Abstract
The rapid increase in knowledge in tumour biology and tumour pathogenesis of triple-negative breast cancer (TNBC) has resulted in new therapeutic approaches and new therapeutic concepts for treatment. For years, TNBC has been considered to be a difficult-to-treat tumour due to its generally aggressive tumour biology and in view of limited therapeutic options. The risk of recurrence and metastasis is higher than in the case of other breast cancer subtypes of the same stage. In addition to surgery and radiation in the curative situation, systemic chemotherapy with anthracyclines and/or taxanes is still the therapy of choice. New therapeutic approaches are based on the knowledge that TNBC is a molecularly very heterogeneous disease. Research groups are working to classify TNBC better and better on a molecular level and use this molecular subtyping as the basis for new therapeutic strategies. The most promising new approaches and considerations regarding the therapy of TNBCs are shown below. In addition, the current therapeutic strategies are discussed using a fictitious case history, taking the current data and the resultant therapeutic consequence into account.
Key words: triple-negative breast cancer, BRCA mutation, subtyping of TNBC, diagnosis and therapy of TNBC
Abbreviations
- adj.
Adjuvant
- BCT
Breast-conserving therapy
- Beva
Bevacizumab
- BRCA
Breast cancer genes
- CT
Chemotherapy
- ECOG
Eastern Cooperative Oncology Group
- ECOG PS
Scale for assessing the overall condition according to the ECOG criteria
- ER−
Estrogen receptor negative
- Gem
Gemcitabine
- HER2−
Negative HER2 status (human epidermal growth factor receptor 2)
- IC
Immune cells
- Ki-67
Proliferation index; antigen for the evaluation of tumour aggressiveness
- Low-risk patient
Patient without indication for chemotherapy
- MRI
Magnetic resonance imaging
- Nab-Pac
Nab-paclitaxel
- NACT
Neoadjuvant chemotherapy
- Non-pCR
No pathological complete remission
- OP
Operation
- Pac
Paclitaxel
- PARPi
Poly-(ADP-ribose) polymerase inhibitor
- pCR
Pathological complete remission
- PD-L1
Programmed cell death ligand 1
- PD-L1 IC+ ≥ 1%
PD-L1 expression on the immune cells
- PgR−
Progesterone receptor negative
- PS
Performance status
- RANKL
Receptor Activator of NF-kB Ligand [protein from the tumour necrosis factor family]
- SLN
Sentinel lymph nodes
- TILs
Tumour-infiltrating lymphocytes
- TNM
TNM system to determine tumour stage (T = Tumour, N = Node status; M = Metastases)
- VRB
Vinorelbin
- 1L
First line
- 2L
Second line
- 3L
Third line
Introduction
Depending on the tumour mass and tumour biology, breast cancer patients have highly varied prognoses 1 , 2 . In about 15% of breast cancers, there is neither expression of the estrogen (ER) and progesterone receptor (PgR) (≤ 1%) nor an overexpression or amplification of the human epidermal growth factor receptor (HER) 2 3 . These triple-negative breast cancers (TNBC: ER−, PgR−, HER2−) generally have an aggressive tumour biology which is associated with a high risk of recurrence and metastasis. Most TNBCs not only metastasise early on in the course of the disease but tend to develop prognostically unfavourable visceral and CNS metastases 4 , 5 , 6 .
The therapeutic options for patients with TNBC are limited. Anthracycline- and/or taxane-based chemotherapy are still the systemic therapy of choice for neoadjuvant and adjuvant treatment as well as in the metastatic situation 3 , 7 , 8 . New perspectives could arise from the rapidly increasing knowledge on the pathogenesis and tumour biology of breast cancer overall and of TNBC in particular. Even the pathogenesis provides information about the heterogeneous development. In addition to BRCA1 , several other germ line variants have been discovered which favour the development of a TNBC 9 , 10 , 11 . TNBC itself is also a very heterogeneous disease on a molecular level. Research groups are working to identify clinically relevant molecular subgroups of TNBC. The objective is to develop therapies matched to the molecular subtype 12 , 13 , 14 , 15 , 16 .
Molecular subtyping of TNBC
Molecular subtyping of the biologically very heterogeneous TNBC was performed based on new molecular findings. The classification of Lehmann et al. (known as the Vanderbilt classification or Lehmann classification) 13 differentiates four molecular subtypes using gene expression analyses – two basal-like subtypes (BL1, BL2), a subtype characterised by tumour-infiltrating lymphocytes and tumour-associated mesenchymal cells (M) as well as a luminal type likely controlled via the androgen receptors (LAR). At approximately 35%, BL1 is the most common molecular TNBC subtype followed by the M subtype, at 25%, the BL2 subtype, at 22%, and the LAR subtype, at 16%. While TNBC generally occurs comparably frequently in premenopausal women, patients with LAR-TNBC at initial diagnosis are significantly older, on average 12 , 13 .
The molecular TNBC subtypes differ not only with regard to their histopathology and gene expression, but also with regard to their tumour biology and prognosis as well as the response to current therapies. The BL1-TNBCs respond to identical neoadjuvant chemotherapy with anthracyclines and taxanes with the highest rate of pathological complete remissions (pCR) of up to 50%. The pCR rate of the other subtypes is to some extent far lower 12 , 13 . This is significant because the pCR rate is a surrogate marker for a longer survival time 17 , 18 , 19 . The authors assume that in the medium term, gene expression analyses and multigene testing will become important in everyday clinical practice in order to better classify the heterogeneous group of TNBCs on a molecular level pretherapeutically and then orient the therapy strategy accordingly. Controlled clinical studies are needed to validate this for routine clinical practice.
Therapeutic Perspectives
Based on the molecular subtyping of TNBC, attempts are made to medically block specific target structures and signalling pathways which are responsible for cell proliferation, invasion, angiogenesis and thus for the survival of the cells 12 , 13 , 15 , 16 :
In up to 25% of TNBC, there is a hyperactivation of the PI3K/AKT/mTOR signalling pathway as the result of an oncogenic alteration. Activating PIK3CA alterations 20 , which occur in particular in the case of the mesenchymal (M) and the LAR subtype, are the most common 12 , 13 . AKT inhibitors have shown good efficacy signals in patients with an AKT/PTEN-disrupted signalling pathway 21 , 22 .
Preclinical investigations indicate specific alterations in the NOTCH signalling pathway which may play a role in TNBC. Affected tumours appear to be particularly sensitive towards gamma secretase inhibitors. To date there have only been preclinical data. There is still a lack of meaningful clinical data 23 , 24 .
The inhibition of the JAK2/STAT3 signalling pathway is a possible therapeutic option for patients with a TNBC of the M or LAR subtype. The increased activity rate of this signalling pathway was observed following neoadjuvant chemotherapy and this suggests that recurrent patients with TNBC and JAK2 amplification could benefit from the blockade of this signalling pathway 25 .
Overexpressed growth factor receptors can be considered as specific targets in the case of TNBC. Older data have already shown that the blockade of the epidermal (EGF) and the vascular endothelial (VEGF) growth factor receptors achieve only limited activity in non-selected patients with TNBC, however 26 , 27 , 28 . The blockade of the fibroblast growth factor receptor (FGFR) appeared to be an interesting approach in the case of detection of an FGFR amplification, since the signals mediated via the FGFR stimulate cell growth and thus promote the survival, migration and differentiation of tumour cells 29 , 30 . However, this approach also could not be confirmed to date. By contrast, so-called “multitargeted kinase inhibitors”, which are currently undergoing clinical development in all breast cancer subtypes, are promising.
Androgen receptors (AR) are expressed in about 10 – 15% of TNBCs 31 . AR expression is typical for the LAR subtype 12 , 13 . For this subtype, antiandrogen therapy could be a useful approach 32 . There are encouraging results for the non-steroidal androgen synthesis inhibitor TAK-700 33 and currently with the antiandrogen enzalutamide 32 .
A promising target is also the cell surface protein Trop-2 which is overexpressed in various epithelial tumours, however not in normal tissue 34 . The overexpression of the membrane-associated Trop-2 is associated with an unfavourable prognosis in breast cancer 35 . An antibody drug conjugate which consists of a monoclonal antibody against Trop-2 and an active metabolite of irinotecan and which shows promising results in the case of TNBC is undergoing clinical testing 36 . The substance is currently undergoing clinical phase III study testing in refractory and recurrent metastatic TNBC 37 .
Therapeutic Options with Direct Clinical Relevance
Immune checkpoint blockade in focus
A promising new therapeutic principle which already has direct clinical relevance is immuno-oncology which uses the immune system to fight and eliminate tumour cells. So-called immune checkpoints through which the immune system can be modulated with drugs play a key role here. Checkpoint inhibitors which are already the standard of therapy in various tumour diseases have made an impression through long-lasting remissions 38 .
According to a classification by Lehmann et al. based primarily on the immunogenicity 12 , 13 , about 20% of TNBCs can be classified as immunogenic and are significantly enriched with immune cell markers. In view of this – to intervene in a modulating way in the bodyʼs own immune system – a differentiation is made between only three groups in the case of TNBC: a luminal subtype with expression of androgen receptors (22%), a TNBC of the “basal-like” type with only a minor immune response and a micromilieu which tends to be unfavourable (high proportion of M2-like macrophages; 45%) as well as a “basal-like” TNBC with a marked immune response and a favourable micromilieu (low proportion of M2 macrophages; 33%) 16 . The three groups could appear together within a TNBC 39 .
