Abstract
Catecholamines are used to increase cardiac output and blood pressure, aiming ultimately at restoring/improving tissue perfusion. While intuitive in its concept, this approach nevertheless implies to be effective that regional organ perfusion would increase in parallel to cardiac output or perfusion pressure and that the catecholamine does not have negative effects on the microcirculation. Inotropic agents may be considered in some conditions, but it requires prior optimization of cardiac preload. Alternative approaches would be either to minimize exposure to vasopressors, tolerating hypotension and trying to prioritize perfusion but this may be valid as long as perfusion of the organ is preserved, or to combine moderate doses of vasopressors to vasodilatory agents, especially if these are predominantly acting on the microcirculation. In this review, we will discuss the pros and cons of the use of catecholamines and alternative agents for improving tissue perfusion in septic shock.
Keywords: Cardiac output, Microcirculation, Vasopressor agents, Inotropic agents, Fluid therapy
Introduction
Shock, whatever its cause, is characterized by a decrease in tissue perfusion, associated with cellular and metabolic abnormalities. The base of shock resuscitation is to improve tissue perfusion by restoring perfusion pressure of vital organs, ensuring an adequate cardiac output and, if possible, improving microvascular alterations. Several interventions can be considered, including fluids, vasopressor, and inotropic agents. Each of these interventions has adverse effects. A positive fluid balance is associated with an increased risk of death [1]. Interestingly, this study reported that the positive fluid balance was mostly due to a lower fluid output (mostly diuresis) while the amount of fluids administered to the patients did not differ between survivors and non-survivors. Hence, it is impossible to differentiate the impact of disease severity from a direct negative effect of fluids. Nevertheless, it sounds reasonable to avoid excessive fluid administration. As an alternative to fluids, vasoactive agents can be proposed. Vasopressive and inotropic catecholamines are life-saving interventions in several circumstances, but these agents are also associated with important adverse events. Tachycardia, arrhythmias, and metabolic, thermogenic, and immunologic effects are the most noticeable ones, but other adverse effects may also occur. In addition, vasopressive catecholamines may be associated with excessive vasoconstriction which may result in an impairment in tissue perfusion, even when perfusion pressure is restored. However, non-adrenergic vasopressors are also associated with some adverse effects, and switching from adrenergic to non-adrenergic vasopressors is not always optimal. Similarly, non-catecholaminergic inotropic agents may also be associated with adverse effects including tachycardia, digital necrosis, or splanchnic ischemia. Accordingly, the challenge in managing critically ill patients is to find the right balance between fluids, adrenergic and, eventually, non-adrenergic vasopressors, and inotropic agents. In this paper, we will focus on septic shock and discuss the different possibilities to optimize tissue perfusion and catecholamine use.
Manipulating perfusion pressure
Several trials have demonstrated that the severity and duration of hypotension are associated with a poor outcome [2, 3]. It sounds reasonable to introduce vasopressors without delay as the late introduction of vasopressors seems to be associated with an increased risk of death [4].
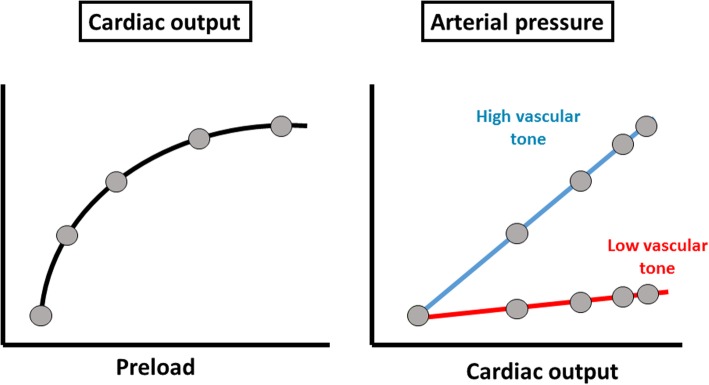
Fluids can increase perfusion pressure, but this effect is rather indirect, as it is mediated by an increase in cardiac output and requires that vascular tone is not too low (Fig. 1). Measurements of arterial elastance (defined as arterial pressure variations divided by stroke volume variations over one respiratory cycle) have been demonstrated to predict the pressure response to fluids [5], but these measurements are not easy to perform in clinical practice. Ideally, a beat-by-beat measurement of cardiac output independent of the arterial waveform should be obtained to prevent mathematical coupling of data using arterial trace to derive pulse pressure and stroke volume. Due to these methodological limitations, this principle is not used at bedside routinely and, in practice, most physicians perform fluid challenges evaluating whether or not perfusion pressure would increase with fluid administration. One may also use diastolic pressure as a witness of the alteration in vascular tone. When diastolic pressure is low, especially in patients with normal heart rates or tachycardia, this finding suggests a decrease in vascular tone, and in these conditions, fluid administration alone is often insufficient to increase perfusion pressure.
Fig. 1.
