Abstract
Context
Coma is a serious manifestation of thyroid storm (TS) about which little is known.
Objective
To describe the features, duration, treatment response, and prognosis of coma in the setting of TS.
Design
Aggregate analysis of individual English-language case reports of coma in the setting of TS from 1935 to January 2019.
Setting
Hospitals.
Patients
Sixty-five cases were identified, 29 from case reports and 36 from case series.
Interventions
Antithyroid drugs, corticosteroids, beta-blockers, iodine, intubation, plasmapheresis, antibiotics, thyroidectomy, radioiodine, dialysis, and l-carnitine.
Main Outcome Measures
Awakening and death rates overall and in relation to administered treatments, day of coma presentation, and time from coma onset; symptoms associated with coma; TS and coma scales; thyroid and cerebrospinal laboratory tests; electroencephalogram; brain imaging; and autopsy results.
Results
Mortality was 38% in the setting of TS-related coma, 11% during the years 1978 to 2019 compared with 70% for 1935 to 1977. Both awakening and death commonly occurred within the first 2 days of coma onset. Reduction in total and free T4 values, and possibly also total T3 value, correlated with awakening from coma. Lower death rates were associated with use of antithyroid drugs, corticosteroids, beta-blockers, and intubation. Plasmapheresis was associated with awakening in 67% of cases but not with lower death rates.
Conclusions
Prognosis of coma associated with TS remains poor. Current guidelines for the early use of plasmapheresis in unresolving TS are advocated and should be considered urgently at the point of confusion or delirium in an effort to abort coma.
Keywords: thyroid storm, coma, unconscious, thyrotoxicosis, plasmapheresis
Thyroid storm (TS) is a rare condition characterized by an extreme, decompensated, life-threatening state of thyrotoxicosis. Diagnostic criteria for TS include, among other features, central nervous system (CNS) effects ranging from agitation to delirium, psychosis, or extreme lethargy to seizure or coma [1, 2]. Coma or even altered mentation has been reported to indicate a more serious outcome of TS [2–6]. Despite this, little is known regarding specific features or the effect of treatments on TS-related coma. For better understanding of this condition, a review of the English-language literature was performed to itemize individually reported cases with coexistent coma and TS, which were then evaluated in aggregate to describe the features, duration, treatment response, and prognosis of coma in the setting of TS.
1. Methods
The English-language literature was reviewed for reports of thyrotoxicosis with coma. This included single case reports and clinical series containing individual case details or reports. Title or abstract words TS, thyrotoxicosis, hyperthyroidism, coma, plasmapheresis, and plasma exchange with text words thyroid, coma, and unconscious were searched in PubMed. Cases were included if they reported diagnosis of both TS and coma or if they had coma and thyrotoxicosis likely meeting Burch-Wartofsky (BW) TS criteria in an independent review and calculation. Coma was as defined by the reporting authors. Specifically, for the report to be included, the reporting authors must have described the patient as having coma, unconsciousness, or unresponsiveness. Confusion, delirium, stupor, and obtundation were not sufficient for inclusion. Cases clinically diagnosed as status epilepticus were excluded. Consistent coma description without internal contradiction within the report was required for inclusion. Reports were reviewed for details pertinent to the thyroid state and coma, all of which were entered into a REDCap database for storage and aggregate analysis [7]. English-language abstracts were included even if the primary article was not in English. BW TS score was calculated from data available for each case [1]. Specifically, a minimum score of 30 was assigned to all coma cases; when detail permitted, additional points were assigned for atrial fibrillation, heart rate, heart failure, temperature, and gastrointestinal dysfunction.
For cases in which treatment details were given, the absence of a particular therapy/intervention was interpreted as indicating that it was not administered. For cases in which no details were given (e.g., no treatment details for any individual cases within some small series), the cases were excluded from the specific analysis.
Statistical analysis was performed using JMP Pro v. 13 software (SAS Institute, Cary, NC). Continuous data were reported as mean ± SD or 95% CIs and categorical data as count and proportions. χ2 or Fisher’s exact test was used to compare categorical data. The Student t test or Wilcoxon two-sample test was used to determine significance for continuous data as appropriate. All tests were two-sided. A P value <0.05 was considered significant.
2. Results
Sixty-five cases of thyrotoxicosis with coma were reported in English-language articles over 84 years between 1935 and 2019. Thirty-six cases were individually reported within small case series [3, 8–16], and 29 were reported as individual case reports [17–45]. The case series (1935 to 2011; median year, 1969) were published earlier than the individual cases (1951 to 2018; median year, 2000). Patients ranged in age from 3.5 to 87 years, with a mean age of 46 (SD, 20) years; 75% of the patients were female. Two patients had more than one episode of coma [32]; in one case, the patient recovered from TS-related coma but later died during the second episode of coma [22].
A. Preexisting Conditions
No differences were noted in the characteristics of the study population or the TS manifestations relative to the origin of the data, whether from case reports or case series (Tables 1 and 2).
Table 1.
Characteristics of Study Population
| Feature | Number of Individual Cases | ||
|---|---|---|---|
| From Whole Series | From Case Reports | From Case Series | |
| n | 65 | 29 | 36 |
| Publication year, range | 1935–2018 (median, 1984) | 1951–2019 (median, 2000) | 1935–2011 (median, 1969) |
| Age, range, y | 3.5–87 mean (SD): 46 (20) | 3.5–87 mean (SD): 48 (21) | 10–74 mean (SD): 44 (19) |
| Female/Malea | 49 (75)/16 (25) | 22 (76)/7 (24) | 27 (75)/9 (26) |
| Preexisting hyperthyroidism | 21 (32) | 9 (31) | 12 (33) |
| Preexisting goiter | 27 (42) | 12 (41) | 15 (42) |
| Preexisting goiter and hyperthyroidism | 15 (23) | 6 (21) | 9 (25) |
| Preexisting weight loss | 19 (29) | 8 (28) | 11 (31) |
| Emaciated | 5 (7.7) | 4 (14) | 1 (2.8) |
| Cardiac history | 7 (11) | 3 (10) | 4 (11) |
| History of atrial fibrillation | 4 (6.2) | 3 (10) | 1 (2.8) |
| History of stroke or brain atrophy | 4 (6.2) | 3 (10) | 1 (2.8) |
| New or known diabetes | 8 (12) | 3 (10) | 5 (14) |
| Pregnancy | 2 (3) | 2 (6.9) | 0 |
| Death reported on case report | 25 (38) | 4 (14) | 21 (58) |
Data are presented as n (%) unless otherwise noted.
Female/Male ratio may exceed 100% because of rounding.
Table 2.
