Introduction
Definitions/purpose
The term “neurogenic bladder” describes lower urinary tract dysfunction that has occurred likely as a result of a neurological injury or disease.1 The International Continence Society (ICS) defines “neurogenic lower urinary tract dysfunction” (NLUTD) as “lower urinary tract dysfunction due to disturbance of the neurological control mechanism.” This broad definition is used to describe a multitude of conditions of varying severity.
Common causes of NLUTD include: spinal cord injury (SCI), multiple sclerosis (MS), and myelomeningocele (MMC). Other causes of NLUTD include: Parkinson’s disease, cerebrovascular accidents, traumatic brain injury, brain or spinal cord tumour, cauda equina syndrome, transverse myelitis, multisystem atrophy, pelvic nerve injury, and diabetes.
It is well-described that neurological disorders can lead to urological complications, including: urinary incontinence, urinary tract infections (UTIs), urolithiasis, sepsis, ureteric obstruction, vesicoureteric reflux (VUR), and renal failure.2 Due to the potential morbidity and even mortality, initial investigation, ongoing management, and surveillance is warranted in this patient population. Despite the frequency and potential severity of NLUTD, there are few high-quality studies in the literature to guide urological practices.
Prior neurogenic guidelines vary in their clinical assessment, investigations used, and surveillance strategies.2–6 The primary reason is that there is limited evidence to support a common strategy. The purpose of this guideline is to help urologists to identify high-risk patients with NLUTD and to provide an approach to the management and surveillance of patients with NLUTD.
Classification
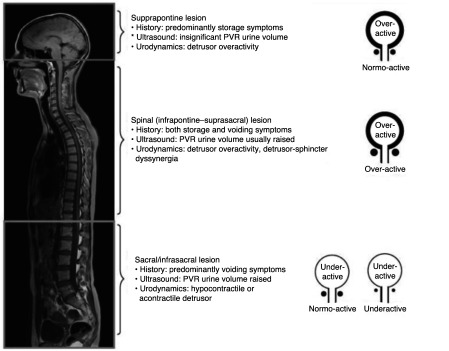
The etiology of a NLUTD is often classified based on whether the primary lesion is suprapontine, suprasacral, sacral, or infrasacral.7 A complementary system was developed by Madersbacher et al based on the function of the detrusor muscle and of the external sphincter.8 These systems allow a physician to have a general idea of how the lower urinary tract is likely to behave in SCI patients with more complete injuries (Fig. 1). Newer systems using magnetic resonance (MR) urography in combination with urodynamics (UDS) have also been proposed.9
Fig. 1.
Classification of lower urinary tract dysfunction based on level of lesion (adapted from Panicker et al7).
Methodology
This review was performed according to the methodology recommended by the Canadian Urologic Association.10 EmBASE and Medline databases were used to identify literature relevant to the early urological care of NLUTD patients. Given the limited literature in this area, no limits were placed on date or study design. Recommendations were developed by consensus and graded using a modified Oxford system, which identifies level of evidence (LOE) and grade of recommendation (GOR). This complete version includes the full text of the guidelines (including the sections in the Executive summary).
We limited our guideline recommendations to adults with NLUTD, although given the small body of literature for certain populations (such as spina bifida), relevant pediatric literature was considered if appropriate. Our initial in-person meeting involved input from various stakeholders, including SCI nurses, neurologists, physiatry, SCI consumers, and urologists. The guideline’s target users are physicians treating and following patients with NLUTD, and the guideline’s goal is to inform standards of clinical care. The guideline and all of the recommendations were reviewed by the authors of this document, and then by invited external reviewers. Comments were solicited from the CUA membership in general prior to finalizing the guideline document. Conflicts were resolved by consensus. The views or interests of the CUA did not influenced the final set of recommendations.
Canadian epidemiology of neurogenic bladder
There are 3.7 million Canadians living with a neurological condition;11,12 three common types of neurological conditions are MS, spina bifida (SB), and SCI. In Canada, there are 100 000 individuals living with a diagnosis of MS, making it the highest prevalence rate of MS in the world.13 There are also 35 000 Canadian’s living with SB, which is the leading cause of disabling birth defect within the country.11 12,14 In Canada, 86 000 people are living with SCI and 4300 new cases of SCI occur each year.15 These numbers are projected to increase to 121 000 individuals, with 5800 new cases a year by 2030.15 Trauma is the most frequent cause of SCI in Canada and most commonly affects men in the 20–29-year age group.15 Compared to international etiology, where the majority of SCI is the result of motor vehicle accidents, in Canada, traumatic spinal cord injury is most commonly caused by falls.15–17 Relevant to our aging population, research also demonstrates that a large proportion of traumatic SCI resulting from falls occurs in the senior population. 16,17 These traumatic SCI cases more frequently result in tetraplegia. Non-traumatic SCI result from disease processes such as MS, SB, tumours, and infections. Of Canadians living with both traumatic and non-traumatic SCI, 30 000 experience paraplegia and 13 000 experience tetraplegia.15,17 The incidence rate of non-traumatic SCI increases concurrently with age.17,18 NLUTD presents a common and important complication following neurological disease. In our aging Canadian population, the amount of traumatic and non-traumatic SCI is expected to increase, along with secondary health complications that accompany SCI, such as neurogenic bladder. Research from the U.S. reports frequency of neurogenic bladder to be 40–90% in MS, 40–61% in SB, and 70–84% in SCI.19 A recent Canadian study looking at the impact of bladder, bowel, and sexual dysfunction in 51 community-dwelling individuals with traumatic SCI reported that 59% of these individuals had bladder dysfunction.20
Neurological conditions often result in physical disability requiring the need for assistance with activities of daily living, including assistance with or requiring devices to manage NLUTD. Giesbrecht et al reported that of Canadians living at home with a physical disability, 51% of individuals using a mobility device required assistance with personal care needs and 36% of these individuals also required assistance with basic medical care.21 Additionally, it was noted that of individuals requiring assistance with care needs at home, those with physical disabilities received an average of 25 hours of care/week vs. 13 hours of care/week given to those without a physical disability.21 Furthermore, for those individuals with physical disability receiving 25 hours of care/week, this reportedly only partially met all the care needs they required.21
Neurogenic disease also places an increased demand for health professional services within our healthcare system and from this, a resulting increase in healthcare costs. For example, a hospital admission for SCI individuals experiencing even uncomplicated UTIs costs the Canadian healthcare system an average of $8000.22 Along with requiring more support with care needs at home, individuals with traumatic and non-traumatic SCI are 2.7 times more likely to contact a physician to address healthcare needs and also 2.6 times more likely to be hospitalized for health complications.15 One of the most common health complications causing specifically traumatic SCI individuals to require these additional healthcare services are UTIs,23 often experienced as a direct result of neurogenic bladder.
The diagnosis of NLUTD
To diagnose someone with NLUTD, a defined neurological condition, or a strong suspicion of an undiagnosed neurologial disease must be present. Potential symptoms that may be suggestive of an undiagnosed acquired neurological disease include those that precede a diagnosis of MS, cauda equina syndrome, and occult neural tube defect.7 In these situations, referral to a neurologist for an evaluation may be warranted.
History and physical exam
In the setting of a diagnosed or probable neurological disease, a careful evaluation must be carried out to identify symptoms and signs associated with neurogenic bladder dysfunction, with an emphasis on identifying common and potentially serious complications. In most cases, investigations followed by appropriate management can minimize this morbidity. The general approach to the clinical history specifically relevant to a patient with NLUTD is shown in Table 1.
Table 1.
Elements of a focused neuro-urological history should be tailored to the disease
| Examples: | |
|---|---|
| History of the neurological disease | SCI: Year and level/completeness of lesion (ASIA level), frequency of autonomic dysreflexia, level of spasticity, mobility/transfers MS: Year and type of MS (primary progressive, secondary progressive, relapsing remitting), mobility level (or Expanded Disability Status Scale) Spinal bifida: Type (i.e., ambulatory lipomyelomeningocele), caregiver, VP shunt, latex allergy, prior reconstructive surgery |
| Bladder management history | Use of catheters (CIC, indwelling [size and frequency of changes], condom), crede/straining/ reflexive bladder emptying, bladder medications, and prior urological surgery history |
| Storage symptoms & voiding symptoms | Frequency, urgency, nocturia, incontinence Weak stream, intermittency, straining, incomplete emptying |
| General components | Allergies, medications, alcohol/drug use/smoking |
| NLUTD complications | UTIs (symptoms, culture status, associated sepsis/fever, response to antibiotics/antibiotic resistance, triggers, hospital admissions) Sequela of incontinence (skin breakdown, ulcers, pad usage, bother) Bladder or renal stone disease Catheter complications (urethral loss in women; urethral erosion, false passages, strictures in men, encrustation/sediment) Renal function (imaging results, renal function) |
| Review of relevant systems | Bowel function Sexual function Coexisting non-NLUTD dysfunction (prostatic enlargement, stress incontinence) Gross hematuria Gynecological/pregnancy history Genitourinary/pelvic pain Motor abilities (hand function, ability to transfer) Cognitive function Support systems/caregivers |
CIC: clean intermittent catheterization; MS: multiple sclerosis; NLUTD: neurogenic lower urinary tract dysfunction; SCI: spinal cord injury; UTI: urinary tract infection.
The timing of this initial evaluation is variable and dependent on the severity of symptoms, underlying risk of serious urological complications, and the etiology of the neurogenic bladder. SB24 and SCI25 have a significant risk of renal dysfunction and are acquired at birth (SB) or often as young adults (SCI); this makes patients particularly susceptible to renal dysfunction in their lifetime. This contrasts with slowly progressive diseases, such as relapsing-remitting MS, or the predominately elderly population with Parkinson’s disease or dementia.
The urological evaluation of a patient with a newly acquired SCI should occur within 3–6 months of the SCI. Efforts should made to assess patients with urological complications or concerns as soon as possible after the acute SCI. Recent evidence has demonstrated that significant bladder dysfunction can appear early after SCI.26 Even ambulatory patients who have experienced a SCI can exhibit significant, and often asymptomatic bladder dysfunction when evaluated with UDS.27 Many patients with MS do not need specialized investigation of their bladder during the initial years after diagnosis. With progression of MS, the risk of bladder dysfunction increases as mobility and functional status decreases, and urological assessment may become more relevant.28,29 When children with SB transition to adulthood, they should be followed by an adult urologist as soon as it is practical to transition them.30 Ideally, transition to an adult care provider should involve more than a referral; a summary of childhood procedures, up-to-date baseline investigations, and a period of overlapping care may be beneficial.31
Voiding diaries should be considered for all patients.32 They allow the patient to self-reflect on their urinary habits and the physician to measure changes over time in a non-invasive manner and interpret urodynamic findings in the context of the patient’s day-to-day urinary patterns. Validated questionnaires are an optional adjunct to the assessment of NLUTD patients; they are generally used for research purposes in this population.33
The specific physical exam to be carried out on patients with NLUTD should include an assessment of body habitus with an abdominal, genital, and rectal exam.7 It may, in certain circumstances, include a focused screening neurological exam (such as lower limb sensory, motor, and reflex function), especially when there is a suspicion of NLUTD without a confirmed neurological disease.
Investigations
Office-based
The initial investigations that should be performed for all NLUTD patients include urine dip (to investigate for infection, microscopic hematuria, and unexpected pyuria or proteinuria), and post-void residual (PVR) volume measurement. Urine dip may need to be followed by a urine microscopy and must be interpreted in the context of catheter usage. In patients who are voiding spontaneously, using reflexive voiding/ crede emptying, or using a condom catheter, the detection of an elevated PVR is important to address potential UTI risk and overflow incontinence and may prompt screening for upper tract deterioration. It is important to recognize that a PVR at the time of renal ultrasound may be artificially elevated secondary to the hydration protocol, resulting in bladder over-distension; an elevated PVR from a renal ultrasound should be confirmed in a more normal setting.
PVR is not clearly defined as a factor associated with increased risk of complications among patients with NLUTD.34 In the non-NLUDT population, a value >300 mL is used to define chronic urinary retention.35 In NLUTD patients with a PVR >300 mL, it is reasonable to follow them for a period of time to determine the stability of their PVR and bladder symptoms. PVR needs to be interpreted based on the proportion of urine voided and method of bladder emptying. The need to treat PVR should be based on patient symptoms rather than an absolute number.
Specific patient populations require further investigation due to a higher risk of serious sequela from bladder dysfunction. The first evaluation of a patient with SB, SCI, or a patient with more advanced MS should include UDS, renal-bladder imaging, and a measurement of renal function.
Urodynamics (UDS)
They are the gold standard for evaluating NLUTD and are necessary due to the absence of normal lower urinary tract sensation and the poor ability of symptoms to predict high-risk features. VideoUDS are preferred, as the additional correlation with imaging allows assessment of VUR, abnormal bladder morphology, and the behaviour of the urinary sphincters during voiding. The availability of videoUDS is not universal, and a voiding cystogram is an acceptable alternative in some cases. Urodynamic diagnoses, such as neurogenic detrusor overactivity (NDO), impaired compliance, reduced bladder capacity, or a high detrusor leak point pressure (DLPP, defined as the lowest vesical pressure at which urine leaks from the bladder in the absence of a detrusor contraction or increased abdominal straining) can identify a patient with potentially higher risk of urological complications (such as renal dysfunction, urinary infections, and incontinence).36–39 Other potential urodynamic characteristics, such as the duration of the NDO contraction, may also predict renal deterioration.40 A DLPP of >40 cmH2O has traditionally been cited as the cutoff above which a patient has a high risk of renal deterioration; however, this is based on a historical study of children with SB, and may not be applicable to adult NLUTD. As DLPP increases, so too does the risk of renal dysfunction due to an increased resting pressure in the bladder being transmitted to the kidneys. If a high DLPP only occurs at a volume greater than the usual capacity during the normal daily voiding pattern, then this DLPP may not be physiologically relevant. A low DLPP maintains low pressure drainage from the kidneys, however, this often results in urinary incontinence.
Imaging
Renal and bladder imaging is necessary to identify hydronephrosis (a late but potentially reversible sign of bladder dysfunction in NLUTD), renal/bladder stone disease, abnormal bladder morphology (for example, thickened bladder wall, diverticula), and both renal atrophy and degree of scarring; both SCI and SB patients are at an increased risk of renal stone disease, and this may present with atypical symptoms (such as nausea or decreased appetite).41–43 Often bladder stones are asymptomatic and early treatment, while they are amendable to endoscopic management, is preferable.