Effects of fluids on arterial pressure. Fluids increase preload which in turn can increase cardiac output if the heart operates on the steep part of the Starling relationship. Whatever the increase in cardiac output, fluids increase arterial pressure mostly in patients with high vascular tone. In patients with low vascular tone, even major increases in cardiac output have minimal impact on arterial pressure
In many patients, administration of vasopressors is thus required for this purpose.
Do vasopressors improve tissue perfusion? Increasing perfusion pressure is not equal to increasing tissue perfusion. First, once the autoregulatory threshold has been reached, further increasing perfusion pressure does not increase perfusion to the organ. Second, vasoconstricting agents may constrict arterioles, thereby decreasing microvascular perfusion. In healthy conditions, both norepinephrine and vasopressin decreased microvascular perfusion [6]. Accordingly, the net result of increasing perfusion pressure on tissue perfusion may depend on the balance between the potential beneficial effects on organ blood flow and negative impact on microvascular perfusion. Experimental evidence provides conflicting results, but factors such as the absence of fluid resuscitation or increased abdominal pressure may act as confounding factors. Evaluating the impact on tissue hypoperfusion in critically ill patients is not easy as it requires to perform advanced measurements before correction of hypotension. Introduction of norepinephrine in severely hypotensive septic shock patients is associated with an increase in cardiac output [7]. Several mechanisms may be implicated, including an increase in contractility, an increase in cardiac preload, and an increase in coronary artery perfusion [8]. The increase in blood pressure and cardiac output was associated with a decrease in lactate levels [7]. In a series of 14 patients with septic shock, correction of severe hypotension with norepinephrine administration resulted in an increase in mean arterial pressure (MAP) from 51 ± 3 mmHg to 79 ± 7 mmHg and was associated in the restoration of urine output in 12 of the 14 patients and an increase in creatinine clearance [9]. Even though evidence remains limited, these results suggest that norepinephrine improves tissue perfusion when used to correct severe hypotension. However, most of these trials evaluated the short-term effects of vasopressors and some of these beneficial effects may vanish over time. In addition, the optimal perfusion pressure may vary between patients.
Optimizing cardiac output
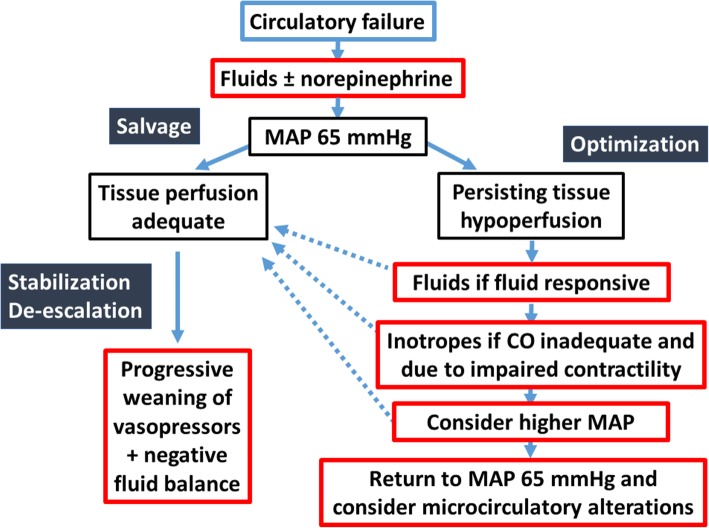
After the salvage phase aiming at restoring a minimal tissue perfusion pressure, optimizing cardiac output should be considered in order to improve tissue perfusion. Interestingly, cardiac output is not a target in itself. Indeed, it is very difficult to define what the ideal value of cardiac output is, as cardiac output fluctuates according to metabolic needs. Hence, instead of targeting given values of cardiac output, it is better to target adapted values of cardiac output. Cardiac output is judged inadequate based on ScvO2 and indices of tissue hypoperfusion. Increasing cardiac output requires a multiple steps approach (Fig. 2).
Fig. 2.
Suggested algorithm for tissue perfusion optimization. According to the phase of therapy (salvage/optimization/stabilization/de-escalation), different targets of resuscitation should be selected. Interventions should be progressively implemented, evaluating their effects and stopping it when ineffective. Once the patient is stabilized, weaning vasoactive agents and achieving a negative fluid balance should be considered
The first step should be to optimize preload in fluid responsive patients. Of note, all patients do not respond to fluids and excessive fluid administration is associated with a poor outcome [10]. Accordingly, it sounds appropriate to identify patients who may respond to fluids before fluid administration. The use of dynamic indices of fluid responsiveness, when applicable, allows the identification of fluid responders [11]. Do fluids also improve tissue perfusion? Obviously, the increase in cardiac output and tissue perfusion may be dissociated. Fluids improve microvascular perfusion only in the first 24 h, but not after 48 h, of sepsis recognition [12]. In a small series of patients, the first bolus improved cardiac output and microvascular perfusion while the second bolus failed to improve microvascular perfusion even though cardiac output further increased [13]. Hence, fluids improve microvascular perfusion in the early phases of sepsis and the optimal amount of fluids required to improve microvascular perfusion remains to be determined.