Systemic Findings Associated With Thyroid Storm in Patients With Coma
| Total (n = 65) | From Case Reports (n = 29) | From Case Series (n = 36) | P | |
|---|---|---|---|---|
| Tachycardia >120 bpm | 57 (88) | 27 (93) | 30 (83) | 1.000 |
| Fever >100.5°F | 54 (83) | 21 (72) | 33 (92) | 0.0689 |
| Hypertension | 19 (29) | 9 (31) | 10 (28) | 0.7907 |
| Heart failure | 20 (30) | 8 (28) | 12 (33) | 0.7877 |
| Atrial fibrillation | 15 (23) | 8 (28) | 7 (19) | 0.7621 |
| Liver dysfunction | 15 (23) | 10 (34) | 5 (14) | 0.4454 |
| Renal dysfunction | 13 (20) | 6 (21) | 7 (19) | 0.1013 |
| RR >20/min | 9 (14) | 7 (24) | 2 (6) | 1.000 |
| Hypotension or shock | 8 (12) | 6 (21) | 2 (6) | 0.2529 |
| Hyperglycemia | 8 (12) | 6 (21) | 2 (6) | 1.000 |
| Hypoglycemia | 5 (8) | 5 (17) | 0 | 1.000 |
| Other arrhythmia | 3 (5) | 2 (7) | 1 (3) | 1.000 |
| DIC | 3 (5) | 3 (10) | 0 | 1.000 |
Data are presented as n (%) unless otherwise noted. The P value shows the comparison for the parameter based on report type, either case reports or from case series. Other arrhythmia = atrial flutter or ventricular fibrillation.
Abbreviations: bpm, beats per min; DIC, disseminated intravascular coagulation; RR, respiratory rate.
Preexisting hyperthyroidism was reported in 21 cases (32%), preexisting goiter in 27 cases (42%), and both goiter and hyperthyroidism in 15 cases (23%). Except for two cases involving noncompliance [23, 24] and one case each in which iodine withdrawal or thyroidectomy was cited as the precipitant for the TS [3], the reports generally lacked detail on whether treatment was given for hyperthyroidism or its duration or efficacy before the TS event.
In 19 cases (29%), patients reportedly lost weight before the TS event, and four were described as emaciated at presentation. New or known diabetes was present in eight cases (12%). Preexisting heart condition (excluding atrial fibrillation) was present in seven cases (11%). Past brain event or abnormality was present in four and history of atrial fibrillation in four (6.2% for each).
Overall, no preexisting condition stood out as a predominant feature in most cases of TS-related coma.
B. Precipitant of TS With Coma
More than one event precipitating the TS-related coma was identified in 21 cases [3, 8, 14–16, 21–24, 33, 35, 36, 38]. No precipitating event could be identified in eight cases [10, 11, 30, 40, 42, 43, 45], and 10 cases had no information on the TS-precipitating event. In some cases, it was not possible to separate precipitating events from consequences of the TS, such as seizure or stroke [15, 20, 24, 26, 35].
Infection was either treated or thought to be likely in 20 cases [3, 8, 10, 12–14, 19, 22, 23, 32], with all other potential precipitating causes reported much less frequently. Six episodes followed an operation (including curettage in two, cesarean section, hysterectomy, thyroid cancer metastasis biopsy, and thyroidectomy) [3, 8, 13, 21, 36, 38]. Iodine exposure from amiodarone was reported in two cases [16, 31] and from CT contrast medium in three cases [15, 34, 38]. There were five cases each of thyroid hormone overdose [14], dehydration [16, 18, 27, 33, 39], and hypoglycemia [22, 29, 32, 35]. Diabetic ketoacidosis or hyperosmotic diabetic coma was reported in four cases [3, 8, 18], and there were three cases each of cardiac events [25, 28, 33], obstetric-related events [13, 21, 36], and trauma [15, 37, 44] or following use of radioactive iodine [3, 35, 41]. Confusion or delirium was reported to precede coma in 38 of 57 cases (67%).
Precipitants of TS-related coma varied, commonly including possible infection. Most cases of coma were preceded by delirium or confusion.
C. Systemic Manifestations of TS During Coma
The most common systemic findings associated with the TS-related coma state were tachycardia (88%) and fever (83%). Cardiovascular abnormalities, including heart failure, hypertension, and atrial fibrillation, were the second-most common events, followed by liver or kidney dysfunction (Table 2). Temperature ≥101°F was reported in 45 cases (69%), and temperature ≥104°F was seen in 18 cases. Heart rate >140 beats per minute was reported in 39 cases (60%). Comorbidities that can also affect the level of consciousness were present in some cases, including hypotension, hypoglycemia, or hepatic failure. Systemic findings associated with TS-related coma were not different between patients reported in case reports and those reported in case series.
The minimum BW score ranged from 30 to 120 points, with a mean (SD) score of 69 (19) points for cases with adequate detail to make the calculation. Not all reports described the thyrotoxic state with coma as a “TS” [9, 14, 15, 18, 23, 24, 29, 33, 44]. Calculation of the BW score of 13 such cases revealed a range of 30 to 110 points, with a mean (SD) score of 59 (23) points, despite having incomplete data for specific calculation of all BW categories in one of the reports (specifically, fever or tachycardia was present in all cases but not in sufficient detail to assign a BW score) [14].
In summary, systemic manifestations associated with TS-related coma were typical of severe hyperthyroidism.
D. Neurologic Findings Associated With Coma in TS
Only eight cases reported Glasgow [46] or other coma scales [15, 23, 27, 31, 32, 34, 37, 38]. In these cases, Glasgow coma scale mean and median values were 7.3 and 7.5, respectively, with a range of 3 to 12. Most reports did not provide sufficient detail for independent calculation of the Glasgow coma scale.
Positive Babinski sign was reported in five cases [20, 25, 30, 40, 42] and clonus in two [40, 42]. Seizure and stroke presented together in two patients [24], including one pediatric patient [35]. Four others had seizure alone [29], three of whom were pediatric patients [15, 45]. Three patients had a stroke [14, 20, 26]. None of these patients had known preexisting structural brain disease.
Neurologic testing in association with TS-related coma may have included electroencephalogram (EEG), lumbar puncture (LP), or brain CT or MRI. EEG was performed in 11 cases [11, 15, 20, 37], seven of which reported diffuse slowing [19, 30, 33, 38, 39, 42, 45]. LP was performed in 15 cases [11, 15, 19, 20, 22, 29, 30, 37, 38, 40, 42, 43, 45]. Low cerebrospinal fluid glucose level was reported in two cases (as 18 and 27 mg/dL) [22, 45].
Eleven patients had abnormal results from either head CT or brain MRI [20, 22, 24, 26, 32, 34, 35, 37], three of whom had a preexisting structural abnormality [25, 27, 30]. Head CT was reported in 18 cases and was read as normal in 11 cases (65%). Brain MRI was reported in only seven cases [33, 38] and had abnormal results in five, including reversible lesion of the splenium in the corpus callosum [37], diffuse atrophy [34], chronic lacunar infarcts in bilateral pons [30], and stroke [35]. There are no reports of functional MRI or fluorodeoxyglucose positron emission tomography in TS-related coma.
Two patients merit special comment because each had two bouts of coma, the second of whom had been under prolonged treatment after the TS. Harada et al. [32] reported a patient with a stroke on day 24 followed by a second bout of coma. Homma et al. [22] reported a patient with seizure and a second coma associated with posterior leukoencephalopathy syndrome diagnosed at 29 days, followed by death within 2 days.
In summary, only a small proportion of patients with TS-related coma had detailed descriptions of the coma or associated brain testing or imaging. When performed, EEG commonly showed diffuse slowing, and LP cerebrospinal fluid was mostly normal. Brain MRI was performed in too few patients to draw conclusions on TS-related coma associations.
