Abstract
Introduction
Data from a randomized trial suggest transfusion rates are similar for robotic and open prostatectomy. The objective of this study was to compare perioperative outcomes of robotic and open prostatectomy at a Canadian academic centre.
Methods
A retrospective review of all prostatectomies performed by all surgeons at The Ottawa Hospital between 2009 and 2016 was completed. Cases and outcomes were identified using an administrative data warehouse. Extracted data included patient factors (age, body mass index, American Society of Anesthesiologists score, Elixhauser comorbidity score), operative factors (length of operation, surgical approach, anesthesia type), and perioperative outcomes (length of recovery room and hospital stay, transfusion rate, hospital cost). Baseline characteristics and outcomes were compared between robotic and open surgical approaches. The primary outcome was transfusion during the index admission.
Results
A total of 1606 prostatectomies were performed by 12 surgeons during the study period (840 robotic, 766 open). The rate of transfusion was lower in patients undergoing robotic compared to open surgery (0.6% vs. 11.2%; p<0.001). The robotic prostatectomy cohort had a shorter length of stay in the recovery room (155.7 vs. 231.1 minutes; p<0.001) and shorter length of hospital admission (1.4 vs. 2.8 days; p<0.001). Hospital costs per case were approximately $800 more for robotic prostatectomy ($11 475 vs. $10 656; p<0.001).
Conclusions
This hospital-wide analysis revealed that robotic prostatectomy is associated with a lower transfusion rate compared to the open approach. Further studies emphasizing patient-reported outcomes are needed.
Introduction
Robotic prostatectomy is the most common surgical approach for prostate cancer in many countries and is being used increasingly in Canada.1–3 The use of robotic surgery has increased in Ontario since 2004 and currently, at least 11 hospitals in the province have one or more robotic surgical systems.1,4 Despite an increase in the proportion of prostatectomies being performed robotically, the majority of cases in Canada are performed open and there remains debate regarding the true benefits of the robotic approach for patients and the healthcare system.2 Concern regarding the cost to purchase and maintain robotic surgical systems is a barrier to the use of robotic surgery in Canada.2
Health Quality Ontario (HQO) recently assessed the effectiveness, safety, and cost-benefit ratio of robotic prostatectomy. 2 Based on the findings of this report, the Ontario Health Technology Advisory Committee recommended against publicly funding robotic prostatectomy due to a lack of clinical benefits and increased costs.2 The majority of studies included in the HQO report were from the U.S., Australia, and Europe.2 Some Canadian studies were included but given little weight in decision-making due to study limitations.2,4–8 A plethora of Canadian administrative data is available for patients receiving prostatectomy, however, these data were not incorporated in the HQO analyses as they are not published.2
Given the lack of real-world Canadian data on this topic, we sought to review all cases of open and robotic prostatectomies at our institution. Contrary to many studies that include a few high-volume robotic or open surgeons, our institution adopted a broader inclusion program, including many surgeons with various training backgrounds, case volumes, and at different locations on the learning curve. Outcomes may, therefore, be more generalizable to a typical patient experience. We hypothesized that patients receiving robotic prostatectomy would receive fewer blood transfusions and would have shorter hospital admissions compared to patients undergoing open prostatectomy. Our primary outcome was the rate of transfusion during the index admission.
Methods
The Ottawa Health Science Network Research Ethics Board approved this historical cohort study examining all patients who underwent radical prostatectomy at The Ottawa Hospital between January 1, 2009 and December 31, 2016. Eligible cases were identified through The Ottawa Hospital Data Warehouse (OHDW). The OHDW is an administrative data repository of clinical, laboratory, and health services information collected through the hospital’s major operational information systems. This includes data on patient demographics, patient comorbidities, admissions, discharges, laboratory results, imaging results, operative procedures, medications, and hospital costs.9,10 Hospital cost data in the OHDW do not include the cost of blood transfusions, as blood products are obtained from Canada Blood Services in a different funding model. Transfusion cost was determined by calculating the number of units of blood each patient received multiplied by the estimated cost of a unit of blood.11,12 The total societal cost of transfusing a single unit of packed red blood cells in hospital ranges from $600–1200 based on recently published cost analyses.11,12 We used $900 as the estimated cost of transfusing a unit of blood in this study.