The second step is to consider inotropic agents when contractility is impaired and results into inadequate cardiac output and impaired tissue perfusion. Adrenergic inotropic agents are used to increase cardiac output aiming ultimately at restoring/improving tissue perfusion. While intuitive in its concept, this approach nevertheless implies several prerequisites. First, it implies that regional organ perfusion would increase in parallel to the increase in cardiac output. In patients with septic shock, increasing cardiac output with dobutamine was associated with an increase in hepato-splanchnic [14] or in cerebral perfusion [15]. The effects of dobutamine on other beds remain less studied.
Importantly, the use of inotropic agents requires prior optimization of cardiac preload.
The final step to consider is the optimization of cardiac afterload. While on the one hand one may consider to increase MAP in order to increase perfusion pressure, the increase in MAP also increases left ventricular afterload which may impair cardiac function and thus cardiac output, especially in patients with sepsis-associated myocardial depression [16].
Which targets?
The targets of resuscitation include perfusion pressure and various indices of tissue hypoperfusion.
What is the optimal blood pressure target? Varpula et al. [2] first demonstrated in 111 patients with septic shock that the time spent with MAP below 65 mmHg was associated with a worse outcome. Several trials not only confirmed this finding [3, 17, 18], but even further expanded this reporting that MAP threshold levels lower than 65 were associated with even more severe outcomes. Interestingly, mortality already increases with shorter periods of time when thresholds of MAP lower than 65 mmHg are considered compared to the 65 mmHg threshold. On the contrary, there was no association between mortality and time spent with below MAP thresholds 80–85 mmHg (but above 65 mmHg) [3, 17, 18]. Altogether, these observational studies support a MAP threshold around 65 mmHg for the majority of the patients.
However, the optimal MAP threshold may require some individualization. In a series of patients with sepsis but without clinical signs of hypoperfusion, a MAP between 55 and 65 mmHg was well tolerated [19]. In an interventional trial evaluating a MAP threshold of 65–70 versus 80–85 mmHg, no differences in survival were observed. In the subgroup of patients previously hypertensive, the higher MAP target was associated with a reduction in acute kidney injury. However, there was also a significant increase in arrhythmic events and a trend toward an increase in acute myocardial infarction. This trial had two major limitations. First, MAP was much higher than the predefined target value in the lower threshold group so that the safety of MAP 65 was not really evaluated. Second, the trial attributed patients to one group or the other without considering the response to therapy. Interestingly, different MAP targets had variable effects on various indices of tissue hypoperfusion and organ function in several small size interventional studies [20, 21]. Hence, the recommendations of the Surviving Sepsis Campaign guidelines to initially target MAP of 65 mmHg remain valid [22]. In selected patients, a higher MAP may be considered, but the effects of increasing MAP above 65 mmHg should be carefully evaluated and returning to lower MAP thresholds should be considered if the patient does not respond to this MAP challenge.
Indices of tissue perfusion include cardiac output, SvO2 and ScvO2, veno-arterial differences in PCO2 (PvaCO2), skin mottling and capillary refill time, blood lactate levels, and urine output. While cardiac output is a major determinant of tissue perfusion, it is remarkable that no specific target value can be derived from the literature. However, it is important to ensure that interventions aiming at increasing cardiac output effectively increase it, to prevent the risk of excessive or even useless administration of fluids and inotropic agents.
Observational trials have shown that the time spent with SvO2 below 70% is associated with an increased risk of death. Trials targeting ScvO2 for resuscitation have been associated with variable outcomes [23, 24]. While the Rivers’ trial [23] showed some benefit with early goal-directed therapy based on ScvO2, these results were not confirmed in subsequent trials [24]. Of note, ScvO2 was already in the target at baseline in the latter trials so that the impact of goal-directed therapy could not be evaluated. On the other hand, the persistence of low ScvO2 is associated with a poor outcome [25]. Accordingly, even if the impact of goal-directed therapy can be questioned, the usefulness of ScvO2 to interpret hemodynamic data, and in particular adequacy of cardiac output, remains [26]. PvaCO2 can also be helpful in identifying patients with impaired tissue perfusion, especially when ScvO2 is close to the normal range [12, 27]. While PvaCO2 has a strong prognostic value [28], its use as a guide for resuscitation has not been tested in large-scale randomized studies. Nevertheless, it provides interesting additional information that should not be neglected. Clinical indices of tissue perfusion such as skin mottling and temperature or capillary refill time [29] also provide important information but, at this stage, have not been tested as a target to therapy. Lactate is an excellent marker of the balance between oxygen demand and actual oxygen consumption, the latter being affected by tissue perfusion. While increased blood lactate levels have consistently been associated with increased mortality rates [30], its hypoxic origin cannot be ascertained. Measuring repeatedly lactate and pyruvate, it has been shown that lactate is mostly of hypoxic origin in the first few hours after the onset of shock but that the proportion of patients presenting hyperlactatemia of hypoxic origin decreased over time so that the majority of the hyperlactatemia at 24 h are of non-hypoxic origin [31]. Hospital mortality significantly decreased in a trial targeting lactate decreases by 20% every 2 h for 8 h [32]. However, lactate decreases may be slow, especially in patients with impaired liver function. It sounds thus reasonable to target lactate decreases [22], but it should be coupled with other markers of tissue hypoperfusion [33]. Hence, many indices can be used to assess tissue hypoperfusion, but none seems superior to the other and it is difficult to have a predefined target value for most of these. Accordingly, it sounds wise to guide resuscitation aiming at improving multiple indices (i.e., ScvO2 + PvaCO2 + lactate + skin perfusion), acknowledging that some may fail to rapidly improve [33].