Tumour-infiltrating lymphocytes in TNBC
Tumour-infiltrating lymphocytes (TILs) are detected more frequently in TNBC than in other breast cancer subgroups. The TILs in the TNBC tissue are associated with certain gene expression profiles and characteristics, such as the expression of programmed cell death (PD)-1 receptors and their ligands PD-ligand (L) 1 + 2, which suggest a targeted cellular immune response. A precondition for a targeted cellular immune response is a tumour-specific antigen which is identified by the immune system as “foreign” 16 . In TNBC, TILs have not only a prognostic but also a predictive significance 40 , 41 , 42 , 43 .
The higher the proportion of TILs in the tumour tissue and the higher the immunogenicity of the tumour, the better the tumours respond to chemotherapy 44 , 45 . On neoadjuvant chemotherapy in early breast cancer, pCR rates of 50% are achieved, for example in the case of high TIL levels (≥ 60%) 46 . This correlation is particularly clear on platinum-based chemotherapy 47 .
Initial clinical data on immunotherapy in TNBC
The German Breast Group (GBG) and AGO Mamma (Consortium for Gynaecological Oncology, Breast Study Group) conducted the randomised phase II study GeparNuevo on patients with early TNBC in which the PD-L1 inhibitor durvalumab was combined neoadjuvantly with an anthracycline/taxane-based chemotherapy 48 . The patients received nab-paclitaxel 125 mg/m 2 per week for twelve weeks ± durvalumab followed by four cycles of epirubicin/cyclophosphamide (EC) 90/600 mg/m 2 every 14 days, ± durvalumab. The patients mostly had an unfavourable tumour biology: More than 80% had a poorly differentiated carcinoma and the mean Ki-67 value was 49%. The initial results show a higher pCR rate (ypT0 ypN0) of 53.4% in the durvalumab arm versus 44.2% in the placebo arm (adjusted OR 1.53; p = 0.182). The pCR rates with durvalumab were particularly high in the case of the young patients (< 40 years: pCR 69.2 vs. 42.9%), starting from tumour stage IIA (pCR: 55.8 vs. 38.6%) as well as in the case of administration of durvalumab beforehand as induction monotherapy over 14 days (so-called window cohort: pCR 61.0 vs. 41.4%). However, there was no association with the extent of TILs in the tumour tissue. Further studies to evaluate this combination are planned.
The combination of atezolizumab plus nab-paclitaxel already has direct clinical relevance in metastatic TNBC which achieved significant advantages with regard to efficacy in the first-line situation in the randomised phase III study IMpassion 130 49 , 50 . The initial results were presented at the European Cancer Conference of the European Society of Medical Oncology (ESMO) 2018 49 as well as at the San Antonio Breast Cancer Symposium (SABCS) 2018 50 . Following a median follow-up observation period of 12.9 months, the combination of nab-paclitaxel plus atezolizumab achieved a significantly longer median PFS for the intention-to-treat (ITT) population, as compared to monotherapy with nab-paclitaxel (7.2 vs. 5.5 months; HR 0.80; p = 0.0025). However, the effect was triggered exclusively by patients with a PD-L1 expression on the immune cells infiltrating the tumour tissue (IC; PD-L1 IC+ ≥ 1%). The patients with PD-L1 IC+ remained free of progression a median of 2.5 months longer (median PFS: 7.5 vs. 5.0 months; HR 0.62; p < 0.001). The assessment of overall survival which has not yet been finalized additionally shows a clinically significant survival advantage of a median of 9.5 months for this group with metastatic PD-L1 IC+ TNBC (25.0 vs. 15.5 months; HR 0.62). In the USA, the FDA (Food and Drug Administration) approved the combination of nab-paclitaxel plus atezolizumab at the start of the year as first-line therapy in metastatic PD-L1 IC+ TNBC. In Europe, approval is expected at the end of 2019.
Significance of the breast cancer gene (BRCA) mutation status
In the case of TNBC, there is an increased incidence of a BRCA mutation, particularly in the case of younger patients. In 15 – 20% of unselected TNBC, there is a BRCA germ line mutation ( gBRCA -mt) 51 , 52 , 53 . A significant proportion of TNBCs without gBRCA mutation have somatic mutations in the homologous recombination (HR) signalling pathway which generate a phenotype, known as BRCA ness, which is very similar to the BRCA -mutated TNBC 54 . Both phenotypes are associated with an increased sensitivity to cytotoxic substances 51 , 53 , 55 , 56 .
In everyday clinical practice, the detection of a BRCA mutation currently does not have any predictive value for the primary therapy of early TNBC, however it is of prognostic significance. A study from England 57 shows that patients with TNBC and a BRCA mutation have a better prognosis than BRCA wild type (wt) patients. The authors explain this with the higher immunogenicity of these tumours and the associated better response to the chemotherapy. Therapeutically, this currently has no consequences in the case of early TNBC: Independent of the BRCA status, the patients receive standard chemotherapy with anthracyclines and taxanes. In the neoadjuvant situation, the addition of carboplatin increases the pCR rate 51 , 53 . The use of carboplatin is considered to be an option here, independent of BRCA status, when there is an additionally increased risk, such as in the case of lymph node involvement 3 .
In the metastatic situation, study data show that patients with a g BRCA mutation on carboplatin-based treatment have a higher likelihood of response than on docetaxel 58 . In addition, in the metastatic setting, poly(ADP-ribose) polymerase (PARP) inhibitors are an option, validated in two randomised studies, for patients with dysfunction of the BRCA1/2 proteins and an associated HR deficiency 59 , 60 , 61 . This leads to the activation of alternative DNA repair pathways which are regulated via the PARP enzymes. This can be prevented by PARP inhibitors. The tumour cells go into apoptosis. However, there are still no positive study data available for primary therapy. The combination with veliparib, the PARP inhibitor with the lowest PARP-trapping activity, did not achieve a higher pCR rate than the combination with a platinum salt 62 . After approval of the first PARP inhibitor testing for a BRCA mutation has therapeutic relevance.
TNBC: Procedure in Routine Clinical Practice
Diagnostic clarification and staging
The estrogen (ER) and progesterone (PgR) receptor status as well as the HER2 status are determined during primary diagnostic measures – preferably on the core biopsy. In addition, the proliferation marker Ki-67 should be determined immunohistochemically. A high Ki-67 value stands for high proliferation activity. It is in this respect a predictor of chemosensitivity and a rather good response to neoadjuvant chemotherapy and does not primarily indicate a poor prognosis 63 . In addition, the Ki-67 value can also provide useful information on the aggressiveness of the disease and on the underlying risk in the case of TNBC. Since the Ki-67 determination is still not sufficiently standardised and thus only inadequately reproducible, the S3 guideline calls for certain requirements for the Ki-67 determination 7 .
Other predictors of a response to neoadjuvant chemotherapy are the detection of TILs and, in the case of TNBC, the detection of a g BRCA mutation 6 , 12 , 16 , 44 , 46 . At present, there is fundamentally an indication for chemotherapy starting at a T1b/c carcinoma or in the case of lymph node involvement. The indication is less strong in case of an older and more comorbid patient and/or a non-aggressive TNBC subtype..
Because of the increased risk of metastasis early on, staging by means of a chest/abdomen CT and skeletal scintigraphy is recommended within the scope of the initial diagnosis of TNBC – independent of whether or not there are clinical symptoms 7 .
Diagnostic measures in the further course of the disease
If a local or loco-regional recurrence is suspected, a histological clarification and staging with a contrast-enhanced CT of the chest/abdomen/pelvis as well as a bone scintigram must be performed 7 . At the metastatic stage, the hormone receptor and HER2 status as well as the Ki-67, if applicable, should be determined once again by means of a biopsy of metastatic tissue. Here as well, the staging includes a CT of the chest/abdomen/pelvis as well as a skeletal scintigraphy 3 (see also Fig. 1 ). If the g BRCA status is not known, the BRCA status of the tumour should be determined, since the first PARP inhibitor is now available for the metastatic situation 60 .
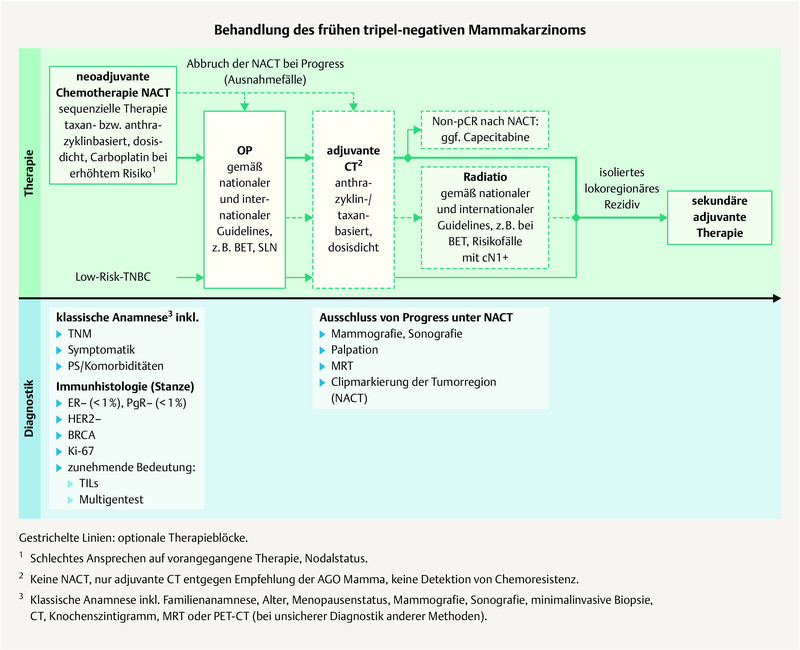
Fig. 1.