Pure laparoscopic prostatectomies were excluded. Robotic cases that were converted to open were included in the robotic cohort to maintain an intention-to-treat analysis. Cases were excluded if radical prostatectomy was not the primary procedure code and prostate cancer was not the primary diagnostic code.
Extracted data included patient and procedure characteristics, as well as perioperative outcomes and hospital costs. Patient characteristics included age, body mass index (BMI, weight in kg/metre2) categorized as <25, 25–30, 30–35, and >35 kg/m2, Elixhauser comorbidity score, and American Society of Anesthesiologists (ASA) score (I–IV). Operative information included the year of surgery, surgical approach (open vs. robotic), anesthetic technique used (spinal or epidural with general vs. general alone), and length of the operation (in minutes). Perioperative outcomes included length of stay in the recovery room in minutes, length of stay in the hospital in days, any transfusion during the index admission, number of units of blood received during the index admission, maximum pain score in the recovery room (scale of 1–10), total hospital cost (Canadian dollars), return to the emergency room (ER) within 30 days of surgery, and readmission to hospital within 30 days of surgery. Year of surgery was grouped into three time periods to assess for temporal trends: pre-robotic (2009–2010), robotic introduction (2011–2013), and contemporary (2014–2016).
Descriptive analyses compared baseline characteristics between the robotic and open cohorts. Postoperative outcomes, including length of hospital stay, hospital costs, and readmission/return to the ER, were further stratified by transfusion status. Reported outcomes were grouped for all surgeons. Sensitivity analyses were performed examining outcomes for individual surgeons who performed at least five cases using either surgical approach.
Univariable and multivariable analyses were performed using log binomial regression to determine associations between patient and procedural characteristics with postoperative outcomes. The primary outcome was red blood cell transfusion during the index admission (yes vs. no). Transfusion was selected as the primary outcome because transfusions are important to patients, are expensive, and may be associated with short- and long-term health risks. Furthermore, the difference in transfusion rates between robotic and open approaches was called into question in a recent two-surgeon randomized trial and in the subsequent HQO report comparing these approaches.2,13 Sensitivity analyses were performed to assess for differences in transfusion rate by time period of surgery (pre-robotic, robotic introduction, contemporary). A second sensitivity analysis was performed to examine between surgeon variability using a random effect model. Secondary outcomes included number of red blood cell transfusions, total length of hospital admission, length of stay in the recovery room, operative time, and total hospital cost. For all analyses, no adjustment was made for multiple testing, and a p≤0.05 was considered statistically significant. SAS software version 9.4 for Windows was used for analyses (Cary, NC, U.S.).
Results
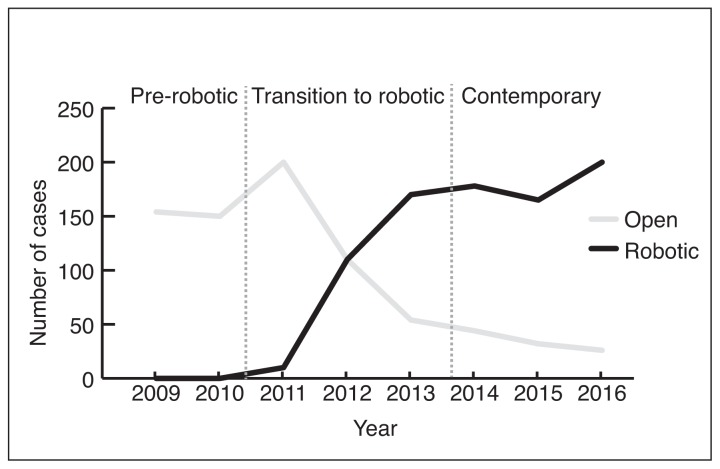
During the study period, 1606 prostatectomies were performed by 12 surgeons, including 766 (48%) open and 840 (52%) robotic cases. The first robotic prostatectomy performed at The Ottawa Hospital was on October 31, 2011. All prostatectomies performed during the pre-robotic time period (2009–2010) were done with an open approach (302/302, 100%). During the robotic introduction time period (2011–2013), 55% of prostatectomies were performed open (360/656) and 45% were robotic (296/656). The majority of prostatectomies in the contemporary time period (2014–2016) were done with a robotic approach (544/646, 84%) (Fig. 1).