Renal function
Patients with SCI and SB are at increased risk of renal dysfunction; a serum creatinine can be used to assess renal function, however, it may not accurately reflect renal function in these two populations.16 Evaluating the creatinine in the context of previous readings is potentially useful, although it is important to note that changes within the normal range may still be significant. Either a nuclear medicine glomerular filtration rate (GFR), or a 24-hour urine collection for creatinine clearance will better reflect renal function, and allow the identification of early renal dysfunction. While renal dysfunction secondary to bladder dysfunction can occur with MS, it is quite uncommon (estimated at 0.5%).44
Cystoscopy
This should be reserved for situations where there is a clinical indication to assess either the urethra or bladder (such as suspicion of urethral strictures or false passages, bladder stones, or bladder cancer). Screening cystoscopy has historically been recommended among patients with indwelling catheters or after SCI, however, there is no evidence that screening programs are effective.45 Cystoscopy has a poor sensitivity for bladder cancer in SCI patients; the higher-risk cancers after SCI are rarely detected at an early enough stage, which would affect their natural history, and there is very low real-world compliance with cystoscopy screening programs. However, there does seem to be an increased risk of bladder cancer in patients after SCI, potentially as a result of indwelling catheter usage, and cystoscopy should be used when there is suspicion of a bladder tumour.45 Patients with NLUTD and bladder cancer may present late due to hematuria being attributed to catheter usage and atypical presentations, such as frequent UTIs, urethral discharge, or abdominal mass. A recent systematic review suggests that urine cytology outperforms cystoscopy in select populations.46
Summary
The initial history, physical exam, and investigations serve to identify high-risk features in patients with SCI, SB, or more advanced MS patients (Table 2). Assignment of risk is based on relevant abnormalities within one of five domains; two are determined from the patient history (etiology of NLUTD and bladder management) and three are determined based on the initial investigations (UDS, renal imaging, and renal function).
Table 2.
Indicators of NLUTD patient characteristics potentially at higher risk of urological morbidity
| High-risk diagnoses/features | |
|---|---|
| Etiology of neurogenic bladder | SCI, spina bifida, advanced MS |
| Bladder management method | Valsalva/crede/reflexive bladder emptying, indwelling catheter SCI patients with autonomic dysreflexia associated with bladder function |
| Urodynamics | DSD, NDO*, impaired compliance (<20 mL/ cmH2O), DLPP >40 cmH2O), vesico-ureteral reflux |
| Renal-bladder imaging | New-onset/worsening hydronephrosis, stone disease, renal atrophy/scarring Abnormal bladder morphology |
| Renal function | New-onset/worsening renal insufficiency |
The exact characteristics of NDO that are most concerning for renal dysfunction are not clearly defined. High-risk NDO should be interpreted based on the volume at onset, duration, peak pressure, and associated incontinence. These urodynamic findings should be interpreted in the context of the normal voiding habits of the patient.
DLPP: detrusor leak point pressure; DSD: detrusor-sphincter dyssynergia; MS: multiple sclerosis; NDO: neurogenic detrusor overactivity; NLUTD: neurogenic lower urinary tract dysfunction; SCI: spinal cord injury.
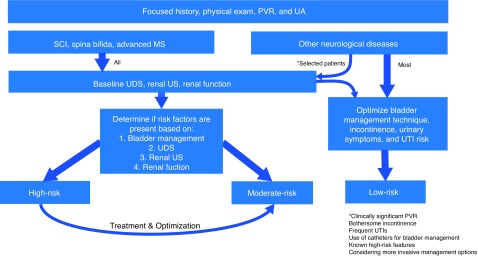
Among patients with NLUTD due to other etiologies (or early stage MS), the majority can be managed with history, physical exam, urinalysis, and PVR (Fig. 2). The subset of these patients with a clinically significant PVR, bothersome incontinence, frequent UTIs, need for catheters as part of their bladder management, known high-risk features on UDS, renal imaging and renal function testing, or those considering more invasive management options may require UDS, renal-bladder imaging, and renal function measurement.
Fig. 2.
Initial investigations and risk stratification for neurogenic lower urinary tract dysfunction (NLUTD) patients. High-risk patients are considered those with spinal cord injury (SCI), spina bifida, advanced multiple sclerosis (MS), or select other neurogenic diseases with evidence of significant urological complications or morbidity in addition to: 1) bladder management technique: Valsalva/crede/reflexive voiding; or 2) known high-risk features on urodynamics (UDS) without confirmation of appropriate attenuation after treatment (detrusor-sphincter dyssynergia [DSD], neurogenic detrusor overactivity [NDO], impaired compliance (<20 ml/cmH2O), detrusor leak point pressure [DLPP] >40 cmH2O, vesico-ureteral reflex); or 3) new/worsening renal imaging (hydronephrosis, atrophy, scarring); or 4) new/worsening renal insufficiency. Patients with SCI, spina bifida, or advanced MS without high-risk features are considered moderate-risk. PVR: post-void residual; UA: urinalysis; US: ultrasound; UTI: urinary tract infection.
Genitourinary sequelae of NLUTD
Risk of upper urinary tract deterioration
Upper urinary tract preservation is a priority when managing patients with NLUTD. Remarkable progress has been made in urological prevention and management to improve renal prognosis in the last decades. Historically, the mortality rate due to renal insufficiency in SCI patients was as high as 50% in the 1960s and dropped to less than 3% currently. In contemporary series, reported rates of chronic kidney disease (CKD) vary from 0.6–3.3%44,47 for MS, 1.3–5.6%47,48 for SCI, and up to 8%49 for MMC patients, which is higher than that of the general population.50,51
Recommendations.
– When referred a new patient with neurogenic bladder, a focused history and physical exam, relevant to the neurogenic condition, should be performed (GOR A, LOE expert opinion).
– All patients with NLUTD should have a urinalysis and PVR as part of their initial evaluation (GOR B, LOE 3).
– After a SCI, patients should have a baseline urological assessment within six months of SCI, or earlier if clinical concerns exist (GOR A, LOE 2).
– Patients with SCI, SB, or advanced MS should have a baseline UDS, renal ultrasound, and measurement of renal function. Selected patients with NLUTD due to other diagnoses may undergo these investigations when referred for specific urological concerns (GOR A, LOE 3).
– The treating clinician should identify patients as either being high-, moderate-, or low-risk, offer the patient appropriate initial therapy, and consider a urological surveillance program as outlined below (GOR B, LOE 3).
In terms or risk factors for CKD, several studies have investigated the prognostic value of urodynamic parameters on renal function deterioration. In 1981, McGuire et al studied 42 myelodysplastic children followed for a mean of 7.1 years and reported that higher intravesical pressure (DLPP >40 cmH2O) was associated with VUR and ureteral dilatation.52 In 1989, another groundbreaking study from Ghoniem et al studied 32 children with MMC and noted that low bladder compliance on UDS predicted risk of upper urinary tract deterioration (UUTD).53
Weld et al studied 316 SCI patients over 18.3 years and observed that low bladder compliance (<12.5 mL/cmH2O) was associated with VUR, radiographic upper tract abnormality, pyelonephritis, and upper tract stones.33 In a retrospective study of 73 patients with traumatic SCI followed for a median of 41 years after injury, Elmelund et al found that the duration of detrusor contractions (DO/cystometry ratio) was associated with renal deterioration. Indeed, patients with and without renal deterioration had the same maximum DLPP (60 cmH2O).40
Interestingly, increased maximum detrusor pressure during voiding (75–115 cmH2O) has been reported as a risk indicator of renal deterioration in SCI patients with NDO.54,55
Despite the lack of strong evidence identifying risk factors for UUTD, causes for UUTD in neurogenic bladder include bladder outlet obstruction (BOO), ureteral obstruction, UTIs, stones and most importantly, persistent high intravesical pressures. 56 High pressures could be from NDO, poor bladder compliance, detrusor-sphincter dyssynergia (DSD) (simultaneous detrusor and urinary sphincter contractions), or a combination.
The pathophysiology of CKD in neurogenic bladder is not well-understood. In some cases, it appears that a sustained high storage pressure results in prolonged compression of the ureteric orifices, leading to obstructed urine outlet during a prolonged period and, consequently, renal damage.57 In other situations, high intravesical pressure causes a defective overwhelmed ureterovesical junction with subsequent VUR and UUTD.
Such secondary VUR may appear as hydroureteronephrosis (HUN) on imaging. Since VUR and HUN may be manifestations of high bladder pressures in neurogenic bladder, treatment should focus first on ensuring low storage pressure. Anti-reflux surgery or double-J ureteral stenting should be avoided in these cases.
Most agree that some bladder methods (reflex triggering and Valsalva or Credé manoeuvres) should be strongly discouraged due their threat for the upper tract (GOR B, LOE 3). In some cases, carefully monitored patients may be able to use these methods successfully. Clean intermittent catheterization (CIC) is a superior method for preserving bladder compliance compared to chronic suprapubic or urethral catheterization.2,33
Symptoms of high intravesical pressure are rarely present (e.g., leakage between CIC) and UDS are required to properly identify it. Compliance must be assessed over the range typically seen by the bladder.58 Despite the fact that patients with a chronic indwelling catheter have an empty bladder most the time, they still warrant followup for urological complications and hydronephrosis.
Overall, patients at higher risk of UUTD are MMC, suprasacral SCI, and men with MS.2 Clinically stable MS patients have lower rates of UUTD compared to those with SCI and MMC, even in the setting of DSD.44
Serum creatinine (sCr) has been criticized as a reliable early marker of renal function in patients with NLUTD, as patients often have muscle atrophy from disuse and denervation. Renography and 24-hour urine creatinine clearance may be preferred to sequentially assess renal function in neurogenic bladder patients.59 Another marker of renal damage is the presence of proteinuria, which can be screened for and warrants a nephrology referral, as it is potentially a prognostic risk factor for mortality due to renal insufficiency.
Renal function decline can occur up to 45 years after injury, making lifelong upper tract surveillance of utmost importance.60
Incontinence and urethral damage
Urinary incontinence is unfortunately commonly observed in patients with neurogenic bladder, with 20–70% of adult neurogenic patients being incontinent to some degree.2 Incontinence highly impacts not only patients’ quality of life (QoL), causing depression and social isolation,61 but can also have other significant consequences.
Freedom from indwelling catheters is a priority in the management of neurogenic bladder. Although long-term indwelling catheters should be avoided, they may be inevitable in some patients with poor manual dexterity, mental deficits, or patients non-compliant with self-catheterization.62
Reports on urethral complications from indwelling catheters are scarce, but they are definitely more common than for patients on CIC.63 Urethral complications, such as strictures, false passages, urethral diverticuli, periurethral abscesses, urethrocutaneous fistula, and iatrogenic traumatic hypospadias may be seen in males with an indwelling catheter.64
In females, urethral dilation, erosion, and potentially destruction may be observed in patients with a long-term indwelling urethral catheter. This is a devastating and difficult-to-treat complication representing a surgical reconstruction challenge with potentially serious secondary consequences, such osteitis pubis or non-healing decubiti ulcers from continued urinary leakage.65
Prevention of these urethral complications is crucial. Daily surveillance of the catheter position to prevent traction down on the leg (ideally positioning the catheter on the abdomen while avoiding kinking and vigilance to sacral and perineal wounds) and use of suprapubic catheters are of utmost importance. Urethral urinary leakage (catheter bypassing) should be addressed by ruling out bladder stones and infection, avoiding increasing the catheter size, and aggressively treating with oral medications or onabotulinumtoxinA injections.66 Patients with indwelling urethral catheters should be offered conversion to a suprapubic catheter in the setting of significant urethral damage (GOR A, LOE 3) and ideally before the urethra has been irreversibly damaged and there is a risk of stress incontinence.62
Sexuality is adversely affected for 40–91% of patients with neurogenic bladder,67 and incontinence is a significant contributing factor due to fear of leakage during intercourse, embarrassment, concerns about odours, dyspareunia from vulvar irritation, or dermatitis from chronic leakage.68 Side effects from medications and surgeries to treat urinary incontinence may also secondarily cause sexual dysfunction from erectile dysfunction, to inadequate vaginal lubrication, or even halitosis from xerostomia.69 Strategies to help prevent urinary incontinence during intercourse include urinating before sex, favouring some positions, or pre-medication with an anti-muscarinic. Slings may help to improve coital incontinence70 and phosphodiesterase type-5 inhibitors may improve lower urinary tract symptoms,71 but these modalities have been mostly reported for non-NLUTD dysfunction and little is known for neurological patients.
Urge urinary incontinence has been identified as an independent risk factor for recurrent falls in MS patients, suggesting that managing “wet” NDO should be included in fall prevention strategies.72
Flack and Powell underlined the economic impact of neurogenic bladder for patients and healthcare systems. Aspects to consider include direct costs related to supplies needed to stay dry (pads, diapers, liners) and to empty fully (catheters, drainage supplies, and lubricant), time lost from work from medical appointments, and cost of procedural interventions. 73 Choosing a bladder care regimen that is cost-effective will help improve patients’ compliance to treatment.73
Patients with neurogenic bladder may also experience fecal incontinence, fecal urgency, and/or chronic obstipation, which may cause significant social distress, hence requiring an individualized bowel regimen.74
In order to achieve continence, different methods of management are available. The key is to individualize treatment and monitor effectiveness and patient acceptability of chosen method taking into consideration activities of daily living, cognition, and disability (including hand function) while protecting the upper tract.
UTIs
UTIs are common in patients with NLUTD and, unfortunately, remain difficult to diagnosis, treat, and prevent. The heterogeneity of this patient population and lack of quality evidence continue to impede the development of comprehensive guidelines.
It has been estimated that the overall rate of UTI in patients with NLUTD is 2.5 episodes per patient year and that one in five patients suffer from recurrent UTIs.75,76 In this population, UTIs are one of the leading causes of emergency department visits, hospitalizations, and potentially life-threatening septicemia.47 In addition to local infectious sequelae, UTIs can lead to acute disease exac erbations (e.g., MS) and are associated with decreased health-related QoL.76
The Enterobacteriaceae family represents the most commonly isolated organism in the NLUTD population, with E.coli comprising 50% of all strains. This is lower than that reported in non-neurogenic UTI and is in part explained by the increased incidence of Pseudomonas, Acinetobacter, Enterococcus, and fungi such as Candida.77,78 Antimicrobial resistance appears to be on the rise, with multidrug resistance found in greater than 50% of uropathogens isolated from SCI patients.77
Accurate diagnosis of UTI in persons with NLUTD is of paramount importance but is often clouded by the high rate of lower urinary tract colonization and difference in clinical presentation. Presently, the accepted definition of UTI in persons with NLUTD requires the presence of leukocyturia, bacteriuria, and clinical symptoms (GOR A, LOE 3).79 There are no evidence-based cutoff values for bacteriuria but the following are generally accepted guidelines:
– >104 cfu/ml (clean voided)
– >102 cfu/ml (clean catheterized sample)
– Any detectable concentration for suprapubic aspirate.
The consensus cutoff value used for leukocyturia is 100 leukocytes/mL or any leukocyte esterase activity on dipstick. Depending on the underlying pathology or level and degree of injury, persons with NLUTD may exhibit vastly different UTI signs and symptoms. The International Spinal Cord Injury Society has developed a UTI data set that outlines these signs and symptoms and includes fever, urinary incontinence/failure of control or leaking around catheter, increased spasticity, malaise, lethargy or sense of unease, cloudy urine, malodorous urine, back pain, bladder pain, dysuria, and autonomic dysreflexia.80
Numerous studies clearly demonstrate that screening and treatment of asymptomatic bacteriuria in persons with NLUTD should be avoided (aside from pregnancy and prior to urological interventions where mucosal bleeding is expected), as it promotes microbe resistance and can increase the likelihood of symptomatic UTI (GOR A, LOE 2).75,81
Acute UTIs in individuals with NLUTD require judicious antimicrobial therapy in addition to basic primary care and/ or sepsis management principles. Urine cultures should always be obtained prior to antimicrobial therapy due to the increased risk of nosocomial and multidrug-resistant microorganisms (GOR A, LOE 2). Any catheter in place for >2 weeks should be removed immediately and replaced and the urine specimen should only be obtained from the new catheter before the initiation of antimicrobial therapy.81
NLUTD persons with UTI must undergo careful clinical assessment to determine the optimal route, spectrum of coverage, and duration of antimicrobial therapy. If UTIs persist, then additional investigations, such as UDS or three-dimensional imaging (ultrasound or computed tomography [CT]), should be considered to rule out further complicating factors (e.g., elevated PVRs or bladder stones). Antibiotic stewardship must be observed in NLUTD UTI and, when possible, narrow spectrum antimicrobials should be used for the shortest duration deemed clinically safe. A seven-day course of antimicrobials is recommended for patients with prompt clinical response and 10–14 days for those with significant infection or a delayed response (GOR A, LOE 3).75 Antimicrobial selection following culture collection should be based on local resistance patterns and antibiograms should be consulted when determining empiric therapy if required.