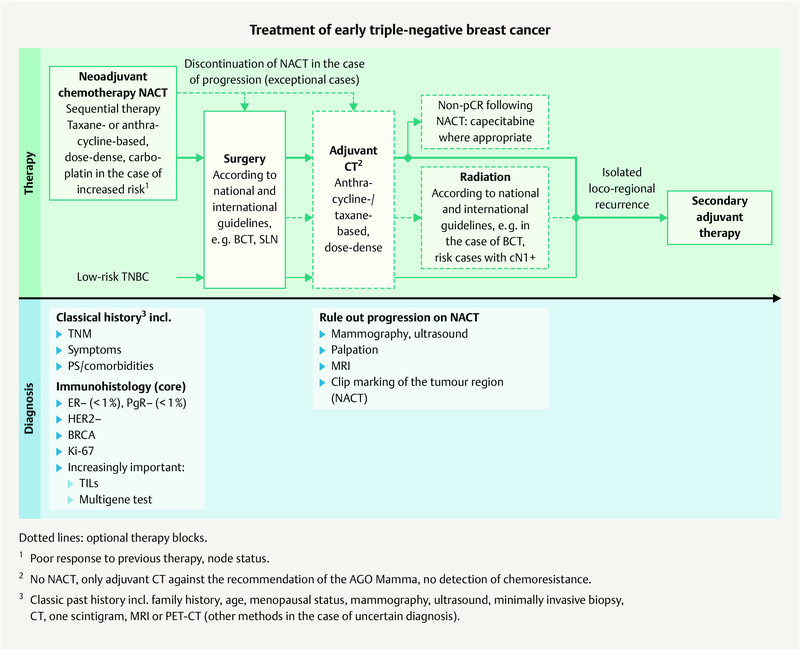
Possible treatment regimen for the treatment of early TNBC (mod. acc. to: 3 , 7 ).
TNBC: Therapeutic options after initial diagnosis
The treatment of breast cancer patients in Germany follows the S3 guideline 7 as well as the annually updated therapeutic recommendations of the AGO Mamma committee 3 ( Figs. 1 and 2 ). Thereafter the standard chemotherapy in early TNBC without distant metastases is currently anthracycline-/taxane-based and is preferably administered neoadjuvantly (AGO recommendation: 1a A ++) 3 . It should be administered over 18 – 24 weeks. The established anthracycline-/taxane-based regimens are currently mentioned in the current AGO recommendation and this also includes the option for dose-dense administration (every 14 days) for patients with lymph node involvement 64 , 65 . In the case of TNBC, paclitaxel should be administered weekly 66 , 67 .
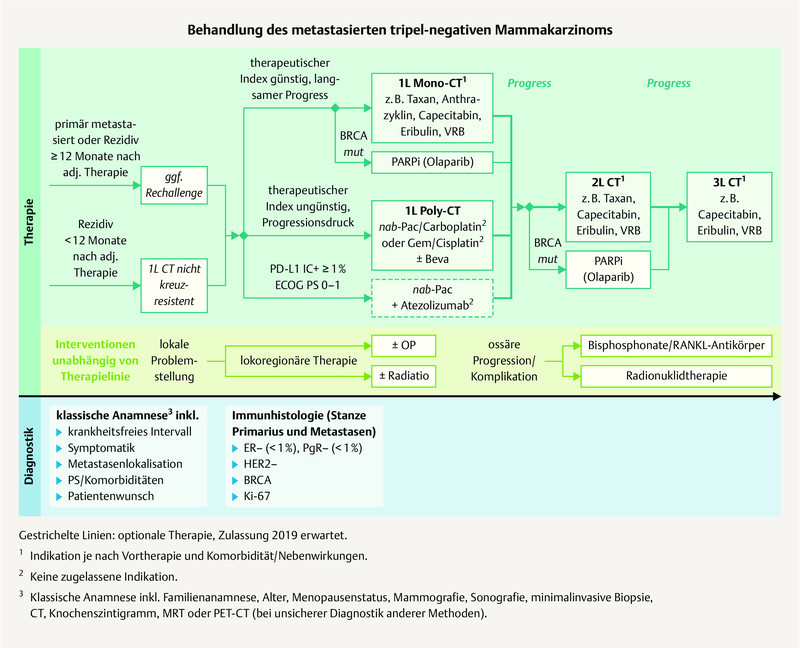
Fig. 2.
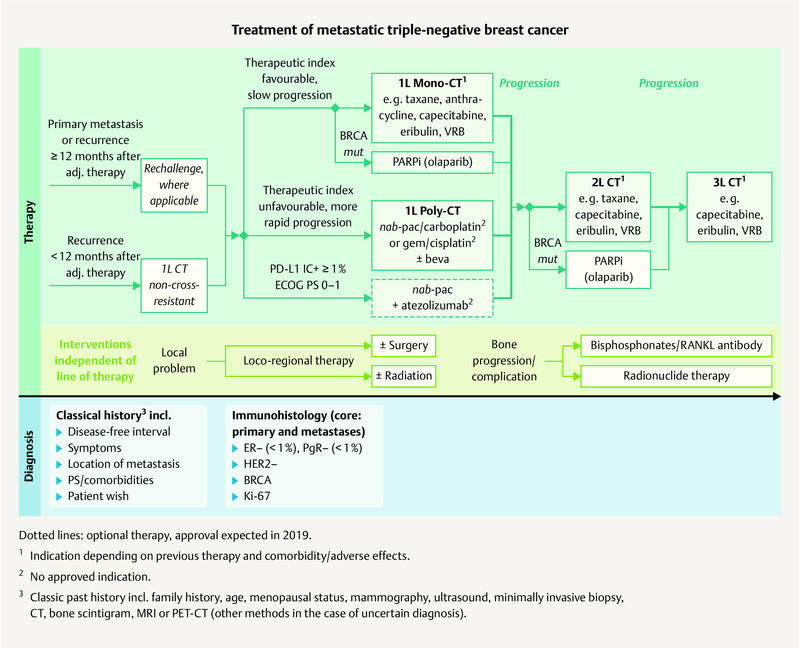
Possible treatment regimen for the treatment of metastatic TNBC (mod. acc. to: 3 , 7 ).
The recommendation for dose-dense/dose-intensified administration of anthracyline-/taxane-based chemotherapy in the case of an elevated risk is based on a meta-analysis of the “Early Breast Cancer Trialistsʼ Collaborative Group” (EBCTCG) 64 as well as on the 10-year data of the ETC study 65 . The latter demonstrated a median survival advantage in favour of adjuvant dose-dense/dose-intensified anthracycline-/taxane-based chemotherapy in patients with more than three affected axillary lymph nodes in comparison to standard therapy with epirubicin/cyclophosphamide-(EC-)paclitaxel which was administered only every three weeks 65 .
Addition of carboplatin?
Based on the data from the GeparSixto 68 and the CALGB 40603 study 69 , the addition of carboplatin to anthracycline-/taxane-based neoadjuvant therapy is also an option in the case of TNBC, according to the AGO Mamma 3 . In both studies, the addition of carboplatin to anthracycline-/taxane-based chemotherapy increased the pCR rate in breast and axilla significantly (GeparSixto [ypT0 ypN0]: 53.2 vs. 36.9%; p = 0.005. CALGB 40603 [ypT0/is ypN0]: 54 vs. 41%; p = 0.0029). However, the higher pCR rate translated only in the GeparSixto study into a significantly higher disease-free survival rate (DFS) after three years for the TNBC patients (86.1 vs. 75.8%; HR 0.56; p = 0.0244) 68 .
Whether the addition of platinum in the scope of a dose-dense/dose-intensified regimen also offers prognostic advantages for the TNBC patients is currently unclear. In the neoadjuvant GeparOcto study 70 , TNBC patients were treated either with a dose-dense platinum-based therapy in combination with anthracyclines and taxanes or with the dose-dense/dose-intensified ETC regimen. For the TNBC patients, no higher pCR was seen for the platinum-based regimen and there was an overall more favourable adverse effect spectrum on the platinum-free treatment.
Neoadjuvant concept to be preferred
An important advantage of the neoadjuvant therapeutic approach is the possibility of in vivo chemosensitivity testing, which enables individualisation of the therapy following the first interim response or even in the post-neoadjuvant setting 3 , 71 , 72 , 73 . If there is a poor response (no pCR) on neoadjuvant chemotherapy, a switch can be made in the adjuvant (postneoadjuvant) situation to a non-cross-resistant regimen in view of the CREATE X study 74 , 75 , after surgery. This option does not exist in the case of primary surgery with subsequent adjuvant chemotherapy. Progression on neoadjuvant chemotherapy is extremely rare. In the GeparSepto study, the rate was < 2% 76 . In this case, the neoadjuvant chemotherapy is generally discontinued and surgery is performed immediately. Postoperatively, adjuvant chemotherapy with a non-cross-resistant regimen should be discussed 3 .