Fig. 1.
Number of open and robotic prostatectomies performed at The Ottawa Hospital from 2009–2016 by year.
Demographic and procedure information for patients receiving open and robotic prostatectomy are presented in Table 1. Patient age and BMI were similar. There were more ASA III patients in the open group (p<0.001), however, Elixhauser comorbidity scores were similar between groups (p=0.7). Regional anesthesia was added to general anesthesia in many cases for open prostatectomy but no robotic cases (p<0.001).
Table 1.
Patient and procedure characteristics of open and robotic prostatectomies performed at The Ottawa Hospital from 2009–2016
| Patient and procedure characteristic | Open prostatectomy n=766 | Robotic prostatectomy n=840 | p |
|---|---|---|---|
| Patient characteristics | |||
| Median age at surgery, years (IQR) | 63 (58–67) | 63 (58–67) | 0.7 |
| Average BMI ± SD (kg/m2) | 28.5±4.2 | 28.4±4.1 | 0.7 |
| BMI (kg/m2) | |||
| <25 | 117 (20.0%) | 153 (18.8%) | 0.4 |
| 25–30 | 285 (48.5%) | 418 (51.4%) | |
| 30–35 | 151 (25.7%) | 186 (22.9%) | |
| >35 | 35 (6.0%) | 57 (7.0%) | |
| ASA score | |||
| I | 25 (3.3%) | 31 (3.7%) | <0.001 |
| II | 370 (48.3%) | 503 (60.0%) | |
| III | 365 (47.7%) | 303 (36.1%) | |
| IV | 6 (0.8%) | 3 (0.4%) | |
| Average Elixhauser comorbidity score | 4.4±2.1 | 4.4±2.1 | 0.7 |
| Procedure characteristics | |||
| Anesthetic technique | |||
| General + regional | 462 (60.3%) | 0 (0%) | <0.001 |
| General alone | 304 (39.7%) | 840 (100%) | |
| Time period of surgery | |||
| Pre-robotic (2009–2010) | 302 (100%) | 0 (0%) | <0.001 |
| Robotic introduction (2011–2013) | 360 (55%) | 296 (45%) | |
| Contemporary (2014–2016) | 102 (16%) | 544 (84%) | |
ASA: American Society of Anesthesiologists; BMI: body mass index; IQR: interquartile range; SD: standard deviation.