Prevention of UTI by method of bladder management
Bladder evacuation method is the main predictor of NLUTD UTI and, as such, must be optimized. When possible, CIC should be used over other methods (GOR A, LOE 2). Transurethral indwelling catheterization carries >5-fold increase risk of recurrent UTIs when compared to suprapubic catheterization (SPC) and CIC.82 While UTI risk between SPC and CIC appears comparable, there is a significantly increased risk of bladder calculi with SPC.83 Condom catheters are effective and safe in select NLUTD patients (low PVRs and bladder storage pressures) but are significantly associated with Pseudomonas and Klebsiella bacteriuria and an incidence of UTI comparable to CIC.
In those patients with SPC and indwelling catheters, frequent violation of the closed drainage system increases the risk of UTI and, as such, should be avoided. In addition, the drainage bag and tubing should always be situated below the level of the bladder to avoid retrograde contamination from the urinary bag.81,84 Catheter placement with a preconnected urinary bag junction does decrease the risk of colonization and should be used when possible.85 It is generally recommended that indwelling catheters be changed every 2–4 weeks, with monthly being the most common interval. These practices are not evidence-based and insufficient evidence exists for guideline recommendations.
Antibiotic and silver-coated catheters have been shown to reduce bacteriuria and UTI but only in the very short-term. In addition, concern exists regarding antimicrobial resistance and silver toxicity with long-term use.86 Routine use is therefore not recommended.
Strong evidence exists against the use of antimicrobials or antiseptics in urinary drainage bags, enhanced meatal care, and routine catheter irrigation with normal saline. Recent evidence supports the use of daily gentamicin bladder irrigation in NLUTD patients performing CIC with recurrent UTI. A 75% reduction in symptomatic UTI recurrence was noted, along with decreased systemic antimicrobial use and subsequent antimicrobial resistance.87
Antimicrobial prophylaxis
A meta-analysis of 15 randomized controlled trials did not support the use of oral antibiotic prophylaxis for NLUTD UTI. Three of the included studies reported an approximately two-fold increase in antimicrobial resistance with oral antimicrobial prophylaxis.88 Therefore, at this time, routine antimicrobial prophylaxis for NLUTD UTI is not recommended for most patients (GOR A, LOE 1).
Currently, evidence is insufficient to recommend routine use of any non-antimicrobial prophylaxis measure, including phytotherapy (e.g., cranberry), probiotics, methenamine salts, urine acidification, D-Mannose, oral immunostimulation, or bacterial interference.
Autonomic dysreflexia
Autonomic dysreflexia (AD) a well-known clinical emergency in subjects who have had an SCI. It typically occurs in patients with an injury at level T6 or above. Physiologically, AD is caused by a massive sympathetic discharge triggered by either a noxious or non-noxious stimulus originating below the level of the SCI. Strategies for acute treatment of emergent AD events have been thoroughly addressed elsewhere.89 Recent data suggests that intravesical injection of onabotulintoxinA decreases the frequency and severity of AD episodes.90
Treatment of NLUTD
Assisted bladder drainage
NLUTD can result in impaired bladder emptying. Over 75% of SCI patients are unable to void on their own.91 The best method of bladder emptying, which preserves renal function and minimizes the risks of urinary tract complications such as UTIs and renal or bladder stones, must be balanced against QoL implications, such as comfort, convenience, and continence.92 QoL cannot be ignored, as highlighted in a review by McIntyre where SCI patients who could void normally had the highest QoL ratings followed by those who could micturate with assistance or perform CIC themselves, while the worst QoL came when an indwelling catheter (IC or SP) or CIC by an attendant was required.93 This is an important reminder to continuously re-evaluate NLUTD patients’ selected drainage method and balance the risks and benefits of their choice.
Non-catheter mechanisms
The non-catheter mechanisms rely on involuntary emptying that is either induced or spontaneous. The Crede manoeuver (external pressure on the bladder) and Valsalva voiding induces bladder drainage via an increase in abdominal pressure that can overcome the external urethral sphincter. It can be inefficient and risk high pressures5 and cause hemorrhoids, hernias, and VUR.94 Spontaneous reflex voiding can occur with stimulation of the sacral or lumbar dermatomes by suprapubic tapping in some patients with upper motor neuron lesions. Condom catheter drainage is often used to collect urine in these non-catheter methods and, therefore, are more common in male patients. Additionally, males with cervical level lesions without the dexterity for CIC may select condom drainage. For patients using these non-catheter methods, regular screening with ultrasound and UDS should be done to avoid complications such as incomplete emptying causing UTIs or stones, as well as dangerous elevated detrusor pressures.5,95
Catheter mechanisms
The options for catheter mechanisms to provide bladder drainage include: CIC, indwelling urethral catheterization and SP. While every attempt should be made to use the gold standard of CIC introduced by Lapides96 in 1972, practitioners must understand the limitations of CIC outlined by Elliot,91 which include: 1) limited upper extremity motor function; 2) anatomic limitations (female or obese); and 3) limited functional bladder capacity (poor compliance or DO). In a review by Binard, the ideal person for CIC has a low Pdet at capacity; a minimum volume of 350–400 cc; an unobstructed urethra; and is compliant, understanding, continent, and cooperative with adequate hand function.97 Practitioners may need to use medical means, such as anticholinergics, beta-3 agonists, or onabotulinumtoxin A, or surgical means, such as augmentation cystoplasty or catheterizable stoma, to facilitate successful CIC. While CIC is the gold standard, it isn’t without complications, including pain for those with sensation, UTI,94 and stricture formation estimated at 4–13% from recent reports despite using hydrophilic catheters.98
The debate regarding the ideal catheter for those performing CIC does not have a clear winner. Options for patients include: single-use disposable catheters that may be non-hydrophilic (uncoated), hydrophilic (coated), or include a gel reservoir. Alternatively, due to financial limitations, many patients still reuse uncoated catheters by various unstudied cleaning protocols (such as washing with warm soapy water and allowing to air dry, and replacing the catheter after a week or when there is visible wear). A recent Cochrane review from 2014 on the issue of catheter reuse was withdrawn after Christison et al identified several flaws in the data extraction and conclusions; their revised analysis found that hydrophilic catheters offered a small but significantly lower incidence of UTI and they reported a trend that favours single-use catheters over repeated multiple use. The authors clearly state that, “until evidence can confidently demonstrate that multiple use is as safe as single-use catheters, healthcare providers should advocate a single use of catheters in individuals with SCI.”99 There may be other benefits of hydrophilic catheters, such as lower risk of hematuria, stricture rates and improved urinary QoL. Unfortunately, current evidence is generally of a low-quality, and likely particular patient characteristics, such as hand function and coverage options, will play a large role in dictating how CIC is carried out.
While guidelines promote the use of CIC, many switch to indwelling catheters (IC or SP), as reported by Pannek with many predictors of likelihood: female gender (2.5x), age >45 (3x), and both severity (AIS A-C tetraplegia) and duration from injury (4x).11 Indwelling catheters allow for some bladder independence but often functional, physical, mental, or social factors trigger this decision.
Indwelling catheter methods (IC or SP) are often felt to be the last choice. Practitioners should advise patients of the risks and benefits; however, the data regarding whether indwelling catheters are more dangerous has been questioned. Authors have promoted the safety of both indwelling catheters 92,100,101 and SP102 with no renal deterioration and a low incidence of incontinence. For example, provided an indwelling catheter is draining all the time, it seems less likely that high storage pressures or low compliance would matter.103 SP tubes allow patients to engage in sexual activities and may carry less of a risk of epididymitis over indwelling catheters.92 Additionally, patients should be investigated for bladder cancer or bladder stones when appropriate.103 Those patients living with indwelling catheters or SP do colonize with polymicrobial and dynamic bacteria at a rapid rate of 5–10% per day104 and is often the cause of stones and symptomatic UTIs. This remains an ongoing frustration for patients and care providers alike.
Selection of an assisted bladder drainage method (CIC, urethral, or suprapubic catheter) should be individualized to the patient’s motor functions, anatomic limitations, bladder characteristics, prior urological complications, and QoL (GOR A, LOE 3).
Oral and transcutaneous medical therapy
Treatment of NLUTD aims to lower detrusor storage pressure and increase bladder capacity in order to protect upper tract function and to decrease urinary incontinence.
Anticholinergics
A meta-analysis in NDO reviewed all randomized controlled trials between 1966 and 2011 (total 960 patients). They demonstrated that anticholinergic administration in this population was associated with statistically significant differences in patient-reported cure/improvement, bladder capacity, and detrusor pressure compared to placebo. Studies that compared one medication to another (usually oxybutynin IR), did not reveal statistically significant differences. The optimal drug dosage was not identified.105 Madersbacher et al extended their review to include other non-randomized controlled studies and found an approximate decrease of 30–40% in maximal detrusor pressures and an increase of maximum cystometric bladder capacity of 30–40% for oxybutynin IR, propiverine IR, propiverine ER, and trospium chloride IR compared to placebo.106 Antimuscarinics should, therefore, be offered to people with urodynamic findings of NDO or those with SCI and symptoms of overactive bladder (OAB) (GOR A, LOE 1a). The preferential drug of choice should be individualized but evidence for efficacy exists for oxybutynin IR and ER, tolterodine IR and ER, propiverine IR, darifenacin, and solifenacin. Antimuscarinic dosage should be escalated to optimize improvement of symptoms or urodynamic parameters, as tolerated by the patient, with the possibility of increasing adverse events. Supratherapeutic dosages may be considered according to tolerability but should be used cautiously.107 Combining antimuscarinics may be beneficial for patients who are refractory to dose-escalation antimuscarinic monotherapy,108,109 and is suggested by the European Association of Urology guidelines.110
The administration of antimuscarinics should be considered whether or not patients are using assisted bladder drainage (GOR C, LOE 4). The absence of its usage has been shown to be a risk factor for upper tract deterioration.111 If the bladder is being drained, there is less of a concern of elevated PVR. In patients with indwelling catheters, oxybutynin use was associated with less risk of hydronephrosis and should be considered.112
Recommendations.
– Oral antimuscarinics with dose-escalation are the first-line pharmacological treatment for patients with NLUTD in order to improve OAB symptoms and NDO, decrease urgency urinary incontinence, and lower detrusor pressures (GOR A, LOE 1a).
– There is very limited data supporting the use of transdermal oxybutynin or mirabegron in NLUTD (GOR C, LOE 4).
B3 adrenergic agonist therapy
There is limited evidence for the use of mirabegron for the treatment of NDO or NLUTD. A retrospective review found an improvement in urodynamic parameters in 15 patients with NDO on mirabegron.113 There are currently trials underway to assess its efficacy in this patient population.114 Mirabegron may be a useful alternative to anticholinergics for patients with symptoms of OAB and NLUTD, but further evidence of urodynamic changes are needed in this population (GOR C, LOE 4).
Intravesical therapy
OnabotulinumtoxinA (Botox®) intradetrusor injection has been proven to be an effective and safe long-term therapy for the management of NLUTD secondary to SCI or MS. Results of powered, placebo-controlled, multicentre, phase 3 randomized controlled trials and meta-analyses demonstrated clinically significant outcomes and sustained efficacy in terms of reduced incontinence episodes, enhanced bladder function, as well as substantial improvements in key urodynamic parameters and QoL (GOR A, LOE 1a).115–119 Achieved therapeutic effects are comparable between both onabotulinumtoxinA doses (200 units and 300 units) in terms of efficacy and durability, but catheter initiation rates were dose-dependent (GOR B, LOE 1b);116,120 200 units is the standard recommended dose by Health Canada with more favourable safety profile.121 Safety assessments identified UTIs and large urine residual or urinary retention as the most frequent adverse events. These findings are more predominant among 300 units groups and patients not using CIC at baseline. Therefore, the likelihood of future need of CIC is increased (GOR A, LOE 1b).116,118,120 Muscle weakness and respiratory problems are other serious complications that are rarely reported.116,120,122
Intravesical oxybutnin by CIC
Intravesical oxybutynin treatment has been shown to be safe and effective short-term therapy in patients suffering from NDO who remain incontinent or are intolerant of oral anticholinergic medication (GOR C, LOE 3).123–127 A recent multicentre, open-label randomized controlled trial confirmed the efficacy and safety of intravesical administration of 0.1% oxybutynin hydrochloride with a significant increase in bladder capacity and fewer adverse drug reactions (GOR B, LOE 2).123 In general, this approach avoids systemic side effects, as the drug bypassed first pass metabolism,126 and is mainly suitable in patients already using CIC.125
Intravesical vanilloids, such as capsaicin and resiniferatoxin, reduce NDO by reversible desensitization of the afferent C-fibers, and thereby increase bladder capacity. Their positive clinical and urodynamic benefits last for a period of a few months without systemic side effects.128–130 Resiniferatoxin is an ultrapotent analogue of capsaicin, with the advantage of less pain during initial administration and superior clinical efficacy.131 Results of randomized controlled trials have demonstrated that botulinumtoxinA intradetrusor injection achieved superior clinical outcomes compared to those of resiniferatoxin instillation.132 Recently, meta-analyses of relevant randomized controlled trials showed poor overall quality of evidence with unfavourable safety profile and no existing licensed substance (GOR C, LOE 3).130
Recommendations.
– OnabotulinumtoxinA injection (200 units) in the detrusor is an effective, minimally invasive treatment that can achieve continence, improve bladder function, and diminish NDO in individuals with SCI or MS who have an inadequate response to or are intolerant of an anticholinergic medication (GOR A, LOE 1).
– AbobotulinumtoxinA is also efficacious in NLUTD, with the optimal dose of 750 units (GOR B, LOE 1b).
– Intravesical oxybutynin is a safe alternative approach to managing NDO and NLUTD in patients who are doing CIC (GOR B, LOE 2).
Neural stimulation & neuromodulation therapy
Neuromodulation represents a promising tertiary treatment option for managing patients with refractory NLUTD. It appears to involve modulation of spinal cord reflexes and brain centres via peripheral afferents (genital, tibial, and sacral afferents).133 A recent review on the use of this modality reports that it can be successful in certain carefully selected neurological populations. However, no definitive conclusions can be drawn from the available evidence at this point. Current data supporting the use of sacral neuromodulation (SNM) and peripheral tibial nerve stimulation (PTNS) in this cohort are limited by observational nature, small sample sizes, heterogeneous populations with differing symptom profiles, and outcomes measured.