Course of Therapy Using the Example of a Patient with TNBC
The course of therapy and the therapeutic options which are currently possible in the case of TNBC are shown and discussed using a fictional patient as an example: The premenopausal patient, aged 42 at initial diagnosis, has histologically confirmed, poorly differentiated, invasive triple-negative breast cancer without evidence of distant metastases (cT2 [2.5 cm] cN0 cM0, ER−, PgR− HER2−, G3, Ki-67 60%). In view of the current recommendations 3 , 7 , she is receiving neoadjuvant chemotherapy. The young age of the patient and the diagnosis of TNBC plus G3 tumour and high Ki-67 value (> 20%) stand for an increased risk, and for this reason, the patient is receiving a dose-dense, anthracycline-based therapy initially with four cycles of epirubicin/cyclophosphamide (EC) every 14 days.
Since the young age and the diagnosis of “TNBC” suggest a possible g BRCA mutation, there is also an indication for genetic counselling and testing, according to the criteria of the “German Consortium for Familial Breast and Ovarian Cancer” 3 , 7 . This is performed in parallel to the neoadjuvant chemotherapy (fast track procedure). In this case, the testing reveals a g BRCA1 mutation. In addition, after four therapy cycles, the tumour demonstrates inadequate response (< PR following RECIST v1.1) to the anthracycline-based neoadjuvant chemotherapy at the first ultrasound follow-up.
A neoadjuvant concept is standard
In consultation with the patient, the neoadjuvant therapy regimen is intensified. The pCR rate in TNBC can be significantly increased through the additional use of carboplatin to taxane 62 , 67 , 68 , 77 , 78 . This is currently shown for example by the randomised phase III study BrighTNess in patients with TNBC 62 . Here the neoadjuvant addition of carboplatin to paclitaxel followed by four cycles of doxorubicin/cyclophosphamide (AC) statistically significantly increased the pCR rate versus paclitaxel monotherapy followed by AC (pCR: 58 vs. 31%; p < 0.0001). The additional neoadjuvant treatment with the PARP inhibitor veliparib did not provide any additional advantage.
Significance of the BRCA status
The therapeutic significance of the BRCA status is currently unclear. In the GeparSixto study 51 , 77 , patients without a BRCA mutation on carboplatin benefited in particular from a higher pCR rate (pCR: ypT0/is ypN0). Patients with a BRCA mutation had no advantage, likely due to the overall increased chemosensitivity, however also no disadvantage from carboplatin 77 . The higher pCR rate translated into a longer disease-free survival (DFS) for all of the TNBC patients – independent of BRCA 1/2 status (p < 0.001) 51 . Moreover, the additional use of bevacizumab was also investigated in the GeparSixto study. Angiogenesis inhibition is also discussed as a therapeutic option in the event a BRCA mutation is detected 53 . It is still not clearly defined which patients with TNBC have an advantage through the use of platinum since the positive correlation between pCR and DFS in other studies was not able to be demonstrated 69 .
However, it is undisputed that pCR is a prognostically favourable factor. Particularly in the case of an increased risk, the neoadjuvant use of platinum – independent of the BRCA status – should therefore be considered. Nonetheless, the prognostic role of the BRCA status in the neoadjuvant setting must be further validated: While a study from Erlangen 79 corroborates the results of the GeparSixto study 51 , according to which the pCR rate, independent of the BRCA status, is the most important predictor for long disease-free and overall survival, this could not be clearly demonstrated in other investigations. Despite a high degree of chemosensitivity and increased pCR rate in comparison to the BRCA wild type patients, this was not reflected in a prognostic benefit in the case of the pCR patients with BRCA 1/2 mutation 53 , 80 .
Importance of nab-paclitaxel in the neoadjuvant setting
The albumin-bound nab-paclitaxel also shows a high degree of efficacy in the neoadjuvant setting. In the GeparSepto study 18 , 76 , the neoadjuvant use of four cycles of nab-paclitaxel was compared to four cycles of conventional paclitaxel, followed in each case by four cycles of epirubicin/cyclophosphamide (EC). Nab-paclitaxel statistically significantly increased the pCR rate of TNBC patients (p > 0.001). After four years, 10% more patients were still disease-free in absolute terms in the nab-paclitaxel arm (78.7 vs. 68.6%; HR 0.66, p = 0.0694) 76 .
The randomised phase II study ADAPT-TN of the WSG (West German Study Group) 81 shows that nab-paclitaxel can be combined well with carboplatin neoadjuvantly. In the study, a total of 336 patients with early TNBC (ER/PgR < 1%; HER2−; cT1c-4c, cN0/+) were randomised and treated for only twelve weeks neoadjuvantly with nab-paclitaxel/carboplatin versus nab-paclitaxel/gemcitabine. In the arm containing carboplatin, the pCR rate after twelve weeks was significantly higher than in the comparative arm (ypT0/is ypN0: 45.9 vs. 28.7%; p = 0.002; OR 2.11; tpCR [ypT0/ypN0]: 54.2 vs. 25.8%; p < 0.001) 81 . In addition, the pCR rate was confirmed as a prognostic factor in TNBC. The data indicate that the only 12-week neoadjuvant treatment with nab-paclitaxel/carboplatin is a possible concept for deescalating the neoadjuvant chemotherapy. After a median observation period of 36 months, the predicted 3-year survival rate was 92.2% in the nab-paclitaxel/carboplatin arm versus 84.7% in the comparison arm (p = 0.08) 82 .
In view of this, the AGO Mamma also considers the neoadjuvant use of nab-paclitaxel to be a therapeutic option in TNBC – despite a lack of approval 3 . In the present case, the patient is receiving neoadjuvant carboplatin in combination with conventional paclitaxel. The use of nab-paclitaxel would be possible and warranted, if accordingly justified.
Procedure following neoadjuvant chemotherapy
The surgical procedure following neoadjuvant chemotherapy as well as postoperative radiation and (post-neo-)adjuvant systemic therapy are performed in each case according to the national and international guidelines and recommendations 3 , 7 , 83 , 84 , 85 . Whenever possible, the patient should undergo breast-conserving surgery and a sentinel lymph node dissection following NACT. The indication for a mastectomy and axillary lymphadenectomy is found in the guideline 3 . There is no differentiation for patients with TNBC or with no TNBC. Postoperative chemotherapy is an option in the case of an increased risk of recurrence, for example, an invasive residual tumour in the breast and/or axilla after NACT 3 , 7 . Where applicable, the adjuvant further treatment with non-cross-resistant substances is an option in the case of TNBC, such as capecitabine, according to the CREATE-X study 74 , 75 .
In the case of our fictitious patient, an ipsilateral ablation plus sentinel node dissection (SND) plus a contralateral prophylactic mastectomy is performed due to the existing g BRCA1 mutation and following appropriate patient information and consent – in accordance with the S3 guideline 7 and AGO recommendation 3 . The sentinel lymph node is unremarkable. However, an invasive residual tumour measuring 3 mm is seen in the breast following neoadjuvant chemotherapy. The histological examination confirms a TNBC. There is no indication for adjuvant radiation since there is no lymph node involvement, the patient underwent mastectomy (R0) and there are no prognostically relevant additional risk factors. Within the scope of systemic adjuvant treatment, the patient now receives six cycles of capecitabine – corresponding to the AGO recommendation 3 and based on the CREATE X study 74 .
If a patient is not treated neoadjuvantly, contrary to the favoured recommendation of the AGO Mamma committee, but rather undergoes primary surgery, there is an indication postoperatively for adjuvant anthracycline/taxane-based chemotherapy which in the present case could be given in a dose-dense manner, in view of the risk constellation (young age, TNBC) 3 , 7 . It is stressed again at this point that in the case of primary surgery plus adjuvant chemotherapy, the option of “in vivo” chemosensitivity testing is eliminated. In the present case, the patient would have possibly received suboptimal adjuvant systemic therapy according to the current state of knowledge.
Isolated loco-regional recurrence
If the patient develops an isolated loco-regional recurrence, there is still a curative chance. She is therefore treated with curative intent, analogously to an initial disease – but taking the previous therapy into account. If there is a good response to the previous anthracycline-/taxane-based systemic therapy and a disease-free interval (DFI) of more than a year (DFI > 1 year), taxane-based chemotherapy is once again indicated. The use of free anthracyclines should be avoided due to the risk of long-term cardiac damage. In the case of a short DFI (< 1 year), a regimen that is not cross-resistant to the previous therapy is recommended 3 .
The recommendation for a further chemotherapy is based among others on the results of the CALOR study 86 which showed after a median follow-up of ten years that patients with HR-negative breast cancer have an advantage with regard to overall survival (HR 0.48) and breast-cancer-specific survival (HR 0.29; interaction p value = 0.034) if they receive chemotherapy in addition to surgery in the event of an isolated loco-regional recurrence. There is currently no indication for the use of a PARP inhibitor or a PD(L)1 inhibitor in the situation of an isolated loco-regional recurrence. Both options are subject to the metastatic situation.
Metastatic TNBC in focus
At present, in the metastatic situation, a cure is generally no longer possible. In addition to oncological systemic therapy directed against the tumour, attention should be paid to additional interventions ( Fig. 2 ). These include supportive measures, the use of bisphosphonates or a receptor activator of NF-kB (RANK) ligand in the case of bone metastases or bone complications as well as local surgical or radiation therapy measures in the event of marked symptoms or impending local complications 3 , 7 . What is important is that loco-regional therapy is indicated only in the case of a local problem. As long as single metastases are not directly threatening for the patient, the local treatment of individual metastases does not substantially change the course of the disease 87 . Currently there ist no precise definition of when there is still so-called limited metastasis for which a more aggressive therapeutic approach could be justified. The indication for an aggressive, multimodal approach in the case of limited metastasis requires interdisciplinary discussion in the tumour board with inclusion of the informed patient.