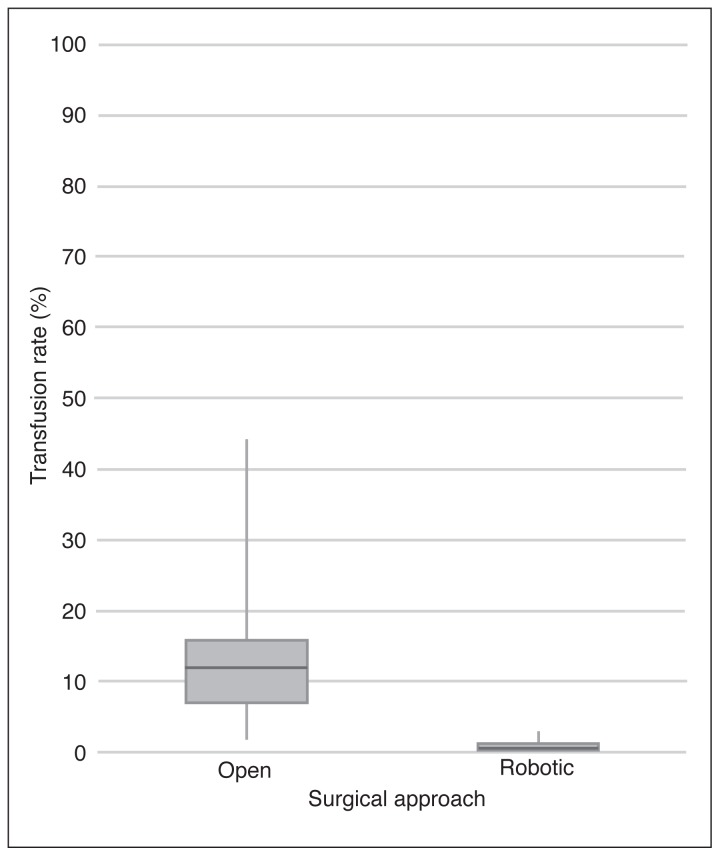
The transfusion rate was significantly lower in the robotic group at 0.6% (5/840) compared to 11.2% (86/766) in the open group (p<0.001). Patients who received a transfusion had longer hospital stays (3.8 vs. 2.0; p<0.001) and were more likely to return to the ER within 30 days of their operation (27% vs. 17%; p=0.02). For patients who received a blood transfusion, the average number of units of blood received was three. The maximum number of units of blood received by a single patient was 12, and 26% (24/91) of patients who received a transfusion received ≥3 units. Sensitivity analyses of individual surgeons who performed both approaches during the study period indicated all had a decrease in the rate of transfusion when they changed to the robotic approach. The variability in transfusion rates between surgeons was also decreased with a robotic surgical approach to prostatectomy (Fig. 2).
Fig. 2.
Box plot of surgeons’ transfusion rates grouped by surgical approach for prostatectomies performed at The Ottawa Hospital from 2009–2016.
Length of stay in the recovery room (231.1 minutes for open and 155.7 minutes for robotic; p<0.001) and overall length of stay in hospital (2.8 days for open and 1.4 days for robotic; p<0.001) were less for robotic prostatectomy (Table 2). The risk of return to the ER (17.0% open vs; 18.1% robotic) and readmission to hospital (3.4% open vs. 2.9% robotic) within 30 days of surgery were similar between groups (p=0.5 and p=0.6, respectively). Mean operative time was eight minutes longer for the robotic cohort (275.9 minutes vs. 267.8 minutes; p=0.01).
Table 2.
Operative outcomes of open and robotic prostatectomies performed at The Ottawa Hospital from 2009–2016
| Operative outcome | Open prostatectomy n=766 | Robotic prostatectomy n=840 | p |
|---|---|---|---|
| Procedure length, mean ± SD (min) | 267.8±57.3 | 275. 9±68.6 | 0.01 |
| Any transfusion during admission | 86 (11.2%) | 5 (0.6%) | <0.001 |
| Number of units of red blood cells transfused | |||
| 1 | 20 (2.6%) | 1 (0.1%) | <0.001 |
| 2 | 44 (5.7%) | 2 (0.2%) | |
| 3 | 7 (0.9%) | 0 | |
| 4 | 6 (0.8%) | 1 (0.1%) | |
| ≥5 | 9 (1.2%) | 1 (0.1%) | |
| Recovery room length of stay, mean ± SD (min) | 231.1±228.5 | 155.7±236.6 | <0.001 |
| Maximum pain score, mean ± SD (scale 1–10) | 3.5±2.3 | 3.7±2.2 | 0.05 |
| Length of stay in hospital, mean ± SD (days) | 2.8±1.2 | 1.4±1.1 | <0.001 |
| Total hospital cost, mean ± SD (CAD) | $10 656±$2601 | $11 475±$2904 | <0.001 |
| Readmission to hospital within 30 days | 26 (3.4%) | 24 (2.9%) | 0.5 |
| Return to ER within 30 days | 130 (17.0%) | 152 (18.1%) | 0.6 |
ER: emergency room; LOS: length of stay; SD: standard deviation.