E. Thyroid Tests With Coma and Recovery
At least one thyroid function test (TFT) was reported in 43 cases (Table 3). A range of TFTs was reported. Most cases reported either total T4 [13–15, 21, 24, 25, 37–43, 45] or free T4 [15, 16, 19, 20, 22–24, 26–28, 30, 31, 33–36, 47]. The highest blood levels were noted in the acute levothyroxine overdose cases, with total T4 level of 600 μg/dL in a patient with preexisting chronic renal failure, an outlier that highly influenced the mean total T4 and T3 values [14]. Cross-sectional analysis of available TFTs showed major reduction in mean total and free T4 and total T3 levels at awakening compared with coma onset. Only a few cases reported longitudinal TFTs both at onset of coma and at awakening in the same patient [15, 30, 31, 34, 38–40, 42, 45]. These cases showed substantial reduction in total and free T4 levels at the time of awakening from coma. Protein-bound iodine was reported in five cases [12, 13, 29] and high radioiodine uptake in six cases [12, 13, 18, 39].
Table 3.
Thyroid Function Tests at Coma Onset and at Awakening
| Test | Cross-Sectional | Mean [CI] | P | Long | Mean [CI] | P |
|---|---|---|---|---|---|---|
| Total T4, µ/dL | Coma onset (n = 23) | 77 [26, 128] | 0.0083 | 5 | 67 [−0.7, 135] | 0.0216 |
| Awakening (n = 5) | 15 [−94, 124] | 15 (−53, 82] | ||||
| Free T4, ng/dL | Coma onset (n = 18) | 5.8 [5.0, 6.5] | 0.0009 | 4 | 5.9 [4.0, 7.8] | 0.0432 |
| Awakening (n = 4) | 2.9 [1.5, 4.2] | 3.2 [1.5, 5.0] | ||||
| Total T3, ng/dL | Coma onset (n = 18) | 2295 [−4897, 5132] | 0.0020 | 4 | 376 [195, 557] | 0.1124 |
| Awakening (n = 4) | 118 [−347, 4939] | 110 [−71, 291] | ||||
| Free T3, pg/dL | Coma onset (n = 12) | 1547 [980, 2116] | 0.1390 | 3 | 1426 [−67.2, 2920] | 0.3493 |
| Awakening (n = 3) | 621 [−516, 1757] | 621 [−873, 2114] | ||||
| No TFTs | (n = 22) | |||||
Cross-sectional average thyroid hormone levels at coma onset and awakening. Most cases did not have measurement at both times. Long = mean of longitudinal TFTs performed in the same patient at coma onset and at awakening.
Abbreviation: CI, 95% confidence interval.
To summarize, available cross-sectional TFT data suggest an association between awakening and major improvement in total and free T4 and total T3 levels. Limited longitudinal data showed an association between awakening and improvement in only total and free T4 levels.
F. Death or Awakening From Coma
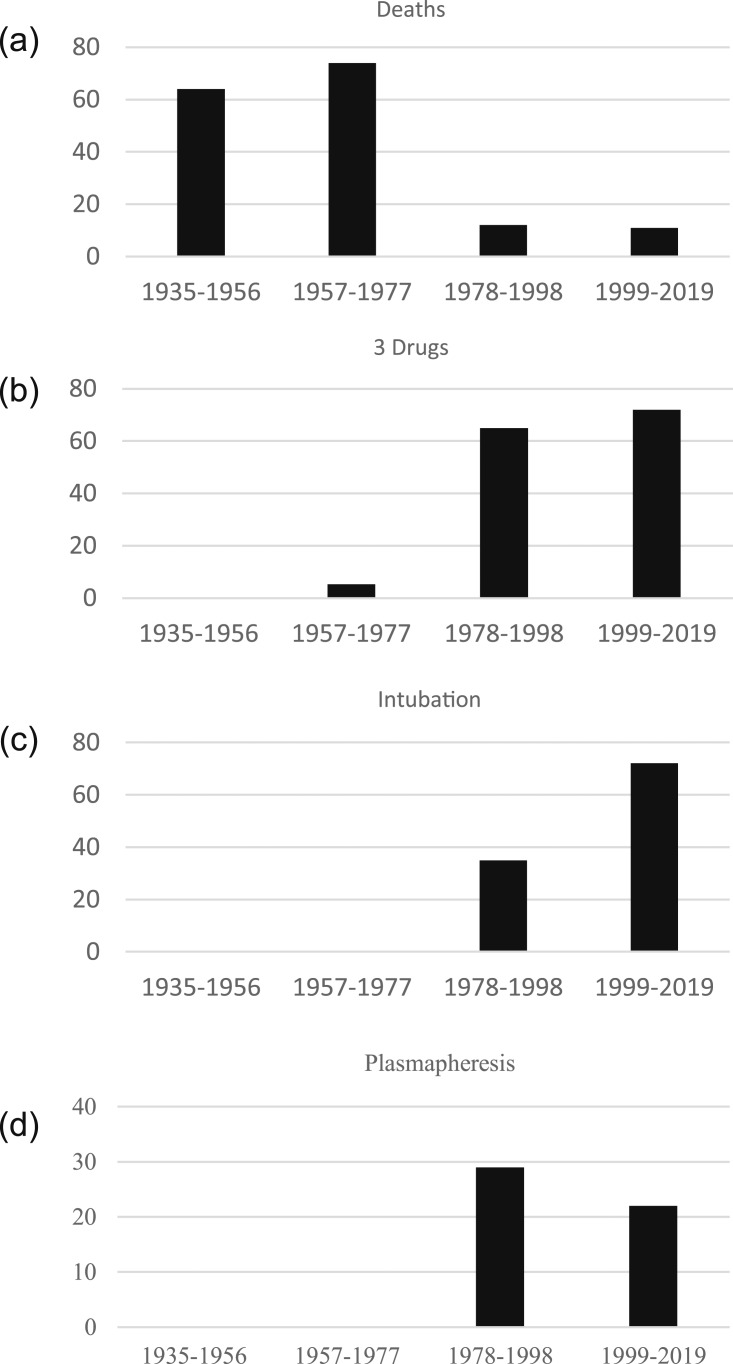
The overall death rate for the group was 38% (25/65). The death rate was 58% in the group extracted from the case series compared with 14% in the group of single cases. Death rates were significantly different over time, with lower mortality (11% to 12%) in the last two quartiles of the study report time (covering the period from 1978 to 2019) [Fig. 1(a)].
Figure 1.
Percentage of events associated with coma in the setting of thyroid storm as reported in the English-language literature from 1935 to 2019, divided by 21-y quartiles. (a) Deaths: 1935‒1956, 64% (7/11); 1957‒1977, 74% (14/19); 1978‒1998, 12% (2/17); 1999‒2019, 11% (2/18); P < 0.0001. Death rate: 1935‒1976, 70% (21/30) and 1978‒2019, 11% (4/35). (b) Three-drug therapy involving antithyroid drugs, corticosteroids, and beta-blockers: 1935‒1956, 0; 1957‒1977, 5.3% (1/19); 1978‒1998, 65% (11/17); 1999‒2019, 72% (13/18); P < 0.0001. (c) Intubation: 1935‒1956, 64% 0; 1957‒1977, 0; 1978‒1998, 35% (6/17); 1999‒2019, 72%; (13/18); P < 0.0001. (d) Plasmapheresis: 1935‒1956, 0; 1957‒1977, 0; 1978‒1998, 29% (5/17); 1999‒2019, 22% (4/18); P = 0.0874.