Dorsal rhizotomy (sacral deafferentation S2-S4/5) and sacral anterior root stimulation (SARS) by an implantable device can achieve safe storage detrusor pressure and voluntary emptying of bladder and bowel in patients with complete SCI.134–137 Furthermore, it diminishes AD.135,138–141 This technique has good variable success rates in specialized centres, but comes with long-term complications and a very high rate of surgical revisions (GOR C, LOE 3). Although the striated muscle fibers of the urethral sphincter are stimulated, it relaxes sooner than the detrusor smooth muscle, resulting in post-stimulation voiding. This approach can also improve bowel and erectile dysfunction.139,142 Alternatives to surgical posterior rhizotomy are investigated in this treatment combination.143–145 Charcot spinal arthropathy as a potential long-term complication and a possible cause for SARS dysfunction can occur.146
There are few studies on PTNS applicability in the NLUTD population, and they are limited by their heterogeneity, small sample size, and retrospective/prospective non-randomized nature. Results of prospective non-randomized trials and meta-analysis demonstrated significant improvements on clinical and urodynamic outcomes after a 12-week period in the MS and Parkinson’s disease patient populations. PTNS appears to be well-tolerated and effective in small studies, with minimal reported adverse events, mainly mild to moderate pain at the puncture site (GOR C, LOE 4).147–150 Recent randomized controlled trials including 100 patients with NDO following SCI reported significant improvements in bladder diary variables within four weeks after PTNS. However, there was no difference when compared to solifenacin therapy.151 PTNS therapy is limited by the need for weekly repeated office-based procedure and the need for long-term or lifelong maintenance.
Recommendations.
– SNM could be considered for the treatment of NDO or non-obstructive urinary retention in carefully selected individuals with NLUTD, as it can be a safe and effective option. It should be preceded by an adequate testing phase and may not be a good alternative to decrease detrusor pressures or improve bladder compliance.
– PTNS can be efficacious in NLUTD resulting from MS, but requires initial frequent weekly visits. It remains unclear which subgroups of neurogenic voiding dysfunction and which underlying neurological disease will respond best to these different therapies.
Surgical management of LUTD
Surgical intervention may be required in a variety of clinical scenarios in managing patients with NLUTD. It is indicated when conservative measures, medical therapy, and minimally invasive interventions alone fail to achieve the objectives of: 1) protecting kidney function and mitigating AD by maintaining bladder storage at safely low pressures; 2) ensuring adequate and timely bladder emptying to mitigate the risks of overflow incontinence, recurrent UTIs, bladder stones, and kidney damage; 3) preventing the adverse effects of incontinence (e.g., dermatitis); and 4) improving QoL by relieving bothersome symptoms of OAB and incontinence.
Bladder augmentation (BA)
BA is indicated in cases of reduced compliance or NDO refractory to all other non-surgical treatments, or reduced bladder capacity necessitating an indwelling catheter or CIC to be done too frequently (GOR B, LOE 2).6,152–154 Compliance is increased in 69–100% of cases, continence restored in 75–100%, and QoL improved in >90%.155–163 Contraindications include bladder malignancy or stones, significant renal dysfunction, bowel disease and/or prior resection, and inability or unwillingness to maintain CIC. Where CIC per urethra is not feasible, patients should be offered continent cutaneous urinary diversion (CCUD).
In cases of thick, fibrous, low-capacity bladders, supratrigonal cystectomy is recommended over clam cystoplasty in preparing the bladder for augmentation.152,164 The ileum is the recommended segment where possible given a lower risk of complications, good efficacy, and ease of use.155,162,163,165 In cases of grade IV–V reflux, ureteric re-implant may be necessary.152,166 Long-term risks include adeno- or urothelial carcinoma (1–4.6%), bladder calculi, and perforation (5–13%).167–172 Careful education and long-term cystoscopic surveillance are therefore recommended; however, the most cost-effective frequency is not established.
Catheterizable channels and continent cutaneous urinary diversion (CCUD)
In cases where urethral catheterization is precluded, a catheterizable channel may be offered after careful consideration and multidisciplinary evaluation.
Most commonly, a simple channel is created from the bladder to the abdominal wall with a valve mechanism to prevent incontinence. Concomitant bladder augmentation is performed only as necessary as indicated above. The most commonly used tube is the appendix (Mitrofanoff appendicovesicostomy). 154,173,174 Where the appendix is unavailable or unsatisfactory (must be 8–10 cm in length for adult patients), a segment of terminal ileum can be employed (Yang-Monti or Casale technique), albeit with slightly poorer outcomes.173,175,176 Where additional length is required, the technique proposed by Casale or a tapered ileal channel with nipple valve may be preferred over the “double Monti” procedure.154,177
In cases where it is not prudent to preserve and use the native bladder (e.g., severely contracted, high-grade VUR, concern for malignancy, devastated outlet), a continent catherizable pouch may be preferred. These procedures can be associated with higher risk of metabolic complications, especially if the ileo-cecal junction is used.178–180
Incontinent urinary diversion (ileovesicostomy and ileal conduit)
Incontinent diversion is a last resort in managing the complications of NLUTD, and indicated in patients who are not candidates for the techniques outlined above or when expertise is not available. Most commonly, these are offered to a patient at high-risk (impaired compliance) who is unable to perform CIC due to upper limb dysfunction.
Ileovesicostomy may be appropriate in select patients. It has the advantages of being technically simple, avoiding the potential complications related to both cystectomy and uretero-ileal anastomoses, obviating the need for indwelling catheters, avoiding any risk for pyocystis, and maintaining native anti-reflux mechanism and sexual/reproductive function. It has the disadvantages of preserving the native bladder and outlet with risks of malignancy or ongoing urethral incontinence if this is not also surgically addressed.152,181 The technique is described by Schwartz et al and further reviewed by Westney.62,182 Few small series are available for review and robust long-term followup and QoL data is lacking.152,181,183–187 Complication rates are high (up to 75%) and include impaired emptying, stomal stenosis, parastomal hernia, and renal and bladder stones (up to 25%).183,184,186
Along with the indications above, ileal conduit may be appropriate in cases of severe incontinence (e.g., devastated outlet) with low likelihood of successful reconstruction, end-stage bladder with high-grade VUR, chronic UTIs with impaired compliance, chronic bladder fistulisation, or malignancy. It is the preferred method of incontinent diversion. The bladder should be removed at the time of surgery to reduce the risks of pyocystis (21–61%), chronic symptomatic cystitis, and malignancy.157,188–190 Minor complications may develop in 46% and major complications in 11%, with overall complication rates of 30–70%.191–194 Upper tract functional preservation is reported in >90% of patients. Significant improvement in urinary-specific QoL but not overall QoL have been reported.191,195,196
External urethral sphincterotomy
External urethral sphincterotomy aims to allow reflex micturition into a reservoir via condom catheter. Surgery is irreversible and multiple procedures may be required. Patients must be carefully counselled about their options. Long-term followup is required given a high rate of recurrent DSD and/or stricture. Patients must be able to retain a condom catheter. A semi-rigid penile prosthesis can be offered to facilitate this; however, there is a 20–30% risk of erosion in this population. 197,198 Female gender, detrusor underactivity, and desire to preserve fertility are also contraindications. Up to 82% of patients will develop recurrent DSD and require at least one repeat procedure, thus annual upper tract imaging and UDS are recommended.199–210 Improvements in PVR, hydronephrosis, recurrent UTIs, and AD have been reported in many small series.152,199
Bladder neck closure (BNC)
BNC, combined with some type of continent or incontinent channel, is indicated in cases of severe outlet damage. It may be accomplished by a retropubic or transvaginal approach. The former is recommended if augmentation and/or ureteric reimplantation are required, if perineal access is unsatisfactory, or if surgeon expertise dictates. When possible, transvaginal BNC offers satisfactory outcomes with reduced morbidity, operating time, and hospital stay.211–214 To minimize risk of failure/ fistulisation, it is critical that patients are counselled about proper bladder drainage (CIC or continuous depending on their diversion) and that low bladder pressures are maintained.
Surveillance studies for NLUTD patients in the community setting
After initial assessment and treatment to optimize bladder function, NLUTD patients are followed with regular clinical assessment and, in some cases, surveillance investigations. NLUTD surveillance is stratified based on the risk of NLUTD sequelae. Although it is suggested that clinical examination alone is not sufficient to determine individual urological management strategies in patients with NLUTD,215 data demonstrating the value of surveillance investigations in the setting of NLUTD is lacking.216 Similarly, urodynamic risk-stratification has been suggested based on high pressure storage and voiding features, but characterization of overall risk groups for NLUTD sequelae remains largely undefined to date52,54,217 Typically, surveillance protocols suggest either on-demand or regularly scheduled UDS, upper tract imaging, and cystoscopy, but there is little consensus on specific approach.3–5,218 Consequently, practice patterns vary with regard to the type and frequency of studies used in NLUTD surveillance.48,218–220 Our suggested approach for NLUTD stratifies patients based on their urological risk factors and specific investigations are recommended.
Surveillance clinical assessment
The primary goal of clinical assessment is to stratify patients based on their risk of NLUTD sequelae. Patients deemed low-risk are followed with a simple clinical assessment, while those deemed higher-risk undergo a more detailed evaluation of the urinary tract function and anatomy. Depending on the specific risk factors involved, this may include urodynamic evaluation, renal-bladder imaging, and renal function assessment. The detailed evaluation of the higher-risk groups is intended to address modifiable factors that may allow the patient to be reclassified as a lower-risk patient. Relevant findings on history include bladder management technique (particularly high-risk groups including condom drainage, valsalva/crede/ reflexive bladder emptying), incontinence pattern, UTI profile, AD, and most recent urodynamic evaluation and upper tract imaging. We recommend regular yearly clinical assessment of all NLUTD patients with their physiatrist, neurologist, or family physician; we recommend that a urologist is involved in the assessment of patients who are in the moderate- or high-risk categories as described in Table 3 (for example SCI, SB, advanced MS) (GOR C, LOE 4).
Table 3.
Surveillance strategy for neurogenic lower urinary tract dysfunction (NLUTD) based on patient risk-stratification
| Risk group | Description | Suggested surveillance strategy |
|---|---|---|
| High-risk | Underlying high-risk disease (SCI, spina bifida, advanced MS) or select other neurogenic diseases with evidence of significant urological complications or morbidity) in addition to:
|
|
| Moderate-risk | Underlying high-risk disease (SCI, spina bifida, advanced MS) or select other neurogenic diseases with evidence of significant urological complications or morbidity) in addition to:
|
|
| Low-risk | No evidence of high-risk disease and no features on initial evaluation that would be considered high-risk |
|
DLPP: detrusor leak point pressure; DSD: detrusor-sphincter dyssynergia; GP: general practitioner; MS: multiple sclerosis; NDO: neurogenic detrusor overactivity; PVR: post-void residual; SCI: spinal cord injury; UDS: urodynamic study; UT: urinary tract.
Surveillance investigations
Imaging
Routine surveillance imaging provides interval evaluation of the anatomy of the urinary tract and characterizes hydronephrosis, renal atrophy, scars, urinary stones, diverticula, trabeculation, large bladder lesions, and quantifies PVR. A recent systematic review concluded that there is sufficient evidence to recommend yearly ultrasound of the kidneys and urinary tract as a useful, cost-effective, non-invasive method for routine long-term followup to detect upper urinary tract problems in all individuals with SCI. Although the findings have been applied to other underlying pathologies within NLUTD, the benefit has not been quantified.41 We suggest yearly renal and bladder ultrasound in high- and moderate-risk NLUTD patients as described in Table 3 (for example SCI, SB, advanced MS) (GOR C, LOE 4).
Cystoscopy
While historically used for concerns of increased bladder cancer risk, cystoscopy can be a valuable tool in the evaluation of urethral or bladder integrity and can provide an estimate of external sphincter function. The value of surveillance cystoscopy for bladder cancer surveillance in the SCI population was addressed in a recent systematic review by Cameron et al.41 The investigators believed that the incidence of bladder cancer was too low to be well-evaluated in these studies, and screening cystoscopy and biopsy did not fit the criteria for a screening test of the general NLUTD population. Patients with prior augmentation cystoplasty have historically been followed with yearly surveillance cystoscopy due to increased risk of bladder cancer.221 Recent studies demonstrate no benefit from surveillance cystoscopy in the augmented population.170,222,223 We support the use of cystoscopy for the assessment of suspected urethral or bladder pathology. We do not support routine surveillance cystoscopy for bladder cancer screening in NLUTD with or without augmentation cystoplasty (GOR C, LOE 4).
UDS
Attempts at establishing a risk vs. benefit ratio for regularly scheduled surveillance UDS are limited by heterogeneous populations and varying surveillance strategies. Some authors demonstrate benefit of regularly scheduled yearly urodynamic evaluation.224,225 Conversely, others establish a safe lower urinary tract with baseline UDS, and subsequently perform annual renal ultrasonography for surveillance. UDS in this strategy is repeated only when patients presented with changing incontinence patterns or alarming radiological changes. 226 Existing guidelines have little consensus on the specific strategy of implementation and high enrollment studies are not currently available. We support the use of surveillance UDS in moderate-risk patients every 2–5 years and high-risk patients every year (GOR C, LOE 4). VideoUDS or a cystogram should be performed in patients where further knowledge of the urinary tract anatomy is needed.
Proposed surveillance strategy
There is a lack of evidence to establish any clear strategy of surveillance for NLUTD, as evidenced by the varying recommendations of numerous prior guidelines.3–5,218 The primary goals of surveillance screening studies are to mitigate NLUTD sequelae and we propose a strategy based on risk-stratification. Our proposed surveillance strategy is included in Table. 3. The integrity of this strategy has not been verified empirically; it represents the consensus opinion of our contributors (GOR C, LOE 4).
Acknowledgements
The authors would like to thank the following for their contributions, review, and feedback during the development process: Magdy Hassouna, Greg Bailey, Jerzy Gajewski, Stephen Steele, Tara Jeji, and Emily Deegan. The authors also thank Dr. Samer Shamout for his assistance with the neuromodulation, intravesical, oral and transcutaneous therapy sections.