The overall hospital cost per procedure was $10 656 (standard deviation [SD] $2601) for open prostatectomy and $11 475 (SD $2904) for robotic prostatectomy (p<0.001). The average total hospital cost for prostatectomy patients who received a transfusion was $14 289 (SD $3954) compared to $10 892 (SD $2585) for patients that did not receive a transfusion (p<0.001).
Univariable analyses identified an increased risk of transfusion with increased patient age, open surgical approach, ASA class IV vs. I, and time period of surgery. In multivariable analyses, increased patient age (relative risk [RR] 1.1; 95% confidence interval [CI] 1.0–1.1; p=0.01), ASA class IV vs. I (RR 8.3; 95% CI 1.0–69.7; p=0.05), and open surgical approach (RR 17.7; 95% CI 7.3–43.2; p<0.001) were associated with transfusion (Table 3).
Table 3.
Multivariable analyses results for transfusion during index admission for open and robotic prostatectomies at The Ottawa Hospital from 2009–2016
| Variable | Comparison | RR (95% CI) | p |
|---|---|---|---|
| Multivariate analysis | |||
| Age | Increase by 1 year | 1.1 (1.0–1.1) | 0.01 |
| BMI (kg/m2) | 25–30 vs. <25 | 0.7 (0.4–1.1) | 0.1 |
| 30–35 vs. <25 | 0.8 (0.5–1.2) | 0.3 | |
| >35 vs. <25 | 0.7 (0.3–1.7) | 0.4 | |
| ASA score | II vs. I | 1.3 (0.2–8.0) | 0.8 |
| III vs. I | 2.2 (0.4–14.1) | 0.4 | |
| IV vs. I | 8.3 (1.0–69.7) | 0.05 | |
| Surgical approach | Open vs. robotic | 17.7 (7.3–43.2) | <0.001 |
| Time period of surgery | Pre-robotic (2009–2010) vs. contemporary (2014–2016) | 1.5 (0.8–3.0) | 0.3 |
| Robotic introduction (2011–2013) vs. contemporary (2014–2016) | 1.2 (0.7–2.1) | 0.6 | |
ASA: American Society of Anesthesiologists; BMI: body mass index; CI: confidence interval; RR: relative risk.
Discussion
This study evaluated short-term outcomes of all patients undergoing open and robotic prostatectomies at a single, large, academic centre in Canada. There were significant differences in transfusion rates (0.6% vs. 11.2%; p <0.001) and overall length of hospital admission (1.4 days vs. 2.8 days; p<0.001) in favour of robotic prostatectomy. These findings are consistent with previous literature, including a recent Cochrane review comparing robotic and open prostatectomy.14,15 Our transfusion rates post-prostatectomy are also consistent with those reported the National Surgical Quality Improvement Program (NSQIP), with over 700 contributing institutions internationally.16 NSQIP transfusion rates are 11.9% following open prostatectomy and 1.5% following minimally invasive prostatectomy (includes laparoscopic and robotic).16
The HQO’s technology assessment of robotic prostatectomy, publicly available in July 2017, synthesized existing literature and performed analyses of relevant outcomes using available evidence.2 The report was reviewed by the Ontario Health Technology Advisory Committee, who recommended against publicly funding robotic prostatectomies in Ontario because they concluded the clinical benefits were negligible and the costs were increased.17 These recommendations caused consternation for many health professionals, policy makers, and patients, many of whom believe robotic prostatectomy offers significant advantages compared to open surgery.
The HQO report was comprehensive and included many sensitivity analyses to test the robustness of results. However, we believe the data included in the analyses, as well as the interpretation of the included evidence could be improved. Many of the conclusions appear to heavily weigh evidence from a single randomized controlled trial (RCT) comparing robotic and open prostatectomy based out of Australia.13 This trial compared short-term outcomes of a single, very experienced open surgeon who had performed 1500 open prostatectomies to those of a single robotic surgeon who had performed 200 robotic prostatectomies and found that early oncological and functional outcomes were similar in the two groups.13 They reported increased complications and adverse events in the open group; however, these differences were not statistically significant. The risk of transfusion was 4% for open patients and 1% for robotic patients (p=0.12). In addition, the open surgeon used a Cell Saver device to reduce transfusions, a technique that is not used in urological cancer surgery at many centres in Canada, including ours. While these results appear to have been interpreted by the Ontario Health Technology Assessment Committee to demonstrate similar transfusion outcomes for the two approaches, this conclusion ignores the dramatic difference in surgeon experience in the two study arms, which makes the generalizability of the trial’s results limited. There is a proven learning curve to prostatectomy with all approaches and previous reports have shown improvement in outcomes even after 1000 cases performed.18,19 We interpret the Australian trial results as proof that a relatively new surgeon using robotic technology can achieve similar or slightly better outcomes than a very experienced open surgeon. Indeed, in the Ottawa cohort, 12 surgeons with various experience and training performed prostatectomy during the study period and all perioperative outcomes were improved for patients receiving a robotic approach. Furthermore, this study includes data during the time period when the robot was introduced in Ottawa. During this time, experienced open surgeons transitioned to robotics and all surgeons performed their first independent robotic case. Despite including these multiple learning curves, outcomes improved. These data are likely more generalizable to the experience most patients would have.
The main argument against robotic prostatectomy in Canada is cost. This study found the difference in average total hospital costs per case was approximately $800 in favour of open surgery. This does not include the one-time capital costs of purchasing the robotic system or annual maintenance of the robotic system; however, these costs are incurred if a hospital performs any surgical procedures robotically. As surgeons become more experienced with the robotic approach, surgical times and operating room costs are expected to decrease. Additionally, as other robotic surgical systems enter the consumer market, competition among suppliers will provide incentive to lower costs of robotic systems and maintenance. Previous cost-benefit analyses comparing approaches have been limited by a lack of Canadian data and data on long-term outcomes and cost forecasting.2,20 More recently, a Health Technology Assessment was released by Alberta Health, which found lower costs per quality-adjusted life-year gained compared to previous reports for the robotic approach.2,20
The significant decrease in transfusions with robotic surgery is important for patients and surgeons. While open prostatectomy remains a good surgical approach for many patients, especially those who are treated by a surgeon with sufficient case volumes and experience, this study shows benefits in the perioperative course for patients undergoing robotic surgery. We found that open surgical approach increases the risk of transfusion 18-fold, and was by far the greatest risk factor for blood transfusion after adjusting for possible confounders (RR 17.7; 95% CI 7.3–43.2). Furthermore, many patients received multiple units of blood and 26% of patients who required a blood transfusion required at least three units of blood. Limiting blood loss has a number of important benefits for surgeons and patients. Intra-operative blood loss may impair visibility and make dissection more challenging. Previous studies have also found an association between transfusions at surgery and worse oncological outcomes.21
Efficiency is also an important component of surgery in the operating room and during the perioperative course. Our study shows that robotic prostatectomy resulted in a significantly more efficient perioperative course for patients and the hospital. Robotic cases spent less time in the recovery room after surgery (155.7 vs. 231.1 minutes; p <0.001) and had similar maximum pain scores despite 60% of open cases receiving spinal anesthetics that block pain in the operating field. This allows for better workflow through the surgical suites and recovery room, freeing up essential resources for other patients. Perhaps more importantly, the average length of hospital admission was half as long following robotic prostatectomy compared to the open approach (1.4 vs. 2.8 days; p<0.001). With hospitals across the province constantly reporting supra-capacity number of patient admissions and increasing ER wait times, the ability to safely discharge patients in a more efficient manner has positive downstream effects for the entire healthcare system. While the cost of extra days in hospital is easy to quantify, the benefits of freeing hospital beds and potentially reducing ER wait times and “hallway medicine” are harder to capture in studies focused on prostatectomy.
This study has several strengths, most notably, the results represent a real-world Canadian experience, including a large number of surgeons with various training backgrounds and experience during a long study period, making the results highly generalizable. Most previous studies report outcomes of a few high-volume surgeons. We did not compare outcomes based on surgeons’ experience (number of cases), as there were very few surgeons in our cohort who had performed >200 cases for either approach to prostatectomy, and the objective of this study was to report the mean outcomes a patient can expect to experience as opposed to the outcomes of a few high-volume surgeons. Second, all patients treated during the learning curve of robotics were included, possibly biasing the results against the robotic approach. Limitations of this study include a non-randomized design, which could theoretically cause an imbalance in patient or tumour characteristics between groups. There were more ASA III patients in the open group; however, we attribute this to increased use of active surveillance in more recent years when most patients received robotic surgery. Furthermore, the Elixhauser comorbidity scores were similar in the two groups, therefore, it is likely any difference in patient risk factors for transfusion were minor. Despite a thorough means of capturing patient and operational data, administrative data is not primarily captured for research and data may be missed or incorrectly classified, which could alter the results. We did audit important variables, including surgical approach and transfusion outcomes, to confirm validity. Finally, we did not examine oncological factors or functional outcomes, however, these have previously been shown to be equivalent or superior using the robotic approach in many studies, and a previous report from our centre showed similar results during the introduction of robotic surgery in Ottawa.22 Inclusion of the societal costs associated with earlier return to work with the robotic approach may have further decreased the difference in cost between open and robotic prostatectomy, but was beyond the scope of this study.23,24
Conclusion
This study shows that robotic prostatectomy reduces transfusions and length of hospital stay. More Canadian data that includes long-term and patient-reported outcomes are needed to help policy makers evaluate the true benefits of robotic surgery for prostate cancer in Canada.
Footnotes
See related commentary on page 190
Competing interests: Dr. Cagiannos has been an advisory board member for Abbvie and Ferring; and has received speaker honoraria from Abbvie, Acerus, and Ferring. Dr. Morash has been an advisory board member for Abbvie, Astellas, Ferring, Janssen, and Sanofi; and participated in the CRONOS II clinical trial supported by Abbvie. Dr. Lavallée has been an advisory board member for Ferring and Sanofi; and has received a grant from Sanofi. The remaining authors report no competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.Kapoor A. The robotic invasion of Canada. Can Urol Assoc J. 2014;8:E466–7. doi: 10.5489/cuaj.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Health Quality Ontario. Robotic surgical system for radical prostatectomy: A health technology assessment. Ont Heal Technol Assess Ser. 2017;17:1–172. [PMC free article] [PubMed] [Google Scholar]
- 3.NHS England. Clinical Commissioning Policy: Robotic-assisted surgical procedures for prostate cancer. 2015. [Accessed April 22, 2019]. pp. 1–14. Available at: http://www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/2015/10/b14pa-rbtic-asstd-srgry-prostate-cancer-oct15.pdf.
- 4.Chin JL, Luke PP, Pautler SE. Initial experience with robotic-assisted laparoscopic radical prostatectomy in the Canadian healthcare system. Can Urol Assoc J. 2007;1:97–101. doi: 10.5489/cuaj.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuller A, Pautler SE. Complications following robot-assisted radical prostatectomy in a prospective Canadian cohort of 305 consecutive cases. Can Urol Assoc J. 2012;7:1–6. doi: 10.5489/cuaj.11116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gagnon LO, Goldenberg SL, Lynch K, et al. Comparison of open and robotic-assisted prostatectomy: The University of British Columbia experience. Can Urol Assoc J. 2014;8:92–7. doi: 10.5489/cuaj.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tholomier C, Bienz M, Hueber PA, et al. Oncological and functional outcomes of 722 robot-assisted radical prostatectomy (RARP) cases: The largest Canadian 5-year experience. Can Urol Assoc J. 2014;8:195–201. doi: 10.5489/cuaj.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rush S, Alibhai SMH, Xu L, et al. Health-related quality of life in robotic vs. open radical prostatectomy. Can Urol Assoc J. 2015;9:179–87. doi: 10.5489/cuaj.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Walraven C, Jennings A, Wong J, et al. Influence of house-staff experience on teaching-hospital mortality: The “July Phenomenon” revisited. J Hosp Med. 2011;6:389–94. doi: 10.1002/jhm.917. [DOI] [PubMed] [Google Scholar]
- 10.Forster AJ, Taljaard M, Oake N, et al. The effect of hospital-acquired infection with Clostridium difficile on length of stay in hospital. CMAJ. 2012;184:37–42. doi: 10.1503/cmaj.110543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shander A, Hofmann A, Ozawa S, et al. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50:753–65. doi: 10.1111/j.1537-2995.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- 12.Lagerquist O, Poseluzny D, Werstiuk G, et al. The cost of transfusing a unit of red blood cells: A costing model for Canadian hospital use. ISBT Sci Ser. 2017;12:375–80. doi: 10.1111/voxs.12355. [DOI] [Google Scholar]
- 13.Yaxley JW, Coughlin GD, Chambers SK, et al. Robot-assisted laparoscopic prostatectomy vs. open radical retropubic prostatectomy: Early outcomes from a randomized controlled phase 3 study. Lancet. 2016;388:1057–66. doi: 10.1016/S0140-6736(16)30592-X. [DOI] [PubMed] [Google Scholar]
- 14.Ilic D, Sm E, Ca A, et al. Laparoscopic and robotic-assisted vs. open radical prostatectomy for the treatment of localized prostate cancer. Cochrane Database Syst Rev. 2017. p. CD009625. www.cochranelibrary.com. [DOI] [PMC free article] [PubMed]
- 15.De Carlo F, Celestino F, Verri C, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: Surgical, oncological, and functional outcomes: A systematic review. Urol Int. 2014;93:373–83. doi: 10.1159/000366008. [DOI] [PubMed] [Google Scholar]
- 16.National Surgical Quality Improvement Program. American College of Surgeons; 2014. [Accessed May 6, 2016]. Available at: www.acsnsqip.org. [Google Scholar]
- 17.Ontario Health Technology Advisory Committee. Robotic surgical system for radical prostatectomy: OHTAC Recommendation. 2017. pp. 1–4. [PMC free article] [PubMed] [Google Scholar]
- 18.Sooriakumaran P, John M, Wiklund P, et al. Learning curve for robotic assisted laparoscopic prostatectomy: A multi-institutional study of 3794 patients. Minerva Urol Nefrol. 2011;63:191–8. [PubMed] [Google Scholar]
- 19.Vickers A, Bianco F, Cronin A, et al. The learning curve for surgical margins after open radical prostatectomy: Implications for margin status as an oncological endpoint. J Urol. 2010;183:1360–5. doi: 10.1016/j.juro.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Health Technology & Policy Unit G of A. Alberta Heal Technol Decis Process. 2017. Robot-assisted laparoscopic prostatectomy; pp. 1–488. [Google Scholar]
- 21.Goubran HA, Elemary M, Radosevich M, et al. Impact of transfusion on cancer growth and outcome. Cancer Growth Metastasis. 2016;9:1–8. doi: 10.4137/CGM.S32797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montroy J, Elzayat E, Morash C, et al. Long-term patient outcomes from the first year of a robotic surgery program using multi-surgeon implementation. Can Urol Assoc J. 2017;12:38–43. doi: 10.5489/cuaj.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geraerts I, Van Poppel H, Devoogdt N, et al. Prospective evaluation of urinary incontinence, voiding symptoms, and quality of life after open and robot-assisted radical prostatectomy. BJU Int. 2013;112:936–43. doi: 10.1111/bju.12258. [DOI] [PubMed] [Google Scholar]
- 24.Plym A, Chiesa F, Voss M, et al. Work disability after robot-assisted or open radical prostatectomy: A nationwide, population-based study. Eur Urol. 2016;70:64–71. doi: 10.1016/j.eururo.2015.12.049. [DOI] [PubMed] [Google Scholar]