Even though promising, microcirculation assessment is not yet part of our clinical armamentarium. Several trials have shown that the microcirculation is altered, especially in sepsis, and that the severity of the alterations is associated with a poor outcome [34–36]. As alterations in microvascular perfusion cannot be predicted from systemic hemodynamics [37], it sounds interesting to evaluate the microcirculation. The main limitations to its current use are the lack of therapeutic intervention specifically targeting the microcirculation and the difficulty to apply these handheld microscopes on the sublingual area in certain conditions. Interestingly, PvaCO2 seems to be a good surrogate of microvascular perfusion [38] and may at least address one of these limitations.
Finally, different thresholds should be considered according to time. Globally, the care of the patient in shock can be divided into four phases: salvage, optimization, stabilization, and de-escalation [39]. The targets for therapy and interventions should be adapted according to these different phases (Fig. 2).
Which agents?
For increasing perfusion pressure, administration of norepinephrine is associated with variable effects. Correcting severe hypotension with norepinephrine was associated with an improved organ function; however, increasing MAP from 65 to 75 or 85 mmHg was associated with variable effects. Second, and even more importantly, it implies that the catecholamine does not have negative effects on the microcirculation. While capillaries do not have adrenergic receptors, alpha- and beta-adrenergic receptors are present in resistive arterioles, at the entrance of capillary network. Administration of beta-adrenergic compounds is associated usually with an improvement in capillary perfusion. On the other hand, administration of alpha-adrenergic agents may be associated with a decrease in microvascular perfusion. The resulting effect of alpha-adrenergic administration on tissue perfusion will reflect the balance between increasing total organ blood flow and impairing microvascular perfusion.
Interestingly, epinephrine is associated with impaired splanchnic perfusion compared to norepinephrine [40]. In patients with cardiogenic shock, epinephrine was also associated with an impaired splanchnic perfusion. More importantly, epinephrine was associated with an increased incidence of refractory shock and a trend toward an increased mortality [41]. Even though the regional effects of norepinephrine and dopamine did not differ, dopamine is associated with more adverse effects and an increased risk of death [42, 43]. Accordingly, norepinephrine is considered as the first-line vasopressor agent [22].
Theoretically, it may be interesting to consider non-adrenergic vasopressors. Importantly, vasopressin derivatives as well as angiotensin behave similarly to norepinephrine. Indeed, even though these three agents stimulate different receptors at the surface of vascular smooth muscle cells, the downstream pathways are very similar with the implication of G protein, phospholipase C, and protein kinase C to promote increases in intracellular calcium through mobilization from sarcoplasmic reticulum and opening of voltage calcium channels. The difference between these agents is thus determined more by the density and sensitivity of the receptors than by the nature of the agent. At usual doses, the effects of norepinephrine and vasopressin do not differ; however, at high doses, vasopressin derivatives may be associated with digital necrosis [44] and splanchnic hypoperfusion [45]. Angiotensin administration may be associated with less requirements of renal support in patients with acute kidney injury [46], but these effects were noticed in a very small subgroup of patients and should be confirmed in larger trials.
Alternative approaches would be either to minimize exposure to vasopressors, tolerating hypotension and trying to prioritize perfusion but this approach can be valid as long as perfusion of the organs is preserved. Alternatively, one may consider to combine moderate doses of vasopressors, aiming at preserving organ perfusion, to vasodilatory agents in order to improve microvascular perfusion. In this context, nitroglycerin was shown to improve microvascular in eight patients with septic shock [47]. However, nitroglycerin was given together with a fluid bolus in order to minimize the risks of hypotension so that the effects of the fluid bolus in the improvement in microcirculatory perfusion cannot be excluded. A randomized trial failed to demonstrate any significant effects of nitroglycerin on the microcirculation in fluid-resuscitated septic shock patients. This may reflect the non-selective effect of nitroglycerin. More selective vasodilatory agents acting preferentially on non-perfused vessels have shown promising results in experimental conditions [48], but clinical evidence is still lacking.