Footnotes
Competing interests: Dr. Kavanagh has been an advisory board member for Paladin Labs, and has received a research grant from Astellas. Dr. Baverstock has been a speaker for Allergan, Astellas, BSCI, and Pfizer; and has participated in clinical trials supported by Astellas and Pfizer. Dr. Campeau has been an advisory board and speaker bureau member for Asetllas and Pfizer; has received grants/honoraria from Allergan, Astellas, and Pfizer; and has participated in clinical trials supported by Pfizer. Dr. Cox has been an advisory board member for Pfizer; a speakers bureau member for Astellas and Pfizer; has received grants/honoraria from Astellas and Pfizer; and has participated in clinical trials supported by Aquinox. Dr. Hickling has been an advisory board member for Pfizer; a speakers bureau member for Allergan, Astellas and Pfizer; has received grants/honoraria from Allergan, Astellas and Pfizer; and has participated in clinical trials supported by Astellas. Dr. Nadeau has been an advisory board member for Allergan, AMS, Astellas, Boston Scientific, Ferring, and Pfizer; a speakers bureau member for, Allergan, Astellas, Ferring Laborie, and Pfizer; and and has participated in clinical trials supported by Astellas. The remaining authors report no competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.Cameron AP. Pharmacologic therapy for the neurogenic bladder. Urol Clin North Am. 2010;37:495–506. doi: 10.1016/j.ucl.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Nseyo U, Santiago-Lastra Y. Long-term complications of the neurogenic bladder. Urol Clin North Am. 2017;44:355–66. doi: 10.1016/j.ucl.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Abrams P, Agarwal M, Drake M, et al. A proposed guideline for the urological management of patients with spinal cord injury. BJU Int. 2008;101:989–94. doi: 10.1111/j.1464-410X.2008.07457.x. [DOI] [PubMed] [Google Scholar]
- 4.Collins CW, Winters JC American Urological Association, Society of Urodynamics Female Pelvic Medicine and Urogenital Reconstruction. AUA/SUFU adult urodynamics guideline: A clinical review. Urol Clin North Am. 2014;41:353–62. vii. doi: 10.1016/j.ucl.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Stohrer M, Blok B, Castro-Diaz D, et al. EAU guidelines on neurogenic lower urinary tract dysfunction. Eur Urol. 2009;56:81–8. doi: 10.1016/j.eururo.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 6.Stohrer M, Castro-Diaz D, Chartier-Kastler E, et al. Guidelines on neurogenic lower urinary tract dysfunction. Prog Urol. 2007;17:703–55. [PubMed] [Google Scholar]
- 7.Panicker JN, Fowler CJ, Kessler TM. Lower urinary tract dysfunction in the neurological patient: Clinical assessment and management. Lancet Neurol. 2015;14:720–32. doi: 10.1016/S1474-4422(15)00070-8. [DOI] [PubMed] [Google Scholar]
- 8.Madersbacher H. The various types of neurogenic bladder dysfunction: An update of current therapeutic concepts. Paraplegia. 1990;28:217–29. doi: 10.1038/sc.1990.28. [DOI] [PubMed] [Google Scholar]
- 9.Liao L. A new comprehensive classification system for both lower and upper urinary tract dysfunction in patients with neurogenic bladder. Urol Int. 2015;94:244–8. doi: 10.1159/000365056. [DOI] [PubMed] [Google Scholar]
- 10.Abrams P, Khoury S. International Consultation on Urological Diseases: Evidence-based medicine overview of the main steps for developing and grading guideline recommendations. Neurourol Urodyn. 2010;29:116–8. doi: 10.1002/nau.20845. [DOI] [PubMed] [Google Scholar]
- 11.Canada Go. Neurological conditions, by age group and sex, household population aged 0 and over 2010/2011. [Accessed May 30, 2019]. Available at: http://www5.statcan.gc.ca/cansim/a26?lang=eng&id=1051300.
- 12.Canada Go. Neurological conditions in institutions, by age, sex, and number of residents, Canada, provinces and territories 2011/2012. [Accessed May 30, 2019]. Available at: http://www5.statcan.gc.ca/cansim/a26?lang=eng&id=1051305.
- 13.Canada MSSo. About MS: What Is MS. 2017. [Accessed May 30, 2019]. Available at: https://mssociety.ca/about-ms/what-is-ms.
- 14.Spina Bifida and Hydrocephalus Association of Canada. About spina bifida. [Accessed May 30, 2019]. Available at: http://sbhac.ca/about-spina-bifida/
- 15.Noonan VK, Fingas M, Farry A, et al. Incidence and prevalence of spinal cord injury in Canada: A national perspective. Neuroepidemiology. 2012;38:219–26. doi: 10.1159/000336014. [DOI] [PubMed] [Google Scholar]
- 16.Dryden DM, Saunders LD, Rowe BH, et al. The epidemiology of traumatic spinal cord injury in Alberta, Canada. Can J Neurol Sci. 2003;30:113–21. doi: 10.1017/S0317167100053373. [DOI] [PubMed] [Google Scholar]
- 17.Farry ABD. The Incidence and Prevalence of Spinal Cord Injury in Canada: Overview and Estimates Based on Current Evidence. Rick Hansen Institute and Urban FutuMres: Strategic Research to Manage Change; 2010. [Google Scholar]
- 18.New PW, Sundararajan V. Incidence of non-traumatic spinal cord injury in Victoria, Australia: A population-based study and literature review. Spinal Cord. 2008;46:406–11. doi: 10.1038/sj.sc.3102152. [DOI] [PubMed] [Google Scholar]
- 19.Dorsher PT, McIntosh PM. Neurogenic bladder. Adv Urol. 2012;2012 doi: 10.1155/2012/816274. 816274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park SE, Elliott S, Noonan VK, et al. Impact of bladder, bowel and sexual dysfunction on health status of people with thoracolumbar spinal cord injuries living in the community. J Spinal Cord Med. 2017;40:548–59. doi: 10.1080/10790268.2016.1213554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giesbrecht EM, Smith EM, Mortenson WB, et al. Needs for mobility devices, home modifications, and personal assistance among Canadians with disabilities. Health Rep. 2017;28:9–15. [PubMed] [Google Scholar]
- 22.White BAB, Dea N, Street JT, et al. The economic burden of urinary tract infection and pressure ulceration in acute traumatic spinal cord injury admissions: Evidence for comparative economics and decision analytics from a matched case-control study. J Neurotrauma. 2017;34:2892–900. doi: 10.1089/neu.2016.4934. [DOI] [PubMed] [Google Scholar]
- 23.Marion TE, Rivers CS, Kurban D, et al. Previously identified common post-injury adverse events in traumatic spinal cord injury – validation of existing literature and relation to selected potentially modifiable comorbidities: A prospective Canadian cohort study. J Neurotrauma. 2017;34:2883–91. doi: 10.1089/neu.2016.4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouyang L, Bolen J, Valdez R, et al. Characteristics and survival of patients with end-stage renal disease and spina bifida in the United States renal data system. J Urol. 2015;193:558–64. doi: 10.1016/j.juro.2014.08.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer MJ, Krishnamoorthi VR, Smith BM, et al. Prevalence of chronic kidney disease in patients with spinal cord injuries/disorders. Am J Nephrol. 2012;36:542–8. doi: 10.1159/000345460. [DOI] [PubMed] [Google Scholar]
- 26.Bywater M, Tornic J, Mehnert U, et al. Detrusor acontractility after acute spinal cord injury –myth or reality? J Urol. 2018;199:1565–70. doi: 10.1016/j.juro.2018.01.046. [DOI] [PubMed] [Google Scholar]
- 27.Bellucci CH, Wollner J, Gregorini F, et al. Acute spinal cord injury--do ambulatory patients need urodynamic investigations? J Urol. 2013;189:1369–73. doi: 10.1016/j.juro.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Ineichen BV, Schneider MP, Hlavica M, et al. High EDSS can predict risk for upper urinary tract damage in patients with multiple sclerosis. Mult Scler. 2018;24:529–34. doi: 10.1177/1352458517703801. [DOI] [PubMed] [Google Scholar]
- 29.Wiedemann A, Kaeder M, Greulich W, et al. Which clinical risk factors determine a pathological urodynamic evaluation in patients with multiple sclerosis? An analysis of 100 prospective cases. World J Urol. 2013;31:229–33. doi: 10.1007/s00345-011-0820-y. [DOI] [PubMed] [Google Scholar]
- 30.Snow-Lisy DC, Yerkes EB, Cheng EY. Update on urological management of spina bifida from prenatal diagnosis to adulthood. J Urol. 2015;194:288–96. doi: 10.1016/j.juro.2015.03.107. [DOI] [PubMed] [Google Scholar]
- 31.Grimsby GM, Burgess R, Culver S, et al. Barriers to transition in young adults with neurogenic bladder. J Pediatr Urol. 2016;12:258 e1–5. doi: 10.1016/j.jpurol.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 32.(UK). NCGC. Management of Lower Urinary Tract Dysfunction in Neurological Disease. Urinary Incontinence in Neurological Disease. National Institute for Health and Clinical Excellence: Guidance; London: 2012. [Google Scholar]
- 33.Weld KJ, Graney MJ, Dmochowski RR. Differences in bladder compliance with time and associations of bladder management with compliance in spinal cord injured patients. J Urol. 2000;163:1228–33. doi: 10.1016/S0022-5347(05)67730-0. [DOI] [PubMed] [Google Scholar]
- 34.Dray E, Cameron AP, Clemens JQ, et al. Does post-void residual volume predict worsening urological symptoms in patients with multiple sclerosis? J Urol. 2018;200:868–74. doi: 10.1016/j.juro.2018.04.068. [DOI] [PubMed] [Google Scholar]
- 35.Stoffel JT, Peterson AC, Sandhu JS, et al. AUA white paper on non-neurogenic chronic urinary retention: Consensus definition, treatment algorithm, and outcome endpoints. J Urol. 2017;198:153–60. doi: 10.1016/j.juro.2017.01.075. [DOI] [PubMed] [Google Scholar]
- 36.Ku JH, Choi WJ, Lee KY, et al. Complications of the upper urinary tract in patients with spinal cord injury: A long-term followup study. Urol Res. 2005;33:435–9. doi: 10.1007/s00240-005-0504-4. [DOI] [PubMed] [Google Scholar]
- 37.Shin JC, Lee Y, Yang H, et al. Clinical significance of urodynamic study parameters in maintenance of renal function in spinal cord injury patients. Ann Rehabil Med. 2014;38:353–9. doi: 10.5535/arm.2014.38.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veenboer PW, Bosch JL, Rosier PF, et al. Cross-sectional study of determinants of upper and lower urinary tract outcomes in adults with spinal dysraphism – new recommendations for urodynamic followup guidelines? J Urol. 2014;192:477–82. doi: 10.1016/j.juro.2014.02.2566. [DOI] [PubMed] [Google Scholar]
- 39.Musco S, Padilla-Fernandez B, Del Popolo G, et al. Value of urodynamic findings in predicting upper urinary tract damage in neuro-urological patients: A systematic review. Neurourol Urodyn. 2018;37:1522–40. doi: 10.1002/nau.23501. [DOI] [PubMed] [Google Scholar]
- 40.Elmelund M, Klarskov N, Bagi P, et al. Renal deterioration after spinal cord injury is associated with length of detrusor contractions during cystometry – a study with a median of 41 years followup. Neurourol Urodyn. 2017;36:1607–15. doi: 10.1002/nau.23163. [DOI] [PubMed] [Google Scholar]
- 41.Cameron AP, Rodriguez GM, Schomer KG. Systematic review of urological followup after spinal cord injury. J Urol. 2012;187:391–7. doi: 10.1016/j.juro.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 42.Ramachandra P, Palazzi KL, Holmes NM, et al. Children with spinal abnormalities have an increased health burden from upper tract urolithiasis. Urology. 2014;83:1378–82. doi: 10.1016/j.urology.2013.12.050. [DOI] [PubMed] [Google Scholar]
- 43.Welk B, Fuller A, Razvi H, et al. Renal stone disease in spinal-cord-injured patients. J Endourol. 2012;26:954–9. doi: 10.1089/end.2012.0063. [DOI] [PubMed] [Google Scholar]
- 44.Fletcher SG, Dillon BE, Gilchrist AS, et al. Renal deterioration in multiple sclerosis patients with neurovesical dysfunction. Mult Scler. 2013;19:1169–74. doi: 10.1177/1352458512474089. [DOI] [PubMed] [Google Scholar]
- 45.Welk B, McIntyre A, Teasell R, et al. Bladder cancer in individuals with spinal cord injuries. Spinal Cord. 2013;51:516–21. doi: 10.1038/sc.2013.33. [DOI] [PubMed] [Google Scholar]
- 46.Alimi Q, Hascoet J, Manunta A, et al. Reliability of urinary cytology and cystoscopy for the screening and diagnosis of bladder cancer in patients with neurogenic bladder: A systematic review. Neurourol Urodyn. 2018;37:916–25. doi: 10.1002/nau.23395. [DOI] [PubMed] [Google Scholar]
- 47.Manack A, Motsko SP, Haag-Molkenteller C, et al. Epidemiology and healthcare utilization of neurogenic bladder patients in a US claims database. Neurourol Urodyn. 2011;30:395–401. doi: 10.1002/nau.21003. [DOI] [PubMed] [Google Scholar]
- 48.Cameron AP, Lai J, Saigal CS, et al. Project NUDiA. Urological surveillance and medical complications after spinal cord injury in the United States. Urology. 2015;86:506–10. doi: 10.1016/j.urology.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang HH, Lloyd JC, Wiener JS, et al. Nationwide trends and variations in urological surgical interventions and renal outcome in patients with spina bifida. J Urol. 2016;195:1189–94. doi: 10.1016/j.juro.2015.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sung BM, Oh DJ, Choi MH, et al. Chronic kidney disease in neurogenic bladder. Nephrology (Carlton) 2018;23:231–6. doi: 10.1111/nep.12990. [DOI] [PubMed] [Google Scholar]
- 51.Lawrenson R, Wyndaele JJ, Vlachonikolis I, et al. Renal failure in patients with neurogenic lower urinary tract dysfunction. Neuroepidemiology. 2001;20:138–43. doi: 10.1159/000054774. [DOI] [PubMed] [Google Scholar]
- 52.McGuire EJ, Woodside JR, Borden TA, et al. Prognostic value of urodynamic testing in myelodysplastic patients. J Urol. 1981;126:205–9. doi: 10.1016/S0022-5347(17)54449-3. [DOI] [PubMed] [Google Scholar]
- 53.Ghoniem GM, Bloom DA, McGuire EJ, et al. Bladder compliance in meningomyelocele children. J Urol. 1989;141:1404–6. doi: 10.1016/S0022-5347(17)41323-1. [DOI] [PubMed] [Google Scholar]
- 54.Gerridzen RG, Thijssen AM, Dehoux E. Risk factors for upper tract deterioration in chronic spinal cord injury patients. J Urol. 1992;147:416–8. doi: 10.1016/S0022-5347(17)37254-3. [DOI] [PubMed] [Google Scholar]
- 55.Wyndaele JJ. Urology in spinal cord injured patients. Paraplegia. 1987;25:267–9. doi: 10.1038/sc.1987.49. [DOI] [PubMed] [Google Scholar]
- 56.Chartier-Kastler E, Ruffion A. [Treatment of vesicoureteric reflux and neurogenic bladder]. Prog Urol. 2007;17:470–2. doi: 10.1016/S1166-7087(07)92350-7. [DOI] [PubMed] [Google Scholar]
- 57.Verpoorten C, Buyse GM. The neurogenic bladder: Medical treatment. Pediatr Nephrol. 2008;23:717–25. doi: 10.1007/s00467-007-0691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dray EV, Cameron AP. Identifying patients with high-risk neurogenic bladder: Beyond detrusor leak point pressure. Urol Clin North Am. 2017;44:441–52. doi: 10.1016/j.ucl.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 59.Ruffion A, Villar E, Denys P, et al. [Renal failure and neurogenic bladder]. Prog Urol. 2007;17:424–30. doi: 10.1016/S1166-7087(07)92341-6. [DOI] [PubMed] [Google Scholar]