Table 4 shows the data for the 44 cases reporting details on the relationship between time of coma onset relative to days to awakening or death. Coma was present in 13 patients (30%) at hospital presentation [15, 17–19, 24, 27, 28, 35, 40, 42, 44, 45], in 17 (39%) within the first 2 days of hospitalization [8, 11, 14, 21–23, 25, 26, 29, 30, 33, 37, 41, 43], and in 14 (32%) from day 3 or later of hospitalization. For those presenting with coma, three were found unconscious [40, 42, 45] and one had a witnessed collapse [19]. Of the 17 patients who developed coma within the first 2 days of hospitalization, many were described as developing the condition rapidly or over minutes [8, 21, 29] to hours [8, 11, 25, 26, 30, 41, 43]. Eight percent (1/13) of patients who presented with coma, 24% (4/17) who developed coma within 2 days of hospitalization, and 43% (6/14) who developed coma on day 3 of hospitalization or later died (P = 0.1138).
Table 4.
Relationship of Coma Presentation Time to Awakening and Death
| Coma Onset Relative to Hospital Presentation | Group, n (% Total) | Survived Coma Days to Awakening n (% of Presentation Group) | Died, n (%) | Coma Days to Death, n |
|---|---|---|---|---|
| Coma at presentation [15, 17–19, 24, 27, 28, 35, 40, 42, 44, 45] | 13 (30) | N = 12 (92) | 1 (7.7) | <1 d: 1 |
| <1 d: 3 (25) | ||||
| 1–2 d: 6 (50) | ||||
| >2 d: 1 (8) | ||||
| No data: 2 (17) | ||||
| Coma onset day 1 or 2 of/after hospitalization [8, 11, 14, 21, 23, 25, 26, 29, 30, 33, 37, 39, 41, 43] | 17 (39) | N = 13 total (76)a | 4 (24)a | 2 d:1a |
| <1 d: 3 (23) | 5 d: 1 | |||
| 1–2 d:1 (8) | >14 d:1 | |||
| >2 d: 8 (62) | Unclear:1 | |||
| No data: 1 (8) | ||||
| Coma onset day 3 or later after hospitalization [9–12, 14, 20, 32, 34, 36, 38] | 14 (32) | N = 9 (57)b | 6 (43)b | <1 d: 3 |
| <1 d: 1 | 1–2 d: 2 | |||
| 1–2 d: 1 | >14 d: 1b | |||
| >2 d: 7 | ||||
| Total | 44 (100) | 34 (77) | 11 (25) |
P = 0.1138 for death by coma day of presentation.
The patient had coma day 1, recovered, and then had recurrent coma associated with posterior leukoencephalopathy syndrome nearly 1 mo later, with death day 2 on the second episode [22]. This case is listed in both the awakening and death columns.
The patient awoke from coma but then died later from another cause [38]. This case is listed in both the awakening and death columns.
The duration from coma onset to death was not clearly reported in 12 of 25 deaths. For 10 cases, coma ended in death within <1 day in four cases (40%), 1 to 2 days in three cases (30%), 3 to 7 days in 1 case (10%), and >14 days in two cases (20%). One death followed resolution of coma [38], and one death followed a second bout of coma nearly 1 month after hospital presentation [22].
For the 40 patients who did not die and for whom there were data, the duration of coma to awakening was <1 day in seven patients (22%), 1 to 2 days in nine patients (22.5%), and >2 days in 18 patients. Specifically, the duration of coma to awakening was >14 days in five patients [14, 16, 20, 31, 37].
To summarize, both awakening and death occurred most commonly within the first 2 days of coma onset. Mortality appeared to be higher in patients who developed coma after hospitalization than in those who presented with coma, though this did not achieve statistical significance.
G. Treatment of TS With Coma
Data on the cases for which treatment information is available are shown on Table 5. No treatment information was available on 12 cases. The most common forms of treatment were antithyroid drugs (68% of cases), corticosteroids (64% of cases), beta-blockers (64% of cases), and iodine (60% of cases). All four drug classes were used in 29% of cases, and all three drug classes except iodine were used in 38% of cases. Death rates were lower in patients who were treated with antithyroid drugs, corticosteroids, beta-blockers, all three of these drugs, all four drugs, and intubation. Treatment with iodine alone was not associated with improved outcome related to death. Intubation was reported in 26% of cases, all after 1978.
Table 5.
Treatment Given for Thyroid Storm and Coma
| Treatment,n (% of Population) | Death,n (% of Treatment Group) | Death % of Without Treatment Group (n) | P | |
|---|---|---|---|---|
| Antithyroid drug | 36 (68) | 6 (17) | 52% (11/21) | 0.0070 |
| Corticosteroid | 34 (64) | 4 (12) | 52% (11/21) | 0.0017 |
| Beta-blocker | 34 (64) | 4 (12) | 52% (11/21) | 0.0017 |
| Three drugs | 25 (47) | 3 (12) | 55% (22/40) | 0.0006 |
| Iodine | 32 (60) | 8 (25) | 30% (7/23) | 0.7621 |
| Four drugs | 19 (36) | 2 (11) | 50% (23/46) | 0.0043 |
| Intubation during coma | 19 (26) | 3 (16) | 48% (22/46) | 0.0239 |
| Antibiotic | 14 (26) | 4 (29) | 21% (6/28) | 0.7071 |
| Plasmapheresis | 9 (17) | 1 (11) | 32% (15/47) | 0.4210 |
| Thyroidectomy | 9 (17) | 1 (11) | 33% (16/48) | 0.2540 |
| Radioiodine | 4 (7.5) | 1 (25) | 29% (15/52) | 1.000 |
| Dialysis | 3 (5.7) | 1 (33) | 38% (23/61) | 1.000 |
| l-carnitine | 2 (3.8) | 1 (50) | 29% (15/51) | 0.5167 |
| Other | 17 (32) | 6 (35) | 23% (3/13) | 0.6908 |
Subgroup analysis of cases for which treatment information is available. Data are shown for all available cases and then further subdivided by those who died with the treatment vs the percentage of those who died without the treatment. “Three drugs” indicate the patient received antithyroid drug, corticosteroid, and beta-blocker; “four drugs” indicates the patient received antithyroid drug, corticosteroid, beta-blocker, and iodine. The P statistic represents Fisher exact test for the presence or absence of the treatment vs the presence or absence of death.
One or more sessions of plasmapheresis were performed in nine cases [16, 23, 26, 38, 40, 41], including three cases with thyroid hormone overdose [14]. It was performed within 1 or 2 days of onset of coma in five cases, within 3 days in one, within 4 days in two, and within 10 days in one. Plasmapheresis was associated with awakening in six of nine cases (67%) in which it was used [14, 23, 38, 40, 41]. In four of the six cases, the plasmapheresis-attributed awakening was also associated with normalization of either T3 or T4 value. In one case, the TFTs were said to be normal the day after awakening. One patient who was treated with plasmapheresis after 10 days of coma and who had awakening associated with the treatment, later died, but not from TS [38]. Plasmapheresis was not associated with a reduced death rate (P = 0.4210) (Table 5). One patient was treated with whole blood exchange transfusion, which was also associated with awakening [13, 48].