Metastases should undergo core biopsy once again in order to detect molecular changes from the primary tumour and take them into account in the treatment 3 , 7 . The biopsy of the metastasis is necessary to determine the established factors (HR status, HER2 status, Ki-67) which are relevant for the therapeutic decision. In addition, the (neo-)adjuvant previous therapy, duration of response to the previous treatment, metastasis localisation, symptoms and overall condition as well as the patientʼs preference should be incorporated in further decisions regarding therapy. If there is still no g BRCA mutation testing, this should be performed definitely in the case of metastatic TNBC, since PARP inhibitors represent an effective new therapeutic option for patients with g BRCA -mutated TNBC 3 .
First-line therapy in the metastatic stage
The standard in the metastatic situation is cytostatic monotherapy. Combinations with bevacizumab or a second cytostatic substance are primarily indicated when rapid remission is highly necessary or in the case of a short disease-free interval (DFI < 12 months) following neoadjuvant prior therapy. A high need for rapid remission is defined by the ESMO as a “visceral crisis” 85 . The AGO Mamma committee describes “imminent organ function” 3 .
Established monotherapies for the first-line treatment of patients with metastatic TNBC are anthracyclines and taxanes, according to the AGO recommendation 3 . A precondition is that the patient has not yet received any anthracycline and/or taxane within the scope of previous therapy or, given acceptable tolerability, was without therapy at least one year 3 . If there is an indication for a taxane-based first-line therapy, the use of nab-paclitaxel is a preferred option. In TNBC, nab-paclitaxel achieves good response rates which are higher than on conventional paclitaxel 76 . If there is a high need for rapid remission, nab-paclitaxel can be combined with bevacizumab and/or carboplatin 88 , 89 , 90 , 91 . In the tnAcity study 91 , the first-line treatment with nab-paclitaxel/carboplatin prolonged the median PFS of patients with metastatic TNBC significantly versus gemcitabine/carboplatin (8.3 vs. 6.0 months; HR 0.58; p = 0.02) and also versus nab-paclitaxel/gemcitabine (8.3 vs. 5.5 months; HR 0.59; p = 0.02). Nab-paclitaxel is currently approved but only as monotherapy in pretreated metastatic breast cancer 92 .
Promising new first-line option
A promising new first-line option is the combination of nab-paclitaxel plus atezolizumab which is currently not yet approved, however. With overall good tolerability, the PD-L1 inhibitor increases the efficacy of nab-paclitaxel. In the randomised phase III study IMpassion130 49 , the median PFS in patients with evidence of expression of PD-L1 on the tumour-infiltrating lymphocytes (PD-L1 IC+ ≥ 1%) could be prolonged by 2.5 months (HR 0.62; p < 0.001) and the median overall survival prolonged by 9.5 months (HR 0.62) through the additional administration of atezolizumab. In the combination of nab-paclitaxel plus atezolizumab, the authors see an effective first-line therapy and new standard therapy option in PD-L1-IC+ metastatic TNBC 49 . It is important that PD-L1 in the case of TNBC is expressed almost exclusively on the immune cells and not on the tumour cells. Thus just under 10% of the tumour cells demonstrate PD-L1 expression. Since these tumours generally also demonstrate PD-L1 expression on the immune cells, the PD-L1 testing on the tumour cells currently has no clinical relevance in the case of TNBC 50 .
The subgroup analyses confirm the PD-L1 expression on the immune cells (PD-L1 IC+: PD-L1 expression ≥ 1% on the immune cells) as a predictive marker for an advantage with regard to PFS and overall survival on nab-paclitaxel plus atezolizumab versus chemotherapy alone 50 . Facing other biomarkers, such as CD8 expression (CD8+), the detection of TILs in the stroma or the BRCA status, the PD-L1 expression on the immune cells proved to be a superior predictor. In the case of patients with PD-L1 IC+ metastatic TNBC, the BRCA status has no relevance with regard to the indication for therapy with nab-paclitaxel plus atezolizumab 50 . For patients with newly diagnosed metastatic or inoperable locally advanced TNBC, the authors recommend PD-L1 testing on the immune cells routinely for clinical practice to possibly consider the use of nab-paclitaxel plus atezolizumab.
Brief response following (neo)adjuvant therapy
In the case of only a brief DFI following (neo)adjuvant therapy – that is, progression within the first twelve months – there is generally also an increased need for rapid remission which justifies the use of combination therapy. If no platinum was given yet, the use of gemcitabine/platinum (cisplatin or carboplatin) is a first-line option. If there is no indication for combination therapy, systemic monotherapy is also possible – optionally with platinum, capecitabine, eribulin or vinorelbin 3 . If no platinum was given yet, a mono- or combination therapy containing carboplatin should be considered, at least in the second-line situation.
In the case of the fictitious patient, more than five liver metastases in terms of a disseminated liver metastasis were diagnosed one year after the last adjuvant capecitabine dose. The liver function is not impaired (transaminase increase, grade 1). The metastasis core biopsies once again confirm TNBC. As the chemosensitivity testing in the neoadjuvant setting has not shown a good response to the dose-dense, anthracycline-based combination therapy, another anthracycline-based first-line therapy is not considered.
The first-line treatment with taxane monotherapy is a valid therapeutic option. Since the patient has already received conventional paclitaxel neoadjuvantly, the use of nab-paclitaxel is an option. In view of the current data from the IMpassion130 study 49 , the use of the combination of nab-paclitaxel plus atezolizumab is an interesting alternative to monotherapy with nab-paclitaxel in PD-L1 IC expression in the tumour tissue. In the case of the fictional patient, the TILs express PD-L1 (> 5%). In consultation with the informed patient (reference to data and “off-label” use), she will therefore receive first-line treatment with nab-paclitaxel plus atezolizumab. The TNBC initially responds to the treatment. However, after seven months, progression of the liver metastases is seen. In addition, new bone metastases which are associated with a risk of fracture in the region of the lumbar spine are diagnosed.
Second-line therapy of metastatic TNBCs
If there is progression on first-line therapy, further cytostatic monotherapies can be considered, according to the S3 guideline and AGO Mamma committee, depending on the need for rapid remission and pretreatment 3 , 7 . For patients with g BRCA -mutated metastatic TNBC, PARP inhibitors represent a new class of substances which are additionally available and which should become established as the therapy of choice, based on the data available. Olaparib is approved in Europe since april 2019. The OlympiAD 60 and also the EMBRACA study 61 showed that PARP inhibition in this situation is a preferable therapeutic option. The AGO Mamma committee recommends the PARP inhibitor olaparib as monotherapy, following anthracycline and taxane treatment in patients with metastatic HER2-negative breast cancer and g BRCA1/2 mutation (1b B +) 3 . According to AGO Mamma monotherapy with carboplatin is an alternative to olaparib if a g BRCA mutation ist detected 3 , 59 .
Course of therapy starting from the second line of therapy
The fictional patient receives bone-protecting and bone-stabilising treatment with a bisphosphonate and continues to be treated with the PARP inhibitor olaparib due to the detected g BRCA mutation in accordance with the recommendation of the AGO Mamma. She responds to monotherapy with olaparib and remains without progression for eight months. The re-biopsy of metastases confirm TNBC.
In the case of new progression, there are further lines of therapy for metastatic TNBC available for this patient, such as eribulin, vinorelbin, gemcitabine, mitomycin-C plus 5-fluorouracil (5-FU) or even metronomic chemotherapy with cyclophosphamide and methotrexate. Whenever possible, patients with TNBC should be treated within a clinical study in order to validate soon new therapeutic options for this disease, which is associated with a very limited prognosis to date 3 .
Outlook
PARP inhibition and immuno-oncology represent new promising therapeutic options for the treatment of TNBC which are available and which must be further validated. In the IMpassion132 study 93 , for example, patients with advanced/metastatic TNBC and short DFI (< 12 months) – pretreated (neo)adjuvantly with anthracyline and taxane – receive a first-line-treatment with the PD-L1-inhibitor atezolizumab in combination with an non-taxane-/anthracycline-based first-line-chemotherapy. Checkpoint inhibitors are also increasingly being investigated in early TNBC within clinical studies. In the phase III study NeoTrip 94 , atezolizumab is combined in the neoadjuvant setting with nab-paclitaxel/carboplatin in patients with early TNBC and a high risk of recurrence and compared to chemotherapy alone. In the adjuvant setting all patients receive combination chemotherapy containing anthracyclines (AC, EC or FEC). The study addresses the consideration of increasing the potential of immunotherapy with chemotherapy and improving the tumour response. The phase-III study GeparDouze 95 is also testing the additional administration of atezolizumab to standard chemotherapy in the neoadjuvant setting in TNBC.
Additional therapeutic approaches which are emerging from the molecular subtyping in TNBC are validated in clinical studies. This also includes the use of antibody-drug conjugates such as CDX-011 (glembatumumab vedotin) 96 and sacituzumab govitecan-hziy 97 , 98 .
It is evident that the accurate determination of tumour biology is becoming more important in TNBC. To depict the rapid evolution of tumour biology in the course of the disease, tumour or metastasis biopsies should be considered at each progression. However, since the results have no clinical impact yet, the indication for multiple biopsies should be made with caution outside of studies. The so-called “liquid biopsy”, in which tumour-related markers are measured in the blood, could be an alternative. This is currently being tested, for example, in the PRAEGNANT study 99 , 100 . To tap the potential of molecular tumour characterisation, the “Breast International Group” (BIG) established AURORA, a comprehensive, multinational molecular screening program to research molecular aberrations in patients with metastatic breast cancer 101 . The options of study participation should be used.