- 60.Elmelund M, Oturai PS, Toson B, et al. Forty-five-year followup on the renal function after spinal cord injury. Spinal Cord. 2016;54:445–51. doi: 10.1038/sc.2015.242. [DOI] [PubMed] [Google Scholar]
- 61.Ginsberg D. The epidemiology and pathophysiology of neurogenic bladder. Am J Manag Care. 2013;19:s191–6. [PubMed] [Google Scholar]
- 62.Westney OL. The neurogenic bladder and incontinent urinary diversion. Urol Clin North Am. 2010;37:581–92. doi: 10.1016/j.ucl.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 63.Ruffion A, Chartier-Kastler E. [Specific features of urethral diseases in spinal cord injury patients]. Prog Urol. 2007;17:436–9. doi: 10.1016/S1166-7087(07)92343-X. [DOI] [PubMed] [Google Scholar]
- 64.Gormley EA. Urologic complications of the neurogenic bladder. Urol Clin North Am. 2010;37:601–7. doi: 10.1016/j.ucl.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 65.Stoffel JT, McGuire EJ. Outcome of urethral closure in patients with neurologic impairment and complete urethral destruction. Neurourol Urodyn. 2006;25:19–22. doi: 10.1002/nau.20146. [DOI] [PubMed] [Google Scholar]
- 66.Lekka E, Lee LK. Successful treatment with intradetrusor botulinum-A toxin for urethral urinary leakage (catheter bypassing) in patients with end-staged multiple sclerosis and indwelling suprapubic catheters. Eur Urol. 2006;50:806–9. doi: 10.1016/j.eururo.2005.12.015. discussion 9–10. [DOI] [PubMed] [Google Scholar]
- 67.Fragalà E, Russo GI, Di Rosa A, et al. Relationship between urodynamic findings and sexual function in multiple sclerosis patients with lower urinary tract dysfunction. Eur J Neurol. 2015;22:485–92. doi: 10.1111/ene.12595. [DOI] [PubMed] [Google Scholar]
- 68.Wehbe SA, Kellogg S, Whitmore K. Urogenital complaints and female sexual dysfunction. Part 2. J Sex Med. 2010;7:2304–17. doi: 10.1111/j.1743-6109.2010.01951.x. quiz 18–9. [DOI] [PubMed] [Google Scholar]
- 69.Nadeau G. Incontinence urinaire et sexualité. In: Lavoisier, editor. Médecine sexuelle Fondements et pratique. Paris: 2016. pp. 412–8. [Google Scholar]
- 70.Serati M, Salvatore S, Uccella S, et al. Female urinary incontinence during intercourse: A review on an understudied problem for women’s sexuality. J Sex Med. 2009;6:40–8. doi: 10.1111/j.1743-6109.2008.01055.x. [DOI] [PubMed] [Google Scholar]
- 71.Martínez-Salamanca JI, Carballido J, Eardley I, et al. Phosphodiesterase type 5 inhibitors in the management of non-neurogenic male lower urinary tract symptoms: Critical analysis of current evidence. Eur Urol. 2011;60:527–35. doi: 10.1016/j.eururo.2011.05.054. [DOI] [PubMed] [Google Scholar]
- 72.Zelaya JE, Murchison C, Cameron M. Associations between bladder dysfunction and falls in people with relapsing-remitting multiple sclerosis. Int J MS Care. 2017;19:184–90. doi: 10.7224/1537-2073.2016-049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Flack C, Powell CR. The worldwide economic impact of neurogenic bladder. Curr Bladder Dysfunct Rep. 2015;10:350–4. doi: 10.1007/s11884-015-0323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Benevento BT, Sipski ML. Neurogenic bladder, neurogenic bowel, and sexual dysfunction in people with spinal cord injury. Phys Ther. 2002;82:601–12. [PubMed] [Google Scholar]
- 75.Siroky MB. Pathogenesis of bacteriuria and infection in the spinal cord injured patient. Am J Med. 2002;113(Suppl 1A):67S–79S. doi: 10.1016/S0002-9343(02)01061-6. [DOI] [PubMed] [Google Scholar]
- 76.Biering-Sorensen F, Nielans HM, Dorflinger T, et al. Urological situation five years after spinal cord injury. Scand J Urol Nephrol. 1999;33:157–61. doi: 10.1080/003655999750015925. [DOI] [PubMed] [Google Scholar]
- 77.Togan T, Azap OK, Durukan E, et al. The prevalence, etiologic agents and risk factors for urinary tract infection among spinal cord injury patients. Jundishapur J Microbiol. 2014;7:e8905. doi: 10.5812/jjm.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yoon SB, Lee BS, Lee KD, et al. Comparison of bacterial strains and antibiotic susceptibilities in urinary isolates of spinal cord injury patients from the community and hospital. Spinal Cord. 2014;52:298–301. doi: 10.1038/sc.2014.10. [DOI] [PubMed] [Google Scholar]
- 79.Groen J, Pannek J, Castro Diaz D, et al. Summary of European Association of Urology (EAU) guidelines on neuro-urology. Eur Urol. 2016;69:324–33. doi: 10.1016/j.eururo.2015.07.071. [DOI] [PubMed] [Google Scholar]
- 80.Goetz LL, Cardenas DD, Kennelly M, et al. International spinal cord injury urinary tract infection basic data set. Spinal Cord. 2013;51:700–4. doi: 10.1038/sc.2013.72. [DOI] [PubMed] [Google Scholar]
- 81.Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 international clinical practice guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625–63. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 82.Krebs J, Wollner J, Pannek J. Risk factors for symptomatic urinary tract infections in individuals with chronic neurogenic lower urinary tract dysfunction. Spinal Cord. 2016;54:682–6. doi: 10.1038/sc.2015.214. [DOI] [PubMed] [Google Scholar]
- 83.Esclarin De Ruz A, Garcia Leoni E, Herruzo Cabrera R. Epidemiology and risk factors for urinary tract infection in patients with spinal cord injury. J Urol. 2000;164:1285–9. doi: 10.1016/S0022-5347(05)67157-1. [DOI] [PubMed] [Google Scholar]
- 84.Siddiq DM, Darouiche RO. New strategies to prevent catheter-associated urinary tract infections. Nat Rev Urol. 2012;9:305–14. doi: 10.1038/nrurol.2012.68. [DOI] [PubMed] [Google Scholar]
- 85.Hartstein AI, Garber SB, Ward TT, et al. Nosocomial urinary tract infection: A prospective evaluation of 108 catheterized patients. Infect Control. 1981;2:380–6. doi: 10.1017/S0195941700055533. [DOI] [PubMed] [Google Scholar]
- 86.Salameh A, Al Mohajer M, Daroucihe RO. Prevention of urinary tract infections in patients with spinal cord injury. CMAJ. 2015;187:807–11. doi: 10.1503/cmaj.141044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cox L, He C, Bevins J, et al. Gentamicin bladder instillations decrease symptomatic urinary tract infections in neurogenic bladder patients on intermittent catheterization. Can Urol Assoc J. 2017;11:E350–4. doi: 10.5489/cuaj.4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morton SC, Shekelle PG, Adams JL, et al. Antimicrobial prophylaxis for urinary tract infection in persons with spinal cord dysfunction. Arch Phys Med Rehabil. 2002;83:129–38. doi: 10.1053/apmr.2002.26605. [DOI] [PubMed] [Google Scholar]
- 89.Krassioukov A, Warburton DE, Teasell R, et al. Spinal Cord Injury Rehabilitation Evidence Research Team. A systematic review of the management of autonomic dysreflexia after spinal cord injury. Arch Phys Med Rehabil. 2009;90:682–95. doi: 10.1016/j.apmr.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Walter M, Kran S, Nigro M, et al. OnabotulinumtoxinA for neurogenic detrusor overactivity not only reduces the frequency and severity of autonomic dysreflexia safely but significantly improves quality of life for individuals with spinal cord injury. Euro Urol. 2018;17:e1357. doi: 10.1016/S1569-9056(18)31782-2. [DOI] [Google Scholar]
- 91.Zlatev DV, Shem K, Elliott CS. How many spinal cord injury patients can catheterize their own bladder? The epidemiology of upper extremity function as it affects bladder management. Spinal Cord. 2016;54:287–91. doi: 10.1038/sc.2015.169. [DOI] [PubMed] [Google Scholar]
- 92.Weld KJ, Dmochowski RR. Effect of bladder management on urological complications in spinal cord injured patients. J Urol. 2000;163:768–72. doi: 10.1016/S0022-5347(05)67800-7. [DOI] [PubMed] [Google Scholar]
- 93.McIntyre A, Cheung KY, Kwok C, et al. Quality of life and bladder management post-spinal cord injury: A systematic review. Appl Res Qual Life. 2014;9:1081–96. doi: 10.1007/s11482-013-9289-8. [DOI] [Google Scholar]
- 94.Jamil F. Towards a catheter-free status in neurogenic bladder dysfunction: A review of bladder management options in spinal cord injury (SCI) Spinal Cord. 2001;39:355–61. doi: 10.1038/sj.sc.3101132. [DOI] [PubMed] [Google Scholar]
- 95.Gao Y, Danforth T, Ginsberg DA. Urologic management and complications in spinal cord injury patients: A 40- to 50-year followup study. Urology. 2017;104:52–8. doi: 10.1016/j.urology.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 96.Lapides J, Diokno AC, Silber SJ, et al. Clean, intermittent self-catheterization in the treatment of urinary tract disease. J Urol. 1972;107:458–61. doi: 10.1016/S0022-5347(17)61055-3. [DOI] [PubMed] [Google Scholar]
- 97.Binard JE, Persky L, Lockhart JL, et al. Intermittent catheterization the right way! (Volume vs. time-directed) J Spinal Cord Med. 1996;19:194–6. doi: 10.1080/10790268.1996.11719432. [DOI] [PubMed] [Google Scholar]
- 98.Cornejo-Davila V, Duran-Ortiz S, Pacheco-Gahbler C. Incidence of urethral stricture in patients with spinal cord injury treated with clean intermittent self-catheterization. Urology. 2017;99:260–4. doi: 10.1016/j.urology.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 99.Christison K, Walter M, Wyndaele JJM, et al. Intermittent catheterization: The devil is in the details. J Neurotrauma. 2018 Feb 1; doi: 10.1089/neu.2017.5413. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Talbot HS, Mahoney EM, Jaffee SR. The effects of prolonged urethral catheterization. I. Persistence of normal renal structure and function. J Urol. 1959;81:138–45. doi: 10.1016/S0022-5347(17)65979-2. [DOI] [PubMed] [Google Scholar]
- 101.Dewire DM, Owens RS, Anderson GA, et al. A comparison of the urological complications associated with long-term management of quadriplegics with and without chronic indwelling urinary catheters. J Urol. 1992;147:1069–71. doi: 10.1016/S0022-5347(17)37472-4. discussion 71–2. [DOI] [PubMed] [Google Scholar]
- 102.MacDiarmid SA, Arnold EP, Palmer NB, et al. Management of spinal cord injured patients by indwelling suprapubic catheterization. J Urol. 1995;154:492–4. doi: 10.1016/S0022-5347(01)67083-6. [DOI] [PubMed] [Google Scholar]
- 103.Sugimura T, Arnold E, English S, et al. Chronic suprapubic catheterization in the management of patients with spinal cord injuries: Analysis of upper and lower urinary tract complications. BJU Int. 2008;101:1396–400. doi: 10.1111/j.1464-410X.2007.07404.x. [DOI] [PubMed] [Google Scholar]
- 104.Warren JW. Catheter-associated urinary tract infections. Infect Dis Clin North Am. 1987;1:823–54. [PubMed] [Google Scholar]
- 105.Madhuvrata P, Singh M, Hasafa Z, et al. Anticholinergic drugs for adult neurogenic detrusor overactivity: A systematic review and meta-analysis. Eur Urol. 2012;62:816–30. doi: 10.1016/j.eururo.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 106.Madersbacher H, Murtz G, Stohrer M. Neurogenic detrusor overactivity in adults: A review on efficacy, tolerability, and safety of oral anti-muscarinics. Spinal Cord. 2013;51:432–41. doi: 10.1038/sc.2013.19. [DOI] [PubMed] [Google Scholar]
- 107.Bennett N, O’Leary M, Patel AS, et al. Can higher doses of oxybutynin improve efficacy in neurogenic bladder? J Urol. 2004;171:749–51. doi: 10.1097/01.ju.0000103274.38694.b1. [DOI] [PubMed] [Google Scholar]
- 108.Horstmann M, Schaefer T, Aguilar Y, et al. Neurogenic bladder treatment by doubling the recommended anti-muscarinic dosage. Neurourol Urodyn. 2006;25:441–5. doi: 10.1002/nau.20289. [DOI] [PubMed] [Google Scholar]
- 109.Amend B, Hennenlotter J, Schäfer T, et al. Effective treatment of neurogenic detrusor dysfunction by combined high-dosed anti-muscarinics without increased side-effects. Eur Urol. 2008;53:1021–8. doi: 10.1016/j.eururo.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 110.Stöhrer M, Blok B, Castro-Diaz D, et al. European Association of Urology. Guidelines on neurogenic lower urinary tract dysfunction. Eur Urol. 2009;56:81–8. doi: 10.1016/j.eururo.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 111.Çetinel B, Önal B, Can G, et al. Risk factors predicting upper urinary tract deterioration in patients with spinal cord injury: A retrospective study. Neurourol Urodyn. 2017;36:653–8. doi: 10.1002/nau.22984. [DOI] [PubMed] [Google Scholar]
- 112.Kim YH, Bird ET, Priebe M, et al. The role of oxybutynin in spinal cord injured patients with indwelling catheters. J Urol. 1997;158:2083–6. doi: 10.1016/S0022-5347(01)68161-8. [DOI] [PubMed] [Google Scholar]
- 113.Wollner J, Pannek J. Initial experience with the treatment of neurogenic detrusor overactivity with a new [beta]-3 agonist (mirabegron) in patients with spinal cord injury. Spinal Cord. 2016;54:78–82. doi: 10.1038/sc.2015.195. [DOI] [PubMed] [Google Scholar]
- 114.Welk B. Ongoing study: Clinical Trials. Urodynamic and clinical efficacy of mirabegron for neurogenic bladder patients. Gov Identifier NCT02044510. [Google Scholar]
- 115.Schurch B, de Seze M, Denys P, et al. Botulinum toxin type A is a safe and effective treatment for neurogenic urinary incontinence: Results of a single treatment, randomized, placebo controlled 6-month study. J Urol. 2005;174:196–200. doi: 10.1097/01.ju.0000162035.73977.1c. [DOI] [PubMed] [Google Scholar]
- 116.Cruz F, Herschorn S, Aliotta P, et al. Efficacy and safety of onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity: A randomized, double-blind, placebo-controlled trial. Eur Urol. 2011;60:742–50. doi: 10.1016/j.eururo.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 117.Mehta S, Hill D, McIntyre A, et al. Meta-analysis of botulinum toxin A detrusor injections in the treatment of neurogenic detrusor overactivity after spinal cord injury. Arch Phys Med Rehabil. 2013;94:1473–81. doi: 10.1016/j.apmr.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 118.Mangera A, Apostolidis A, Andersson KE, et al. An updated systematic review and statistical comparison of standardized mean outcomes for the use of botulinum toxin in the management of lower urinary tract disorders. Eur Urol. 2014;65:981–90. doi: 10.1016/j.eururo.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 119.Cheng T, Shuang WB, Jia DD, et al. Efficacy and safety of onabotulinumtoxinA in patients with neurogenic detrusor overactivity: A systematic review and meta-analysis of randomized controlled trials. PLoS One. 2016;11:e0159307. doi: 10.1371/journal.pone.0159307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ginsberg D, Gousse A, Keppenne V, et al. Phase 3 efficacy and tolerability study of onabotulinumtoxinA for urinary incontinence from neurogenic detrusor overactivity. J Urol. 2012;187:2131–9. doi: 10.1016/j.juro.2012.01.125. [DOI] [PubMed] [Google Scholar]
- 121.Food and Drug Administration. Highlights of prescribing information Botox® 2016. [Accessed May 30, 2019]. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2016/103000s5252lbl.pdf.