To summarize, patients with TS-related coma treated with antithyroid drugs, corticosteroids, beta-blockers, and intubation had lower death rates. Plasmapheresis was performed in a limited number of patients, in whom it was often followed by awakening but not by lower death rates.
3. Discussion
This report, based on an analysis of aggregated English-literature case reports, describes the characteristics and treatment of 65 patients with coexistent TS and coma, the largest series to date on this specific population. Fifty-one percent of the patients (24/47) either died or awoke within 2 days of coma onset. The overall mortality rate of the group was 39%, lower for those described in case reports (14%) than for those described in case series (58%), probably because the case reports were from a later median time period than the case series. Specifically, the mortality rate was also lower during the last two quartiles (42 years) of the study period (1978 to 2019), when it was 12% and 11%, compared with 64% and 74% for the first two 21-year quartiles (1935 to 1977). Mortality appeared to be higher in patients who developed coma after hospitalization than in those who presented with coma, though this did not reach statistical significance. Factors associated with coma in TS were similar in cases extracted from individual reports or case series. Reduction in total and free T4 values, and possibly also total T3 level, correlated with awakening from coma. Death rates were lowest for patients treated with antithyroid drugs, corticosteroids, beta-blockers, and intubation. Plasmapheresis was credited with awakening in a high percentage of cases in which it was used, but it was not associated with lower death rates.
CNS manifestations are common in TS diagnostic criteria and associated scoring systems [1, 2]. In the BW system, coma or seizure is assigned the maximum CNS section of 30 points, where a total score of 45 or more points is highly suggestive of TS. In the Japanese TS scoring system, a score of 1 or higher on the Japan Coma scale (for which coma has a score of 100 to 300) or 14 or lower on the Glasgow Coma Scale is sufficient CNS manifestation to qualify as TS1 [2].
Coma may occur in the setting of critical illness without thyrotoxicosis, where it has a high associated mortality. In a prospective cohort study of adults who were admitted to 361 intensive care units (ICUs) and who received mechanical ventilation, coma was present in 17%, for whom it was associated with a 36% ICU mortality rate [49]. In a retrospective study of 2189 ICU patients with coma, overall 58% died, including 20% in the “metabolic brain dysfunction” group, for whom TS was not listed specifically as a cause of coma [50].
In contrast, coma was noted in 7.9% of 356 TS cases in a Japanese survey and in 7.4% of 1324 TS cases in a Japanese nationwide database [2, 5]. Review of 16 TS series (Table 6) showed a wide range of individual study rates of coma in TS, death in TS, and death in patients with TS-related coma. Combining the data from 1922 patients with TS, 294 died (15%), 164 had coma (8.5%), and 61 of 164 patients with TS-related coma (37%) died [3–5, 10, 12–14, 16, 51–57]. As shown in Table 6, these results are heavily influenced by the two large Japanese cohorts compared with all other studies, which had 36 or fewer cases. Excluding the data from the two large cohorts, 122 of 242 patients (50%) died, 38 of 242 patients (16%) had coma, and 23 of 38 patients with TS-related coma (61%) died. Comparison of death rates across the individual studies as well as the aggregate data might lead to the conclusion that the mortality rate is higher in patients with coma in TS than in the general population with TS.
Table 6.
Historical Rates of Coma and Death in Thyroid Storm
| TS (n) | TS With Coma (n) | TS Deaths (n) | Coma Deaths (n) | |
|---|---|---|---|---|
| 1934, Bayley [56] | 51 | Not specified | 51 | Not specified |
| 1935, Zondek [9] | 3 | 2 (67%) | 2 (67%) | 2 (100%) |
| 1944, Waldenstrom [10] | 10 | 4 (40%) | 3 (30%) | 3 (75%) |
| 1947 McArthur et al. [57] | 36 | 0 | 24 (67%) | 0 |
| 1951, Rives [51] | 25 | Not specified | 10 | Not specified |
| 1960 Waldstein et al. [52] | 21 | 4 (19%) | 6 (29%) | 2 (50%) |
| 1969, Nelson and Becker [12] | 21 | 8 (38%) | 16 (76%) | 8 (100%) |
| 1969, Mazzaferri and Skillman [3] | 20 | 4 (20%) | 4 (20%) | 3 (75%) |
| 1971 Roizen and Becker [53] | 8 | 6 (75%) | 3 (38%) | 3 (50%) |
| 1972 Ashkar et al. [13] | 6 | 3 (50%) | 3 (50%) | 2 (67%) |
| 1999, Weber et al. [54] | 4 | 0 | 0 | 0 |
| 2011, Muller et al. [16] | 3 | 1 (33%) | 0 | 0 |
| 2012, Akamizu et al. [22] | 356 | 28 (7.9%) | 38 (11%) | Not specified |
| 2015, Swee et al. [55] | 28 | 0 | 7 (25%) | 0 |
| 2015, Angell et al. [4] | 25 | 1 (4%) | 0 | Not specified |
| 2016, Ono et al. [5] | 1324 | 98 (7.4%) | 134 (10%) | 38 (39%) |
| Total | 1922 | 164 (8.5%) | 294 (15%) | 61 (37%) |
| Present study | 65 | 25(38%) |
n = number of patients, not n of coma episodes.
The 65 TS coma cases from the current report had a death rate (38%) similar to that of past TS series. However, the current results may provide important timeline discrimination that could not be seen in past reports. Specifically, death rates associated with TS-related coma have been lower in the last 42 years than before then, now in the 11% to 12% range, similar to the overall death rate of TS reported by Ono and Akamizu [2, 5] , covering dates starting in 2004. This same period was associated with higher use of combined treatment with antithyroid drugs, beta-blockers, and corticosteroids; intubation; and plasmapheresis [Fig. 1(b)‒1(d)].
Treatment of TS-related coma most commonly included antithyroid drugs, corticosteroids, and beta-blockers, and a high percentage of patients received drugs from all three classes, especially during the last 42 years (since 1978) of the study period. The death rate was lower in patients who received all of the three drug classes than in patients who did not receive them; it was not possible to separate out the effect strength of individual drug classes because a majority of patients who received any of the three drug classes also received all three drugs classes (up to 25 of 36 patients). In contrast, a statistically beneficial TS-related coma mortality effect of iodine or antibiotic treatment was not shown. The TS‒related coma death rate was lower in those who underwent intubation and mechanical respiratory support than in those who did not receive this treatment, demonstrating an unsurprising beneficial effect of modern day supportive medical care. Very small numbers of subjects received other treatments not associated with improved mortality, including dialysis, thyroidectomy, radioactive iodine, and l-carnitine.