Acknowledgements
The authors are independent and fully responsible for the content as well as the editorial decisions of this manuscript. The authors thank Birgit-Kristin Pohlmann, Nordkirchen for editorial collaboration in the drafting of the manuscript (support from Celgene GmbH Munich).
Danksagung
Die Autoren sind unabhängig und voll verantwortlich für den Inhalt sowie die editorischen Entscheidungen dieses Manuskripts. Für die redaktionelle Mitarbeit bei der Erstellung des Manuskripts (Unterstützung der Celgene GmbH München) danken die Autoren Birgit-Kristin Pohlmann, Nordkirchen.
Footnotes
Conflict of Interest/Interessenkonflikt Andreas Schneeweiss received honoraria from Roche, Celgene, AstraZeneca, Pfizer, Novartis, Amgen and a travel grant from Roche and Celgene. Carsten Denkert received honoraria from AstraZeneca, Pfizer, Celgene, Teva, Myriad, Roche, Daiichi, MSD and was the co-founder of Sividon Diagnostics, Cologne. Peter A. Fasching received honoraria from Amgen, Novartis, Roche, Pfizer and conducted research with support from Novartis. Carlo Fremd received honoraria from Roche, Celgene and Amgen. Oleg Gluz received honoraria and conducted research with support from Celgene, Roche, Novartis, Daiichi, GHI, Pfizer, Amgen, Esai, MSD. Travel grant from Roche, Daiichi, Celgene. Cornelia Kolberg-Liedtke received honoraria from Roche, AstraZeneca, Eisai, Celgene, Amgen, Novartis, Pfizer, Lilly, Phaon Scientific, Hexal, SonoScape and conducted research with support from Roche, Novartis, Pfizer. Travel grant from Roche. Sibylle Loibl conducted research with support from AstraZeneca, Abbee, Celgene, Amgen, Pfizer, Novartis and Roche. Hans-Joachim Lück received honoraria from Amgen, Pfizer, Roche, Novartis, AstraZeneca, Tesaro, Daiichi Sankyo, Lilly and a travel grant from Tesaro.
Andreas Schneeweiss erhielt Honoraria von Roche, Celgene, AstraZeneca, Pfizer, Novartis, Amgen und Travel Grant von Roche und Celgene. Carsten Denkert erhielt Honoraria von AstraZeneca, Pfizer, Celgene, Teva, Myriad, Roche, Daiichi, MSD und war Mitbegründer von Sividon Diagnostics, Cologne. Peter A. Fasching erhielt Honoraria von Amgen, Novartis, Roche, Pfizer und führte Forschung durch mit Unterstützung von Novartis. Carlo Fremd erhielt Honoraria von Roche, Celgene und Amgen. Oleg Gluz erhielt Honoraria und führte Forschung durch mit Unterstützung von Celgene, Roche, Novartis, Daiichi, GHI, Pfizer, Amgen, Esai, MSD. Travel Grant von Roche, Daiichi, Celgene. Cornelia Kolberg-Liedtke erhielt Honoraria von Roche, AstraZeneca, Eisai, Celgene, Amgen, Novartis, Pfizer, Lilly, Phaon Scientific, Hexal, SonoScape und führte Forschung durch mit Unterstützung von Roche, Novartis, Pfizer. Travel Grant von Roche. Sibylle Loibl führte Forschung durch mit Unterstützung von AstraZeneca, Abbee, Celgene, Amgen, Pfizer, Novartis und Roche. Hans-Joachim Lück erhielt Honoraria von Amgen, Pfizer, Roche, Novartis, AstraZeneca, Tesaro, Daiichi Sankyo, Lilly und Travel Grant von Tesaro.
References/Literatur
- 1.Zeichner S B, Ambros T, Zaravinos J. Defining the survival benchmark for breast cancer patients with systemic relapse. Breast Cancer (Auckl) 2015;9:9–17. doi: 10.4137/BCBCR.S23794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeichner S B, Herna S, Mani A. Survival of patients with de-novo metastatic breast cancer: analysis of data from a large breast cancer-specific private practise, a university-based cancer center and review of the literature. Breast Cancer Res Treat. 2015;153:617–624. doi: 10.1007/s10549-015-3564-3. [DOI] [PubMed] [Google Scholar]
- 3.Arbeitsgemeinschaft Gynäkologische Onkologie (AGO) Kommission ‚Mamma‘ (vertreten durch Wolfgang Janni) Diagnose und Behandlung von Patienten mit primärem und metastasiertem Mammakarzinom. Version 2019 v1 München: W. Zuckschwerdt; 2019. Online:www.ago-online.delast access: 27.03.2019 [Google Scholar]
- 4.Mersin H, Yildirim E, Berberoglu U. The prognostic importance of triple negative breast carcinoma. Breast. 2008;17:341–346. doi: 10.1016/j.breast.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 5.Dawood S, Lei X, Litton J K. Incidence of brain metastases as a first site of recurrence among women with triple negative breast cancer. Cancer. 2012;118:4652–4659. doi: 10.1002/cncr.27434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin N U, Vanderplas A, Hughes M E. Clinicopathologic features, patterns of recurrence, and survival among women with triple negative breast cancer in the National Comprehensive cancer Network. Cancer. 2012;118:5463. doi: 10.1002/cncr.27581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF) Interdisziplinäre S3-Leitlinie für die Früherkennung, Diagnostik, Therapie und Nachsorge des Mammakarzinoms, Langversion 4.0Dezember 2017. AWMF Registernummer: 032-045OL. Online:http://www.leitlinienprogramm-onkologie.de/leitlinien/mammakarzinom/last access: 08.10.2018
- 8.Wöckel A, Festl J, Stüber T. Interdisciplinary Screening, Diagnosis, Therapy and Follow-up of Breast Cancer. Guideline of the DGGG and the DKG (S3-Level, AWMF Registry Number 032/045OL, December 2017) – Part 1 with Recommendations for the Screening, Diagnosis and Therapy of Breast Cancer. Geburtsh Frauenheilk. 2018;78:927–948. doi: 10.1055/a-0646-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Häberle L, Hein A, Rübner M. Predicting Triple-Negative Breast Cancer Subtype Using Multiple Single Nucleotide Polymorphisms for Breast Cancer Risk and Several Variable Selection Methods. Geburtsh Frauenheilk. 2017;77:667–678. doi: 10.1055/s-0043-111602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevens K N, Fredericksen Z, Vachon C M. 19p13.1 is a triple-negative-specific breast cancer susceptibility locus. Cancer Res. 2012;72:1795–1803. doi: 10.1158/0008-5472.CAN-11-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Purrington K S, Slager S, Eccles D. Genome-wide association study identifies 25 known breast cancer susceptibility loci as risk factors for triple-negative breast cancer. Carcinogenesis. 2012;35:1012–1019. doi: 10.1093/carcin/bgt404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehmann B D, Bauer J A, Chen X. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehmann B C, Jovanovic B, Chen X. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. doi:10.13717/journal.pone.0157368. PLoS One. 2016;11:e0157368. doi: 10.1371/journal.pone.0157368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreike B, van Kouwenhove M, Horlings H. Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast Cancer Res. 2007;9:R65. doi: 10.1186/bcr1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burstein M D, Tsimelzon A, Poage G M. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res. 2015;21:1688–1698. doi: 10.1158/1078-0432.CCR-14-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jézéquel P, Loussouarn D, Guérin-Charbonnel C. Gene-expression molecular subtyping of triple-negative breast cancer tumours. Importance of immune response. Breast Cancer Res. 2015;17:43. doi: 10.1186/s13058-015-0550-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liedtke C, Mazouni C, Hess K R. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 18.Schneeweiss A, Jackisch C, Schmatloch S. Survival analysis of the prospectively randomized phase III GeparSepto trial comparing neoadjuvant chemotherapy with weekly nab-paclitaxel with solvent-based paclitaxel followed by anthracycline/cyclophosphamide for patients with early breast cancer – GBG 69. SABCS 2017; Abstr. GS3-05
- 19.Cortazar P, Zhang L, Untch M. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 20.Marty B, Maire V, Gravier E. Frequent PTEN genomic alterations and activated phosphatidylinositol 3-kinase pathway in basal-like breast cancer cells. Breast Cancer Res. 2008;10:R101. doi: 10.1186/bcr2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmid P, Abraham J, Chan S.AZD5363 plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (PAKT): A randomised, double-blind, placebo-controlled, phase II trial J Clin Oncol 201836 (no.15_suppl)1007–1007.doi:10.1200/JCO.2018.36.15_suppl.100729432078 [Google Scholar]
- 22.Dent R, Im S A, Espie M. Overall survival update of the double-blind placebo-controlled randomised phase 2 LOTUS trial of first-line Ipataserib + paclitaxel for locally advanced/metastatic triple-negative breast cancer. doi:10.1200/JCO.2018.36.15_suppl.1008 J Clin Oncol. 2018;36 (no.15_suppl):1008. [Google Scholar]