- 122.Del Popolo G, Filocamo MT, Li Marzi V, et al. Neurogenic detrusor overactivity treated with english botulinum toxin a: 8-year experience of one single centre. Eur Urol. 2008;53:1013–9. doi: 10.1016/j.eururo.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 123.Schroder A, Albrecht U, Schnitker J, et al. Efficacy, safety, and tolerability of intravesically administered 0.1% oxybutynin hydrochloride solution in adult patients with neurogenic bladder: A randomized, prospective, controlled, multicentre trial. Neurourol Urodyn. 2016;35:582–8. doi: 10.1002/nau.22755. [DOI] [PubMed] [Google Scholar]
- 124.Haferkamp A, Staehler G, Gerner HJ, et al. Dosage escalation of intravesical oxybutynin in the treatment of neurogenic bladder patients. Spinal Cord. 2000;38:250–4. doi: 10.1038/sj.sc.3100995. [DOI] [PubMed] [Google Scholar]
- 125.Pannek J, Sommerfeld HJ, Botel U, et al. Combined intravesical and oral oxybutynin chloride in adult patients with spinal cord injury. Urology. 2000;55:358–62. doi: 10.1016/S0090-4295(99)00540-3. [DOI] [PubMed] [Google Scholar]
- 126.Buyse G, Waldeck K, Verpoorten C, et al. Intravesical oxybutynin for neurogenic bladder dysfunction: Less systemic side effects due to reduced first pass metabolism. J Urol. 1998;160:892–6. doi: 10.1016/S0022-5347(01)62828-3. [DOI] [PubMed] [Google Scholar]
- 127.Mizunaga M, Miyata M, Kaneko S, et al. Intravesical instillation of oxybutynin hydrochloride therapy for patients with a neuropathic bladder. Spinal Cord. 1994;32:25–9. doi: 10.1038/sc.1994.5. [DOI] [PubMed] [Google Scholar]
- 128.Kim JH, Rivas DA, Shenot PJ, et al. Intravesical resiniferatoxin for refractory detrusor hyperreflexia: A multicentre, blinded, randomized, placebo-controlled trial. J Spinal Cord Med. 2003;26:358–63. doi: 10.1080/10790268.2003.11753706. [DOI] [PubMed] [Google Scholar]
- 129.Geirsson G, Fall M, Sullivan L. Clinical and urodynamic effects of intravesical capsaicin treatment in patients with chronic traumatic spinal detrusor hyperreflexia. J Urol. 1995;154:1825–9. doi: 10.1016/S0022-5347(01)66793-4. [DOI] [PubMed] [Google Scholar]
- 130.Phé V, Schneider MP, Peyronnet B, et al. Intravesical vanilloids for treating neurogenic lower urinary tract dysfunction in patients with multiple sclerosis: A systematic review and meta- analysis. A report from the Neuro-Urology Promotion Committee of the International Continence Society (ICS) Neurourol Urodyn. 2018;37:67–82. doi: 10.1002/nau.23314. [DOI] [PubMed] [Google Scholar]
- 131.Giannantoni A, Di Stasi SM, Stephen RL, et al. Intravesical capsaicin vs. resiniferatoxin in patients with detrusor hyperreflexia: A prospective randomized study. J Urol. 2002;167:1710–4. doi: 10.1016/S0022-5347(05)65183-X. [DOI] [PubMed] [Google Scholar]
- 132.Giannantoni A, Di Stasi SM, Stephen RL, et al. Intravesical resiniferatoxin vs. botulinum-A toxin injections for neurogenic detrusor overactivity: A prospective randomized study. J Urol. 2004;172:240–3. doi: 10.1097/01.ju.0000132152.53532.5d. [DOI] [PubMed] [Google Scholar]
- 133.Kessler TM, Wollner J, Kozomara M, et al. [Sacral neuromodulation for neurogenic bladder dysfunction]. Urologe A. 2012;51:179–83. doi: 10.1007/s00120-011-2779-0. [DOI] [PubMed] [Google Scholar]
- 134.Brindley GS. The first 500 patients with sacral anterior root stimulator implants: General description. Paraplegia. 1994;32:795–805. doi: 10.1038/sc.1994.126. [DOI] [PubMed] [Google Scholar]
- 135.Krasmik D, Krebs J, van Ophoven A, et al. Urodynamic results, clinical efficacy, and complication rates of sacral intradural deafferentation and sacral anterior root stimulation in patients with neurogenic lower urinary tract dysfunction resulting from complete spinal cord injury. Neurourol Urodyn. 2014;33:1202–6. doi: 10.1002/nau.22486. [DOI] [PubMed] [Google Scholar]
- 136.Martens FM, den Hollander PP, Snoek GJ, et al. Quality of life in complete spinal cord injury patients with a Brindley bladder stimulator compared to a matched control group. Neurourol Urodyn. 2011;30:551–5. doi: 10.1002/nau.21012. [DOI] [PubMed] [Google Scholar]
- 137.Benard A, Verpillot E, Grandoulier AS, et al. Comparative cost-effectiveness analysis of sacral anterior root stimulation for rehabilitation of bladder dysfunction in spinal cord injured patients. Neurosurgery. 2013;73:600–8. doi: 10.1227/NEU.0000000000000033. discussion 8. [DOI] [PubMed] [Google Scholar]
- 138.Sauerwein D. [Surgical treatment of spastic bladder paralysis in paraplegic patients. Sacral deafferentation with implantation of a sacral anterior root stimulator]. Urologe A. 1990;29:196–203. [PubMed] [Google Scholar]
- 139.Van Kerrebroeck PE, Koldewijn EL, Rosier PF, et al. Results of the treatment of neurogenic bladder dysfunction in spinal cord injury by sacral posterior root rhizotomy and anterior sacral root stimulation. J Urol. 1996;155:1378–81. doi: 10.1016/S0022-5347(01)66272-4. [DOI] [PubMed] [Google Scholar]
- 140.Kutzenberger J. Surgical therapy of neurogenic detrusor overactivity (hyperreflexia) in paraplegic patients by sacral deafferentation and implant driven micturition by sacral anterior root stimulation: Methods, indications, results, complications, and future prospects. Acta Neurochir Suppl. 2007;97:333–9. doi: 10.1007/978-3-211-33079-1_44. [DOI] [PubMed] [Google Scholar]
- 141.Vignes JR, Bauchet L, Ohanna F. Dorsal rhizotomy combined with anterior sacral root stimulation for neurogenic bladder. Acta Neurochir Suppl. 2007;97:323–31. doi: 10.1007/978-3-211-33079-1_43. [DOI] [PubMed] [Google Scholar]
- 142.Kutzenberger J, Domurath B, Sauerwein D. Spastic bladder and spinal cord injury: Seventeen years of experience with sacral deafferentation and implantation of an anterior root stimulator. Artificial Organs. 2005;29:239–41. doi: 10.1111/j.1525-1594.2005.29043.x. [DOI] [PubMed] [Google Scholar]
- 143.Bhadra N, Grunewald V, Creasey G, et al. Selective suppression of sphincter activation during sacral anterior nerve root stimulation. Neurourol Urodyn. 2002;21:55–64. doi: 10.1002/nau.2068. [DOI] [PubMed] [Google Scholar]
- 144.Kirkham AP, Knight SL, Craggs MD, et al. Neuromodulation through sacral nerve roots 2 to 4 with a Finetech-Brindley sacral posterior and anterior root stimulator. Spinal Cord. 2002;40:272–81. doi: 10.1038/sj.sc.3101278. [DOI] [PubMed] [Google Scholar]
- 145.Schumacher S, Bross S, Scheepe JR, et al. Extradural cold block for selective neurostimulation of the bladder: Development of a new technique. J Urol. 1999;161:950–4. doi: 10.1016/S0022-5347(01)61827-5. [DOI] [PubMed] [Google Scholar]
- 146.Krebs J, Grasmucke D, Potzel T, et al. Charcot arthropathy of the spine in spinal cord injured individuals with sacral deafferentation and anterior root stimulator implantation. Neurourol Urodyn. 2016;35:241–5. doi: 10.1002/nau.22706. [DOI] [PubMed] [Google Scholar]
- 147.Kabay SC, Kabay S, Yucel M, et al. Acute urodynamic effects of percutaneous posterior tibial nerve stimulation on neurogenic detrusor overactivity in patients with Parkinson’s disease. Neurourol Urodyn. 2009;28:62–7. doi: 10.1002/nau.20593. [DOI] [PubMed] [Google Scholar]
- 148.Kabay S, Kabay SC, Yucel M, et al. The clinical and urodynamic results of a 3-month percutaneous posterior tibial nerve stimulation treatment in patients with multiple sclerosis-related neurogenic bladder dysfunction. Neurourol Urodyn. 2009;28:964–8. doi: 10.1002/nau.20733. [DOI] [PubMed] [Google Scholar]
- 149.Gobbi C, Digesu G, Khullar V, et al. Percutaneous posterior tibial nerve stimulation as an effective treatment of refractory lower urinary tract symptoms in patients with multiple sclerosis: Preliminary data from a multicentre, prospective, open-label trial. Mult Scler. 2011;17:1514–9. doi: 10.1177/1352458511414040. [DOI] [PubMed] [Google Scholar]
- 150.Gaziev G, Topazio L, Iacovelli V, et al. Percutaneous tibial nerve stimulation (PTNS) efficacy in the treatment of lower urinary tract dysfunctions: A systematic review. BMC Urol. 2013;13:61. doi: 10.1186/1471-2490-13-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen G, Liao L, Li Y. The possible role of percutaneous tibial nerve stimulation using adhesive skin surface electrodes in patients with neurogenic detrusor overactivity secondary to spinal cord injury. Int Urol Nephrol. 2015;47:451–5. doi: 10.1007/s11255-015-0911-6. [DOI] [PubMed] [Google Scholar]
- 152.6th International Consultation on Incontinence. Neurologic Urinary and Fecal Incontinence. In: Abrams P, Cardozo L, Wagg A, Wein A, editors. Incontinence. 16 ed. International Continence Society; 2017. pp. 1093–308. [Google Scholar]
- 153.Game X, Karsenty G, Chartier-Kastler E, et al. [Treatment of neurogenic detrusor hyperactivity: enterocystoplasty]. Prog Urol. 2007;17:584–96. doi: 10.1016/S1166-7087(07)92373-8. [DOI] [PubMed] [Google Scholar]
- 154.Gor RA, Elliott SP. Surgical management of neurogenic lower urinary tract dysfunction. Urol Clin North Am. 2017;44:475–90. doi: 10.1016/j.ucl.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 155.Biers SM, Venn SN, Greenwell TJ. The past, present and future of augmentation cystoplasty. BJU Int. 2012;109:1280–93. doi: 10.1111/j.1464-410X.2011.10650.x. [DOI] [PubMed] [Google Scholar]
- 156.Cetinel B, Kocjancic E, Demirdag C. Augmentation cystoplasty in neurogenic bladder. Investig Clin Urol. 2016;57:316–23. doi: 10.4111/icu.2016.57.5.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chartier-Kastler EJ, Mozer P, Denys P, et al. Neurogenic bladder management and cutaneous non-continent ileal conduit. Spinal Cord. 2002;40:443–8. doi: 10.1038/sj.sc.3101346. [DOI] [PubMed] [Google Scholar]
- 158.Hasan ST, Marshall C, Robson WA, et al. Clinical outcome and quality of life following enterocystoplasty for idiopathic detrusor instability and neurogenic bladder dysfunction. Br J Urol. 1995;76:551–7. doi: 10.1111/j.1464-410X.1995.tb07777.x. [DOI] [PubMed] [Google Scholar]
- 159.Sajadi KP, Goldman HB. Bladder augmentation and urinary diversion for neurogenic LUTS: Current indications. Curr Urol Rep. 2012;13:389–93. doi: 10.1007/s11934-012-0271-z. [DOI] [PubMed] [Google Scholar]
- 160.Scales CD, Jr, Wiener JS. Evaluating outcomes of enterocystoplasty in patients with spina bifida: A review of the literature. J Urol. 2008;180:2323–9. doi: 10.1016/j.juro.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 161.Shekarriz B, Upadhyay J, Demirbilek S, et al. Surgical complications of bladder augmentation: Comparison between various enterocystoplasties in 133 patients. Urology. 2000;55:123–8. doi: 10.1016/S0090-4295(99)00443-4. [DOI] [PubMed] [Google Scholar]
- 162.Gurung PM, Attar KH, Abdul-Rahman A, et al. Long-term outcomes of augmentation ileocystoplasty in patients with spinal cord injury: A minimum of 10 years of followup. BJU Int. 2012;109:1236–42. doi: 10.1111/j.1464-410X.2011.10509.x. [DOI] [PubMed] [Google Scholar]
- 163.Kilic N, Celayir S, Elicevik M, et al. Bladder augmentation: urodynamic findings and clinical outcome in different augmentation techniques. Eur J Pediatr Surg. 1999;9:29–32. doi: 10.1055/s-2008-1072208. [DOI] [PubMed] [Google Scholar]
- 164.Krebs J, Bartel P, Pannek J. Functional outcome of supratrigonal cystectomy and augmentation ileocystoplasty in adult patients with refractory neurogenic lower urinary tract dysfunction. Neurourol Urodyn. 2016;35:260–6. doi: 10.1002/nau.22709. [DOI] [PubMed] [Google Scholar]
- 165.Mundy AR, Stephenson TP. “Clam” ileocystoplasty for the treatment of refractory urge incontinence. Br J Urol. 1985;57:641–6. doi: 10.1111/j.1464-410X.1985.tb07023.x. [DOI] [PubMed] [Google Scholar]
- 166.Misseri R, Rosenbaum DH, Rink RC. Reflux in cystoplasties. Arch Esp Urol. 2008;61:213–7. doi: 10.4321/S0004-06142008000200015. [DOI] [PubMed] [Google Scholar]
- 167.DeFoor W, Tackett L, Minevich E, et al. Risk factors for spontaneous bladder perforation after augmentation cystoplasty. Urology. 2003;62:737–41. doi: 10.1016/S0090-4295(03)00678-2. [DOI] [PubMed] [Google Scholar]
- 168.McInerney PD, DeSouza N, Thomas PJ, et al. The role of urodynamic studies in the evaluation of patients with augmentation cystoplasties. Br J Urol. 1995;76:475–8. doi: 10.1111/j.1464-410X.1995.tb07749.x. [DOI] [PubMed] [Google Scholar]
- 169.Welk B, Herschorn S, Law C, et al. Population based assessment of enterocystoplasty complications in adults. J Urol. 2012;188:464–9. doi: 10.1016/j.juro.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 170.Higuchi TT, Granberg CF, Fox JA, et al. Augmentation cystoplasty and risk of neoplasia: Fact, fiction, and controversy. J Urol. 2010;184:2492–6. doi: 10.1016/j.juro.2010.08.038. [DOI] [PubMed] [Google Scholar]
- 171.Metcalfe PD, Cain MP, Kaefer M, et al. What is the need for additional bladder surgery after bladder augmentation in childhood? J Urol. 2006;176:1801–5. doi: 10.1016/j.juro.2006.03.126. discussion 5. [DOI] [PubMed] [Google Scholar]