A comment on the mortality rate timeline reported in this series on TS-related coma compared with that of a population with TS, relative to beta-blocker treatment, is merited. A relationship between the availability of propranolol and the outcome of TS was noted by Mazzaferri and Skillman [3] in their 1969 series involving 22 episodes of TS in 20 patients; they observed that before 1960, patients did not get adrenergic blockade, whereas all but one received adrenergic blockade after 1960. The TS mortality rate was 43% before the use of beta blockade and 7% after beta-blockers were added to the treatment regimen [3]. This contrasts with the current report, which showed a 74% TS-related coma death rate in the 21-year quartile extending to 1978, suggesting that beta blockade availability starting in 1964 was insufficient to significantly affect outcomes in TS-related coma.
No improvement in the death rate was seen with plasmapheresis in this series, in which only nine patients underwent the treatment; however, plasmapheresis was associated with awakening in six (67%). Others have also observed the association of awakening with plasmapheresis treatment [58]. Plasmapheresis has been recommended as treatment of TS-related coma on the basis of a few individual reports [16, 38, 48] and also for TS not clinically improving within 24 to 48 hours in the guidelines of the Japan Thyroid Association TS and American Society for Apheresis [6, 59].
What is it about TS that causes or increases susceptibility to coma? The time course of coma onset in patients with massive levothyroxine overdose may help answer this question [14]. Here, classic symptoms of thyrotoxicosis occurred within 3 days, but coma onset did not occur until 7 to 10 days after massive thyroid hormone ingestion, suggesting that the thyrotoxic state may take time to affect (i.e., deplete or accumulate) a process or substance related to brain function. Likewise, awakening from coma associated with reduction in high T4 levels may support the direct or indirect effect of high thyroid levels on brain function. This was demonstrated in a high percentage of patients treated with plasmapheresis, which may have also removed other CNS effectors (e.g., autoimmune, catecholamine, cytokine) beyond thyroid hormones. Systemic findings expected to affect brain function, including hypotension (12%) and hypoglycemia (8%), were noted in a minority of TS-related coma cases; once reversed, they should have resolved coma, but did not. Renal dysfunction requiring dialysis was present in only three patients (4.7%). Hepatic failure‒related encephalopathy was not typical of most reports describing abnormal liver tests. Most TS-related coma cases have not had a structural brain abnormality, or if they did, it was attributed to concomitant stroke. EEG most often showed diffuse slowing, a nonspecific finding typical of coma. Autopsy was performed in 16 of the 25 coma-related deaths reported here, but brain findings were specifically described in only three, two with cerebral edema [12] and one with “leptomeningitis chronica” [10]. Others have described focal brain edema in the limbic system, attributed to limbic encephalitis, associated with altered mental status and drowsiness followed by seizure in the setting of TS, but not with coma [60]. Finally, past CNS events may convey a susceptibility to TS-related coma or recurrent coma.
Other possible mechanisms may include alterations in brain hemodynamics, energy metabolism [61, 62], neurotransmitters [63], or brain network connectivity [64]. Relative to these mechanisms, no data specific to TS-related coma exist, but rather only hypothesis might be extrapolated from studies in hyperthyroid animals and humans.
The limitations of this study include the retrospective design and nonuniform and potentially incomplete information across cases. All parameters were as defined by the original reporting authors and may not have been consistent across the study. The denominator was less than the total n of the study cases for many of the evaluated parameters. It was impossible to ensure that the absence of a reported detail indicated the feature was not present, only that it was not reported. Time to awakening from coma was limited by descriptions that may have lacked details relative to the degree of mental status abnormality. The analysis does not include control groups of TS cases without coma or critical illness coma cases without TS. Nevertheless, this report demonstrates the utility of REDCap-indexed analysis of aggregated case reports as a rich source of primary human clinical data, providing a foundation upon which to build an evidence base [65].
4. Conclusions
Prognosis of TS-related coma remains poor but has improved with modern day treatments, including antithyroid drugs, beta-blockers, corticosteroids, and intubation. Both awakening and death occurred most commonly within the first 2 days of coma onset. Plasmapheresis was associated with awakening from coma in a high percentage of cases in which it was used. Current Japan Thyroid Association TS guidelines for the early use of plasmapheresis in unresolved TS are recommended and we also suggest its urgent use for TS-related coma and also at the onset of confusion or delirium to abort coma. Modern day neuroimaging investigations or treatments, which have been reported in other types of coma, have not yet been reported in TS-related coma. Future investigations or investigational treatments of TS-related coma may enhance understanding of coma-related mechanisms and target improved outcomes for this patient population; these interventions include fMRI or PET, therapeutic hypothermia, and more detailed neurologic autopsy, focusing on brain regions relevant to awakeness. Future case reports on TS-related coma should record longitudinal Glasgow coma scale subparameters, as well as details on duration of coma in response to specific therapies and thyroid hormone levels at awakening.
Acknowledgments
Financial Support: This research was supported by the National Institutes of Health’s National Center for Advancing Translational Sciences, grant no. UL1TR002494. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health’s National Center for Advancing Translational Sciences.
Disclosure Summary: The author has nothing to disclose.
Glossary
Abbreviations:
- BW
Burch-Wartofsky
- CNS
central nervous system
- EEG
electroencephalogram
- ICU
intensive care unit
- LP
lumbar puncture
- TFT
thyroid function test
- TS
thyroid storm
References and Notes
- 1. Burch HB, Wartofsky L. Life-threatening thyrotoxicosis: thyroid storm. Endocrinol Metab Clin North Am. 1993;22(2):263–277. [PubMed] [Google Scholar]
- 2. Akamizu T, Satoh T, Isozaki O, Suzuki A, Wakino S, Iburi T, Tsuboi K, Monden T, Kouki T, Otani H, Teramukai S, Uehara R, Nakamura Y, Nagai M, Mori M; Japan Thyroid Association. Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys. Thyroid. 2012;22(7):661–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mazzaferri EL, Skillman TG. Thyroid storm: a review of 22 episodes with special emphasis on the use of guanethidine. Arch Intern Med. 1969;124(6):684–690. [DOI] [PubMed] [Google Scholar]
- 4. Angell TE, Lechner MG, Nguyen CT, Salvato VL, Nicoloff JT, LoPresti JS. Clinical features and hospital outcomes in thyroid storm: a retrospective cohort study. J Clin Endocrinol Metab. 2015;100(2):451–459. [DOI] [PubMed] [Google Scholar]
- 5. Ono Y, Ono S, Yasunaga H, Matsui H, Fushimi K, Tanaka Y. Factors associated with mortality of thyroid storm: analysis using a national inpatient database in Japan. Medicine (Baltimore). 2016;95(7):e2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Satoh T, Isozaki O, Suzuki A, Wakino S, Iburi T, Tsuboi K, Kanamoto N, Otani H, Furukawa Y, Teramukai S, Akamizu T. 2016Guidelines for the management of thyroid storm from The Japan Thyroid Association and Japan Endocrine Society (first edition). Endocr J. 2016;63(12):1025–1064. [DOI] [PubMed] [Google Scholar]
- 7. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Root HF. Treatment of combined diabetic coma and acute hyperthyroidism (“thyroid storm”). Trans Am Clin Climatol Assoc. 1935;51:1–20. [PMC free article] [PubMed] [Google Scholar]
- 9. Zondek H. The different forms of Graves’ disease. In: The Diseases of the Endocrine Glands. Edward Arnold and Co; 1935:149–161.