- 23.Harrison H, Farnie G, Howell S J. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res. 2010;70:709–718. doi: 10.1158/0008-5472.CAN-09-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoeck A, Lejnine S, Truong A. Discovery of biomarkers predictive of GSI response in triple-negative breast cancer and adenoid cystic carcinoma. Cancer Discov. 2014;4:1154–1167. doi: 10.1158/2159-8290.CD-13-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balko J M, Giltnane J M, Wang K. Molecular profiling of the residual disease of triple-negative breast cancers after neoadjuvant chemotherapy identifies actionable therapeutic targets. Cancer Discov. 2014;4:232–245. doi: 10.1158/2159-8290.CD-13-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baselga J, Gomez P, Greil R. Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J Clin Oncol. 2013;31:2586–2592. doi: 10.1200/JCO.2012.46.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carey L A, Rugo H S, Marcom P K. TBCRC 001: randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J Clin Oncol. 2012;30:2615–2623. doi: 10.1200/JCO.2010.34.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brufsky A, Valero V, Tiango B. Second-line bevacizumab-containing therapy in patients with triple-negative breast cancer: subgroup analysis of the RIBBON-2 trial. Breast Cancer Res Treat. 2012;133:1067–1075. doi: 10.1007/s10549-012-2008-6. [DOI] [PubMed] [Google Scholar]
- 29.Turner N, Lambros M B, Horlings H M. Integrative molecular profiling of triple negative breast cancers identifies amplicon drivers and potential therapeutic targets. Oncogene. 2010;29:2013–2023. doi: 10.1038/onc.2009.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharpe R, Pearson A, Herrera-Abreu M T. FGFR signaling promotes the growth of triple-negative and basal-like breast cancer cell lines both in vitro and in vivo. Clin Cancer Res. 2011;17:5275–5286. doi: 10.1158/1078-0432.CCR-10-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niemeier L A, Dabbs D J, Beriwal S. Androgen receptor in breast cancer: expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod Pathol. 2010;23:205–212. doi: 10.1038/modpathol.2009.159. [DOI] [PubMed] [Google Scholar]
- 32.Traina T A, Miller K, Yardley D A. Enzalutamide for the Treatment of Androgen Receptor-Expressing Triple-Negative Breast Cancer. J Clin Oncol. 2018;36:884–890. doi: 10.1200/JCO.2016.71.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamaoka M, Hara T, Hitaka T. Orteronel (TAK-700), a novel non-steroidal 17,20-lyase inhibitor: effects on steroid synthesis in human and monkey adrenal cells and serum steroid levels in cynomolgus monkeys. J Steroid Biochem Mol Biol. 2012;129:115–128. doi: 10.1016/j.jsbmb.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Stepan L P, Trueblood E S, Hale K. Expression of Trop2 cell surface glycoprotein in normal and tumor tissues: potential implications as a cancer therapeutic target. J Histochem Cytochem. 2011;59:701–710. doi: 10.1369/0022155411410430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ambrogi F, Fornili M, Boracchi P. Trop2 is a determinant of breast cancer survival. PLoS One. 2014;9:e96993. doi: 10.1371/journal.pone.0096993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bardia A, Starodub A, Moroose R L. IMMUN-132, a new antibody-drug-conjugate against Trop2, as a novel therapeutic for patients with relapsed/refractory, metastatic, triple-negative breast cancer: results from phase I/II clinical trial ( NCT01631552). 37th San Antonio Breast Cancer Symposium (SABCS) 2014; P5-19-27
- 37.ClinicalTrials.gov Identifier: NCT02574455
- 38.Emens L A, Kok M, Ojalvo L S. Targeting the programmed cell death-1 pathway in breast and ovarian cancer. Curr Opin Obstet Gynecol. 2016;28:142–147. doi: 10.1097/GCO.0000000000000257. [DOI] [PubMed] [Google Scholar]
- 39.Gao R, Davis A, McDonald T O. Punctuated copy number evolution and clonal stasis in triple-negative breast cancer. Nat Genet. 2016;48:1119–1130. doi: 10.1038/ng.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pruneri G, Vingiani A, Bagnardi V. Clinical validity of tumor-infiltrating lymphocytes analysis in patients with triple-negative breast cancer. Ann Oncol. 2016;27:249–256. doi: 10.1093/annonc/mdv571. [DOI] [PubMed] [Google Scholar]
- 41.Pruneri G, Vingiani A, Denkert C. Tumor infiltrating lymphocytes in early breast cancer. doi:10.1016/j.breast.2017.03.010. The Breast. 2018;37:2.07E216. doi: 10.1016/j.breast.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 42.Loi S, Michiels S, Salgado R. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER Trial. Ann Oncol. 2014;25:1544–1550. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 43.Adams S, Gray R J, Demaria S. Prognostic Value of Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer From Two Phase III Randomized Adjuvant Breast Cancer Trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32:2959–2966. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Denkert C, von Minckwitz G, Brase J C. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with and without carboplatin in human epidermal growth factor receptor 1-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33:983–991. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 45.Würfel F, Erber R, Huebner H. TILGen: A Program to Investigate Immune Targets in Breast Cancer Patients – First Results on the Influence of Tumor-Infiltrating Lymphocytes. Breast Care (Basel) 2018;13:8–14. doi: 10.1159/000486949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Denkert C, von Minckwitz G, Darb-Esfahani S. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40–50. doi: 10.1016/S1470-2045(17)30904-X. [DOI] [PubMed] [Google Scholar]
- 47.Mayer I A, Abramson V G, Lehmann B D. New strategies for triple-negative cancer – deciphering the heterogeneity. Clin Cancer Res. 2014;20:787–790. doi: 10.1158/1078-0432.CCR-13-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loibl S, Untch M, Burchardi N.Randomized phase II neoadjuvant study (GeparNuevo) to investigate the addition of durvalumab to a taxane-anthracycline containing chemotherapy in triple negative breast cancer ASCO J Clin Oncol 201836(Suppl.)Abstr. 104 [Google Scholar]
- 49.Schmid P, Adams S, Rugo H S. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 50.Emens L A, Loi S, Rugo H S. Impassion130: Efficacy in immune biomarker subgroups from the global, randomized, double-blind, placebo-controlled, Phase III study of Atezolizumab + nab-paclitaxel in patients with treatment-naïve, locally advanced or metastatic triple-negative breast cancer. SABCS 2018; abstract/oral presentation GS1-04
- 51.Hahnen E, Lederer B, Hauke J. Germline Mutation Status, Pathological Complete Response, and Disease-free Survival in Triple-Negative Breast Cancer: Secondary Analysis of the GeparSixto Randomized Clinical Trial. JAMA Oncol. 2017;3:1378–1385. doi: 10.1001/jamaoncol.2017.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Couch F J, Hart S N, Sharma P. Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J Clin Oncol. 2015;33:304–311. doi: 10.1200/JCO.2014.57.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fasching P A, Loibl S, Hu C. BRCA1/2 Mutations and Bevacizumab in the Neoadjuvant Treatment of Breast Cancer: Response and Prognosis Results in Patients With Triple-Negative Breast Cancer From the GeparQuinto Study. J Clin Oncol. 2018;36:2281–2287. doi: 10.1200/JCO.2017.77.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lord C J, Ashworth A. BRCAness revisited. Nat Rev Cancer. 2016;16:110–120. doi: 10.1038/nrc.2015.21. [DOI] [PubMed] [Google Scholar]
- 55.Telli M L, Timms K M, Reid J. Homologous Recombination Deficiency (HRD) Score Predicts Response to Platinum-Containing Neoadjuvant Chemotherapy in Patients with Triple-Negative Breast Cancer. Clin Cancer Res. 2016;22:3764–3773. doi: 10.1158/1078-0432.CCR-15-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loibl S, Weber K E, Timms K M. Survival analysis of carboplatin added to an anthracycline/taxane-based neoadjuvant chemotherapy and HRD score as predictor of response – final results from GeparSixto. Ann Oncol. 2018 doi: 10.1093/annonc/mdy460. [DOI] [PubMed] [Google Scholar]
- 57.Copson E R, Maishman T C, Tapper W J. Germline BRCA mutation and outcome in young-onset breast cancer (POSH). A prospective cohort study. Lancet Oncol. 2018;19:169–180. doi: 10.1016/S1470-2045(17)30891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tutt A, Tovey H, Cheang M CU. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nature Medicine. 2018;24:628–637. doi: 10.1038/s41591-018-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodler E T, Kurland B R, Griffin M. Phase I Study of Veliparib Combined with Cisplatin and Vinorelbine in Advanced Triple-Negative Breast Cancer and/or BRCA Mutation-Associated Breast Cancer. Clin Cancer Res. 2016;22:2855–2864. doi: 10.1158/1078-0432.CCR-15-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robson M, Im S A, Senkus E. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 61.Litton J K, Rugo H S, Ettl J. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med. 2018;379:753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Loibl S, OʼShaughnessy J, Untch M. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess). A randomised, phase 3 trial. Lancet Oncol. 2018;19:497–509. doi: 10.1016/S1470-2045(18)30111-6. [DOI] [PubMed] [Google Scholar]
- 63.Denkert C, Loibl S, Müller B M. Ki67 levels as predictive and prognostic parameters in pretherapeutic breast cancer core biopsies: a translational investigation in the neoadjuvant GeparTrio trial. Ann Oncol. 2013;24:2786–2793. doi: 10.1093/annonc/mdt350. [DOI] [PubMed] [Google Scholar]