For increasing cardiac output, dobutamine is the agent of choice. It is characterized by a short half-life and has minimal adverse effects at usual doses. In patients with septic shock, dobutamine increased oxygen delivery with minimal impact on MAP [49]. In most cases, the increase in cardiac output was related to an increase in stroke volume more. In another trial, it was nevertheless noted that the increase in cardiac output was in some case related more to an increase in heart rate than in stroke volume [50]. In addition, one should note that metabolic effects can be observed mostly when high doses of beta-adrenergic agents are used. Hence, one should always use the minimal dose associated with the desired hemodynamic effect and wean it as soon as possible. Non-adrenergic alternatives include phosphodiesterase inhibitors and levosimendan. Phosphodiesterase inhibitors like enoximone and milrinone are characterized by a long half-life and associate vasodilatory to the inotropic properties. Hypotension is often encountered and often limit/prevent the use of these agents in septic shock. Levosimendan, a calcium sensitizer, is an attractive alternative. Unfortunately, it is also associated with vasodilatory properties and with a very long half-life. This latter property makes this agent not optimal for therapy in septic patients as its action (positive inotropy as well as adverse effects) would remain present for 5–7 days. Of note, a prolonged effect is not desired in septic shock as myocardial depression usually resolves over a few days, and hence, indication for inotropic stimulation also disappears. In a small size trial, levosimendan was effective in increasing cardiac output and improving gastric perfusion [51]. Even though it has limited impact on blood pressure, the amount of fluids administered was also higher in patients receiving levosimendan. In a randomized trial, levosimendan was ineffective in preventing new-onset organ dysfunction [52]. Unfortunately, the indication to administrate levosimendan in that trial was not triggered on hemodynamic endpoints (no cardiac output measurements and no assessment of cardiac function). Indeed, as for other inotropic agents, levosimendan should only be administered in patients with tissue hypoperfusion related to an inadequate cardiac output associated with altered contractility. Accordingly, the hypothesis of whether levosimendan could improve, or not, tissue perfusion in patients with inadequate cardiac output in sepsis was not tested.
Dobutamine remains thus the inotropic agent of choice in sepsis, even though other inotropic agents may offer interesting alternatives in some conditions.
Conclusions
The main goal of hemodynamic resuscitation in shock is to improve tissue perfusion and oxygenation. As these cannot be directly evaluated at bedside in routine practice, physicians are left with surrogates such as perfusion pressure, cardiac output, SvO2, ScVO2, PvaCO2, cardiac output, diuresis, lactate, and skin mottling. As none of these can be considered ideal and as no single target value can be identified for all patients, the management of the patients with septic shock patient should be individualized taking into consideration several indices of tissue hypoperfusion.
While catecholamines are often used to increase blood pressure and cardiac output, these have adverse effects that may be associated with increased risk of death especially when used at high doses. Accordingly, these agents should be wisely used, minimizing the dose by optimizing fluid resuscitation, considering alternative agents when possible, and taking into account the response to therapy.
Acknowledgements
None
Funding
None declared by the authors. Publication of this supplement was supported by Fresenius Kabi.
Availability of data and materials
Not applicable
About this supplement
This article has been published as part of Critical Care, Volume 23 Supplement 1, 2019: Future of Critical Care Medicine (FCCM) 2018. The full contents of the supplement are available at https://ccforum.biomedcentral.com/articles/supplements/volume-23-supplement-1.
Abbreviations
- MAP
Mean arterial pressure
- PCO2
Partial pressure in CO2
- PvaCO2
Veno-arterial difference in PCO2
- ScvO2
Central venous oxygen saturation
- SvO2
Mixed-venous oxygen saturation
Authors’ contributions
All authors provided intellectual contributions and read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Daniel De Backer, Phone: 32.2.434.8976, Email: ddebacke@ulb.ac.be.
Pierre Foulon, Email: pfoulon.pro@gmail.com.