- 10. Waldenström J. Acute thyrotoxic encephalo- or myopathy, its cause and treatment. Acta Med Scand. 1945;121(2-3):251–294. [Google Scholar]
- 11. Weaver JA, Jones A, Smith RA. Thyrotoxic coma (apathetic crisis). BMJ. 1956;1(4957):20–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nelson NC, Becker WF. Thyroid crisis: diagnosis and treatment. Ann Surg. 1969;170(2):263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ashkar FS, Miller R, Gilson AJ. Thyroid function and serum thyroxine in thyroid storm. South Med J. 1972;65(3):372–374. [DOI] [PubMed] [Google Scholar]
- 14. Binimelis J, Bassas L, Marruecos L, Rodriguez J, Domingo ML, Madoz P, Armengol S, Mangues MA, de Leiva A. Massive thyroxine intoxication: evaluation of plasma extraction. Intensive Care Med. 1987;13(1):33–38. [DOI] [PubMed] [Google Scholar]
- 15. Radetti G, Dordi B, Mengarda G, Biscaldi I, Larizza D, Severi F. Thyrotoxicosis presenting with seizures and coma in two children. Am J Dis Child. 1993;147(9):925–927. [DOI] [PubMed] [Google Scholar]
- 16. Muller C, Perrin P, Faller B, Richter S, Chantrel F. Role of plasma exchange in the thyroid storm. Ther Apher Dial. 2011;15(6):522–531. [DOI] [PubMed] [Google Scholar]
- 17. Troen P, Taymor RC, Goldberg BI. Thyroid crisis associated with diabetic coma. N Engl J Med. 1951;244(11):394–398. [DOI] [PubMed] [Google Scholar]
- 18. Singh I, Srivastava MC. Hyperglycemia, keto-acidosis and coma in a nondiabetic hyperthyroid patient. Metabolism. 1968;17(10):893–895. [DOI] [PubMed] [Google Scholar]
- 19. Gilbert RE, Thomas GW, Hope RN, Sasse AC, Seeman E, Doolan L, Jerums G. Coma and thyroid dysfunction. Anaesth Intensive Care. 1992;20(1):86–87. [DOI] [PubMed] [Google Scholar]
- 20. Page SR, Scott AR. Thyroid storm in a young woman resulting in bilateral basal ganglia infarction. Postgrad Med J. 1993;69(816):813–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pugh S, Lalwani K, Awal A. Thyroid storm as a cause of loss of consciousness following anaesthesia for emergency caesarean section. Anaesthesia. 1994;49(1):35–37. [DOI] [PubMed] [Google Scholar]
- 22. Homma M, Shimizu S, Ogata M, Yamada Y, Saito T, Yamamoto T. Hypoglycemic coma masquerading thyrotoxic storm. Intern Med. 1999;38(11):871–874. [DOI] [PubMed] [Google Scholar]
- 23. Enghofer M, Badenhoop K, Zeuzem S, Schmidt-Matthiesen A, Betz C, Encke A, Usadel KH. Fulminant hepatitis A in a patient with severe hyperthyroidism: rapid recovery from hepatic coma after plasmapheresis and total thyroidectomy. J Clin Endocrinol Metab. 2000;85(5):1765–1769. [DOI] [PubMed] [Google Scholar]
- 24. Rau CS, Lui CC, Liang CL, Chen HJ, Kuo YL, Chen WF. Superior sagittal sinus thrombosis induced by thyrotoxicosis: case report. J Neurosurg. 2001;94(1):130–132. [DOI] [PubMed] [Google Scholar]
- 25. Ghobrial MW, Ruby EB. Coma and thyroid storm in apathetic thyrotoxicosis. South Med J. 2002;95(5):552–554. [PubMed] [Google Scholar]
- 26. Kokuho T, Kuji T, Yasuda G, Umemura S. Thyroid storm-induced multiple organ failure relieved quickly by plasma exchange therapy. Ther Apher Dial. 2004;8(4):347–349. [DOI] [PubMed] [Google Scholar]
- 27. Trasciatti S, Prete C, Palummeri E, Foppiani L. Thyroid storm as precipitating factor in onset of coma in an elderly woman: case report and literature review. Aging Clin Exp Res. 2004;16(6):490–494. [DOI] [PubMed] [Google Scholar]
- 28. Iwańczuk W. [Myocardial infarction and shock associated with thyrotoxicosis]. Anestezjol Intens Ter. 2010;42(3):142–146. [PubMed] [Google Scholar]
- 29. Petheram IS. Letter: death in thyrotoxicosis. Lancet. 1973;302(7838):1151. [DOI] [PubMed] [Google Scholar]
- 30. Yang SPL, Wu PH, Tey BH, Tan CK. A patient with thyroid storm presenting with apathetic thyrotoxicosis and features of meningoencephalitis. Thyroid. 2011;21(6):675–678. [DOI] [PubMed] [Google Scholar]
- 31. Kimmoun A, Munagamage G, Dessalles N, Gerard A, Feillet F, Levy B. Unexpected awakening from comatose thyroid storm after a single intravenous injection of l-carnitine. Intensive Care Med. 2011;37(10):1716–1717. [DOI] [PubMed] [Google Scholar]
- 32. Harada Y, Akiyama H, Yoshimoto T, Urao Y, Ryuzaki M, Handa M. Thyroid storm with multiple organ failure, disseminated intravascular coagulation, and stroke with a normal serum FT3 level. Intern Med. 2012;51(17):2379–2383. [DOI] [PubMed] [Google Scholar]
- 33. Sato Y, Taki K, Honda Y, Takahashi S, Yoshimura A. Lithium toxicity precipitated by thyrotoxicosis due to silent thyroiditis: cardiac arrest, quadriplegia, and coma. Thyroid. 2013;23(6):766–770. [DOI] [PubMed] [Google Scholar]
- 34. Shimoda Y, Satoh T, Takahashi H, Katano-Toki A, Ozawa A, Tomaru T, Horiguchi N, Kaira K, Nishioka M, Shibusawa N, Hashimoto K, Wakino S, Mori M, Yamada M. A case of thyroid storm with a markedly elevated level of circulating soluble interleukin-2 receptor complicated by multiple organ failure and disseminated intravascular coagulation syndrome. Endocr J. 2014;61(7):691–696. [DOI] [PubMed] [Google Scholar]
- 35. Rohrs HJ III, Silverstein JH, Weinstein DA, Amdur RJ, Haller MJ. Thyroid storm following radioactive iodine (RAI) therapy for pediatric graves disease. Am J Case Rep. 2014;15:212–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kofinas JD, Kruczek A, Sample J, Eglinton GS. Thyroid storm-induced multi-organ failure in the setting of gestational trophoblastic disease. J Emerg Med. 2015;48(1):35–38. [DOI] [PubMed] [Google Scholar]