- 64.Gray R, Bradley R, Braybrooke J. Increasing the dose density of adjuvant chemotherapy by shortening intervals between courses or by sequential drug administration significantly reduces both disease recurrence and breast cancer mortality: An EBCTCG meta-analysis. SABCS 2017; Abstr. GS1-01
- 65.Moebus V, Jackisch C, Lück H J. Ten-year results of intense dose-dense chemotherapy show superior survival compared with a conventional schedule in high-risk primary breast cancer: final results of AGO phase III iddEPC trial. Ann Oncol. 2018;29:178–185. doi: 10.1093/annonc/mdx690. [DOI] [PubMed] [Google Scholar]
- 66.Sparano J A, Wang M, Martino S. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–1671. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sparano J A, Zhao F, Martino S. Long-Term Follow-Up of the E1199 Phase III Trial Evaluating the Role of Taxane and Schedule in Operable Breast Cancer. J Clin Oncol. 2015;33:2353–2360. doi: 10.1200/JCO.2015.60.9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.von Minckwitz G, Schneeweiss A, Loibl S. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15:747–756. doi: 10.1016/S1470-2045(14)70160-3. [DOI] [PubMed] [Google Scholar]
- 69.Sikov W M, Berry D A, Perou C M. Impact of the Addition of Carboplatin and/or Bevacizumab to Neoadjuvant Once-per-Week Paclitaxel Followed by Dose-Dense Doxorubicin and Cyclophosphamide on Pathologic Complete Response Rates in Stage II to III Triple-Negative Breast Cancer: CALGB 40603 (Alliance) J Clin Oncol. 2015;33:13–21. doi: 10.1200/JCO.2014.57.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schneeweiss A, Möbus V, Tesch H. Intense dose-dense epirubicin, paclitaxel, cyclophosphamide versus weekly paclitaxel, liposomal doxorubicin (plus carboplatin in triple-negative breast cancer) for neoadjuvant treatment of high-risk early breast cancer (GeparOcto-GBG 84): A randomised phase III trial. Eur J Cancer. 2018;106:181–192. doi: 10.1016/j.ejca.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 71.von Minckwitz G, Untch M, Blohmer J U. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 72.Berruti A, Amoroso V, Gallo F. Pathologic complete response as a potential surrogate for the clinical outcome in patients with breast cancer after neoadjuvant therapy. A metaanalysis of 29 randomized prospective studies. J Clin Oncol. 2014;32:3883–3891. doi: 10.1200/JCO.2014.55.2836. [DOI] [PubMed] [Google Scholar]
- 73.Symmans W F, Wei C, Gould R. Long-Term Prognostic Risk After Neoadjuvant Chemotherapy Associated With Residual Cancer Burden and Breast Cancer Subtype. J Clin Oncol. 2017;35:1049–1060. doi: 10.1200/JCO.2015.63.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Masuda N, Lee S-J, Ohtani S. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med. 2017;376:2147–2156. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 75.Zujewski J A, Rubinstein L. CREATE-X a role for capecitabine in early-stage breast cancer: an analysis of available data. NPJ Breast Cancer. 2017;3:27. doi: 10.1038/s41523-017-0029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Untch M, Jackisch C, Schneeweiss A. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): a randomised, phase 3 trial. Lancet Oncol. 2016;17:345–356. doi: 10.1016/S1470-2045(15)00542-2. [DOI] [PubMed] [Google Scholar]
- 77.von Minckwitz G. Early survival analysis of the randomised phase II trial investigating the addition of carboplatin to neoadjuvant therapy for triple-negative and HER2-positive early breast cancer (GeparSixto). SABCS2015; Abstr. S2-04
- 78.Petrelli F, Coinu A, Borgonovo K. The value of platinum agents as neoadjuvant chemotherapy in triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res Treat. 2014;144:223–232. doi: 10.1007/s10549-014-2876-z. [DOI] [PubMed] [Google Scholar]
- 79.Wunderle M, Gass P, Häberle L. BRCA mutations and their influence on pathological complete response and prognosis in a clinical cohort of neoadjuvantly treated breast cancer patients. Breast Cancer Res Treat. 2018;171:85–94. doi: 10.1007/s10549-018-4797-8. [DOI] [PubMed] [Google Scholar]
- 80.Paluch-Shimon S, Friedman E, Berger R. Neo-adjuvant doxorubicin and cyclophosphamide followed by paclitaxel in triple-negative breast cancer among BRCA1 mutation carriers and non-carriers. Breast Cancer Res Treat. 2016;157:157–165. doi: 10.1007/s10549-016-3800-5. [DOI] [PubMed] [Google Scholar]
- 81.Gluz O, Nitz U, Liedtke C. Comparison of Neoadjuvant Nab-Paclitaxel+Carboplatin vs Nab-Paclitaxel+Gemcitabine in Triple-Negative Breast Cancer: Randomized WSG-ADAPT-TN Trial Results. J Natl Cancer Inst. 2018;110:628–637. doi: 10.1093/jnci/djx258. [DOI] [PubMed] [Google Scholar]
- 82.Gluz O, Nitz U, Liedtke C. Prognostic impact of anthracyclines and immune/proliferation markers in TNBC according to pCR after de-escalated neoadjuvant chemotherapy with 12 weeks og nab-paclitaxel/carboplatin or gemcitabine: Survival results of WSG-ADAPT-TN phase II trial. ESMO 2018; München, Poster
- 83.National Comprehensive Cancer Network (NCCN) Breast CancerOnline:https://www.nccn.org/professionals/physician_gls/default.aspxlast access: 15.01.2019
- 84.Therapieempfehlungen der Amerikanischen Gesellschaft für klinische Onkologie (ASCO)Online:https://www.asco.org/practice-guidelines/quality-guidelines/guidelines/breast-cancerEuropean Society for medical oncology; last access: 15.01.2019
- 85.ESMO Cardoso F, Costa A, Senkus E.International Consensus Guidelines for Advanced Breast Cancer (ABC3)Online:https://www.esmo.org/Guidelines/Breast-Cancerlast access: 15.01.2019oder:3rd ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC3) Ann Oncol 20172816–33.doi:10.1093/annonc/mdw544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wapnir I R, Price K N, Anderson S J. Efficacy of Chemotherapy for ER-Negative and ER-Positive Isolated Locoregional Recurrence of Breast Cancer. Final Analysis of the CALOR Trial. N Engl J Med. 2018;36:1073–1079. doi: 10.1200/JCO.2017.76.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Coombe R, Lisy K, Campbell J. Survival outcomes following aggressive treatment of oligometastatic breast cancer: a systematic review protocol. JBI Database System Rev Implement Rep. 2017;15:2013–2019. doi: 10.11124/JBISRIR-2016-002954. [DOI] [PubMed] [Google Scholar]
- 88.Rugo H S, Barry W T, Moreno-Aspitia A. Randomized phase III trial of paclitaxel once per week compared with nanoparticle albumin-bound nab-paclitaxel once per week or ixabepilone with bevacizumab as first-line chemotherapy for locally recurrent or metastatic breast cancer: CALGB 40502/NCCTG N063H (alliance) J Clin Oncol. 2015;33:2361–2369. doi: 10.1200/JCO.2014.59.5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hamilton E, Kimmick G, Hopkins J. Nab-paclitaxel/bevacizumab/carboplatin chemotherapy in first-line triple negative metastatic breast cancer. Clin Breast Cancer. 2013;13:416–420. doi: 10.1016/j.clbc.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 90.Lobo C, Lopes G, Baez O. Final results of a phase II study of nab-paclitaxel, bevacizumab and gemcitabine as first-line therapy for patients with HER2-negative metastatic breast cancer. Breast Cancer Res Treat. 2010;123:427–435. doi: 10.1007/s10549-010-1002-0. [DOI] [PubMed] [Google Scholar]
- 91.Yardley D A, Coleman R, Conte P. nab-Paclitaxel plus carboplatin or gemcitabine versus gemcitabine plus carboplatin as first-line treatment of patients with triple-negative metastatic breast cancer: results from the tnAcity trial. Ann Oncol. 2018;29:1763–1770. doi: 10.1093/annonc/mdy201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fachinformation Abraxane ® 5 mg/ml, Stand: Juli 2018
- 93.Dent R, Andre F, Goncalves A.IMpassion132: A double-blind randomized phase 3 trial evaluating chemotherapy (CT) ± atezolizumab (atezo) for early progressing locally advanced/metastatic triple-negative breast cancer (mTNBC) ASCO J Clin Oncol 201836(Suppl.)TPS1115 [Google Scholar]
- 94.ClinicalTrials.gov Identifier: NCT02620280
- 95.Online:https://www.gbg.de/de/studien/gepardouze.phplast access: 20.01.2019
- 96.ClinicalTrials.gov Identifier: NCT01997333
- 97.Stirrups R. Sacituzumab govitecan-hziy for triple-negative breast cancer. doi:10.1016/S1470-2045(19)30074-9. Lancet Oncol. 2019:pii:S1470-2045(19)30074-9. doi: 10.1016/S1470-2045(19)30074-9. [DOI] [PubMed] [Google Scholar]
- 98.Bardia A, Mayer I A, Vahdat L T. Sacituzumab Govitecan-hiziy in Refractory Metastatic Triple-Negative Breast Cancer. N Engl J Med. 2019;380:741–751. doi: 10.1056/NEJMoa1814213. [DOI] [PubMed] [Google Scholar]
- 99.ClinicalTrials.gov Identifier: NCT02338167
- 100.Fasching P A, Brucker S Y, Fehm T N. Biomarkers in Patients with Metastatic Breast Cancer and the PRAEGNANT Study Network. Geburtsh Frauenheilk. 2015;75:41–50. doi: 10.1055/s-0034-1396215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Online:http://www.gbg.de/de/Studien/aurora.phplast access: 20.01.2019