References
- 1.Sakr Y, Rubatto Birri PN, Kotfis K, Nanchal R, Shah B, Kluge S, et al. Higher fluid balance increases the risk of death from sepsis: results from a large international audit. Crit Care Med. 2017;45(3):386–394. doi: 10.1097/CCM.0000000000002189. [DOI] [PubMed] [Google Scholar]
- 2.Varpula M, Tallgren M, Saukkonen K, Voipio-Pulkki LM, Pettila V. Hemodynamic variables related to outcome in septic shock. Intensive Care Med. 2005;31(8):1066–1071. doi: 10.1007/s00134-005-2688-z. [DOI] [PubMed] [Google Scholar]
- 3.Dunser MW, Takala J, Ulmer H, Mayr VD, Luckner G, Jochberger S, et al. Arterial blood pressure during early sepsis and outcome. Intensive Care Med. 2009;35(7):1225–1233. doi: 10.1007/s00134-009-1427-2. [DOI] [PubMed] [Google Scholar]
- 4.Bai X, Yu W, Ji W, Lin Z, Tan S, Duan K, et al. Early versus delayed administration of norepinephrine in patients with septic shock. Crit Care. 2014;18(5):532. doi: 10.1186/s13054-014-0532-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monge Garcia MI, Gil CA, Gracia RM. Dynamic arterial elastance to predict arterial pressure response to volume loading in preload-dependent patients. Crit Care. 2011;15(1):R15. doi: 10.1186/cc9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friesenecker BE, Tsai AG, Martini J, Ulmer H, Wenzel V, Hasibeder WR, et al. Arteriolar vasoconstrictive response: comparing the effects of arginine vasopressin and norepinephrine. Crit Care. 2006;10(3):R75. doi: 10.1186/cc4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamzaoui O, Jozwiak M, Geffriaud T, Sztrymf B, Prat D, Monnet X, et al. Norepinephrine exerts an inotropic effect at the early phase of human septic shock. Br J Anaesth. 2018;120(3):517–524. doi: 10.1016/j.bja.2017.11.065. [DOI] [PubMed] [Google Scholar]
- 8.De Backer D, Pinsky M. Norepinephrine improves cardiac function during septic shock, but why? Br J Anaesth. 2018;120(3):421–424. doi: 10.1016/j.bja.2017.11.069. [DOI] [PubMed] [Google Scholar]
- 9.Albanese J, Leone M, Garnier F, Bourgoin A, Antonini F, Martin C. Renal effects of norepinephrine in septic and nonseptic patients. Chest. 2004;126(2):534–539. doi: 10.1378/chest.126.2.534. [DOI] [PubMed] [Google Scholar]
- 10.Brandt S, Regueira T, Bracht H, Porta F, Djafarzadeh S, Takala J, et al. Effect of fluid resuscitation on mortality and organ function in experimental sepsis models. Crit Care. 2009;13(6):R186. doi: 10.1186/cc8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cecconi M, De Backer D, Antonelli M, Beale RJ, Bakker J, Hofer C, et al. Consensus on circulatory shock and hemodynamic monitoring. Task Force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40(12):1795–1815. doi: 10.1007/s00134-014-3525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ospina-Tascon G, Neves AP, Occhipinti G, Donadello K, Buchele G, Simion D, et al. Effects of fluids on microvascular perfusion in patients with severe sepsis. Intensive Care Med. 2010;36(6):949–955. doi: 10.1007/s00134-010-1843-3. [DOI] [PubMed] [Google Scholar]
- 13.Pottecher J, Deruddre S, Teboul JL, Georger J, Laplace C, Benhamou D, et al. Both passive leg raising and intravascular volume expansion improve sublingual microcirculatory perfusion in severe sepsis and septic shock patients. Intensive Care Med. 2010;36:1867–1874. doi: 10.1007/s00134-010-1966-6. [DOI] [PubMed] [Google Scholar]
- 14.De Backer D, Creteur J, Noordally O, Smail N, Gulbis B, Vincent JL. Does hepato-splanchnic VO2/DO2 dependency exist in critically ill septic patients? Am J Respir Crit Care Med. 1998;157:1219–1225. doi: 10.1164/ajrccm.157.4.9705075. [DOI] [PubMed] [Google Scholar]
- 15.Berre J, De Backer D, Moraine JJ, Melot C, Kahn RJ, Vincent JL. Dobutamine increases cerebral blood flow velocity and jugular bulb hemoglobin saturation. Crit Care Med. 1997;25:392–398. doi: 10.1097/00003246-199703000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Vieillard-Baron A, Caille V, Charron C, Belliard G, Page B, Jardin F. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med. 2008;36(6):1701–1706. doi: 10.1097/CCM.0b013e318174db05. [DOI] [PubMed] [Google Scholar]