- 37. Namatame C, Sonoo T, Fukushima K, Naraba H, Hashimoto H, Nakamura K. A thyroid storm patient with protracted disturbance of consciousness and reversible lesion in the splenium of corpus callosum: a case report. Medicine (Baltimore). 2018;97(7):e9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pinto A, Drake T, Cayci Z, Burmeister LA. Thyroid storm with coma in a patient with metastatic thyroid carcinoma and Graves’ disease: won the battle but lost the war: AACE Clin Case Reports. 2019;5(1): e7–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Serri O, Gagnon RM, Goulet Y, Somma M. Coma secondary to apathetic thyrotoxicosis. Can Med Assoc J. 1978;119(6):605–607. [PMC free article] [PubMed] [Google Scholar]
- 40. Newcomer J, Haire W, Hartman CR. Coma and thyrotoxicosis. Ann Neurol. 1983;14(6):689–690. [DOI] [PubMed] [Google Scholar]
- 41. Tajiri J, Katsuya H, Kiyokawa T, Urata K, Okamoto K, Shimada T. Successful treatment of thyrotoxic crisis with plasma exchange. Crit Care Med. 1984;12(6):536–537. [DOI] [PubMed] [Google Scholar]
- 42. Laman DM, Berghout A, Endtz LJ, van der Vijver JC, Wattendorff AR. Thyroid crisis presenting as coma. Clin Neurol Neurosurg. 1984;86(4):295–298. [DOI] [PubMed] [Google Scholar]
- 43. Anaissie E, Tohmé JF. Reserpine in propranolol-resistant thyroid storm. Arch Intern Med. 1985;145(12):2248–2249. [PubMed] [Google Scholar]
- 44. Howton JC. Thyroid storm presenting as coma. Ann Emerg Med. 1988;17(4):343–345. [DOI] [PubMed] [Google Scholar]
- 45. Aiello DP, DuPlessis AJ, Pattishall EG III, Kulin HE. Thyroid storm: presenting with coma and seizures in a 3-year-old girl. Clin Pediatr (Phila). 1989;28(12):571–574. [DOI] [PubMed] [Google Scholar]
- 46. Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;304(7872):81–84. [DOI] [PubMed] [Google Scholar]
- 47. Hull K, Horenstein R, Naglieri R, Munir K, Ghany M, Celi FS. Two cases of thyroid storm-associated cholestatic jaundice. Endocr Pract. 2007;13(5):476–480. [DOI] [PubMed] [Google Scholar]
- 48. Ashkar FS, Katims RB, Smoak WM III, Gilson AJ. Thyroid storm treatment with blood exchange and plasmapheresis. JAMA. 1970;214(7):1275–1279. [PubMed] [Google Scholar]
- 49. Esteban A, Anzueto A, Frutos F, Alía I, Brochard L, Stewart TE, Benito S, Epstein SK, Apezteguía C, Nightingale P, Arroliga AC, Tobin MJ; Mechanical Ventilation International Study Group. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA. 2002;287(3):345–355. [DOI] [PubMed] [Google Scholar]
- 50. Weiss N, Regard L, Vidal C, Luque Y, Taldir G, Vallet H, Diehl JL, Fagon JY, Guerot E. Causes of coma and their evolution in the medical intensive care unit. J Neurol. 2012;259(7):1474–1477. [DOI] [PubMed] [Google Scholar]
- 51. Rives JD, Shepard RM. Thyroid crisis. Am Surg. 1951;17(5):406–418. [PubMed] [Google Scholar]
- 52. Waldstein SS, Slodki SJ, Kaganiec GI, Bronsky D. A clinical study of thyroid storm. Ann Intern Med. 1960;52(3):626–642. [Google Scholar]
- 53. Roizen M, Becker CE. Thyroid storm: a review of cases at University of California, San Francisco. Calif Med. 1971;115(4):5–9. [PMC free article] [PubMed] [Google Scholar]
- 54. Weber C, Scholz GH, Lamesch P, Paschke R. Thyroidectomy in iodine induced thyrotoxic storm. Exp Clin Endocrinol Diabetes. 1999;107(7):468–472. [DOI] [PubMed] [Google Scholar]
- 55. Swee S, Chng CL, Lim A. Clinical characteristics and outcome of thyroid storm: a case series and review of neuropsychiatric derangements in thyrotoxicosis. Endocr Pract. 2015;21(2):182–189. [DOI] [PubMed] [Google Scholar]
- 56. Bayley R. Thyroid crisis. Surg Gynecol Obstet. 1934;59:41–47. [Google Scholar]
- 57. McArthur JW, Rawson RW, Means JH, Cope O. Thyrotoxic crisis: an analysis of the thirty-six cases at the Massachusetts General Hospital during the past twenty-five years. J Am Med Assoc. 1947;134(10):868–874. [DOI] [PubMed] [Google Scholar]
- 58. Horn K, Brehm G, Habermann J, Pickardt CR, Scriba PC. Successful treatment of thyroid storm by continuous plasmapheresis with a blood-cell separator [in German]. Klin Wochenschr. 1976;54(20):983–986. [DOI] [PubMed] [Google Scholar]
- 59. Schwartz J, Padmanabhan A, Aqui N, Balogun RA, Connelly-Smith L, Delaney M, Dunbar NM, Witt V, Wu Y, Shaz BH. Guidelines on the use of therapeutic apheresis in clinical practice‒evidence-based approach from the Writing Committee of the American Society for Apheresis: the seventh special issue. J Clin Apher. 2016;31(3):149–162. [DOI] [PubMed] [Google Scholar]
- 60. Miyazaki Y, Fukuoka K, Murase T, Yamamori I, Mano K. Abnormal MRI and EEG findings in thyroid storm resulting from Graves’ disease. Thyroid. 2008;18(10):1131–1132. [DOI] [PubMed] [Google Scholar]
- 61. Schreckenberger MF, Egle UT, Drecker S, Buchholz HG, Weber MM, Bartenstein P, Kahaly GJ. Positron emission tomography reveals correlations between brain metabolism and mood changes in hyperthyroidism. J Clin Endocrinol Metab. 2006;91(12):4786–4791. [DOI] [PubMed] [Google Scholar]
- 62. Miao Q, Zhang S, Guan YH, Ye HY, Zhang ZY, Zhang QY, Xue RD, Zeng MF, Zuo CT, Li YM. Reversible changes in brain glucose metabolism following thyroid function normalization in hyperthyroidism. AJNR Am J Neuroradiol. 2011;32(6):1034–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang Q, Bai Z, Gong Y, Liu X, Dai X, Wang S, Liu F. Monitoring glutamate levels in the posterior cingulate cortex of thyroid dysfunction patients with TE-averaged PRESS at 3T. Magn Reson Imaging. 2015;33(6):774–778. [DOI] [PubMed] [Google Scholar]
- 64. Liu B, Wen L, Ran Q, Zhang S, Hu J, Gong M, Zhang D. Dysregulation within the salience network and default mode network in hyperthyroid patients: a follow-up resting-state functional MRI study [published online ahead of print 26 September 2018]. Brain Imaging Behav. doi: 10.1007/s11682-018-9961-6. [DOI] [PubMed] [Google Scholar]
- 65. Jackson D, Daly J, Saltman DC. Aggregating case reports: a way for the future of evidence-based health care? Clin Case Rep. 2014;2(2):23–24. [DOI] [PMC free article] [PubMed] [Google Scholar]