leone A, Belotti LM, Fortuna D, Gabbieri D, Zussa C, et al. Acute type A aortic dissection: significance of multiorgan malperfusion. Eur J Cardiothorac Surg 2013;43:820–6. [DOI] [PubMed] [Google Scholar]
- 9.Hofferberth SC, Newcomb AE, Yii MY, Yap KK, Boston RC, Nixon IK, et al. Hybrid proximal surgery plus adjunctive retrograde endovascular repair in acute DeBakey type I dissection: superior outcomes to conventional surgical repair. J Thorac Cardiovasc Surg 2013;145:349–54; discussion 354-5. [DOI] [PubMed] [Google Scholar]
- 10.Tsagakis K, Konorza T, Dohle DS, Kottenberg E, Buck T, Thielmann M, et al. Hybrid operating room concept for combined diagnostics, intervention and surgery in acute type A dissection. Eur J Cardiothorac Surg 2013;43:397–404. [DOI] [PubMed] [Google Scholar]
- 11.Leontyev S, Legare JF, Borger MA, Buth KJ, Funkat AK, Gerhard J, et al. Creation of a scorecard to predict in-hospital death in patients undergoing operations for acute type A aortic dissection. Ann Thorac Surg 2016;101:1700–6. [DOI] [PubMed] [Google Scholar]
- 12.Grimm JC, Magruder JT, Crawford TC, Sciortino CM, Zehr KJ, Mandal K, et al. Differential outcomes of type A dissection with malperfusion according to affected organ system. Ann Cardiothorac Surg 2016;5:202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deeb GM, Williams DM, Bolling SF, Quint LE, Monaghan H, Sievers J, et al. Surgical delay for acute type A dissection with malperfusion. Ann Thorac Surg 1997;64:1669–75; discussion 1675-7. [DOI] [PubMed] [Google Scholar]
- 14.Williams DM, Lee DY, Hamilton BH, Marx MV, Narasimham DL, Kazanjian SN, et al. The dissected aorta: percutaneous treatment of ischemic complications--principles and results. J Vasc Interv Radiol 1997;8:605–25. [DOI] [PubMed] [Google Scholar]
- 15.Williams DM, Lee DY, Hamilton BH, Marx MV, Narasimham DL, Kazanjian SN, et al. The dissected aorta: part III. Anatomy and radiologic diagnosis of branch-vessel compromise. Radiology. 1997;203:37–44. [DOI] [PubMed] [Google Scholar]
- 16.Patel HJ, Williams DM, Dasika NL, Suzuki Y, Deeb GM. Operative delay for peripheral malperfusion syndrome in acute type A aortic dissection: a long-term analysis. J Thorac Cardiovasc Surg 2008;135:1288–95; discussion 1295-6. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention; National Center for Health Statistics. National Death Index. Available at: https://www.cdc.gov/nchs/ndi/index.htm Accessed December 27, 2017.
- 18.McMurry TL, Hu Y, Blackstone EH, Kozower BD. Propensity scores: Methods, considerations, and applications in the Journal of Thoracic and Cardiovascular Surgery. J Thorac Cardiovasc Surg 2015;150:14–9. [DOI] [PubMed] [Google Scholar]
- 19.Winger DG, Nason KS. Propensity-score analysis in thoracic surgery: When, why, and an introduction to how. J Thorac Cardiovasc Surg 2016;151:1484–7. [DOI] [PubMed] [Google Scholar]
- 20.Lee J, Little TD. A practical guide to propensity score analysis for applied clinical research. Behav Res Ther 2017;98:76–90. [DOI] [PubMed] [Google Scholar]
- 21.Chiu P, Tsou S, Goldstone AB, Louie M, Woo YJ, Fischbein MP. Immediate operation for acute type A aortic dissection complicated by visceral or peripheral malperfusion, J Thorac Cardiovasc Surg 2018. doi: 10.1016/j.jtcvs.2018.01.096. [DOI] [PubMed] [Google Scholar]
- 22.Midulla M, Renaud A, Martinelli T, Koussa M, Mounier-Vehier C, Prat A, et al. Endovascular fenestration in aortic dissection with acute malperfusion syndrome: immediate and late follow-up. J Thorac Cardiovasc Surg 2011;142:66–72. [DOI] [PubMed] [Google Scholar]
- 23.Yamashiro S, Arakaki R, Kise Y, Inafuku H, Kuniyoshi Y. Management of visceral malperfusion complicated with acute type A aortic dissection. Interact Cardiovasc Thorac Surg 2015;21:346–51. [DOI] [PubMed] [Google Scholar]
- 24.Uchida K, Karube N, Yasuda S, Miyamoto T, Matsuki Y, Isoda S, et al. Pathophysiology and surgical treatment of type A acute aortic dissection. Ann Vasc Dis 2016;9:160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bossone E, Corteville DC, Harris KM, Suzuki T, Fattori R, Hutchison S, et al. Stroke and outcomes in patients with acute type A aortic dissection. Circulation. 2013;128:S175–9. [DOI] [PubMed] [Google Scholar]
- 26.Morimoto N, Okada K, Okita Y. Lack of neurologic improvement after aortic repair for acute type A aortic dissection complicated by cerebral malperfusion: predictors and association with survival. J Thorac Cardiovasc Surg 2011;142:1540–4. [DOI] [PubMed] [Google Scholar]
- 27.Badreldin AM, Doerr F, Elsobky S, Brehm BR, Abul-dahab M, Lehmann T, et al. Mortality prediction after cardiac surgery: blood lactate is indispensible. Thorac Cardiovasc Surg 2013;61:708–17 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Discussion of the management of ATAAD patients with mesenteric malperfusion syndrome (MesMPS), including indications for upfront endovascular revascularization, detailed technique of fenestration and stenting of the dissected aorta and its branches, timing for delayed open aortic repair, and short- and long-term outcomes.
Supplemental Figure S1: CONSORT flow diagram of patients’ selection.