- 17.Maheshwari K, Nathanson BH, Munson SH, Khangulov V, Stevens M, Badani H, et al. The relationship between ICU hypotension and in-hospital mortality and morbidity in septic patients. Intensive Care Med. 2018;44:857–867. doi: 10.1007/s00134-018-5218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vincent JL, Nielsen ND, Shapiro NI, Gerbasi ME, Grossman A, Doroff R, et al. Mean arterial pressure and mortality in patients with distributive shock: a retrospective analysis of the MIMIC-III database. Ann Intensive Care. 2018;8:107. doi: 10.1186/s13613-018-0448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavillegrand JR, Dumas G, Bige N, Zafimahazo D, Guidet B, Maury E, et al. Should we treat mild hypotension in septic patients in the absence of peripheral tissue hypoperfusion? Intensive Care Med. 2018;44(9):1593–1594. doi: 10.1007/s00134-018-5315-5. [DOI] [PubMed] [Google Scholar]
- 20.Deruddre S, Cheisson G, Mazoit JX, Vicaut E, Benhamou D, Duranteau J. Renal arterial resistance in septic shock: effects of increasing mean arterial pressure with norepinephrine on the renal resistive index assessed with Doppler ultrasonography. Intensive Care Med. 2007;33(9):1557–1562. doi: 10.1007/s00134-007-0665-4. [DOI] [PubMed] [Google Scholar]
- 21.Dubin A, Pozo MO, Casabella CA, Palizas F, Jr, Murias G, Moseinco MC, et al. Increasing arterial blood pressure with norepinephrine does not improve microcirculatory blood flow: a prospective study. Crit Care. 2009;13(3):R92. doi: 10.1186/cc7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 23.Rivers E, Nguyen B, Havstadt S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 24.Angus DC, Barnato AE, Bell D, Bellomo R, Chong CR, Coats TJ, et al. A systematic review and meta-analysis of early goal-directed therapy for septic shock: the ARISE, ProCESS and ProMISe investigators. Intensive Care Med. 2015;41(9):1549–1560. doi: 10.1007/s00134-015-3822-1. [DOI] [PubMed] [Google Scholar]
- 25.Protti A, Masson S, Latini R, Fumagalli R, Romero M, Pessina C, et al. Persistence of central venous oxygen desaturation during early sepsis is associated with higher mortality: a retrospective analysis of the ALBIOS trial. Chest. 2018;15:1291–1300. doi: 10.1016/j.chest.2018.04.043. [DOI] [PubMed] [Google Scholar]
- 26.De Backer D. Detailing the cardiovascular profile in shock patients. Crit Care. 2017;21(Suppl 3):311. doi: 10.1186/s13054-017-1908-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perner A, Gordon AC, De Backer D, Dimopoulos G, Russell JA, Lipman J, et al. Sepsis: frontiers in diagnosis, resuscitation and antibiotic therapy. Intensive Care Med. 2016;42(12):1958–1969. doi: 10.1007/s00134-016-4577-z. [DOI] [PubMed] [Google Scholar]
- 28.Ospina-Tascon GA, Umana M, Bermudez W, Bautista-Rincon DF, Hernandez G, Bruhn A, et al. Combination of arterial lactate levels and venous-arterial CO to arterial-venous O content difference ratio as markers of resuscitation in patients with septic shock. Intensive Care Med. 2015;41:796–805. doi: 10.1007/s00134-015-3720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ait-Oufella H, Bakker J. Understanding clinical signs of poor tissue perfusion during septic shock. Intensive Care Med. 2016;42(12):2070–2072. doi: 10.1007/s00134-016-4250-6. [DOI] [PubMed] [Google Scholar]
- 30.Casserly B, Phillips GS, Schorr C, Dellinger RP, Townsend SR, Osborn TM, et al. Lactate measurements in sepsis-induced tissue hypoperfusion: results from the Surviving Sepsis Campaign database. Crit Care Med. 2015;43(3):567–573. doi: 10.1097/CCM.0000000000000742. [DOI] [PubMed] [Google Scholar]
- 31.Rimachi R, Bruzzi dC, Orellano-Jimenez C, Cotton F, Vincent J, De Backer D. Lactate/pyruvate ratio as a marker of tissue hypoxia in circulatory and septic shock. Anaesth Intensive Care. 2012;40(3):427–432. doi: 10.1177/0310057X1204000307. [DOI] [PubMed] [Google Scholar]
- 32.Jansen TC, van Bommel J, Schoonderbeek J, Sleeswijk Visser SJ, van der Klooster JM, Lima AP, et al. Early lactate-guided therapy in ICU patients: a multicenter, open-label, randomized, controlled trial. Am J Respir Crit Care Med. 2010;182(6):752–761. doi: 10.1164/rccm.200912-1918OC. [DOI] [PubMed] [Google Scholar]
- 33.Bakker J, De Backer D, Hernandez G. Lactate-guided resuscitation saves lives: we are not sure. Intensive Care Med. 2016;42(3):472–474. doi: 10.1007/s00134-016-4220-z. [DOI] [PubMed] [Google Scholar]
- 34.De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. 2002;166:98–104. doi: 10.1164/rccm.200109-016OC. [DOI] [PubMed] [Google Scholar]
- 35.De Backer D, Donadello K, Sakr Y, Ospina-Tascon GA, Salgado DR, Scolletta S, et al. Microcirculatory alterations in patients with severe sepsis: impact of time of assessment and relationship with outcome. Crit Care Med. 2013;41(3):791–799. doi: 10.1097/CCM.0b013e3182742e8b. [DOI] [PubMed] [Google Scholar]