- 172.Shaw J, Lewis MA. Bladder augmentation surgery – what about the malignant risk? Eur J Pediatr Surg. 1999;9(Suppl1):39–40. doi: 10.1055/s-2008-1072313. [DOI] [PubMed] [Google Scholar]
- 173.Mitrofanoff P. [Trans-appendicular continent cystostomy in the management of the neurogenic bladder]. Chir Pediatr. 1980;21:297–305. [PubMed] [Google Scholar]
- 174.Ardelt PU, Woodhouse CR, Riedmiller H, et al. The efferent segment in continent cutaneous urinary diversion: A comprehensive review of the literature. BJU Int. 2012;109:288–97. doi: 10.1111/j.1464-410X.2011.10242.x. [DOI] [PubMed] [Google Scholar]
- 175.Casale AJ. A long continent ileovesicostomy using a single piece of bowel. J Urol. 1999;162:1743–5. doi: 10.1016/S0022-5347(05)68228-6. [DOI] [PubMed] [Google Scholar]
- 176.Monti PR, Lara RC, Dutra MA, et al. New techniques for construction of efferent conduits based on the Mitrofanoff principle. Urology. 1997;49:112–5. doi: 10.1016/S0090-4295(96)00503-1. [DOI] [PubMed] [Google Scholar]
- 177.Narayanaswamy B, Wilcox DT, Cuckow PM, et al. The Yang-Monti ileovesicostomy: A problematic channel? BJU Int. 2001;87:861–5. doi: 10.1046/j.1464-410x.2001.02208.x. [DOI] [PubMed] [Google Scholar]
- 178.Akerlund S, Delin K, Kock NG, et al. Renal function and upper urinary tract configuration following urinary diversion to a continent ileal reservoir (Kock pouch): A prospective 5–11-year followup after reservoir construction. J Urol. 1989;142:964–8. doi: 10.1016/S0022-5347(17)38954-1. [DOI] [PubMed] [Google Scholar]
- 179.Roth S, Semjonow A, Waldner M, et al. Risk of bowel dysfunction with diarrhea after continent urinary diversion with ileal and ileocecal segments. J Urol. 1995;154:1696–9. doi: 10.1016/S0022-5347(01)66754-5. [DOI] [PubMed] [Google Scholar]
- 180.Stein R, Lotz J, Fisch M, et al. Vitamin metabolism in patients with a Mainz pouch I: Long-term followup. J Urol. 1997;157:44–7. doi: 10.1016/S0022-5347(01)65276-5. [DOI] [PubMed] [Google Scholar]
- 181.Sorokin I, De E. Options for independent bladder management in patients with spinal cord injury and hand function prohibiting intermittent catheterization. Neurourol Urodyn. 2015;34:167–76. doi: 10.1002/nau.22516. [DOI] [PubMed] [Google Scholar]
- 182.Schwartz SL, Kennelly MJ, McGuire EJ, et al. Incontinent ileo-vesicostomy urinary diversion in the treatment of lower urinary tract dysfunction. J Urol. 1994;152:99–102. doi: 10.1016/S0022-5347(17)32826-4. [DOI] [PubMed] [Google Scholar]
- 183.Atan A, Konety BR, Nangia A, et al. Advantages and risks of ileovesicostomy for the management of neuropathic bladder. Urology. 1999;54:636–40. doi: 10.1016/S0090-4295(99)00192-2. [DOI] [PubMed] [Google Scholar]
- 184.Hellenthal NJ, Short SS, O’Connor RC, et al. Incontinent ileovesicostomy: Long-term outcomes and complications. Neurourol Urodyn. 2009;28:483–6. doi: 10.1002/nau.20695. [DOI] [PubMed] [Google Scholar]
- 185.Leng WW, Faerber G, Del Terzo M, et al. Long-term outcome of incontinent ileovesicostomy management of severe lower urinary tract dysfunction. J Urol. 1999;161:1803–6. doi: 10.1016/S0022-5347(05)68808-8. [DOI] [PubMed] [Google Scholar]
- 186.Tan HJ, Stoffel J, Daignault S, et al. Ileovesicostomy for adults with neurogenic bladders: Complications and potential risk factors for adverse outcomes. Neurourol Urodyn. 2008;27:238–43. doi: 10.1002/nau.20467. [DOI] [PubMed] [Google Scholar]
- 187.Vanni AJ, Stoffel JT. Ileovesicostomy for the neurogenic bladder patient: Outcome and cost comparison of open and robotic assisted techniques. Urology. 2011;77:1375–80. doi: 10.1016/j.urology.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 188.Kato H, Hosaka K, Kobayashi S, et al. Fate of tetraplegic patients managed by ileal conduit for urinary control: Long-term followup. Int J Urol. 2002;9:253–6. doi: 10.1046/j.1442-2042.2002.00463.x. [DOI] [PubMed] [Google Scholar]
- 189.Fazili T, Bhat TR, Masood S, et al. Fate of the leftover bladder after supravesical urinary diversion for benign disease. J Urol. 2006;176:620–1. doi: 10.1016/j.juro.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 190.Singh G, Wilkinson JM, Thomas DG. Supravesical diversion for incontinence: A long-term followup. Br J Urol. 1997;79:348–53. doi: 10.1046/j.1464-410X.1997.01007.x. [DOI] [PubMed] [Google Scholar]
- 191.Legrand G, Roupret M, Comperat E, et al. Functional outcomes after management of end-stage neurological bladder dysfunction with ileal conduit in a multiple sclerosis population: A monocentric experience. Urology. 2011;78:937–41. doi: 10.1016/j.urology.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 192.Cheng JN, Lawrentschuk N, Gyomber D, et al. Cystectomy in patients with spinal cord injury: Indications and long-term outcomes. J Urol. 2010;184:92–8. doi: 10.1016/j.juro.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 193.Cohn JA, Large MC, Richards KA, et al. Cystectomy and urinary diversion as management of treatment-refractory benign disease: The impact of preoperative urological conditions on perioperative outcomes. Int J Urol. 2014;21:382–6. doi: 10.1111/iju.12284. [DOI] [PubMed] [Google Scholar]
- 194.DeLong J, Tighiouart H, Stoffel J. Urinary diversion/reconstruction for cases of catheter intolerant secondary progressive multiple sclerosis with refractory urinary symptoms. J Urol. 2011;185:2201–6. doi: 10.1016/j.juro.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 195.Guillotreau J, Castel-Lacanal E, Roumiguie M, et al. Prospective study of the impact on quality of life of cystectomy with ileal conduit urinary diversion for neurogenic bladder dysfunction. Neurourol Urodyn. 2011;30:1503–6. doi: 10.1002/nau.21121. [DOI] [PubMed] [Google Scholar]
- 196.Stein R, Fisch M, Ermert A, et al. Urinary diversion and orthotopic bladder substitution in children and young adults with neurogenic bladder: A safe option for treatment? J Urol. 2000;163:568–73. doi: 10.1016/S0022-5347(05)67933-5. [DOI] [PubMed] [Google Scholar]
- 197.Gross AJ, Sauerwein DH, Kutzenberger J, et al. Penile prostheses in paraplegic men. Br J Urol. 1996;78:262–4. doi: 10.1046/j.1464-410X.1996.08021.x. [DOI] [PubMed] [Google Scholar]
- 198.Perkash I, Kabalin JN, Lennon S, et al. Use of penile prostheses to maintain external condom catheter drainage in spinal cord injury patients. Paraplegia. 1992;30:327–32. doi: 10.1038/sc.1992.76. [DOI] [PubMed] [Google Scholar]
- 199.Barbalat Y, Rutman M. Detrusor-external sphincter dyssynergia: Review of minimally invasive and endoscopic management. Urology. 2016;90:3–7. doi: 10.1016/j.urology.2015.11.049. [DOI] [PubMed] [Google Scholar]
- 200.Ricottone AR, Pranikoff K, Steinmetz JR, et al. Long-term followup of sphincterotomy in the treatment of autonomic dysreflexia. Neurourol Urodyn. 1995;14:43–6. doi: 10.1002/nau.1930140108. [DOI] [PubMed] [Google Scholar]
- 201.Vainrib M, Reyblat P, Ginsberg DA. Long-term efficacy of repeat incisions of bladder neck/external sphincter in patients with spinal cord injury. Urology. 2014;84:940–5. doi: 10.1016/j.urology.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 202.Vapnek JM, Couillard DR, Stone AR. Is sphincterotomy the best management of the spinal cord injured bladder? J Urol. 1994;151:961–4. doi: 10.1016/S0022-5347(17)35134-0. [DOI] [PubMed] [Google Scholar]
- 203.Chancellor MB, Bennett C, Simoneau AR, et al. Sphincteric stent versus external sphincterotomy in spinal cord injured men: Prospective, randomized, multicentre trial. J Urol. 1999;161:1893–8. doi: 10.1016/S0022-5347(05)68837-4. [DOI] [PubMed] [Google Scholar]
- 204.Fontaine E, Hajri M, Rhein F, et al. Reappraisal of endoscopic sphincterotomy for post-traumatic neurogenic bladder: A prospective study. J Urol. 1996;155:277–80. doi: 10.1016/S0022-5347(01)66618-7. [DOI] [PubMed] [Google Scholar]
- 205.Juma S, Mostafavi M, Joseph A. Sphincterotomy: Long-term complications and warning signs. Neurourol Urodyn. 1995;14:33–41. doi: 10.1002/nau.1930140107. [DOI] [PubMed] [Google Scholar]
- 206.Kim YH, Kattan MW, Boone TB. Bladder leak point pressure: the measure for sphincterotomy success in spinal cord injured patients with external detrusor-sphincter dyssynergia. J Urol. 1998;159:493–6. doi: 10.1016/S0022-5347(01)63957-0. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 207.Noll F, Sauerwein D, Stohrer M. Transurethral sphincterotomy in quadriplegic patients: Long-term followup. Neurourol Urodyn. 1995;14:351–8. doi: 10.1002/nau.1930140409. [DOI] [PubMed] [Google Scholar]
- 208.Pan D, Troy A, Rogerson J, et al. Long-term outcomes of external sphincterotomy in a spinal injured population. J Urol. 2009;181:705–9. doi: 10.1016/j.juro.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 209.Pannek J, Hilfiker R, Goecking K, et al. Preoperative urodynamic assessment in patients with spinal cord lesions undergoing sphincterotomy: Is success predictable? Urol Int. 2009;83:386–91. doi: 10.1159/000251176. [DOI] [PubMed] [Google Scholar]
- 210.Yang CC, Mayo ME. External urethral sphincterotomy: Long-term followup. Neurourol Urodyn. 1995;14:25–31. doi: 10.1002/nau.1930140106. [DOI] [PubMed] [Google Scholar]
- 211.Eckford SB, Kohler-Ockmore J, Feneley RC. Long-term followup of transvaginal urethral closure and suprapubic cystostomy for urinary incontinence in women with multiple sclerosis. Br J Urol. 1994;74:319–21. doi: 10.1111/j.1464-410X.1994.tb16619.x. [DOI] [PubMed] [Google Scholar]
- 212.Ginger VA, Miller JL, Yang CC. Bladder neck closure and suprapubic tube placement in a debilitated patient population. Neurourol Urodyn. 2010;29:382–6. doi: 10.1002/nau.20751. [DOI] [PubMed] [Google Scholar]
- 213.Rovner ES, Goudelocke CM, Gilchrist A, et al. Transvaginal bladder neck closure with posterior urethral flap for devastated urethra. Urology. 2011;78:208–12. doi: 10.1016/j.urology.2010.11.054. [DOI] [PubMed] [Google Scholar]
- 214.Willis H, Safiano NA, Lloyd LK. Comparison of transvaginal and retropubic bladder neck closure with suprapubic catheter in women. J Urol. 2015;193:196–202. doi: 10.1016/j.juro.2014.07.091. [DOI] [PubMed] [Google Scholar]
- 215.Wyndaele JJ. Correlation between clinical neurological data and urodynamic function in spinal cord injured patients. Spinal Cord. 1997;35:213–6. doi: 10.1038/sj.sc.3100391. [DOI] [PubMed] [Google Scholar]
- 216.Bycroft J, Hamid R, Bywater H, et al. Variation in urological practice amongst spinal injuries units in the UK and Eire. Neurourol Urodyn. 2004;23:252–6. doi: 10.1002/nau.20005. discussion 7. [DOI] [PubMed] [Google Scholar]
- 217.McGuire EJ, Woodside JR, Borden TA, et al. Prognostic value of urodynamic testing in myelodysplastic patients 1981. J Urol. 2002;167:1049–53. doi: 10.1016/S0022-5347(02)80338-X. discussion 54. [DOI] [PubMed] [Google Scholar]
- 218.Blok BF, Karsenty G, Corcos J. Urological surveillance and management of patients with neurogenic bladder: Results of a survey among practicing urologists in Canada. Can J Urol. 2006;13:3239–43. [PubMed] [Google Scholar]
- 219.Razdan S, Leboeuf L, Meinbach DS, et al. Current practice patterns in the urologic surveillance and management of patients with spinal cord injury. Urology. 2003;61:893–6. doi: 10.1016/S0090-4295(02)02518-9. [DOI] [PubMed] [Google Scholar]
- 220.Welk B, Liu K, Shariff SZ. The use of urologic investigations among patients with traumatic spinal cord injuries. Res Rep Urol. 2016;8:27–34. doi: 10.2147/RRU.S99840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Soergel TM, Cain MP, Misseri R, et al. Transitional cell carcinoma of the bladder following augmentation cystoplasty for the neuropathic bladder. J Urol. 2004;172:1649–51. doi: 10.1097/01.ju.0000140194.87974.56. discussion 51–2. [DOI] [PubMed] [Google Scholar]
- 222.Higuchi TT, Fox JA, Husmann DA. Annual endoscopy and urine cytology for the surveillance of bladder tumors after enterocystoplasty for congenital bladder anomalies. J Urol. 2011;186:1791–5. doi: 10.1016/j.juro.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 223.Hamid R, Greenwell TJ, Nethercliffe JM, et al. Routine surveillance cystoscopy for patients with augmentation and substitution cystoplasty for benign urological conditions: Is it necessary? BJU Int. 2009;104:392–5. doi: 10.1111/j.1464-410X.2009.08401.x. [DOI] [PubMed] [Google Scholar]
- 224.Linsenmeyer TA, Linsenmeyer MA. Impact of annual urodynamic evaluations on guiding bladder management in individuals with spinal cord injuries. J Spinal Cord Med. 2013;36:420–6. doi: 10.1179/2045772313Y.0000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Nosseir M, Hinkel A, Pannek J. Clinical usefulness of urodynamic assessment for maintenance of bladder function in patients with spinal cord injury. Neurourol Urodyn. 2007;26:228–33. doi: 10.1002/nau.20319. [DOI] [PubMed] [Google Scholar]
- 226.Edokpolo LU, Foster HE., Jr Renal tract ultrasonography for routine surveillance in spinal cord injury patients. Top Spinal Cord Inj Rehabil. 2013;19:54–60. doi: 10.1310/sci1901-54. [DOI] [PMC free article] [PubMed] [Google Scholar]