- 36.Massey MJ, Hou PC, Filbin M, Wang H, Ngo L, Huang DT, et al. Microcirculatory perfusion disturbances in septic shock: results from the ProCESS trial. Crit Care. 2018;22(1):308. doi: 10.1186/s13054-018-2240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Backer D, Creteur J, Dubois MJ, Sakr Y, koch M, Verdant C, et al. The effects of dobutamine on microcirculatory alterations in patients with septic shock are independent of its systemic effects. Crit Care Med. 2006;34(2):403–408. doi: 10.1097/01.CCM.0000198107.61493.5A. [DOI] [PubMed] [Google Scholar]
- 38.Ospina-Tascon GA, Umana M, Bermudez WF, Bautista-Rincon DF, Valencia JD, Madrinan HJ, et al. Can venous-to-arterial carbon dioxide differences reflect microcirculatory alterations in patients with septic shock? Intensive Care Med. 2016;42(2):211–221. doi: 10.1007/s00134-015-4133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vincent JL, De Backer D. Circulatory shock. N Engl J Med. 2013;369(18):1726–1734. doi: 10.1056/NEJMra1208943. [DOI] [PubMed] [Google Scholar]
- 40.De Backer D, Creteur J, Silva E, Vincent JL. Effects of dopamine, norepinephrine, and epinephrine on the splanchnic circulation in septic shock: which is best? Crit Care Med. 2003;31:1659–1667. doi: 10.1097/01.CCM.0000063045.77339.B6. [DOI] [PubMed] [Google Scholar]
- 41.Levy B, Clere-Jehl R, Legras A, Morichau-Beauchant T, Leone M, Frederique G, et al. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2018;72(2):173–182. doi: 10.1016/j.jacc.2018.04.051. [DOI] [PubMed] [Google Scholar]
- 42.De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362(9):779–789. doi: 10.1056/NEJMoa0907118. [DOI] [PubMed] [Google Scholar]
- 43.Vail E, Gershengorn HB, Hua M, Walkey AJ, Rubenfeld G, Wunsch H. Association between US norepinephrine shortage and mortality among patients with septic shock. JAMA. 2017;317(14):1433–1442. doi: 10.1001/jama.2017.2841. [DOI] [PubMed] [Google Scholar]
- 44.Liu ZM, Chen J, Kou Q, Lin Q, Huang X, Tang Z, et al. Terlipressin versus norepinephrine as infusion in patients with septic shock: a multicentre, randomised, double-blinded trial. Intensive Care Med. 2018;44(11):1816–1825. doi: 10.1007/s00134-018-5267-9. [DOI] [PubMed] [Google Scholar]
- 45.Klinzing S, Simon M, Reinhart K, Bredle DL, Meier-Hellmann A. High-dose vasopressin is not superior to norepinephrine in septic shock. Crit Care Med. 2003;31:2646–2650. doi: 10.1097/01.CCM.0000094260.05266.F4. [DOI] [PubMed] [Google Scholar]
- 46.Tumlin JA, Murugan R, Deane AM, Ostermann M, Busse LW, Ham KR, et al. Outcomes in patients with vasodilatory shock and renal replacement therapy treated with intravenous angiotensin II. Crit Care Med. 2018;46(6):949–957. doi: 10.1097/CCM.0000000000003092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spronk PE, Ince C, Gardien MJ, Mathura KR, Oudemans-van Straaten HM, Zandstra DF. Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet. 2002;360:1395–1396. doi: 10.1016/S0140-6736(02)11393-6. [DOI] [PubMed] [Google Scholar]
- 48.He X, Su F, Velissaris D, Salgado DR, de Souza BD, Lorent S, et al. Administration of tetrahydrobiopterin improves the microcirculation and outcome in an ovine model of septic shock. Crit Care Med. 2012;40(10):2833–2840. doi: 10.1097/CCM.0b013e31825b88ba. [DOI] [PubMed] [Google Scholar]
- 49.Vincent JL, Roman A, De Backer D, Kahn RJ. Oxygen uptake/supply dependency: effects of short-term dobutamine infusion. Am Rev Respir Dis. 1990;142:2–8. doi: 10.1164/ajrccm/142.1.2. [DOI] [PubMed] [Google Scholar]
- 50.Jellema WT, Groeneveld AB, Wesseling KH, Thijs LG, Westerhof N, van Lieshout JJ. Heterogeneity and prediction of hemodynamic responses to dobutamine in patients with septic shock. Crit Care Med. 2006;34(9):2392–2398. doi: 10.1097/01.CCM.0000233871.52553.CD. [DOI] [PubMed] [Google Scholar]
- 51.Morelli A, De Castro S, Teboul JL, Singer M, Rocco M, Conti G, et al. Effects of levosimendan on systemic and regional hemodynamics in septic myocardial depression. Intensive Care Med. 2005;31(5):638–644. doi: 10.1007/s00134-005-2619-z. [DOI] [PubMed] [Google Scholar]
- 52.Gordon AC, Perkins GD, Singer M, McAuley DF, Orme RM, Santhakumaran S, et al. Levosimendan for the prevention of acute organ dysfunction in sepsis. N Engl J Med. 2016;375:1638–1648. doi: 10.1056/NEJMoa1609409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable